Abstract
Cocaine self-administration in rats results in dysfunctional neuroadaptations in the prelimbic (PrL) cortex during early abstinence. Central to these adaptations is decreased phospho-extracellular signal-regulated kinase (p-ERK), which plays a key role in cocaine-seeking. Normalizing ERK phosphorylation in the PrL cortex immediately after cocaine self-administration decreases subsequent cocaine-seeking. The disturbance in ERK phosphorylation is accompanied by decreased phosphorylation of striatal-enriched protein tyrosine phosphatase (STEP), indicating increased STEP activity. STEP is a well-recognized ERK phosphatase but whether STEP activation during early abstinence mediates the decrease in p-ERK and is involved in relapse is unknown. Here we show that a single intra-PrL cortical microinfusion of the selective STEP inhibitor, TC-2153, immediately after self-administration suppressed post-abstinence context-induced relapse under extinction conditions and cue-induced reinstatement, but not cocaine prime-induced drug-seeking or sucrose-seeking. Moreover, an intra-PrL cortical TC-2153 microinfusion immediately after self-administration prevented the cocaine-induced decrease in p-ERK within the PrL cortex during early abstinence. Interestingly, a systemic TC-2153 injection at the same time point failed to suppress post-abstinence context-induced relapse or cue-induced reinstatement, but did suppress cocaine prime-induced reinstatement. These data indicate that the STEP-induced ERK dephosphorylation in the PrL cortex during early abstinence is a critical neuroadaptation that promotes relapse to cocaine-seeking and that systemic versus intra-PrL cortical inhibition of STEP during early abstinence differentially suppresses cocaine-seeking.
Keywords: Cocaine, Extracellular signal-regulated kinase, Prelimbic cortex, Self-administration, STEP, TC-2153
2. Introduction
Cocaine self-administration (SA) causes a transient dephosphorylation of critical neuronal plasticity-related proteins in the prelimbic (PrL) cortex of rats that leads to subsequent relapse to cocaine-seeking. The phosphoproteins affected by cocaine include extracellular signal-regulated kinase (ERK), cAMP response element-binding protein (CREB), and the NMDA receptor subunits GluN2AY1325 and Glun2BY1472 (Whitfield et al. 2011; Go et al. 2016). A single intra-PrL cortical microinfusion of brain-derived neurotrophic factor (BDNF) immediately following the final SA session normalizes all phosphoproteins, prevents cocaine SA-depressed basal levels of glutamate within the nucleus accumbens core (NAc core), and suppresses relapse to cocaine-seeking for weeks after the infusion (Berglind et al. 2007, 2009; Whitfield et al. 2011; Go et al. 2016). Further, infusing a mitogen-activated protein kinase kinase (MEK) inhibitor (U0126) into the PrL cortex prior to BDNF prevents BDNF’s ability both to normalize ERK phosphorylation during early abstinence and to suppress subsequent cocaine-seeking (Whitfield et al. 2011). Collectively, these findings indicate that early abstinence from cocaine SA is a critical time point when transient hypoactivity of ERK signaling in the PrL cortex promotes dysfunctional corticostriatal glutamatergic neurotransmission, facilitating relapse to cocaine-seeking.
A major question is how cocaine SA causes ERK dephosphorylation in the PrL cortex. Of several phosphatases that dephosphorylate ERK, the STriatal-Enriched protein tyrosine Phosphatase (STEP) has emerged as a fundamental regulator of the ERK cascade in response to dopamine and glutamate interactions in the striatum triggered by psychostimulants (Valjent et al. 2005). STEP is a CNS-specific tyrosine phosphatase localized to dopaminoceptive neurons with high expression in the striatum (Lombroso et al. 1993) and moderate expression in the medial prefrontal cortex (PFC) (Boulanger et al. 1995). Canonically, STEP is activated by Ca2+ influx through GluN2B-containing NMDA receptors (Paul et al. 2003), leading to a calcineurin-dependent phosphatase cascade that culminates in the dephosphorylation and activation of STEP (Paul et al. 2003; Valjent et al. 2005; Goebel-Goody et al. 2012). Following activation, STEP dephosphorylates regulatory tyrosine residues on various substrates implicated in neuronal plasticity, including ERK (Paul et al. 2003; Paul & Connor 2010), the Src family kinase, Fyn (Nguyen et al. 2002), GluN2A (Tian et al. 2016), and GluN2B (Braithwaite et al. 2006).
STEP activity is augmented in several neuropsychiatric and neurodegenerative disorders. For example, STEP activity is elevated in human postmortem samples and in animal models of Alzheimer’s Disease (AD) (Snyder et al. 2005; Kurup et al. 2010), Parkinson’s Disease (Kurup et al. 2015), and schizophrenia (Xu et al. 2016b). Moreover, inhibition of STEP with systemic injections of the novel, relatively selective inhibitor, TC-2153, significantly improves memory deficits in a mouse model of AD (Xu et al. 2014) as well as PCP-induced hyperlocomotion in mice (Xu et al. 2016a). TC-2153 treatment reverses the biochemical, electrophysiological, and cognitive deficits in mouse models of schizophrenia and in glutamatergic neurons derived from human-induced pluripotent stem cells from schizophrenic patients (Xu et al. 2016b). As expected, TC-2153 acutely increases ERK phosphorylation in vivo (Xu et al. 2016a,b).
Regarding preclinical models of addiction, dephosphorylation events within the PrL cortex during early abstinence from cocaine SA are accompanied by decreased phosphorylation, and thus increased activation, of STEP (Sun et al. 2013), suggesting STEP is a likely mediator of the ERK dephosphorylation in the PrL cortex during early abstinence from cocaine SA. Therefore, we investigated whether inhibition of STEP with an intra-PrL cortical microinfusion of TC-2153 immediately following the final SA session would prevent ERK dephosphorylation in the PrL cortex during early abstinence and decrease relapse to cocaine-seeking. We also tested whether systemic injections of TC-2153 at the same time point would reduce relapse. Our results indicate that intra-PrL administration of TC-2153 immediately following the final SA session suppressed context-induced relapse and cue-, but not cocaine prime-, induced reinstatement. Intra-PrL TC-2153 also prevented the cocaine-induced ERK dephosphorylation within the PrL cortex during early abstinence. However, systemic TC-2153 injections at the same time point had the opposite effects. Overall, our results indicate that the cocaine-induced activation of STEP is an important regulator of neuroadaptations occurring within the PrL cortex during early abstinence that lead to subsequent cocaine-seeking.
3. Materials and Methods
3.1 Animal subjects and surgery
Adult male Sprague Dawley rats (n=166) Charles Rivers Laboratories; Wilmington, MA) were individually housed on a 12-hr reverse light/dark cycle (lights off at 6AM). Upon arrival, rats were allowed at least 3 days of acclimation to the vivarium. During this time, they were provided standard rat chow (Harlan; Indianapolis, IN) and water ad libitum. All animal use protocols were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed., 2011). All rats weighed 275–325g at the time of surgery. On the day of surgery, rats were injected i.p. with a ketamine (66 mg/kg) and xylazine (1.33 mg/kg) mixture for anesthesia and ketorolac (2.0 mg/kg) for analgesia. Rats that underwent self-administration were implanted in the right jugular vein with chronic indwelling i.v. silastic catheters (Fisher Scientific; Hampton, NH). Catheters were attached by subcutaneous tubing to a backmount which exited from an incision in the back. Following catheterization, rats were either secured in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) or underwent post-operative recovery care (specified below). Following securement in the stereotaxic apparatus, 26-gauge bilateral stainless steel guide cannulae (Plastics One, Roanoke, VA) were aimed 1 mm above the PrL cortex (+2.8mm anteroposterior, +/− 0.6mm mediolateral, −3.0mm dorsoventral relative to bregma). Guide cannulae were fixed to the skull with cranioplastic cement and anchored with steel screws. Following surgery, a bilateral 10 mm stylet was inserted into the guide cannula to prevent blockage, and rats were infused i.v. with 0.1 ml of cefazolin and 0.05 ml of Taurolidine-Citrate Solution (TCS; Access Technologies, Skokie, IL). Post-operative care was conducted for 5 days, during which rats were inspected for abnormalities, and catheters were flushed with 0.05 ml TCS.
3.2 Self-administration
Rats were trained to self-administer cocaine on a fixed-ratio 1 schedule of reinforcement for 12–14 days (2 hr/day; criterion of ≥ 10 infusions/day), during which rats were food restricted to 20g of chow to motivate learning. SA was conducted in standard MedPC operant chambers which contained two retractable levers (Fairfax, VT), and were housed within sound-attenuating cubicles fitted with a fan for airflow and masking noise. Active lever presses elicited a light and tone cue complex followed by a 0.2 mg/50 μl infusion of cocaine hydrochloride (NIDA, Research Triangle Park, NC), followed by a 20 second timeout period. Yoked-saline controls received a non-contingent infusion of 0.9% saline (with light and tone cues) when their cocaine partner received a contingent cocaine infusion. For all experiments, inactive lever presses had no programmed consequence. Sucrose SA was conducted in the same manner as cocaine SA experiments, except active lever presses were reinforced with a single 45 mg chocolate flavored sucrose pellet (BioServe-F07256; Flemington, NJ).
3.3 Intracranial microinfusions
Prior to intracranial microinfusions, rats were habituated to a different behavioral room equipped with an infusion pump (Harvard Apparatus) containing two gas-tight Hamilton syringes (10 μl) for at least 2 days immediately following their SA sessions. The day prior to their microinfusions, a stylet extending 1 mm past the tip of the guide cannula was inserted. Immediately following their final SA session, rats were infused (0.5 Ml/hemisphere, 0.25 μl/minute) with either vehicle (0.01% DMSO in filtered 0.1M PBS) or 1 μM of TC-2153. Injectors (33 gauge) were left in place for 1 minute after infusions to facilitate diffusion. Following microinfusions, rats were either returned to the vivarium for 6 days of homecage abstinence or were rapidly decapitated two hours later for phospho-protein analysis.
3.4 Systemic injections
TC-2153 (2.0 mg/ml) was dissolved in 18% DMSO/2% Tween-20 in filtered 0.1M PBS, and rats were injected (i.p.) with a volume 2.5X their body weight to achieve a final dose of 5 mg/kg, immediately after the final SA session. Six and 10 mg/kg have been shown to increase p-ERK in mice in vivo (Xu et al. 2014, 2016a). However, due to solubility restrictions (4 mg/ml did not stay in solution in our hands), we tested only 5 mg/kg. Following injections, rats were returned to the vivarium for 6 days of homecage abstinence.
3.4 Post-abstinence relapse test, extinction, cue- and cocaine prime-induced reinstatement
Following abstinence, rats underwent a post-abstinence (PA) relapse test under extinction conditions (active lever presses had no programmed consequence). After at least 6 days (no more than 21 days) of further extinction training to a criterion of an average of ≤15 presses on the active lever over the last 3 days, rats underwent a 2-hour cue-induced reinstatement test. During this test, active lever presses elicited the cocaine-associated cue complex, but no infusion. Following further extinction, rats underwent a 2-hour cocaine prime-induced reinstatement test (10 mg/kg, i.p.) under extinction conditions. Following the cocaine prime test, rats were decapitated with anesthesia to confirm accuracy of cannula placements.
3.5 Histology
Following decapitation, brains were extracted, rapidly frozen in isopentane, and stored at −80°C. Brains were either coronally sectioned (40 μM) with a cryostat and Nissl-stained to confirm cannula placements, or a single 3mm wide × 2mm deep tissue punch through the dorsomedial PFC (AP +~4.68–2.76) was taken using a modified biopsy punch (Braintree Scientific; Braintree, MA), and stored at −80°C until processing. Cannula placements were confirmed during dissection (Fig. 1A).
Figure 1.
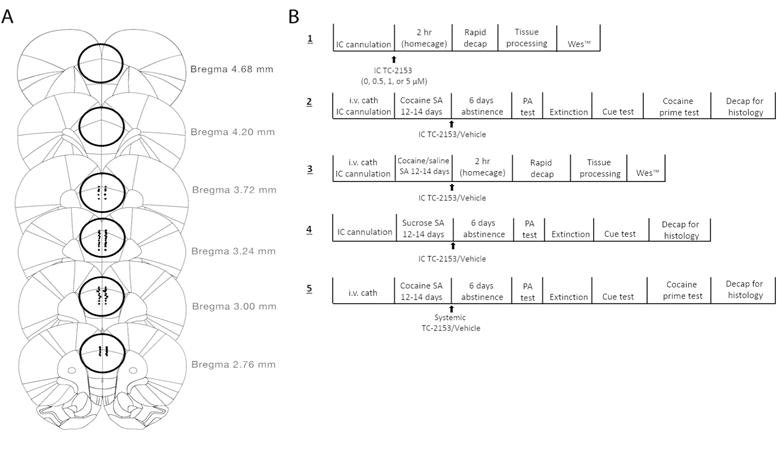
A. Map of coronal sections through the PFC showing all cannula placements for experiments 2, 4, and 5. The circled regions correspond to areas where tissue punches were taken for experiments 1 and 3 (AP ~+4.68mm–+2.76mm relative to bregma). B. Schematic of the experimental timelines for all experiments. Final sample sizes: Experiment 1-(0 μM: n=5, 0.5 μM: n=7, 1 μM: n=6, 5 μM: n=7); Experiment 2- (TC-2153 (1μM): n=9, vehicle: n=13); Experiment 3- (Saline+Vehicle: n=10, Saline+TC- 2153: n=7, Cocaine+Vehicle: n=8, Cocaine+TC-2153: n=9); Experiment 4- (TC-2153 (1 μM): n=7, Vehicle: n=4); Experiment 5- (TC-2153 (5 mg/kg): n=10, Vehicle: n=11).
3.6 Tissue processing
Tissue processing was performed as previously described (Sun et al. 2013). Briefly, 115 μl of ice-cold RIPA buffer (in mM: 50 Tris-HCL, pH 7.4, 150 NaCl, 2 EDTA, pH 7.4, and 10% glycerol, 1% Triton X, 1% NP-40, and 1% sodium deoxycholate) with a complete set of protease and phosphatase inhibitors, was added to each punch. Punches were briefly sonicated, incubated in ice-cold buffer for 30 minutes, centrifuged at 10,000 × g for 20 minutes at 4°C, and the supernatant was used for all analyses. Protein concentrations were determined using a standard BCA assay. Lysates were then diluted to 2 μg/μl in 0.1X Sample Buffer (Protein Simple; San Jose, CA) and individually aliquoted.
3.7 Wes™ immunoassay
The Wes™ capillary electrophoresis system (Protein Simple, Bio-Techne, San Jose, CA) was used for protein quantification. Aliquots of lysates (2 μg/μl) were thawed and further diluted in 0.1X Sample Buffer to 0.8 μg/μl for analysis of p-ERK and 0.4 μg/μl for analysis of t-ERK using a combination of ¾ 0.1x Sample Buffer and ¼ 4X Master Mix (1:1 mix of 40 mM DTT and 10X Sample Buffer, Protein Simple, San Jose, CA) according to the manufacturer’s instructions. Samples were denatured at 95°C for 5 minutes. A rabbit primary antibody against p-ERK1/2 (1:50, Cell Signaling Technology-9101S RRID:AB_331646) was multiplexed with a primary antibody against calnexin (Enzo life sciences-ADI-SPA-860-F RRID:AB_11178981) at a concentration of 1:2000. A rabbit primary antibody against t-ERK1/2 (1:100, Cell Signaling Technology-9102S RRID:AB_10695746) was multiplexed with 1:1000 calnexin. Supplier anti-rabbit secondary antibodies were used as instructed (Protein Simple, San Jose, CA).
Each plate contained samples from all experimental groups, and both p-ERK2 and t-ERK2 were analyzed for each sample from the same aliquot. Analysis of electropherogram peaks was performed using Compass™ software (Protein Simple) using the Gaussian distribution of the Area Under the Curve (AUC) of the peak luminol-peroxidase signal in the capillaries. Analysis was done by dividing the AUC of p-ERK2 by t-ERK2. Data are expressed as a fold change relative to the respective controls.
3.8 Experimental design
Fig. 1B shows the timeline for all experimental procedures conducted. Experiment 1 was conducted to determine the optimum concentration of TC-2153 that increased p-ERK when microinfused into the PrL cortex. Rats received a single intra-PrL cortical microinfusion of vehicle or TC-2153 (0.5, 1, or 5 μM) and were rapidly decapitated two hours later. These concentrations were chosen based on previous work showing that treatment of cortical cultures with concentrations of TC-2153 ranging from 1–5 μM increased p-ERK (Xu et al. 2014). The time point was chosen because the cocaine-induced dephosphorylation of ERK in the PrL cortex occurring two hours after the end of SA is necessary for subsequent cocaine-seeking because normalizing ERK dephosphorylation with a single intra-PrL cortical BDNF infusion suppresses cocaine-seeking (Whitfield et al. 2011).
Experiment 2 was designed to determine whether an intra-PrL microinfusion of TC-2153 immediately following the final SA session would suppress relapse after abstinence and extinction. Rats were either microinfused with vehicle or the optimal concentration of TC-2153 (1 μM) from Experiment 1 immediately following the final of 12–14 cocaine SA sessions. Following 6 days of forced abstinence, rats underwent a PA context-induced relapse test, further extinction, a cue-induced reinstatement test, further extinction, and a cocaine prime-induced reinstatement test. In Experiment 3, rats either underwent cocaine self-administration or received yoked-saline infusions, and received either an intra-PrL microinfusion of TC-2153 (1 μM) or vehicle immediately after the final SA session. Rats were rapidly decapitated two hours later to determine the effect of TC-2153 on the cocaine-induced ERK dephosphorylation within the PrL cortex during early abstinence. Experiment 4 was conducted in the same manner as Experiment 2, except active lever presses were reinforced with chocolate sucrose pellets instead of cocaine infusions and there was no sucrose-primed test. The design of Experiment 5 was similar to Experiment 2, except rats received a systemic injection of TC-2153 (5 mg/kg, ip) or vehicle immediately following the final cocaine SA session.
3.9 Statistical analysis
All analyses were performed using GraphPad Prism 7 software (La Jolla, CA). All behavioral data were analyzed with a mixed-model ANOVA with time (SA/extinction versus PA/Cue/cocaine prime test) as the within-subject variable and treatment (TC-2153 versus Vehicle) as the between-subject variable. If significant main effects or an interaction was observed, Bonferroni-corrected pairwise comparisons were used to compare groups during and across time points. Grubbs’ test was used to determine statistical outliers and these were removed from final analyses (n=4). Data in Experiment 1 were analyzed with a one-way ANOVA followed by Dunnett’s multiple comparison test to compare all TC-2153 concentrations to the vehicle control group. Data in Experiment 3 were analyzed with a two-way ANOVA followed by Student- Neuman-Keuls pairwise comparison tests. All data are expressed as the mean +/− SEM, and statistically significant differences were determined at p<0.05.
4. Results
4.1 Experiment 1. Intra-PrL microinfusion of TC-2153 increased p-ERK in naïve rats
Naïve rats (n=32; n=8/group) received a bilateral intra-PrL microinfusion of vehicle or one of three concentrations of TC-2153 (0.5, 1, or 5 μM). Rats were rapidly decapitated two hours after the infusion to determine the optimal concentration of TC-2153 that increased p-ERK in the PrL cortex in vivo. Of the 32 rats initially used, 2 died after surgery and 4 brain samples were incorrectly punched. One sample was not analyzed due to lysate discoloration.
A one-way ANOVA indicated a significant effect of intra-PrL TC-2153 on p-ERK (F(3,21)=3.21, p<0.05). Dunnett’s multiple comparison test indicated that only the 1 μM concentration of TC-2153 significantly increased p-ERK relative to vehicle controls (p=0.04; Fig. 2A,C). There was no effect of TC-2153 on t-ERK normalized to calnexin (F(3,21)=0.08, p=0.97; Fig. 2B,D). Thus, for all future microinfusion experiments, we used a 1 μM concentration of TC-2153.
Figure 2.
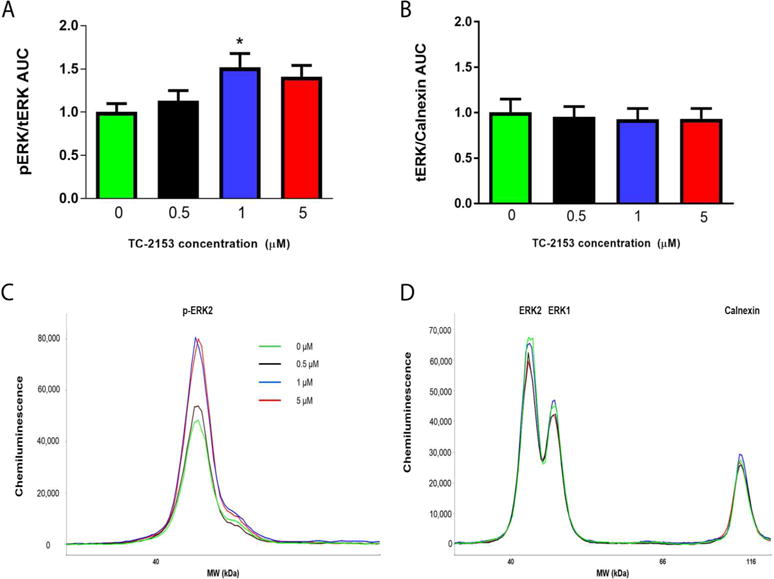
A single intra-PrL microinfusion of TC-2153 (1 μM) increased p-ERK in naïve rats. Rats were infused with vehicle (0) or 0.5, 1, or 5 μM of TC-2153 and rapidly decapitated two hours later to determine the optimal concentration of TC-2153 that increased p-ERK when microinfused into the PrL cortex. A. Only 1 μM TC-2153 increased p-ERK relative to vehicle-infused rats (*p<0.05). B. There was no difference in t-ERK/calnexin. Representative chemiluminescent peaks for p-ERK (C) and t-ERK multiplexed with calnexin (D) for groups in experiment 1.
4.2 Experiment 2. Intra-PrL microinfusion of TC-2153 suppressed context-induced relapse and cue-, but not cocaine prime-, induced reinstatement
We next investigated the effect of a bilateral intra-PrL microinfusion of TC-2153 (1 μM) immediately following SA on context-induced relapse following abstinence, cue-, and cocaine prime-, induced reinstatement following extinction. Two rats died during surgery, 5 rats died before finishing SA, 11 rats were removed from the final analysis due to cannula placements outside of the PrL cortex, and 2 rats were outliers according to Grubbs’ test during the cue and cocaine prime test in the TC-2153 and vehicle group, respectively. Final group sizes were: n=13 (vehicle) and n=9 (TC-2153). Infusions earned over the last 3 days of SA did not differ between groups (p=0.16). After infusions, rats were exposed to 6 days of abstinence followed by a PA test. Results revealed a significant main effect of time (F(1,20)=6.09, p=0.02) and treatment (F(1,20)=8.53, p=0.009), but no treatment by time interaction (F(1,20)=3.30, p=0.08). Bonferroni-corrected pairwise comparisons indicated that active lever presses were similar between groups during the last 3 days of SA before microinfusions (p>0.99). Rats infused with vehicle (p<0.01), but not TC-2153 (p>0.99), pressed the active lever significantly more during the PA test than during the last 3 days of SA and rats infused with TC-2153 pressed the active lever during the PA test significantly less than vehicle-infused controls (p<0.01; Fig. 3A).
Figure 3.
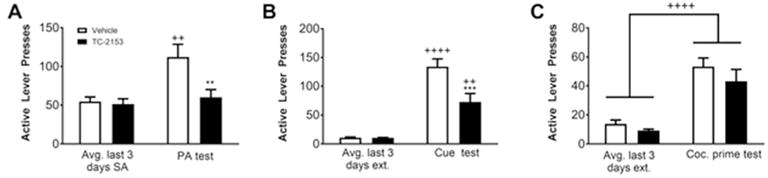
A single intra-PrL TC-2153 microinfusion immediately following the final SA session suppressed cocaine-seeking following abstinence and extinction training. Rats were either infused in the PrL cortex with TC-2153 (1 μM) or vehicle immediately following the final SA session. TC-2153 suppressed active lever pressing during (A) the PA test following abstinence as well as (B) during the cue-induced reinstatement test after extinction, but not (C) the cocaine prime-induced reinstatement test (++p<0.01, ++++p<0.0001 relative to the last 3 days of SA/extinction; **p<0.01, ***p<0.001 relative to vehicle).
After completion of extinction, rats underwent a cue-induced reinstatement test. There was a significant main effect of time (F(1,20)=88.67, p<0.0001) and treatment (F(1,20)=8.82, p<0.01), as well as a significant treatment by time interaction (F(1,20)=9.80, p<0.01; Fig. 3B). Bonferroni-corrected pairwise comparisons indicated that active lever pressing over the last 3 days of extinction did not differ between groups (p>0.99). Both vehicle- (p<0.0001) and TC-2153- (p<0.01) infused rats pressed the active lever significantly more during the cue test than during the last 3 days of extinction and rats infused with TC-2153 pressed the active lever significantly less than vehicle-infused rats (p<0.001).
Following re-extinction to criterion, rats underwent a cocaine prime-induced reinstatement test (Fig. 3C). One rat removed its headcap prior to the cocaine prime test and was excluded from the analysis. There was a significant main effect of time (F(1,19)=54.86, p<0.0001), indicating that both groups had increased active lever pressing during the cocaine prime-induced reinstatement test relative to the last 3 days of extinction. However there was no significant main effect of treatment (F(1,19)=1.857, p=0.19) or a significant treatment by time interaction (F(1,19)=0.33, p=0.57). These results show that a single intra-PrL cortical microinfusion of TC-2153 immediately following SA suppresses cocaine-seeking in the PA and cue-, but not cocaine prime-, induced reinstatement tests.
4.3 Experiment 3. Intra-PrL cortical microinfusion of TC-2153 prevented the cocaine-induced ERK dephosphorylation within the PrL cortex during early abstinence
In order to determine the effect of STEP inhibition on the cocaine-induced ERK dephosphorylation within the PrL cortex during early abstinence, rats (n=48) were randomly assigned to either cocaine SA or yoked-saline groups following surgery. One rat was removed prior to the end of SA after a catheter failure. Immediately following the final of 12–14 cocaine/yoked saline SA sessions, the PrL cortex of rats was microinfused bilaterally with TC-2153 or vehicle and rapidly decapitated two hours after the infusion. Of the remaining 47 rats, 13 were excluded from analyses for the following reasons: cannula placements that were outside of the PrL cortex (n=6), catheter failure on the final day of SA (n=1), or tissue samples were punched incorrectly (n=6). Following exclusion, final group sizes were as follows: Saline+Vehicle (n=10), Saline+TC-2153 (n=7), Cocaine+Vehicle (n=8), Cocaine+TC-2153 (n=9).
A two-way ANOVA revealed a significant main effect of treatment (TC-2153 versus vehicle) as well as SA (cocaine versus saline) on p-ERK (Treatment:F(1,30)=14.84, p<0.001, SA:F(1,30)=10.28, p<0.01), but no significant treatment by SA interaction (F(1,30)=0.08, p=0.78). Student-Neuman-Keuls tests revealed that p-ERK levels were significantly lower in the cocaine + vehicle group versus the yoked-saline + vehicle group. TC-2153 significantly increased p-ERK in yoked-saline rats (p<0.05; Fig. 4A,C) and prevented the cocaine SA-induced decrease. There was no effect of treatment, SA, or a significant treatment by SA interaction on t-ERK/calnexin (Treatment:F(1,30)=2.28, p=0.14, SA:F(1,30)=2.43, p=0.13, treatment X SA:F(1,30)=3.12, p=0.09; Fig. 4B,D). These results indicate that intra-PrL TC-2153 infused immediately following the final SA session prevented the cocaine-induced ERK dephosphorylation within the PrL cortex during early abstinence.
Figure 4.
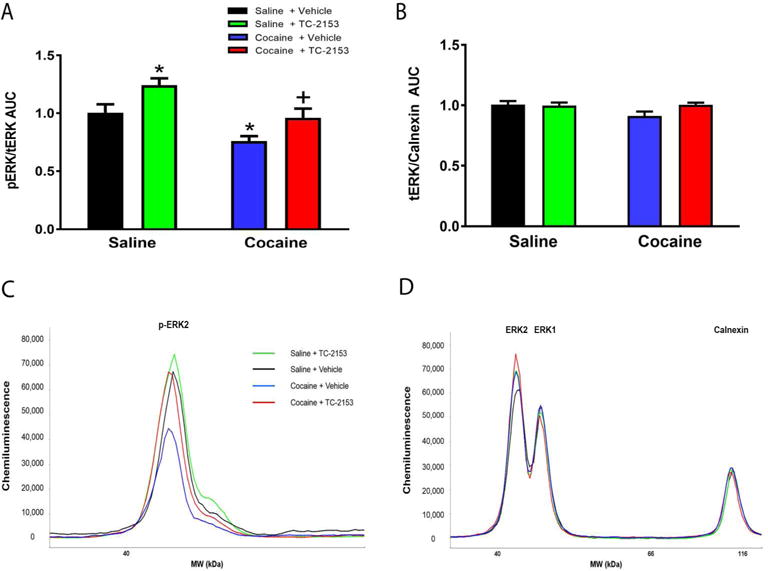
A single intra-PrL TC-2153 microinfusion prevented the cocaine-induced dephosphorylation of ERK within the PrL cortex during early abstinence. A. Rats microinfused with vehicle immediately following the final cocaine SA session show decreased p-ERK relative to yoked-saline rats infused with vehicle. This decrease is prevented in rats that received a TC-2153 microinfusion immediately following cocaine SA. Additionally, yoked-saline rats microinfused with TC-2153 showed augmented p-ERK relative to saline-vehicle rats (*p<0.05 relative to Saline-Vehicle, +p<0.05 relative to Cocaine-Vehicle). B. There were no differences between groups in t-ERK/calnexin. Representative chemiluminescent peaks for p-ERK (C) and t-ERK multiplexed with calnexin (D) for groups in experiment 3.
4.4 Experiment 4. Intra-PrL cortical microinfusion of TC-2153 did not affect context-induced relapse or cue-induced reinstatement of sucrose seeking
In order to determine whether the suppressive effect of intra-PrL infusion of TC-2153 on relapse was cocaine-specific, rats (n=16) underwent 12–14 days of sucrose self-administration, and were microinfused with either TC-2153 or vehicle immediately following the final SA session. One rat died after surgery and 3 rats removed their headcaps prior to the PA test. Of the remaining 12 rats, one was removed from analysis because its cannula placement was outside of the PrL cortex. Following infusions, rats underwent 6 days of forced abstinence followed by a PA test. A two-way ANOVA revealed a significant main effect of time (F(1,9)=35.69, p<0.001; Fig. 5A), but no main effect of treatment (F(1,9)=0.00005, p>0.99) or a significant treatment by time interaction (F(1,9)=0.01, p=0.93), indicating that both groups had reduced active lever pressing during the PA test relative to the last 3 days of sucrose SA. Following further extinction to criterion, rats underwent a cue-induced reinstatement test. Results indicated a significant main effect of time (F(1,9)=37.79, p<0.001; Fig. 5B), but not treatment (F(1,9)=0.31, p=0.59) or a significant treatment by time interaction (F(1,9)=1.08, p=0.33). These data indicate that both groups had increased active lever pressing during the cue-induced reinstatement test relative to the last 3 days of extinction, which was not altered by a prior TC-2153 microinfusion.
Figure 5.
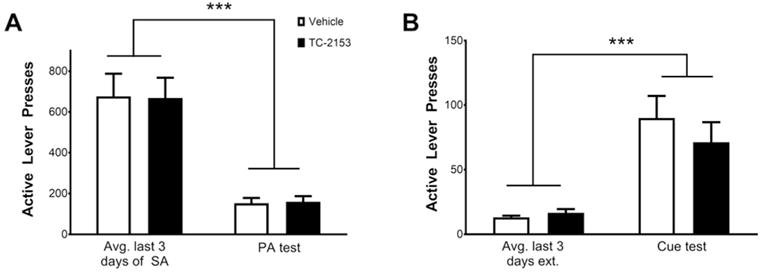
A single intra-PrL cortical microinfusion of TC-2153 fails to suppress sucrose seeking after abstinence and extinction. A. Animals microinfused with TC-2153 or vehicle show equal responding on the active lever during the PA test as well as (B) during the cue-induced reinstatement test, but both groups had reduced responding during the PA test relative to the last 3 days of SA and increased responding during the cue test relative to the last 3 days of extinction (***p<0.001 relative to the last 3 days of SA or extinction, two-way ANOVA).
4.5 Experiment 5. A systemic injection of TC-2153 preferentially suppressed cocaine prime-induced reinstatement
In order to determine the effect of systemic administration of TC-2153 on relapse, rats (n=28) underwent cocaine SA for 12–14 days as above. Three rats became ill and were euthanized prior to the end of SA. Two rats were removed for catheter failure prior to finishing SA. One rat per group was an outlier according to Grubbs’ test in the cocaine prime test and was removed from all analyses. The remaining rats were injected i.p. with TC-2153 (5 mg/kg, n=10) or vehicle (n=11) immediately following the last of 12–14 cocaine SA sessions. The number of infusions earned over the last 3 days of SA was similar between groups before i.p. injections (p=0.66). Following 6 days of abstinence, rats were returned to their SA chambers for the PA test. A two-way ANOVA revealed a significant main effect of time (F(1,19)=23.10, p=0.0001; Fig. 6A), indicating that both groups had increased active lever pressing in the PA test relative to the last 3 days of SA, but there was no significant main effect of treatment (F(1,19)=0.18, p=0.67) or a significant treatment by time interaction (F(1,19)=0.003, p=0.96).
Figure 6.
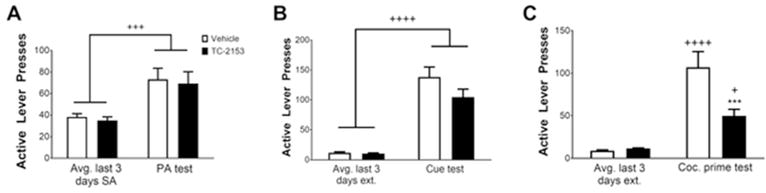
Systemic injection of TC-2153 suppressed cocaine prime-induced reinstatement but not post-abstinence context-induced relapse or cue-induced reinstatement. Rats were either injected with TC-2153 (5 mg/kg, i.p.) or vehicle immediately following the final cocaine SA session. Both groups had increased active lever pressing during (A) the PA test following 6 days of forced abstinence as well as (B) during the cue-induced reinstatement test following extinction. There was no effect of TC-2153 on active lever pressing in either test. However, TC-2153 injections suppressed (C) cocaine prime-induced reinstatement (++++p<0.0001, +++p<0.001, +p<0.05 relative to the last 3 days of SA/extinction, ***p<0.001 relative to vehicle).
Following completion of extinction, rats underwent a cue-induced reinstatement test. Results showed a significant main effect of time (F(1,19)=110.50, p<0.0001; Fig. 6B), indicating that both groups pressed the active lever significantly more during the cue- induced reinstatement test compared to the last 3 days of extinction, but there was no main effect of treatment (F(1,19)=2.50, p=0.13) or a treatment by time interaction (F(1,19)=2.38, p=0.14).
Following re-extinction to criterion, rats underwent a cocaine prime-induced reinstatement test. There were significant main effects of time (F(1,19)=42.66, p<0.0001), treatment (F(1,19)=7.065, p=0.02), as well as a significant treatment by time interaction (F(1,19)=8.26, p<0.01; Fig. 6C). Bonferroni-corrected pairwise comparisons indicated that active lever presses were equal between groups over the last 3 days of extinction (p>0.99), but rats injected with TC-2153 pressed the active lever significantly less than vehicle-injected rats during the cocaine prime test (p<0.001). Collectively, these data indicate that injections of TC-2153 immediately following SA preferentially suppress cocaine prime-induced reinstatement.
5. Discussion
5.1 Summary of findings
The results of the current experiments are the first to show that the cocaine-induced activation of STEP in the PrL cortex during early abstinence from cocaine SA (Sun et al. 2013) plays a role in relapse. Interestingly, we found that an intra-PrL cortical TC-2153 microinfusion immediately after SA suppressed context-induced relapse and cue-, but not cocaine prime-, induced reinstatement. However, systemic TC-2153 injections had the opposite effect. Additionally, intra-PrL cortical TC-2153 prevented the cocaine-induced ERK dephosphorylation in the PrL cortex during early abstinence. These results corroborate previous findings that early abstinence from cocaine SA is a critical time point whereby an intervention, like an infusion of BDNF or TC-2153, that normalizes ERK and/or GluN2 signaling in the PrL cortex produces an enduring suppression of relapse (Whitfield et al. 2011; Go et al. 2016). This finding coupled with the finding that BDNF does not alter food-seeking (Berglind et al. 2007) and TC-2153 does not alter sucrose-seeking indicates that intra-PrL cortical interventions during early abstinence selectively attenuate cocaine-seeking without affecting the motivation to seek natural rewards.
5.2 Differential effect of systemic vs. intra-PrL cortical TC-2153 on cocaine-seeking
Recent studies indicate that systemic delivery of TC-2153 normalizes cognitive/behavioral dysfunction in animal models of disorders with augmented STEP activity (Xu et al. 2014; Xu et al. 2016a,b). However, our experiments are the first to microinfuse TC-2153 and find that systemic versus intra-PrL cortical TC-2153 differentially suppressed cocaine-seeking. Several potential explanations exist for the different effects based on route of administration. The lack of effect of systemic TC-2153 on context-induced relapse and cue-induced reinstatement could be due to the inability of 5 mg/kg TC-2153 to prevent the dephosphorylation of ERK within the PrL cortex during early abstinence from cocaine SA. However, this is unlikely because 5 mg/kg TC-2153 increased p-ERK within the PrL cortex two hours after an injection in naïve rats (Fig. S1). Alternatively, it is likely that systemic TC-2153 increased p-ERK, as well as other STEP targets, in numerous brain regions outside of the PrL cortex, such as the hippocampus, striatum, and amygdala, that countered the elevation of p-ERK in the PrL cortex during context and cue-induced reinstatement and enabled preferential inhibition of cocaine prime-induced reinstatement.
5.3 Intra-PrL cortical TC-2153 prevented the cocaine-induced ERK dephosphorylation during early abstinence
The results of the current study are in agreement with previous studies indicating that cocaine SA decreases p-ERK in the PrL cortex during early abstinence (Whitfield et al. 2011; Go et al. 2016). Consistent with our hypothesis, we show here that this effect was prevented by intra-PrL cortical microinfusions of TC-2153. ERK is central to neuroadaptations occurring within neural circuits that mediate drug-seeking (Lu et al. 2006; Zhai et al. 2008). Therefore, it is not surprising that time-course experiments indicate that p-ERK changes dynamically within the PFC during various stages of abstinence from cocaine SA (Miszkiel et al. 2014; Whitfield et al. 2011). For example, cocaine-mediated decreases in p-ERK in the PrL cortex two hours after SA normalize by 22 hours and remain unaltered after six days of abstinence (Whitfield et al. 2011). Importantly, intra-PrL infusion of BDNF is unable to suppress relapse when microinfused after six days of forced abstinence (Berglind et al. 2007). However, the suppressive effect of BDNF on cocaine-seeking when it is microinfused immediately after SA depends on normalizing p-ERK during early abstinence (Whitfield et al. 2011).
5.4. Potential mechanism of STEP activation during early abstinence
STEP has been shown to be dephosphorylated and activated by Ca2+ influx through GluN2B-containing NMDA receptors via a PP2B-PP1 dependent mechanism in corticostriatal cultures (Paul et al. 2003). Moreover, extrasynaptic NMDA receptor stimulation inactivates ERK in cultured hippocampal and cortical neurons (Kim et al. 2005; Ivanov et al. 2006; Leveille et al. 2008) whereas synaptic NMDA receptor stimulation leads to STEP ubiquitination, facilitating sustained ERK activation (Hardingham et al. 2001,2002; Xu et al, 2009). Thus, an attractive hypothesis is that a hyperglutamatergic tone arises in the PrL cortex after chronic SA which facilitates extrasynaptic NMDA receptor stimulation, and subsequent activation of STEP, inhibiting NMDA function and associated ERK signaling during early abstinence. In support, not only does cocaine SA lead to the dephosphorylation of ERK and CREB, but also GluN2AY1325 and GluN2BY1472 (Go et al. 2016), and STEP dephosphorylates GluN2BY1472 (Braithwaite et al. 2006).
TC-2153 also enhances GluN2A pan-tyrosine phosphorylation in hippocampal cultures. Interestingly, STEP−/− mice have increased GluN2A pan-tyrosine, but not GluN2AY1325, phosphorylation (Tian et al. 2016), suggesting a different tyrosine site in GluN2A may be dephosphorylated by STEP. However, STEP may indirectly dephosphorylate GluN2AY1325 via dephosphorylation and inactivation of Fyn as demonstrated in vitro (Nguyen et al. 2002). Thus, future experiments should investigate whether TC-2153 prevents the cocaine-induced GluN2A/B dephosphorylation. Furthermore, an intra-PrL cortical microinfusion of Ro-25-6981, a selective GluN2B antagonist, immediately after SA does not reduce relapse (Go et al. 2016). However, treatment of cortical cultures with memantine, a non-selective NMDA antagonist, preferentially blocks extrasynaptic NMDA receptors (Leveille et al. 2008), suggesting that preferential inhibition of extrasynaptic GluN2B-containing NMDA receptors may prevent the cocaine-induced activation of STEP, ERK dephosphorylation, and subsequent cocaine-seeking.
5.5 Conclusions
ERK dephosphorylation in the PrL cortex during early abstinence represents a critical neuroadaptation that promotes relapse. Normalizing p-ERK with a single intra-PrL cortical BDNF (Whitfield et al. 2011) or TC-2153 microinfusion immediately after cocaine SA suppresses cocaine-seeking. Although BDNF and TC-2153 have a similar effect on cocaine-seeking, they are likely producing this effect in different ways. Intra-PrL cortical BDNF immediately after SA is likely acting upstream of STEP by directly increasing activity of MEK signaling during early abstinence (Whitfield et al. 2011), as well as potentially inducing the degradation of STEP (Saavedra et al. 2015). However, TC-2153 acts only at the level of STEP to prevent the catalytic activity of STEP following activation (Xu et al. 2014). Both of these interventions culminate in normalized p-ERK, highlighting the importance of ERK dephosphorylation as a neuroadaptation occurring in the PrL cortex during early abstinence that leads to cocaine-seeking. In conclusion, our results reveal an important role of STEP activation in the PrL cortex in regulating cocaine-seeking, as well as associated adaptations in p-ERK that occur during early abstinence from cocaine SA.
Supplementary Material
Acknowledgments
We thank Sarah M. Barry, Stephen Saunier, and Kailey Ray for excellent technical assistance. This work was supported by P50 DA15369, R01 DA033579, T32 DA007288 (JFM), F31 DA041021 (BMS), and R01 MH091037-05 (PJL).
Footnotes
Author contributions
BMS, JFM, and PJL were responsible for the study concept and design. BMS performed all experiments, analyzed all data, and drafted the manuscript. JFM provided critical review of the study design and data analyses, revisions of the manuscript, and funded the research. PJL provided critical revision of the manuscript and provided TC-2153. All authors critically reviewed content and approved the final version for publication.
The authors declare no competing financial interests.
References
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeing in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Lalumiere RT, Kalivas PW, McGinty JF. A single Intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;75:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, Oksenberg D, Nikolich K. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006;23:2847–2856. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go BS, Barry SM, McGinty JF. Glutamatergic neurotransmission in the prefrontal cortex mediates the suppressive effect of intra-prelimbic cortical infusion of BDNF on cocaine-seeking. Eur Neuropsychopharmacol. 2016;26:1989–1999. doi: 10.1016/j.euroneuro.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H. A calcium microdomainn near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kurup PK, Xu J, Videira RA, Ononenyi C, Baltazar G, Lombroso PJ, Nairn AC. STEP61 is a substrate of the E3 ligase parkin and is upregulated in Parkinson’s disease. Proc Natl Acad Sci USA. 2015;112:1202–1207. doi: 10.1073/pnas.1417423112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani D, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Aß-Mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of erk in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Detka J, Cholewa J, Frankowska M, Nowak E, Budziszewska B, Przegalinski E, Filip M. The effect of active and passive intravenous cocaine administration on the extracellular signal-regulated kinase (erk) activity in the rat brain. Pharmacol Rep. 2014;66:630–637. doi: 10.1016/j.pharep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal-enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Paul S, Connor JA. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem. 2010;114:1107–1118. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Puigdellivol M, Tyebji S, Kurup P, Xu J, Gines S, Alberch J, Lombroso PJ, Perez-Navarro E. BDNF induces striatal-enriched protein tyrosine phosphatase 61 degradation through the proteasome. Mol Neurobiol. 2015;53:4261–4273. doi: 10.1007/s12035-015-9335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Sun WL, Zelek-Molik A, Mcginty JF. Short and long access to cocaine selfadministration activates tyrosine phosphatase STEP and attenuates GluN expression but differentially regulates GluA expression in the prefrontal cortex. Psychopharmacology. 2013;229:603–613. doi: 10.1007/s00213-013-3118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Xu J, Lei G, Lombroso PJ, Jackson MF, MacDonald JF. STEP activation by Gaq coupled GPCRs opposes Src regulation of NMDA receptors containing the GluN2A subunit. Sci Rep. 2016 doi: 10.1038/srep36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen activated protein kinase dependent. J Neurosci. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chatterjee M, Baguley TD, Brouillette J, Kurup P, Ghosh D, Kanyo J, Zhang Y, Seyb K, Ononenyi C, Foscue E, Anderson GM, Gresack J, Cuny GD, Glicksman MA, Greengard P, Lam TT, Tautz L, Nairn AC, Ellman JA, Lombroso PJ. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer’s disease. PLoS Biol. 2014;12:e1001923. doi: 10.1371/journal.pbio.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Baguley TD, Foscue E, Ellman JA, Nairn AC, Lombroso PJ. Inhibition of the tyrosine phosphatase STEP61 restores BDNF expression and reverses motor and cognitive deficits in phencyclidine-treated mice. Cell Mol Life Sci. 2016a;73:1503–1514. doi: 10.1007/s00018-015-2057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Simineri N, Gupta S, Ononenyi C, Foscue E, Baguley TD, Carty N, Barros C, Mueller U, Ellman JA, Nairn AC, Aronow B, Lombroso PJ, Brennand KJ. Inhibition of STEP61 activity ameliorates deficits in mouse and hiPSC-based schizophrenia models. Mol Psychiatry. 2016b doi: 10.1038/mp.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in extracellular signal-regualted kinase (erk) signaling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


