Abstract
The ADH1B (Alcohol Dehydrogenase 1B (class I), Beta Polypeptide) gene and its best-known functional alleles, Arg48His (rs1229984, ADH1B*2) and Arg370Cys (rs2066702, ADH1B*3), have been investigated in relation to many phenotypic traits; most frequently including alcohol metabolism and alcohol drinking behaviors, but also human evolution, liver function, cancer, and, recently, the comprehensive human phenome. To understand ADH1B functions and consequences, we provide here a bioinformatic analysis of its gene regulation and molecular functions, literature review of studies focused on this gene, and a discussion regarding future research perspectives. ADH1B alleles have large effects on alcohol metabolism, and this relationship particularly encourages further investigations in relation to alcoholism and alcohol-associated cancer to understand better the mechanisms by which alcohol metabolism contributes to alcohol abuse and carcinogenesis. We also observed that ADH1B has complex mechanisms that regulate its expression across multiple human tissues, and these may be involved in cardiac and metabolic traits. Evolutionary data strongly suggest that the selection signatures at the ADH1B locus are primarily related to effects other than those on alcohol metabolism. This is also supported by the involvement of ADH1B in multiple molecular pathways and by the findings of our recent phenome-wide association study. Accordingly, future studies should also investigate other functions of ADH1B potentially relevant for the human phenome.
Keywords: alcohol dehydrogenase 1B, gene regulation, molecular function, complex traits
Introduction
A drink of beverage alcohol presents the organism with an important physiological task – the alcohol must be metabolized, and the resulting calories prepared for use. Alcohol is highly caloric (7kcal/g) and heavy drinkers can supply much of their energy needs just from alcohol, although the displacement in the diet of fats, carbohydrates, and proteins by alcohol can create another host of problems. For the discussion below, it will be helpful to keep the magnitude of this metabolic task in mind: this is not a trivial amount of material to be dealt with. Issues related to alcohol metabolism can have profound effects on multiple physiological systems, as will be discussed below.
The focus of the present article, the ADH1B gene, encodes the beta subunit of class I ADH. The functional enzyme consists of homo- and heterodimers of alpha, beta, and gamma subunits; the corresponding genes (ADH1A, ADH1B, and ADH1C) map to chromosome 4q23 together with the other human ADH genes (ADH4, ADH5, ADH6, and ADH7). The clones of the full-length cDNA coding for class I ADH subunits were identified by Ikuta and colleagues, providing the first information regarding their molecular structures [Ikuta and others 1985]. However, studies of ADH began many years before: an ADH protein was firstly purified from Saccharomyces cerevisiae in 1937 [Negelein and Wulff 1937]. Initially, this enzyme attracted interest focused on the need to understand the ability of different organisms to oxidize alcohol [Lutwak-Mann 1938]. Later, numerous molecular studies investigated the role of ADH in a wide range of situations, including alcohol metabolism, human behavior, liver function, and human evolution [Brooks and Zakhari 2014; Buhler and others 2015; Carr and others 2002; Edenberg 2000; Edenberg 2007; Li and others 2011a; Li and others 2011b]. In recent years, omic studies based on high-throughput technologies confirmed the key role of ADH in multiple molecular mechanisms [Gelernter and others 2014; Kropotova and others 2014; Winnier and others 2015]. In particular, variation in the ADH1B gene was demonstrated to have a large effect in the predisposition to several complex traits, including alcoholism and (primarily GI tract) cancer [Gelernter and others 2014; McKay and others 2011; Wu and others 2012]. The relevance of the ADH1B locus was further confirmed by genomic analyses that highlighted how its genetic variation was shaped by selective pressures during human evolution [Galinsky and others 2016]. Due to its clear involvement in the major alcohol metabolic pathway, different authors have hypothesized that ADH1B phenotypic associations are related to alcohol use and its downstream consequences [Holmes and others 2014; Silverwood and others 2014]. However, recent findings have shown that ADH1B may affect the human phenome through alcohol-independent mechanisms also [Polimanti and others 2016a]. To understand the network of ADH1B activities and consequences, we provide here a bioinformatic analysis of gene regulation and the molecular functions of its protein product, a literature review of the studies conducted on this gene, and a discussion of future perspectives of ADH1B research. It is our intention to help scientists who are interested in the ADH1B locus to connect multiple functional aspects that they should might consider in their research.
Gene Regulation and Variation and Protein Structure
Bioinformatic Analysis
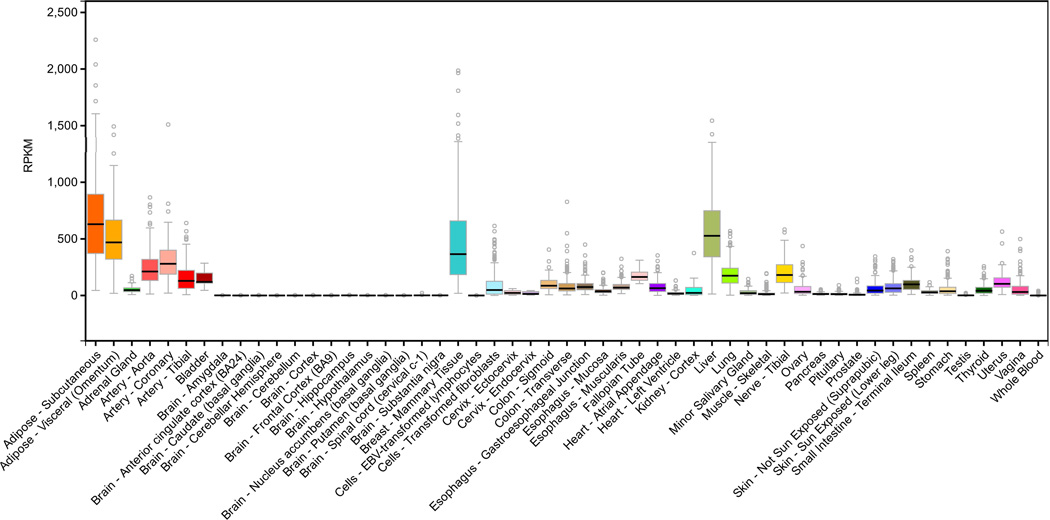
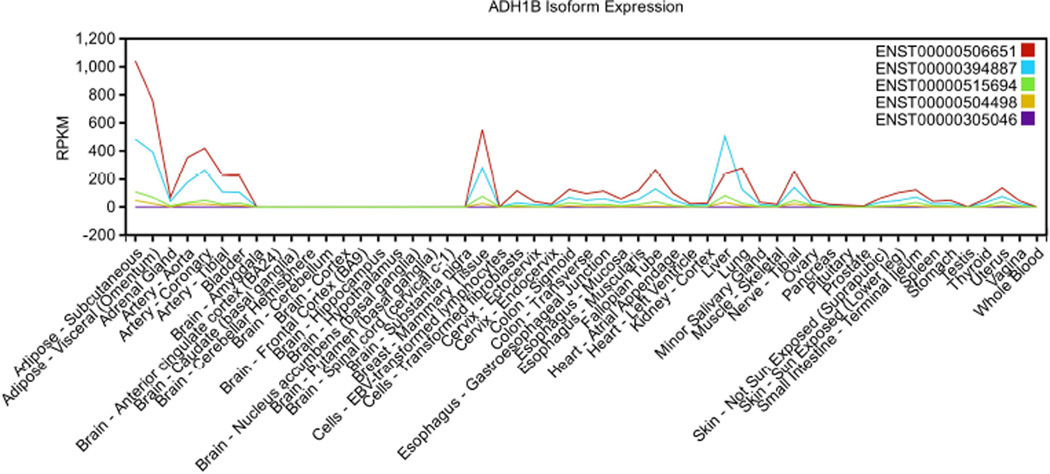
According to COMPARTMENTS, a subcellular localization database (available at http://compartments.jensenlab.org/Search; [Binder and others 2014]) considering multiple information sources regarding different cell types, the ADH1B protein product was identified with the highest confidence in the cytosol, with low confidence in the mitochondrion and the nucleus, and with the lowest confidence in the extracellular space and the peroxisome. Regarding gene expression distribution, the early studies mainly focused their attention on the liver where the ADH1B protein product plays a role in hepatic alcohol oxidation [Zakhari 2006]. However, ADH1B is also expressed in other human tissues. In the RNA-sequencing analysis conducted by the GTEx consortium (Release V6 data available at http://www.gtexportal.org/home/; [Mele and others 2015]), ADH1B showed expression in several human tissues, with the highest values (>200 Reads Per Kilobase) observed in subcutaneous adipose tissue, liver, omentum, coronary arteries, and aorta (Figure 1). Five different transcripts are expressed by ADH1B and they showed tissue-specific distribution (Figure 2). Specifically, ENST00000506651 and ENST0000039488 account for the most ADH1B expression, but ENST00000506651 is the most expressed isoform in all tissues with the exception of liver, where ENST00000394887 is the most expressed (Supplemental Figure 1). According to the CCDS (Consensus Coding Sequence) database (available at https://www.ncbi.nlm.nih.gov/CCDS/CcdsBrowse.cgi; [Farrell and others 2014]), ENST00000305046 and ENST00000394887 were considered coding sequences with high-quality annotation (CCDS ID: 34033.1 and 68761.1, respectively) and their protein products are annotated in the UniProt database (Uniprot ID: P00325; data available at http://www.uniprot.org/; [Magrane and UniProt Consortium 2011]). ENST00000305046 (CCDS ID: 34033.1) corresponds to the canonical ADH1B isoform (P00325-1; Length: 375 aa) whereas ENST00000394887 corresponds to the ADH1B isoform 2 (P00325-2; Length: 345 aa). ADH1B isoform 2 differs from the ADH1B canonical isoform in that it lacks the initial 40 amino acids (Supplemental Figure 2).
Figure 1.
ADH1B expression across human tissues (data available at http://www.gtexportal.org/home/).
Figure 2.
ADH1B isoform expressions (ENST00000506651, ENST00000394887, ENST00000515694, ENST00000504498, and ENST00000305046) across human tissues (data available at http://www.gtexportal.org/home/).
Structurally, the ADH1B protein presents three binding sites: two metal-binding sites for the catalytic zinc and the structural zinc, respectively; and a nucleotide biding site for the NAD (Nicotinamide Adenine Dinucleotide) coenzyme. Most studies of ADH1B enzymatic activities mainly focused on three alleles (which used to be designated ADH1B*1, ADH1B*2, and ADH1B*3) based on two missense substitutions (Arg48His, rs1229984; Arg370Cys, rs2066702 – the “*1” variant has neither of these two possible substitutions) commonly present in various human populations (Table 1). ADH1B*2 (Arg48His, rs1229984) occurs mostly in Asian and European-ancestry populations, while ADH1B*3 (Arg370His, rs2066702) is seen almost exclusive in African-ancestry populations. Both protein products of ADH1B rs1229984 and rs2066702 facilitate the release of the NAD coenzyme at the end of the reaction with a consequent 70- to 80-fold higher turnover rate than the protein product of ADH1B reference sequences [Edenberg 2007; Hurley and others 2003]. That is, the minor alleles are in both cases more active than the common alleles.
Table 1.
Most-investigated functional alleles in ADH1B locus. Minor Allele Frequencies are reported from the 1,000 Genomes Project Phase 3 (AFR: Africa; EAS: East-Asia; EUR: European: SAS: South Asia).
| Allele | rsID | Amino acid change | Enzyme Activity | AFR | AMR | EAS | EUR | SAS |
|---|---|---|---|---|---|---|---|---|
| ADH1B*1 | - | - | - | - | - | - | - | - |
| ADH1B*2 | rs1229984 | Arg48His | ↑ | 0 | 0.06 | 0.70 | 0.03 | 0.02 |
| ADH1B*3 | rs2066702 | Arg370Cys | ↑ | 0.19 | 0.02 | 0 | 0 | 0 |
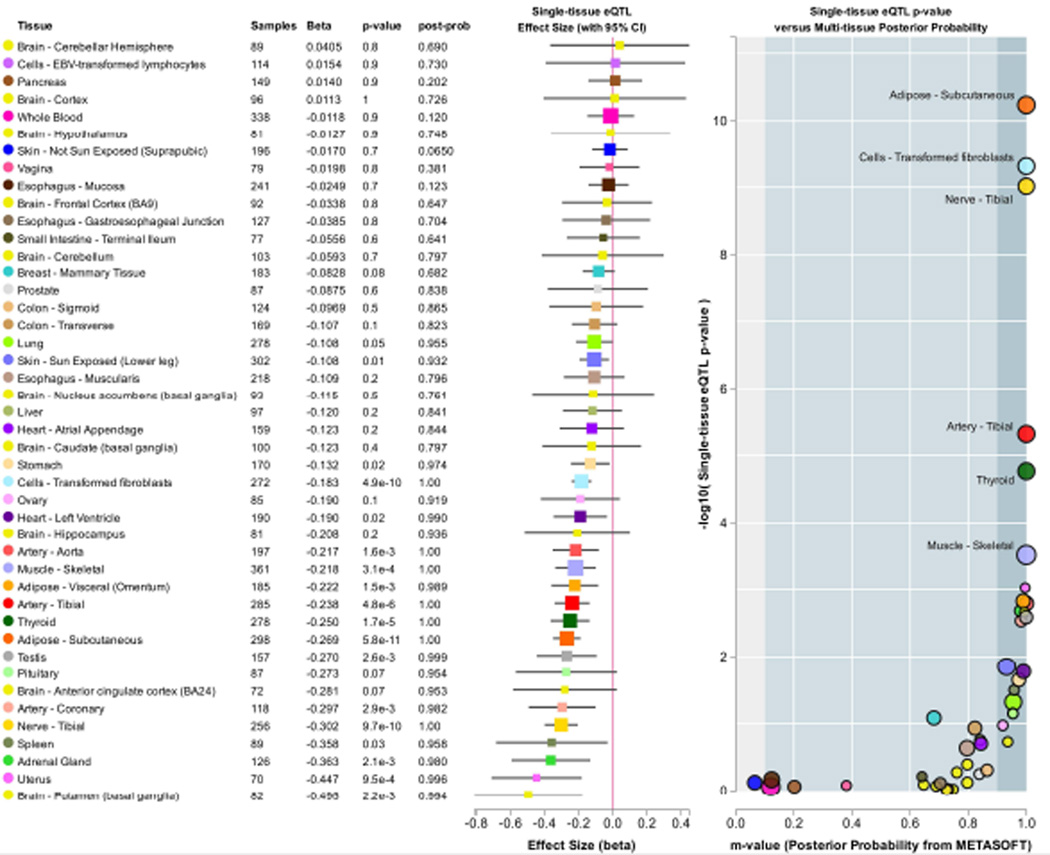
These are not the only possible functional variants, and the explosion of the next-generation sequencing has uncovered a more comprehensive understanding of human genome variation, including at ADH1B. Considering the worldwide populations included in the 1,000 Genomes Project Phase 3 dataset (available at http://browser.1000genomes.org/index.html; [1000 Genomes Project Consortium and others 2015]), 145 missense variants have now been identified considering the canonical ADH1B transcript, but the total number of coding and non-coding variants is much higher, 1,110 SNPs (Supplemental Table 1). Non-coding variants may play very important roles in gene regulation, and consequently in the expression of phenotypic traits. Analyzing GTEx data (Release V6), we observed 165 independent ADH1B expression quantitative trait loci (eQTL) considering a false discovery rate (FDR) < 5% and a linkage disequilibrium (LD) r2 cutoff ≥ 0.1. These ADH1B eQTLs are related to 43 SNPs and 5 tissues, including subcutaneous adipose tissue, tibial artery, transformed fibroblast, tibial nerve, and thyroid (Supplemental Table 2). Considering multi-tissue eQTL posterior probabilities for ADH1B available in the GTEx dataset, rs10516440 showed effects on multiple tissues, including subcutaneous adipose tissue, tibial artery, heart (left ventricle), lung, skeletal muscle, tibial nerve, sun-exposed skin (lower leg), thyroid, and whole blood (Figure 3). Rs10516440 is located in the upstream region of ADH gene cluster, and it affects gene expression of all ADH1 genes in multiple tissues (FDR < 5%; Supplemental Table 3). In our genome-wide association study (GWAS) of alcohol dependence (AD) [Gelernter and others 2014], the minor allele rs10516440*G, which correlated with reduced expression of ADH1 genes, was nominally associated with increased AD risk (N = 8,788, z = 3.952, p = 7.74*10−5) with nearly equal contribution from both African-Americans (N = 4,141, z = 2.63, p = 8.52*10−3) and European-Americans (N = 4647, z = 2.96, p=3.07*10−3), although, lacking the associated informatics, this was not stressed in that prior publication. This observation may serve to highlight the possible advantages of identifying functional SNPs based on various annotations.
Figure 3.
Effects of rs10516440 on ADH1B expression across human tissues (data available at http://www.gtexportal.org/home/).
Molecular Functions
Literature Review
ADH1B is mainly known for its involvement in the major human ethanol metabolic pathway (Figure 4). There are four distinct human ethanol degradation pathways, three oxidative pathways and one non-oxidative pathway [Zakhari 2006]. The oxidative mechanisms differ for the first step where ethanol is converted to acetaldehyde: 1) cytosolic ADH (e.g., ADH1B); 2) Cytochrome P450 2E1 (CYP2E1); 3) Peroxisomal catalase. Acute ethanol consumption induces the hepatic oxidative pathways, predominantly the ADH-mediated pathway [Zakhari 2006]. Conversely, chronic ethanol consumption increases the contribution of hepatic CYP2E1 activity and non-oxidative pathways with respect to ADH. Inhibition of oxidative ethanol metabolism increases FAEE levels, indicating that oxidative and non-oxidative mechanisms are alternative metabolically linked pathways [Zakhari 2006]. In all oxidative pathways, the second step, where acetaldehyde is converted to acetate, is mediated by mitochondrial aldehyde dehydrogenase (ALDH). The non-oxidative metabolism is not completely understood, but its final products are fatty acid ethyl esters (FAEEs) and phosphatidyl ethanol. Ethanol metabolic mechanisms have been observed mainly in hepatic tissue, but also occurs in other organs, including stomach, pancreas, lung, and brain [Deitrich and others 2006; Zakhari 2006].
Figure 4.
ADH1B protein interactive network (data available at http://string-db.org/).
Bioinformatic Analysis
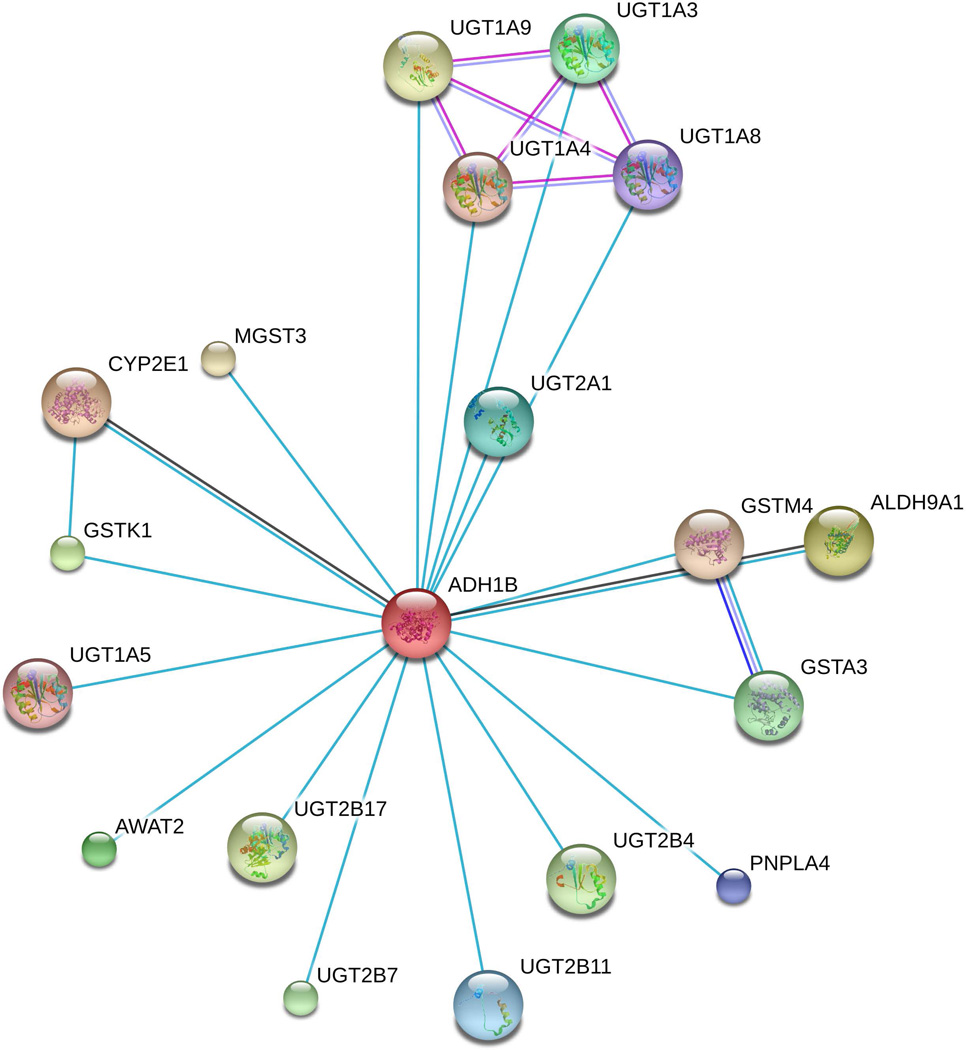
ADH1B shows enzymatic activities besides those related to ethanol. According to the HumanCyc database (available at http://humancyc.org/; [Romero and others 2005]), ADH1B catalyzes 19 different reactions interacting with different substrates and cofactors (Table 2). Considering multiple databases (i.e., Biosystems [Geer and others 2010], Reactome [Fabregat and others 2016], PharmGKB [Whirl-Carrillo and others 2012], KEGG [Kanehisa and others 2016]), ADH1B is reported to be involved in the metabolic pathways of many compounds besides ethanol, including fatty acids, acetone, epinephrine, glucose, retinol, tyrosine, tryptophan, ifosfamide, cyclophosphamide, abacavir, and celecoxib; and notably, neurotransmitters serotonin and norepinephrine (Supplemental Table 4). To understand further the interaction of ADH1B with other proteins and the related molecular mechanisms, we investigated the STRING v.10.0 database (available at http://string-db.org/; [Kanehisa and others 2016]) considering interaction score > 0.9 (highest confidence) and excluding textmining from the interaction sources. We observed that ADH1B shows highest-confidence interactions with 18 known proteins (Figure 5; Supplemental Table 5). We then conducted a Gene Ontology (GO) enrichment analysis considering the ADH1B protein interactive network, and observed 10 significant GO results (FDR < 5%; Supplemental Table 6) related to metabolic processes (GO~0006805, GO~0044281, GO~0044710, GO~0071704, GO~0044237, and GO~0008152), cellular response (GO~0071466, GO~0070887, and GO~0042221), and catalytic activity (GO~0003824).
Table 2.
Chemical reactions catalyzed by ADH1B protein product (EC: Enzyme Commission number).
| EC | Reaction |
|---|---|
| 1.1.1.1 | ethanol + NAD+ ↔ acetaldehyde + NADH + H+ |
| 3-methylbutanol + NAD+ ↔ 3-methylbutanal + NADH + H+ | |
| 5-hydroxytryptophol + NAD+ ← 5-hydroxyindole acetaldehyde + NADH + H+ | |
| 3-methoxy-4-hydroxyphenylglycol + NAD+ ↔ 3-methoxy-4-hydroxyphenylglycolaldehyde + NADH + H+ | |
| a primary alcohol + NAD+ ↔ an aldehyde + NADH + H+ | |
| 3,4-dihydroxyphenylglycol + NAD+ ← 3,4-dihydroxyphenylglycolaldehyde + NADH + H+ | |
| a secondary alcohol + NAD+ ↔ a ketone + NADH + H+ | |
| 1-propanol + NAD+ ↔ 1-propanal + NADH + H+ | |
| indole-3-glycol + NAD+ ↔ indole-3-glycol aldehyde + NADH + H+ | |
| isobutanol + NAD+ ↔ isobutanal + NADH + H+ | |
| 2-methylbutanol + NAD+ ↔ 2-methylbutanal + NADH + H+ | |
| 2-phenylethanol + NAD+ ↔ phenylacetaldehyde + NADH + H+ | |
| methionol + NAD+ ↔ 3-methylthiopropanal + NADH + H+ | |
| 4-tyrosol + NAD+ ↔ (4-hydroxyphenyl)acetaldehyde + NADH + H+ | |
| 1.1.1.2 | an alcohol + NADP+ ↔ an aldehyde + NADPH + H+ |
| 1.1.1.80 | propan-2-ol + NADP+ → acetone + NADPH + H+ |
| 1.1.2.7 | a primary alcohol + 2 an oxidized cytochrome cL ↔ an aldehyde + 2 a reduced cytochrome cL + 2 H+ |
| 1.1.2.8 | a primary alcohol + 2 an oxidized cytochrome c550 ↔ an aldehyde + 2 a reduced cytochrome c550 |
| 1.1.9.1 | a primary alcohol + an oxidized azurin ↔ an aldehyde + a reduced azurin |
Figure 5.
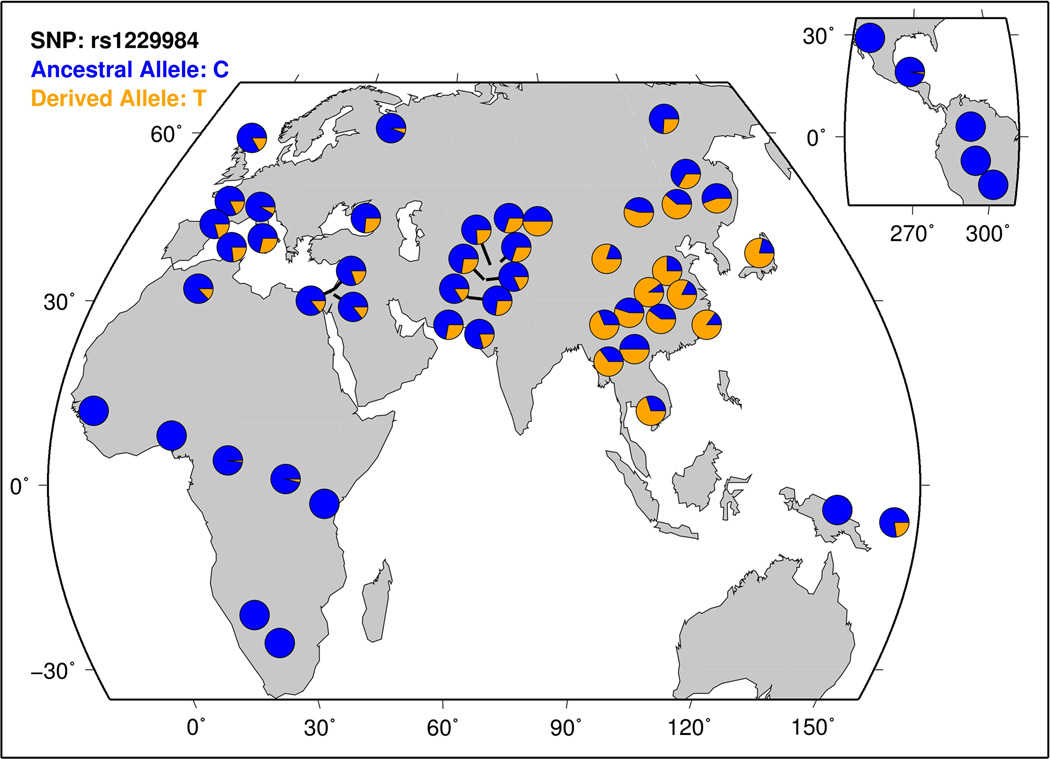
ADH1B rs1229984 variation across human populations (data available at http://hgdp.uchicago.edu/).
Alcoholism
Literature Review
The initial studies about the role of alcohol metabolism genes in alcohol sensitivity explained, at first, some of the population differences in alcohol intoxication symptoms (e.g., facial flushing, elevation of skin temperature, increase in pulse rate, and ventilation): many Asian-ancestry subjects showed an increased sensitivity to alcohol drinking compared to European-ancestry individuals [Wolff 1972]. Population screenings determined that Asian populations present higher frequencies of a highly-active hepatic ADH isoform and a highly-inactive hepatic ALDH isoform than those observed in European populations [Goedde and others 1979; Stamatoyannopoulos and others 1975]. As noted above, ADH and ALDH enzymes catalyze different steps in the process of alcohol degradation. The intermediate product of this two-step reaction is acetaldehyde, which is more toxic than ethanol itself (while more reinforcing in the CNS) and it is mainly responsible for alcohol intoxication symptoms [Brooks and Zakhari 2014]. Since both highly-active ADHs and highly-inactive ALDHs have the potential to cause increased circulating acetaldehyde levels, several authors hypothesized their possible involvement in population differences in alcohol sensitivity [Goedde and others 1979; Stamatoyannopoulos and others 1975]. These variant ADH and ALDH isoforms are encoded by gene alleles with nonsynonymous substitutions in the encoded proteins (those in ADH1B are discussed above) and in 1991, in one of the first studies in psychiatric genetics to use a molecular approach, Thomasson and colleagues demonstrated that ADH1B*2 (Arg48His, rs1229984) and ALDH2*2 (Glu504Lys, rs671) are associated with reduced risk of alcoholism in an Asian sample [Thomasson and others 1991]. Notwithstanding the small size of the sample used in this study, the findings have been confirmed many times since. Subsequently, numerous gene-candidate studies showed that, although ADH1B rs1229984 minor allele frequency (MAF) is lower in non-Asian populations than that observed in Asians, it is protective with respect to alcohol drinking behaviors also in other ancestries [Li and others 2011a; Luo and others 2006]. Conversely, ALDH2 rs671 is very rare in non-Asian populations (MAF < 1% in accordance with 1,000 Genomes Project Phase 3 data; [1000 Genomes Project Consortium and others 2015]) and no informative analysis can be conducted in European-ancestry subjects. In African and Native American populations, ADH1B rs2066702 (Arg370Cys) showed protective effect similar to that observed for ADH1B rs1229984 [Ehlers and others 2012; McCarthy and others 2010]. Our meta-analysis of candidate gene studies confirmed the strong involvement of ADH1B rs1229984 in AD risk, and also for alcohol abuse and alcohol-induced diseases in multiple ethnic populations [Li and others 2011a]. The same result was also confirmed by an independent meta-analysis of genetic studies of alcohol drinking behaviors [Buhler and others 2015]. Genomic studies have demonstrated that candidate gene analysis and their meta-analyses can produce false positive results due to publication bias [Sullivan 2013]. Although the relationship between ADH1B and alcohol-related traits is on much firmer biological and statistical ground than most other such associations [Bierut and others 2012], it was also important to demonstrate the relationship in a genome-wide context. GWAS (hypothesis-free investigations) can be powerful in investigation of the genetic architecture of complex traits, such as alcohol drinking behaviors. GWAS of AD and maximum number of alcoholic drinks confirmed the protective effects of ADH1B rs1229984 and rs2066702 with respect to alcohol drinking behaviors in European-Americans and African-Americans [Gelernter and others 2014; Xu and others 2015]. On the basis of these previous GWAS, ADH1B rs1229984 and rs2066702 appear to have relatively large effect sizes on AD symptom count and maximum daily number of alcoholic drinks in European-Americans and African-Americans respectively (Table 3). Although these effect sizes are substantially larger than the average for alleles discovered in GWAS of complex traits, they explain very little of the variance of the alcohol use behaviors. Indeed, it is widely recognized that the predisposition to complex traits, such as alcohol use disorder, is highly polygenic with hundreds to thousands of loci likely involved [Loh and others 2015].
Table 3.
Effects of ADH1B rs1229984 and rs2066702 on AD symptom count and number of alcoholic drinks in European-Americans and African-Americans, respectively.
| Trait | Allele-Ancestry | N | Allele Frequency |
Effect (Beta) |
P value | Reference |
|---|---|---|---|---|---|---|
| AD symptom count |
rs1229984*A-Europe | 6,875 | 0.06 | −0.03 | 2.91*10−18 | Gelernter et al., 2014 |
| rs2066702*A-Africa | 5,432 | 0.19 | −0.02 | 2.24*10−13 | ||
| Number of alcoholic drinks |
rs1229984*A-Europe | 5,064 | 0.06 | −0.26 | 5.96*10−15 | Xu et al., 2015 |
| rs2066702*A-Africa | 4,491 | 0.19 | −0.16 | 2.50*10−10 |
A further analysis of the ADH1B locus also highlighted that non-coding variants contribute to AD risk and the gene haplotype structure to population differences [Polimanti and others 2015a]. GWAS conducted in Asian populations also confirmed the protective role of ALDH2 rs671 [Baik and others 2011; Quillen and others 2014; Takeuchi and others 2011]. Recent studies also reported that ADH1B rs1229984 and rs2066702 are associated directly with the accumulation of blood acetaldehyde [Kang and others 2014] as predicted by knowledge of their physiological functions, in agreement with their effects on enzymatic activities [Chiang and others 2016] and the results of genetic studies of alcoholism. However, ALDH2 rs671, rather than ADH1B rs1229984, seems more responsible for acetaldehyde concentrations and facial flushing in Asians populations [Peng and others 2014]. In this scenario, where genotype affects the enzymatic activity which increases the symptoms which reduce alcohol drinking behaviors, other factors also seem to moderate the protective effect of ADH1B rs1229984 on alcohol drinking behaviors. Two studies conducted in independent samples observed a reduced protective effect of ADH1B rs1229984 on alcohol drinking behaviors in subjects exposed to childhood adversity [Meyers and others 2015; Sartor and others 2014]. A further study observed a reduced protective effect of ADH1B rs1229984 on alcohol drinking behaviors in adolescents reporting most or all best friends drinking [Olfson and others 2014].
Further pathogenic mechanisms could be related to methylation. In the ADH gene cluster, DNA methylation, which is also modulated by genetic variation, appeared to at least partially underlie the association of genetic variation with AD [Zhang and others 2014a]. Finally, our recent study extended our understanding of the ADH1B association with symptoms related to alcohol use disorders considering DSM-IV and DSM-5 diagnostic systems [Hart and others 2016]. We observed that ADH1B rs1229984 was related to a range of alcohol-related social/interpersonal problems and the associations were mediated by the maximum number of drinks consumed in a 24-hour period (a measure of innate tolerance), suggesting that variation in ADH1B affects the adaptation to heavy drinking [Hart and others 2016].
Human Evolutionary History
Literature Review
As discussed above, ADH1B rs1229984 MAF shows very strong differences between Asian and non-Asian populations. Figure 5 reports the allele frequencies in the 53 populations from seven continental groups of Human Genome Diversity Project (available at http://hgdp.uchicago.edu/cgi-bin/gbrowse/HGDP/; [Li and others 2008]). Initial candidate gene studies highlighted that variation in ADH gene cluster presents unusual patterns of linkage disequilibrium and diversity in Asian populations, and, in particular, ADH1B rs1229984 frequencies are not driven by random genetic drift but are instead attributable to positive selection in these human groups [Han and others 2007; Li and others 2007; Osier and others 2002]. Further analyses determined that the emergence of the ADH1B rs1229984 minor allele occurred about 10,000~7,000 years ago, which coincides with the time of origin and expansion of neolithic agriculture (rice domestication) in southern China [Peng and others 2010]. However, a subsequent study demonstrated that the expansion of the selected ADH1B rs1229984 haplogroup is more recent, around 2,800 years ago [Li and others 2011b]. Furthermore, although ADH1B rs1229984 originated in the ancestors of Sino-Tibetan populations and the high diversity is present in Tibetan ADH1B haplotypes, no selection was observed in modern Tibetans [Lu and others 2012]. Methods based to different selection statistics (e.g., population differentiation and haplotype lengths) were applied to genome-wide data and confirmed the positive selection signatures in ADH1B gene in Asians [Peter and others 2012; Wang and others 2014c]. Some authors also hypothesized that, within Asian ancestry, other types of natural selection for ADH1B rs1229984, together with positive selection, are also present, including stabilizing selection and divergent selection [Evsyukov and Ivanov 2013]. A recent investigation based on principal component analysis also reported a genome-wide significant signal of selection for ADH1B locus in Europeans, suggesting convergent evolution in Europe and East Asia [Galinsky and others 2016].
Although multiple kinds of evidence strongly indicate that evolutionary pressures shaped ADH1B genetic diversity, the mechanisms are not well understood. Although some authors suggest it is due to the concomitant occurrence of ADH1B rs1229984 and rice domestication (and the consequent use of rice-fermented beverages) [Peng and others 2010], the protective effect of ADH1B on alcoholism, a “modern” phenotypic trait, is unlikely to be the force responsible for the selection of ADH1B rs1229984, especially since ADH1B rs1229984 expansion and selection seem to be more recent than rice domestication (rice domestication: 10,000~7,000 years ago; ADH1B rs1229984 expansion: 2,800 years ago) [Li and others 2011b]. A recent study demonstrated that ALDH2 rs671, the other locus associated with increased acetaldehyde levels in Asians [Kang and others 2014], is also associated with reduced risk of tuberculosis [Park and others 2014]. Although in the same study no association was found between tuberculosis and ADH1B and there is no genome-wide evidence for natural selection at the ALDH2 locus, this finding may suggest a role of alcohol-metabolism genes in the predisposition to infectious diseases, which are the most-recognized environmental factors that have shaped the human genome during its evolutionary history [Daub and others 2013]. A phenome-wide association study (PheWAS) in a large Asian cohort could help to direct future evolutionary investigations of ADH1B locus.
Further support for the evolutionary role of ADH1 genes is provided by investigations of primate evolution. Although there is still an open debate, primates showed multiple independent gene conversions among ADH1 paralogous genes in marmoset, macaque, and human lineages [Carrigan and others 2012]. Analyzing ADH1B cDNA from mouse, chimpanzee, and human samples, the synonymous and non-synonymous substitution (dN/dS) ratios was significantly low in all pairs, suggesting the presence of purifying selection [Oota and others 2007]. This supports that the ADH1 system was evolutionarily selected to be highly efficient, most obviously to permit a higher consumption of fermented fruits.
Predisposition to Cancer
Literature Review
Alcohol consumption is a risk factor associated to several forms of cancer and the International Agency for Research on Cancer (IARC) has defined acetaldehyde (the first intermediate product of alcohol degradation) associated with consumption of alcoholic beverages as “carcinogenic to humans” (Group 1) [IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2010; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2012]. In particular, alcohol carcinogenesis mostly affects tissues directly exposed to alcohol ingestion (e.g., the oral cavity, pharynx, esophagus, larynx, and colon), tissues involved in alcohol metabolism (e.g., liver), and tissue exposed to alcohol-associated oxidative stress (e.g., breast) [Persson and others 2013; Toh and others 2010; Varela-Rey and others 2013]. The full set of carcinogenic mechanisms of alcohol, including formation of acetaldehyde-DNA adducts, hyperregeneration, and epigenetic changes, is still to be elucidated and confirmed [Brooks and Zakhari 2014]. Genetic studies (candidate gene studies, meta-analyses, and GWAS) of alcohol-associated cancers (AAC; e.g., upper aerodigestive tract cancers, hepatocellular carcinoma, breast cancer, colon cancer, and thyroid cancer) have repeatedly implicated risk alleles in alcohol metabolism genes, including ADH1B, ALDH2, and other ADH genes, especially in Asian populations [Hidaka and others 2015; Liu and others 2016; McKay and others 2011; Wang and others 2014a; Wang and others 2014b; Wu and others 2012; Zhang and others 2015]. Furthermore, ADH1B and ALDH2 alleles showed interaction with alcohol use behaviors in determining AAC risk [Masaoka and others 2016; Maurya and others 2014; Siegert and others 2013; Zhang and others 2014b]. Beyond alcohol consumption, ADH and ALDH alleles seem to interact with other factors in relation to cancer risk, such oral hygiene [Tsai and others 2014]. The effects of ADH1B and ALDH2 do not appear to be limited to cancer onset risk, but also contribute to the cancer prognosis [Kagemoto and others 2016; Tucker and others 2014]. To understand better the mechanisms that link ADH1B and ALDH2 with the predisposition to cancer, different authors have investigated the genomic features of AAC tissue. Both the ADH1B and ALDH2 genes showed downregulation in different types of tumor tissues [Kropotova and others 2014; Liu and others 2015] and this seems to be due to epigenetic changes, such as hypermethylation of the promoter regions and non-hystonic acetylation [Shen and others 2016; Udali and others 2015].
Human Phenome
Literature Review
Since ADH1B rs1229984 has a large effect on alcohol drinking behaviors, many studies have investigated its association with additional phenotypes. Due to its role in liver detoxification, the ADH1B protective allele was tested with respect to alcoholic liver disease in multiple independent candidate gene studies, and our meta-analysis confirmed its strong association [Li and others 2011a]. However, a large study conducted in cohorts (N = 9,080) from the Copenhagen City Heart Study showed that ADH1B rs1229984 genotypes were not associated with and did not modify the effect of alcohol on biochemical tests or risk of liver disease [Tolstrup and others 2009]. The same study cohort was also used to conduct a large Mendelian-randomization study (N = 54,604) to estimate the causal effects of long-term alcohol consumption on coronary heart disease risk factors [Lawlor and others 2013]. The authors observed effects of long-term alcohol consumption, calculated on the basis of ADH1B genotype (instrumental variable), on blood pressure, body mass index (BMI), and triglyceride levels [Lawlor and others 2013]. A larger Mendelian-randomization study (N = 261,991) replicated these results, reporting that the carriers of the protective allele had a more favorable cardiovascular profile and a reduced risk of coronary heart disease than those without the genetic variant [Holmes and others 2014]. Since there is a U-shaped relationship between alcohol use and cardiovascular events – that is, moderate alcohol consumption leads to better outcome than very low or very high – the Alcohol-ADH1B Consortium tested this hypothesis using ADH1B rs1229984 as a genetic instrument and they confirmed the presence non-linear causal effects of alcohol intake [Silverwood and others 2014]. This non-linear relationship was also confirmed by the ADH1B genotype-differential effect of initiating moderate red wine consumption on 24-h blood pressure observed in a randomized trial of patients with type-2 diabetes [Gepner and others 2016]. In Asian alcoholic individuals, both ADH1B and ALDH2 protective alleles showed association with high serum triglyceride levels and low serum cholesterol levels, leukocyte, granulocyte, and monocyte counts [Yokoyama and others 2016; Yokoyama and others 2015]. Our GWAS of BMI in subjects with AD identified ALDH1A1 as a risk locus, supporting the role of alcohol risk genes in the predisposition to metabolic traits [Polimanti and others 2015b]; this should be considered in light of our comments in the Introduction, namely that in alcohol-dependent individuals, alcohol can not only account for a substantial part of the individual’s caloric intake, but can displace other nutrients. A transcriptomic analysis also identified ADH1B as involved in the differentiation of brown adipose tissue [Tews and others 2014] and its transcriptional changes in adipose tissue are associated with waist circumference, BMI, and fasting plasma insulin [Winnier and others 2015].
Another large Mendelian-randomization study (N = 34,452) was conducted using ADH1B rs1229984 as the instrumental variable to understand the relationship between alcohol consumption and cognitive performance; there was no significant association [Kumari and others 2014]. Negative results were also observed in independent studies of cognitive impairment and depression conducted in older men (N = 3,542 and 3,873, respectively) [Almeida and others 2014a; Almeida and others 2014b]. However, different studies reported a protective effect of ADH1B genotype with respect to educational achievements [Borinskaya and others 2013; Latvala and others 2014; von Hinke Kessler Scholder and others 2014] – that is, the minor allele, protective with respect to alcohol use disorders, also is associated to higher educational attainment. ADH1B rs2066702 and rs1229984 showed also protective effects with respect to prenatal alcohol exposure in relation to school performance and attention in subjects of African and European descends, respectively [Dodge and others 2014; Zuccolo and others 2013]. Our recent PheWAS increased the spectrum of phenotypic traits potentially associated with ADH1B variation [Polimanti and others 2016a]. We identified multiple findings related to psychological traits, socioeconomic status, vascular/metabolic conditions, and reproductive health and, applying Bayesian network learning algorithms to investigate the causative relationships among ADH1B, alcohol use, there novel traits, we observed that some of these observations may be independent from the role of ADH1B in alcohol metabolism and due to other ADH1B functions [Polimanti and others 2016a].
Future Perspectives
Here we presented a comprehensive review of the information available from molecular databases and current literature regarding the role of ADH1B in alcohol use disorders and more generally, in the human phenome. The majority of the evidence is focused on how ADH1B non-synonymous substitutions (rs1229984 and rs2066702), associated with increased catalytic activity, are associated with large effects on alcohol sensitivity, which consequently affects drinking behaviors and long-term consequences of alcohol use. However, some aspects of this alcohol-related cascade need additional study. For instance, ADH1B protective alleles seem to have a reduced effect on alcohol drinking behaviors in subjects exposed to a negative social environment [Meyers and others 2015; Olfson and others 2014; Sartor and others 2014]. Investigating subjects with high alcohol sensitivity may facilitate the identification of loci that interact with social environment in determining alcohol drinking behaviors. Another example is related to the role of ADH1B in the predisposition to cancer: it is not clear whether this association is mediated by alcohol use, by alcohol metabolism in non-hepatic tissues, or by both mechanisms. Furthermore, the analysis of ADH1B protein networks with genome-wide data can contribute to clarifying the pathogenic pathways by which alcohol contributes to carcinogenesis.
We refer to these genes as alcohol metabolism loci, but their protein products have other functions as well. Beyond these alcohol metabolism-related aspects, there are other issues, which may open new routes in ADH1B research. Beyond the hepatic ADH1B isoform (ENST00000394887), there is another ADH1B transcript (ENST00000506651) highly expressed in adipose and cardiac tissues (GTEx data). Since transcriptomic analysis demonstrated that ADH1B expression in adipose tissues is associated with metabolic traits [Winnier and others 2015], further investigations are necessary to understand the mechanisms related to non-hepatic ADH1B expression. One important aspect of ADH1B gene regulation is surely the role of non-coding variants. In GTEx data, we observed that non-coding variants regulate ADH1B expression in multiple tissues and, in particular, rs10516440 seems to coordinate the gene expression of ADH1 genes in multiple tissues and to be associated with alcohol dependence in African-Americans and European-Americans. Important information could be provided by understanding how coding and non-coding variations interact in determining the ADH1B function and how ADH1B regulatory mechanisms are shared with the other ADH genes. Investigating ADH1B molecular pathways, we observed that this gene is involved in metabolism of multiple drugs, including ifosfamide, cyclophosphamide, abacavir, and celecoxib (PharmGKB data). To our knowledge, no study has investigated the effect of the known ADH1B functional alleles on the pharmacokinetics/pharmacodynamics of these drugs. Future studies should also deepen our understanding of ADH1B functions not related to alcohol metabolism. Our recent PheWAS highlighted that ADH1B rs1229984 is associated with a wide range of phenotypic traits and some of these appear not to be mediated by alcohol use [Polimanti and others 2016a]. Additional support regarding non-alcohol-related functions of ADH1B is provided by evolutionary studies. The strong selection signatures observed in the ADH1B locus in Asian populations (with suggestive evidence of convergent evolution in Europeans; [Galinsky and others 2016]) are very likely not related to alcohol consumption, mainly because ALDH2, the other locus affecting alcohol drinking behaviors in Asians (and with an even stronger effect), does not show any genomic selection signature. Both our PheWAS and the evolutionary evidence strongly suggest that ADH1B should present other functions with relevant effects on the human phenome and further studies based on phenome-scan and polygenic adaptation [Polimanti and others 2016c] are needed to clarify the role of ADH1B in human evolution. Our recent genome-wide gene-by alcohol dependence analysis of risky sexual behaviors also indicated that alcohol dependence and its risk alleles may moderate the predisposition to risky behaviors [Polimanti and others 2016b]. Due to its large effects on alcohol dependence, ADH1B is a strong candidate to be investigated in relation to risky behaviors.
Supplementary Material
Supplemental Figure 1: Exon expression for ADH1B gene in subcutaneous adipose tissue and liver (data available at http://www.gtexportal.org/home/).
Supplemental Figure 2: Canonical amino acid sequence of ADH1B protein product. Amino acids highlighted in light blue are those lacking in the ADH1B isoform 2 (data available at http://www.uniprot.org/).
Acknowledgments
This study was supported by National Institutes of Health grants RC2 DA028909, R01 DA12690, R01 DA12849, R01 DA18432, R01 AA11330, R01 AA017535, P50 AA012870, the Connecticut MIRECC, and a NARSAD Young Investigator Award (to RP) from the Brain & Behavior Research Foundation.
References
- 1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Alcohol consumption and cognitive impairment in older men: a mendelian randomization study. Neurology. 2014a;82(12):1038–1044. doi: 10.1212/WNL.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. The triangular association of ADH1B genetic polymorphism, alcohol consumption and the risk of depression in older men. Mol Psychiatry. 2014b;19(9):995–1000. doi: 10.1038/mp.2013.117. [DOI] [PubMed] [Google Scholar]
- Baik I, Cho NH, Kim SH, Han BG, Shin C. Genome-wide association studies identify genetic loci related to alcohol consumption in Korean men. Am J Clin Nutr. 2011;93(4):809–816. doi: 10.3945/ajcn.110.001776. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, Agrawal A, Bucholz KK, Grucza R, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Porjesz B, Saccone NL, Schuckit M, Tischfield J, Wang JC, Foroud T, Rice JP, Edenberg HJ. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17(4):445–450. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JX, Pletscher-Frankild S, Tsafou K, Stolte C, O’Donoghue SI, Schneider R, Jensen LJ. COMPARTMENTS: unification and visualization of protein subcellular localization evidence. Database (Oxford) 2014:bau012. doi: 10.1093/database/bau012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borinskaya SA, Kim AA, Rubanovich AV, Yankovsky NK. The Impact of ADH1B Alleles and Educational Status on Levels and Modes of Alcohol Consumption in Russian Male Individuals. Acta Naturae. 2013;5(3):99–106. [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ, Zakhari S. Acetaldehyde and the genome: beyond nuclear DNA adducts and carcinogenesis. Environmental and molecular mutagenesis. 2014;55(2):77–91. doi: 10.1002/em.21824. [DOI] [PubMed] [Google Scholar]
- Buhler KM, Gine E, Echeverry-Alzate V, Calleja-Conde J, de Fonseca FR, Lopez-Moreno JA. Common single nucleotide variants underlying drug addiction: more than a decade of research. Addict Biol. 2015;20(5):845–871. doi: 10.1111/adb.12204. [DOI] [PubMed] [Google Scholar]
- Carr LG, Foroud T, Stewart T, Castelluccio P, Edenberg HJ, Li TK. Influence of ADH1B polymorphism on alcohol use and its subjective effects in a Jewish population. Am J Med Genet. 2002;112(2):138–143. doi: 10.1002/ajmg.10674. [DOI] [PubMed] [Google Scholar]
- Carrigan MA, Uryasev O, Davis RP, Zhai L, Hurley TD, Benner SA. The natural history of class I primate alcohol dehydrogenases includes gene duplication, gene loss, and gene conversion. PloS one. 2012;7(7):e41175. doi: 10.1371/journal.pone.0041175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CP, Lai CL, Lee SP, Hsu WL, Chi YC, Gao HW, Yao CT, Chau GY, Yin SJ. Ethanol-metabolizing activities and isozyme protein contents of alcohol and aldehyde dehydrogenases in human liver: phenotypic traits of the ADH1B*2 and ALDH2*2 variant gene alleles. Pharmacogenet Genomics. 2016 doi: 10.1097/FPC.0000000000000205. [DOI] [PubMed] [Google Scholar]
- Daub JT, Hofer T, Cutivet E, Dupanloup I, Quintana-Murci L, Robinson-Rechavi M, Excoffier L. Evidence for polygenic adaptation to pathogens in the human genome. Mol Biol Evol. 2013;30(7):1544–1558. doi: 10.1093/molbev/mst080. [DOI] [PubMed] [Google Scholar]
- Deitrich R, Zimatkin S, Pronko S. Oxidation of ethanol in the brain and its consequences. Alcohol Res Health. 2006;29(4):266–273. [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Jacobson SW. Protective effects of the alcohol dehydrogenase-ADH1B*3 allele on attention and behavior problems in adolescents exposed to alcohol during pregnancy. Neurotoxicol Teratol. 2014;41:43–50. doi: 10.1016/j.ntt.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ. Regulation of the mammalian alcohol dehydrogenase genes. Prog Nucleic Acid Res Mol Biol. 2000;64:295–341. doi: 10.1016/s0079-6603(00)64008-4. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liang T, Gizer IR. ADH and ALDH polymorphisms and alcohol dependence in Mexican and Native Americans. Am J Drug Alcohol Abuse. 2012;38(5):389–394. doi: 10.3109/00952990.2012.694526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsyukov A, Ivanov D. Selection variability for Arg48His in alcohol dehydrogenase ADH1B among Asian populations. Hum Biol. 2013;85(4):569–577. doi: 10.3378/027.085.0404. [DOI] [PubMed] [Google Scholar]
- Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, Jassal B, Jupe S, Korninger F, McKay S, Matthews L, May B, Milacic M, Rothfels K, Shamovsky V, Webber M, Weiser J, Williams M, Wu G, Stein L, Hermjakob H, D’Eustachio P. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2016;44(D1):D481–D487. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell CM, O’Leary NA, Harte RA, Loveland JE, Wilming LG, Wallin C, Diekhans M, Barrell D, Searle SM, Aken B, Hiatt SM, Frankish A, Suner MM, Rajput B, Steward CA, Brown GR, Bennett R, Murphy M, Wu W, Kay MP, Hart J, Rajan J, Weber J, Snow C, Riddick LD, Hunt T, Webb D, Thomas M, Tamez P, Rangwala SH, McGarvey KM, Pujar S, Shkeda A, Mudge JM, Gonzalez JM, Gilbert JG, Trevanion SJ, Baertsch R, Harrow JL, Hubbard T, Ostell JM, Haussler D, Pruitt KD. Current status and new features of the Consensus Coding Sequence database. Nucleic Acids Res. 2014;42(Database issue):D865–D872. doi: 10.1093/nar/gkt1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky KJ, Bhatia G, Loh PR, Georgiev S, Mukherjee S, Patterson NJ, Price AL. Fast Principal-Component Analysis Reveals Convergent Evolution of ADH1B in Europe and East Asia. Am J Hum Genet. 2016;98(3):456–472. doi: 10.1016/j.ajhg.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer LY, Marchler-Bauer A, Geer RC, Han L, He J, He S, Liu C, Shi W, Bryant SH. The NCBI BioSystems database. Nucleic Acids Res. 2010;38(Database issue):D492–D496. doi: 10.1093/nar/gkp858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Zhao H, Farrer LA. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19(1):41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner Y, Henkin Y, Schwarzfuchs D, Golan R, Durst R, Shelef I, Harman-Boehm I, Spitzen S, Witkow S, Novack L, Friger M, Tangi-Rosental O, Sefarty D, Bril N, Rein M, Cohen N, Chassidim Y, Sarusi B, Wolak T, Stampfer MJ, Rudich A, Shai I. Differential Effect of Initiating Moderate Red Wine Consumption on 24-h Blood Pressure by Alcohol Dehydrogenase Genotypes: Randomized Trial in Type 2 Diabetes. Am J Hypertens. 2016;29(4):476–483. doi: 10.1093/ajh/hpv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedde HW, Harada S, Agarwal DP. Racial differences in alcohol sensitivity: a new hypothesis. Human genetics. 1979;51(3):331–334. doi: 10.1007/BF00283404. [DOI] [PubMed] [Google Scholar]
- Han Y, Gu S, Oota H, Osier MV, Pakstis AJ, Speed WC, Kidd JR, Kidd KK. Evidence of positive selection on a class I ADH locus. Am J Hum Genet. 2007;80(3):441–456. doi: 10.1086/512485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Lynch KG, Farrer L, Gelernter J, Kranzler HR. Which alcohol use disorder criteria contribute to the association of ADH1B with alcohol dependence? Addict Biol. 2016;21(4):924–938. doi: 10.1111/adb.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka A, Sasazuki S, Matsuo K, Ito H, Sawada N, Shimazu T, Yamaji T, Iwasaki M, Inoue M, Tsugane S, Group JS. Genetic polymorphisms of ADH1B, ADH1C and ALDH2, alcohol consumption, and the risk of gastric cancer: the Japan Public Health Center-based prospective study. Carcinogenesis. 2015;36(2):223–231. doi: 10.1093/carcin/bgu244. [DOI] [PubMed] [Google Scholar]
- Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, Prieto-Merino D, Dehghan A, Trompet S, Wong A, Cavadino A, Drogan D, Padmanabhan S, Li S, Yesupriya A, Leusink M, Sundstrom J, Hubacek JA, Pikhart H, Swerdlow DI, Panayiotou AG, Borinskaya SA, Finan C, Shah S, Kuchenbaecker KB, Shah T, Engmann J, Folkersen L, Eriksson P, Ricceri F, Melander O, Sacerdote C, Gamble DM, Rayaprolu S, Ross OA, McLachlan S, Vikhireva O, Sluijs I, Scott RA, Adamkova V, Flicker L, Bockxmeer FM, Power C, Marques-Vidal P, Meade T, Marmot MG, Ferro JM, Paulos-Pinheiro S, Humphries SE, Talmud PJ, Mateo Leach I, Verweij N, Linneberg A, Skaaby T, Doevendans PA, Cramer MJ, van der Harst P, Klungel OH, Dowling NF, Dominiczak AF, Kumari M, Nicolaides AN, Weikert C, Boeing H, Ebrahim S, Gaunt TR, Price JF, Lannfelt L, Peasey A, Kubinova R, Pajak A, Malyutina S, Voevoda MI, Tamosiunas A, Maitland-van der Zee AH, Norman PE, Hankey GJ, Bergmann MM, Hofman A, Franco OH, Cooper J, Palmen J, Spiering W, de Jong PA, Kuh D, Hardy R, Uitterlinden AG, Ikram MA, Ford I, Hypponen E, Almeida OP, Wareham NJ, Khaw KT, Hamsten A, Husemoen LL, Tjonneland A, Tolstrup JS, Rimm E, Beulens JW, Verschuren WM, Onland-Moret NC, Hofker MH, Wannamethee SG, Whincup PH, Morris R, Vicente AM, Watkins H, Farrall M, Jukema JW, Meschia J, Cupples LA, Sharp SJ, Fornage M, Kooperberg C, LaCroix AZ, Dai JY, Lanktree MB, Siscovick DS, Jorgenson E, Spring B, Coresh J, Li YR, Buxbaum SG, Schreiner PJ, Ellison RC, Tsai MY, Patel SR, Redline S, Johnson AD, Hoogeveen RC, Hakonarson H, Rotter JI, Boerwinkle E, de Bakker PI, Kivimaki M, Asselbergs FW, Sattar N, Lawlor DA, Whittaker J, Davey Smith G, Mukamal K, Psaty BM, Wilson JG, Lange LA, Hamidovic A, Hingorani AD, Nordestgaard BG, Bobak M, Leon DA, Langenberg C, Palmer TM, Reiner AP, Keating BJ, Dudbridge F, Casas JP, InterAct C. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley TD, Edenberg HJ, Li T-K. Pharmacogenomics of Alcoholism. Pharmacogenomics. KGaA: Wiley-VCH Verlag GmbH & Co; 2003. pp. p417–p441. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. Lyon, France. Geneva: International Agency for Research on Cancer; Distributed by WHO Press; 2010. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal Habits and Indoor Combustions. Lyon, France. Geneva: International Agency for Research on Cancer; Distributed by WHO Press; 2012. [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Fujiyoshi T, Kurachi K, Yoshida A. Molecular cloning of a full-length cDNA for human alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1985;82(9):2703–2707. doi: 10.1073/pnas.82.9.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagemoto K, Urabe Y, Miwata T, Oka S, Ochi H, Kitadai Y, Tanaka S, Chayama K. ADH1B and ALDH2 are associated with metachronous SCC after endoscopic submucosal dissection of esophageal squamous cell carcinoma. Cancer Med. 2016;5(7):1397–1404. doi: 10.1002/cam4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Bae KY, Kim SW, Kim J, Shin HY, Kim JM, Shin IS, Yoon JS, Kim JK. Effect of the allelic variant of alcohol dehydrogenase ADH1B*2 on ethanol metabolism. Alcoholism, clinical and experimental research. 2014;38(6):1502–1509. doi: 10.1111/acer.12427. [DOI] [PubMed] [Google Scholar]
- Kropotova ES, Zinovieva OL, Zyryanova AF, Dybovaya VI, Prasolov VS, Beresten SF, Oparina NY, Mashkova TD. Altered expression of multiple genes involved in retinoic acid biosynthesis in human colorectal cancer. Pathol Oncol Res. 2014;20(3):707–717. doi: 10.1007/s12253-014-9751-4. [DOI] [PubMed] [Google Scholar]
- Kumari M, Holmes MV, Dale CE, Hubacek JA, Palmer TM, Pikhart H, Peasey A, Britton A, Horvat P, Kubinova R, Malyutina S, Pajak A, Tamosiunas A, Shankar A, Singh-Manoux A, Voevoda M, Kivimaki M, Hingorani AD, Marmot MG, Casas JP, Bobak M. Alcohol consumption and cognitive performance: a Mendelian randomization study. Addiction. 2014;109(9):1462–1471. doi: 10.1111/add.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latvala A, Rose RJ, Pulkkinen L, Dick DM, Korhonen T, Kaprio J. Drinking, smoking, and educational achievement: cross-lagged associations from adolescence to adulthood. Drug Alcohol Depend. 2014;137:106–113. doi: 10.1016/j.drugalcdep.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Nordestgaard BG, Benn M, Zuccolo L, Tybjaerg-Hansen A, Davey Smith G. Exploring causal associations between alcohol and coronary heart disease risk factors: findings from a Mendelian randomization study in the Copenhagen General Population Study. Eur Heart J. 2013;34(32):2519–2528. doi: 10.1093/eurheartj/eht081. [DOI] [PubMed] [Google Scholar]
- Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011a;70(6):504–512. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gu S, Han Y, Xu Z, Pakstis AJ, Jin L, Kidd JR, Kidd KK. Diversification of the ADH1B gene during expansion of modern humans. Ann Hum Genet. 2011b;75(4):497–507. doi: 10.1111/j.1469-1809.2011.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mukherjee N, Soundararajan U, Tarnok Z, Barta C, Khaliq S, Mohyuddin A, Kajuna SL, Mehdi SQ, Kidd JR, Kidd KK. Geographically separate increases in the frequency of the derived ADH1B*47His allele in eastern and western Asia. Am J Hum Genet. 2007;81(4):842–846. doi: 10.1086/521201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, Cann HM, Barsh GS, Feldman M, Cavalli-Sforza LL, Myers RM. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319(5866):1100–1104. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang HI, Lee MH, Jen CL, Hu HH, Lu SN, Wang LY, You SL, Huang YT, Chen CJ. Alcohol Drinking Mediates the Association between Polymorphisms of ADH1B and ALDH2 and Hepatitis B-Related Hepatocellular Carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(4):693–699. doi: 10.1158/1055-9965.EPI-15-0961. [DOI] [PubMed] [Google Scholar]
- Liu X, Gao Y, Zhao B, Li X, Lu Y, Zhang J, Li D, Li L, Yin F. Discovery of microarray-identified genes associated with ovarian cancer progression. Int J Oncol. 2015;46(6):2467–2478. doi: 10.3892/ijo.2015.2971. [DOI] [PubMed] [Google Scholar]
- Loh PR, Bhatia G, Gusev A, Finucane HK, Bulik-Sullivan BK, Pollack SJ, Schizophrenia Working Group of Psychiatric Genomics C de Candia TR, Lee SH, Wray NR, Kendler KS, O’Donovan MC, Neale BM, Patterson N, Price AL. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet. 2015;47(12):1385–1392. doi: 10.1038/ng.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Kang L, Hu K, Wang C, Sun X, Chen F, Kidd JR, Kidd KK, Li H. High diversity and no significant selection signal of human ADH1B gene in Tibet. Investig Genet. 2012;3(1):23. doi: 10.1186/2041-2223-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Schork NJ, Gelernter J. Diplotype trend regression analysis of the ADH gene cluster and the ALDH2 gene: multiple significant associations with alcohol dependence. American journal of human genetics. 2006;78(6):973–987. doi: 10.1086/504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutwak-Mann C. Alcohol dehydrogenase of animal tissues. Biochem J. 1938;32(8):1364–1374. doi: 10.1042/bj0321364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane M UniProt Consortium. UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford) 2011;2011:bar009. doi: 10.1093/database/bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka H, Ito H, Soga N, Hosono S, Oze I, Watanabe M, Tanaka H, Yokomizo A, Hayashi N, Eto M, Matsuo K. Aldehyde dehydrogenase 2 (ALDH2) and alcohol dehydrogenase 1B (ADH1B) polymorphisms exacerbate bladder cancer risk associated with alcohol drinking: gene-environment interaction. Carcinogenesis. 2016;37(6):583–588. doi: 10.1093/carcin/bgw033. [DOI] [PubMed] [Google Scholar]
- Maurya SS, Anand G, Dhawan A, Khan AJ, Jain SK, Pant MC, Parmar D. Polymorphisms in drug-metabolizing enzymes and risk to head and neck cancer: evidence for gene-gene and gene-environment interaction. Environmental and molecular mutagenesis. 2014;55(2):134–144. doi: 10.1002/em.21837. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Pedersen SL, Lobos EA, Todd RD, Wall TL. ADH1B*3 and response to alcohol in African-Americans. Alcoholism, clinical and experimental research. 2010;34(7):1274–1281. doi: 10.1111/j.1530-0277.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JD, Truong T, Gaborieau V, Chabrier A, Chuang SC, Byrnes G, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Bucur A, Bencko V, Holcatova I, Janout V, Foretova L, Lagiou P, Trichopoulos D, Benhamou S, Bouchardy C, Ahrens W, Merletti F, Richiardi L, Talamini R, Barzan L, Kjaerheim K, Macfarlane GJ, Macfarlane TV, Simonato L, Canova C, Agudo A, Castellsague X, Lowry R, Conway DI, McKinney PA, Healy CM, Toner ME, Znaor A, Curado MP, Koifman S, Menezes A, Wunsch-Filho V, Neto JE, Garrote LF, Boccia S, Cadoni G, Arzani D, Olshan AF, Weissler MC, Funkhouser WK, Luo J, Lubinski J, Trubicka J, Lener M, Oszutowska D, Schwartz SM, Chen C, Fish S, Doody DR, Muscat JE, Lazarus P, Gallagher CJ, Chang SC, Zhang ZF, Wei Q, Sturgis EM, Wang LE, Franceschi S, Herrero R, Kelsey KT, McClean MD, Marsit CJ, Nelson HH, Romkes M, Buch S, Nukui T, Zhong S, Lacko M, Manni JJ, Peters WH, Hung RJ, McLaughlin J, Vatten L, Njolstad I, Goodman GE, Field JK, Liloglou T, Vineis P, Clavel-Chapelon F, Palli D, Tumino R, Krogh V, Panico S, Gonzalez CA, Quiros JR, Martinez C, Navarro C, Ardanaz E, Larranaga N, Khaw KT, Key T, Bueno-de-Mesquita HB, Peeters PH, Trichopoulou A, Linseisen J, Boeing H, Hallmans G, Overvad K, Tjonneland A, Kumle M, Riboli E, Valk K, Vooder T, Metspalu A, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Blanche H, Gut IG, Galan P, Heath S, Hashibe M, Hayes RB, Boffetta P, Lathrop M, Brennan P. A genome-wide association study of upper aerodigestive tract cancers conducted within the INHANCE consortium. PLoS genetics. 2011;7(3):e1001333. doi: 10.1371/journal.pgen.1001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, Young TR, Goldmann JM, Pervouchine DD, Sullivan TJ, Johnson R, Segre AV, Djebali S, Niarchou A GTEx Consortium. Wright FA, Lappalainen T, Calvo M, Getz G, Dermitzakis ET, Ardlie KG, Guigo R. Human genomics. The human transcriptome across tissues and individuals. Science. 2015;348(6235):660–665. doi: 10.1126/science.aaa0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Shmulewitz D, Wall MM, Keyes KM, Aharonovich E, Spivak B, Weizman A, Frisch A, Edenberg HJ, Gelernter J, Grant BF, Hasin D. Childhood adversity moderates the effect of ADH1B on risk for alcohol-related phenotypes in Jewish Israeli drinkers. Addict Biol. 2015;20(1):205–214. doi: 10.1111/adb.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negelein E, Wulff HJ. Diphosphopyridinproteid ackohol, acetaldehyd. Biochem Z. 1937:351–389. [Google Scholar]
- Olfson E, Edenberg HJ, Nurnberger J, Jr, Agrawal A, Bucholz KK, Almasy LA, Chorlian D, Dick DM, Hesselbrock VM, Kramer JR, Kuperman S, Porjesz B, Schuckit MA, Tischfield JA, Wang JC, Wetherill L, Foroud TM, Rice J, Goate A, Bierut LJ. An ADH1B variant and peer drinking in progression to adolescent drinking milestones: evidence of a gene-by-environment interaction. Alcoholism, clinical and experimental research. 2014;38(10):2541–2549. doi: 10.1111/acer.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oota H, Dunn CW, Speed WC, Pakstis AJ, Palmatier MA, Kidd JR, Kidd KK. Conservative evolution in duplicated genes of the primate Class I ADH cluster. Gene. 2007;392(1–2):64–76. doi: 10.1016/j.gene.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Osier MV, Pakstis AJ, Soodyall H, Comas D, Goldman D, Odunsi A, Okonofua F, Parnas J, Schulz LO, Bertranpetit J, Bonne-Tamir B, Lu RB, Kidd JR, Kidd KK. A global perspective on genetic variation at the ADH genes reveals unusual patterns of linkage disequilibrium and diversity. Am J Hum Genet. 2002;71(1):84–99. doi: 10.1086/341290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Park CS, Lee HS, Park KS, Park BL, Cheong HS, Shin HD. Functional polymorphism in aldehyde dehydrogenase-2 gene associated with risk of tuberculosis. BMC Med Genet. 2014;15:40. doi: 10.1186/1471-2350-15-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng GS, Chen YC, Wang MF, Lai CL, Yin SJ. ALDH2*2 but not ADH1B*2 is a causative variant gene allele for Asian alcohol flushing after a low-dose challenge: correlation of the pharmacokinetic and pharmacodynamic findings. Pharmacogenet Genomics. 2014;24(12):607–617. doi: 10.1097/FPC.0000000000000096. [DOI] [PubMed] [Google Scholar]
- Peng Y, Shi H, Qi XB, Xiao CJ, Zhong H, Ma RL, Su B. The ADH1B Arg47His polymorphism in east Asian populations and expansion of rice domestication in history. BMC Evol Biol. 2010;10:15. doi: 10.1186/1471-2148-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson EC, Schwartz LM, Park Y, Trabert B, Hollenbeck AR, Graubard BI, Freedman ND, McGlynn KA. Alcohol consumption, folate intake, hepatocellular carcinoma, and liver disease mortality. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(3):415–421. doi: 10.1158/1055-9965.EPI-12-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BM, Huerta-Sanchez E, Nielsen R. Distinguishing between selective sweeps from standing variation and from a de novo mutation. PLoS genetics. 2012;8(10):e1003011. doi: 10.1371/journal.pgen.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Kranzler HR, Gelernter J. Phenome-Wide Association Study for Alcohol and Nicotine Risk Alleles in 26394 Women. Neuropsychopharmacology. 2016a doi: 10.1038/npp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Wang Q, Meda SA, Patel KT, Pearlson GD, Zhao H, Farrer L, Kranzler HR, Gelernter J. The interplay between risky sexual behaviors and alcohol dependence: genome-wide association and neuroimaging support for LHPP as a risk gene. Neuropsychopharmacology. 2016b doi: 10.1038/npp.2016.153. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Yang BZ, Zhao H, Gelernter J. Evidence of Polygenic Adaptation in the Systems Genetics of Anthropometric Traits. PloS one. 2016c;11(8):e0160654. doi: 10.1371/journal.pone.0160654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Yang C, Zhao H, Gelernter J. Dissecting ancestry genomic background in substance dependence genome-wide association studies. Pharmacogenomics. 2015a;16(13):1487–1498. doi: 10.2217/pgs.15.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Zhang H, Smith AH, Zhao H, Farrer LA, Kranzler HR, Gelernter J. Genome-wide association study of body mass index in subjects with alcohol dependence. Addict Biol. 2015b doi: 10.1111/adb.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen EE, Chen XD, Almasy L, Yang F, He H, Li X, Wang XY, Liu TQ, Hao W, Deng HW, Kranzler HR, Gelernter J. ALDH2 is associated to alcohol dependence and is the major genetic determinant of “daily maximum drinks” in a GWAS study of an isolated rural Chinese sample. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(2):103–110. doi: 10.1002/ajmg.b.32213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P, Wagg J, Green ML, Kaiser D, Krummenacker M, Karp PD. Computational prediction of human metabolic pathways from the complete human genome. Genome Biol. 2005;6(1):R2. doi: 10.1186/gb-2004-6-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Wang Z, Xu K, Kranzler HR, Gelernter J. The joint effects of ADH1B variants and childhood adversity on alcohol related phenotypes in African-American and European-American women and men. Alcoholism, clinical and experimental research. 2014;38(12):2907–2914. doi: 10.1111/acer.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Wang B, Luo J, Jiang K, Zhang H, Mustonen H, Puolakkainen P, Zhu J, Ye Y, Wang S. Global-scale profiling of differential expressed lysine acetylated proteins in colorectal cancer tumors and paired liver metastases. J Proteomics. 2016;142:24–32. doi: 10.1016/j.jprot.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Siegert S, Hampe J, Schafmayer C, von Schonfels W, Egberts JH, Forsti A, Chen B, Lascorz J, Hemminki K, Franke A, Nothnagel M, Nothlings U, Krawczak M. Genome-wide investigation of gene-environment interactions in colorectal cancer. Human genetics. 2013;132(2):219–231. doi: 10.1007/s00439-012-1239-2. [DOI] [PubMed] [Google Scholar]
- Silverwood RJ, Holmes MV, Dale CE, Lawlor DA, Whittaker JC, Smith GD, Leon DA, Palmer T, Keating BJ, Zuccolo L, Casas JP, Dudbridge F Alcohol ADHBC. Testing for non-linear causal effects using a binary genotype in a Mendelian randomization study: application to alcohol and cardiovascular traits. Int J Epidemiol. 2014;43(6):1781–1790. doi: 10.1093/ije/dyu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G, Chen SH, Fukui M. Liver alcohol dehydrogenase in Japanese: high population frequency of atypical form and its possible role in alcohol sensitivity. Am J Hum Genet. 1975;27(6):789–796. [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. Questions about DISC1 as a genetic risk factor for schizophrenia. Mol Psychiatry. 2013;18(10):1050–1052. doi: 10.1038/mp.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi F, Isono M, Nabika T, Katsuya T, Sugiyama T, Yamaguchi S, Kobayashi S, Ogihara T, Yamori Y, Fujioka A, Kato N. Confirmation of ALDH2 as a Major locus of drinking behavior and of its variants regulating multiple metabolic phenotypes in a Japanese population. Circ J. 2011;75(4):911–918. doi: 10.1253/circj.cj-10-0774. [DOI] [PubMed] [Google Scholar]
- Tews D, Schwar V, Scheithauer M, Weber T, Fromme T, Klingenspor M, Barth TF, Moller P, Holzmann K, Debatin KM, Fischer-Posovszky P, Wabitsch M. Comparative gene array analysis of progenitor cells from human paired deep neck and subcutaneous adipose tissue. Mol Cell Endocrinol. 2014;395(1–2):41–50. doi: 10.1016/j.mce.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Thomasson HR, Edenberg HJ, Crabb DW, Mai XL, Jerome RE, Li TK, Wang SP, Lin YT, Lu RB, Yin SJ. Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. Am J Hum Genet. 1991;48(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S, Egashira A, Saeki H, Kakeji Y, Morita M, Sakaguchi Y, Okamura T, Maehara Y. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: molecular mechanisms of carcinogenesis. International journal of clinical oncology. 2010;15(2):135–144. doi: 10.1007/s10147-010-0057-6. [DOI] [PubMed] [Google Scholar]
- Tolstrup JS, Gronbaek M, Tybjaerg-Hansen A, Nordestgaard BG. Alcohol intake, alcohol dehydrogenase genotypes, and liver damage and disease in the Danish general population. Am J Gastroenterol. 2009;104(9):2182–2188. doi: 10.1038/ajg.2009.370. [DOI] [PubMed] [Google Scholar]
- Tsai ST, Wong TY, Ou CY, Fang SY, Chen KC, Hsiao JR, Huang CC, Lee WT, Lo HI, Huang JS, Wu JL, Yen CJ, Hsueh WT, Wu YH, Yang MW, Lin FC, Chang JY, Chang KY, Wu SY, Liao HC, Lin CL, Wang YH, Weng YL, Yang HC, Chang JS. The interplay between alcohol consumption, oral hygiene, ALDH2 and ADH1B in the risk of head and neck cancer. Int J Cancer. 2014;135(10):2424–2436. doi: 10.1002/ijc.28885. [DOI] [PubMed] [Google Scholar]
- Tucker SL, Gharpure K, Herbrich SM, Unruh AK, Nick AM, Crane EK, Coleman RL, Guenthoer J, Dalton HJ, Wu SY, Rupaimoole R, Lopez-Berestein G, Ozpolat B, Ivan C, Hu W, Baggerly KA, Sood AK. Molecular biomarkers of residual disease after surgical debulking of high-grade serous ovarian cancer. Clin Cancer Res. 2014;20(12):3280–3288. doi: 10.1158/1078-0432.CCR-14-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udali S, Guarini P, Ruzzenente A, Ferrarini A, Guglielmi A, Lotto V, Tononi P, Pattini P, Moruzzi S, Campagnaro T, Conci S, Olivieri O, Corrocher R, Delledonne M, Choi SW, Friso S. DNA methylation and gene expression profiles show novel regulatory pathways in hepatocellular carcinoma. Clin Epigenetics. 2015;7:43. doi: 10.1186/s13148-015-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Rey M, Woodhoo A, Martinez-Chantar ML, Mato JM, Lu SC. Alcohol, DNA methylation, and cancer. Alcohol research : current reviews. 2013;35(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- von Hink Kessler Scholder S, Wehby GL, Lewis S, Zuccolo L. Alcohol Exposure In Utero and Child Academic Achievement. Econ J (London) 2014;124(576):634–667. doi: 10.1111/ecoj.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Zhou PY, Liu P, Zhang Y. ALDH2 and ADH1 genetic polymorphisms may contribute to the risk of gastric cancer: a meta-analysis. PloS one. 2014a;9(3):e88779. doi: 10.1371/journal.pone.0088779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang J, Wei J, Xu X, Pan W, Ge Y, Zhou C, Liu C, Gao J, Yang M, Mao W. Replication study of ESCC susceptibility genetic polymorphisms locating in the ADH1B–ADH1C–ADH7 cluster identified by GWAS. PloS one. 2014b;9(4):e94096. doi: 10.1371/journal.pone.0094096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Huang X, Li R, Xu H, Jin L, He Y. Detecting recent positive selection with high accuracy and reliability by conditional coalescent tree. Mol Biol Evol. 2014c;31(11):3068–3080. doi: 10.1093/molbev/msu244. [DOI] [PubMed] [Google Scholar]
- Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, Altman RB, Klein TE. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier DA, Fourcaudot M, Norton L, Abdul-Ghani MA, Hu SL, Farook VS, Coletta DK, Kumar S, Puppala S, Chittoor G, Dyer TD, Arya R, Carless M, Lehman DM, Curran JE, Cromack DT, Tripathy D, Blangero J, Duggirala R, Goring HH, DeFronzo RA, Jenkinson CP. Transcriptomic identification of ADH1B as a novel candidate gene for obesity and insulin resistance in human adipose tissue in Mexican Americans from the Veterans Administration Genetic Epidemiology Study (VAGES) PloS one. 2015;10(4):e0119941. doi: 10.1371/journal.pone.0119941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff PH. Ethnic differences in alcohol sensitivity. Science. 1972;175(4020):449–450. doi: 10.1126/science.175.4020.449. [DOI] [PubMed] [Google Scholar]
- Wu C, Kraft P, Zhai K, Chang J, Wang Z, Li Y, Hu Z, He Z, Jia W, Abnet CC, Liang L, Hu N, Miao X, Zhou Y, Liu Z, Zhan Q, Liu Y, Qiao Y, Zhou Y, Jin G, Guo C, Lu C, Yang H, Fu J, Yu D, Freedman ND, Ding T, Tan W, Goldstein AM, Wu T, Shen H, Ke Y, Zeng Y, Chanock SJ, Taylor PR, Lin D. Genome-wide association analyses of esophageal squamous cell carcinoma in Chinese identify multiple susceptibility loci and gene-environment interactions. Nat Genet. 2012;44(10):1090–1097. doi: 10.1038/ng.2411. [DOI] [PubMed] [Google Scholar]
- Xu K, Kranzler HR, Sherva R, Sartor CE, Almasy L, Koesterer R, Zhao H, Farrer LA, Gelernter J. Genomewide Association Study for Maximum Number of Alcoholic Drinks in European Americans and African Americans. Alcoholism, clinical and experimental research. 2015;39(7):1137–1147. doi: 10.1111/acer.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Brooks PJ, Yokoyama T, Mizukami T, Matsui T, Kimura M, Matsushita S, Higuchi S, Maruyama K. Blood Leukocyte Counts and Genetic Polymorphisms of Alcohol Dehydrogenase-1B and Aldehyde Dehydrogenase-2 in Japanese Alcoholic Men. Alcoholism, clinical and experimental research. 2016;40(3):507–517. doi: 10.1111/acer.12983. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Yokoyama T, Matsui T, Mizukami T, Kimura M, Matsushita S, Higuchi S, Maruyama K. Alcohol Dehydrogenase-1B (rs1229984) and Aldehyde Dehydrogenase-2 (rs671) Genotypes Are Strong Determinants of the Serum Triglyceride and Cholesterol Levels of Japanese Alcoholic Men. PloS one. 2015;10(8):e0133460. doi: 10.1371/journal.pone.0133460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29(4):245–254. [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang F, Kranzler HR, Yang C, Xu H, Wang Z, Zhao H, Gelernter J. Identification of methylation quantitative trait loci (mQTLs) influencing promoter DNA methylation of alcohol dependence risk genes. Human genetics. 2014a;133(9):1093–1104. doi: 10.1007/s00439-014-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jiang Y, Wu Q, Li Q, Chen D, Xu L, Zhang C, Zhang M, Ye L. Gene-environment interactions on the risk of esophageal cancer among Asian populations with the G48A polymorphism in the alcohol dehydrogenase-2 gene: a meta-analysis. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014b;35(5):4705–4717. doi: 10.1007/s13277-014-1616-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu N, Miao L, Yuan H, Wang R, Jiang H. Alcohol dehydrogenase-1B Arg47His polymorphism is associated with head and neck cancer risk in Asian: a meta-analysis. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(2):1023–1027. doi: 10.1007/s13277-014-2727-x. [DOI] [PubMed] [Google Scholar]
- Zuccolo L, Lewis SJ, Smith GD, Sayal K, Draper ES, Fraser R, Barrow M, Alati R, Ring S, Macleod J, Golding J, Heron J, Gray R. Prenatal alcohol exposure and offspring cognition and school performance. A ‘Mendelian randomization’ natural experiment. Int J Epidemiol. 2013;42(5):1358–1370. doi: 10.1093/ije/dyt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Exon expression for ADH1B gene in subcutaneous adipose tissue and liver (data available at http://www.gtexportal.org/home/).
Supplemental Figure 2: Canonical amino acid sequence of ADH1B protein product. Amino acids highlighted in light blue are those lacking in the ADH1B isoform 2 (data available at http://www.uniprot.org/).