Abstract
The formation of axon collateral branches from the pre-existing shafts of axons is an important aspect of neurodevelopment and the response of the nervous system to injury. This article provides an overview of the role of the cytoskeleton and signaling mechanisms in the formation of axon collateral branches. Both the actin filament and microtubule components of the cytoskeleton are required for the formation of axon branches. Recent work has begun to shed light on how these two elements of the cytoskeleton are integrated by proteins that functionally or physically link the cytoskeleton. While a number of signaling pathways have been determined as having a role in the formation of axon branches, the complexity of the downstream mechanisms and links to specific signaling pathways remain to be fully determined. The regulation of intra-axonal protein synthesis and organelle function are also emerging as components of signal-induced axon branching. Although much has been learned in the last couple of decades about the mechanistic basis of axon branching we can look forward to continue elucidating this complex biological phenomenon with the aim of understanding how multiple signaling pathways, cytoskeletal regulators and organelles are coordinated locally along the axon to give rise to a branch.
Keywords: interstitial branch, axon sprouting, neuronal morphogenesis, neurite, filopodia
Introduction
The function of the nervous system requires complex neuronal circuitry. The formation of circuitry depends on the establishment of synaptic contacts between single neurons and multiple targets. During development, axon branching allows each neuron to establish synaptic contacts with multiple targets and is crucial for the assembly of highly interconnected network (Gallo, 2011; Gibson and Le Ma, 2011; Lewis et al., 2013; Rockland, 2013; Kalil and Dent, 2014; Petrovic and Schmucker, 2015). Therefore, understanding the mechanisms underlying the control of axonal branching is crucial in the study of neuronal circuit development (Gibson and Le Ma, 2011). In the adult nervous system axon branches also emerge in response to injury and neurodegeneration, and are repressed by extracellular signals (Onifer et al., 2011; Akbik et al., 2012; Carmel and Martin, 2014; Kadomatsu and Sakamoto, 2014). The injury induced axon branching/sprouting contributes to the endogenous circuitry repair mechanisms (Carmel and Martin, 2014).
Axon branches can arise from two distinct mechanisms. (1) The growth cone can split and give rise two Y or T shaped axon branches. For example, live imaging of individual zebrafish motor axons reveals that the first axonal branches are generated via bifurcation of the growth cone (Sainath and Granato, 2013). Although growth cone bifurcation can contribute to the formation of branches in specific instances, this is not the major mechanism that contributes to axon branching (discussed in Gallo, 2011). (2) Axon collateral branches emerge from protrusive filopodia and lamellipodia initiated locally along the shaft of the axon independent of the growth cone. Studies of the dynamics of collateral branching revealed three mechanisms of axon collateral branch initiation primarily involving either formation of filopodia, lamellipodia or growth cone pausing (reviewed in Gallo, 2011). In the filopodia based mechanism, branches initiate as axonal filopodia protrusions from the quiescent axon shaft. Time-lapse imaging of fluorescently labeled corticospinal axons showed that in the vicinity of the pons the axon shaft exhibits several dynamic behaviors including the de novo formation of filopodia extensions, and some of the filopodia mature into a branch (Bastmeyer and O’Leary, 1996). Similarly, in vitro studies demonstrated that along the axons of sensory neurons, branches are initiated by the emergence of filopodia followed by stabilization of the filopodia and maturation into a branch (Figure 1; Gallo and Letourneau, 1998, 1999; Ketschek and Gallo, 2010; Spillane et al., 2012). In the lamellipodial mechanism, branches arise from lamellipodia that form at the base of the axon, and move anterograde along the axon. These lamellipodial precursors of branches are termed growth cone like “waves” and have been observed in hippocampal neurons (Ruthel and Banker, 1999; Flynn et al., 2009). Branches then emerge from locations along the axon where the waves stop. Moreover, lamellipodia can also arise de novo along the axon shaft in the absence of growth cone like “waves”. In the growth cone pausing mechanism, the collateral branch is formed from sites along the axon shaft representative of locations where the growth cone stalled, leaving behind a domain of persistent protrusive activity, followed by resumption of its advance (Halloran and Katherine, 1994; Kalil et al., 2000). The importance of axonal protrusive activity in the form of filopodia and lamellipodia is underscored in all of these mechanisms for the initiation of a collateral branch.
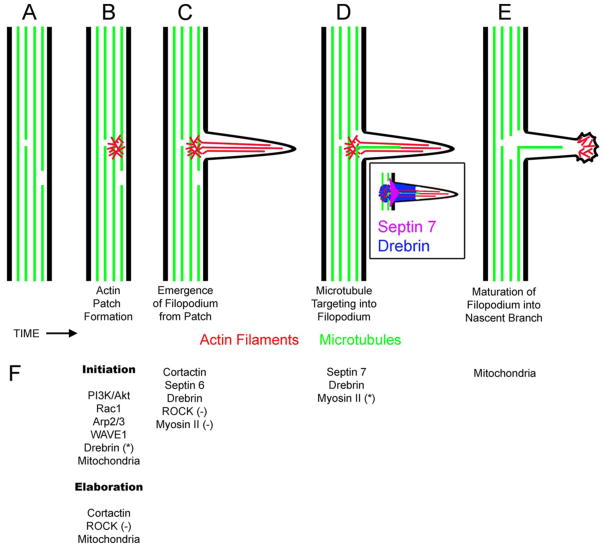
Figure 1.
Sequence of cytoskeletal events leading to the formation of axon collateral branches. The schematic shows the formation of a collateral branch, in time (left to right), along an axon (A). The first event in the formation of a branch is the emergence of an axonal filopodium (B–C). The emergence of an axonal filopodium is preceded by the formation of an axonal actin filament patch (B). The actin patch is a meshwork of actin filaments that gives rise to the bundle of actin filaments that defines the core of the filopodium (C). The targeting of an axonal microtubule into the filopodium is the next necessary step in the formation of a branch from a filopodium (D). The inset shows the general distributions of Drebrin and Septin 7 at axonal filopodia. Septin 7 localizes specifically to the base of filopodia and Drebrin localizes to actin patches and the proximal half of filopodia. Note that although not specifically shown, actin patches have shorter lifespans than filopodia and often are not present at the base of existing filopodia, although remnants may persist. Filopodia containing microtubules that remain in place have the potential to mature into nascent branches. The process of maturation involves the disassembly of the filopodial actin filament bundle, and the establishment of a distally polarized actin filament cytoskeleton giving rise a small growth cone-like structure at the tip of the nascent branch (E). Once the branch if established it then has the potential to continue elongating or undergo retraction back into the main axon. Each of the steps B–E has a given probability of occurring. In other words, only subsets of actin patches give rise to filopodia, only subsets of filopodia are targeted by microtubules, and only a subset of filopodia containing microtubules undergo maturation. Ultimately a branch arises from a segment of the axon that has successfully met the criteria for each of these steps. Not shown in this schematic is the local splaying of the microtubule array at sites of potential branching that is however discussed in the main text. The splaying occurs early in the process between steps A–B. (F) Summary of molecules discussed in the main text with specific identified roles in steps A–E. Each set of regulators is shown below the relevant step. Other regulators of branching which have not been assigned specific loci of regulation in the cytoskeletal basis of branching are not shown, but discussed in the text. Under (B) regulators are shown depending on whether they control the initiation of patches or their subsequent development (i.e., increase in size and lifespan). Unless denoted by (−) the role of the regulator is positive. If denoted by (−) the role is inhibitory. For example, Myosin II acts to suppress the emergence of filopodia from actin patches (C). In (B) Drebrin is denoted with a (*) to note that while it is required for patch initiation it is not sufficient to drive initiation. In (E) myosin II is denoted with (*). In this case, Myosin II does not regulate the entry of microtubules into filopodia but it serves to decrease the distance the microtubules penetrate into the filopodium.
The formation of axon branches involves the regulation of the neuronal cytoskeleton. The major constituents of the axonal cytoskeleton include actin filaments and microtubules that are highly dynamic and undergo rapid cycles of polymerization and depolymerization (Figure 1; Gallo, 2011; Kalil and Dent, 2014; Zhang and Rasband, 2016). Branching initiates through the protrusion of actin filament-based filopodia and lamellipodia that are subsequently invaded by axonal microtubules as the branch matures and continues extending (Figure 1). In this review, we will discuss the role of cytoskeletal dynamics and their regulators in the formation of branches. Moreover, we will consider the signaling pathways known to regulate cytoskeletal dynamics and modulate axonal branching, and conclude with a discussion regarding the necessity to elucidate how the many cellular events underlying branching are coordinated in space and time. The basic sequence of cytoskeletal events and a summary of the identified cytoskeletal regulators underlying the formation of an axon branch at a specific point along the axon shaft are shown in Figure 1.
Cytoskeletal dynamics and reorganization underlying the early stages of branch formation
Actin dynamics in axonal branching
Unlike the highly dynamic growth cone, the consolidated axon shaft contains relatively low levels of actin filaments and exhibits minimal protrusive activity. The formation of axon branches is preceded by the dynamic polymerization and reorganization of the cytoskeleton along the axon shaft. This includes the local accumulation of actin filaments that is required for the formation of actin-driven protrusions and subsequent branch formation. The first step in the formation of collateral branches involves the actin filament dependent initiation of axonal filopodia, and in some cases lamellipodia (as discussed above). Since the formation of axonal filopodia is the most common first step for branching, this review will focus on this process. Filopodia are thin finger like membrane protrusions mainly composed of a bundle of parallel actin filaments and actin associated proteins. The rapidly polymerizing barbed ends of filaments are oriented toward the tip of the filopodium, generating forces that push the membrane forward. Formation of filopodia can be understood in terms of three basic events: actin filament nucleation driving the initiation of new actin filaments, rapid elongation of nucleated actin filaments through barbed-end polymerization, and the bundling of elongating actin filaments (Svitkina et al., 2003; Mattila and Lappalainen, 2008).
Axonal actin patches serve as precursors to the emergence of axonal filopodia
Focal accumulations of actin filaments along the axon, termed actin patches, are precursors to the emergence of filopodia (Figures 1A–C, 2A; reviewed in Gallo, 2011, 2013), a form of filopodial emergence that is similar to that described for filopodia arising from non-neuronal lamellipodia (Svitkina et al., 2003). Actin patches consist of localized highly dynamic domains of actin filaments with general organization similar to lamellipodial structures (Spillane et al., 2011). Live imaging of chicken sensory neurons transfected with eYFP-actin, revealed that actin patches form spontaneously and are transient (Loudon et al., 2006; Ketschek and Gallo, 2010; Spillane et al., 2011, 2012, 2013; Sainath et al., 2016), and similar structures have been reported in other neuronal systems in vitro and in vivo (Korobova and Svitkina, 2008; Mingorance-Le Meur and O’Connor, 2009; Andersen et al., 2011; Spillane et al., 2011; Chia et al., 2014; Chetta et al., 2015; Hand et al., 2015). Actin patches serving as precursors to the formation of axonal filopodia and branches have also been imaged in vivo (Andersen et al., 2011; Spillane et al., 2011; Hand et al., 2015). Although filopodia emerge from actin patches, only some patches give rise to axonal filopodia. Along the axons of embryonic sensory neurons approximately twenty percent of patches give rise to filopodia (Ketschek and Gallo, 2010). The emergence of the filopodia from the actin patches, involves the reorganization of some of the actin filaments in the patch into a bundle of filaments. The branch inducing signal Nerve Growth Factor (NGF) promotes formation of axonal filopodia by increasing the rate of formation of actin patches through localized microdomains of PI3K signaling without altering the probability that an actin patch will give rise to a filopodium (Ketschek and Gallo, 2010; Spillane et al., 2012). Thus, at least in the context of NGF signaling, the regulation of the formation of axonal actin patches is a crucial aspect of the mechanism of the initiation of axon branches. If and how other branch inducing signals similarly impact the rate of formation of actin patches, or the probability of filopodia emergence from patches, remains to be determined.
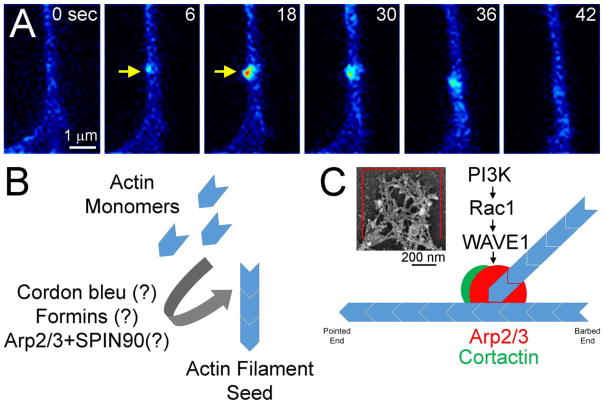
Figure 2.
Axonal actin filament patch formation and dynamics. (A) Example of actin patch formation (yellow arrow at 6 sec), elaboration (6–18 sec) and dissipation (30–42 sec) as imaged along the axon of an embryonic sensory neuron expressing eYFP-β-actin. (B) Possible actin filament nucleators involved in the initiation of actin patches. The specific nucleators required for patch initiation remains to be determined, as reflected by the (?). (C) The Arp2/3 complex is required for the detection and elaboration of axonal actin filament patches. The inset shows an example of the complex network of actin filaments in axonal actin patches, as detected using platinum replica electron microscopy (from a collaboration with Dr. T. Svitkina, University of Pennsylvania). PI3K activity drives the Rac1 GTPase that in turn activates the Arp2/3 complex through WAVE1. Cortactin serves to stabilize Arp2/3 mediated filament branches and positively regulates the elaboration of patches.
Actin filaments are highly regulated by actin associated proteins that have important roles in axonal branching. An important regulator at the earliest steps of collateral branch formation is the Arp2/3 complex (Strasser et al., 2004; Spillane, et al., 2011, 2012). The Arp2/3 complex nucleates new filaments from the sides of existing filaments, giving rise to networks of branched actin filaments that can then be bundled together to give rise to the actin filament bundle and serving as the core of the emerging filopodium (Svitkina et al., 2003; Korobova and Svitkina, 2008). Spillane et al. (2011) demonstrated that in sensory neurons the Arp2/3 complex is required for the formation of actin patches, axonal filopodia and in turn branches (Figure 2C). (Spillane et al., 2011, 2012). WAVE1 and cortactin drive activation of the Arp2/3 complex and stabilization of Arp2/3-mediated branched filaments, respectively. The role of these two proteins in filopodia formation during branching has been studied (Kim, et al., 2006; Mingorance-Le Meur and O’Connor, 2009; Spillane, et al., 2012). In chicken sensory neurons, WAVE1 activity promotes Arp2/3-dependent actin patch initiation (Spillane et al., 2012), while Cortactin positively regulates actin patch duration and promotes the emergence of filopodia from actin patches (Spillane, et al., 2012). The Arp2/3 complex is generally considered to require binding to the sides of pre-existing filaments to nucleate branched filaments. Therefore, additional actin filament nucleators may also operate in actin patches to generate the mother filaments that the Arp2/3 complex subsequently binds (Figure 2B). The actin nucleation factor cordon-bleu is a likely candidate as it positively contributes to the formation of axon collateral branches in hippocampal neurons, and can cooperate with Arp2/3 in the formation of actin filaments (Ahuja et al., 2007; Hou et al., 2015). Nevertheless, the specific role of cordon bleu in actin patch and filopodia initiation during axonal branching needs further studies. It is also likely that additional members of the actin nucleating formin family will have roles in the formation of axonal filopodia and branches, but this remains to be determined (Yang and Svitkina, 2011). A genetic analysis of actin nucleators in Drosophila revealed that both the DAAM formin and the Arp2/3 complex partially contribute to the regulation of the number of growth cone filopodia, and when both DAAM and Arp2/3 are ablated very few filopodia form (Gonçalves-Pimentel et al., 2011). However, a role for Arp2/3 in mediating the entirety of filament nucleation should not be readily discounted, as recent work has determined that the Arp2/3 complex can be activated to nucleate filaments independent of pre-existing filaments when the complex is activated by binding to WISH/DIP1/SPIN90 (Wagner et al., 2013). SPIN90 is expressed by neurons, localizes to axons and positively regulates the number of growth cone filopodia (Kim et al., 2011). Given the relatively large cohort of identified actin filament nucleation systems, elucidation of the ones relevant to axon branching will be a fruitful venue of future research.
Emergence and elongation of axonal filopodia
The emergence of a filopodium involves the rapid elongation of actin filaments through barbed-end polymerization, and the bundling of actin filaments to give rise to the core of filaments supporting the shaft of the filopodium (Lebrand et al., 2004; Mattila and Lappalainen, 2008). These processes require the activity of different multiple actin binding proteins. For example, the Ena/VASP family proteins are multifunctional actin binding proteins that directly regulate assembly of the actin filament network and modulate the morphology and behavior of lamellipodia and filopodia (Dent et al., 2007; Mattila and Lappalainen, 2008; Bear et al., 2009). Ena/VASP proteins bind to the barbed ends of actin filaments and enhance actin filament elongation. Subcellular depletion of Ena/VASP, by targeting the proteins to the mitochondria, decreases the number of filopodia in hippocampal neurons and inhibits collateral branch formation along retinotectal axons (Lebrand et al., 2004; Dwivedy et al., 2007). The actin-severing protein actin-depolymerizing factor (ADF)/cofilin increases actin depolymerization, severs filaments and increases the turnover of actin filaments, thereby promoting filament assembly by increasing the available pool of actin monomers and free barbed ends (Fass et al., 2004). ADF/cofilin is required for Brain Derived Nerve Factor (BDNF)-induced filopodia at retinal growth cones (Chen et al., 2006; Flynn et al., 2012). Fascin is a filament bundling protein that increases the stiffness of filopodial bundles, and promotes extension and maintenance of the filopodia beyond the leading edge in non-neuronal cells (Vignjevic D et al., 2006; Mattila and Lappalainen, 2008). However, the specific contribution of fascin to axonal branching has not been addressed. Cortactin and the actin filament binding protein Drebrin have both been shown to promote the formation of axonal filopodia and axon collateral branching. Analysis of the roles of these proteins in actin patch dynamics, revealed that both contribute to the emergence of filopodia from actin patches (Figure 1; Spillane et al., 2012; Ketschek et al., 2016) and are discussed further in subsequent sections.
Microtubule dynamics in axon branching
Although axons generate multiple filopodia during the process of branching, only a subset of these filopodia mature into collateral branches. The maturation of a filopodium into a branch requires the invasion of the filopodium by microtubules (Gallo, 2011; Kalil and Dent, 2014; Figure 3A). The targeting of microtubules into filopodia provides cytoskeletal support for the nascent branch, and allows the delivery of axonal transport cargoes into the branch. Microtubules are hollow tubular structures composed of linear arrays of α and β tubulin dimers. Microtubules exhibit polarity and the two ends are referred to as the plus and minus ends, respectively. The majority of the plus ends/tips in axons are directed distally toward the axon terminus. The plus end of microtubules is highly dynamic undergoing repeated bouts of polymerization and depolymerization, collectively referred to as plus end dynamic instability. In developing branches, tubulin dimers are added and subtracted at the microtubule plus end that is directed distally toward the membrane of the filopodium or branch (Conde and Caceres, 2009; Kalil and Dent, 2014). In the axon, microtubules are organized in a parallel bundled array. However, at the site of nascent branches microtubules become unbundled and can also undergo local fragmentation along the axon shaft (Yu et al., 1994; Dent et al., 1999; Gallo and Letourneau, 1998, 1999; Ketschek et al., 2015). The process of branching thus involves both microtubule plus end dynamic instability and the local reorganization of the axonal microtubule array.
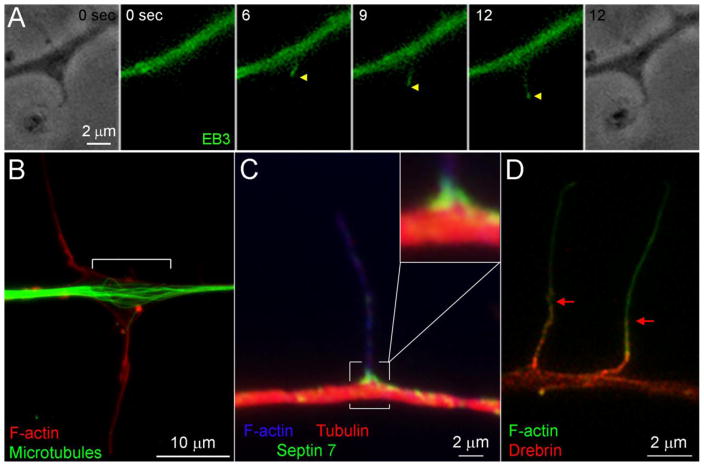
Figure 3.
Microtubule dynamics, organization and microtubule-actin interactors. (A) Example of a microtubule plus tip decorated with eGFP-EB3 (denoted by yellow arrowheads) entering an axonal filopodium. The phase contrast images show the filopodium at 0 and 12 secs. The microtubule tip enters the filopodium at 6 sec and by 12 sec has traveled almost to the tip of the filopodium. (B) Example of the splaying of the microtubule array at sites of axonal protrusive activity, reflected by filopodia. The splaying of microtubules is denoted by the white]. Note that the adjacent axon segments exhibit a uniformly bundled array. (C) Example of Septin 7 localization to the base of an axonal filopodium (from a collaboration with Dr. E. Spiliotis, Drexel University). The inset shows a 2x empty magnification view of the base of the filopodium. (D) Example of the distribution of Drebrin restricted to the proximal 4–5 microns of axonal filopodia (denoted by red arrows) (from a collaboration with Dr. J. Chilton, University of Exeter). All examples are from embryonic chicken sensory neurons.
Observations at early stages of branch formation revealed localized disruption of the bundled microtubule array where the microtubules splay apart. (Figure 3B; Dent et al., 1999; Gallo and Letourneau, 1998, 1999; Kornack and Giger, 2005; Ketschek et al., 2015). The mechanism that promotes the splaying apart (debundling) of microtubules is not understood. However, a recent study suggests that actomyosin contractibility along the axon negatively regulates the ability of microtubules to undergo splaying/debundling (Ketschek et al., 2015). The same study reported that in chicken sensory neurons NGF promotes the localized debundling of axonal microtubules along the axon prior to the emergence of collateral branches. Depolymerization of actin filaments, or inhibition of myosin II, potentiated the effects of NGF on microtubule debundling. Sites of microtubule debundling strongly correlated with the entry of microtubules into axonal filopodia, suggesting that debundling may assist the entry of microtubule tips into filopodia. Furthermore, axonal microtubule debundling during branching might promote the stalling or capture of axonal transport cargoes using microtubule based mechanisms. Nevertheless, the mechanistic function of microtubule debundling during axon branching needs further investigation. In addition, localized severing of long microtubules into smaller fragments may also contribute to branching by providing a supply of small microtubules that could then undergo active transport into nascent branches (Dent et al., 1999; Gallo and Letourneau, 1998, 1999; Yu et al., 1994, 2008). Microtubules can enter into axonal filopodia through either polymerization or active transport, and both mechanisms can contribute to branching depending on the experimental model systems (Dent et al., 1999; Gallo and Letourneau, 1999, 1998; Ketschek et al., 2015). Molecules with identified roles in promoting the entry or retention of microtubules in filopodia are discussed below (i.e., Septin 7 and Drebrin). Myosin II is found in axonal filopodia and does not regulate the entry of microtubules into filopodia, but negatively regulates the ability of the microtubule tip to penetrate deep into the filopodial shaft following entry (Figure 1; Ketschek et al., 2016).
Microtubule severing proteins
During branching the properties of microtubules are regulated by diverse cellular factors, including microtubule associated proteins (MAPs) which bind microtubules along their lengths (Kornack and Giger, 2005; Gallo, 2011; Kalil and Dent., 2014). Spastin and Katanin are severing proteins that fragment microtubules and differentially promote the formation of collateral branching (Qiang et al., 2006; Yu et al., 2008). In cultured hippocampal neurons, Spastin overexpression enhances the formation of axon branches, whereas its depletion decreases branch formation (Yu et al., 2008). The MAP Tau protects microtubules from the severing activity of Katanin. Depletion of Tau from axons potentiates the induction of axon branching by Katanin (Qiang et al., 2006). This suggests two different models of branch formation involving microtubule severing. In the first model, the local concentration of Spastin along the axon may drive microtubule severing, and in the second model the local regulation of Tau binding to microtubules may determine the ability of Katanin to sever microtubules. Each of these mechanisms leading to the severing of axonal microtubules are likely under the regulation of signaling cascades that regulate events such as the phosphorylation of Tau, the attachment or detachment of other proteins, and the localization of Spastin/Katanin (Kornack and Giger; 2005; Qiang et al., 2006; Yu et al., 2008), but these issues remain largely unexplored. Finally, in the context of NGF-induced axon branching, and the localized splaying of the axonal microtubule array, there does not seem to be any severing of microtubules or decreases in microtubule content at sites of axon branching (Ketschek et al., 2016). This observation indicates that microtubule severing is not necessary, but can have an important role in branching in a cell type or context dependent manner. Whether microtubules populate nascent branches following severing and transport or through plus end polymerization, could simply reflect two different means to achieve the same end.
Microtubule plus-end associated proteins
The microtubule plus end proteins (+Tips) are proteins that specifically accumulate at the plus ends of polymerizing microtubules, allowing control of microtubule dynamics and growth directionality. Different studies have shown the importance of +Tips in growth cone mediated axon extension (Kornack and Giger, 2005; Conde and Caceres, 2009). The adenomatous polyposis coli protein (APC) binds to the plus end of microtubules and stabilizes their growing ends (Slep et al., 2005). APC is essential for microtubule organization within the axon and growth cone of early developing mouse cortical neurons (Zhou et al., 2004, Purro et al., 2008; Yokota et al., 2009). Genetic deletion of APC leads to excessive branching that correlates with disruption of microtubule organization at branch points. Furthermore, many of the exuberant branches arise from growth cone splitting and not collateral formation along the axon shaft. The exact mechanisms of how APC contributes to axonal branching remain to be elucidated (Chen et al., 2011; Pacheco and Gallo, 2016).
Kinesins
Kinesins are a large family of motor proteins that move cargoes along microtubules. However, some members of the Kinesin 1 subfamily, including KIF2A, do not function as motor proteins but possess microtubule-destabilization activity (Kornack and Giger, 2005). Studies in knockout mice showed that KIF2A suppresses the elongation of established axon branches, resulting in abnormally long collateral branches (Homma et al., 2003; Noda et al., 2012). Thus, KIF2A plays an important role in suppression of collateral branch extension, but a role in branch initiation was not observed. Similarly, a recent study found that Kinesin-1 (KIF5) accumulates at the tips of neurites within the axon arbor of cerebellar granule neurons and suppresses their retraction, resulting in stabilization and subsequent increased growth (Seno et al., 2016). Moreover, a role for microtubule motors in the formation of collateral branches along sensory axons in response to treatment with NGF is suggested by a study using ciliobrevin D, an inhibitor of the retrograde motor protein dynein (Sainath and Gallo, 2015). As also observed in some other studies (see discussion in Sainath and Gallo, 2015), manipulation of dynein activity was found to also impact the activity of kinesin driven anterograde transport and block overall axonal transport. Thus, the effects of ciliobrevin D on the inhibition of axon branching cannot be readily attributed to inhibition of dynein, but do provide general evidence for a role of microtubule based motors in the initiation of axon branches, as well as the regulation of the later stages of branch elongation.
Additional microtubule associated proteins (MAPs)
Doublecortin (DCX) binds microtubules and influences microtubule stability and bundling (Tint et al., 2009; Brouhard and Rice, 2014). DCX is required for neuronal migration and axonal wiring (Koizumi et al., 2006). Studies in hippocampal neurons showed that DCX is preferentially associated with microtubules in regions enriched in actin filaments (Tint et al., 2009). Furthermore, DCX knockdown triggers excessive branching of cortical and granule neurons, an effect attributed to a failure in the maintenance of microtubule crosslinking (Bilimoria et al., 2010; Li et al., 2014). MAP1B is one of the earliest MAPs to be expressed during development. In vitro studies highlight a key role of MAP1B in multiple processes that are essential for neuronal regeneration, including axon guidance, retraction and branching (Bouquet et al., 2007; Stroissnigg et al., 2007; Barnat et al., 2010; Tymanskyj et al., 2012). Deletion of MAP1B in mice increases axonal branching (Bouquet et al., 2007; Tymanskyj et al., 2012). Live imaging analysis of branch formation demonstrated that in the absence of MAP1B axonal protrusions are more likely to be stabilized and therefore mature into a branch (Bouquet et al 2004; Barnat et al., 2016). The intra-axonal translation of MAP1B was also found to be negatively regulated by microRNA9 and downregulation of MAP1B translation promoted axon branching (Dajas-Bailador et al., 2012).
Interactions between microtubules and actin filaments
Interaction between dynamic microtubules and actin filaments underlie many fundamental processes including axonal branching (Kalil and Dent, 2014). Time lapse imaging studies demonstrated that at branch points the splaying of microtubules is accompanied by focal accumulation of actin filaments. Application of drugs that decrease either actin or microtubule dynamics also inhibit polymerization of the other cytoskeleton element (Dent and Kalil, 2001, Rodriguez et al., 2003). These observations support the notion that interactions between dynamic microtubules and actin filaments are required for axon branching and axon outgrowth. However, the mechanisms that coordinate the remodeling of actin and microtubules during branching are poorly understood. Although there are multiple possible molecules involved in branching that have the potential to bind actin filaments and microtubules, few studies to date have addressed the role of any of those molecules in the coordination of the actin and microtubule cytoskeleton during branching. For a comprehensive treatment of microtubule-actin interactions during branching readers are directed to a recent review focused on this topic (Pacheco and Gallo, 2016).
Septins
Septins comprise a family of GTP binding proteins that polymerize into higher order structures. There are four main groups of Septins defined by their founding members (Septin 2,3,6 and 7 groups; Mostowy and Cossart, 2012). The first described Septin hetero-oligomer consists of two Septin 2 subunits that bind each other and are flanked by Septin 6 subunits that in turn bind Septin 7 subunits, resulting in a 762267 oligomer. However, Septin hetero-oligomers vary in subunit composition as dictated by the rules of interaction between Septin family members (see Nakahira et al., 2010), thereby giving rise to a large number of possible oligomers. Septins regulate actin and microtubule organization, their crosstalk and their binding to others effectors (Sheffield et al., 2003; Weirich et al., 2008). Septin 6 and Septin 7 have been shown to be required for the morphogenesis of dendrites and axonal branching through regulation of the axonal cytoskeleton (Tada et al., 2007; Xie et al., 2007; Cho et al., 2011; Hu et al., 2012; Ageta-Ishihara et al., 2013). Furthermore, a recent investigation noted that Septin7 negatively regulates the levels of acetylated tubulin in axons (Ageta-Ishihara et al., 2013). Studies in chicken sensory neurons suggest that the combined action of Septin 6 and Septin 7 provides a mechanism for coordinating the cytoskeleton during collateral branching along axons (Hu et al., 2012). This study demonstrated that Septin 6 localizes to actin patches and likely acts as a scaffold to locally recruit cortactin to promote the formation of filopodia from patches. Septin 7 targets specifically to the base of axonal filopodia where the initial interactions of microtubule tips with filopodia are expected to take place (Figure 1, 3C). Septin7 alters the organization of axonal microtubules, and was found to be required and sufficient for the promotion of the localization of microtubules into axonal filopodia. Septin 6 was also found to be required for the targeting of microtubules into axonal filopodia, as evidenced by shRNA depletion, but overexpression of Septin 6 did not promote microtubule targeting. This required but not sufficient role of Septin 6 may relate to its function as a Septin 7 binding partner. However, relatively low colocalization was observed for Septin 6 and 7 in patches and at the base of filopodia. This observation suggests that at sites of filopodia formation these Septins may not represent the canonical 762267 oligomer, and clarification of the Septin oligomers of relevance to axon branching awaits further consideration. Collectively, these studies suggest that Septin 6 and 7 comprise a module that regulates the reorganization of actin filaments and microtubules, thereby affecting the development of axonal collateral branches (Hu et al., 2012).
Drebrin
Drebrin is an actin filament binding protein, highly expressed in neuronal cells, involved in the regulation of actin filament organization. Debrin can bundle actin filaments and regulate the binding of additional actin binding proteins (Dun and Chilton, 2010; Wort, et al., 2010). A previous study pointed to an important role for Drebrin as a link between the actin and microtubule systems. Drebrin binds to EB3, a microtubule plus end binding protein that associates with the tips of polymerizing microtubules (Geraldo et al., 2008). Furthermore, Drebrin increases the number of axonal filopodia and branches generated by oculomotor neurons in vivo (Dun et al., 2012). Ketschek et al (2016) reported that in sensory neurons Drebrin coordinates the actin filament and microtubule cytoskeleton during the initial stages of axon branching. This study revealed that Drebrin is involved in the formation of the filopodia bundle from actin patches. Additionally, Drebrin normally localizes to actin patches and the proximal but not distal half of filopodia (Figure 3D). Drebrin is thus well placed to promote the entry of microtubule plus ends into the base of filopodia and guide their tips into the filopodia shaft. Consistently, overexpression of Drebrin greatly increased instances of microtubule plus tips entering axonal filopodia. Interestingly, myosin II was found to negatively regulate the localization of Drebrin into the distal most parts of filopodia, as inhibition of myosin II resulted in the presence of Drebrin throughout filopodia instead of just the most proximal half of the filopodium. By directly monitoring the effects of manipulating Drebrin function on both the axonal actin filament and microtubule cytoskeleton, this report identified Drebrin as the first known coordinator of both cytoskeletal systems during axon branching.
Microtubule-actin crosslinking factor (MACF1)
MACF1 is a cytoskeletal crosslinking protein that interacts with actin filaments and microtubules, and plays an important role in neuronal development (Fuchs and Karakesisoglou, 2001; Lin et al., 2005). A recent study addressed the functional consequences of knocking out MACF1 in cortical and hippocampal neurons, in vivo and in vitro, on dendritic and axonal development (Ka and Kim, 2015). When MACF1 was knocked out, axons grew shorter and formed less axonal branches. MACF1 deletion disrupted actin and microtubule organization. However, the effects of MACF1 knockout on the specifics of the dynamics of the axonal cytoskeleton and branch formation remain to be specifically addressed.
Signaling mechanisms that regulate cytoskeletal dynamics during axonal branching
Nerve Growth Factor (NGF) induced branching
Extracellular signals, including neurotrophins, regulate the formation of collateral branches (Gallo, 2011, 2013; Gibson and Ma, 2011; Bilimoria and Bonnie, 2013; Kalil and Dent, 2014). Neurotrophins are growth factors essential for the development of the vertebrate nervous system. Each neurotrophin can signal through two different types of cell surface receptors, Trk and p75. Activation of Trk receptor trigger activation of the phosphatidylinositol-3 kinase (PI3K), mitogen-activated protein kinase pathways, and others (Huang and Reichardt, 2001, 2003). NGF stimulates formation of sensory axonal collateral branching in sensory neurons through activation of the PI3K signaling pathway (Gallo and Letourneau, 1998; Markus et al., 2002; Jones et al., 2003; Ketschek and Gallo, 2010). PI3K is a lipid kinase that generates PIP3 (phosphatidylinositol [3,4,5] triphosphate) from PIP2 (phosphatidylinositol [4,5] biphosphate). PIP3 serves to target a variety of proteins to the membrane. PIP3 recruits Akt Kinase, which can inhibit GSK3-β, and activate other signaling pathways such as mTOR (Pan et al., 2005; Quian et al., 2005; Toker and Marmiroli, 2014; Kriplani et al., 2015). The PI3K pathway is a major regulator of axonal growth, controlling cellular cytoskeletal dynamics and expression of genes functioning during neuron morphogenesis (Atwal et al., 2000; Arévalo et al., 2006; Cosker and Eickholt, 2007; Eickholt, et al 2007). Conversely, PTEN phosphatase converts PIP3 back to PIP2, and genetic deletion of PTEN increases axonal branching (Kwon et al., 2006; Drinjakovic et al., 2010; Geoffroy et al., 2015).
The current understanding of the mechanism of NGF-induced branching involves the activation of the TrkA receptor by NGF, resulting in activation of PI3K signaling which in turns activates the Rac1 GTPase to drive WAVE1 activity, thereby activating the actin nucleating complex Arp2/3 (Figure 2C; Spillane et al., 2011, 2012). Spillane et al (2012) showed that in chicken sensory neurons, PI3K activity increases the axonal levels of the actin associated protein cortactin that promotes the emergence of the filopodia from the actin patches, and also the levels of WAVE1. The NGF-induced increases in these proteins is mediated by intra-axonal translation of axonally targeted mRNAs. Through live imaging of sites of PIP3 formation along axons, Ketschek and Gallo (2010) identified localized microdomains of PI3K signaling that spatio-temporally correlate with the locations of actin patch formation along axons.
Live imaging of microtubule tip polymerization in sensory neurons, transfected with microtubule plus tip associated GFP-EB3, showed that NGF promotes microtubule polymerization in distal axons, and treatment with NGF increases the percentage of filopodia that contain microtubules (Ketschek and Gallo, 2010; Spillane, et al., 2012). Ketschek et al (2016) reported that NGF promotes the localized debundling of axonal microtubules prior to the emergence of collateral branches. These investigations determined that NGF promotes the formation of collateral branches through the regulation of both the actin filament and microtubule cytoskeleton.
Glycogen synthase kinase-3 (GSK3-α and β)
GSK3 is a serine/threonine kinase primarily regulated through inhibition of its activity by phosphorylation of its amino-terminal serine residue (Ser9). This protein is a downstream effector of multiple extracellular cues including Wnts and NGF (Kim, et al., 2011b). GSK3 has emerged as an important regulator of axon extension and branching by regulating the reorganization of microtubules (Zhou et al., 2004; Purro et al., 2008; Bilimoria et al., 2010; Barnat et al., 2016). Pharmacological inhibition of GSK3 activity was shown to induce axon branching in embryonic sensory neurons (Kim et al., 2006b). Moreover, Wnt3 signaling directly affects the microtubule cytoskeleton in the growth cone by inducing APC loss from the microtubule plus-end through inhibition of GSK3-β (Purro et al., 2008). Interestingly, a recent study demonstrated that GSK3-β mediated phosphorylation of MAP1B negatively regulates neurite branching in adult sensory neurons (Barnat et al, 2016). GSK3-β also phosphorylates DCX contributing to DCX function in the restriction of axonal branching in granule neurons of the cerebellar cortex (Bilimoria et al, 2010).
3′-5′-cyclic adenosine monophosphate (cAMP) signaling
cAMP has been suggested to serve as an important mediator in axonal growth (Averaimo and Nicol, 2014). By using photo-activated adenylyl cyclase (PAC) to locally increment cAMP levels, a recent study revealed that short term elevation of intracellular cAMP induces axonal branching via Protein Kinase A (PKA) activation (Zhou et al 2016). Furthermore, a study performed in hippocampal neurons indicates that Netrin promotes filopodia formation along the axon through PKA induced phosphorylation of the actin regulators Ena/VASP (Lebrand et al 2004). Calpain protease activity is essential for the repression of protrusive activity along the axon by limiting Cortactin levels and inhibiting actin polymerization (Mingorance-Le Meur and O’Connor, 2009). cAMP-PKA signaling inhibits the activity of Calpain in hippocampal neurons, resulting in decreased proteolysis of Cortactin and increased axonal protrusive activity and branching (Mingorance-Le Meur and O’Connor, 2009). The theme of the regulation of axonal plasticity by Calpain mediated mechanisms was also determined in studies involving the effect of SDF1 on axon development. SDF1 binds to the G-protein-coupled receptor C-X-C motif chemokine receptor 4 (CXCR4) (Liu et al., 2016), and regulates the development of the nervous system through effects on cell migration and axon guidance (Mithal, et al, 2012). A recent report provides evidence that SDF1 reduces branching in cortical interneuron through dual regulation of actin and microtubules. Upon SDF1 signaling, Calpain becomes active and cleaves Cortactin, reducing Arp2/3 function, consolidating the actin network and reducing branch formation. Simultaneously, SDF1 signaling activates DCX to bind and bundle microtubules, stabilizing the microtubule array and reducing neuronal branching (Lysko et al., 2011, 2014). Whether the regulation of calpain by SDF1 is mediated by cAMP signaling remains to be determined.
The role of GTPases in axonal branching
Rho GTPases (Rac1, RhoA and Cdc42) are monomeric G proteins that can switch between an inactive GDP bound state and active GTP-bound state. Guanine nucleotide exchange factor (GEFs) interact with Rho GTPases and stimulate the exchange of GDP to GTP to generate an active form. In contrast, GTPase activating proteins (GAPs) activate the intrinsic GTPase activity, resulting in inactivation (Schmidt and Hall, 2002). A major role of Rho GTPases is to control the assembly, disassembly and dynamic rearrangement of the actin filament and microtubule cytoskeleton. Rho GTPases play crucial roles in axon growth, guidance and branching (Hall and Lalli, 2010). Rac1 has been shown to positively regulate axon branching (Moon and Gomez, 2010; Spillane, et al., 2012). Overexpression of vav2, a Rac1/Cdc42 GEF, or constitutively active Rac1 in cultured Xenopus neurons promotes branching, and In vivo vav2 increases branching in commissural interneurons (Moon and Gomez, 2010). In sensory neurons, Rac1 is activated by NGF-PI3K pathway, and its activity promotes the formation of actin patches. The effect of Rac1 is likely due to the activation of its downstream effector WAVE1 that in turn activates the Arp2/3 complex and promotes actin polymerization (Spillane et al., 2012). RhoA-Kinase (ROCK) is activated downstream of RhoA and in neurons it has generally been associated with the promotion of actomyosin driven contractility and the suppression of the formation of protrusive actin filament based structures (Gallo, 2011; Spillane and Gallo, 2014), while promoting the formation of cytoplasmic bundles of actin filaments that serve contractile functions (Gallo, 2006). Analysis of eYFP-actin dynamics in the distal axon of chicken sensory neurons revealed that RhoA-ROCK negatively regulates the elaboration of actin patches (Loudon et al., 2006). RhoA-ROCK signaling positively regulates the activity of myosin II by increasing the phosphorylation of myosin II regulatory light chains, thereby inhibiting the emergence of filopodia from actin patches by the activation of Myosin II (Gallo, 2006; Loudon et al., 2006). Focal Adhesion Kinase (FAK), negatively regulates axonal branching in hippocampal neurons, in part by the activation and recruitment of p190RhoGEF (Rico et al., 2004). In Drosophila mushroom body, activation of RhoA or inactivation of its effector DROCK/ROCK, also inhibit the formation of branching (Billuart et al., 2001). Conversely, another study presented evidence that RhoA activity in cortical neurons acts as a positive mediator of activity dependent axon branching (Ohnami et al., 2008). In addition to the previously discussed role of RhoA in driving actomyosin contractility, RhoA can also drive actin polymerization through the regulation of formins (Kühn and Geyer, 2014), a role that may be assumed in the context of activity mediated axonal plasticity. It is possible that neuronal activity may bias the relative contribution of RhoA toward filament polymerization and away from the actomyosin system, thereby resulting in the promotion of protrusive activity.
Calcium
Cytoplasmic calcium levels are major regulators of a multitude of cellular functions including cytoskeletal dynamics. The initiation of axonal filopodia and branches induced by the local application of NGF along sensory axons was found to require neither extracellular nor intracellular sources of calcium (Gallo and Letourneau, 1998). Unexpectedly, lowering calcium levels instead promoted the formation of axonal filopodia in response to NGF along sensory axons. Consistently, a recent study of the NGF induced branching of sympathetic axons in vitro and in vivo indicates that coronin-1 suppresses NGF-induced branching through a calcium dependent mechanism (Suo et al., 2015). In contrast, the induction of branches along cortical neuron axons by Netrin-1, correlates spatiotemporally with localized calcium fluxes that are required for the emergence of branches (Tang and Kalil, 2005). Focal elevation of calcium levels along the axons of buccal ganglion neurons from the snail Helisoma trivolvis induced the formation of filopodia (Williams et al, 1995), albeit in a developmental stage dependent manner. Similarly, using cultures of grasshopper central nervous system neurons, Lau et al (1999) found that localized released of caged calcium induced the formation of filopodia along axons from previously established accumulations of actin filaments, likely analogous to actin patches. The role of calcium in the formation of axonal filopodia and subsequently branches appears to be dependent on the neuron type (e.g., peripheral vs central nervous system neurons) or signal inducing the response (e.g., NGF vs Netrin-1), and also the developmental stage of the neuron. Clearly, additional investigation into the role of calcium in the formation of axonal filopodia and branches is warranted and will likely uncover multiple possible scenarios and underlying mechanisms.
Intra-axonal protein synthesis
Localized translation of mRNAs in axons contributes to neuronal morphogenesis (Holt and Schuman, 2013). The mRNA binding protein FMRP, that is involved in axonal translation, negatively regulates axon branching (Pan et al., 2004; Tucker et al., 2006) through a mechanism remains to be fully elucidated. Multiple mRNAs coding for axonal cytoskeletal regulators (β-Actin, Cofilin, Gap43, Arp2, Cortactin, WAVE1, Fascin, Tubulin) are targeted into axons, through their 3′UTR sequences (Yoo et al., 2010; Gumy et al., 2011; Spillane et al., 2012, 2013). The PI3K pathway promotes translation through the mammalian target of rapamycin (mTOR) pathway (Martelli et al., 2011; Nandagopal and Roux, 2015). Spillane et al (2012) provided evidence that in cultured chicken sensory axons NGF signaling through PI3K-mTOR signaling promotes collateral branch formation dependent on the intra-axonal protein synthesis of Arp2 subunit of the Arp2/3 complex, WAVE1, and Cortactin. This study supports the important role of intra-axonal protein synthesis to locally increase cytoskeletal regulators that are involved in axonal branching (Spillane et al., 2012). The observation that microRNA-9 locally suppresses the axonal translation of MAP1B in cortical neurons, and that this suppression promotes axon branching (see prior section on MAPs; Dajas-Bailador et al., 2012), is also consistent with the notion that neurotrophins regulate branching through the regulation of intra-axonal protein synthesis dependent mechanism. Collectively, these studies indicate that increases in the translation of branch promoting proteins and the suppression of the translation of branch inhibitory proteins both contribute to the mechanism. Consistent with this notion, chondroitin sulfate proteoglycans (CSPGs) have been reported to promote the intra-axonal translation of RhoA (Walker et al., 2012), which is generally inhibitory to branching (see prior section on GTPases), while also suppressing the translation of Cortactin which promotes branching (Sainath et al., 2016). In general, these observations are consistent with the originally proposed idea that the role of intra-axonal protein synthesis is to locally regulate the axonal proteome to generate specific responses to extracellular signals (Alvarez et al., 2000).
Involvement of mitochondria in determining axon branching
The positioning of mitochondria along axons has emerged as a required component of the mechanism that determines the sites of axon branching (Courchet et al., 2013; Spillane et al., 2013; Tao et al., 2014). While mitochondria and their respiration are required for the formation of axonal filopodia and branches, the role of the mitochondrion seems to be “permissive” in determining where branches can form. Indeed, although branches form from axon segments containing mitochondria, not all axonal segments containing mitochondria give rise to a branch. The mitochondria dependent component of axon branching is in part mediated by AMPK, LKB1 and NUAK signaling (Courchet et al., 2013; Tao et al, 2014) that serve to regulate mitochondria stalling, positioning and transport within axons. In the context of NGF induced axon branching, mitochondria positioning and respiration also serve to define sites along the axon of preferential intra-axonal protein synthesis that is in turn required for the ensuing branching (Spillane et al., 2013). Mitochondria positioning and respiration also define axon segments that preferentially give rise to actin patches and filopodia, and the presence of a respiring mitochondrion at the base of a filopodium is also required for the maturation of a filopodium into a branch (Spillane et al., 2013). A recent study reports that CSPGs, which inhibit axon branching (reviewed in Kadomatsu and Sakamoto, 2014), suppress mitochondrial respiration and in turn mitochondria associated actin filament dynamics and the intra-axonal protein synthesis of Cortactin (Sainath et al., 2016), providing evidence for the bidirectional regulation of mitochondria by branch promoting (e.g., NGF) and inhibitory extracellular signals. The study by Sainath et al (2016) also determined that actin patches associated with mitochondria exhibit more pronounced dynamics than those not associated with mitochondria, in a manner dependent on mitochondrial respiration, further emphasizing the role of axonal mitochondria as determinants of local actin dynamics along axons. For additional treatment of the role of organelles in branch formation the reader is directed to a recent review focusing on this topic (Winkle et al., 2016).
Concluding Statement
The research to date has provided important insights on multiple cellular processes operative during axonal branching. Future investigation into additional aspects of established mechanisms and the elucidation of novel mechanistic perspectives will continue to add to our understanding of the process of axon branching. A few main themes have emerged that are shared by all forms of axon branching. First, localized signaling events along the axon shaft determine the ability of that segment of the axon to gives rise to a branch (e.g., localized PI3K and/or calcium signaling). Second, the first step in the formation of an axon branch involves actin filaments generating filopodia and/or lamellipodia that then have the potential to mature into branches. Third, the maturation of a branch from a filopodium or lamellipodium requires the targeting and maintenance of microtubules into the protrusion. Fourth, stalled mitochondria are a component of the mechanism that determines the segments of the axon that have the potential to generate a branch.
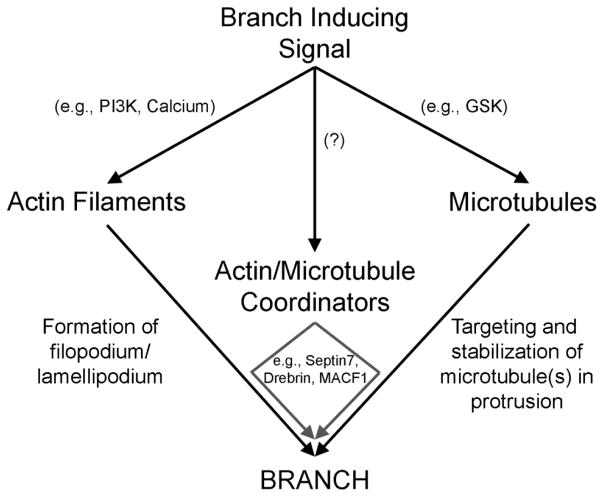
A fundamental theme that remains to be clarified revolves around understanding why a particular segment of the axon gives rise to a branch but not the adjoining segments. The discovery of mitochondria positioning as a determinant of sites along the axon with the potential to generate a branch has added much to this issue. However, while the presence of a mitochondrion is permissive for the formation of a branch it is not strictly speaking instructive as most other sites populated by mitochondria do not give rise to branches. Localized signaling events (e.g., by PI3K) along the axon shaft drive the dynamics of actin patches and filopodia. (Ketschek and Gallo, 2010; Spillane, et al., 2012, 2011) a role also observed in dendritic filopodia (Luikart et al., 2008). The sites of actin patch formation along the axon are also delineated by the presence of respiring mitochondria (Ketschek and Gallo, 2010). However, the mitochondria dependent regulation of actin filaments is only one component of axon branching. In contrast, the targeting of microtubules into filopodia is independent of mitochondria respiration (Spillane et al., 2013). Similarly, although the actin cytoskeleton requires intra-axonal protein synthesis during NGF-induced axon branching, the enhanced polymerization of axonal microtubules and their targeting to filopodia is independent of protein synthesis (Spillane et al., 2012). Collectively, these considerations indicate that the actin filament and microtubule cytoskeleton are regulated independently (Figure 4), at least in some forms of axon branching. However, whether the mechanisms that regulate the crosstalk and interactions between actin filaments and microtubules (e.g., Drebrin and MACF1) are regulated remains to be addressed (Figure 4). These mechanisms may operate at baseline levels without additional regulation, while the two cytoskeletal elements are independently regulated. This issue awaits further investigation.
Figure 4.
General summary model of the regulation of the axonal cytoskeleton during branching. Branch inducing signals activate multiple pathways that independently regulate the actin filament and microtubule cytoskeleton. The ensuing cytoskeletal dynamics promote branching by increasing actin filament dependent protrusive activity and microtubule tip polymerization or transport into protrusions. Molecules that coordinate the actin filament and microtubule cytoskeleton, in turn serve to promote interactions between the cytoskeletal elements. It remains to be determined if and by which signaling pathways cytoskeletal coordinators may be under regulation by branch inducing signals. The formation of a branch requires the formation of protrusive structures along the axon through the regulation of actin filaments, and the targeting and retention of microtubules in these protrusions. The means through which these ends are accomplished, at the molecular level, are likely diverse relying on different signaling pathways and effectors, as discussed in the concluding statement.
A fruitful future direction for the cohort of investigators addressing axon branching, will be to determine how the axonal cytoplasm becomes reorganized at sites of branching. To date, multiple signaling pathways have been involved in branching, but often times the exact roles of these signaling pathways in the regulation of the various components of the cellular mechanism of branching remain to be determined. It seems likely that individual pathways will have orchestrational roles. For example, the PI3K pathway directly impacts the dynamics of the actin filament cytoskeleton through downstream actin binding proteins, while also having the potential to regulate mitochondrial respiration that in turn regulates local actin filament dynamics and intra-axonal translation (Sainath et al., 2016). Similarly, GSK3 signaling can impact a variety of cellular processes related to microtubules. Understanding how these multiple pathways converge and diverge in the regulation of specific aspects of the mechanism of branching remains a frontier. Investigations addressing if and how cytoskeletal motor systems serve to locally target the required organelles, signaling and effector molecules to sites of branching are lacking but may yield interesting novel observations regarding how the neuron organizes its axonal cytoplasm.
Finally, it will also be of interest to further consider the possibility that there may be a multiplicity of mechanisms capable of driving the formation of axon branches. While the basic sequence of cytoskeletal events underlying the formation of a branch does not vary (e.g., formation of protrusive structures, subsequent population by microtubules and maturation into a branch; Figure 4), the evidence reviewed herein indicates that different upstream signals can regulate the process across different cells or contexts (e.g., calcium signaling, GTPase signaling). Also, as previously noted, microtubules can be targeted into nascent branches through either plus tip mediated polymerization or active transport. Similarly, there are models and examples of filopodia formation independent of the network convergence mechanism and Arp2/3 (reviewed in Gallo, 2013). Therefore, it would not be unexpected if in some contexts the formation of filopodia leading to branches may be operating independent of the Arp2/3 complex. Ultimately, the unifying elements of the process of branching are the cytoskeletal rearrangements themselves, and these may be accomplished through more than one mechanism. Furthermore, studies of the cellular mechanism of axon branching are almost invariably performed on embryonic or early postnatal neurons due to technical reasons revolving around the inability to culture adult neurons, the dorsal root ganglion being an exception. Thus, the extrapolation of the current knowledge base to the branching/sprouting observed in the adult nervous system in injury and disease states may not be warranted, and the field would benefit from continued analysis of the mechanism of branching in adult neuron model systems.
HIGHLIGHTS.
Branching requires coordination of the cytoskeleton
Coordination of the cytoskeleton is mediated by actin-microtubule binding proteins
Mitochondria have a pivotal role in determining sites of branching
Mitochondria function regulates the axonal actin filament cytoskeleton
Intra-axonal protein synthesis is required for NGF-induced branching
Acknowledgments
Funding: This work was supported by NIH awards to GG (NS095471, NS078030) and a fellowship from the Chilean National Scholarship Program for Graduate Studies (Conicyt).
Footnotes
Declaration of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ageta-Ishihara N, Miyata T, Ohshima C, Watanabe M, Sato Y, Hamamura Y, Higashiyama T, Mazitschek R, Bito H, Kinoshita M. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat Commun. 2013;4:2532. doi: 10.1038/ncomms3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja R, Pinyol R, Reichenbach N, Custer L, Klingensmith J, Kessels MM, Qualmann B. Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell. 2007;131:337–350. doi: 10.1016/j.cell.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbik F, Cafferty WB, Strittmatter SM. Myelin associated inhibitors: a link between injury-induced and experience-dependent plasticity. Exp Neurol. 2012;235:43–52. doi: 10.1016/j.expneurol.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. doi: 10.1016/s0301-0082(99)00062-3. [DOI] [PubMed] [Google Scholar]
- Andersen EF, Asuri NS, Halloran MC. In vivo imaging of cell behaviors and F-actin reveals LIM-HD transcription factor regulation of peripheral versus central sensory axon development. Neural Dev. 2011;6:27. doi: 10.1186/1749-8104-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo JC, Wu SH. Neurotrophin signaling: many exciting surprises! Cell. Mol Life Sci. 2006;63:1523–1537. doi: 10.1007/s00018-006-6010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Averaimo S, Nicol X. Intermingled cAMP, cGMP and calcium spatiotemporal dynamics in developing neurons. Front Cell Neurosci. 2014;8:376. doi: 10.3389/fncel.2014.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnat M, Benassy MN, Vincensini L, Soares S, Fassier C, Propst F, Andrieux A, von Boxberg Y, Nothias F. The GSK3-MAP1B pathway controls neurite branching and microtubule dynamics. Mol Cell Neurosci. 2016;72:9–21. doi: 10.1016/j.mcn.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F. Distinct roles of c- Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J Neurosci. 2010;30:7804–7816. doi: 10.1523/JNEUROSCI.0372-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastmeyer M, O’Leary DD. Dynamics of target recognition by interstitial axon branching along developing cortical axons. J Neurosci. 1996;16:1450–1459. doi: 10.1523/JNEUROSCI.16-04-01450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122:1947–1953. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria PM, Bonni A. Molecular control of axon branching. Neuroscientist. 2013;19:16–24. doi: 10.1177/1073858411426201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria PM, Torre-Ubieta L, Ikeuchi Y, Becker EBE, Reiner O, Azad Bonni A. A JIP3-regulated GSK3beta/DCX signaling pathway restricts axon branching. J Neurosci. 2010;30:16766–16776. doi: 10.1523/JNEUROSCI.1362-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billuart P, Winter CG, Maresh A, Zhao X, Luo L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. doi: 10.1016/s0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- Bouquet C, Ravaille-Veron M, Propst F, Nothias F. MAP1B coordinates microtubule and actin filament remodeling in adult mouse Schwann cell tips and DRG neuron growth cones. Mol Cell Neurosci. 2007;36:235–247. doi: 10.1016/j.mcn.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Bouquet C, Soares S, von Boxberg Y, Ravaille-Veron M, Propst F, Nothias F. Microtubule-associated protein 1B controls directionality of growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons. J Neurosci. 2004;24:7204–7213. doi: 10.1523/JNEUROSCI.2254-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard GJ, Rice LM. The contribution of alphabeta-tubulin curvature to microtubule dynamics. J Cell Biol. 2014;207:323–334. doi: 10.1083/jcb.201407095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Martin JH. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front Integr Neurosci. 2014;18:8–51. doi: 10.3389/fnint.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TJ, Gehler S, Shaw AE, Bamburg JR, Letourneau PC. Cdc42 participates in the regulation of ADF/cofilin and retinal growth cone filopodia by brain derived neurotrophic factor. J Neurobiol. 2006;66:103–114. doi: 10.1002/neu.20204. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tian X, Kim WY, Snider WD. Adenomatous polyposis coli regulates axon arborization and cytoskeleton organization via its N-terminus. PLoS One. 2011;6:e24335. doi: 10.1371/journal.pone.0024335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetta J, Love JM, Bober BG, Shah SB. Bidirectional actin transport is influenced by microtubule and actin stability. Cell Mol Life Sci. 2015;72:205–220. doi: 10.1007/s00018-015-1933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PH, Chen B, Li P, Rosen MK, Shen K. Local F-actin network links synapse formation and axon branching. Cell. 2014;156:208–220. doi: 10.1016/j.cell.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SJ, Lee H, Dutta S, Song J, Walikonis R, Moon IS. Septin 6 regulates the cytoarchitecture of neurons through localization at dendritic branch points and bases of protrusions. Mol Cells. 2011;32:89–98. doi: 10.1007/s10059-011-1048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Cosker KE, Eickholt BJ. Phosphoinositide 3-kinase signalling events controlling axonal morphogenesis. Biochem Soc Trans. 2007;35:207–210. doi: 10.1042/BST0350207. [DOI] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Jr, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N. microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nature Neurosci. 2012;15:697–699. doi: 10.1038/nn.3082. [DOI] [PubMed] [Google Scholar]
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, Alberts AS, Mori S, Gertler FB. Filopodia are required for cortical neurite initiation. Nat Cell Biol. 2007;9:1347–1359. doi: 10.1038/ncb1654. [DOI] [PubMed] [Google Scholar]
- Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. 2010;65:341–357. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun XP, Bandeira de Lima T, Allen J, Geraldo S, Gordon-Weeks P, Chilton JK. Drebrin controls neuronal migration through the formation and alignment of the leading process. Mol Cell Neurosci. 2012;49:341–350. doi: 10.1016/j.mcn.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun XP, Chilton JK. Control of cell shape and plasticity during development and disease by the actin binding protein Drebrin. Histol Histopathol. 2010;25:533–540. doi: 10.14670/HH-25.533. [DOI] [PubMed] [Google Scholar]
- Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development. 2007;134:2137–2146. doi: 10.1242/dev.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML, Bilancio A, Need AC, Smith AJ, Hall SM, Hamers FP, Giese KP, Bradbury EJ, Vanhaesebroeck B. Control of axonal growth and regeneration of sensory neurons by the p110delta PI 3-kinase. PLoS One. 2007;2:e869. doi: 10.1371/journal.pone.0000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass J, Gehler S, Sarmiere P, Letourneau P, Bamburg JR. Regulating filopodial dynamics through actin-depolymerizing factor/cofilin. Anat Sci Int. 2004;79:173–183. doi: 10.1111/j.1447-073x.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Flynn KC, Hellal F, Neukirchen D, Jacob S, Tahirovic S, Dupraz S, Stern S, Garvalov BK, Gurniak C, Shaw AE, Meyn L, Wedlich-Söldner R, Bamburg JR, Small JV, Witke W, Bradke F. ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron. 2012;76:1091–1107. doi: 10.1016/j.neuron.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Flynn KC, Pak CW, Shaw AE, Bradke F, Bamburg JR. Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev Neurobiol. 2009;69:761–779. doi: 10.1002/dneu.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Karakesisoglou I. Bridging cytoskeletal intersections. Genes Dev. 2001;15:1–14. doi: 10.1101/gad.861501. [DOI] [PubMed] [Google Scholar]
- Gallo G. Mechanisms underlying the initiation and dynamics of neuronal filopodia: from neurite formation to synaptogenesis. Int Rev Cell Mol Biol. 2013;301:95–156. doi: 10.1016/B978-0-12-407704-1.00003-8. [DOI] [PubMed] [Google Scholar]
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol. 2011;71:201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A induced axon retraction. J Cell Sci. 2006;119:3413–3423. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J Neurosci. 1999;19:3860–3873. doi: 10.1523/JNEUROSCI.19-10-03860.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy CG, Lorenzana AO, Kwan JP, Lin K, Ghassemi O, Ma A, Xu N, Creger D, Liu K, He Z, Zheng B. Effects of PTEN and Nogo Codeletion on Corticospinal Axon Sprouting and Regeneration in Mice. J Neurosci. 2015;35:6413–6428. doi: 10.1523/JNEUROSCI.4013-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol. 2008;10:1181–1189. doi: 10.1038/ncb1778. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development. 2011;138:183–195. doi: 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves-Pimentel C, Gombos R, Mihály J, Sánchez-Soriano N, Prokop A. Dissecting regulatory networks of filopodia formation in a Drosophila growth cone model. PLoS One. 2011;6:e18340. doi: 10.1371/journal.pone.0018340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy LF, Yeo GS, Tung YC, Zivraj KH, Willis D, Coppola G, Lam BY, Twiss JL, Holt CE. Fawcett JW Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 2011;17:85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. http://dx.doi.org/10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci. 1994;14:2161–2177. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand RA, Khalid S, Tam E, Kolodkin AL. Axon Dynamics during Neocortical Laminar Innervation. Cell Rep. 2015;12:172–182. doi: 10.1016/j.celrep.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Schuman EM. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron. 2013;80:648–657. doi: 10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, Noda Y. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003;114:229–239. doi: 10.1016/s0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- Hou W, Izad M, Nemitz S, Haag N, Kessels MM, Qualmann B. The Actin Nucleator Cobl Is Controlled by Calcium and Calmodulin. PLoS Biology. 2015;13:e1002233. doi: 10.1371/journal.pbio.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, Spiliotis ET. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol. 2012;22:1109–1115. doi: 10.1016/j.cub.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: Roles in Neuronal Development and Function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DM, Tucker BA, Rahimtula M, Mearow KM. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J Neurochem. 2003;86:116–1128. doi: 10.1046/j.1471-4159.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- Ka M, Kim WY. Microtubule-Actin Crosslinking Factor 1 Is Required for Dendritic Arborization and Axon Outgrowth in the Developing. Brain Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadomatsu K, Sakamoto K. Sulfated glycans in network rewiring and plasticity after neuronal injuries. Neurosci Res. 2014;78:50–54. doi: 10.1016/j.neures.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Kalil K, Dent EW. Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat Rev Neurosci. 2014;15:7–18. doi: 10.1038/nrn3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, Szebenyi G, Dent EW. Common mechanisms underlying growth cone guidance and axon branching. J Neurobiol. 2000;44:145–158. [PubMed] [Google Scholar]
- Ketschek A, Spillane M, Dun XP, Hardy H, Chilton J, Gallo G. Drebrin coordinates the actin and microtubule cytoskeleton during the initiation of axon collateral branches. Dev Neurobiol. 2016;76:1092–1110. doi: 10.1002/dneu.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek A, Jones S, Spillane M, Korobova F, Svitkina T, Gallo G. Nerve growth factor promotes reorganization of the axonal microtubule array at sites of axon collateral branching. Dev Neurobiol. 2015;75:1441–1461. doi: 10.1002/dneu.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 2010;30:12185–12197. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ryu SH, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature letters. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, Snider WD. Essential roles of GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron. 2006b;52:981–996. doi: 10.1016/j.neuron.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Bae J, Cho IH, Choi KY, Park YJ, Ryu JH, Chun JS, Song WK. Control of growth cone motility and neurite outgrowth by SPIN90. Exp Cell Res. 2011;2317:2276–2287. doi: 10.1016/j.yexcr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Kim YT, Hur EM, Snider WD, Zhou FQ. Role of GSK3 signaling in neuronal morphogenesis. Front Mol Neurosci. 2011b;4:48. doi: 10.3389/fnmol.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell. 2008;19:1561–1574. doi: 10.1091/mbc.E07-09-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Giger RJ. Probing microtubule +TIPs: regulation of axon branching. Curr Opin Neurobiol. 2005;15:58–66. doi: 10.1016/j.conb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kriplani N, Hermida MA, Brown ER, Leslie NR. Class I PI 3-kinases: Function and evolution. Adv Biol Regul. 2015;59:53–64. doi: 10.1016/j.jbior.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Kühn S, Geyer M. Formins as effector proteins of Rho GTPases. Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PM, Zucker RS, Bentley D. Induction of filopodia by direct local elevation of intracellular calcium ion concentration. J Cell Biol. 1999;145:1265–1275. doi: 10.1083/jcb.145.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, Gertler FB. Critical role of Ena/ VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Jr, Courchet J, Polleux F. Cell biology in neuroscience: Cellular and molecular mechanisms underlying axon formation, growth, and branching. J Cell Biol. 2013;202:837–848. doi: 10.1083/jcb.201305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mao M, Wang H, Zen K, Zhang C, Li L. MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons. Protein Cell. 2014;5:160–169. doi: 10.1007/s13238-014-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CM, Chen HJ, Leung CL, Parry DA, Liem RK. Microtubule actin crosslinking factor 1b: a novel plakin that localizes to the Golgi complex. J Cell Sci. 2005;118:3727–3738. doi: 10.1242/jcs.02510. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ma C, Shen J, Wang D, Hao J, Hu Z. SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus pulposus cells via the NF-κB pathway. Mol Med Rep. 2016;14:783–789. doi: 10.3892/mmr.2016.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Jr, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66:847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci. 2008;28:7006–7012. doi: 10.1523/JNEUROSCI.0195-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko DE, Putt M, Golden JA. SDF1 Reduces Interneuron Leading Process Branching through Dual Regulation of Actin and Microtubules. J Neurosci. 2014;34:4941–4962. doi: 10.1523/JNEUROSCI.4351-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko DE, Putt M, Golden JA. SDF1 regulates leading process branching and speed of migrating interneurons. J Neurosci. 2011;31:1739–1745. doi: 10.1523/JNEUROSCI.3118-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: Molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Chappell W, Abrams SL, Bäsecke J, Stivala F, Donia M, Fagone P, Nicoletti F, Libra M, Ruvolo V, Ruvolo P, Kempf CR, Steelman LS, McCubrey JA. Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia. 2011;25:064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- Mingorance-Le Meur A, O’Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2009;28:248–260. doi: 10.1038/emboj.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithal DS, Banisadr G, Miller RJ. CXCL12 Signaling in the Development of the Nervous System. J Neuroimmune Pharmacol. 2012;7:820–834. doi: 10.1007/s11481-011-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon MS, Gomez TM. Balanced Vav2 GEF activity regulates neurite outgrowth and branching in vitro and in vivo. Mol Cell Neurosci. 2010;44:118–128. doi: 10.1016/j.mcn.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- Nakahira M, Macedo JN, Seraphim TV, Cavalcante N, Souza TA, Damalio JC, Reyes LF, Assmann EM, Alborghetti MR, Garratt RC, Araujo AP, Zanchin NI, Barbosa JA, Kobarg J. A draft of the human septin interactome. PLoS One. 2010;5:e13799. doi: 10.1371/journal.pone.0013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandagopal N, Roux PP. Regulation of global and specific mRNA translation by the mTOR signaling pathway. Translation (Austin) 2015;3:e983402. doi: 10.4161/21690731.2014.983402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Niwa S, Homma N, Fukuda H, Imajo-Ohmi D, Hirokawa N. Phosphatidylinositol 4-phosphate 5-kinase alpha (PIPKα) regulates neuronal microtubule depolymerase kinesin, KIF2A and suppresses elongation of axon branches. Proc Natl Acad Sci USA. 2012;109:1725–1730. doi: 10.1073/pnas.1107808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnami S, Endo M, Hirai S, Uesaka N, Hatanaka Y, Yamashita T, Yamamoto N. Role of RhoA in activity-dependent cortical axon branching. J Neurosci. 2008;28:9117–9121. doi: 10.1523/JNEUROSCI.1731-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Smith GM, Fouad K. Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics. 2011;8:283–93. doi: 10.1007/s13311-011-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco A, Gallo G. Actin filament-microtubule interactions in axon initiation and branching. Brain Res Bull. 2016 doi: 10.1016/j.brainresbull.2016.07.013. http://dx.doi.org/10.1016/j.brainresbull.2016.07.013. [DOI] [PMC free article] [PubMed]
- Pan J, Kao YL, Joshi S, Jeetendran S, Dipette D, Singh US. Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 2005;93:571–583. doi: 10.1111/j.1471-4159.2005.03106.x. [DOI] [PubMed] [Google Scholar]
- Pan L, Zhang YQ, Woodruff E, Broadie K. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol. 2004;14:1863–1870. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- Petrovic M, Schmucker D. Axonal wiring in neural development: Target-independent mechanisms help to establish precision and complexity. Bioessays. 2015;37:996–1004. doi: 10.1002/bies.201400222. [DOI] [PubMed] [Google Scholar]
- Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J Neurosci. 2008;28:8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Zhong X, Flynn DC, Zheng JZ, Qiao M, Wu C, Dedhar S, Shi X, Jiang BH. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene. 2005;24:3154–3165. doi: 10.1038/sj.onc.1208525. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS. Collateral branching of long-distance cortical projections in monkey. J Comp Neurol. 2013;521:4112–4123. doi: 10.1002/cne.23414. [DOI] [PubMed] [Google Scholar]
- Rodriguez OC, Schaefe AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Banker G. Role of moving growth cone like “wave” structures in the outgrowth of cultured hippocampal axons and dendrites. J Neurobiol. 1999;39:97–106. doi: 10.1002/(sici)1097-4695(199904)39:1<97::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sainath R, Ketschek A, Grandi L, Gallo G. CSPGs inhibit axon branching by impairing mitochondria-dependent regulation of actin dynamics and axonal translation. Dev Neurobiol. 2016 doi: 10.1002/dneu.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainath R, Gallo G. The dynein inhibitor ciliobrevin D inhibits the bi-directional transport of organelles along sensory axons and impairs NGF-mediated regulation of growth cones and axon branches. Dev Neurobiol. 2015;75:757–777. doi: 10.1002/dneu.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainath R, Granato M. Plexin A3 and turnout regulate motor axonal branch morphogenesis in zebrafish. PLoS One. 2013;8:e54071. doi: 10.1371/journal.pone.0054071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno T, Ikeno T, Mennya K, Kurishita M, Sakae N, Sato M, Takada H, Konishi Y. Kinesin-1 sorting in axons controls the differential retraction of arbor terminals. J Cell Sci. 2016;129:3499–3510. doi: 10.1242/jcs.183806. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;6:587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Sheffield PJ, Oliver CJ, Kremer BE, Sheng S, Shao Z, Macara IG. Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J Biol Chem. 2003;278:3483–3488. doi: 10.1074/jbc.M209701200. [DOI] [PubMed] [Google Scholar]
- Slep KC, Rogers SL, Elliott SL, Ohkura H, Kolodziej PA, Vale RD. Structural determinants for EB1-mediated recruitment ofAPC and spectraplakins to the microtubule plus end. J Cell Biol. 2005;168:587–598. doi: 10.1083/jcb.200410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Gallo G. Involvement of Rho-family GTPases in axon branching. Small GTPases. 2014;5:e27974. doi: 10.4161/sgtp.27974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci. 2012;32:17671–17689. doi: 10.1523/JNEUROSCI.1079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol. 2011;71:747–758. doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004;43:81–94. doi: 10.1016/j.neuron.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Stroissnigg H, Trancikova A, Descovich L, Fuhrmann J, Kutschera W, Kostan J, Meixner A, Nothias F, Propst F. S-nitrosylation ofmicrotubule-associated protein 1B mediates nitric-oxide-induced axon retraction. Nat Cell Biol. 2007;9:1035–1045. doi: 10.1038/ncb1625. [DOI] [PubMed] [Google Scholar]
- Suo D, Park J, Young S, Makita T, Deppmann CD. Coronin-1 and calcium signaling governs sympathetic final target innervation. J Neurosci. 2015;35:3893–3902. doi: 10.1523/JNEUROSCI.4402-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Kalil K. Netrin-1 induces axon branching in developing cortical neurons by frequency-dependent calcium signaling pathways. J Neurosci. 2005;25:6702–6715. doi: 10.1523/JNEUROSCI.0871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K, Matsuki N, Koyama R. AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Dev Neurobiol. 2014;74:557–573. doi: 10.1002/dneu.22149. [DOI] [PubMed] [Google Scholar]
- Tint I, Jean D, Baas PW, Black MM. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. J Neurosci. 2009;29:10995–11010. doi: 10.1523/JNEUROSCI.3399-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A, Marmiroli S. Signaling specificity in the Akt pathway in biology and disease. Adv Biol Regul. 2014;55:28–38. doi: 10.1016/j.jbior.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B, Richards RI, Lardelli M. Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum Mol Genet. 2006;15:3446–3458. doi: 10.1093/hmg/ddl422. [DOI] [PubMed] [Google Scholar]
- Tymanskyj SR, Scales TM, Gordon-Weeks PR. MAP1B enhances microtubule assembly rates and axon extension rates in developing neurons. Mol Cell Neurosci. 2012;49:110–119. doi: 10.1016/j.mcn.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Kojima SC, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174:863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR, Luan Q, Liu SL, Nolen BJ. Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments Curr. Biol. 2013;23:1990–1998. doi: 10.1016/j.cub.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BA, Ji SJ, Jaffrey SR. Intra-axonal translation of RhoA promotes axon growth inhibition by CSPG. J Neurosci. 2012;32:14442–14447. doi: 10.1523/JNEUROSCI.0176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich CS, Erzberger JP, Barral Y. The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol. 2008;9:478–489. doi: 10.1038/nrm2407. [DOI] [PubMed] [Google Scholar]
- Williams CV, Davenport RW, Dou P, Kater SB. Developmental regulation of plasticity along neurite shafts. J Neurobiol. 1995;27:127–140. doi: 10.1002/neu.480270202. [DOI] [PubMed] [Google Scholar]
- Winkle CC, Taylor KL, Dent EW, Gallo G, Greif KF, Gupton SL. Beyond the cytoskeleton: The emerging role of organelles and membrane remodeling in the regulation of axon collateral branches. Dev Neurobiol. 2016 doi: 10.1002/dneu.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth DC, Daly CN, Geraldo S, Oozeer F, Gordon-Weeks PR. Drebrin contains a cryptic F-actin-bundling activity regulated by Cdk5 phosphorylation. J Cell Biol. 2013;202:793–806. doi: 10.1083/jcb.201303005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol. 2007;17:1746–1751. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Yang C, Svitkina T. Filopodia initiation: focus on the Arp2/3 complex and formins. Cell Adh Migr. 2011;5:402–408. doi: 10.4161/cam.5.5.16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Kim WY, Chen Y, Wang X, Stanco A, Komuro Y, Snider W, Anton ES. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61:42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S, van Niekerk EA, Merianda TT, Twiss JL. Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp Neurol. 2010;223:19–27. doi: 10.1016/j.expneurol.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol Biol Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ahmad FJ, Baas PW. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J Neurosci. 1994;14:5872–5884. doi: 10.1523/JNEUROSCI.14-10-05872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Rasband MN. Cytoskeletal control of axon domain assembly and function. Curr Opin Neurobiol. 2016;39:116–121. doi: 10.1016/j.conb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Tanaka KF, Matsunaga S, Iseki M, Watanabe M, Matsuki N, Ikegaya Y, Koyama R. Photoactivated adenylyl cyclase (PAC) reveals novel mechanisms underlying cAMP-dependent axonal morphogenesis. Sci Rep. 2016;5:19679. doi: 10.1038/srep19679. [DOI] [PMC free article] [PubMed] [Google Scholar]