Abstract
In this review, we focus on the role of orexin signaling in blood pressure control and its potential link to hypertension by summarizing evidence from several experimental animal models of hypertension. Studies using the spontaneously hypertensive rat (SHR) animal model of human essential hypertension show that pharmacological blockade of orexin receptors reduces blood pressure in SHRs but not in Wistar-Kyoto rats. In addition, increased activity of the orexin system contributes to elevated blood pressure and sympathetic nerve activity (SNA) in dark-active period Schlager hypertensive (BPH/2J) mice, another genetic model of neurogenic hypertension. Similar to these two models, Sprague-Dawley rats with stress-induced hypertension display an overactive central orexin system. Furthermore, upregulation of the orexin receptor 1 increases firing of hypothalamic paraventricular nucleus neurons, augments SNA, and contributes to hypertension in the obese Zucker rat, an animal model of obesity-related hypertension. Finally, we propose a hypothesis for the implication of the orexin system in salt-sensitive hypertension. All of this evidence, coupled with the important role of elevated SNA in increasing blood pressure, strongly suggests that hyperactivity of the orexin system contributes to hypertension.
Keywords: Orexin, Hypertension, Sympathetic Nerve Activity, Salt-Sensitive Hypertension
Introduction
Orexin A and orexin B are two neuropeptides (also known as hypocretin 1 and hypocretin 2) produced from the same precursor polypeptide (prepro-orexin) by proteolytic processing that were independently discovered in 1998 by two different groups (de Lecea et al. 1998; Sakurai et al. 1998). Two G-protein coupled receptors mediate the actions of these neuropeptides, the orexin receptor 1 (OX1R) and orexin receptor 2 (OX2R) (Sakurai et al. 1998). The cell bodies of orexin producing neurons are strictly localized in the hypothalamus, particularly in the lateral hypothalamic area, perifornical nucleus, dorsomedial hypothalamic nucleus, dorsal hypothalamic area, and posterior hypothalamic area in the rat brain (Date et al. 1999; Nambu et al. 1999; Peyron et al. 1998). However, these orexin-containing neurons have extensive projections throughout the entire brain including the cerebral cortex, thalamus, circumventricular organs, limbic system, and brainstem, as well as throughout the hypothalamus (Date et al. 1999; Nambu et al. 1999; Peyron et al. 1998). As expected from this extensive projection pattern, the orexin system contributes to the regulation of several physiological processes, which have been extensively reviewed elsewhere (de Lecea 2012; Sakurai 2007; Sakurai 2014; Tsujino and Sakurai 2009). Included in the range of functions that the orexins regulate is the central control of sympathetic nerve activity (SNA) and blood pressure. The anatomy of orexin producing neurons in relation to regions of the brain involved in SNA and blood pressure control as well as functional in vivo studies, which support that the orexin system participates in SNA and blood pressure regulation, have recently been reviewed (Carrive 2013; Li and Nattie 2014). These studies show that orexin-containing nerve terminals (Baldo et al. 2003; Ciriello and de Oliveira 2003; Ciriello et al. 2003; Nambu et al. 1999; Peyron et al. 1998; Shahid et al. 2012; Zheng et al. 2005) and receptors from in-situ hybridization studies (Lu et al. 2000; Marcus et al. 2001; Trivedi et al. 1998) and immunohistochemistry studies (Backberg et al. 2002; Cluderay et al. 2002; Hervieu et al. 2001) reside in many cardiovascular control regions of the brain. In addition, it has been shown that orexin-producing neurons also innervate as well as drive sympathetic preganglionic neurons in the spinal cord (Antunes et al. 2001; Date et al. 2000). Accumulating evidence indicates that hyperactivity of the orexin system contributes to several animal models of hypertension. In this review, we focus on the role of the orexin system in several animal models of hypertension studied to date as well as propose our hypothesis that the orexin system may be implicated in the Dahl animal model of salt-sensitive hypertension.
Orexin and the Spontaneous Hypertensive Rat (SHR) Model of Essential Hypertension
The majority of studies to date, examining the role of the orexin system in hypertension have used the SHR model, a commonly used animal model for human essential hypertension. Similar to essential hypertension in humans in which the development of hypertension is age-dependent, SHRs develop an age-dependent increase in blood pressure starting around 6 weeks (Andresen et al. 1980). Elevated SNA is a critical mechanism underlying the development and maintenance of hypertension in this model (Judy et al. 1976). Since the orexin system is involved in SNA and blood pressure regulation, initial studies sought to determine if alterations in the orexin system contribute to hypertension in this model via pharmacological blockade of orexin receptors or endogenous application of the orexins.
In one of the first studies linking orexin to hypertension, Li et al. showed that blocking orexin signaling by oral administration of the dual orexin receptor antagonist, almorexant, significantly decreased blood pressure and heart rate (HR) in SHRs but not Wistar Kyoto (WKY) rats in both wakefulness and sleep during dark and light periods of the diurnal cycle (Li et al. 2013). Although no studies to date have directly measured the SNA response to orexin receptor blockade in SHRs, Li et al. indirectly measured SNA in response to almorexant. They found that oral administration of almorexant significantly reduced the low-frequency and low-frequency/high-frequency ratio of systolic blood pressure in conscious SHRs in both wakefulness and sleep during dark and light periods of the diurnal cycle compared to pretreatment (Li et al. 2013). They also found that almorexant significantly decreased cerebrospinal fluid (CSF), noradrenaline, and both noradrenaline and adrenaline plasma levels in SHRs (Li et al. 2013). Taking all into account, this study suggests that overactivation of the orexin system contributes to the maintenance of hypertension in the SHR model potentially via increasing sympathetic vasomotor tone (Li et al. 2013).
Another study by Lee et al. corroborated these findings and additionally showed that overactivation of the orexin system in the brain contributes to hypertension in the SHR model (Lee et al. 2013). Instead of oral administration, this study administered centrally, via intracerebroventricular (ICV) injection, specific OX1R and OX2R antagonists. They found that only OX2R antagonist TCS-OX2-29 decreased mean arterial pressure (MAP) and HR in SHRs but not in WKY rats compared to vehicle control injection rats (Lee et al. 2013). Surprisingly, out of all the brain regions involved in SNA and blood pressure control tested in this study: hypothalamic paraventricular nucleus (PVN), dorsomedial hypothalamic area, perifornical hypothalamic area, rostral ventrolateral medulla (RVLM), and caudal nucleus tractus solitarius, only the RVLM had a significantly reduced total and transmembrane OX2R protein expression in SHRs compared to WKY rats (Lee et al. 2013). Subsequent OX2R blockade in the RVLM resulted in a significant reduction in MAP and HR in SHRs but not in WKY rats (Lee et al. 2013). Overall, this study suggests that the OX2R may play a more important role than the OX1R in the SHR model and that elevated OX2R activation in the RVLM contributes to the maintenance of hypertension in the SHR model.
Recently, Lee et al. further investigated the mechanisms responsible for elevated RVLM OX2R activation in SHRs (Lee et al. 2015). They found RVLM injection of orexin A (agonist for OX1R and OX2R) and OX2R specific agonist ALOXB resulted in a greater increase in MAP in SHRs compared to WKY rats (Lee et al. 2015). The neuronal nitric oxide synthase (nNOS) specific inhibitor 7-Nitro-indazole but not the inducible NOS (iNOS) inhibitor aminoguanidine significantly attenuated the pressor response to RVLM OX2R activation (Lee et al. 2015). This suggests that despite lower OX2R expression in the RVLM, increased pressor sensitivity of the OX2R mediated by nNOS signaling may underlie the enhanced OX2R activation supporting hypertension in SHRs.
Multiple studies have quantified either the number of orexin-containing neurons or orexin A and B levels in SHRs compared to WKY rats in order to further investigate the mechanisms that mediate orexin system overactivation in this model (Clifford et al. 2015; Li et al. 2016). Notably, Lee et al. reported that both 4 and 16 week-old SHRs had a greater total number of immunoreactive orexin A neurons in the hypothalamus than age-matched WKY rats (Lee et al. 2015). They also found the total number of RVLM projecting orexin A immunoreactive neurons to be significantly greater in 16 week-old SHRs compared to WKY rats (Lee et al. 2015). Immunoreactive orexin A neurons as well as RVLM projecting immunoreactive orexin A neurons were greater in 16 week-old SHRs compared to age-matched WKY rats in the dorsomedial hypothalamus and the perifornical hypothalamus, regions where the cell bodies of orexin-producing neurons are located (Lee et al. 2015). They also found that hypothalamus levels of orexin A and orexin B and RVLM levels of orexin A were significantly increased in SHRs compared to WKY rats (Lee et al. 2015). These results offer an additional explanation to the increased RVLM OX2R activation mediating hypertension in SHRs. Lee et al. suggests that more orexin containing neuron projections to the RVLM as well as higher levels of orexin A in the RVLM may cause enhanced OX2R activation (Lee et al. 2015). However, rather than the number of orexin-containing neurons being greater in SHRs compared to WKY rats, it is more likely that the existing neurons are producing orexins at a higher rate in SHRs so that more stain.
It has been demonstrated that the orexin system contributes to regulation of the CO2 chemoreflex (Li and Nattie 2014). In addition to a lower resting blood pressure, orexin knockout mice have a decreased respiratory hypercapnic chemoreflex (Kayaba et al. 2003; Nakamura et al. 2007). Antagonism of orexin receptors significantly decreases the CO2 chemoreflex in normal rats (Dias et al. 2010; Dias et al. 2009; Li and Nattie 2010). In addition, orexin neurons are sensitive to changes in CO2 and PH in vitro (Williams et al. 2007). The role of the orexin system in cardio-respiratory function as well as the anatomy of the orexin system in relation to regions of the brain involved in respiration and central chemoreception has recently been reviewed (Li and Nattie 2014). SHRs have an enhanced peripheral (Simms et al. 2009) as well as central chemoreflex (Li et al. 2016). Therefore, a recent study by Li et al. investigated the contribution of the overactive orexin system to the augmented CO2 chemoreflex and associated hypertension in SHRs (Li et al. 2016). They showed that the augmented CO2 chemoreflex and MAP in young and adult SHRs was significantly attenuated by oral administration of almorexant in wakefulness and sleep (Li et al. 2016). In adult SHRs the augmented pressor response to hypercapnia was significantly attenuated by almorexant treatment in wakefulness and non-rem sleep (Li et al. 2016). Also both young and adult SHRs had an increased number of orexin A immunoreactive neurons in the hypothalamus that were activated in response to hypercapnia than age-matched WKY rats (Li et al. 2016). These experiments demonstrate that overactivation of the orexin system also contributes to hypertension in SHRs via augmenting the CO2 chemoreflex.
Orexin and the Schlager High Blood Pressure Mouse (BPH/2J)
Similar to SHRs, elevated SNA is a mechanism that contributes to hypertension in the Schlager high blood pressure mouse (BPH/2J) (Davern et al. 2009; Jackson et al. 2013). Gene array studies showed that mRNA expression of prepro-orexin, which encodes the precursor orexin polypeptide, was increased in early and established phases of hypertension in BPH/2J mice compared to normotensive BPN/3J control mice (Marques et al. 2011b). Also mRNA expression of the prepro-orexin gene was greater in dark-active BPH/2J mice compared to BPH/2J light-inactive mice (Marques et al. 2011a). Given that hypertension is more pronounced in BPH/2J compared to BPN/3J control mice during the dark-active period (Jackson et al. 2016), a recent study investigated whether increased activity of the orexin system contributes to hypertension in BPH/2J mice (Jackson et al. 2016). They found that intraperitoneal injection of two doses (30 and 100 mg/Kg) and oral dose (300 mg/kg) of almorexant significantly reduced MAP, HR, and locomotor activity compared to vehicle control in BPH/2J mice but not in BPN/3J control mice in the dark-active phase (Jackson et al. 2016). Maximum decreases in MAP were −16 mmHg from 100 mg/Kg intraperitoneal injection and −11 mmHg from oral administration 300 mg/kg almorexant. However, they found that intraperitoneal administration of almorexant (100 mg/Kg) did not change MAP from baseline in BPH/2J or BPN/3J control mice during the light-inactive period (Jackson et al. 2016). Although not measured directly, indirect measurements of SNA were reduced in dark-active period BPH/2J mice but not in BPN/3J mice indicating the orexin system contributes to augmented sympathetic outflow in dark-active period BPH/2J mice (Jackson et al. 2016). Similar to the SHR model, Jackson et al. also found that BPH/2J mice had more neurons immunoreactive for orexin than BPN/3J mice in the lateral hypothalamus (Jackson et al. 2016). Overall, this study suggests that increased activity of the orexin system contributes to hypertension and increased SNA in dark-active period BPH/2J mice further establishing a role for the orexin system in neurogenic hypertension.
Orexin and Stress-Induced Hypertension
The orexin system is involved in cardiovascular responses to certain forms of stresses (Furlong et al. 2009; Johnson et al. 2010). It has also previously been shown that a stress-induced hypertension rat (SIHR) established by 14 days of foot shocks and loud noises in Sprague Dawley (SD) rats changes NOS (nitric oxide synthase) isotype expression in the RVLM, a key region that mediates the cardiovascular effects of orexin A in the brain (Chen et al. 2000). Since the NOS/nitric oxide (NO) system in the RVLM contributes to blood pressure regulation (Kishi 2013), Xiao et al. investigated whether orexin A is involved in stress-induced hypertension established in adult male SD rats with 14 consecutive days of foot shocks and loud noises by modulating the NOS/NO system in the RVLM (Xiao et al. 2013). They reported that the number of immunoreactive orexin A neurons in the lateral hypothalamic-perifornical area as well as the protein expression of OX1R in the RVLM was greater in SIHRs compared to control rats (Xiao et al. 2013). As mentioned above, rather than the number of orexin-containing neurons being greater in SIHRs compared to control rats, it is more likely that the existing neurons in SIHRs produce orexins at a higher rate. It was found that RVLM microinjection of orexin A resulted in a dose-dependent increase in systolic blood pressure and HR in SIHRs and control rats, but the increase was more pronounced in SIHRs (Xiao et al. 2013). OX1R or OX2R blockade with SB-408124 or TCS-OX2-29 partially attenuated the HR and systolic blood pressure response to RVLM orexin A microinjection in both SIHR and control rats and also lowered the elevated systolic blood pressure and HR of SIHRs (Xiao et al. 2013). RVLM inhibition of nNOS and guanylate cyclase attenuated the systolic blood pressure and HR response to RVLM injection of orexin A in both control and SIHRs (Xiao et al. 2013). This study suggests that the cardiovascular actions of orexin A in the RVLM of SIHRs is mediated by NO derived from nNOS and associated soluble guanylate cyclase pathway. These findings are consistent with the results from Lee et al. discussed above (Lee et al. 2015), which showed that enhanced OX2R-nNOS signaling in the RVLM contributes to hypertension in SHRs suggesting nNOS may be an important signal pathway for the orexin system in the RVLM in several forms of hypertension.
Orexin and Obesity-Related Hypertension
The orexin system may also be a link between obesity and hypertension as alterations in this system have also been implicated in the obese Zucker rat model of obesity-related hypertension. Augmented SNA contributes to hypertension in this model (Carlson et al. 2000), therefore orexin is a logical target given that it regulates SNA and energy homeostasis. Specifically, central administration of orexin stimulates feeding in rats (Sakurai et al. 1998). Furthermore, orexin neurons are sensitive to peripheral metabolic signals specifically they are inhibited by leptin and excited by ghrelin (Yamanaka et al. 2003). To investigate the contribution of the orexin system to obesity-induced hypertension, Zhou et al. first compared the protein levels of OX1R and OX2R in the PVN of Zucker obesity rats and their lean control rats using Western blot. They found that PVN OX1R expression was significantly greater in 15 week-old obese Zucker rats compared to age and sex-matched lean Zucker rats (Zhou et al. 2015), while no significant difference in OX2R expression was observed in the PVN between these two group of rats. Consistent with this result, they found that microinjection of the OX1R antagonist SB334867 into the PVN significantly decreased MAP and renal sympathetic nerve activity (RSNA) in obese Zucker rats but not in lean Zucker rats (Zhou et al. 2015). PVN OX2R blockade did not affect MAP or RSNA in obese Zucker rats or lean Zucker rats. Additionally, orexin A induced a greater increase in the firing rate of spinal cord projecting PVN neurons in obese Zucker rats compared to lean Zucker rats, which was attenuated by OX1R antagonist SB334867, but not OX2R antagonist TCS OX2 29 (Zhou et al. 2015). This study suggests that upregulation of the OX1R contributes to increased firing of PVN neurons and contributes to augmented SNA and hypertension in the obese Zucker rat model of obesity related hypertension.
Orexin and Salt-Sensitive Hypertension
Despite the importance of hyperactivity of the orexin system in several animal models of hypertension, there have been no studies to date linking the orexin system to salt-sensitive hypertension. Our recent preliminary data showed that OX1R expression is significantly increased in the Dahl salt-sensitive (Dahl S) rat with a high-salt diet compared to their cohorts with a normal diet. This observation coupled with the studies reviewed above which suggest that the orexin system contributes to neurogenic hypertension in several forms of hypertension have led us to hypothesize that the orexin system may be implicated in salt-dependent forms of hypertension where the central nervous system plays a contributing role via augmented SNA. In particular, we hypothesize that the orexin system may contribute to hypertension in the Dahl S rat, an animal model of salt-sensitive hypertension.
Studies have demonstrated that the central nervous system contributes to the Dahl model of salt-sensitive hypertension via elevations in sympathetic nervous system activity (Gordon et al. 1981; Takeshita et al. 1979). The PVN is a key regulator of SNA and its function is essential to maintain elevated blood pressure in Dahl S rats on a high-salt diet (Goto et al. 1981). It has previously been shown that orexin A and B have excitatory actions in the PVN by depolarizing both parvocellular and magnocellular neurons (Follwell and Ferguson 2002; Samson et al. 2002; Shirasaka et al. 2001). Therefore, we hypothesize the PVN may be a region where potential upregulation or enhanced sensitivity of components of the orexin system may contribute to the development or maintenance of high-salt induced hypertension in Dahl S rats via activation of the sympathetic nervous system.
Presently, it is unknown how a high-salt diet could activate the orexin system in Dahl S rats. There have been no studies that have directly determined if orexin-producing neurons are sensitive to changes in osmolality. Indirect evidence shows that prepro-orexin mRNA is upregulated in the hypothalamus of 48-hour water deprived rats (Kunii et al. 1999), which suggests that orexin producing neurons may be sensitive to changes in osmolality. In addition, the lamina terminalis, which is the location of the primary central osmoreceptors, is reported to have efferent projections to the lateral hypothalamus where orexin-producing neurons are located (Hahn and Swanson 2010; Hurley and Johnson 2014). Previous studies from other groups have demonstrated that a high-salt diet induces an increase in CSF Na+ concentration in Dahl S rats (Huang et al. 2004; Nakamura and Cowley 1989; Simchon et al. 1999), which may be due to an abnormal Na+ transport system in this rat strain (Amin et al. 2009). Therefore, we hypothesize that an increase in CSF Na+ in response to a high-salt diet may subsequently activate the orexin system either by directly activating orexin-producing neurons or indirectly through activation of central osmoreceptors in Dahl S rats. This may affect neuronal activity of parvocellular presympathetic neurons by either increased orexin release in the PVN, upregulation of orexin receptors, or enhanced sensitivity of orexin receptors resulting in activation of the sympathetic nervous system eventually leading to the development or maintenance of salt-sensitive hypertension (Fig. 1). Future studies will test this hypothesis and determine if blocking orexin receptors in the PVN can decrease SNA and blood pressure in hypertensive Dahl S rats as well as prevent the development of salt-sensitive hypertension. In addition, the molecular mechanisms that mediate the orexin system control of SNA and blood pressure in salt-sensitive hypertension will be investigated.
Fig. 1.
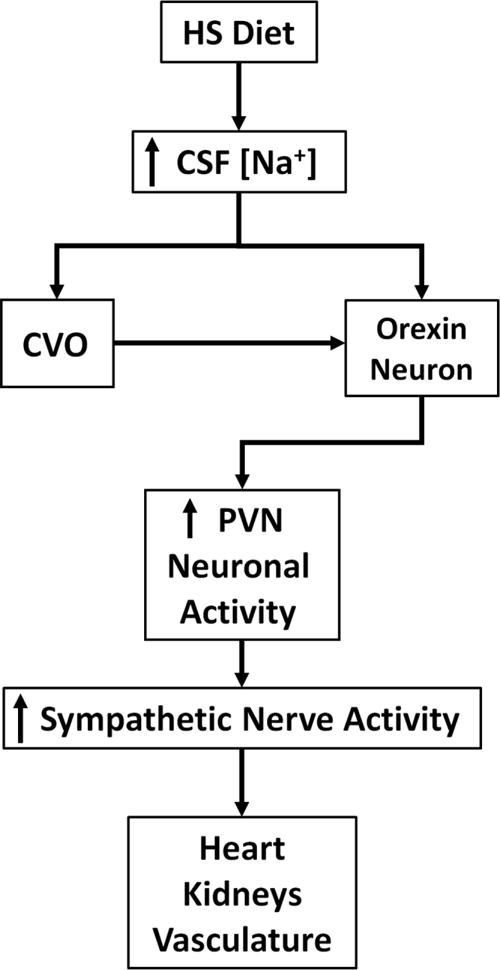
Hypothesis for high-salt (HS) induced activation of the orexin system in the hypothalamic paraventricular nucleus (PVN) contributing to hypertension in Dahl S rats. A HS diet may increase cerebrospinal fluid (CSF) sodium concentration in Dahl S rats. Circumventricular organs (CVO) in the lamina terminalis may sense increased osmolality and activate orexin-producing neurons. Alternatively, orexin-producing neurons may be directly sensitive to changes in CSF sodium concentration. This may increase orexin release to the PVN and augment PVN neuronal activity resulting in increased sympathetic nerve activity targeting the heart, kidneys, and vasculature and contribute to hypertension. Reduced ability of natriuresis by the kidneys of Dahl S rats may support chronic activation of the orexin system and may explain why the orexin system would not be chronically activated in SD or Dahl salt resistant rats in response to a high-salt diet.
Summary and conclusions
The reviewed experiments suggest that the orexin system contributes to several experimental animal models of hypertension including the SHR model of essential hypertension, the Schlager high blood pressure mouse (BPH/2J), stress-induced hypertension established by foot shock and noise in SD rats, and the obese Zucker rat model of obesity-related hypertension.
Enhanced OX2R activation in the RVLM due to increased sensitivity of the OX2R mediated by nNOS signaling as well as enhanced production of orexins have been identified as mechanisms by which the orexin system contributes to neurogenic hypertension in the SHR model. Overactivation of the orexin system may also contribute to neurogenic hypertension in SHRs by augmenting the CO2 chemoreflex. Increased production of orexins may also contribute to hypertension and increased SNA in dark-active period Schlager high blood pressure (BPH/2J) mice. Both the OX1R and OX2R are involved in the cardiovascular actions of orexin A in the RVLM of stress-induced hypertension established by foot shock and noise in SD rats and is mediated by nNOS signaling. This suggests that nNOS may be an important signal pathway for the orexin system in the RVLM in several forms of hypertension. Furthermore, upregulation of the OX1R increases firing of hypothalamic PVN neurons, augments SNA, and leads to hypertension in the obese Zucker rat. Based on this accumulating evidence that suggests the orexin system contributes to several models of neurogenic hypertension, there is strong rationale to investigate the contribution of the orexin system in other models of hypertension where the central nervous system contributes via augmented SNA.
Acknowledgments
NIHR15HL129213 (Shan); Michigan Technological University Research Excellence Fund (Shan).
Footnotes
Contributions:
Michael J. Huber wrote the manuscript, with editorial comments from Qing-Hui Chen and Zhiying Shan.
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- Amin MS, Reza E, Wang H, Leenen FH. Sodium transport in the choroid plexus and salt-sensitive hypertension. Hypertension. 2009;54:860–867. doi: 10.1161/hypertensionaha.108.125807. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kuraoka S, Brown AM. Baroreceptor function and changes in strain sensitivity in normotensive and spontaneously hypertensive rats. Circulation research. 1980;47:821–828. doi: 10.1161/01.res.47.6.821. [DOI] [PubMed] [Google Scholar]
- Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281:R1801–1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. The European journal of neuroscience. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. The Journal of comparative neurology. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension. 2000;35:403–408. doi: 10.1161/01.hyp.35.1.403. [DOI] [PubMed] [Google Scholar]
- Carrive P. Orexin, orexin receptor antagonists and central cardiovascular control. Frontiers in neuroscience. 2013;7:257. doi: 10.3389/fnins.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. American journal of physiology Regulatory, integrative and comparative physiology. 2000;278:R692–697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. American journal of physiology Regulatory, integrative and comparative physiology. 2003;284:R1611–1620. doi: 10.1152/ajpregu.00719.2002. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain research. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Clifford L, Dampney BW, Carrive P. Spontaneously hypertensive rats have more orexin neurons in their medial hypothalamus than normotensive rats. Experimental physiology. 2015;100:388–398. doi: 10.1113/expphysiol.2014.084137. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regulatory peptides. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Date Y, Mondal MS, Matsukura S, Nakazato M. Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neuroscience letters. 2000;288:87–90. doi: 10.1016/s0304-3940(00)01195-2. [DOI] [PubMed] [Google Scholar]
- Date Y, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern PJ, Nguyen-Huu TP, La Greca L, Abdelkader A, Head GA. Role of the sympathetic nervous system in Schlager genetically hypertensive mice. Hypertension. 2009;54:852–859. doi: 10.1161/hypertensionaha.109.136069. [DOI] [PubMed] [Google Scholar]
- de Lecea L. Hypocretins and the neurobiology of sleep-wake mechanisms. Progress in brain research. 2012;198:15–24. doi: 10.1016/b978-0-444-59489-1.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respiratory physiology & neurobiology. 2010;170:96–102. doi: 10.1016/j.resp.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. The Journal of physiology. 2009;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. The Journal of physiology. 2002;545:855–867. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. The European journal of neuroscience. 2009;30:1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Matsuguchi H, Mark AL. Abnormal baroreflex control of heart rate in prehypertensive and hypertensive Dahl genetically salt-sensitive rats. Hypertension. 1981;3:I135–141. doi: 10.1161/01.hyp.3.3_pt_2.i135. [DOI] [PubMed] [Google Scholar]
- Goto A, Ikeda T, Tobian L, Iwai J, Johnson MA. Brain lesions in the paraventricular nuclei and catecholaminergic neurons minimize salt hypertension in Dahl salt-sensitive rats. Clinical science (London, England: 1979) 1981;61(Suppl 7):53s–55s. doi: 10.1042/cs061053s. [DOI] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain research reviews. 2010;64:14–103. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. American Journal of Physiology-Heart and Circulatory Physiology. 2004;287:H1160–H1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- Hurley SW, Johnson AK. The role of the lateral hypothalamus and orexin in ingestive behavior: a model for the translation of past experience and sensed deficits into motivated behaviors. Frontiers in systems neuroscience. 2014;8:216. doi: 10.3389/fnsys.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KL, Dampney BW, Moretti JL, Stevenson ER, Davern PJ, Carrive P, Head GA. Contribution of Orexin to the Neurogenic Hypertension in BPH/2J Mice. Hypertension. 2016;67:959–969. doi: 10.1161/hypertensionaha.115.07053. [DOI] [PubMed] [Google Scholar]
- Jackson KL, et al. A novel interaction between sympathetic overactivity and aberrant regulation of renin by miR-181a in BPH/2J genetically hypertensive mice. Hypertension. 2013;62:775–781. doi: 10.1161/hypertensionaha.113.01701. [DOI] [PubMed] [Google Scholar]
- Johnson PL, et al. A key role for orexin in panic anxiety. Nature medicine. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy WV, Watanabe AM, Henry DP, Besch HR, Jr, Murphy WR, Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circulation research. 1976;38:21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- Kayaba Y, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285:R581–593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kishi T. Regulation of the sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 Academic Conference Award from the Japanese. Society of Hypertension Hypertension research: official journal of the Japanese Society of Hypertension. 2013;36:845–851. doi: 10.1038/hr.2013.73. [DOI] [PubMed] [Google Scholar]
- Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T. Orexins/hypocretins regulate drinking behaviour. Brain research. 1999;842:256–261. doi: 10.1016/s0006-8993(99)01884-3. [DOI] [PubMed] [Google Scholar]
- Lee YH, Dai YW, Huang SC, Li TL, Hwang LL. Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats. Experimental physiology. 2013;98:1145–1155. doi: 10.1113/expphysiol.2013.072298. [DOI] [PubMed] [Google Scholar]
- Lee YH, Tsai MC, Li TL, Dai YW, Huang SC, Hwang LL. Spontaneously hypertensive rats have more orexin neurons in the hypothalamus and enhanced orexinergic input and orexin 2 receptor-associated nitric oxide signalling in the rostral ventrolateral medulla. Experimental physiology. 2015;100:993–1007. doi: 10.1113/ep085016. [DOI] [PubMed] [Google Scholar]
- Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. The Journal of physiology. 2013;591:4237–4248. doi: 10.1113/jphysiol.2013.256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. The Journal of physiology. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. Orexin, cardio-respiratory function, and hypertension. Frontiers in neuroscience. 2014;8:22. doi: 10.3389/fnins.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Roy SH, Nattie EE. An augmented CO2 chemoreflex and overactive orexin system are linked with hypertension in young and adult spontaneously hypertensive rats. The Journal of physiology. 2016;594:4967–4980. doi: 10.1113/jp272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Hormones and behavior. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. The Journal of comparative neurology. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Marques FZ, Campain AE, Davern PJ, Yang YH, Head GA, Morris BJ. Genes influencing circadian differences in blood pressure in hypertensive mice. PloS one. 2011a;6:e19203. doi: 10.1371/journal.pone.0019203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques FZ, Campain AE, Davern PJ, Yang YH, Head GA, Morris BJ. Global identification of the genes and pathways differentially expressed in hypothalamus in early and established neurogenic hypertension. Physiological genomics. 2011b;43:766–771. doi: 10.1152/physiolgenomics.00009.2011. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. Journal of applied physiology (Bethesda, Md: 1985) 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Cowley AW. Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension. 1989;13:243–249. doi: 10.1161/01.hyp.13.3.243. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain research. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness Nature reviews. Neuroscience. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The role of orexin in motivated behaviours Nature reviews. Neuroscience. 2014;15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regulatory peptides. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. British journal of pharmacology. 2012;165:2292–2303. doi: 10.1111/j.1476-5381.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281:R1114–1118. doi: 10.1152/ajpregu.2001.281.4.R1114. [DOI] [PubMed] [Google Scholar]
- Simchon S, Manger W, Golanov E, Kamen J, Sommer G, Marshall CH. Handling 22NaCl by the blood-brain barrier and kidney: its relevance to salt-induced hypertension in dahl rats. Hypertension. 1999;33:517–523. doi: 10.1161/01.hyp.33.1.517. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? The Journal of physiology. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita A, Mark AL, Brody MJ. Prevention of salt-induced hypertension in the Dahl strain by 6-hydroxydopamine. The American journal of physiology. 1979;236:H48–52. doi: 10.1152/ajpheart.1979.236.1.H48. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS letters. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacological reviews. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, et al. Orexin A regulates cardiovascular responses in stress-induced hypertensive rats. Neuropharmacology. 2013;67:16–24. doi: 10.1016/j.neuropharm.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. The Journal of comparative neurology. 2005;485:127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology. 2015;99:481–490. doi: 10.1016/j.neuropharm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


