Abstract
Background and aims
Aortic atherosclerosis is an aggregate marker of vascular risk factor exposure and has been associated with intracranial atherosclerosis and stroke. We hypothesized that atherosclerosis of the descending aorta (DAo) could be a risk marker for brain aging and injury.
Methods
We evaluated 1527 participants (mean age 59.9 years, 53.5% women) in the Framingham Offspring cohort who underwent both aortic and brain MRI. Participants were free of clinical stroke, dementia, or other neurological illness at the time of axial MRI of the thoracic and abdominal DAo and subsequent brain MRI. We related the prevalence and burden of aortic plaque to total cerebral brain volume (TCBV) and white matter hyperintensity volume (WMHV). An additional analysis compared incidence of stroke or TIA in participants with and without DAo plaques.
Results
Presence of thoracic DAo plaque (8%) was associated with decreased TCBV in sex-pooled analysis (−0.77, SE 0.25, p=0.002, equivalent to 4.5 years of aging) and with increased WMHV only in men (0.26, SE 0.12, p=0.032, equivalent to 6.5 years aging). We observed similar associations of DAo plaque burden with TCBV and WMHV. There were 43 strokes and 11 TIAs in prospective follow-up (median 7 years). Presence of DAo plaque was not associated with subsequent stroke or TIA.
Conclusions
In this cross-sectional community-based study, we found DAo plaque is associated with accelerated brain aging. These data underscore the potential implications of incidentally identified subclinical aortic atherosclerosis and question whether targeted intervention in these high risk individuals can modulate cognitive decline.
Keywords: Aorta, Atherosclerosis, Brain, Cerebrovascular Disorders, Magnetic Resonance Imaging, Neuroimaging, White Matter
Introduction
Previous studies have linked the presence and burden of aortic atherosclerosis with cognitive impairment, magnetic resonance imaging (MRI) indices of brain aging and injury, and stroke.(1–4) Vascular injury to the brain can occur because of embolism from atheroma in the aortic arch or may be related to a chronic disruption of central hemodynamics. The role of the latter mechanism is emphasized by investigations of aortic wall stiffness and the damaging effects of excessive pressure and flow in the cerebral microcirculation (5).
Aortic plaque may be a marker of overall atherosclerotic plaque burden (6), concomitant intracranial atherosclerotic disease (7), or systemic phenomena that can affect brain structure through neuronal loss. In the setting of large vessel atherosclerosis, cerebral damage may be mediated by elevated homocysteine levels or increased von Willebrand factor and hypercoagulability (8, 9). The severity of thoracic aortic intimal changes has been associated with both antioxidants and inflammatory markers in the serum, such as uric acid and high sensitive C-reactive protein (10), implicating chronic systemic oxidative and inflammatory processes, potentially damaging to the brain.
Few investigations have associated descending aortic plaque of the thorax or abdomen to brain changes or injury (11, 12). Blood flow in the descending aorta is separate from cervical and cerebral circulation, thus plaques in the descending aorta may serve as more specific markers of generalized atherosclerosis or microvascular damage, relative to ascending aortic plaques. Additionally, aortic atherosclerosis may not be fully correlated with results of other non-invasive measures of subclinical atherosclerosis, such as coronary artery calcification and carotid intima-media thickness by ultrasound (13).
We sought to evaluate the cross-sectional association between descending aorta (DAo) plaque prevalence and burden and total cerebral brain volume (TCBV) and white matter hyperintensity volume (WMHV). In additional analysis, we studied the prospective relation of DAo plaque prevalence to incidence of stroke in a community-based sample.
Patients and methods
Study population
The Framingham Offspring cohort was recruited in 1971 and comprises 5,124 participants who have been evaluated nine times. Non-simultaneous brain MRI and cardiovascular magnetic resonance (CMR) imaging were performed during and after the seventh (‘baseline’) examination cycle (1998–2001) between 1999 and 2005. Persons with clinical stroke, dementia or other neurological conditions that could affect brain MRI measures were excluded. Participants with a history of atrial fibrillation were excluded as well as those with a prosthetic valve. Of the 2,219 participants with available brain MRI data, 1,576 had attended the baseline examination and had undergone CMR; 49 were excluded because of missing data on education and other risk factors, yielding a study sample of 1,527 for the present investigation. The median length of time between brain MRI and cardiac MRI was 3.7 years (Q1 3.0, Q3 4.6). The Institutional Review Boards of Boston University Medical Center and Beth Israel Deaconess Medical Center approved the study protocol and written informed consent was obtained from all study participants.
Baseline examinations
Educational achievement was dichotomized at high school graduation. Body mass index (BMI) was the ratio of measured weight in kilograms to the square of height in meters (kg/m2). Serum total cholesterol, high-density lipoprotein cholesterol levels, and triglycerides were measured at the baseline exam. We determined whether lipid lowering therapy was used. Plasma total homocysteine levels were measured at the prior exam (1995–1998) using high-performance liquid chromatography with fluorescence detection.
Information on vascular risk factors was collected at the baseline exam, including the components of the Framingham Stroke Risk Profile (FSRP), which has been described and validated for predicting stroke risk (14). The FSRP risk factors include systolic blood pressure, use of antihypertensive therapy, diabetes mellitus (defined as a fasting blood glucose of ≥126 mg/dL, a previous diagnosis of diabetes, or using hypoglycemic medication or insulin), current smoking status, atrial fibrillation, and previous cardiovascular disease including diagnoses of coronary heart disease, heart failure, or peripheral vascular disease.
Carotid intima-media thickness (IMT) was measured using carotid ultrasound studies, acquired following a standard protocol l(15) during the sixth examination cycle (1995–1998). Methods for ultrasound imaging of the carotid arteries, and measurement of IMT, are described in a previous report (16). Coronary artery calcium score (CAC) was calculated in participants who underwent a non-contrast cardiac multidetector computed tomography (MDCT) scan during the seventh examination cycle (1998–2001). MDCT protocols and methods for calculation of Agatston Score for prevalence of coronary artery calcium are previously described (17, 18).
Cardiovascular Magnetic Resonance (CMR)
CMR acquisition, measurement techniques, and inter-rater reliability of aortic plaque measurement have been described previously (19). Briefly, a commercial 1.5T whole-body CMR system (Gyroscan ACS-NT or Achieva, Philips Medical Systems) was used to perform thoraco-abdominal aortic CMR. Approximately thirty-six transverse slices were obtained; this included twelve 10-mm thick slices, with a 10-mm gap, spanning the aortic arch and thoracic DAo and twenty-four 5-mm thick slices spanning the abdominal DAo to the origin of the common iliac arteries. Atherosclerotic plaque was defined as a characteristic luminal protrusion of ≥1 mm in radial thickness that could be distinguished from the minimal residual blood signal (Fig. 1) by a single blinded observer (NO-M). DAo plaques were divided according to their location into thoracic or abdominal plaques, above and under the diaphragm. For plaque prevalence, participants were dichotomized by presence or absence of plaque. Plaque burden was defined as the percent of axial slices demonstrating plaque.
Figure 1.
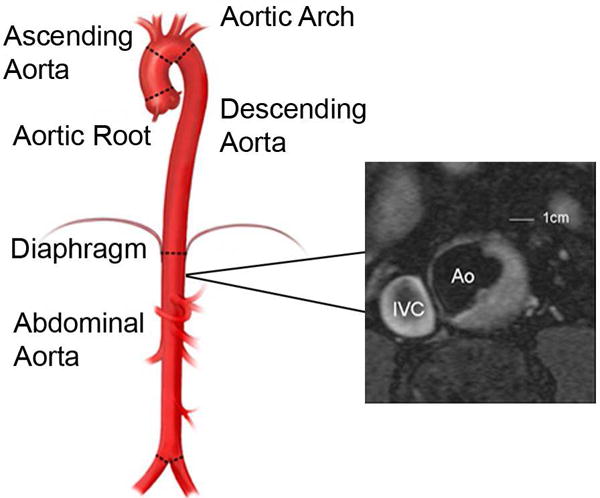
Transverse cardiovascular magnetic resonance image of the abdominal aorta and an aortic plaque, from a Framingham Offspring Cohort participant.
IVC, inferior vena cava; Ao, aorta. Illustration adapted from www.mountsinai.org with permission from Dr. Allan Stewart. Copyright 2016, Icahn School of Medicine at Mount Sinai.
Brain MRI
Acquisition and data processing of brain MRI scans, as well as volumetric measurement techniques and inter-rater reliability have been previously reported (20, 21). The TCBV is the ratio of total brain parenchymal volume to total cranial volume to correct for differences in head size. The measurements of brain volume were done on coronal sections. TCBV was measured through a mathematical modeling of the resulting images after a manual removal of all non-brain elements from the images. This was done by operator-guided tracing of the dura mater within the cranial vault (including the middle cranial fossa but above the posterior fossa and cerebellum). The measurements of TCBV included the supra-tentorial gray and white matter without the CSF.
WMHV was measured by summation of first and second echo images from T2 sequences after removal of the CSF, and was also reported as a proportion of the total cranial volume, allowing correction for head size. The summed data were adjusted for the heterogeneous imaging intensities and a log normal distribution was fitted.
Statistical analysis
Our primary analysis investigated two dichotomous predictor variables: 1) presence or absence of plaque in the thoracic DAo and 2) presence or absence of any DAo plaque (thoracic, abdominal, or both). Multivariable linear regression analysis was used to relate the two predictor variables to continuous measures of TCBV and WMHV (outcome variables; separate analyses for each). Crude associations were determined, adjusting for age and sex only (Model 1). Previous studies have shown that levels of stroke risk factors are related to brain volume and WMHV (22), so such stroke risk factors were included as additional covariates. Specifically, covariates in the regression models were age, sex, and baseline systolic blood pressure, smoking, diabetes, education, BMI, total cholesterol:high density lipoprotein ratio, triglycerides, use of antihypertensives, lipid lowering therapy, prior cardiovascular disease, and homocysteine (Model 2). To gain further insight into the added value of CMR measurements of aortic plaque prevalence in the descending aorta, a final model additionally adjusted for CAC and carotid IMT (Model 3). Previous studies have suggested a difference by sex associated with aortic atherosclerosis (19, 23), so analysis was stratified by sex a priori. Sensitivity analyses were performed to relate plaque burden in the DAo (continuous variable) to TCBV and WMHV, using the above models. All analyses were pre-specified.
In additional analysis, sex-specific Cox models were used to study the association of DAo plaque with probability of stroke after verifying the assumption of proportionality of hazards. The predictor variables were: 1) presence or absence of plaque in the thoracic DAo and 2) presence or absence of any DAo plaque (thoracic, abdominal, or both). We adjusted for age, sex, education, diabetes, BMI, systolic blood pressure, smoking, lipid lowering therapy, prior cardiovascular disease, and homocysteine. Participants were followed for incident stroke from the date of CMR to December 2012.
p-values presented are two-sided, and were declared significant at a two-sided 0.025 level of significance. Analyses were carried out using SAS version 9.3.
Results
Plaque in the thoracic DAo was observed in 60/710 (8.4%) of men and 58/817 (7.1%) of women. All participants with plaque in the thoracic DAo had plaque in the abdominal DAo. The prevalence of plaque in either the thoracic or abdominal DAo in men was 333/710 (47%) and in women 392/817 (48%). Baseline characteristics are presented in Table 1, displaying the overall sample and the sample stratified by sex. Table 2 displays baseline characteristics stratified by presence of plaque in the thoracic DAo and sex. Among those with any DAo plaque, the mean percentage of MRI axial slices with plaques (“plaque burden”) was 11.7% (SD 11.3%. min 3.1%, max 77.7%). Participants with CMR measurements of aortic plaque who also had measurements of CAC and carotid IMT included 371 males and 447 females.
Table 1.
Study sample characteristics, overall and by sex.
| Overall | Men | Women | |
|---|---|---|---|
|
| |||
| N (%) | 1527 | 710 (46.5) | 817 (53.5) |
|
| |||
| Age, years, mean±SD | 59.9±9.1 | 60.0±9.2 | 59.8±9.0 |
|
| |||
| Systolic BP, mmHg, mean±SD | 125±18 | 126±16 | 124±19 |
|
| |||
| Antihypertensive treatment, % | 28 | 32 | 25 |
|
| |||
| Diabetes mellitus, % | 9 | 12 | 6 |
|
| |||
| Triglycerides, mg %, mean±SD | 134±92 | 144±109 | 125±72 |
|
| |||
| Total cholesterol/HDL ratio, mean±SD | 4.1±1.4 | 4.6±1.4 | 3.6±1.2 |
|
| |||
| Lipid treatment, % | 18 | 21 | 15 |
|
| |||
| Prevalent CVD, % | 8 | 12 | 6 |
|
| |||
| tHcy, μmol/liter, mean±SD | 8.2±2.6 | 8.9±2.4 | 7.5±2.5 |
|
| |||
| BMI, kg/m2, mean±SD | 27.9±5.0 | 28.5±4.2 | 27.3±5.5 |
|
| |||
| Current smoking, % | 10 | 10 | 10 |
|
| |||
| Education levela, mean±SD | 3.1±0.9 | 3.2±0.9 | 3.0±0.9 |
|
| |||
| Plaque present in the thoracic DAo, % | 8 | 8 | 7 |
|
| |||
| Any plaque present in the DAob, % | 47 | 47 | 48 |
|
| |||
| Thoracic DAo plaque burdenc, %, mean±SD | 1.87±8.22 | 2.22±9.26 | 1.57±7.19 |
|
| |||
| Any DAo plaque burdend, %, mean±SD | 5.56±9.77 | 5.49±10.26 | 5.62±9.33 |
|
| |||
| TCBV, mean±SD | 78.26±3.07 | 77.80±3.27 | 78.67±2.82 |
|
| |||
| WMHV, mean±SD | −3.15±0.99 | −3.21±0.96 | −3.10±1.01 |
|
| |||
| Incident stroke/TIAe | |||
| Ischemic stroke, n (%) | 36 (2.4) | 20 (2.8) | 16 (2.0) |
| Hemorrhagic stroke, n (%) | 7 (0.5) | 2 (0.3) | 5 (0.6) |
| TIA, n (%) | 11 (0.7) | 8 (1.1) | 3 (0.4) |
BMI,body mass index; BP,blood pressure; DAo,descending aorta; CVD,cardiovascular disease; HDL,high density lipoprotein; WMHV,log-transformed white matter hyperintensity volume:total cranial volume ratio; SD,standard deviation; TCBV,total cerebral brain volume:total cranial volume ratio; tHcy,total homocysteine; TIA,transient ischemic attack.
Education grading on a scale of 1 to 4 for (1) no high school education, (2) high school education, (3) some college education, and (4) college degree.
Thoracic DAo, abdominal DAo, or both.
Percent of MRI axial slices with plaque in the thoracic DAo.
Percent of MRI axial slices with plaque in the thoracic or abdominal DAo.
Stroke or TIA ascertained in prospective follow up (median duration of follow up 7.0 years, Q1 5.9, Q3 7.9).
Table 2.
Study sample characteristics, by prevalence of plaque in the descending thoracic aorta and sex.
| Thoracic plaque present in the DAo | No plaque in the thoracic DAo | |||
|---|---|---|---|---|
|
| ||||
| Men | Women | Men | Women | |
|
| ||||
| N (%) | 60 (8.4) | 58 (7.1) | 650 (91.5) | 759 (92.9) |
|
| ||||
| Age, years, mean±SD | 67.8±7.0 | 66.3±7.5 | 59.3±9.0 | 59.3±8.9 |
|
| ||||
| Systolic BP, mmHg, mean±SD | 132±19 | 134±23 | 125±16 | 123±18 |
|
| ||||
| Antihypertensive treatment, % | 55 | 43 | 30 | 24 |
|
| ||||
| Diabetes mellitus, % | 10 | 12 | 12 | 6 |
|
| ||||
| Triglycerides, mg %, mean±SD | 157±152 | 136±65 | 143±105 | 124±73 |
|
| ||||
| Total cholesterol/HDL ratio, mean±SD | 4.6±1.4 | 4.0±1.3 | 4.6±1.4 | 3.6±1.2 |
|
| ||||
| Lipid treatment, % | 33 | 26 | 20 | 14 |
|
| ||||
| Prevalent CVD, % | 25 | 19 | 10 | 4 |
|
| ||||
| tHcy, μmol/liter, mean±SD | 9.9±3.3 | 8.3±2.6 | 8.8±2.3 | 7.5±2.5 |
|
| ||||
| BMI, kg/m2, mean±SD | 28.2±4.4 | 26.0±4.4 | 28.5±4.2 | 27.4±5.6 |
|
| ||||
| Current smoking, % | 20 | 26 | 9 | 9 |
|
| ||||
| Education levela, mean±SD | 2.9±0.9 | 2.7±0.8 | 3.2±0.9 | 3.0±0.9 |
|
| ||||
| Thoracic DAo plaque burdenb, %, mean±SD | 26.28±19.68 | 22.10±16.65 | 0 | 0 |
|
| ||||
| Any DAo plaque burdenc, %, mean±SD | 22.53±19.52 | 18.84±17.30 | 3.92±7.14 | 4.61±7.53 |
|
| ||||
| TCBV, mean±SD | 75.58±3.32 | 76.80±2.94 | 78.00±3.19 | 78.81±2.76 |
|
| ||||
| WMHV, mean±SD | −2.58±1.04 | −2.72±0.95 | −3.27±0.93 | −3.13±1.01 |
|
| ||||
| Incident stroke/TIAd | ||||
| Ischemic stroke, n (%) | 2 (3.3) | 1 (1.7) | 18 (2.8) | 15 (2.0) |
| Hemorrhagic stroke, n (%) | 0 (0.0) | 1 (1.7) | 2 (0.3) | 4 (0.5) |
| TIA, n (%) | 2 (3.3) | 0 (0.0) | 6 (0.9) | 3 (0.4) |
BMI, body mass index; BP, blood pressure; DAo, descending aorta; CVD, cardiovascular disease; HDL, high density lipoprotein; WMHV, log-transformed white matter hyperintensity volume:total cranial volume ratio; SD, standard deviation; TCBV, total cerebral brain volume:total cranial volume ratio; tHcy, total homocysteine; TIA, transient ischemic attack.
Education grading on a scale of 1 to 4 for (1) no high school education, (2) high school education, (3) some college education, and (4) college degree.
Percent of MRI axial slices with plaque in the thoracic DAo.
Percent of MRI axial slices with plaque in the thoracic or abdominal DAo.
Stroke or TIA ascertained in prospective follow up (median duration of follow up 7.0 years, Q1 5.9, Q3 7.9)
Associations between DAo plaque prevalence and TCBV are presented in Table 3. Compared to persons without DAo plaque in the thorax, persons with plaque had a significantly lower mean TCBV, after controlling for vascular risk factors (−0.77%, SE=0.25; p=0.002). The association remained after additional adjustment for CAC and carotid IMT (−0.98%, SE=0.39; p=0.012). In sex-stratified analysis, controlling for vascular risk factors, mean TCBV was lower in both men and women with a thoracic DAo plaque (−0.39%, SE=0.35, p=0.27 and −1.13%, SE=0.35, p<0.001, respectively), and the association remained significant in women with additional adjustment for CAC and carotid IMT (−1.69%, SE=0.58; p=0.004). Observed brain volume loss in women with a thoracic DAo plaque, controlling for vascular risk factors, was equivalent to that associated with 8.8 years of aging in women in the overall study sample. In the sex-pooled analysis, lower mean TCBV was equivalent to 4.5 years of aging in the men and women of the overall study sample.
Table 3.
Association of plaque prevalence in the descending aorta with brain volume
| TCBV | |||||||
|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | ||
| Plaque present in the thoracic DAo | Overall (n=118) | −0.85 (0.25) | <0.001 | −0.77 (0.25) | 0.002 | −0.98 (0.39) | 0.012 |
| Interaction with sex | 0.748 | 0.875 | 0.387 | ||||
| Men (n=60) | −0.58 (0.36) | 0.105 | −0.39 (0.35) | 0.27 | −0.28 (0.53) | 0.601 | |
| Women (n=58) | −1.04 (0.35) | 0.003 | −1.13 (0.35) | <0.001 | −1.69 (0.58) | 0.004 | |
| Any plaque present (thoracic, abdominal, or both) in the DAo | Overall (n=722) | −0.22 (0.13) | 0.101 | −0.19 (0.13) | 0.15 | −0.15 (0.18) | 0.405 |
| Interaction with sex | 0.008 | 0.029 | 0.670 | ||||
| Men (n=332) | −0.44 (0.20) | 0.026 | −0.28 (0.19) | 0.15 | −0.09 (0.26) | 0.304 | |
| Women (n=390) | 0.02 (0.18) | 0.913 | −0.04 (0.17) | 0.80 | 0.11 (0.25) | 0.664 | |
β,beta value; DAo, descending aorta; SE, standard error; TCBV, total cerebral brain volume:total cranial volume ratio.
Model 1: Age and sex.
Model 2: Model 1, plus adjustment for education, body mass index, systolic blood pressure, smoking, diabetes, total cholesterol:high density lipoprotein ratio, triglycerides, antihypertensive use, lipid lowering therapy, prior cardiovascular disease, and homocysteine.
Model 3: Model 2, plus adjustment for carotid artery calcium score (CAC) and carotid intima-media thickness (IMT); 26/371 men and 23/447 women had plaque present in the thoracic DAo on cardiovascular MRI (CMR) and measures for CAC and carotid IMT; 165/371 men and 223/447 women had any plaque present in the DAo on CMR and measures for CAC and carotid IMT
Table 4 displays results for the analysis associating DAo plaque prevalence with WMHV. Overall, plaque prevalence in the thoracic DAo was not associated with WMHV. In sex-stratified analysis, only men with thoracic DAo plaque had a mean increase in WMHV (0.26%, SE=0.12, p=0.03). The association was attenuated, but still reached significance, with adjustment for CAC and carotid IMT (0.38%, SE=0.19; p=0.040). The quantified increase in WMHV in men with thoracic DAo plaque, controlling for vascular risk factors, was equivalent to 6.5 years of aging in males in the overall study sample. We found no association between prevalence of any DAo plaque (thoracic, abdominal, or both) and WMHV.
Table 4.
Association of plaque prevalence in the descending aorta with white matter hyperintensity volume.
| WMHV | |||||||
|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | ||
| Plaque present in the thoracic DAo | Overall (n=118) | 0.16 (0.09) | 0.061 | 0.11 (0.08) | 0.21 | 0.11 (0.13) | 0.408 |
| Interaction with sex | 0.199 | 0.133 | 0.043 | ||||
| Men (n=60) | 0.29 (0.12) | 0.015 | 0.26 (0.12) | 0.03 | 0.38 (0.19) | 0.040 | |
| Women (n=58) | 0.04 (0.12) | 0.751 | −0.05 (0.13) | 0.67 | −0.17 (0.20) | 0.39 | |
| Any plaque present (thoracic, abdominal, or both) in the DAo | Overall (n=722) | 0.01 (0.05) | 0.786 | 0.00 (0.05) | 0.93 | −0.05 (0.06) | 0.418 |
| Interaction with sex | 0.424 | 0.470 | 0.290 | ||||
| Men (n=332) | 0.06 (0.07) | 0.376 | 0.05 (0.07) | 0.47 | 0.03 (0.09) | 0.750 | |
| Women (n=390) | −0.02 (0.06) | 0.701 | −0.05 (0.06) | 0.47 | −0.11 (0.08) | 0.177 | |
Β, beta value; DAo, descending aorta; SE, standard error; WMHV, log-transformed white matter hyperintensity volume:total cranial volume ratio
Model 1: Age and sex.
Model 2: Model 1, plus adjustment for education, body mass index, systolic blood pressure, smoking, diabetes, total cholesterol:high density lipoprotein ratio, triglycerides, antihypertensive use, lipid lowering therapy, prior cardiovascular disease, and homocysteine.
Model 3: Model 2, plus adjustment for carotid artery calcium score (CAC) and carotid intima-media thickness (IMT); 26/371 men and 23/447 women had plaque present in the thoracic DAo on cardiovascular MRI (CMR) and measures for CAC and carotid IMT; 165/371 men and 223/447 women had any plaque present in the DAo on CMR and measures for CAC and carotid IMT.
Supplemental Tables 1 and 2 display results for the sensitivity analyses associating DAo plaque burden with TCBV and WMHV, respectively. Compared to the analyses with aortic plaque prevalence, similar associations were observed. Adjusting for vascular risk factors, plaque burden in the thoracic DAo was associated with a lower mean TCBV in sex-pooled analysis (−0.018%, SE=0.008, p=0.02) and lower mean TCBV in both men and women (−0.006%, SE=0.01, p=0.52, and −0.037%, SE=0.01, p=0.004, respectively). The associations in sex-pooled analysis and in women persisted when additionally adjusting for CAC and carotid IMT. Overall DAo plaque burden (thoracic, abdominal, or both) was associated with lower mean TCBV in sex-pooled analysis adjusting for vascular risk factors (−0.015, SE=0.007, p=0.03) and in the final model adjusting for CAC and carotid IMT (−0.021, SE=0.011; p=0.027). Plaque burden in the thoracic DAo in men only was associated with an increase in WMHV in both the models adjusting for vascular risk factors (0.007, SE=0.003, p=0.04) and additionally CAC and carotid IMT (0.012, SE=0.006; p=0.027). We found no association between overall DAo plaque burden and WMHV.
We performed additional analysis for the association of DAo plaque with subsequent incident stroke. Median duration of follow up for incident stroke was 7.0 years (Q1 5.9, Q3 7.9). There were 43 strokes (36 ischemic and 7 hemorrhagic) ascertained during follow up and 11 TIAs (Table 1). We did not observe a statistically significant association between the presence of plaques in the DAo and stroke/TIA, stroke alone, or ischemic stroke alone. Results for risk of stroke are presented in Supplemental Table 3.
Discussion
In our cross-sectional analysis of the aorta and brain, we observed that the presence and burden of thoracic DAo plaque was associated with lower TCBV. The loss of brain volume associated with thoracic DAo plaque was equivalent to 8.8 years of aging in women and, in sex-pooled analysis, 4.5 years of brain aging. We observed an increase in WMHV equivalent to 6.5 years of aging in men who had thoracic DAo plaque.
There was no statistically significant effect modification by sex, perhaps due to limited power to detect an interaction. Previous research with the FHS Offspring cohort identified greater aortic plaque burden in older women than in men of corresponding age and greater burden in women than in men in the absence of hypertension.(19, 23) Women and men have differences in the presence or progression of white matter hyperintensities and cerebral brain volume.(22, 24–26) Differences may be due to variation in heritability of WMHV,(27) menopause and hormonal effects, or sex differences in cardiovascular risk profile,(28) with implications for clinical outcomes.(29)
Previous studies suggest that arterial disease at varying locations might differentially influence the presence or progression of brain aging or cerebrovascular disease.(1, 28, 30) A report from the Second Manifestations of ARTerial disease (SMART) Study observed that the presence of white matter lesions and silent infarcts on brain MRI may be higher in patients with symptomatic atherosclerotic disease, compared with findings from community-based studies.(30) The investigators analyzed brain changes in patients with coronary artery disease, peripheral arterial disease, and abdominal aortic aneurysm. A prior report from the Framingham Study associated the prevalence of carotid artery calcification with a decrease in global and regional brain volumes and an increase in WMHV.(16)
Occasional studies have identified the DAo as a source for retrograde embolism, typically from complex atheromas (size > 4 mm) in close proximity to the arteries of the neck. (31, 32) Generally, atherosclerosis in the DAo is a marker of atherosclerotic burden and ongoing systemic processes that cause brain injury, such as microvascular damage from increased vascular pulsatility pressure,(5) as opposed to a direct source of cerebral embolism.(32, 33) In the Rotterdam Scan Study, abdominal aortic atherosclerosis at mid-life, measured on radiographs, was associated with the presence of periventricular white matter lesions in late-life, but not with subcortical white matter lesions.(11)
Aortic atherosclerosis has been strongly associated with arterial stiffness.(34) Stiffness in the large arteries may promote cerebral microvascular dysfunction through hypertension, increased blood pressure variability, or excessive flow pulsatility, manifesting as white matter disease in the brain.(5, 35) We showed a cross-sectional association of aortic atherosclerosis with markers of brain injury in a relatively young study sample, with a mean age of 60 years old. Thus, the cumulative adverse effects of impaired autoregulation and intermittent relative ischemia may have onset at an earlier age, perhaps in mid-life. This mechanism is supported by recent studies from the FHS Third Generation cohort showing associations between aortic stiffness and both decreased white matter integrity and the presence of white matter hyperintensities.(35, 36)
The strengths of our study include the large community-based sample evaluated and the sensitivity and specificity of brain MRI for quantifying brain volume and WMHV. CMR is comparable to transesophageal echocardiography and computed tomography for imaging aortic plaques(37) but less invasive and free of radiation exposure. CMR is increasingly being used in clinical and research settings.(38, 39)
Our study had a cross-sectional design, so we were unable to establish a temporal relationship between the development of the aortic plaques and changes in brain MRI traits. Because MRI markers of brain aging and injury tend to remain stable or progress over time, we would expect that the time lag between brain MRI and cardiac MR performed in our study would underestimate the strength of the associations. Future studies may confirm a relationship between aortic atherosclerosis and progression of brain changes over time, including the occurrence of silent cerebral infarctions and cerebral microbleeds.
The imaging protocol did not measure ascending aortic or arch plaque, limiting the analysis to plaque of the DAo. Our cohorts are middle-aged and mainly of European descent, so further prospective confirmatory studies using diverse population samples are warranted. Relationships with grey and white matter volumes can be studied in future investigations. The current study was likely underpowered to detect modest associations with stroke prospectively, given the low number of stroke and TIA events. This was likely due to the young mean age of our sample and the exclusion of participants with atrial fibrillation.
In conclusion, descending thoracic aortic plaque is associated with measures of accelerated brain aging. These observations underscore the potential implications of incidentally identified subclinical aortic atherosclerosis for brain morphology, and perhaps, downstream function. Further studies are needed to clarify a causal and temporal relationship between large vessel disease and brain changes and to determine whether targeted intervention in these high risk individuals modulates cognitive decline.
Supplementary Material
Highlights.
Atheromatous plaque in the descending aorta was associated with lower brain volume
Brain volume loss was equivalent to 8.8 years of aging in women, 4.5 years overall
White matter hyperintensity volume was increased in men with thoracic aortic plaque
Plaque in the descending aorta was not associated with subsequent stroke/TIA
Acknowledgments
Financial support
The FHS and research supported by the Boston University School of Medicine are funded by the National Heart, Lung, and Blood Institute (N01-HC-25195; HHSN268201500001I), and by grants from the National Institute of Neurologic Disorders and Stroke (NINDS R01NS017950). HJA is supported by grants from the National Institute of Aging (NIA T32-AG036697) and the American Heart Association (AHA 15GPSPG23770000). This project was supported in part by contracts from the NHLBI RO1-HL70279 (WJM) and NIA R01-AG008122 (SS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
HJA and REP initiated and designed the study, derived the hypotheses, performed the literature search, interpreted the results, and wrote the report. SS and JMM designed the study, planned and supervised analyses, and acquired and interpreted results. SS obtained funding. All authors critically revised the report for intellectual content, approved the final version of the report, and vouch for the integrity of the data and analyses.
References
- 1.Bos D, Vernooij MW, Elias-Smale SE, Verhaaren BF, Vrooman HA, Hofman A, et al. Atherosclerotic calcification relates to cognitive function and to brain changes on magnetic resonance imaging. Alzheimers Dement. 2012;8(5 Suppl):S104–11. doi: 10.1016/j.jalz.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Tugcu A, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, et al. Atherosclerotic Plaques in the Aortic Arch and Subclinical Cerebrovascular Disease. Stroke. 2016;47(11):2813–9. doi: 10.1161/STROKEAHA.116.015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The French Study of Aortic Plaques in Stroke Group. N Engl J Med. 1996;334(19):1216–21. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 4.Harloff A, Simon J, Brendecke S, Assefa D, Helbing T, Frydrychowicz A, et al. Complex plaques in the proximal descending aorta: an underestimated embolic source of stroke. Stroke. 2010;41(6):1145–50. doi: 10.1161/STROKEAHA.109.577775. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134(Pt 11):3398–407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meissner I, Khandheria BK, Sheps SG, Schwartz GL, Wiebers DO, Whisnant JP, et al. Atherosclerosis of the aorta: Risk factor, risk marker, or innocent bystander?: A prospective population-based transesophageal echocardiography study. Journal of the American College of Cardiology. 2004;44(5):1018–24. doi: 10.1016/j.jacc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 7.Nam HS, Han SW, Lee JY, Ahn SH, Ha JW, Rim SJ, et al. Association of aortic plaque with intracranial atherosclerosis in patients with stroke. Neurology. 2006;67(7):1184–8. doi: 10.1212/01.wnl.0000238511.72927.3c. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65(5):642–9. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonneveld MA, van Dijk AC, van den Herik EG, van Loon JE, de Lau LM, van der Lugt A, et al. Relationship of Von Willebrand Factor with carotid artery and aortic arch calcification in ischemic stroke patients. Atherosclerosis. 2013;230(2):210–5. doi: 10.1016/j.atherosclerosis.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 10.Gur M, Sahin DY, Elbasan Z, Kalkan GY, Yildiz A, Kaya Z, et al. Uric acid and high sensitive C-reactive protein are associated with subclinical thoracic aortic atherosclerosis. J Cardiol. 2013;61(2):144–8. doi: 10.1016/j.jjcc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 11.de Leeuw FE, De Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, et al. Aortic atherosclerosis at middle age predicts cerebral white matter lesions in the elderly. Stroke. 2000;31(2):425–9. doi: 10.1161/01.str.31.2.425. [DOI] [PubMed] [Google Scholar]
- 12.Hermann DM, Lehmann N, Gronewold J, Bauer M, Mahabadi AA, Weimar C, et al. Thoracic aortic calcification is associated with incident stroke in the general population in addition to established risk factors. Eur Heart J Cardiovasc Imaging. 2015;16(6):684–90. doi: 10.1093/ehjci/jeu293. [DOI] [PubMed] [Google Scholar]
- 13.Kathiresan S, Larson MG, Keyes MJ, Polak JF, Wolf PA, D’Agostino RB, et al. Assessment by cardiovascular magnetic resonance, electron beam computed tomography, and carotid ultrasonography of the distribution of subclinical atherosclerosis across Framingham risk strata. Am J Cardiol. 2007;99(3):310–4. doi: 10.1016/j.amjcard.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 15.Polak JF, O’Leary DH, Kronmal RA, Wolfson SK, Bond MG, Tracy RP, et al. Sonographic evaluation of carotid artery atherosclerosis in the elderly: relationship of disease severity to stroke and transient ischemic attack. Radiology. 1993;188(2):363–70. doi: 10.1148/radiology.188.2.8327679. [DOI] [PubMed] [Google Scholar]
- 16.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–6. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102(9):1136–41, 41.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Jaffer FA, O’Donnell CJ, Larson MG, Chan SK, Kissinger KV, Kupka MJ, et al. Age and sex distribution of subclinical aortic atherosclerosis: a magnetic resonance imaging examination of the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22(5):849–54. doi: 10.1161/01.atv.0000012662.29622.00. [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35(8):1857–61. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 22.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63(9):1591–9. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 23.Oyama N, Gona P, Salton CJ, Chuang ML, Jhaveri RR, Blease SJ, et al. Differential impact of age, sex, and hypertension on aortic atherosclerosis: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28(1):155–9. doi: 10.1161/ATVBAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 24.van den Heuvel DM, Admiraal-Behloul F, ten Dam VH, Olofsen H, Bollen EL, Murray HM, et al. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63(9):1699–701. doi: 10.1212/01.wnl.0000143058.40388.44. [DOI] [PubMed] [Google Scholar]
- 25.Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30(6):946–56. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, et al. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35(7):1609–13. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- 28.van der Veen PH, Muller M, Vincken KL, Witkamp TD, Mali WP, van der Graaf Y, et al. Longitudinal changes in brain volumes and cerebrovascular lesions on MRI in patients with manifest arterial disease: the SMART-MR study. J Neurol Sci. 2014;337(1–2):112–8. doi: 10.1016/j.jns.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Chene G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11(3):310–20. doi: 10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geerlings MI, Appelman AP, Vincken KL, Algra A, Witkamp TD, Mali WP, et al. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis. 2010;210(1):130–6. doi: 10.1016/j.atherosclerosis.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Wehrum T, Guenther F, Vach W, Gladstone BP, Wendel S, Fuchs A, et al. Aortic Atherosclerosis Determines Increased Retrograde Blood Flow as a Potential Mechanism of Retrograde Embolic Stroke. Cerebrovasc Dis. 2017;43(3–4):132–8. doi: 10.1159/000455053. [DOI] [PubMed] [Google Scholar]
- 32.Wehrum T, Kams M, Strecker C, Dragonu I, Gunther F, Geibel A, et al. Prevalence of potential retrograde embolization pathways in the proximal descending aorta in stroke patients and controls. Cerebrovasc Dis. 2014;38(6):410–7. doi: 10.1159/000369001. [DOI] [PubMed] [Google Scholar]
- 33.Katsanos AH, Giannopoulos S, Kosmidou M, Voumvourakis K, Parissis JT, Kyritsis AP, et al. Complex atheromatous plaques in the descending aorta and the risk of stroke: a systematic review and meta-analysis. Stroke. 2014;45(6):1764–70. doi: 10.1161/STROKEAHA.114.005190. [DOI] [PubMed] [Google Scholar]
- 34.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32(2):454–60. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 35.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, et al. Association of Aortic Stiffness With Cognition and Brain Aging in Young and Middle-Aged Adults: The Framingham Third Generation Cohort Study. Hypertension. 2016;67(3):513–9. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maillard P, Mitchell GF, Himali JJ, Beiser A, Fletcher E, Tsao CW, et al. Aortic Stiffness, Increased White Matter Free Water, and Altered Microstructural Integrity: A Continuum of Injury. Stroke. 2017 doi: 10.1161/STROKEAHA.116.016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fayad ZA, Nahar T, Fallon JT, Goldman M, Aguinaldo JG, Badimon JJ, et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation. 2000;101(21):2503–9. doi: 10.1161/01.cir.101.21.2503. [DOI] [PubMed] [Google Scholar]
- 38.Lima JA, Desai MY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol. 2004;44(6):1164–71. doi: 10.1016/j.jacc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Pitcher A, Ashby D, Elliott P, Petersen SE. Cardiovascular MRI in clinical trials: expanded applications through novel surrogate endpoints. Heart. 2011;97(16):1286–92. doi: 10.1136/hrt.2011.225904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


