Abstract
Progress in deriving a spectrum of central nervous system cell phenotypes from human pluripotent stem cells has spurred significant advances in in vitro modeling and development of regenerative therapies for neurological disorders. While the clinical impact of these advances is still being evaluated, their integration with advanced tissue engineering methodologies and therapeutic approaches that induce neural circuit plasticity, respectively, remain underexplored frontiers.
Graphical abstract
Introduction
Over the past 15 years, there has been revolutionary progress in deriving human central nervous system (CNS) cell phenotypes and tissues. Successful cultivation of human embryonic stem cells (hESCs) in 1998 [1] was quickly followed by their differentiation into neuroepithelial cells [2]. These are the germinal neural stem cells (NSCs) that constitute the embryonic neural tube from which all CNS tissues arise. This in vitro NSC phenotype turned out to be remarkably analogous to its in vivo counterpart, and through direct implementation of developmental biology principles, scientists rapidly generated numerous neuronal and glial phenotypes (Fig. 1a). Practical application of these findings was further propelled by the discovery of induced pluripotent stem cells (iPSCs) [3]. This enabled generation of patient-specific CNS cells and 2-D cultures for disease modeling as well as conception of an autologous regenerative cell therapy supply chain. More recently, NSC’s have been observed to display powerful emergent properties. This is evidenced by their ability to spontaneously recapitulate extensive levels of ex vivo morphogenesis to generate 3-D tissues, a.k.a. organoids [4–6], with levels of cell phenotype diversity and microscale cytoarchitectures mimetic of those in the human fetal CNS.
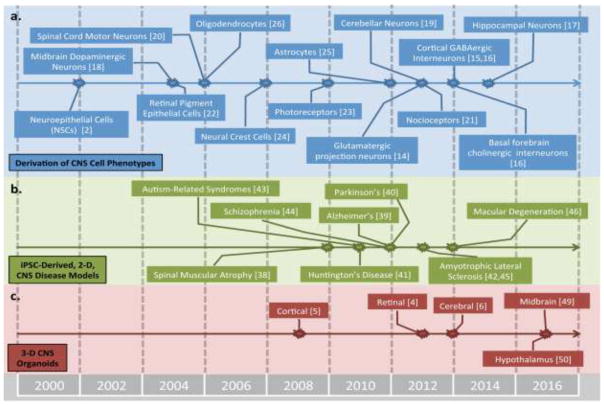
Figure 1.
Chronology of progress in deriving CNS cells and tissues. Timeline list seminal studies describing derivation of human CNS (a) regional cell phenotypes, (b) 2-D disease-in-a-dish models, and (c) 3-D organoids from hPSCs. Includes references cited in the review; it is not a comprehensive list.
Here, we provide a synopsis of progress in deriving human CNS cell phenotypes, translating these cells towards regenerative clinical therapies, and engineering 2-D cultures and 3-D organoids humanoid models of neurological disorders. While these are tremendous advancements, full realization of their therapeutic and modeling potential will require developing interdisciplinary approaches using technologies and methodologies from related fields, e.g. tissue engineering and neuromodulation. Such mergers could enhance engraftment of regenerative cell therapies as well as enable instructed ex vivo morphogenesis of anatomically and physiologically mimetic 3-D CNS tissue units that could one day serve as transplants.
Deriving CNS cell phenotypes
For decades, human neural cells and clinical transplants were solely isolated from fetal tissue sources [7]. Today, this has been almost universally supplanted by the more sustainable human pluripotent stem cell (hPSC, i.e. hESC and iPSC) source. NSC derivation protocols have advanced from re-plating hPSC-derived embryoid bodies and physically isolating polarized NSC structures, a.k.a. neural rosettes [2], to highly efficient differentiations using hPSC monolayers in the absence of [8] or while antagonizing [9] transforming growth factor-beta (TGF-β) and bone morphogenetic protein (BMP) pathway activity. Human PSC-derived NSCs are identified in culture by a polarized, columnar morphology and co-expression of Pax6/Sox2/N-cadherin [8]. Also, they default to a rostral, forebrain fate in the absence of exogenous morphogens [10].
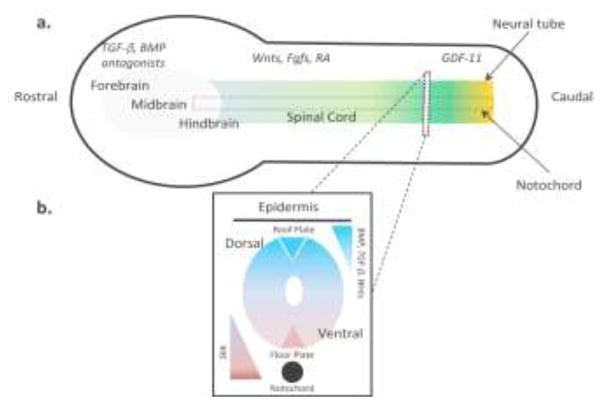
In line with neurodevelopmental biology principles, patterning NSCs to diverse regional phenotypes along the CNS rostrocaudal (R/C) and dorsoventral (D/V) axes requires early morphogenic interventions (Fig. 2). Prior to acquisition of a Pax6+ NSC state, exposure of neurally differentiating cultures to Wnts and Fibroblast growth factors (Fgfs) induces an intermediate Brachyury (T)+/Sox2+/Pax6− neuromesodermal progenitor (NMP) phenotype [11]. In the NMP state, the cells continually transition from a rostral to caudal CNS phenotype, and the extent of caudalization is deterministically patterned by the duration of Wnt/Fgf/Growth differentiation factor-11 (GDF-11) signaling (Fig. 2a). Activating retinoic acid (RA) signaling at any point during the caudalization time course induces full neural conversion to a T−/Sox2+/Pax6+ NSC state and fixes its R/C positioning [11]. Concurrent with RA-induced neural induction, modulation of Wnt [10] and Tgf-β [11] (dorsal) or Sonic Hedgehog (Shh, ventral) [10] signaling can be used to pattern the NSC phenotype along the CNS D/V axis (Fig. 2b). BMP signaling is also known to play a role in dorsalizing NSCs in vivo [12], but has yet to be used analogously in hPSC neural differentiation protocols. Upon completion of regional phenotype patterning, NSCs can be cultured as neurospheres [2] or in monolayers [11] and immediately begin generating further differentiated region-specific neuronal progenitors. Moreover, the NSCs transition from an early epithelial to mid and late phenotype over successive passages [13].
Figure 2.
Schematic of signaling pathways modulated by researchers to mimic CNS development ex vivo. a) Morphogens gradients for patterning regional phenotypes along the rostrocaudal (R/C) axis. Forebrain tissues are patterned in the absence of TGF-β/BMP signaling, and Wnts/Fgfs/RA signaling is modulated to pattern more caudal tissues. GDF-11 is added to access the most caudal spinal cord phenotypes. b) Morphogens gradients for patterning regional phenotypes along the dorsoventral (D/V) axis. The ventral neural tube is patterned by graded Shh first from the notochord and then the floor plate. The dorsal neural tube is patterned by graded BMP, TGFβ, and Wnt signaling first from the epidermis and then the roof plate.
Analogous to their in vivo counterparts, NSCs transition sequentially through neurogenic, astrogenic, and oligodendrogenic progenitor states. The progeny’s regional phenotype is dictated by that of NSCs’, and neurons can be derived after 2–4 weeks of culture [8]. As illustrated in Figure 1a, numerous CNS neuronal subtypes have been derived, including cortical glutamatergic projection neurons [14] and GABAergic interneurons [15,16], basal forebrain cholinergic [16], hippocampal dentate gyrus granular neurons [17], midbrain dopaminergic neurons [18], cerebellar granule neurons [19], and spinal cord motor [20] and sensory neurons [21]. Human PSCs can also be differentiated into retinal pigment epithelium (RPE) [22] and retinal progenitor cells (RPCs) that give rise to rod and cone photoreceptors upon transplantation [23]. Neural crest cells can be isolated shortly after neural induction from the periphery of neural rosettes [24]. Alternatively, astrocytes [25] and oligodendrocytes [26] can only be generated after 1–2 and >2 months of NSCs culture, respectively.
Regenerating CNS tissues
With access to diverse CNS cell phenotypes, clinical translation of hPSC-derived, regenerative therapies began in 2009 with Geron Corporation’s FDA-approved trial of hESC-derived oligodendrocyte progenitor cells (OPCs) to treat spinal cord injury [27]. The trial was abandoned in 2011 for financial reasons, but has since been completed by Asterias Biotherapeutics (NCT01217008) along with an ongoing trial testing their own AST-OPC1 line (NCT02302157). In a January 2017 press release, Asterias announced that complete cervical spinal cord injury (AIS-A) patients administered with 10 million AST-OPC1s showed a positive safety profile and improvements in upper extremity motor function at 6- and 9-month follow-ups [28]. Since Geron’s initial studies, clinical trials testing the regenerative efficacy of hESC-derived therapies for degenerative retinal disorders have predominated. Six different entities have eight different ongoing clinical trials. This includes RPE cell therapy trials by Southwest Hospital in China (NCT02748734, 2016), Astellas Institute for Regenerative Medicine (NCT01344993, NCT01345006, and NCT01469832, 2011), and Cell Cure Neuroscience LTD (NCT02286089, 2014) for various forms of macular degeneration. jCyte Inc. is conducting an RPC cell therapy trial (NCT02320812; 2014) to treat retinitis pigmentosa. Pfizer’s hESC-derived RPE trial (NCT01691261, 2012) is currently on hold, and the first iPSC-derived RPE trial being conducted by the RIKEN Institute (2014) is planning to resume shortly following recent demonstration of successful allogeneic transplantation in HLA-matched primates [29]. Also, the Federal University of São Paulo (NCT02903576, 2016) is testing the regenerative efficacy of an implanted bioengineered RPE layer as compared to a standard bolus injection of a cell suspension.
Outside of the retinal space, only one additional human PSC-derived therapy for CNS regeneration is in clinical trials. International Stem Cell Corporation is currently testing whether human parthenogenetic embryonic stem cell-derived NSCs (ISC-hpNSC) can be used to treat Parkinson’s patients (NCT02452723, 2015). In July 2016, they announced successful intracranial transplant of 30 million ISC-hpNSCs into their first patient [30]. Clinical trials of other hPSC-derived neuronal progenitor cell therapies are on the horizon as indicated by elegant proof-of-principle studies in animal models for Parkinson’s [31], Huntington’s [32], Amyotrophic Lateral Sclerosis (ALS) [33], Epilepsy [34], and learning and memory disorders [16]. A common theme throughout these studies is the critical importance of matching the cell therapy’s regional phenotype with the transplantation site to effectively reconstitute degenerated neuronal circuitry.
Engineering CNS tissues
While still awaiting full clinical implementation, hPSC-derived CNS cells have drastically enhanced our ability to create in vitro models of human neural development, physiology, toxicity, and disease. Novel insights into signaling pathways and cell phenotypes involved in development of various regional CNS tissues have been elucidated through analysis of in vitro hPSC differentiation processes [11,13]. Cells, tissues, and microphysiological systems derived from hPSCs have been shown to recapitulate facets of in vivo CNS physiology, and thus, have been useful in screening for neurotoxicity [35] and investigating molecular underpinnings of traumatic injury [36] and viral infections [37]. Also, since first revealed as feasible in 2009 [38], numerous studies have observed facets of neurodegenerative [39–42], neurodevelopmental [43], and psychiatric disorders [44] in 2-D cultures of CNS cells derived from patient-specific iPSCs (Fig. 1b). Such disease-in-a-dish models have tremendous potential for drug screening applications; however, their utility as clinically predictive screening platforms remains to be demonstrated.
The mere fact that CNS diseases believed to have a mid-to-late adult onset pathology, e.g. Alzheimer’s [39], Parkinson’s [40], and Huntington’s Disease [41], Amyotrophic Lateral Sclerosis (ALS) [42,45], and Macular Degeneration [46], can be modeled by iPSC-derived cultures that do not mature past a fetal phenotype is a ground breaking discovery (Fig. 1b). This enables the possibility of clinically implementing patient-specific disease models for personalized medicine strategies. However, full in vivo disease pathology, e.g. targeted neuronal subtype death, is not routinely and robustly observed, possibly due to the limited biomimicry achievable in standard 2-D culture. A cell death phenotype is generally only observable upon application of an exogenous physiological stressor, and a subset of pathological facets are typically used as disease indicators instead [40,41,45]. Improving standard 2-D disease-in-a-dish models potentially requires overcoming their lack of biomimetic tissue cytoarchitecture and cell phenotype diversity as well as progressing past a fetal maturation state.
To create more biomimetic CNS models, tissue engineering techniques are being integrated with stem cell culture. By shifting from 2- to 3-D Matrigel hydrogel culture, neural tissues derived from fetal NSCs overexpressing pathological Alzheimer’s protein precursors were able to deposit amyloid-β plaques in vitro for the first time [47]. Using 3-D aggregate culture, neurally differentiating hESCs were discovered to possess innate abilities to spontaneously morph, i.e. differentiate and self-organize, and recapitulate remarkable levels of cortic- and retinogenesis in vitro [4,5] (Fig. 1c). The extensive morphogenesis capabilities of hPSC aggregates was further revealed by embedment within 3-D Matrigel hydrogels and long-term culture in stirred-tank bioreactors to enhance interstitial transport of oxygen and nutrients. This allowed the organoids to grow to millimeters in diameter, and within a single organoid, generate diverse cerebral tissues that enable novel disease in a dish models, e.g. microcephaly [6]. Extended culture of cerebral organoids can generate even further biomimetic CNS tissue microenvironments containing laminated, interconnected, and electrophysiologycially active neuronal tissue cytoarchitectures with interspersed astrocytes [48]. Moreover, neurodevelopmental biology principles discussed previously can be applied to morphing, NSC-stage aggregates to derive organoids from other CNS regions, e.g. midbrain-like [49] and hypothalamic [50] organoids.
Conclusion
Advancements in our ability to efficiently derive CNS cells and tissues foreshadow a new era in brain, eye, and spinal cord regenerative medicine. While such hPSC-derived cell therapies are still in the early stages of clinical trials, pre-clinical consideration should be given to their integration with neuromodulation [51] and acute intermittent hypoxia (AIH) approaches [52]. Patients treated with both of these approaches have demonstrated remarkable recovery of function via activation of endogenous plasticity mechanisms that induce adaptive neuronal circuitry changes. Thus, a combinatorial cell therapy utilizing all of these methods would likely create an optimally supportive trophic and plastic microenvironment for facilitating transplant engraftment.
Human PSC-derived organoids have become the premier 3-D platform for investigating human CNS disorders in vitro due to constituent microscale tissue structures displaying unprecedented biomimicry. However, their derivation relies primarily on spontaneous, uncontrolled morphogenesis. This can limit reproducibility in cellular/tissue composition as well as the ability to acquire an anatomically mimetic cytoarchitecture throughout the entire organoid, i.e. at the macroscale [6]. Transitioning to an instructed, controlled morphogenesis will be necessary to reproducibly derive organoids with a biomimetic macroscale anatomy containing multiple CNS tissues that interconnect to make physiologically relevant neurological circuits. In progressing toward this goal, synthetic matrices capable of supporting organoid morphogenesis are being developed to replace widely used but ill-defined and heterogeneous Matrigel hydrogels [53]. Tissue engineered platforms that enable spatiotemporal control of hPSC-derived CNS tissue morphology during morphogenesis are being developed for both 2-D [54] and 3-D culture [55]. In theory, platforms that instruct R/C and D/V morphogenetic patterning of developing organoids by exogenous application of morphogen gradients will also be needed. However, recent work describing spontaneous D/V patterning in mouse ESC-derived cysts may indicate that this is not necessary [56]. Regardless, intimate integration of tissue engineering methodologies with organoid derivation protocols will be needed to advance towards instructed organoid morphogenesis and create next generation CNS in vitro models and potentially even organ transplants.
Highlights.
Developmental biology principles can be applied to generate diverse CNS cell types.
Clinical trials for diverse CNS cell therapies are either ongoing or imminent.
2- and 3-D CNS tissue models expand the scope of clinical relevance.
Acknowledgments
This publication was developed under the Assistant Agreement No. 83573701 awarded by the U.S. Environmental Protection Agency (EPA) to R.S.A. and has not been formally reviewed by the EPA. The views expressed in this document are solely those of R.S.A. and colleagues and do not necessarily reflect those of the Agency, and the EPA does not endorse any products or commercial services mentioned in this publication. The publication was also developed with support from NIH grant 5R21NS082618-02 and an Innovation in Regulatory Science Award from the Burroughs Wellcome Fund (R.S.A.).
Footnotes
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 6••.Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. First application of stirred tank bioreactor culture to CNS organoids. This enables the organoids to grow to millimeters in diameter and recapitulate extensive levels of internal, cerebral tissue morphogenesis. Using microcephaly patient iPSCs, authors demonstrate a strong disease-in-a-dish phenotype due to a significant decrease in outer radial glial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- 8.Lippmann ES, Estevez-Silva MC, Ashton RS. Defined human pluripotent stem cell culture enables highly efficient neuroepithelium derivation without small molecule inhibitors. Stem Cells. 2014;32:1032–1042. doi: 10.1002/stem.1622. [DOI] [PubMed] [Google Scholar]
- 9.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XJ, Zhang X, Johnson MA, Wang ZB, LaVaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Lippmann ES, Williams CE, Ruhl DA, Estevez-Silva MC, Chapman ER, Coon JJ, Ashton RS. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Reports. 2015;4:632–644. doi: 10.1016/j.stemcr.2015.02.018. Demonstrates the use of a biphasic morphogen regime to pattern NSCs and neuronal progeny to distinct and diverse R/C regions throughout the hindbrain and spinal cord. Wnt and FGF signaling are used to differentiate hESCs into neuromesodermal progenitors that express HOX genes in a colinear, combinatorial, and time-dependent manner. Activation of retinoic acid signaling halts caudal progression and induces a NSC phenotype with a defined HOX profile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tozer S, Le Dréau G, Martí E, Briscoe J. Temporal control of BMP signalling determines neuronal subtype identity in the dorsal neural tube. Development. 2013;140:1467–1474. doi: 10.1242/dev.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziller MJ, Edri R, Yaffe Y, Donaghey J, Pop R, Mallard W, Issner R, Gifford CA, Goren A, Xing J, et al. Dissecting neural differentiation regulatory networks through epigenetic footprinting. Nature. 2016;518:355–359. doi: 10.1038/nature13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Kirwan P, Smith J, Robinson HPC, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–86. S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. Directed Differentiation and Functional Maturation of Cortical Interneurons from Human Embryonic Stem Cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol. 2013;31:440–447. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, Mei A, Mchenry L, Lisuk D, Grasmick JM, et al. Modeling Hippocampal Neurogenesis Using Human Pluripotent Stem Cells. Stem Cell Reports. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erceg S, Lukovic D, Moreno-Manzano V, Stojkovic M, Bhattacharya SS. Derivation of cerebellar neurons from human pluripotent stem cells. Curr Protoc Stem Cell Biol. 2012;Chapter 1(Unit 1H.5) doi: 10.1002/9780470151808.sc01h05s20. [DOI] [PubMed] [Google Scholar]
- 20.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 21.Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30:715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 23.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee G, Kim H, Elkabetz Y, Shamy Al G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 25.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 27.Alper J. Geron gets green light for human trial of ES cell derived productin brief. Nat Biotechnol. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- 28.Asterias Biotherapeutics, Inc. 2017 Jan 24; http://www.prnewswire.com/news-releases/asterias-announces-additional-motor-function-improvement-at-6-months-and-9-months-following-treatment-with-ast-opc1-in-patients-with-complete-cervical-spinal-cord-injuries-300395247.html.
- 29.Sugita S, Iwasaki Y, Makabe K, Kamao H, Mandai M, Shiina T, Ogasawara K, Hirami Y, Kurimoto Y, Takahashi M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Reports. 2016;7:635–648. doi: 10.1016/j.stemcr.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Stem Cell Corporation. 2016 Jul 28; https://globenewswire.com/news-release/2016/07/28/859745/0/en/International-Stem-Cell-Corporation-Announces-Successful-Cell-Transplantation-for-the-First-Patient-in-Phase-1-Clinical-Trial-of-ISC-hpNSC.html.
- 31•.Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol. 2015;33:204–209. doi: 10.1038/nbt.3124. Definitive proof-of-principle demonstration that transplanted midbrain dopaminergic neurons derived from hESCs can regenerate nigrostriatal pathways in Parkinsonian rodent models to enable motor function recovery. The authors use an elegant optogenetic approach to orthogonally modulate graft activity and show a direct and coordinate effect on animal recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. Powerful proof-of-principle study showing that transplantation of basal forebrain GABAergic progenitors can regenerate circuitry in a Huntington’s disease rodent model to correct associated motor deficits. Importantly, authors show that only basal forebrain and not spinal GABAergic progenitors could provide a regenerative effect, demonstrating the importance of proper transplant regional specification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nizzardo M, Simone C, Rizzo F. Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet. 2014;23:342–354. doi: 10.1093/hmg/ddt425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PKJ, Han JJ, Azimi N, Kim KS, et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Santos Costa V, Jiang P, Nguyen BK, Bolin JM, Daly W, et al. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci USA. 2015;112:12516–12521. doi: 10.1073/pnas.1516645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman SA, Phillips JK, Costa JT, Cho FS, Oungoulian SR, Finan JD. Stretch Injury of Human Induced Pluripotent Stem Cell Derived Neurons in a 96 Well Format. Sci Rep. 2016;6:34097. doi: 10.1038/srep34097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20:4530–4539. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B, Dolmetsch RE, Langston W, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang N, An MC, Montoro D, Ellerby LM. Characterization of Human Huntington’s Disease Cell Model from Induced Pluripotent Stem Cells. PLoS Curr. 2010;2:RRN1193. doi: 10.1371/currents.RRN1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, et al. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci USA. 2012;109:5803–5808. doi: 10.1073/pnas.1202922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4:145ra104–145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 46.Singh R, Shen W, Kuai D, Martin JM, Guo X, Smith MA, Perez ET, Phillips MJ, Simonett JM, Wallace KA, et al. iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet. 2013;22:593–607. doi: 10.1093/hmg/dds469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Meth. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, Tan ZY, Saw TY, Tan CP, Lokman H, et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Brand R, Mignardot J-B, Zitzewitz von J, Le Goff C, Fumeaux N, Wagner F, Capogrosso M, Moraud EM, Micera S, Schurch B, et al. Neuroprosthetic technologies to augment the impact of neurorehabilitation after spinal cord injury. Ann Phys Rehabil Med. 2015;58:232–237. doi: 10.1016/j.rehab.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- 53.Ranga A, Girgin M, Meinhardt A, Eberle D, Caiazzo M, Tanaka EM, Lutolf MP. Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci USA. 2016;133:E6831–E6839. doi: 10.1073/pnas.1603529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knight GT, Sha J, Ashton RS. Micropatterned, clickable culture substrates enable in situ spatiotemporal control of human PSC-derived neural tissue morphology. Chem Commun (Camb) 2015;51:5238–5241. doi: 10.1039/c4cc08665a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lancaster MA, Corsini NS, Burkard TR, Knoblich JA. Guided self-organization recapitulates tissue architecture in a bioengineered brain organoid model. 2016 doi: 10.1101/049346. bioRxiv. [DOI] [Google Scholar]
- 56••.Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Bräuninger M, Stec A, Schackert G, Lutolf M, Tanaka EM. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports. 2014;3:987–999. doi: 10.1016/j.stemcr.2014.09.020. Astounding demonstration that hydrogel encapsulated mESCs can form neuroepithelial cysts, which spontaneously recapitulate dorsoventral morphogenetic patterning in the absence of ventral Shh signaling center, i.e. a notochord. To date, a similar phenomenon has not been demonstrated using hESC-derived tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]