Abstract
Objective
The purpose of this study was to investigate anticipatory postural adjustments (APA) during the transitional movement task of gait initiation (GI) in individuals acutely following a concussion.
Design
Cohort Study.
Setting
University Research Center.
Participants
A population based sample of 84 participants divided into two equal groups of acutely post-concussion (CONC) and healthy student athletes.
Intervention
Participants were tested on two occasions – a pre-injury baseline test and then the CONC group was retested acutely post-concussion and the control group again at a similar time. All participants completed 5 trials of GI on 4 forceplates.
Main Outcome Measures
The dependent variables were the displacement and velocity of the center of pressure (COP) during the APA phase and initial step kinematics. Comparisons were made with a 2 (Group) × 2 (Time) repeated measures ANOVA.
Results
There was a significant interaction for COP posterior displacement (P<0.001) and lateral displacement (P<0.001). Posteriorly, post-hoc testing identified a significant reduction in CONC (PRE: 5.7 ± 1.6 cm and POST: 2.6 ± 2.1 cm, P<0.001), but no difference in Control (PRE: 4.0 ± 1.6 cm and POST: 4.0 ± 2.5 cm, P=0.921). Laterally, post-hoc testing identified a significant reduction in CONC (PRE: 5.8 ± 2.1 cm and POST: 3.8 ± 1.8 cm, P<0.001), but no difference in Control (PRE: 5.0 ± 2.5 cm and POST: 5.2 ± 2.4 cm, P=0.485).
Conclusions
The results of this study suggest difficulty in the planning and execution of GI acutely post-concussion and posterior APA displacement and velocity are highly effective measures of impaired postural control. Finally, the APA phase is linked to the supplementary motor area which suggests a supraspinal contribution to post-concussion impaired postural control.
Keywords: mild traumatic brain injury, balance, locomotion, postural control
There are an estimated 1.6 – 3.8 million concussions occurring annually in the U. S., however this likely underestimates the true incidence rate as many concussions are unreported or unrecognized.1, 2 Postural control assessment, a common impairment following concussion, is a recommended component of the Concussion in Sport (CIS) consensus statement and is typically assessed with the Balance Error Scoring System (BESS).3–6 However, the BESS has very low sensitivity (0.34) even acutely post-concussion and typically returns to baseline levels within 3 – 5 days despite ongoing cognitive deficits and symptoms.7–9 This is likely due to the numerous limitations of the BESS including improved performance with repeat administration, the influence of test environment and fatigue, as well as poor reliability and high minimum detectable change scores.10–14 Additionally, the BESS is a static test which involves the individual attempting to maintain a quiet upright stance. Conversely, level, over-ground gait is a dynamic task largely controlled by central pattern generators in the spinal cord with overriding cortical control via descending pathways from the motor cortex.15, 16
During gait studies, post-concussion patients, when compared cross-sectionally to healthy controls, display a conservative gait strategy (e.g., reduced gait velocity, restricted Center of Pressure (COP) – Center of Mass (COM) separation, increased COM frontal plane displacement) that is likely designed to maintain stability in individuals following a concussion.17–19 Further, the more challenging and complex the task, such as cognitive-motor challenges or obstacle avoidance, the greater and more prolonged the postural control deficits.17–23 These tasks are regulated by diverse supraspinal structures (e.g., supplementary motor area, dorsal and ventral premotor areas, superior parietal lobe, posterior cingulate cortex) which provides some insight into the neurophysiological post-concussion deficits and these tests batteries have identified deficits in postural control have been noted beyond clinical recovery and up to 1 – 2 months post-concussion.15, 19, 22–27 While these findings are noteworthy for identifying impairments in challenging dynamic tasks following a concussion, they do not address the ability to plan and self-initiate a step during a transitional movement, a task considered more challenging than gait.16, 28
Gait initiation (GI) requires both the generation of propulsive forces to transition from a quiet stance to a rhythmic dynamic state (locomotion) as well as effective postural control to maintain balance during the transition. The control of posture and locomotion, however, are interdependent at several levels of the central nervous system.16 The supraspinal neurological control of GI, based on animal, imaging, and stroke investigations, is suggested to be centralized in the supplementary or premotor area.15, 16, 27, 29 During GI, anticipatory postural adjustments (APAs), via relaxation of the Soleus and activation of the Tibialis Anterior, are responsible for moving the COP posteriorly and laterally towards the initial swing limb while the COM shifts towards the initial stance limb.30, 31 (Figure 1) This inherently destabilizing task is a sensitive indicator of balance dysfunction and has successfully identified impairments in postural control amongst diverse aging and neurological disorder populations.30–34 Specifically, reductions in the displacement and velocity of the COP during the APA phase of GI appears most sensitive to neurological impairments.30–32
Figure 1. Center of Pressure (COP) Displacement.
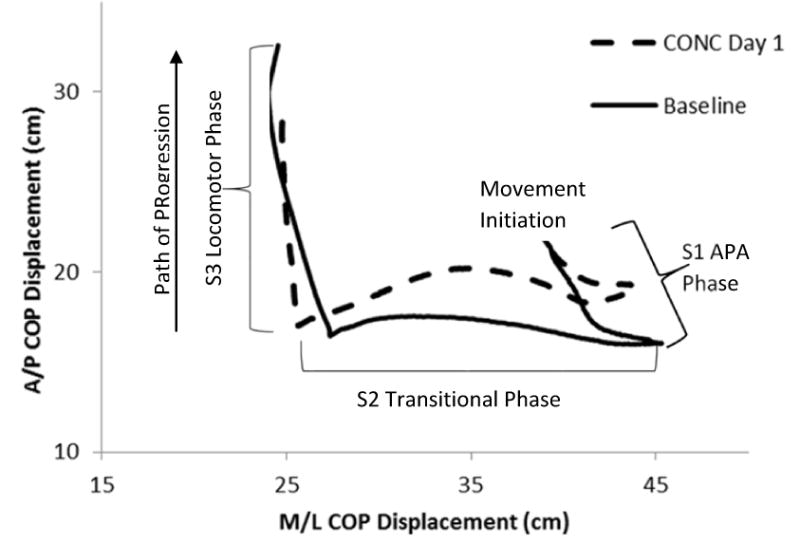
Following movement initiation, the COP translates posterior and lateral towards the initial swing limb (S1 or APA phase). As the heel of the initial swing limb leaves the ground, the COP translates laterally towards the initial stance limb (S2 or Transitional phase). Finally, as the initial stance limb leaves the ground to complete the initial step, the COP translates anteriorly (S3 or Locomotor phase).
Impaired postural control is a cardinal sign of concussion;25 however, there has been limited investigation of dynamic postural control and specifically the dynamically challenging ability to plan, initiate, and execute a step effectively. Therefore, the purpose of this study was to investigate impairments in dynamic postural control during the transitional motor task of GI in individuals acutely post-concussion. We hypothesize that concussed individuals will select a posturally conservative movement strategy by reducing both the displacement and velocity of their COP in an effort to restrict the threats to their postural stability.
METHODS
Participants
There were two participant groups in this study; 1) 42 National Collegiate Athletic Association (NCAA) student-athletes who suffered concussions (CONC), and 2) 42 NCAA student-athletes who served as control participants (Control). (Table 1) The inclusion criteria for both groups was having a baseline GI test performed prior to intercollegiate athletic participation. The CONC group’s injury was initially identified by a certified athletic trainer and the diagnosis was confirmed by a physician with experience in concussion management and consistent with CIS guidelines.35 Participants were excluded from the study if they self-reported a current lower extremity injury, vestibular or neurological pathologies (aside from the current concussion), medication usage which affected balance, or suffered a substantial lower extremity injury between the baseline and follow-up test. All control participants denied prior concussion history including diagnosed, unreported, or potential (e.g., memory loss following head impact) concussion. All participants provided written informed consent prior to participating as approved by the University’s Institutional Review Board.
Table 1. Participant Demographics.
There were no significant differences (P>0.05) for participant demographics expect for Concussion History, expected as per inclusion guidelines, which was significant (P<0.001). The CONC group were acutely classified with an 11.9% loss of consciousness rate and a 26.2% post-traumatic amnesia rate. The Symptom Score was a 22 item 0 – 6 graded symptom checklist with a higher score reflecting greater symptom burden. The Standard Assessment of Concussion is a brief cognitive screen with a score range of 0 – 30 with a higher score reflecting better cognitive performance. These were only collected on the CONC participants.
| CONC | Control | |
|---|---|---|
| Gender | 52.4% Female | 52.4% Female |
| Age (years) | 19.2 ± 2.1 | 20.1 ± 1.3 |
| Height (cm) | 173.5 ± 12.2 | 177.2 ± 11.0 |
| Weight (kg) | 79.0 ± 23.3 | 87.7 ± 24.4 |
| Concussion History | 0.9 ± 1.2 | 0.0 ± 0.0 |
| Symptom Severity (0 – 132) | 27.0 ± 24.0 | NA |
| Standard Assessment of Concussion | 26.2 ± 3.8 | NA |
| Sport Participation | Football: 15 Cheerleading: 6 Women’s Soccer: 6 Women’s Basketball: 6 Men’s Soccer: 4 Men’s Basketball: 2 Softball: 1 Track & Field: 1 Tennis: 1 |
Football: 13 Cheerleading: 9 Women’s Soccer: 7 Women’s Basketball: 5 Men’s Basketball: 3 Men’s Soccer: 3 Track & Field: 2 |
Instrumentation
All trials of GI were performed along a 10-m walkway. Kinetic data was collected using 4 forceplates (FP)a, mounted flush with the walkway surface, at 1000 Hz. The COP location for each FP was calculated using standard biomechanics equations;
where M and F represent moments and forces respectively and the subscripts represent direction. Zoff is the distance between the contact surface and the origin of the force plate.
Procedures
All participants performed baseline concussion tests the summer prior to their first academic and athletic year at the institution and this represented their pre-test (PRE). All CONC participants were tested within 24 hours of suffering their concussion while Control participants were retested as part of subsequent studies and there was no difference in duration between testing sessions by group (CONC: 369 ± 321 days (Range: 5 – 1,254 days) and Control: 435 ± 347 days (Range: 7 – 1,319 days) respectively, P=0.481).
All participants performed 5 trials of GI beginning with a self-selected comfortable stance on two FPs and their position was marked to maintain consistency across trials. In response to a verbal cue, participants stepped onto a third FP and continued walking unobstructed for approximately 10 m. Individuals were allowed to practice the task prior to data collection and no participant took more than 2 practice trials.
Data Analysis
During GI, movement initiation for each trial was determined by the first change (2 standard deviations from the mean of the first 0.5 s) in ground reaction force and confirmed by COP displacement.30, 31 The COP displacement was divided into 3 separate regions based on 2 landmarks consistent with prior GI studies.30, 31, 33 Briefly, the first region, the APA phase (S1), began with movement initiation and ended at Landmark 1, the most posterior and lateral position of the COP towards the initial swing limb. The second region, the transitional phase (S2), represents the transition of the COP from the initial swing limb to the initial stance limb and ends at Landmark 2, when the COP begins to move forward under the initial stance limb. The final region represents the locomotor phase (S3), and is defined by the anterior progression of the COP under the stance limb until toe-off.30, 31, 33 (Figure 1) During each phase, both the displacement and velocity of the COP were calculated for the anterior/posterior (AP) and medial/lateral (ML) directions.
The stepping parameters were also calculated for the initial step. Initial Step Length was calculated as the sagittal distance between the initial position of the COP and its position at the foot-off time of the trailing stance foot. The mean Initial Step Velocity was calculated the first step displacement divided by the time from movement initiation to the initial heel strike on the subsequent FP.
Statistical Analysis
Descriptive statistics were calculated for participant’s demographics along with the means of dependent variables (SPSS 24, IBM, Inc. Armonk, NY). Because this study aimed to identify changes in the APA phase of GI, only the S1 COP components were analyzed along with the initial step length and velocity. A 2 (group) × 2 (time) mixed design ANOVA was performed on each APA dependent variable and significant interactions were followed up with a pairwise comparison using Tukey’s procedure to examine the simple main effect of time for each group. Because there were significant group differences at PRE for both Step Length (p<0.001) and Step Velocity (p<0.001), an ANCOVA was performed with the PRE serving as the covariate. Cohen’s D effect sizes were calculated for CONC pre-post when post-hoc significance was identified. The alpha level was set at 0.05 for COP displacements. However, because the stepping characteristics of initial step length and velocity are not independent, a Bonferonni correction was applied and the alpha value corrected to 0.025.
RESULTS
COP Kinematics
There were significant group by time interactions for S1 A/P Displacement (F=70.680, P<0.001) and S1 M/L Displacement (F=24.850, P<0.001) whereby the CONC group reduced both displacements. (Figure 2) The within CONC effect sizes represented moderate to large (A/P: 0.73 and M/L: 0.45) decreases in COP displacement. There was a significant group by time interaction for S1 A/P Velocity (F=33.411, P<0.001) (Figure 3), but not for S1 M/L velocity (F=3.145, P=0.054). The within CONC effect size was moderate (S1 A/P: 0.59) for decreased COP velocity.
Figure 2. Center of Pressure Displacement during the APA.
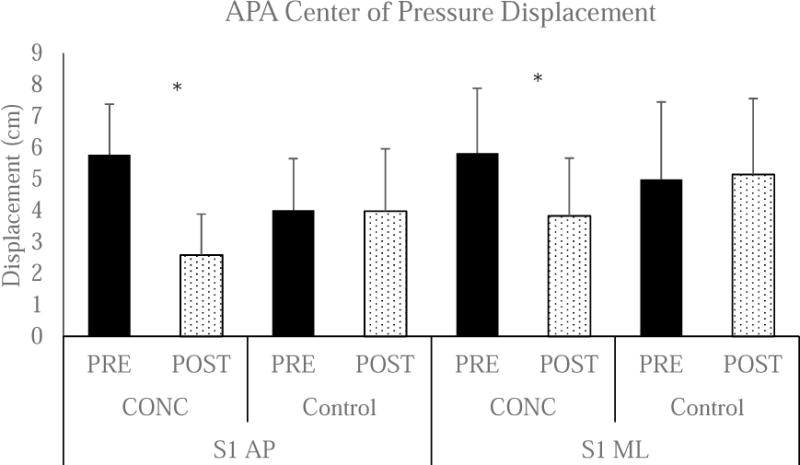
There was a significant Group by Time interaction for S1 AP (P<0.001). *Post-hoc testing identified a signification reduction in CONC (PRE: 5.7 ± 1.6 cm and POST: 2.6 ± 2.1 cm, P<0.001), but no difference in Control (PRE: 4.0 ± 1.6 cm and POST: 4.0 ± 2.5 cm, P=0.921). There was also a significant Group × Time interaction for S1 ML (P<0.001). Post-hoc testing identified a significant reduction in CONC (PRE: 5.8 ± 2.1 cm and POST: 3.8 ± 1.8 cm, P<0.001), but no difference in Control (PRE: 5.0 ± 2.5 cm and POST: 5.2 ± 2.4 cm, P=0.485).
Figure 3. Center of Pressure Velocity during the APA.
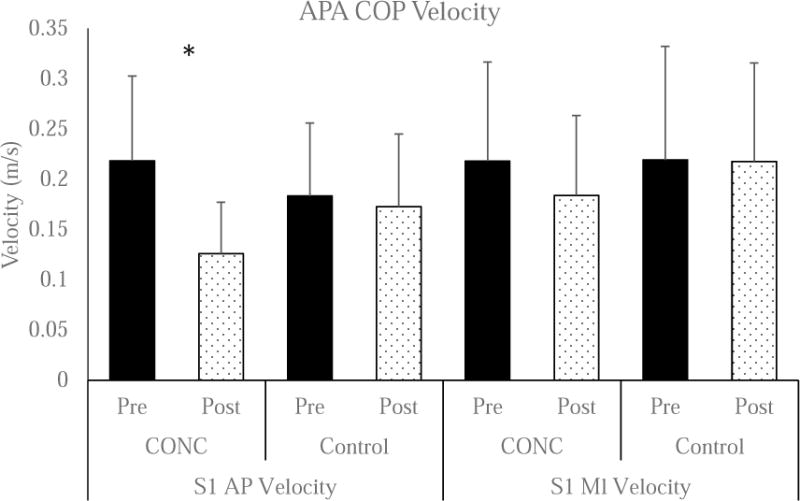
There was a significant Group × Time interaction (P<0.001) for the A/P component. *Post-Hoc testing identified a significant reduction in COP velocity in CONC (PRE: 0.21 ± 0.08 m/s and Post: 0.13 ± 0.05 m/s, P<0.001), but no difference in Control (PRE: 0.18 ± 0.07 m/s and POST: 0.17 ± 0.07 m/s, P=0.341). The Group × Time interaction was not significant for the M/L component (F=3.145, P=0.054).
Stepping Parameters
With the PRE performance used as a covariate, there was not a significant main effects for group for Initial Step Length (F=2.156, p=0.146). There was a significant main effect for group for Initial Step Velocity (F=4.447, p=0.038). (Figure 4) The within CONC effect size were small to moderate (Initial Step Velocity: 0.30) for slower initial step velocity.
Figure 4. Initial Step Kinematics.
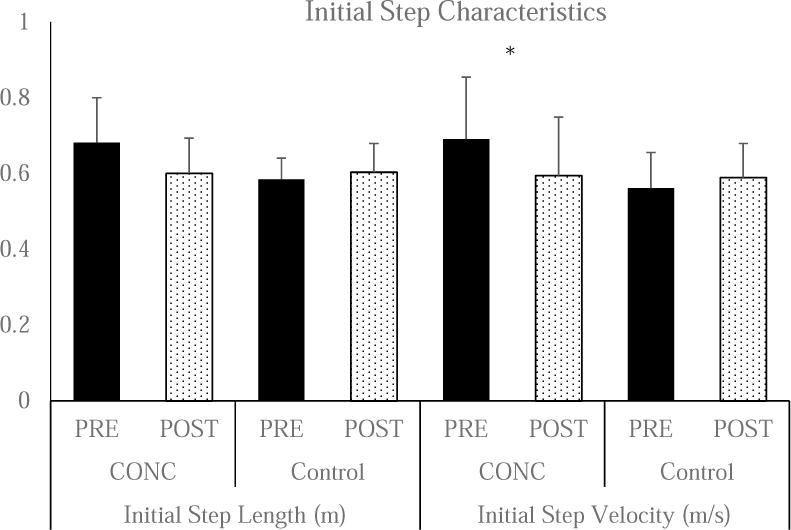
There were significant group differences at PRE for both initial step length (CONC: 0.68 ± 0.11 m and Control: 0.59 ± 0.04 m, p<0.001) and initial step velocity (CONC: 0.69 ± 0.16 m/s and Control: 0.56 ± 0.09 m/s, p<0.001) and therefore the PRE value was used as a covariate. The ANCOVA did not identify significant group differences for POST Initial Step Length (CONC: 0.60 ± 0.09 m and Control: 0.60 ± 0.05 m, p=0.146). There were significant group for POST Initial Step Velocity (CONC: 0.59 ± 0.15 m/s and Control: 0.58 ± 0.09 m/s, p=0.038).
DISCUSSION
Impairments in postural control following a concussion are well documented in quiet stance and gait tasks; however, these tasks primarily assess the integration of the sensory system (visual, vestibular, and somatosensory) and central pattern generators. Therefore, this study investigated postural control during the challenging transitional motor task of GI which is primarily controlled by the supplementary motor area (APA component) and motor cortex (locomotor component), thus it is not surprising that GI is frequently impaired in the elderly and individuals with neurological disorders. The main finding of this study was a noted deficit in the APA phase of GI, with moderate to large effect sizes, suggesting impairments in the planning and execution of this dynamic motor task.
Successful GI requires balancing the competing demands of generating forward momentum while maintaining postural stability, thus it is both a challenging and destabilizing task.36 The APAs function is to stabilize posture prior to the initiation of a voluntary movement.16 The CONC participants in this study significantly reduced both the posterior (↓3.2 cm; 56%) and lateral (↓2.0 cm; 34%) components of the preparatory phase. In studies of elderly and patients with neurological disorders, restrictions in the COP APA results, in part, from inappropriate prolonged activation of the Soleus and Gastrocnemius muscles.37 This muscle activity pattern likely reduces the effectiveness of the Tibialis Anterior activation in generating the forces necessary to propel the body forward. During quiet stance assessments acutely post-concussion, increased co-contraction has been speculated as a motor strategy to reduce postural sway and maintain upright balance.38, 39 Thus, a failure to inhibit the Soleus and Gastrocnemius is a potential explanation of the reduced posterior displacement of the COP in CONC participants and future investigations should include EMG studies. These findings are consistent with a conservative postural control strategy identified both acutely and chronically post-concussion.21, 25, 40–42
Deficits in static postural control following concussion are postulated to result from disrupted sensory interaction problems between the visual, vestibular, and somatosensory systems.25, 43 The results of this study seek to further clarify and specify these disruptions. The preparatory component of the GI motor task is believed to be modulated by the supplementary motor area while the locomotor component is regulated by the primary motor cortex.16 Specifically, the supplementary motor area neurons respond prior to a self-initiated motor sequence.16 The specific neurophysiology of concussion has been described as a neurometabolic cascade of events resulting in a neuronal suppression affecting diffuse areas of the brain simultaneously.44 However, some evidence suggests the supplementary motor area specifically may be adversely affected by a concussion which may help to explain the impaired performance identified herein.45 Indeed, Slobounov suggested impaired neuronal input from the prefrontal cortex into the supplementary motor cortex acutely post-concussion.46 Additional investigations have identified metabolic changes (e.g., lowered glutamate/phosphocreatine ratios) in the primary motor cortex suggesting the motor systems interneurons may be particularly vulnerable to concussions.47 There is some limited evidence suggesting abnormal function of the neurotransmitter dopamine following concussion which plays a key role in motor control outputs from the Basal Ganglia.48 Dopamine reductions in individuals with Parkinson’s disease is also speculated to contribute to the impairments of the control mechanism used to displace the COP posteriorly during GI.30 While we are unable to generate causative relationships from this study, it is likely that these neurometabolic changes are, at least in part, responsible for the impaired performance of GI following concussion. Finally, in light of the recent findings potentially linking multiple concussions to later-life impairments, these potential central neurophysiological deficits should be further investigated.49–51
The two groups PRE GI APA performance was similar to previous reports for healthy young adults; however the CONC participants’ GI APA characteristics closely resembled those of aging or neurologically diseased patient populations.30, 32, 52–55 Specifically, the mean posterior displacement of the COP during the preparatory (S1) phase for CONC participants was only 2.59 cm, a 54% reduction from PRE, and substantially lower than previous reports of elderly (range: 3.2 – 3.5 cm) and comparable to Parkinson disease patients (2.5 – 2.9 cm).30, 32 At PRE, only one CONC participant had a posterior displacement less than 3 cm (2.4%); however post-concussion 31 participants (75.6%) were below 3 cm. Furthermore, half (21/42) of the post-concussion participants were below the lower end (2.5 cm) of Parkinson’s disease performance. It must be clearly stated that this in no way implies a relationship between concussion and Parkinson’s disease, rather it likely reflects a conservative postural control strategy during a challenging transitional task. The 34% reduction in lateral COP displacement towards the initial swing limb, 3.83 cm, was not as impaired as aging or Parkinson’s disease (2.02 – 2.94cm), but was still substantially reduced (34%) from the baseline performance.30, 32 The COP APA posterior mean velocity decreased by 38% (pre: 0.21 m/s and post: 0.13 m/s) whereas the control participants velocity was largely unchanged (<0.01 m/s). There is limited COP velocity data in the literature; however, the baseline values herein exceeded healthy young adults in a prior study, but the COP displacements were also larger herein than reported by Nocera which likely explains the differences between studies.56 There was a significant reduction in Initial Step Velocity (0.09 m/s), but not Initial Step Length (8.1cm), but both measures remained well above the Parkinson’s disease and elderly populations. Both COP lateral and posterior displacements are associated with gait speed, thus the reduction in displacements and initial step velocity are consistent with prior findings.57 This suggests that the APA, particularly the posterior displacement and velocity, are likely the more effective measures of impaired postural control acutely post-concussion and their recovery pattern warrants further investigation.
The results of this study suggest that in healthy collegiate student-athletes, GI is a highly stable task as the control participant’s performance was nearly unchanged between test sessions. The control participant’s APA displacements in the posterior (0.03 cm decrease) and lateral (0.18 cm increase) direction represented changes of 0.7% and 3.5% respectively and did not approach statistical significance. Conversely, 97.6% (41/42) of concussion participants demonstrated a reduction in posterior displacement and velocity during the APA phase and the one remaining participant’s improvement was negligible (displacement: 0.04 cm; velocity 0.01 m/s). Consistent reductions in performance were also observed for most concussion participants in the lateral COP Displacement (83.3%; 35/42), Initial Step Length (76.2%; 32/42) and Initial Step Velocity (69.0%; 29/42). Despite highly similar group demographics, anthropometrics, and homogenous baseline test conditions, there were PRE group differences for both initial step length and velocity which do not have easy explanations. While not recorded within this study, it is possible that either prior/chronic lower extremity musculoskeletal injuries (e.g., chronic ankle instability),58 or the presence of attention-deficit/hyperactivity disorder or other mental health challenge (e.g., depression or anxiety) could have been different between groups; however, it is important to note that these differences were delimited to the stepping characteristics only and not the APA displacements or velocities.
Study Limitations
The GI results presented herein are delimited to FP data and we were unable to identify the location of the COP relative to anatomical landmarks of the foot. The lack of motion capture video kinematics and electromyography is a limitation of the study, however, in order to acquire premorbid data on large groups of participants, time constraints make utilization of these instruments impractical. The utilization of laboratory FPs restricts the clinical application of these findings due to cost and expertise; however, these results are consistent with previous findings that gait related tasks are effective in identifying impaired postural control and comparison to more clinically applicable tasks (e.g., tandem gait, timed up and go) is warranted.59 To further address this area, future investigations could utilize wearable accelerometers thus transitioning from the laboratory to more clinical settings.60 A strength of this study is the relatively large number of participants, however the data was underpowered to perform comparisons between GI performance and concussion history, gender, or initial presentation (e.g., loss of consciousness, post traumatic amnesia). Despite the similar demographics, there were non-significantly better (e.g., larger displacements and faster velocities) PRE APA values for the CONC group.
Conclusions
Impairments in postural control are a cardinal symptom of concussion, however the sensitivity of existing clinical and laboratory tests remains low. The results of this study suggest that acutely post-concussion, individuals demonstrate impairments in the planning and execution of GI, a task previously linked to the supplementary motor area.15, 16, 27, 29 Furthermore, we identified GI as a highly stable motor task in healthy participants, but an effective measure of identifying post-concussion impaired postural control. This stability is important as many existing novel tests are subject to practice or learning effects with repeat administration.7, 10 Future studies should extend these findings with comparison to both clinical test results, self-reported symptoms, and return to participant guidelines.
Acknowledgments
This project was funded, in part, by a grant from the National Institute of Health/Neurological Disorders and Stroke (NINDS 1R15NS070744-01A1). The funding agency had no role in the interpretation of the results, preparation of the manuscript, or the decision to submit the manuscript to Archives of Physical Medicine and Rehabilitation.
Abbreviations
- A/P
Anterior-Posterior
- APA
Anticipatory Postural Control
- CONC
Concussion Group
- COM
Center of Mass
- COP
Center of Pressure
- GI
Gait Initiation
- M/L
Medial-Lateral
- NCAA
National Collegiate Athletic Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suppliers List:
a: Model OR-6, Advanced Mechanical Technology, Inc., 176 Waltham Street, Watertown, MA 02472-4800 USA. 1-617-926-6700
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Llewellyn T, Burdette GT, Joyner AB, Buckley TA. Concussion Reporting Rates at the Conclusion of an Intercollegiate Athletic Career. Clin J Sport Med. 2014;24(1):76–9. doi: 10.1097/01.jsm.0000432853.77520.3d. [DOI] [PubMed] [Google Scholar]
- 3.Buckley TA, Burdette G, Kelly K. Concussion-Management Practice Patterns of National Collegiate Athletic Association Division II and III Athletic Trainers: How the Other Half Lives. J Athl Train. 2015;50(8):879–88. doi: 10.4085/1062-6050-50.7.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly KC, Jordan EM, Joyner AB, Burdette GT, Buckley TA. National Collegiate Athletic Association Division I Athletic Trainers’ Concussion-Management Practice Patterns. J Athl Train. 2014;49(5):665–73. doi: 10.4085/1062-6050-49.3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley T, Baugh C, Meehan W, DiFabio M. Concussion Management Plan Compliance: A Study of NCAA Power 5 Schools. Ortho J Sports Med Epub. 2017 Apr 25; doi: 10.1177/2325967117702606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S. Consensus Statement on Concussion in Sport - the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med Epub. 2017 Apr 26; doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 7.McCrea M, Barr WB, Guskiewicz K, Randolph C, Marshall SW, Cantu R, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11(1):58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 8.McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2556–63. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 9.Buckley TA, Munkasy BA, Clouse BP. Sensitivity and Specificity of the Modified Balance Error Scoring System in Concussed Student-Athletes. Clin J Sport Med. 2017 doi: 10.1097/JSM.0000000000000426. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Burk JM, Munkasy BA, Joyner AB, Buckley TA. Balance Error Scoring System Performance Changes After a Competitive Athletic Season. Clin J Sport Med. 2013;23(4):312–7. doi: 10.1097/JSM.0b013e318285633f. [DOI] [PubMed] [Google Scholar]
- 11.Rahn C, Munkasy BA, Joyner AB, Buckley TA. Sideline Performance of the Balance Error Scoring System during a Live Sporting Event. Clin J Sport Med. 2015;25(3):248–53. doi: 10.1097/JSM.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnoff JT, Peterson VJ, Hollman JH, Smith J. Intrarater and interrater reliability of the Balance Error Scoring System (BESS) PM R. 2009;1(1):50–4. doi: 10.1016/j.pmrj.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Valovich TC, Perrin DH, Gansneder BM. Repeat administration elicits a practice effect with the balance error scoring system but not with the standardized assessment of concussion in high school athletes. J Athl Train. 2003;38(1):51–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Caccese JB, Buckley TA, Kaminski TW. Sway Area and Velocity Correlated with MobileMat Balance Error Scoring System (BESS) Scores. J Appl Biomech. 2016;32(4):329–34. doi: 10.1123/jab.2015-0273. [DOI] [PubMed] [Google Scholar]
- 15.Wang JJ, Wai YY, Weng YH, Ng KK, Huang YZ, Ying LL, et al. Functional MRI in the assessment of cortical activation during gait-related imaginary tasks. J Neural Trans. 2009;116(9):1087–92. doi: 10.1007/s00702-009-0269-y. [DOI] [PubMed] [Google Scholar]
- 16.Massion J. Movement, Posture and Equilibrium - Interaction and Coordination. Prog Neurobio. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 17.Howell DR, Osternig LR, Chou L-S. Adolescents Demonstrate Greater Gait Balance Control Deficits After Concussion Than Young Adults. Am J Sports Med. 2015;43(3):625–32. doi: 10.1177/0363546514560994. [DOI] [PubMed] [Google Scholar]
- 18.Parker TM, Osternig LR, Lee HJ, van Donkelaar P, Chou LS. The effect of divided attention on gait stability following concussion. Clin Biomech. 2005;20(4):389–95. doi: 10.1016/j.clinbiomech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Howell DR, Osternig LR, Christie AD, Chou LS. Return to Physical Activity Timing and Dual-Task Gait Stability Are Associated 2 Months Following Concussion. J Head Trauma Rehabil. 2015;19:19. doi: 10.1097/HTR.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 20.Catena RD, van Donkelaar P, Chou L-S. Altered balance control following concussion is better detected with an attention test during gait. Gait Posture. 2007;25(3):406–11. doi: 10.1016/j.gaitpost.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Martini DN, Sabin MJ, DePesa SA, Leal EW, Negrete TN, Sosnoff JJ, et al. The chronic effects of concussion on gait. Arch Phys Med Rehabil. 2011;92(4):585–9. doi: 10.1016/j.apmr.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Fait P, Swaine B, Cantin J-F, Leblond J, McFadyen BJ. Altered Integrated Locomotor and Cognitive Function in Elite Athletes 30 Days Postconcussion: A Preliminary Study. J Head Trauma Rehabil. 2013;28(4):293–301. doi: 10.1097/HTR.0b013e3182407ace. [DOI] [PubMed] [Google Scholar]
- 23.Cossette I, Ouellet M-C, McFadyen BJ. A Preliminary Study to Identify Locomotor-Cognitive Dual Tasks That Reveal Persistent Executive Dysfunction After Mild Traumatic Brain Injury. Arch Phys Med Rehabil. 2014;95(8):1594–7. doi: 10.1016/j.apmr.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Gao J, Hu J, Buckley T, White K, Hass C. Shannon and Renyi Entropies to Classify Effects of Mild Traumatic Brain Injury on Postural Sway. Plos One. 2011;6(9) doi: 10.1371/journal.pone.0024446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckley TA, Oldham JR, Caccese JB. Postural control deficits identify lingering post-concussion neurological deficits. J Sport Health Sci. 2016;5(1):61–9. doi: 10.1016/j.jshs.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cossette I, Gagne ME, Ouellet MC, Fait P, Gagnon I, Sirois K, et al. Executive dysfunction following a mild traumatic brain injury revealed in early adolescence with locomotor-cognitive dual-tasks. Brain Inj. 2016;30(13–14):1648–55. doi: 10.1080/02699052.2016.1200143. [DOI] [PubMed] [Google Scholar]
- 27.Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: A PET study. Hum Brain Mapp. 2003;19(1):47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang HA, Krebs DE. Dynamic balance control in elders: Gait initiation assessment as a screening tool. Arch Phys Med Rehabil. 1999;80(5):490–4. doi: 10.1016/s0003-9993(99)90187-9. [DOI] [PubMed] [Google Scholar]
- 29.Chang W-H, Tang P-F, Wang Y-H, Lin K-H, Chiu M-J, Chen S-HA. Role of the premotor cortex in leg selection and anticipatory postural adjustments associated with a rapid stepping task in patients with stroke. Gait Posture. 2010;32(4):487–93. doi: 10.1016/j.gaitpost.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson’s disease. Gait Posture. 2012;35(4):669–73. doi: 10.1016/j.gaitpost.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Vallabhajosula S, Buckley TA, Tillman MD, Hass CJ. Age and Parkinson’s disease related kinematic alterations during multi-directional gait initiation. Gait Posture. 2013;37(2):280–6. doi: 10.1016/j.gaitpost.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Halliday SE, Winter DA, Frank JS, Patla AE, Prince F. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture. 1998;8(1):8–14. doi: 10.1016/s0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 33.Hass CJ, Waddell DE, Wolf SL, Juncos JL, Gregor RJ. Gait initiation in older adults with postural instability. Clin Biomech. 2008;23(6):743–53. doi: 10.1016/j.clinbiomech.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melzer I, Goldring M, Melzer Y, Green E, Tzedek I. Voluntary stepping behavior under single- and dual-task conditions in chronic stroke survivors: A comparison between the involved and uninvolved legs. J Electromyogr Kinesiol. 2010;20(6):1082–7. doi: 10.1016/j.jelekin.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 35.McCrory P, Meeuwisse W, Aubry M, Cantu R, Dvorak J, Echemendia RJ, et al. Consensus Statement on Concussion in Sport-the 4th International Conference on Concussion in Sport Held in Zurich, November 2012. Clin J Sport Med. 2013;23(2):89–117. doi: 10.1097/JSM.0b013e31828b67cf. [DOI] [PubMed] [Google Scholar]
- 36.Polcyn AF, Lipsitz LA, Kerrigan DC, Collins JJ. Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Arch Phys Med Rehabil. 1998;79(12):1582–9. doi: 10.1016/s0003-9993(98)90425-7. [DOI] [PubMed] [Google Scholar]
- 37.Elble RJ, Cousins R, Leffler K, Hughes L. Gait initiation by patients with lower-half Parkinsonism. Brain. 1996;119:1705–16. doi: 10.1093/brain/119.5.1705. [DOI] [PubMed] [Google Scholar]
- 38.Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Recovery of postural control after cerebral concussion: New insights using approximate entropy. J Athl Train. 2006;41(3):305–13. [PMC free article] [PubMed] [Google Scholar]
- 39.Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer V, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39(11):805–11. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckley TA, Vallabhajosula S, Oldham JD, Munkasy BA, Evans KM, Krazeise DA, et al. Evidence of a Conservative Gait Strategy in Athletes with a History of Concussions. J Sport Health Sci. 2016;5(4):417–23. doi: 10.1016/j.jshs.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckley TA, Munkasy BA, Tapia-Lovler TG, Wikstrom EA. Altered gait termination strategies following a concussion. Gait Posture. 2013;38(3):549–51. doi: 10.1016/j.gaitpost.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oldham JR, Munkasy BA, Evans KM, Wikstrom EA, Buckley TA. Altered dynamic postural control during gait termination following concussion. Gait Posture. 2016;49:437–42. doi: 10.1016/j.gaitpost.2016.07.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guskiewicz KM. Balance Assessment in the Management of Sport-Related Concussion. Clin Sports Med. 2011;30(1):89–102. doi: 10.1016/j.csm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228–35. [PMC free article] [PubMed] [Google Scholar]
- 45.Jantzen KJ, Anderson B, Steinberg FL, Kelso JAS. A prospective functional MR imaging study of mild traumatic brain injury in college football players. Am J Neuroradiol. 2004;25(5):738–45. [PMC free article] [PubMed] [Google Scholar]
- 46.Slobounov S, Sebastianelli W, Moss R. Alteration of posture-related cortical potentials in mild traumatic brain injury. Neurosci Lett. 2005;383(3):251–5. doi: 10.1016/j.neulet.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic Changes in the Acute Phase after Sports Concussions Correlate with Symptom Severity. J Neurotrauma. 2010;27(1):65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- 48.Chen JK, Johnston KM, Petrides M, Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch Gen Psychiatry. 2008;65(1):81–9. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- 49.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–26. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 51.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903–9. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 52.Honeine JL, Schieppati M, Crisafulli O, Do MC. The Neuro-Mechanical Processes That Underlie Goal-Directed Medio-Lateral APA during Gait Initiation. Front Hum Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cau N, Cimolin V, Galli M, Precilios H, Tacchini E, Santovito C, et al. Center of pressure displacements during gait initiation in individuals with obesity. J Neuroeng Rehabil. 2014;11:8. doi: 10.1186/1743-0003-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, et al. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97(6):4368–79. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- 55.Hartley EM, Hoch MC, McKeon PO. Reliability and responsiveness of gait initiation profiles in those with chronic ankle instability. Gait Posture. 2016;49:86–9. doi: 10.1016/j.gaitpost.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Nocera JR, Roemmich R, Elrod J, Altmann LJP, Hass CJ. Effects of cognitive task on gait initiation in Parkinson disease: Evidence of motor prioritization? J Rehabil Res Dev. 2013;50(5):699–707. doi: 10.1682/jrrd.2012.06.0114. [DOI] [PubMed] [Google Scholar]
- 57.Caderby T, Yiou E, Peyrot N, Begon M, Dalleau G. Influence of gait speed on the control of mediolateral dynamic stability during gait initiation. J Biomech. 2014;47(2):417–23. doi: 10.1016/j.jbiomech.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic Ankle Instability Alters Central Organization of Movement. Am J Sports Med. 2010;38(4):829–34. doi: 10.1177/0363546509351562. [DOI] [PubMed] [Google Scholar]
- 59.Oldham JR, DiFabio MS, Kaminski TW, DeWolf RM, Buckley TA. Normative Tandem Gait in Collegiate Athletes Implications for Clinical Concussion Assessment. Sports Health Epub. 2016 Nov 29; doi: 10.1177/1941738116680999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Howell D, Osternig L, Chou LS. Monitoring recovery of gait balance control following concussion using an accelerometer. J Biomech. 2015;26(15):00351–6. doi: 10.1016/j.jbiomech.2015.06.014. [DOI] [PubMed] [Google Scholar]


