Abstract
A wide range of stress-related pathologies such as post-traumatic stress disorder are thought to arise from aberrant or maladaptive forms of stress adaptation. The hypothalamic-pituitary-adrenal (HPA) axis readily adapts to repeated stressor exposure, yet little is known about adaptation in neuroimmune responses to repeated or sequential stress challenges. In Experiment 1, rats were exposed to ten days of restraint alone (60 min daily), forced swim alone (30 min daily), or daily sequential exposure to restraint (60 min) followed immediately by forced swim (30 minutes), termed sequential stress exposure. Habituation of the corticosterone (CORT) response occurred to restraint by 5 days and swim at 10 days, whereas rats exposed to sequential stress exposure failed to display habituation to the combined challenge. Experiment 2 compared 1 or 5 days of forced swim to sequential stress exposure and examined how each affected expression of several neuroimmune and cellular activation genes in the paraventricular nucleus of the hypothalamus (PVN), prefrontal cortex (PFC), and hippocampus (HPC). Sequential exposure to restraint and swim increased IL-1β in the PVN, an effect that was attenuated after 5 days. Sequential stress exposure also elicited IL-6 and TNF-α responses in the HPC and PFC, respectively, that did not habituate after 5 days. Experiment 3 tested whether prior habituation to restraint (5 days) would alter the IL-1β response evoked by swim exposure imposed immediately after the 6th day of restraint. Surprisingly, a history of repeated exposure to restraint attenuated the PVN IL-1β response after swim in comparison to acutely-exposed subjects despite an equivalent CORT response. Overall, these findings suggest that habituation of neuroimmune responses to stress proceeds (a) independent of HPA axis habituation; (b) likely requires more daily sessions of stress to develop; and that (c) IL-1β displays a greater tendency to habituate after repeated stress challenges than other stress-reactive cytokines.
Keywords: Corticosterone, habituation, interleukin-1, multimodal stress, sequential stress
1.0 Introduction
Exposure to physiological or perceived threats elicits a stress response that redirects physiological systems toward overcoming the challenge at hand. While this response is initially adaptive, in the case of prolonged stress exposure it can become overactive and maladaptive, depleting resources and inhibiting growth and memory (1), altering nutrient preferences (2), and decreasing food intake (3). One of the major consequences of stressor exposure is activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in the release of corticosterone (CORT). Stressors thought to be more psychological in nature, such as restraint and social defeat, have been shown to drive the HPA axis through activation of forebrain and limbic structures, whereas stressors that more directly affect physiological homeostasis, such as hypoxia or dehydration, activate the PVN through brainstem autonomic nuclei (4).
Cytokines are small signaling proteins that are important in regulating the immune response and inflammation. Interleukin-1 beta (IL-1β) in particular is known to stimulate the HPA axis and modify neural plasticity (5), sickness-like behaviors such as fever (6), reduce food and water intake (7), and reduce social interaction (8, 9). Another pro-inflammatory cytokine, TNF-α, has been shown to work in tandem with IL-1β; each can stimulate release of the other, and high local concentrations can induce inflammation, recruit neutrophils, and promote insulin resistance (10). Interleukin-6 (IL-6) is another important mediator of inflammation and energy metabolism, and has both pro-inflammatory (cytokine) and more recently discovered anti-inflammatory (myokine) roles, such as inhibiting expression of TNF-α and IL-1β (11).
Cytokines have emerged as important stress-responsive targets. Stress exposure has been shown to drive the activation of microglia (12), and activated microglia show morphological changes such as a thickening of fine processes, additional branching of processes, and changes in cell-surface markers that indicate a stress-reactive, primed state associated with inflammation (13). Some examples of these markers are CD14, which are found on microglia and are shown to be increased in response to stress (14), and the interaction between CD200, found on neurons, and CD200R, found on microglia, whose decoupling results in microglial activation (15). Microglia may also be a key cellular source of cytokines, since minocycline, a putative microglial inhibitor, blocked stress-induced IL-1β expression (12, 16). IL-1β has been the key focus of many studies because it is reliably elevated in the hypothalamus following multiple types of stressors, including immobilization (17), tailshock (18), and footshock (12, 19). However, forced swim (20), predator odor (21), and social defeat (22) did not induce IL-1β expression. It is of note that the stressors considered to be more intense induced IL-1β, whereas the less-intense stressors did not, which lends support to the notion that IL-1 expression may in part reflect the intensity of the stress challenge (23). Further, while restraint alone did not induce IL-1β expression in the hypothalamus, when it was combined with placement on an orbital shaker or insulin-induced hypoglycemia, thereby increasing the intensity of the stressful experience, IL-1β protein was induced (19). Recent findings have shown that multiple types of stress imposed simultaneously (termed multi-modal stressors) such as restraint, noise, and rotation, may have a greater impact on the stress response than any one modality alone (24). In other studies, the combination of restraint with either insulin-induced hypoglycemia or being placed on an orbital shaker increased IL-1β in hypothalamic blocks, whereas restraint alone had no effect (19). Together, these findings seemed to suggest that a certain threshold of stress intensity may be necessary to incur stress-dependent changes in cytokine expression during an acute bout of stress.
While there is a rich literature on neuroendocrine adaption to stress, few studies have addressed habituation of neuroimmune responses to stress. Our lab examined the effect of social defeat on IL-1β expression, but neither acute, 7 days, nor 21 days of exposure increased IL-1β expression (22). Repeated restraint stress, but not chronic variable stress, has been shown to increase Iba-1 levels, indicative of microglial activity, in the prelimbic and infralimbic portions of the prefrontal cortex (25).
One practical consideration in the design of chronic stress studies is the temporal relationship between individual components of the stress regimen. For instance, repeated restraint is a common stress model that produces robust habituation of the HPA axis. Importantly, there is growing interest in the impact of sequential exposure to stress challenges, such as in the single prolonged stress (SPS) model of post-traumatic stress disorder, in which rats are exposed to a restraint, forced swim, and ether exposure (26–28). In contrast, the chronic variable stress (CVS) models are comprised of multiple stress components that are unpredictable by design. Certainly both approaches have many strengths and limitations, but neither procedure lends itself toward understanding the interaction between stress challenges imposed in rapid succession. Of particular interest is the question of how habituation to a mild stressor is impacted if immediately followed by a robust stressor which is notably resistant to habituation, such as forced swim or footshock (29). It would make sense that, across multiple daily pairings and exposures, the anticipation of the second more intense challenge might (a) disrupt habituation either by reducing robustness or lengthening the number of days required to see an effect; or (b) lead to signs of anticipatory responses as daily exposures (pairings) ensue. Additionally, in this paradigm the initial stressor is positioned to become predictive of the subsequent more robust stressor. Prior work has shown that the psychological factor of predictability can modulate the stress response. A procedure where shock was cued by a CS for 5 days induced a rise in basal CORT levels indicative of the chronically stressed state, and cue presentation induced an anticipatory rise in plasma NE, an effect that was not seen in subjects given unpredictable shocks (30). As stress in daily life is often multimodal and somewhat predictable, the study of these elements may provide an important piece of the chronic stress puzzle.
With these matters in mind, we conducted three experiments to explore the impact of repeated exposure to restraint followed immediately by forced swim, termed sequential stress exposure, on habituation of the CORT response, pro-inflammatory cytokine expression, and markers of cellular activity. In experiment 1, we investigated habituation of the CORT response to sequential stress using a within-subjects design by exposing rats to 10 days of restraint alone, swim alone, or restraint immediately followed by swim and measured CORT at key points that allowed for determination of habituation to individual elements of the sequential stress. Experiment 2 examined mRNA expression levels of key cytokines and cellular activation markers in the PVN, PFC, and HPC after 1 or 5 days of sequential stress. The terminal nature of this design necessitated a between-subjects design, and day 5 was chosen as it allowed for examination of gene targets at a point where differences in habituation between CORT and pro-inflammatory cytokines may be detectable. Experiment 3 examined the potential development of expectancies regarding sequential stress challenges and their impact on neuroimmune processes by comparing acute exposure to sequential stress between subjects that had no previous stress experience and subjects that had previously been exposed to 5 days of the first element of the sequential stressor (restraint). These studies fill an important gap in our knowledge of how stress challenges experienced in rapid succession, across 5–10 consecutive days, might impact both the HPA and neuroimmune consequences of stress.
2.0 Materials and Methods
2.1 Subjects
Adult male Sprague-Dawley rats purchased from Harlan Laboratories (Indianapolis, IN) were pair-housed with access to food and water ad libitum and provided wooden chew sticks for enrichment. For practical purposes, these initial studies were conducted with only male subjects. Ongoing studies examining sex differences are forthcoming. Colony conditions were maintained at 22±1°C with 12:12 light–dark cycle (lights on 07:00 h). Rats were given a minimum of 2 weeks to acclimate prior to experimentation and were handled for 3–5 min on each of two days before experimentation. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Binghamton University and animals were treated in accordance with PHS policy.
2.2 Restraint
Rats were restrained in a Plexiglas tube (length = 21.6 cm, inner diameter (ID) = 6.4 cm) with ample holes for ventilation for either 60 or 90 minutes. The restraint stressor was devoid of any active immobilization, limb/tail tethering, or compression, and allowed sufficient movement so that animals could rotate (barrel roll) within the tube but did not allow for them to turn around head to tail (31).
2.3 Forced Swim Stressor
Rats were transported to a dedicated procedural room and immediately placed in a cylinder (45 cm high, 20 cm diameter) filled 30 cm high with water that was carefully maintained at 25°C, as previously described (20). Rats were forced to swim for 30 min, after which they were towel-dried and either returned to their home cages or tissue was collected.
2.4 Tail Blood Collection and Measurement of Corticosterone
Rats were briefly restrained in Plexiglas tubes (length = 21.6 cm, ID = 6.4 cm) and the tip of the tail (~1 mm) was transected with a razor blade. Blood (50–100 μl) was collected with gentle massaging of the tail in EDTA coated Vacutainers (BD Vacutainers, VWR cat.no. VT6450, Radnor, PA) and samples were immediately placed on ice. All blood samples were collected within 2 min to ensure serum measures of CORT reflected ambient levels untainted by the stress of the blood sampling procedure itself. Rats were returned to their home cages immediately afterwards or remained in restraint as dictated by group assignment. Serum was separated for 15 min at 3220 g in a refrigerated centrifuge and frozen at −20°C until time of assay.
Total serum CORT levels were measured by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Enzo Life Sciences; Farmingdale, NY) according to manufacturer’s instructions, with one exception. Samples were heat-inactivated to denature endogenous corticosteroid binding globulin (CBG) via immersion in a 75°C water bath for 60 min (32, 33). Prior assays show this procedure produces superior denaturation of CBG than the enzyme cleavage step provided in the kit (unpublished observations). Assay sensitivity was 27.0 pg/ml with an inter-assay coefficient of 5.40%.
2.5 Tissue Collection
Tissue was harvested after rapid decapitation and trunk blood was collected in EDTA-coated vacutainers. Plasma was separated in a refrigerated centrifuge and frozen at −20°C until time of assay. Brains were removed immediately after decapitation and whole brains were flash frozen in 2-methylbutane (EMD Millipore, cat. No. MX0760-1, Billerica, MA) and stored at −80°C. Brains were sectioned coronally (60μm) on a cryostat (Leica Model CM1850, Wetzlar, Germany) and bilateral tissue punches were taken from structures of interest, according to the atlas of Paxinos and Watson (2005; see figure 1A, B, and C for details). In the case of the PVN, some additional surrounding tissue was included in the punch to ensure that the entire PVN was collected, and thus these punches are referred to as “PVN-enriched”. Though brains were not saline perfused to ensure that cytokine expression in blood could not contaminate the samples, previous work has shown that perfusion produced no differences in brain IL-1β protein levels as compared to non-perfused tissue (18), and we have found the same results when examining mRNA levels with real-time RT-PCR (unpublished observations). Peripheral organs and glands were quickly harvested and either weighed or flash frozen and then stored at −80°C.
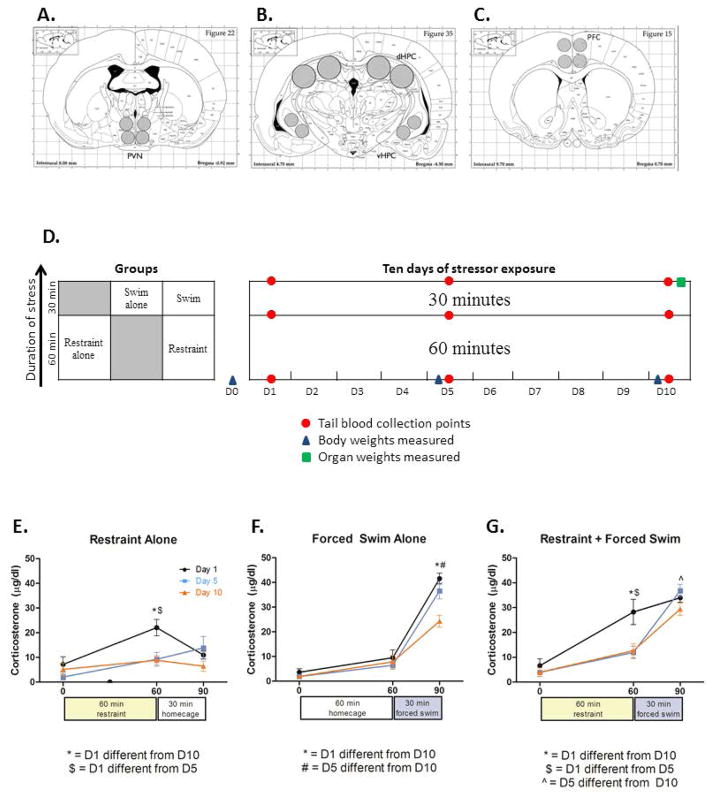
Figure 1.
Locations of brain punches for target structures, Experiment 1 design, and CORT adaptation results. A. Cingulate portion of the prefrontal cortex (Cing/PFC) - four punches, each 1.2 mm punch diameter, 1 mm punch deep. B. Paraventricular nucleus of the hypothalamus (PVN)-enriched punches – four punches, each 1.2 mm punch diameter, 1 mm punch depth. C. Hippocampus (HPC) – dorsal and ventral portions were combined and processed together. Dorsal – four punches, each 2 mm diameter, 1.5 mm depth. Ventral - four punches, each 1.2 mm diameter, 1 mm deep. D. In experiment 1, Rats were exposed to restraint alone, forced swim alone, or restraint immediately followed by forced swim for 10 days. Blood collection timepoints on days 1, 5, and 10 are indicated with red dots, body weight measurements are indicated with blue triangles, and organs were weighed at the point signified by the green square. Results from the corticosterone ELISA (n=8/group) are depicted for (E) the restraint alone group, (F) the forced swim alone group, and (G) the restraint + forced swim group. The * denotes where D1 differs from D10, # where D5 differs from D10, $ where D1 differs from D5, and ^ where D5 differs from D10.
2.6 Tissue Processing
Each tissue sample was placed in a 2.0 ml Eppendorf tube with 500 μL Trizol® RNA reagent and a 5 mm stainless steel bead. Tissue was then homogenized using a Qiagen TissueLyser II™ (Qiagen, Valencia, CA) for 2–4 min at 20 Hz to ensure thorough homogenization of samples. Total cellular RNA was extracted from tissue using Qiagen RNeasy Mini kits according to the manufacturer’s instructions. RNA was separated from the supernatant through chloroform extraction performed at 12,000 g for 15 min at 4°C. Equal volume of 70% ethanol was added to the collected RNA and purified through RNeasy mini columns. Columns were washed and eluted with 30 μL of RNase-free water (65°C). RNA yield and purity was determined using the NanoDrop 2000 spectrophotometer (NanoDrop, Wilmington, DE). RNA was stored at −80°C prior to cDNA synthesis.
2.7 Real-Time RTPCR
Synthesis of cDNA was performed on 0.3–1.0 μg of normalized total RNA from each sample using the QuantiTect Reverse Transcription kit (Cat No. 205313, Qiagen, Valencia, CA) which included a DNase treatment step. All cDNA was stored at −20°C until further processing. Probed cDNA amplification was performed in a 20 μL reaction consisting of 10 μL IQ SYBR Green supermix (Bio-Rad Laboratories), 0.1 μL forward and reverse primer, 2 μL cDNA template, and 8.8 μL ribonuclease-free water run in triplicate in a 384-well plate (BioRad Laboratories) using the BioRad CFX 384 Real Time System C1000 Thermal Cycler (BioRad Laboratories). Relative gene expression was quantified using the delta- delta (2-ΔΔCT) method relative to the stable housekeeping gene β-actin (34). Housekeeping genes were analyzed separately to ensure stability across treatment groups prior to use as a reference. See Table 1 for primer sequences.
Table 1.
Primers, accession numbers, sequences, and functional roles of targets in real-time RT-PCR for all gene expression studies.
| Primer | Accession # | Sequences (forward, reverse) | Functional Role | Ref. |
|---|---|---|---|---|
| B-actin | V01217 | 5′-GTC GTA CCA CTG GCA TTG TG-3′ 5′-GCC ATC TCT TGC TCG AAG TC-3′ |
Stably expressed cytoskeletal actin; used as a housekeeping gene | (51) |
| c-Fos | NM_016992.1 | 5′-CCA AGC GGA GAC AGA TCA AC-3′ 5′-AAG TCC AGG GAG GT CACA GA-3′ |
Immediate early gene, expression signifies cellular activity; used as a general index of cellular activation | (52) |
| IL-1β | NM_031512 | 5′-AGG ACC CAA GC ACCT TCT TT-3′ 5′-AGA CAG CAC GAG GCA TTT TT-3′ |
Inflammatory cytokine, rapidly expressed in response to stressors | (18) |
| IL-6 | NM_012589 | 5′-TAG TCC TTC CTA CCC CAA CTT CC-3′ 5′-TTG GTC CTT AGC CAC TCC TTC-3′ |
Has both pro- and anti-inflammatory roles, expressed in response to infection and stressors | (53) |
| TNF-α | NM_012675 | 5′-GGG GCC ACC ACG CTC TTC TG-3′ 5′-CGA CGT GGG CTA CGG GCT TG-3′ |
Endogenous pyrogen primarily expressed by macrophages in response to infection and stressors | (54) |
| CD14 | NM_021744.1 | 5′-TCC ATC GGT GCT CAC AAA TA-3′ 5′-TTG GGG ATT TAG CTC AGT GG-3′ |
Cell surface molecule, co-receptor for detection of bacterial LPS; often used as a marker of microglial activity | (55) |
| CD200 | NM_031518.2 | 5′-CTG CCA TCT GTC CAC CTA CA-3′ 5′-AAG GGT TCC TGG GTT GTT TT-3′ |
Signaling molecule released by neurons important in regulating the inflammatory response; expression levels reflect neuronal-sourced dampening of inflammatory microglial activity | (56) |
| CD200R | NM_023953.1 | 5′-CTG CTC TGC TGC CCT TCT AT-3′ 5′-ATG GGT CTC CCT TGT GTC TG-3′ |
Cell-surface molecule on microglia important in regulating the inflammatory response; serves as a marker of microglial activity | (56) |
| GFAP | NM_018009.2 | 5′-GAG CCC CTA ACT CTG TGC TG-3′ 5′-GCA CAC CTC ACA TCA CAT CC-3′ |
Expressed by active astrocytes, involved in cell communication and the response to insult | (48) |
2.8 Statistical Analysis
Data were first analyzed with Statistica software using either a between-subjects one-way ANOVA or factorial ANOVA as appropriate to the experimental design (described below). Post-hoc testing was done using Tukey’s test for all observed main effects and interactions. To further control for multiple comparisons within experiments, MANOVAs were conducted on each structure that included all target genes. Since MANOVA outcomes largely supported the outcomes and conclusions of the original ANOVAs, elaboration of results focused solely on ANOVA analyses. In all cases, an α-level of 0.05 was used as the criterion for determining statistical significance.
2.9 Experimental Design
2.9a Experiment 1: Habituation of the corticosterone response to sequential stressor exposure
The aim of Experiment 1 was to examine adaptation of the CORT response in rats when daily restraint was immediately followed by a second stress challenge (forced swim) for 10 consecutive days. Ten total days was chosen as the CORT response has been shown to habituate to restraint by five days of repeated exposure, whereas habituation to a subjectively more intense stressor such as forced swim or restraint followed immediately by swim may require a greater number of exposures (31, 35). Rats (n=8 per group, N=24) were divided into 3 experimental groups of either restraint alone (60 min daily), forced swim alone (30 min daily), or 60 min of restraint followed immediately by 30 min of forced swim (total stress duration = 90 min; see figure 1D). Body weights were taken the day before experimentation and also on the mornings of the fifth and tenth days of stressor exposure. Tail blood samples were collected from all groups on days 1, 5, and 10 at time points corresponding to before restraint (0 min), following restraint (60 min) and immediately after forced swim (90 min; see Figure 1D). On day 10 rats were killed immediately following stressor exposure and peripheral organs and glands were weighed.
2.9b Experiment 2: Neuroimmune changes in response to repeated sequential stressor exposure
The goal of Experiment 2 was to examine cytokine expression and indices of cellular activation in response to sequential stress exposure in stress-reactive brain regions (PVN, PFC and HPC) on either the initial or fifth day of stress exposure via real-time RT-PCR. These time points were chosen based on the finding in Experiment 1 that CORT habituation to forced swim had not occurred by day 5, so any observed cytokine changes were not likely to be due to CORT adaptation. Rats (n=8–10 per group, N=42) were exposed to 1 or 5 days of forced swim, 1 or 5 days of restraint followed immediately by forced swim, or served as home cage controls. Brains, serum, and peripheral tissue were collected immediately after stress termination under no-stress conditions on the last day of stress exposure. The day of stress initiation was varied so that all groups were represented on each day of tissue harvest.
2.9c Experiment 3: Neuroimmune effects of expectancy violation
Experiment 3 examined cytokine expression and indices of cellular activation after repeated daily exposure to 60 minutes of restraint with a subsequent unexpected change in stressor (specifically, 30 minutes of forced swim immediately after the restraint, or an additional 30 minutes of restraint). Expectancy violation has been shown to result in increases in the CORT response and struggling behavior in restraint (36), but cytokine adaptations have yet to be examined. Rats (n=8 per group, N=40) were exposed to either 5 days of restraint or remained in home cage, then were challenged with an extension of the stressor (either 30 minutes of forced swim or 30 additional minutes of restraint) on day 6. Body weights were taken before treatment on days 1 and 6, and brains, tissue, and serum were collected on day 6 immediately after stress termination. The five conditions consisted of acute restraint + swim (exposure to 60 min restraint followed immediately by 30 min swim), restraint history + swim (5 days of restraint, then 60 min restraint followed immediately by 30 min forced swim on day 6), restraint length extension (restraint was extended to 90 minutes on day 6), stress history only (5 days of restraint and remain in homecage on day 6, and homecage controls (see Figure 3A).
3.0 Results
3.1 Experiment 1
3.1a Body Weights
One-way ANOVA analysis of body weight gain across 10 days showed rats that experienced restraint gained more weight than those in the forced swim alone or restraint+swim conditions [F(2,21) = 12.88, p < 0.001]. No differences were found in organ weights that were adjusted to body weight ([organ weight/body weight]*1000) in the thymus [F(2, 21) = 2.56, p > 0.05], adrenals [F(2,21) = 0.718, p>0.05], or spleen [F(2, 21)=0.574, p>0.05]. See Table 2.
Table 2.
Body and organ weights from Experiment 1
| Restraint Alone | Forced Swim Alone | Restraint + Forced Swim | ||
|---|---|---|---|---|
| Body weights (g) | Baseline | 278.9 ± 2.5 | 274.3 ± 2.5 | 275.6 ± 3.1 |
| Day 5 | 301.5 ± 3.4* | 283.6 ± 4.1* | 285.9 ± 3.9 | |
| Day 10 | 322.5 ± 4.6 | 296.5 ± 5.1 | 297.88 ±3.8 | |
| Δ weight (Day 10 – Day 1) | 43.60 ± 3.7 | 22.25 ± 3.53 | 22.25 ± 3.00 | |
| Tissue weights adjusted to body weight (g × 1000) | Spleen | 2.25 ± 0.06 | 2.137 ± 0.09 | 2.20 ± 0.07 |
| Adrenal glands | 0.127 ± 0.009 | 0.137 ± 0.004 | 0.129 ± 0.004 | |
| Thymus | 1.26 ± 0.05 | 1.08 ±0.07 | 1.12 ±0.05 |
Note. Mean ± SEM of body weights by day, change in weight across the experiment, peripheral organ/gland weights, and peripheral tissue weights adjusted to body weight at time of collection (organ weight/body weight at time of tissue collection × 1000). Bold text denotes a significant difference from all other groups. On day 5,
indicates groups that were different from each other.
3.1b Corticosterone
Corticosterone results are illustrated in figure 1E–G. Due to an error in sample processing, some samples were lost (22 out of 216). Rather than deleting the entire record for each affected subject, we ensured missing data were randomly distributed across groups (Little’s MCAR test: Chi-square = 10.432, DF = 7, p=0.165) and utilized an expectation maximization algorithm (tolerance = 0.001, convergence = 0.0001, iterations = 25) using SPSS to substitute in missing values, then carried forward with ANOVA analysis (37). A 3×3×3 (stress condition × timepoint × day) mixed-design ANOVA revealed a significant time by group by day [F(8, 84) = 4.00, p<0.001] interaction. As illustrated in figure 1E, restraint alone led to habituation after 5 days (p<0.0001) and this effect persisted on the tenth day of testing (p<0.0001). Habituation to forced swim alone occurred at 10 days of repeated exposure (p<0.0001) but not at 5 days (p>0.05; see figure 1F). In the Restraint + Forced Swim group, corticosterone was reduced on days 5 (p<0.0001) and 10 (p<0.0001) relative to day 1 at the end of restraint (indicating habituation), and a reduction was seen between day 5 and day 10 after the forced swim (p<0.005; see figure. 2G).
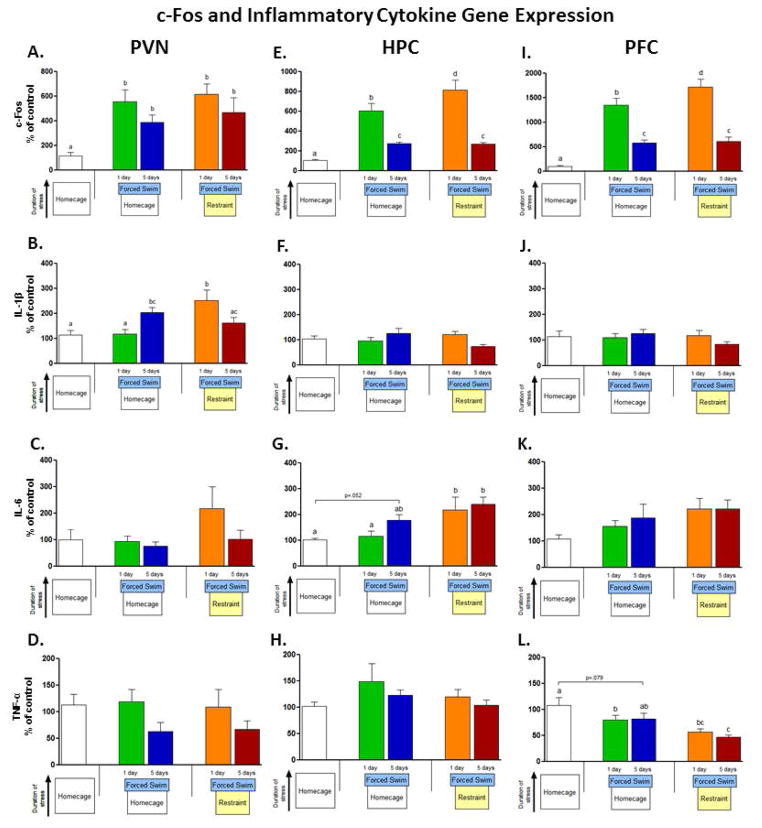
Figure 2.
Inflammatory cytokine and c-Fos real-time RTPCR results from Experiment 2. Rats were exposed to 1 or 5 days of forced swim or restraint immediately followed by forced swim. Immediately after stress on the last day of exposure, rats were rapidly decapitated and brains were collected. All data is expressed relative to the control group (homecage) and normalized to β-actin. Each group consisted of 6–8 subjects. Different letters across groups denote significant differences.
3.2 Experiment 2 Results
3.2a Gene Expression
Housekeeper
Prior to adjusting target genes of interest relative to β-actin, one-way ANOVAs were utilized to assess the stability of β-actin and its suitability for use as a stable reference gene. Analyses revealed no effect of any stress manipulations on β-actin expression in any brain structure (PVN [F(4,36)=0.21, p>0.05]; HPC [F(4,36)=0.51, p>0.05]; PFC [F(4,35)=1.99, p>0.05]), so all subsequent target genes were expressed relative to β-actin.
Cytokines
One-way ANOVAs showed an effect of stress exposure on IL-1β in the PVN [F(4, 35) = 5.43, p<0.01]. In the forced swim only groups, one day of exposure had no effect on IL-1β, but it was increased after 5 days compared to both control and 1 day (p’s<0.05). The restraint+swim groups showed the opposite pattern, with an elevation immediately after 1 day of sequential stressor exposure (p<0.001) and a return to baseline levels after 5 days of exposure illustrating a tendency for habituation (figure 2A). In the HPC, one-way ANOVA analysis found an effect on IL-6 [F(4,36) = 4.93, p<0.01], and post-hoc analysis in revealed that IL-6 was elevated after exposure to one day of restraint+swim (p<0.01) and remained elevated after 5 days of exposure (p<0.01). Swim alone did not elevate IL-6, though after 5 days a trend towards significance was observed (p=.052, figure 2F). In the PFC TNF-α expression was suppressed in response to stress [F(4,34) = 5.76, p<0.01], and post-hoc analysis found that expression was reduced after exposure to one day of forced swim (p<0.05) but not after 5 days, though there was a trend towards reduced expression (p=.079). TNF-α expression was also reduced in response to both 1 and 5 days of restraint+swim (p’s<0.001). Exposure to 5 days of restraint+swim suppressed expression of TNF-α more than either 1 or 5 days of swim alone (p’s<0.05; figure 2K). No other significant effects of stress condition were observed on other cytokines.
Cellular Activation
In all three structures, stress manipulations significantly increased c-Fos expression (PVN [F(4,33)=0.6.08, p<0.001]; HPC [F(4,36) = 27.40, p<0.0001]; PFC [F(4,33)=43.59, p<0.0001]; figure 2D, 2H, 2L). In the PVN, c-Fos expression was elevated in all conditions (p’s<0.05), while in the HPC and PFC c-Fos expression was elevated after 1 day of swim (p<0.0001) and 1 day of restraint+swim (p<0.0001), and in both cases the restraint + swim group’s expression level was higher than that of the swim only group (p<0.05). After 5 days of repeated exposure habituation was observed in both groups in both structures (p’s<0.0001), but expression was still elevated relative to home cage controls (p’s<0.01). An effect of stressor exposure on CD14 was found in the PFC [F(4,34) = 4.81, p<0.01] (see table 3) with expression being elevated only in the repeated swim group. An effect was seen on GFAP expression in the HPC [F(4,36) = 2.95, p<0.05] with GFAP being elevated after one day of restraint+swim and returning to baseline after 5 days of exposure. CD200 and CD200R were unaffected by stress condition in all 3 structures examined.
Table 3.
Real time RTPCR and Corticosterone results from Experiment 2
| Homecage | Homecage-Swim (1 day) | Homecage-Swim (5 days) | Restraint-Swim (1 day) | Restraint-Swim (5 days) | Statistical analyses | |
|---|---|---|---|---|---|---|
| PVN | ||||||
| β-actin | 99.8 ± 17.4 | 101.4 ± 25.1 | 93.3 ± 22.3 | 124.0 ± 19.9 | 120.8 ± 23.9 | F(4,35) = 0.38, p > 0.05 |
| TNF-α | 112.3 ± 19.8 | 118.7 ± 23.1 | 64.6 ± 33.3 | 124.3 ± 34.3 | 67.6 ± 15.9 | F(4,34) = 1.57, p > 0.05 |
| IL-1β | 114.2 ± 18.3a | 118.1 ± 18.0a | 205.1 ± 19.0b | 230.7 ± 41.9b | 162.3 ± 22.3ab | F(4,34) = 4.53, p < 0.01 |
| IL-6 | 100.4 ± 40.0 | 94.9 ± 18.9 | 76.3 ± 16.4 | 152.6 ± 61.4 | 102.5 ± 33.0 | F(4,31) = 0.80, p > 0.05 |
| c-Fos | 118.3 ± 27.3a | 557.8 ± 92.9bc | 388.3 ± 60.0b | 675.4 ± 68.3c | 470.5 ± 117.1bc | F(4,32) = 7.18, p < 0.001 |
| CD14 | 119.5 ± 23.4 | 132.0 ± 42.0 | 113.6 ± 15.4 | 171.1 ± 76.9 | 110.1 ± 21.6 | F(4,32) = 0.33, p > 0.05 |
| CD200 | 142.8 ± 42.4 | 139.9 ± 38.7 | 62.5 ± 32.1 | 152.7 ± 41.3 | 147.1 ± 38.2 | F(4,34) = 0.86, p > 0.05 |
| CD200R | 115.1 ± 21.9 | 114.7 ± 19.2 | 89.3 ± 14.4 | 178.3 ± 71.2 | 90.2 ± 18.1 | F(4,35) = 0.31, p > 0.05 |
| GFAP | 117.1 ± 21.5 | 88.1 ± 14.2 | 87.3 ± 13.5 | 86.4 ± 14.3 | 83.5 ± 15.5 | F(4,34) = 0.76, p > 0.05 |
| HPC | ||||||
| β-actin | 102.1 ± 7.2 | 93.9 ± 16.2 | 89.1 ± 9.8 | 85.9 ± 7.9 | 101.4 ± 7.7 | F(4,36) = 0.51, p > 0.05 |
| TNF-α | 102.4 ± 7.6 | 149.3 ± 34.0 | 123.3 ± 9.6 | 119.8 ± 13.9 | 103.9 ± 9.9 | F(4,35) = 1.12, p > 0.05 |
| IL-1β | 105.1 ± 11.9 | 97.0 ± 12.9 | 126.0 ± 19.9 | 122.2 ± 11.8 | 74.9 ± 7.7 | F(4,36) = 2.33, p > 0.05 |
| IL-6 | 101.7 ± 6.7a | 115.8 ± 21.0a | 179.2 ± 20.9ab | 219.3 ± 38.8b | 240.1 ± 27.4b | F(4,36) = 4.93, p < 0.01 |
| c-Fos | 103.9 ± 9.9a | 605.6 ± 74.2b | 277.8 ± 14.5c | 816.6 ± 100.3d | 270.1 ± 17.9c | F(4,36) = 27.40, p < 0.001 |
| CD14 | 103.6 ± 9.5 | 146.8 ± 40.0 | 137.0 ± 20.8 | 151.2 ± 22.4 | 118.4 ± 9.7 | F(4,36) = 0.75, p > 0.05 |
| CD200 | 101.6 ± 6.2 | 93.5 ± 9.5 | 99.8 ± 9.6 | 111.3 ± 9.1 | 100.6 ± 9.5 | F(4,36) = 0.51, p > 0.05 |
| CD200R | 101.3 ± 6.2 | 113.6 ± 9.5 | 86.1 ± 9.6 | 120.2 ± 9.1 | 88.9 ± 9.5 | F(4,35) = 1.48, p > 0.05 |
| GFAP | 102.6 ± 8.4a | 89.9 ± 8.3a | 86.3 ± 8.5a | 133.1 ± 18.9b | 96.5 ± 4.6a | F(4,36) = 2.95, p < 0.05 |
| PFC | ||||||
| β-actin | 134.4 ± 22.0 | 159.8 ± 27.2 | 76.7 ± 18.9 | 149.7 ± 15.6 | 127.6 ± 13.6 | F(4,35) = 1.99, p > 0.05 |
| TNF-α | 108.2 ± 14.9a | 80.3 ± 8.8b | 81.8 ± 11.1ab | 56.9 ± 6.1bc | 46.8 ± 4.7c | F(4,34) = 5.76, p < 0.01 |
| IL-1β | 113.8 ± 22.2 | 110.6 ± 16.1 | 126.1 ± 16.7 | 118.1 ± 19.9 | 83.9 ± 11.4 | F(4,33) = 0.64, p > 0.05 |
| IL-6 | 108.4 ± 16.0 | 152.1 ± 20.7 | 189.0 ± 50.5 | 222.8 ± 40.5 | 221.5 ± 35.3 | F(4,33) = 2.04, p > 0.05 |
| c-Fos | 105.2 ± 11.5a | 1347.9 ± 142.4b | 576.9 ± 65.9c | 1697.9 ± 157.8d | 614.3 ± 90.4c | F(4,33) = 43.59, p < 0.001 |
| CD14 | 102.3 ± 7.8a | 106.4 ± 13.9a | 160.9 ± 23.0b | 85.2 ± 7.0a | 80.2 ± 8.1a | F(4,34) = 4.81, p < 0.01 |
| CD200 | 106.0 ± 9.9 | 125.9 ± 12.6 | 101.9 ± 16.9 | 122.7 ± 8.5 | 121.1 ± 10.6 | F(4,33) = 0.84, p > 0.05 |
| CD200R | 106.7 ± 12.7 | 110.8 ± 17.2 | 173.6 ± 60.4 | 106.4 ± 10.0 | 94.3 ± 60.4 | F(4,35) = 1.32, p > 0.05 |
| GFAP | 104.2 ± 9.7 | 115.1 ± 23.7 | 131.6 ± 34.5 | 88.5 ± 13.4 | 130.0 ± 18.2 | F(4,33) = 0.87, p > 0.05 |
| Corticosterone (μg/dl) | ||||||
| CORT | 1.18 ± 0.47a | 42.25 ± 8.68b | 49.53 ± 8.02b | 42.79 ± 7.38b | 48.29 ± 6.16b | F(4,35) = 62.45, p < 0.001 |
Note. Means and SEM for Experiment 2. Relative gene expression data in the paraventricular nucleus of the hypothalamus (PVN), prefrontal cortex (PFC), and hippocampus (HPC) were adjusted to β-actin as a housekeeper and expressed as percent change from the control group (homecage). Corticosterone data is in μg/dl. Bold text indicates a significant effect; data points marked with differing letters are significantly different from one another (p < 0.05).
3.2b Corticosterone
A one-way ANOVA revealed a significant effect of stress condition [F(4, 35) = 62.45, p<0.001] with increased CORT levels in all stressed groups (p’s<0.0001). Results are displayed in table 3.
3.3 Experiment 3 Results
Body Weights
Body weights were measured prior to stress onset on day 6. Groups that received the same treatment across the initial 5 days of the experiment were collapsed into home cage and restraint groups and body weight analysis was conducted using an independent T-test. As expected, 5 days of restraint led to a reduction in weight gain, with restrained rats (x̄ = 23.08, SEM = 0.72) having gained less weight than homecage rats (x̄ = 25.88, SEM = 0.75); t(38) = 2.60, p<0.05.
Corticosterone
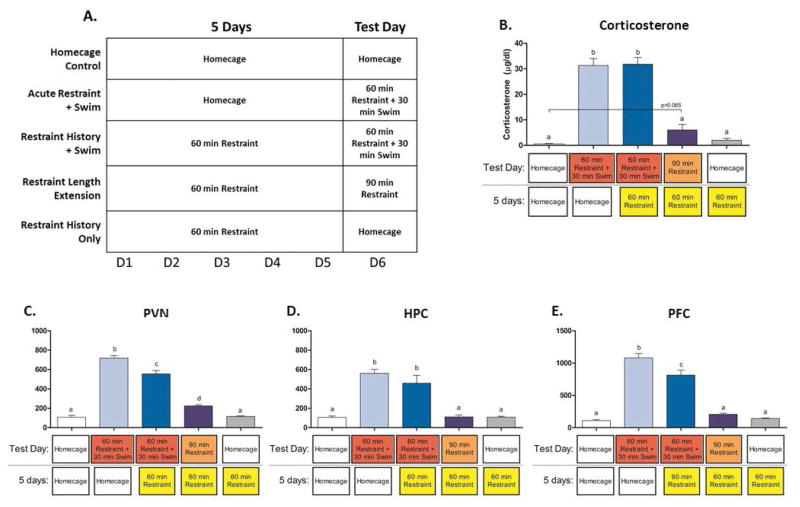
One-way ANOVA analysis revealed a significant effect of stress condition [F(4, 35) = 61.87, p<0.001]. Post-hoc testing revealed that both the acute restraint + swim (p<0.001) and the swim violation (p<0.001) groups were elevated compared to controls, and a trend for an increased CORT response was seen in the restraint duration violation group (p=0.065; see figure 3b).
Figure 3.
Design and results from Experiment 3. A. Rats were exposed to 5 days of repeated daily exposure to 60 minutes of restraint with a subsequent change in stressor (30 minutes of forced swim or an additional 30 minutes of restraint) on day 6, acutely exposed to 60 minutes of restraint followed immediately by 30 minutes of forced swim, received 5 days of restraint with no stress on day 6, or were non-stressed controls. B. Plasma corticosterone levels were assessed in each of the above groups (n=8). Different letters across groups denote significant differences. Real-time RTPCR data from the PVN (C), HPC (D), and PFC (E) expressed relative to the control group (homecage) and normalized to β-actin. Different letters across groups denote significant differences.
3.3a Gene Expression
Housekeeper
As in Experiment 2, β-actin expression was analyzed and no differences were found (PVN [F(4,35)=0.14, p>0.05]; HPC [F(4,35)=0.14, p>0.05]; PFC [F(4,35)=0.62, p>0.05]), thus all subsequent target genes were expressed relative to β-actin.
Cytokines
In the PVN-enriched punches, an effect of stress on IL-1β was found [F(4, 34) = 5.40, p<0.01], with acute restraint+swim being elevated as compared to all other groups (p’s<0.05). An effect of IL-1β was also seen in the HPC [F(4, 35) = 2.88, p<0.05]. In the PFC, IL-1β was found to be elevated [F(4,35) = 6.02, p<0.001], with the restraint history+swim group showing significant higher expression relative to the homecage (p<0.01), restraint history only (p<0.01), and restraint length extension groups (p<0.01). PFC IL-1β was also elevated in the acute restraint+swim group as compared to homecage controls (p<0.01) and restraint history only (p<0.05). IL-6 in the HPC was also found to be elevated [F(4, 35) = 4.14, p<0.01], with the acute restraint+swim and restraint history+swim groups showing greater expression relative to homecage controls (p’s<0.01) and the restraint history only group (p’s<0.05). Finally, TNF-α expression was lower in the acute restraint+swim (p<0.01), swim violation (p<0.01), and restraint length extension (p<0.01) groups relative to homecage controls [F(4,34) = 3.85, p<0.05].
Cellular Activation
When the influence of stress conditions on c-fos expression was examined, significant effects of stress on c-Fos expression were seen in the PVN [F(4, 35) = 155.01, p<0.001], HPC [F(4, 35) = 26.86, p<0.0001], and PFC [F(4, 35) = 87.49, p<0.0001]. Post-hoc analyses revealed that in all structures, both groups that experienced forced swim showed elevations relative to homecage controls, the restraint history only group, and the restraint length extension group (p’s <0.0001). The restraint history+swim group showed a lesser induction of c-Fos in both the PVN (p<0.0001) and PFC (p<0.001) relative to the acute restraint+swim group. The restraint length extension group had elevated c-Fos relative to controls only in the PVN (p<0.001).
4.0 Discussion
These experiments utilizing repeated sequential exposure to restraint and forced swim were performed in order to (a) characterize how the CORT response adapted over the course of repeated exposures, (b) to determine how rapid sequential exposure to restraint followed by swim modifies the course of habituation, and (c) to characterize neuroimmune and cellular activation marker responses to repeated sequential stressor exposure. Understanding how stressors interact to modify the impact of the stress response is a critical step in understanding the interplay of multiple stressors as experienced in daily life, and these studies are among the first towards determining how neuroimmune responses adapt across the experience of chronic stress. Importantly, there is growing interest in the impact of sequential exposure to stress challenges, such as in the single prolonged stress (SPS) model of post-traumatic stress disorder, in which rats are exposed to a restraint, forced swim and ether exposure (26–28).
Consistent with previous studies, daily exposure to restraint (60 min each day) led to significant habituation of the CORT response after 5 days and persisted through day 10 (Figure 1E). In contrast, no evidence of CORT habituation to forced swim (30 min daily) was observed after 5 days of in either experiment 1 or 2, and in experiment 1 it took 10 days of repeated swim exposure for evidence of habituation to emerge. Although some prior studies have concluded that repeated daily exposure to forced swim did not produce signs of CORT habituation, those studies did not include 10 consecutive days of swim exposure (20, 38). In a similar vein, Dal-Zotto et al. (39) demonstrated that 14 days of forced swim led to a more rapid recovery of CORT during the post-stress recovery period, though peak levels did not differ. One tentative conclusion that can be drawn here is that a greater number of daily stress sessions may be necessary for habituation to develop for stress challenges that elicit a stronger initial response as compared to more mild stressors. Indeed, in experiment 1 the peak CORT response to the initial forced swim exposure (Figure 1F) was nearly double the peak response evoked by the initial restraint challenge (Figure 1E), and the outcome was that it took twice as long (10 days instead of 5) to achieve significant habituation of the CORT response.
The primary goal of Experiment 1 was to test two complementary hypotheses regarding adaptation to repeated daily exposure to 2 stress challenges imposed in sequence. First, we predicted that daily restraint would serve as a cue that ultimately predicted the onset of forced swim, a more intense and challenging threat than passive restraint. If this were the case, the restraint+swim group’s plasma CORT concentrations should have exceeded those observed on Day 1 at the 60 min time point. In contrast, we observed that repeated exposure to restraint+swim induced habituation of the CORT response at the intermediate post-restraint time point (60 min; see Figure 1H) comparable to that which is typically observed in response to restraint alone (31, 35). Thus, we must conclude that preceding forced swim with restraint for as much as 10 consecutive days (trials) does not elicit an anticipatory CORT response. Secondly, we predicted that restraint preceding swim (Figure 1H) would disrupt habituation relative to rats that experienced swim alone (Figure 1F), which was supported as the magnitude of CORT habituation on day 10 was reduced. Overall, while certain aspects of habituation (to restraint) remained intact in this sequential stress procedure, other aspects of habituation (to the forced swim component) appeared to be diminished. A better understanding of intra- and inter-stressor variations in stress habituation processes could ultimately reveal a better understanding of the intersection between psychological aspects of stressful experiences and the development of stress pathophysiology.
Given that CORT habituation after restraint was not disrupted by the subsequent swim stressor in either Experiment 1 or Experiment 2, observed changes in other targets of interest were likely to be independent of CORT adaptation. In both the HPC and PFC, sequential stressor exposure resulted in elevated c-Fos expression levels after 1 day of exposure as compared to swim alone, but that effect was no longer present at 5 days (Figure 2). A similar but non-significant trend was observed in the PVN. It makes sense that the higher-order structures showed greater attenuation of activity after repeated exposure as they are involved in the more psychological aspects of stress (including novelty), whereas the PVN consolidates inputs from these structures and those that regulate the more physiological aspects of stress such as brainstem sympathetic nuclei, whose inputs are less likely to habituate (4). Few changes were observed in other cellular activation markers, such as GFAP and CD200, signifying that acute and repeated exposures to these stressors probably exert minimal influences on microglial and astrocytic activation markers.
A major goal of experiment 2 was to determine whether neuroimmune adaptations in response to both forced swim and sequential restraint+swim were occurring by day 5. Forced swim has been fairly well-characterized as a stressor, with studies having examined HPA reactivity, behavior (40), and physiological responses (39), but the IL-1β response to forced swim is not as well-understood. Our lab previously examined whether 1 or 2 days of forced swim can induce central IL-1β expression, and no differences in protein content were observed in whole hypothalamus, hippocampus, or posterior cortex (20). However, subsequent assessments demonstrated modest increases in IL-1β gene expression in the PVN after acute swim challenge (22) which was not replicated in the present studies. Though the reasons for these outcomes remain unclear, it seems that acute forced swim exposure exerts only modest effects on IL-1β expression in Sprague Dawley rats. This is consistent with the current study in which we observed no change in prefrontal or hippocampal IL-1β mRNA expression after acute or repeated forced swim exposure. However, five days of forced swim led to a two-fold increase in IL-1β mRNA expression in the PVN. It is not presently clear why acute forced swim has not consistently induced IL-1β mRNA expression; it may be that repeated exposure is required to induce a reliable IL-1β response. In contrast to the swim only groups, the sequential restraint+swim groups showed the opposite pattern, with acute exposure resulting in a significant increase in PVN IL-1β expression and habituation after 5 days of repeated exposure. Perhaps unsurprisingly, exposure to restraint+swim for 1 day elicited a larger increase in IL-1β expression in the PVN compared to 1 day of forced swim only. As restraint on its own is known to reliably induce a modest increase in IL-1β expression in the PVN (22, 41), it was expected that adding the swim stressor after restraint would induce greater response. Regarding the reduced IL-1β response after 5 days of restraint+swim exposure, we saw in Experiment 1 that the IL-1β response to restraint was habituated in the restraint+swim with repeated exposure. If that is the case, it could partially explain the observed habituation with the sequential stressor. Alternatively, sequential exposure may be more than the sum of its parts and result in a response and course of habituation unique to that of either component in isolation. There is also the question of how lingering effects of restraint could impact subsequent cytokine changes after exposure to the sequential stressors, as there was no group that experienced restraint followed by 30 minutes in the homecage. Previous studies using detailed footshock timecourses have shown that the pro-inflammatory cytokine responses typically return to baseline after 60 minutes (42, 43), and with restraint the CORT response peaks at 30 minutes and then begins to return to baseline (44). Thus, we would expect that the observed effects upon exposure to the sequential stressor are due to a culmination of the restraint and swim challenges, and not sustained cytokine changes or effects of CORT following restraint. However, the present data should be evaluated with this minor limitation in mind.
Although the present studies did not address the mechanism by which CORT and neuroimmune gene expression changes might display differential expression of habituation, one intriguing possibility might be differential adaptations in adrenergic receptor expression. For instance, NE-dependent activation of CRH-expressing neurons within the PVN is known to occur through α1-adrenergic receptors (45), whereas central IL-1β expression has been shown to occur via beta-adrenergic receptor activation (12, 16, 46). In this way, changes in adrenergic receptor isotypes throughout the course of stress challenges could account for the differences in habituation observed in the CORT response and in pro-inflammatory cytokines. Further studies will be necessary to elaborate the mechanisms underlying these changes.
Experiment 3 examined how a history of restraint would modify the responses to the combined restraint+swim stressor. As expected, CORT was increased in rats that experienced swim, and 5 days of restraint history did not affect the CORT response post-swim. This may be in contrast to previous findings where experience of a stressor on day 1 (immobilization) followed by a different stressor on day 2 (tailshock) led to a hyper-responsive CORT response(47). However, in the present study multiple stressors occurred on the same day, and multiple days of testing allowed for the possibility of habituation. The CORT response in the restraint extension was not statistically different from controls despite the extra 30 minutes of restraint, likely indicating that habituation to the stressor remained intact. It is also possible that the CORT response was already resolved at this time, as a previous study found that the CORT response was resolving between one and two hours of restraint (19). Thus a more thorough timecourse would be necessary to definitely address this question.
Across brain structures, c-Fos expression was higher in the acute restraint+swim group versus the restraint history+swim group, indicating that the previous experience of repeated restraint had an attenuating effect on the general response to the sequential stressor on day 6. This may in part be due to the restraint portion of the experience on test day no longer being novel which could result in a habituated c-Fos response (48). Interestingly, the restraint length extension group displayed an increase in c-Fos only in the PVN, which may be reflective of habituated inhibitory inputs from the PFC and HPC as the overall experience was not novel, while inputs from brainstem nuclei remained activated due to the restraint stressor. Regardless, the changes in response to restraint length extension on all other measures were either non-existent or very small in comparison to the restraint history+swim group. Similarly to experiment 2, few changes in activation markers other than c-Fos were observed, suggesting that glial contributions to the observed adaptations are minor.
Once again, the most interesting findings were seen in IL-1β expression, with a replication of the robust increase in the PVN in response to acute restraint + swim that was observed in Experiment 2. In addition, prior experience with 5 days of repeated restraint attenuated that response despite no reduction of the CORT response. This attenuation looks similar to that seen in Experiment 2 when restraint + swim was repeated for 5 days even though in this case the rats experienced swim for the first time. It may be that in the restraint history+swim group, the prior restraint experience resulted in the activation of compensatory mechanisms that dampened the IL-1β response to swim, or it may be that a habituation of the IL-1β response can generalize across stressors whereas the CORT response cannot. Although significant differences in IL-1β expression were observed in the HPC, these effects were of such small magnitude that any physiological consequence is unlikely. In the PFC, two-fold increases were observed in both groups that experienced forced swim with no attenuation due to prior restraint exposure, in contrast to the effect seen in the PVN. These findings imply that the IL-1β habituation seen in the PVN is not driven by parallel IL-1β adaptations in the HPC or PFC.
The studies presented here utilized gene expression as an index of neuroimmune responses to stress, which raises certain issues that require discussion. First, real time RT-PCR offers the advantages of being a cost-effective, rapid approach toward understanding changes in numerous genes utilizing minute quantities of tissue. However, the significant outcomes here should also be examined at the protein level, though it should be noted that tissue content of cytokines in the CNS is generally at or near the floor of sensitivity for most biochemical assays. Another question is the cellular origin of the cytokine mRNA, particularly since few changes in glial activation markers were observed. Though we would predict that the effects would be observed specifically in microglia based on the preponderance of evidence with other stress challenges (16), we cannot rule out the possibility that other cell types may be contributing to the changes observed here. Finally, our experiments were run only in males, and future studies should extend these results to females as well. It can be noted, however, that females display a robust increase in IL-1β expression after footshock, an effect that varies significantly in response to ovarian hormones (49). Though CORT habituation to restraint has been shown to occur similarly in males and females (50), habituation to other stressors, such as the presently-used forced swim, has yet to be examined and may proceed differently between the sexes.
Overall, these experiments fill a gap in our knowledge of how the stress response adapts with repeated exposure to stressors experienced in sequential rapid succession and violations of expectancy. We found that CORT habituation occurs similarly whether or not stressors are sequential, though the magnitude or length of onset of habituation to the last-experienced stressor may be impacted. Stressor expectancy violation does not appear to sensitize the CORT response, and to our surprise even resulted in an attenuation of the IL-1β response in the PVN. The other cytokines examined, IL-6 and TNF-α, did not display habituation over the course of multiple days of exposure in any of our paradigms. It remains to be seen how cytokine expression may change over even longer periods of stressor exposure and how the response to a future intense stress challenge may be modified by compensatory mechanisms already in progress. It is clear that different aspects of the stress response are differentially impacted by repeated stressor exposure, and this preliminary look into major cytokine and cellular activity marker expression opens the door for further exploration of the complex interplay of real-world stressors and the stress response.
Table 4.
Real-time RTPCR results from Experiment 3
| Homecage | Restraint Duration Extension | Acute Restraint + Swim | Restraint History + Swim | Restraint History Only | Statistical analyses | |
|---|---|---|---|---|---|---|
| PVN | ||||||
| β-actin | 109.9 ± 14.6 | 106.8 ± 15.6 | 108.4 ± 12.0 | 111.3 ± 7.9 | 118.6 ± 8.25 | F(4,35) = 0.14, p > 0.05 |
| TNF-α | 103.5 ± 10.0 | 95.9 ± 9.7 | 76.0 ± 5.4 | 71.5 ± 8.6 | 87.6 ± 7.6 | F(4,33) = 2.39, p > 0.05 |
| IL-1β | 110.2 ± 18.7ab | 165.7 ± 49.8ab | 325.6 ± 67.8c | 194.4 ± 37.7b | 71.7 ± 7.0a | F(4,34) = 5.40, p < 0.01 |
| IL-6 | 101.6 ± 6.2 | 134.1 ± 27.0 | 127.3 ± 19.2 | 146.0 ± 24.1 | 100.7 ± 12.0 | F(4,34) = 1.16, p > 0.05 |
| c-Fos | 108.27 ± 18.1a | 224.2 ± 154.9d | 718.0 ± 26.5b | 555.0 ± 35.1c | 116.3 ± 6.7a | F(4,35) = 155.0, p < 0.001 |
| CD14 | 107.4 ± 16.6 | 114.3 ± 22.3 | 107.4 ± 16.6 | 105.6 ± 10.4 | 89.4 ± 5.4 | F(4,35) = 0.36, p > 0.05 |
| CD200 | 103.3 ± 8.7 | 103.4 ± 12.2 | 117.7 ± 7.7 | 118.0 ± 6.2 | 110.9 ± 5.6 | F(4,35) = 0.74, p > 0.05 |
| CD200R | 110.4 ± 16.6 | 95.4 ± 12.8 | 76.7 ± 11.7 | 87.4 ± 8.1 | 116.4 ± 10.1 | F(4,35) = 1.77, p > 0.05 |
| HPC | ||||||
| β-actin | 104.9 ± 10.5 | 111.9 ± 5.7 | 105.2 ± 7.6 | 109.2 ± 7.6 | 106.3 ± 7.8 | F(4,35) = 0.14, p > 0.05 |
| TNF-α | 103.1 ± 9.3 | 82.2 ± 6.8 | 97.9 ± 6.8 | 91.5 ± 11.2 | 92.5 ± 36.2 | F(4,35) = 0.65, p > 0.05 |
| IL-1β | 102.1 ± 8.2abc | 97.6 ± 4.4ac | 117.7 ± 16.4ab | 131.3 ± 12.5b | 86.7 ± 5.0c | F(4,35) = 2.88, p < 0.05 |
| IL-6 | 108.7 ± 17.6a | 148.4 ± 17.2ab | 195.8 ± 18.3b | 184.1 ± 23.9b | 125.1 ± 12.8a | F(4,35) = 4.14, p < 0.01 |
| c-Fos | 106.0 ± 14.4a | 111.4 ± 19.8a | 560.2 ± 42.3b | 458.6 ± 82.1b | 109.0 ± 8.69a | F(4,35) = 26.86, p < 0.001 |
| CD14 | 105.8 ± 14.7 | 116.9 ± 11.9 | 124.9 ± 10.5 | 128.8 ± 12.2 | 126.7 ± 13.4 | F(4,35) = 0.56, p > 0.05 |
| CD200 | 101.4 ± 6.6 | 132.2 ± 8.3 | 109.4 ± 5.9 | 115.7 ± 8.4 | 115.0 ± 6.6 | F(4,35) = 2.45, p > 0.05 |
| CD200R | 105.9 ± 15.5 | 107.4 ± 17.7 | 89.9 ± 9.1 | 79.2 ± 6.3 | 104.7 ± 11.2 | F(4,35) = 1.01, p > 0.05 |
| PFC | ||||||
| β-actin | 101.7 ± 7.3 | 89.2 ± 6.3 | 80.0 ± 12.9 | 91.2 ± 11.5 | 93.9 ± 8.8 | F(4,34) = 0.66, p > 0.05 |
| TNF-α | 109.9 ± 18.3a | 56.7 ± 7.9b | 65.0 ± 7.7b | 55.2 ± 6.9b | 77.4 ± 13.3ab | F(4,34) = 3.85, p < 0.05 |
| IL-1β | 110.2 ± 19.3a | 138.7 ± 9.0ac | 182.7 ± 11.2bc | 212.4 ± 22.1b | 131.1 ± 19.2a | F(4,35) = 6.02, p < 0.001 |
| IL-6 | 113.9 ± 19.0 | 117.6 ± 17.1 | 139.1 ± 14.1 | 130.4 ± 25.8 | 112.8 ± 21.2 | F(4,34) = 0.31, p > 0.05 |
| c-Fos | 109.1 ± 17.7a | 206.6 ± 18.8a | 1082.4 ± 67.2b | 814.8 ± 78.6c | 141.4 ± 9.9a | F(4,35) = 87.49, p < 0.001 |
| CD14 | 109.3 ± 16.5 | 85.6 ± 8.9 | 93.1 ± 9.2 | 86.9 ± 12.1 | 85.2 ± 8.2 | F(4,35) = 0.80, p > 0.05 |
| CD200 | 102.9 ± 8.8 | 87.8 ± 8.9 | 82.9 ± 12.2 | 82.8 ± 11.4 | 91.0 ± 8.7 | F(4,35) = 0.67, p > 0.05 |
| CD200R | 105.4 ± 11.7 | 108.7 ± 19.6 | 117.8 ± 12.8 | 78.9 ± 10.9 | 118.0 ± 15.0 | F(4,35) = 1.25, p > 0.05 |
Note. Means and SEM for Experiment 3. Gene expression data in the paraventricular nucleus of the hypothalamus (PVN), prefrontal cortex (PFC), and hippocampus (HPC) were adjusted to β-actin as a housekeeper and expressed as percent change from the control group (homecage). Bold text indicates a significant effect; data points marked with differing letters are significantly different from one another (p < 0.05).
Acknowledgments
Research reported in this publication was supported by the National Institute of Health Grants P50AA017823 and RO1AG043467 to T. Deak, and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
References
- 1.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui D-H, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Martí O, Martí J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 4.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 5.Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrin. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent S, Rodriguez F, Kelley KW, Dantzer R. Reduction in food and water intake induced by microinjection of interleukin-1β in the ventromedial hypothalamus of the rat. Physiol Behav. 1994;56:1031–1036. doi: 10.1016/0031-9384(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 8.Bluthé R-M, Dantzer R, Kelley KW. Central mediation of the effects of interleukin-1 on social exploration and body weight in mice. Psychoneuroendocrino. 1997;22:1–11. doi: 10.1016/s0306-4530(96)00042-x. [DOI] [PubMed] [Google Scholar]
- 9.Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong M-L, Chrousos GP, Webster E. The impact of the nonpeptide corticotropin-Releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress 1. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- 10.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. BioMed Res Int. 2010 doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Rohan Walker F, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohleb ES, Hanke ML, Corona AW, Powell ND, La’Tonia MS, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki E, Shintani F, Kanba S, Asai M, Nakaki T. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cell Mol Neurobiol. 1997;17:557–562. doi: 10.1023/A:1026319107528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bulletin. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Deak T, Bellamy C, D’Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003;972:53–63. doi: 10.1016/s0006-8993(03)02485-5. [DOI] [PubMed] [Google Scholar]
- 21.Plata-Salamán CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, Merali Z, Anisman H. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1β system, tumor necrosis factor-α, transforming growth factor-β1, and neuropeptide mRNAs in specific brain regions. Brain Res Bulletin. 2000;51:187–193. doi: 10.1016/s0361-9230(99)00204-x. [DOI] [PubMed] [Google Scholar]
- 22.Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol Behav. 2011;104:187–198. doi: 10.1016/j.physbeh.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Berkenbosch F, Van Oers J, Del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 24.Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry. 2014;19:811–822. doi: 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopp BL, Wick D, Herman JP. Differential effects of homotypic vs. heterotypic chronic stress regimens on microglial activation in the prefrontal cortex. Physiol Behav. 2013;122:246–252. doi: 10.1016/j.physbeh.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 27.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrino. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 28.Liberzon I, Lopez J, Flagel S, Vazquez D, Young E. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999 doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitman DL, Natelson B, Ottenweller J, McCarty R, Pritzel T, Tapp W. Effects of exposure to stressors of varying predictability on adrenal function in rats. Behav Neurosci. 1995;109:767. doi: 10.1037//0735-7044.109.4.767. [DOI] [PubMed] [Google Scholar]
- 31.Barnum C, Blandino P, Deak T. Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J Neuroendocrinol. 2007;19:632–642. doi: 10.1111/j.1365-2826.2007.01571.x. [DOI] [PubMed] [Google Scholar]
- 32.Spencer RL, Deak T. A users guide to HPA axis research. Physiol Behav. 2016 doi: 10.1016/j.physbeh.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck HM, Hueston CM, Bishop C, Deak T. Enhancement of the hypothalamic–pituitary–adrenal axis but not cytokine responses to stress challenges imposed during withdrawal from acute alcohol exposure in Sprague–Dawley rats. Psychopharmacology. 2011;218:203–215. doi: 10.1007/s00213-011-2388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC((T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- 36.Kearns RR, Spencer RL. An unexpected increase in restraint duration alters the expression of stress response habituation. Physiol Behav. 2013;122:193–200. doi: 10.1016/j.physbeh.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dellaert F. The expectation maximization algorithm. Georgia Institute of Technology; 2002. [Google Scholar]
- 38.Wotjak C, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 39.Dal-Zotto S, Martí O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav Brain Res. 2000;114:175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 40.Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–134. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 41.Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 1995;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hueston CM, Buck HM, Deak T. The inflamed axis: The interaction between cytokines and HPA hormones in the stress response. Physiol Behav. 2014;124:77–91. doi: 10.1016/j.physbeh.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Blandino P, Jr, Hueston CM, Barnum CJ, Bishop C, Deak T. The impact of ventral noradrenergic bundle lesions on increased IL-1 in the PVN and hormonal responses to stress in male sprague dawley rats. Endocrinology. 2013;154:2489–2500. doi: 10.1210/en.2013-1075. [DOI] [PubMed] [Google Scholar]
- 44.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plotsky P. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121:924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- 46.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 47.Orr TE, Meyerhoff JL, Mougey EH, Bunnell BN. Hyperresponsiveness of the rat neuroendocrine system due to repeated exposure to stress. Psychoneuroendocrino. 1990;15:317–328. doi: 10.1016/0306-4530(90)90057-g. [DOI] [PubMed] [Google Scholar]
- 48.Struthers WM, DuPriest A, Runyan J. Habituation reduces novelty-induced FOS expression in the striatum and cingulate cortex. Exp Brain Res. 2005;167:136–140. doi: 10.1007/s00221-005-0061-7. [DOI] [PubMed] [Google Scholar]
- 49.Arakawa K, Arakawa H, Hueston CM, Deak T. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology. 2014;100:162–177. doi: 10.1159/000368606. [DOI] [PubMed] [Google Scholar]
- 50.Babb JA, Masini CV, Day HE, Campeau S. Habituation of hypothalamic–pituitary–adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress. 2014;17:224–234. doi: 10.3109/10253890.2014.905534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanic N, Perovic M, Mladenovic A, Ruzdijic S, Kanazir S. Effects of aging, dietary restriction and glucocorticoid treatment on housekeeping gene expression in rat cortex and hippocampus—Evaluation by real time RT-PCR. J Mol Neurosci. 2007;32:38–46. doi: 10.1007/s12031-007-0006-7. [DOI] [PubMed] [Google Scholar]
- 52.Cullinan W, Herman J, Battaglia D, Akil H, Watson S. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 53.Shizuya K, Komori T, Fujiwara R, Miyahara S, Ohmori M, Nomura J. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sci. 1997;61:PL135–PL140. doi: 10.1016/s0024-3205(97)00608-5. [DOI] [PubMed] [Google Scholar]
- 54.Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. The increase in TNF-α levels is implicated in NF-κB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 55.Tanga F, Raghavendra V, DeLeo J. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand–receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]