Abstract
Background
Insomnia is common in the elderly, and is associated with chronic disease, but use of hypnotics increases the incidence of falls. Montmorency tart cherry juice has improved insomnia by self-report questionnaire.
Study Question
Is insomnia confirmed by polysomnography, and is tryptophan availability a potential mechanism for treating insomnia?
Study Design
A placebo-controlled, balanced, crossover study with subjects >50 years of age and insomnia were randomized to placebo (2 weeks) or cherry juice (2 weeks) (240ml 2 times/day) separated by a 2-week washout.
Measures and Outcomes
Sleep was evaluated by polysomnography and 5 validated questionnaires. Serum indoleamine 2, 3-dioxygenase (IDO), the kynurenine to tryptophan ratio, and prostaglandin E2 (PGE-2) were measured. In vitro, Caco-2 cells were stimulated with interferon-gamma (IFN-γ) and the ability of cherry juice procyanidin to inhibit IDO which degrades tryptophan and stimulates inflammation was measured. The content of procyanidin B-2 and other major anthocyanins in cherry juice were determined.
Results
Eleven subjects were randomized, 3 with sleep apnea were excluded and referred. The 8 completers with insomnia increased sleep time by 84 minutes on polysomnography (p=0.0182) and sleep efficiency increased on the Pittsburgh Sleep Quality Index (p=0.03). Other questionnaires showed no significant differences. The serum kynurenine to tryptophan ratio decreased, as did the level of prostaglandin E2 (both p<0.05). In vitro, cherry juice procyanidin B-2 dose-dependently inhibited IDO.
Conclusions
Cherry juice increased sleep time and sleep efficiency. Cherry juice procyanidin B-2 inhibited IDO, increased tryptophan availability, reduced inflammation and may be partially responsible for improvement in insomnia.
Keywords: Procyanidin, Indoleamine 2, 3-dioxygenase, Montmorency tart cherry juice, Sleep, Tryptophan, Kynurenine
INTRODUCTION
Insomnia is a common condition in the elderly and is associated with reduced quality of life and adverse outcomes. In a survey, 23% to 34% of 9,000 adults over 65 years of age complained of insomnia.1 Insomnia is associated with increased prevalence of medical disorders including hypertension,2 type-2 diabetes,3 exacerbation of chronic pain4 and a decline in cognitive function.5 Cognitive behavioral therapy is not always effective for chronic primary insomnia, and the short-term use of hypnotics increases the risk of falls in the elderly by over 4-fold.6 Therefore, it is important to identify treatments for insomnia without apparent side effects.
Montmorency (Prunus cerasus) tart cherry juice has been reported to have a positive effect on insomnia in elderly people as measured by the Insomnia Severity Index. The biggest effect seen was on the “waking after sleep onset” subscale.7 Although the authors stated that the mechanism for the beneficial effect of tart cherry juice on insomnia was unknown, they suggested that melatonin contained in the cherries could be responsible. The effective dose of the cherry juice was juice derived from 100g of cherries. The amount of melatonin in the dose of cherry juice used in the study was equivalent to 0.135 μg, and the dose of melatonin recommended for sleep is 0.5 to 5 mg.8 Thus, it would appear that the effect of tart cherry juice on sleep is due to more than its melatonin content.
Tryptophan, a precursor of serotonin, reduces sleep latency in humans at doses of 1.2 to 2.4 grams.9 Since tart cherries contain only 9 mg of tryptophan per 100 grams, one might presume that this small amount of tryptophan could not impact insomnia. Tryptophan degradation, however, parallels and predicts insomnia.10–12 Tryptophan is degraded by the enzyme indoleamine 2, 3 deoxygenase (IDO) to produce kynurenine. Indoleamine 2, 3 deoxygenase is stimulated by inflammation, and inhibition of IDO not only increases serotonin and improves mood, but also decreases inflammation.13–14. Since tryptophan is degraded into kynurenine, the ratio of kynurenine to tryptophan is a measure of tryptophan degradation, a lower value suggesting decreased tryptophan degradation.
Tart cherry juice contains 0.2% procyanidins.15 Since procyanidins can be detected in human serum 2 h after ingestion,16 we hypothesized that tart cherry juice standardized to a specific procyanidin content would decrease the ratio of kynurenine to tryptophan and contribute to the treatment of insomnia.
MATERIALS AND METHODS
Clinical Trial
A randomized, double-blind, placebo controlled clinical trial was conducted to test the effectiveness of tart cherry juice of known procyanidin content as a treatment for insomnia. This study was approved by the Institutional Review Board of Pennington Biomedical Research Center and registered on ClinicalTrials.gov under NCT01669317. Eleven healthy male or female subjects (age ≥50 years) with chronic insomnia and a usual bedtime between 9 p.m. and midnight were included in this study. Insomnia was defined as trouble sleeping on average more than 3 nights per week, with an Insomnia Severity Index score greater than or equal to 10 and meeting the International Classification of Sleep Disorders – 2 (ICSD-2) criteria for insomnia.17–18 ICSD-2 defines insomnia as a complaint of difficulty initiating sleep, difficulty maintaining sleep, waking up too early and sleep that is chronically non-restorative or poor in quality, despite adequate opportunity for sleep and circumstances conducive to sleep. Insomnia includes at least one of the following daytime complaints related to the sleep difficulty: fatigue or malaise; poor attention, concentration or memory impairment; social vocational dysfunction or poor school performance; mood disturbances or irritability; daytime sleepiness; motivation, energy or initiative reduction; proneness for errors or accidents at work or while driving; tension, headaches, or gastrointestinal symptoms in response to sleep loss; or concerns or worries about sleep. Subjects who had diabetes mellitus, were taking sedating or hypnotic medications or were taking chronic medication without a stable dose for one month or longer were excluded.
Subjects fasted for 10 hours except for water prior to screening, and blood was drawn for glucose, creatinine, potassium, uric acid, albumin, calcium, magnesium, creatine phosphokinase, alanine-leucine transaminase, alkaline phosphatase, iron, cholesterol, triglycerides, high density lipoprotein (HDL) cholesterol, and low density lipoprotein (LDL) cholesterol. A health questionnaire and five validated questionnaires were completed (Insomnia Severity Index, the Epworth Sleepiness Scale, the Pittsburgh Sleep Quality Index, the Beck Depression Inventory and the State-Trait Anxiety Inventory).19–22
Subjects who passed screening drank 240ml of tart cherry juice containing a measured level of procyanidin (Table 4) or placebo juice in the morning and 1 to 2 hours before bedtime for 14 days. All cherry juice was from the same batch and was analyzed for its content of phenolic compounds. The placebo juice was made of vapor-distilled water, fructose, dextrose, lemon powder and looked and tasted like cherry juice. After two weeks of cherry juice or placebo, subjects had an over-night polysomnographic sleep study, and blood was drawn that evening for measurement of the kynurenine to tryptophan ratio to evaluate tryptophan degradation, and prostaglandin E-2 (PGE-2) to evaluate inflammation. Upon waking after the sleep study, the 5 validated questionnaires were repeated and subjects were questioned about any adverse events. After a two week washout period, the subjects were crossed-over to the tart cherry juice or the placebo they did not take in the first two-week testing period, and the two-week testing period was repeated. The order of the cherry juice was balanced and assigned randomly.
Table 4.
Contents of procyanidin B-2 and major anthocyanins in Indian Summer® tart cherry juice
| Major compounds in cherry juice | Content (μg/ml) |
|---|---|
| Procyanidin B-2 | 451.56 |
| Cyanidin-3-0-glucosylrutinoside | 123.33 |
| Cyanidin-3-0-rutinoside | 20.26 |
| Cyanidin-3-0-glucoside | 3.51 |
Blood testing, serum preparation and analysis
Blood drawn for testing of free tryptophan, kynurenine and PGE-2 was frozen and stored at −80° C until analysis by high-performance liquid chromatography (HPLC) with ultraviolet (UV) and fluorescence detection. The ratio of kynurenine/tryptophan as an index of IDO activity was calculated using an Agilent 1100 series HPLC with a diode array detector and a fluorescence detector.23 The wavelength of the UV detector was set at 365 nm and fluorescence excitation was at 254 nm with detection at 404 nm. A 6-methyltryptophan internal standard was used, and the fluorescence conditions were set to excitation at 220 nm with detection at 354 nm.24 The stock solutions of tryptophan or kynurenine were 1 mmol L−1 in the mobile phase, and were prepared immediately before use. Serial dilutions of the serum samples were made to produce final (additional) concentrations of 6.25, 12.5, 25, 50, 75 and 100 μmol L−1 for tryptophan, and 0.0625, 0.125, 0.25, 0.75, 1.5 and 6.25 μmol L−1 for kynurenine. After addition of the internal standard, the medium was precipitated with perchloric acid (8% final acid concentration), centrifuged for 10 min at 64 g in a refrigerated centrifuge and 20 μL of the clear supernatant was used for testing. PGE-2 levels in sera before and after cherry juice consumption were determined by enzyme-linked immunosorbent assay (ELISA) using a commercial kit from Peprotech (Rocky Hill, NJ).
Chemicals and Reagents
The Cherry Marketing Institute provided Indian Summer® cherry juice without Vitamin C which was stored at 20°C and used within three months. HPLC grade acetonitrile, methanol, and trifluoroacetic acid were purchased from Fisher Scientific (Fair Lawn, N.J.). C18-Sep-Pak cartridges were purchased from Waters (Milford, MA). Proanthocyanidin B-2 was purchased from Chromadex (Irvine, CA). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum, trypsin and phosphate buffered saline were obtained from Fisher Scientific (Pittsburgh, PA). Bio-Rad DC protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). An ELISA kit for prostaglandin E2 (PGE2) was purchased from Peprotech (Rick Hill, NJ). Polyvinylidene fluoride (PVDF) membranes and 4–12% Bis-Tris gel were obtained from Invitrogen (Carlsbad, CA). Bovine serum albumin (BSA), primary antibodies against IDO, nuclear factor kappa-light-chain-enhancer of activated B cells subunit (NF-κB-p65) and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Primary antibody against cyclooxygenase-2 (COX-2) was purchased from Cayman Chemical (Ann Arbor, MI). Peroxidase-conjugated secondary antibodies were obtained from Jackson Immunoresearch (West Grove, PA). X-ray film was purchased from Phoenix Research (Candler, NC).
Cell culture
Caco-2 colon cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA). The cells were propagated in Invitrogen minimum essential media (MEM) containing sodium bicarbonate, 15 mM Hepes, fetal bovine serum to a final concentration of 10%, Invitrogen Glutamax, and sodium pyruvate. All experiments were with 80–90% confluent cultures. All cells were propagated at 37°C in 5% CO2 in a humidified chamber.
In Vitro effect of proanthocyanidin-B2
Caco-2 (1.8 × 105 cells/well) colon cancer cells were stimulated with 10 ng/mL interferon-gamma (IFN-γ) in the absence or presence of 0–50 μM of procyanidin B-2 at 37°C, 5% CO2 for 24 h. The levels of IDO, NF-κB or COX-2 in the control and procyanidin-treated cells were evaluated by Western blot using anti-IDO, anti-NF-KB, or anti-COX-2 antibodies. Beta actin served as a loading control. The data are representative of three separate experiments.
Determination of the procyanidin B-2 content
The method of Kennedy25 was used to isolate and extract procyanidin-B2 from cherry juice. The method of Kim and Lee26 was used to determine the levels of procyanidin B-2 in cherry juice. Procyanidin B-2 standards were used for quantitative analysis of procyanidin B-2 in cherry juice. The method of Kim and Lee26 was used for the extraction, isolation and analysis of cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside or cyanidin-3-O-glucosylrutinoside in cherry juice. These anthocyanins and procyanidins were selected, because they represent the major anthocyanins and procyanidins present in tart cherries.27 Analyses of procyanidin B-2 or anthocyanins were performed using an Agilent 1100 HPLC equipped with a photodiode detector. Procyanidin B-2 was detected at 280 nm and anthocyanins were detected at 520 nm.
Statistical analysis
Since there were no polysomnographic studies of cherry juice upon which to base a power analysis, the clinical trial was designed as a pilot study to determine power for a larger, adequately powered study that would give statistically significant differences. The differences in the polysomnographic studies and questionnaires were compared by t-test for paired observations. Categorical variables were compared by chi-squared test.
Proanthocyanin-B2, Procyanidin-B2, anthocyanins, procyanins, IDO, NF-κB, COX-2, PGE-2, interferon-γ, tryptophan, kynureinie and the kynurenine to tryptophan ratio were analyzed using analysis of variance (ANOVA) or non-parametric equivalent of ANOVA. Comparisons between the active and the placebo conditions were made on days 35 and 70 using variance analysis with the baseline values on day 0 as a covariate. Probability (p) values < 0.05 were considered statistically significant.
RESULTS
Clinical Trial
Eleven subjects were randomized, but eight subjects completed both arms of the study and data relating to those eight subjects were analyzed. Three subjects had moderate to severe sleep apnea by polysomnography. They were eliminated from the analysis and referred for evaluation and treatment. The eight subjects who completed both arms of the study are described in Table 1. The polysomnographic results are presented in Table 2. The sleep time was extended in the cherry juice condition by 84 minutes (p=0.0182). The sleep efficiency improved in the cherry juice condition, but did not reach statistical significance. The results of the Pittsburgh Sleep Quality Index (PSQI) are presented in Table 3. The Habitual Sleep Efficiency improved (p=0.03), and the sleep duration also improved on the PSQI but did not reach statistical significance. There were no statistically significant differences on the Insomnia Severity Index, the Epworth Sleepiness scale, the Beck Depression Inventory II or the State-Trait Anxiety Inventory. There were no adverse events.
Table 1.
Demographics of the participants in the sleep study
| Number completed | 8 |
|
| |
| Gender | |
| Female | 5 |
| Male | 3 |
|
| |
| Race | |
| Caucasian | 5 |
| Black | 3 |
|
| |
| Age average | 68 |
| SD | 9.2 |
|
| |
| Height average (cm) | 167.4 |
| SD | 14.1 |
|
| |
| Weight average (kg) | 78.2 |
| SD | 11.3 |
|
| |
| SBP average (mmHg) | 124 |
| SD | 4.8 |
|
| |
| DBP average (mmHg)) | 76 |
| SD | 4.1 |
|
| |
| Resting HR average (bpm) | 65 |
| SD | 6.1 |
|
| |
| BMI average (kg/m2) | 28.1 |
| SD | 4.0 |
Table 2.
Polysomnography
| Difference (Cherry Juice – Placebo) | P value | |
|---|---|---|
| Number of awakenings | 0.63 ±6.09 | 0.78 |
| Wake time after sleep onset | −4.9 min ± 42.2 | 0.75 |
| Sleep onset latency | −0.56 min ± 28.38 | 0.96 |
| Total sleep time | 84 min ± 61.7 | 0.0182* |
| Sleep efficiency | 0.046 ± 0.09 | 0.19 |
| Stage REM latency | 32.56 min ± 68.38 | 0.22 |
Table 3.
Questionnaires
| Questionnaires | Difference (Cherry Juice – Placebo) | P value |
|---|---|---|
|
| ||
| Pittsburgh Sleep Quality Index | ||
| Habitual sleep efficiency | 0.5 ± 0.5 | 0.0331* |
| Sleep Duration | 0.125 ±0.083 | 0.6845 |
| Other Questions | NS | |
|
| ||
| Insomnia Severity Index | ||
| All Questions | NS | |
|
| ||
| Epworth Sleepiness Scale | ||
| All Questions | NS | |
|
| ||
| Beck Depression Inventory II | ||
| All Questions | NS | |
|
| ||
| State-Trait Anxiety Inventory | ||
| All Questions | NS | |
Serum kynurenine
The kynurenine to tryptophan ratio was reduced in the cherry juice condition (p<0.05) indicating an inhibition of IDO with a reduction in the degradation of tryptophan (Figure 2). The level of PGE-2, a marker of inflammation, was also dose-dependently reduced (p<0.05) (Figure 3).
Figure 2.
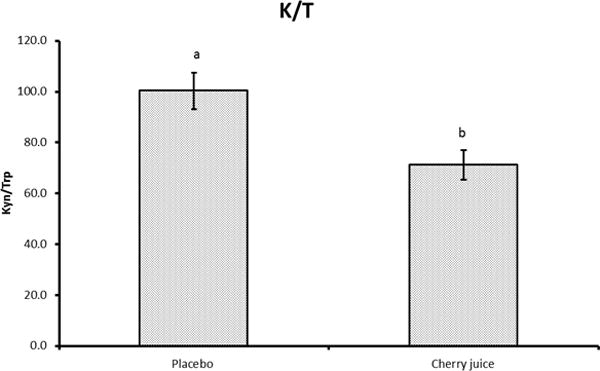
Serum Kyurenine to tryptophan ratio after placebo or cherry juice treatment. *p<0.05
Figure 3.
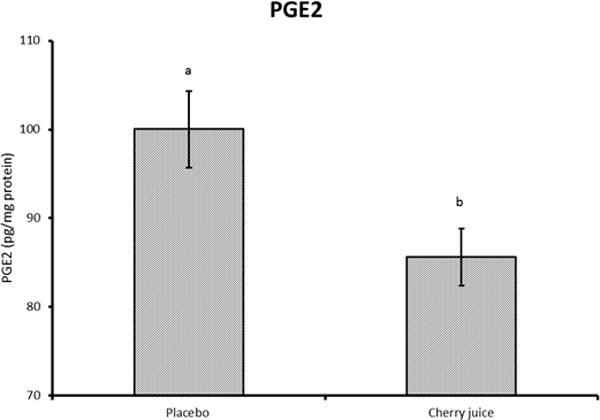
Serum PGE-2 levels after placebo or cherry juice treatments. *p<0.05
Procyanidin B-2 and anthocyanin contents of tart cherry juice
The tart cherry juice contained procyanidin B-2, cyanidin-3-O-glucosylrutinoside, cyaniding-3-O-glucoside and cyanidin-3-O-rutinoside (Table 4). The presence of these bioactives in cherries has been reported.15
Interaction of procyanidin B-2 and inflammatory biomarkers
The efficacy of procyanidin B-2 against IDO, was demonstrated by stimulating Caco-2 cells with IFN-γ. The IDO, NFK-B, and COX-2 levels in cancer cells decreased with progressively higher concentrations of procyanidin B-2. We found that procyanidin B-2 at 24 μM to 50 μM inhibited IFN-γ induced IDO in human Caco-2 colon cancer cell lines (Figure 1)
Figure 1.
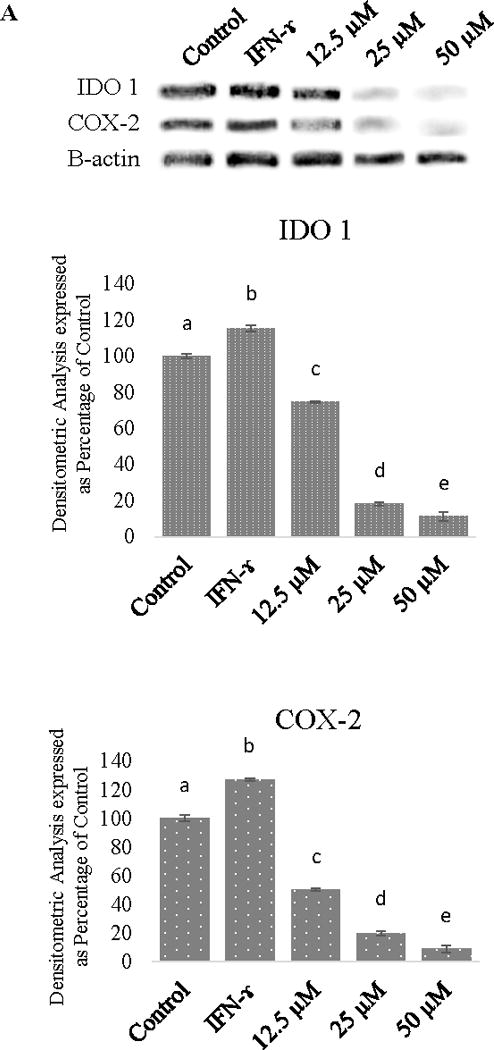
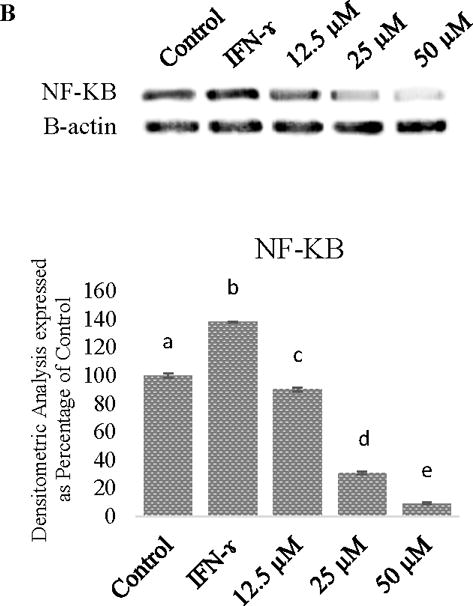
A. Relative induction of indoleamine 2,3-dioxygenase (IDO) and COX-2 in Caco-2 cells stimulated with IFN-γ and decrease of IDO and COX-2 in cells treated with procyanidin B-2. B. Relative induction of NF-KB in Caco-2 cells stimulated with IFN-γ. 1= control, 2= IFN-γ, 3= 12.5 μM procyanidin B-2, 4= 25 μM procyanidin B-2, and 5= 50 μM procyanidin B-2
DISCUSSION
Pigeon et al. performed a pilot cross-over study exploring the effect of tart cherry juice (240ml twice a day) or placebo over two weeks on insomnia in individuals ≥65 years of age with a 2-week washout period between crossover arms.7 They reported statistically significant improvement during the cherry juice consumption in the Insomnia Severity Index and a 62 minute improvement in waking after sleep onset. Our study design was patterned after that of Pigeon et al, but unlike that study, ours used formal polysomnography testing, the gold-standard method for evaluating sleep pathology. Sleep questionnaires used in the Pigeon et al. study represent a weakness, since they rely on self-report to evaluate a condition in which people are not conscious of their surroundings, and sleep questionnaires do not identify participants with sleep apnea. Sleep apnea is a mechanical condition that results in poor sleep quality, but would not be expected to improve without reversal of airway obstruction. Since 3 of 11 subjects in our study meeting the Pigeon et al. screening criteria had sleep apnea rather than insomnia, being able to identify and eliminate sleep apnea is important. Using polysomnography gives a more accurate assessment of sleep and allows selection of subjects with insomnia.
Howatson et al. evaluated sleep quality in 20 healthy exercising volunteers between the ages of 18 and 40 years (26±4.6, mean ± SD).24 They used actigraphy to access the endpoints and gave 30ml of tart cherry juice concentrate twice a day for 7 days or placebo in a cross-over trial with a 2-week washout period between study arms. The cherry juice condition had a statistically significant increase of time in bed (25 minutes), total sleep time (34 minutes) and sleep efficiency (5–6%). The urinary degradation product of melatonin increased statistically (~17%) in the cherry juice condition, but there was no change in the circadian rhythm of melatonin. Melatonin is postulated to treat insomnia, by restoring disturbed circadian rhythms. Cherry juice increased melatonin intake by 85μg/d while the melatonin dose shown to treat insomnia ranges between 0.5 and 5 mg/d, a dose that is 6- to 60-fold higher. Thus, the increase in melatonin may not be the major mechanism responsible for the improving sleep quality. The Howatson et al.24 study does, however, give support to the beneficial effects of tart cherry juice on sleep quality in a younger group of volunteers than those tested in the present study.
Our study included healthy volunteers that were ≥50 years of age. We eliminated three of 11 subjects with sleep apnea that were diagnosed by polysomnography, something that the studies by Pigeon et al. and Howatson et al. did not do. The polysomnography was done on only two nights and some acclimation to the procedure might have been desirable. This weakness should have been overcome by the balanced order of the sleep studies and the cross-over design of the trial. Although the prevalence of sleep apnea may have been lower in the younger subjects studied by Howatson, the subjects studied by Pigeon et al. were all ≥65 years of age. One strength of our study was its accuracy through the use of polysomnography, but since it was a small pilot study, many of the parameters we measured did not demonstrate a statistically significant change. This is a weakness that can be addressed by a larger future study with adequate power to detect the other endpoints. Despite the small size of our pilot study, we demonstrated a statistically significant 84 minute increase in sleep time by polysomnography and reduced plasma levels of kynurenine with increased tryptophan levels. The kynurenine tryptophan ratio (K/T) is the gold standard for determining IDO activity.28–29 The in vitro and in vivo results obtained suggest that the procyanidin B-2 in cherry juice is an inhibitor of IDO and part of the mechanism by which tart cherry juice improves sleep efficiency. These findings suggest that procyanidin B-2-rich cherry juice can improve tryptophan bioavailability for serotonin synthesis and might theoretically contribute to mood-enhancing effects, although this was not detected on the questionnaires evaluated in this study.30 These findings suggest that of all the procyanidins that reach the gastrointestinal tract, the oligomeric procyanidins are converted into dimeric procyanidins and absorbed. The polymeric procyanidins are not bioavailable. Because of the high content of procyanidin B-2 in the tart cherry juice used in this study, we used procyanidin B-2 as a standard for the study.
The other major phenolic compounds in tart cherries such as cyaniding-3-O-glucosylrutinoside are deglycosylated before absorption and metabolized into phenolic acids. We did not evaluate the effects of the phenolic acids on IDO. IFN-γ up regulates COX-2 and IDO and has been shown to correlate with increased production of PGE2 and kynurenine.31 IDO overexpression is linked to COX-2 expression.32 Inhibition of IDO is associated with COX-2 suppression.33 Our in vitro results showed inhibition of IDO and COX-2 (Figure 1) and our in vivo results showed inhibition of PGE2 (Figure 3).
We conclude that tart cherry juice is an effective treatment for insomnia. It has no adverse events as demonstrated in our study as well as in prior studies by others. Procyanidin B-2 is a likely active ingredient in tart cherry juice acting through plasma kynurenine reduction, tryptophan enhancement, and inhibition of IDO. Procyanidin B-2 may be an effective ingredient for improving insomnia. Thus, tart cherry juice and its active ingredients may offer a safe, yet effective, improvement in insomnia that will not increase the prevalence of falls or other side effects associated with hypnotic medications.
Acknowledgments
Sources of support: This trial was supported by the Cherry Marketing Institute which had nothing to do with the design of the trial. This work was partially supported by a NORC Center Grant # 2P30DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK. This work was supported in part by P50AT002776 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) of the National Institutes of Health which funds the Botanical Dietary Supplements Research Center of Pennington Biomedical Research Center and the Department of Plant Biology and Pathology in the School of Environmental and Biological Sciences (SEBS) of Rutgers University. This work was supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center.
Footnotes
Conflicts of interest: none
References
- 1.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–350. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 3.Vgontzas AN, Liao D, Bixler EO, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DJ, Mallory LJ, Lichstein KL, et al. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 5.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu TY, Chie WC, Yang RS, et al. Risk factors for single and recurrent falls: a prospective study of falls in community dwelling seniors without cognitive impairment. Prev Med. 2013;57:511–517. doi: 10.1016/j.ypmed.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Pigeon WR, Carr M, Gorman C, et al. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J Med Food. 2010;13:579–583. doi: 10.1089/jmf.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhardt S, Tan DX, Manchester LC, et al. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus) J Agric Food Chem. 2001;49:4898–4902. doi: 10.1021/jf010321+. [DOI] [PubMed] [Google Scholar]
- 9.George CF, Millar TW, Hanly PJ, et al. The effect of L-tryptophan on daytime sleep latency in normals: correlation with blood levels. Sleep. 1989;12:345–353. doi: 10.1093/sleep/12.4.345. [DOI] [PubMed] [Google Scholar]
- 10.Riemann D, Feige B, Hornyak M, et al. The tryptophan depletion test: impact on sleep in primary insomnia - a pilot study. Psychiatry Res. 2002;109:129–135. doi: 10.1016/s0165-1781(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 11.Arnulf I, Quintin P, Alvarez JC, et al. Mid-morning tryptophan depletion delays REM sleep onset in healthy subjects. Neuropsychopharmacology. 2002;27:843–851. doi: 10.1016/S0893-133X(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 12.Hudson C, Hudson SP, Hecht T, et al. Protein source tryptophan versus pharmaceutical grade tryptophan as an efficacious treatment for chronic insomnia. Nutr Neurosci. 2005;8:121–127. doi: 10.1080/10284150500069561. [DOI] [PubMed] [Google Scholar]
- 13.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 14.Dantzer R, O'Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou B, Bosak KN, Brickner PR, et al. Processed tart cherry products–comparative phytochemical content, in vitro antioxidant capacity and in vitro anti-inflammatory activity. J Food Sci. 2012;77:H105–112. doi: 10.1111/j.1750-3841.2012.02681.x. [DOI] [PubMed] [Google Scholar]
- 16.Sano A, Yamakoshi J, Tokutake S, et al. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67:1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- 17.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Gagnon C, Belanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701–710. doi: 10.3122/jabfm.2013.06.130064. [DOI] [PubMed] [Google Scholar]
- 19.Beaudreau SA, Spira AP, Stewart A, et al. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med. 2012;13:36–42. doi: 10.1016/j.sleep.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnau RC, Meagher MW, Norris MP, et al. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20:112. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 21.Quek K, Low W, Razack A, et al. Reliability and validity of the Spielberger State-Trait Anxiety Inventory (STAI) among urological patients: a Malaysian study. Med J Malaysia. 2004;59:258–267. [PubMed] [Google Scholar]
- 22.Kleinman L, Zodet M, Hakim Z, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Qual Life Res. 2000;9:499–508. doi: 10.1023/a:1008960710415. [DOI] [PubMed] [Google Scholar]
- 23.Vignau J, Jacquemont MC, Lefort A, et al. Simultaneous determination of tryptophan and kynurenine in serum by HPLC with UV and fluorescence detection. Biomed Chromatogr. 2004;18:872–874. doi: 10.1002/bmc.445. [DOI] [PubMed] [Google Scholar]
- 24.Howatson G, Bell PG, Tallent J, et al. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr. 2012;51:909–916. doi: 10.1007/s00394-011-0263-7. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy JA. Proanthocyanidins: Extraction, Purification, and determination of subunit composition by HPLC. John Wiley & Sons, Inc; 2002. pp. I1.4.1–I1.4.11. (Current Protocols in Food Analytical Chemistry). [Google Scholar]
- 26.Kim DO, Lee CY. Extraction and isolation of polyphenolics. John Wiley & Sons, Inc; 2002. pp. I1.2.1–I1.2.12. (Current Protocols in Food Analytical Chemistry). [Google Scholar]
- 27.Seymour EM, Lewis SK, Urcuyo-Llanes DE, et al. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food. 2009;12:935–942. doi: 10.1089/jmf.2008.0270. [DOI] [PubMed] [Google Scholar]
- 28.Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146–152. doi: 10.1097/MOG.0b013e32835c9cb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangge H, Stelzer I, Reininghaus EZ, et al. Disturbed tryptophan metabolism in cardiovascular disease. Curr Med Chem. 2014;21:1931–1937. doi: 10.2174/0929867321666140304105526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosinska A, Andlauer W. Cocoa polyphenols are absorbed in Caco-2 cell model of intestinal epithelium. Food Chem. 2012;135:999–1005. doi: 10.1016/j.foodchem.2012.05.101. [DOI] [PubMed] [Google Scholar]
- 31.Iachininoto MG, Nuzzolo ER, Bonanno G, et al. Cyclooxygenase-2 (COX-2) inhibition constrains indoleamine 2,3-dioxygenase 1 (IDO1) activity in acute myeloid leukaemia cells. Molecules. 2013;18:10132–10145. doi: 10.3390/molecules180910132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei J, Li MQ, Ding D, et al. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int J Clin Exp Pathol. 2013;6:431–444. [PMC free article] [PubMed] [Google Scholar]
- 33.Mei J, Jin LP, Ding D, et al. Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metalloproteinase-9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells. Mol Hum Reprod. 2012;18:467–476. doi: 10.1093/molehr/gas021. [DOI] [PubMed] [Google Scholar]


