Abstract
Polyketide biosynthetic pathways have been engineered to generate natural product analogs for over two decades. However, manipulation of modular type I polyketide synthases (PKSs) to make unnatural metabolites commonly results in attenuated yields or entirely inactive pathways, and the mechanistic basis for compromised production is rarely elucidated since rate limiting or inactive domain(s) remain unidentified. Accordingly, we synthesized and assayed a series of modified pikromycin (Pik) pentaketides that mimic early pathway engineering to probe the substrate tolerance of the PikAIII-TE module in vitro. Truncated pentaketides were processed with varying efficiencies to corresponding macrolactones, while pentaketides with epimerized chiral centers were poorly processed by PikAIII-TE and failed to generate 12-membered ring products. Isolation and identification of extended but prematurely offloaded shunt products suggested that the Pik thioesterase (TE) domain has limited substrate flexibility and functions as a gatekeeper in the processing of unnatural substrates. Synthesis of an analogous hexaketide with an epimerized nucleophilic hydroxyl group allowed for direct evaluation of the substrate stereoselectivity of the excised TE domain. The epimerized hexaketide failed to undergo cyclization and was exclusively hydrolyzed, confirming the TE domain as a key catalytic bottle-neck. In an accompanying paper, we engineer the standalone Pik thioesterase (TE) to yield a thioesterase (TES148C) and module (PikAIII-TES148C) that display gain-of-function processing of substrates with inverted hydroxyl groups.
Graphical Abstract
INTRODUCTION
Polyketides comprise a diverse class of secondary metabolites produced primarily by microorganisms, many of which possess medicinal value. Numerous therapeutically relevant polyketides, including macrolides, are produced biosynthetically by modular type I polyketide synthases (PKSs)1. These megasynthases function as molecular assembly lines whereby the construction of structurally and functionally complex natural products is accomplished through sequential Claisen condensations of simple acyl-ACP building blocks2. Biosynthetic elongation of a growing polyketide chain occurs by a series of catalytic domains that are organized into distinct modules. In turn, each module is responsible for the incorporation of a single monomeric extender unit into the growing polyketide chain with various options for reductive processing at the β-keto position prior to transfer of the intermediate to the downstream module. Each round of chain elongation requires a minimum of three catalytic domains, an acyltransferase (AT) that selects for an acyl-coenzyme A extender unit to prime the acyl carrier protein (ACP) and a ketosynthase (KS) that accepts a growing chain from the ACP of the previous module and catalyzes decarboxylative Claisen condensation to extend the polyketide2. Modules may also contain combinations of ketore-ductase (KR), dehydratase (DH), and enoyl reductase (ER) domains that tailor the β-keto group to a hydroxyl (KR), alkene (DH), or alkane (ER), respectively. Finally, the terminal module typically contains a thioesterase (TE) domain that is responsible for offloading the fully mature intermediate, often as a cyclized product3.
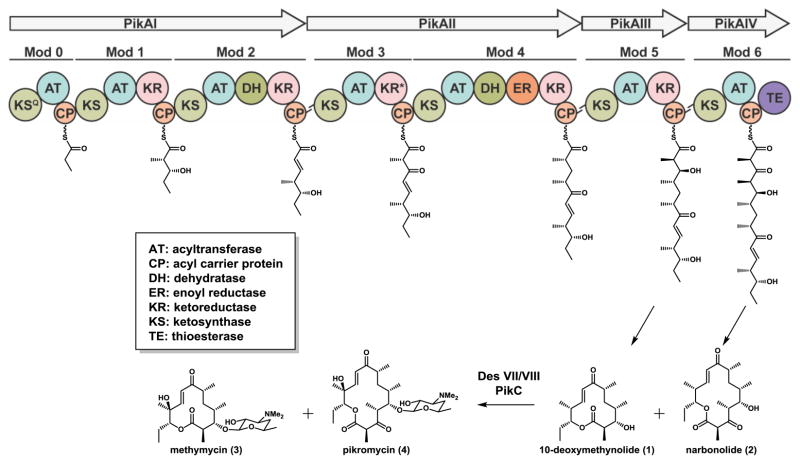
The pikromycin (Pik) biosynthetic pathway (Figure 1) is unique among modular type I PKS in its ability to generate two macrocyclic polyketides, the 12-membered ring aglycone, 10-deoxymethynolide (10-dml, 1) and 14-membered ring aglycone, narbonolide (2)4. Both macrolactones are further diversified through the action of post-assembly line tailoring enzymes to yield a group of five macrolide natural products5. This inherent substrate flexibility of the Pik pathway has made it an attractive target for engineering of both the modules responsible for assembling the polyketide core6 as well as the tailoring enzymes that introduce additional functionality to the polyketide backbone7. Despite promising bioactivities inherent to many polyketide compounds, development of a natural product into a clinically relevant therapeutic is often hindered by suboptimal pharmacological properties, necessitating optimization through structure activity relationship (SAR) studies. However, high structural complexity often requires lengthy semi-synthesis routes that are constrained by accessible functionality and reactivity. An alternative strategy for expanding chemical diversity in a natural product scaffold involves biosynthetic pathway engineering, whereby novel natural product analogs are generated by manipulation of the enzymes responsible for their biosynthesis8. The strategies for genetic engineering of modular PKSs have been the subject of many excellent reviews5,9–12 and include: altering the selection of starter and extender units, modifying the extent of β-keto reductions, and substitution of entire modules or subunits to increase or decrease chain lengths. Unfortunately, these bioengineering attempts often result in attenuated yields or fail to produce new molecules entirely8,13–15.
Figure 1.
The pikromycin (Pik) biosynthetic pathway. The macrolactones 10-dml (1) and narbonolide (2) generated by the Pik PKS undergo further tailoring to the macrolides methymycin (3) and pikromycin (4), respectively. KSQ, KS-like domain; KR*, inactive KR.
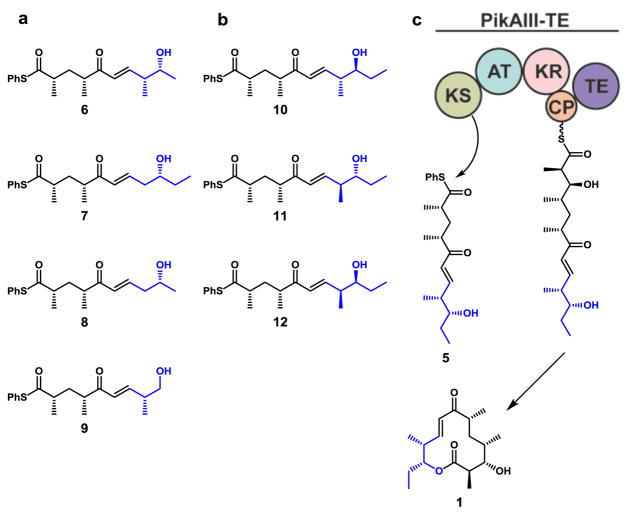
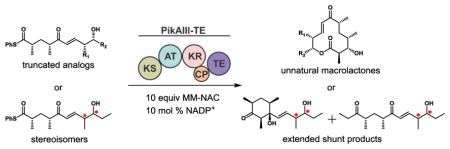
The biochemical basis for low levels or failed production of metabolites from engineered PKSs remains elusive. Typically, genetic modifications have been performed in early pathway PKS modules13,14,16–19, and it has remained unclear whether attenuated product formation stems from the engineered module or the downstream modules (or both) that must accept and process the resulting unnatural intermediates. The lack of detailed mechanistic information is a consequence of engineering pathways in vivo, and highlights the need for more targeted investigations to probe the catalytic details of individual domains. Towards a more general framework for rational engineering of efficient PKSs, this study interrogates the substrate flexibility of a downstream hybrid module, PikAIII-TE20–22, to assess the biochemical function of individual domain(s) as part of the larger module. Accordingly, two classes of pentaketides were envisioned (Figure 2): 1) truncations 6–9 designed to mimic intermediates that would arise from early pathway AT domain engineering to assess the downstream tolerance of substrate chain length and aliphatic regions, and 2) diastereomers 10–12 that imitate intermediates arising from early KR modifications to query the previously observed strict stereo-selectivity of reductive processing domains towards distal stereo-centers.23–25
Figure 2.
Unnatural pentaketides designed to probe flexibility of PikAIII-TE. Pentaketide probes to query (a) the chain length and methyl-branching dependence and (b) stereo-tolerance of PikAIII-TE. (c) Full-module processing of the native pentaketide 5.
With seven unique pentaketide analogs in hand (Figure 2), we employed an in vitro strategy for probing PikAIII-TE. Through detection of minor shunt products, we sought to identify points of catalytic failure and inefficiencies within the PKS module.
RESULTS
Probing of PikAIII-TE with unnatural pentaketides
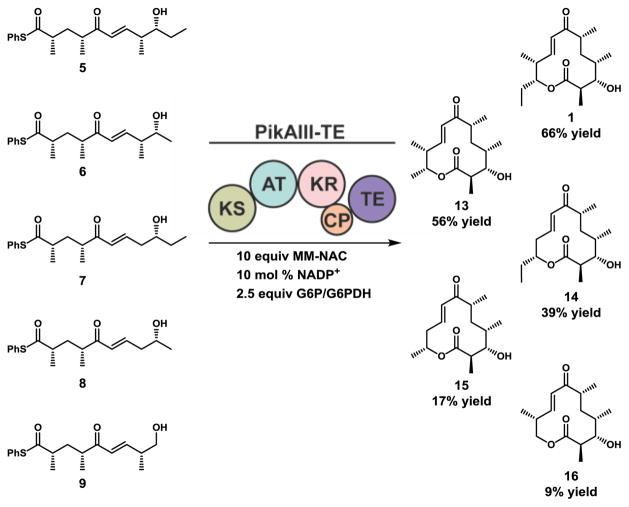
To interrogate the flexibility of the enzymes involved in the late-stage chain extension, processing, and macrolactone ring formation of unnatural linear polyketide intermediates, we synthesized (see Supporting Information for details) a panel of pentaketides using a previously described approach10. Targeted substrates were furnished as thiophenol thioesters as opposed to traditionally employed NAC thioesters. We have previously shown thiophenol thioesters promote full-module catalysis, presumably by favoring KS acylation over TE acylation26. We then assayed the well-characterized hybrid module PikAIII-TE10,21,22 in vitro for the ability to generate analogs of the natural 12-membered macrolac-tone 10-deoxymethynolide (1). To improve cost and scalability, NADPH was recycled using a glucose-6-phosphate/glucose-6-phosphate dehydrogenase system, and methylmalonate (MM) was delivered as MM-NAC as opposed to the native MM-CoA27.
PikAIII-TE displayed moderate flexibility for truncated substrates (6–9, Scheme 1) and was able to process C-10 desmethyl 6 with near wild type efficiency (56% yield), and C-8 desmethyl 7 with a 39% yield. However, C-8,10 didesmethyl 8 was processed less effectively (17% yield), and C-9 desethyl 9 afforded poor conversion to the expected product (9% yield). The significant decrease in product yields from the native substrate 5 to unnatural substrates 8 and 9 is notable as these terminal truncations are distal from the site of the Claisen condensation (KS) and subsequent β-keto reduction (KR) during the chain elongation process, suggesting that the thioesterase domain could be responsible for compromised catalytic efficiency with the unnatural substrates. Under these reaction conditions the substrate was completely consumed, with hydrolysis of starting material and decomposition13,14,16 accounting for the remaining mass balance (vide infra).
Scheme 1.
Evaluation of PikAIII-TE with a panel of truncated pentaketides 6–9. Enzymatic reaction conditions: 4 mM Pik pentaketide, 40 mM (10 equiv) MM-NAC, 20 mM (5 equiv) 2-vinylpyridine, 0.4 mM (10 mol %) NADP+, 10 mM (2.5 equiv) glucose-6-phosphate, glucose-6-phosphate dehydrogenase (2 units/mL), 4 μM (0.1 mol %) cell free PikAIII-TE, 8 h, stationary, RT.
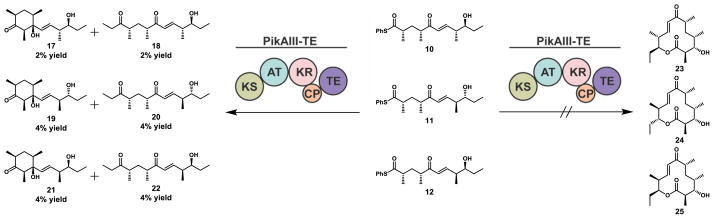
PikAIII-TE failed to yield detectable macrocyclic products with any of the series 10–12 designed to probe the PKS selectivity toward substrates bearing unnatural C-8 and C-9 stereocenters (Scheme 2). In initial experiments, preparatory HPLC of the crude reaction mixture and 1H NMR analysis of all fractions yielded unidentifiable decomposition products along with trace quantities of extended products that were off-loaded as linear shunt products. Scaled-up reactions provided adequate quantities of 17–22 for structural elucidation.
Scheme 2.
Reaction of PikAIII-TE with a panel of stereoisomer pentaketides 10–12. Enzymatic reaction conditions: 4 mM Pik pentaketide, 40 mM (10 equiv) MM-NAC, 20 mM (5 equiv) 2-vinylpyridine, 0.4 mM (10 mol %) NADP+, 10 mM (2.5 equiv) glucose-6-phosphate, glucose-6-phosphate dehydrogenase (2 units/mL), 4 μM (0.1 mol %) cell free PikAIII-TE, 8 h, stationary, RT.
Synthesis of Pik hexaketides
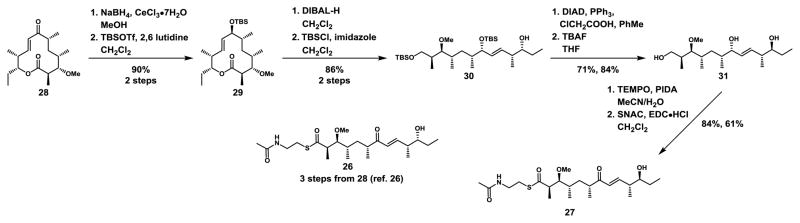
Macrolactonization of natural product analogs are notoriously difficult to perform synthetically28 and formation of the macrocycle is a key feature of many bioactive polyketide and nonribosomal peptide natural products.29 Thus, we were intrigued by the prospect that the TE domain is serving as a gatekeeper domain in engineered PKS pathways. To provide further insight into these observations we synthesized the analogous native 26 and C-11-epimerized 27 methyl protected hexaketides for use as direct probes of the TE domain. Targeted heaxaketides were furnished as traditionally employed NAC thioesters, which we have previously shown26 to promote TE acylation relative to aryl thioesters. 26 was prepared as described previously26 and 27 was synthesized in nine linear steps from 10-dml (28). Luche reduction and TBS protection of the resulting secondary hydroxyl group yielded 29. DIBAL-H reduction and TBS protection of the primary alcohol provided 30, and Mitsunobu inversion of the homoallylic hydroxyl group with chloroacetic acid followed by TBAF deprotection yielded 31. TBAF deprotection proved advantageous, as acidic deprotection methods such as HF and trifluoroacetic acid led to rapid decomposition from either chloroacetate ester or from the free homo-allylic alcohol starting material, respectively. TEMPO/PIDA oxidized the allylic hydroxyl group to an α,β-unsaturated ketone and the primary alcohol to a carboxylic acid without oxidizing the homo-allylic alcohol. Subsequent thioesterification provided NAC thioester 27 (Scheme 3).
Scheme 3.
Synthesis of native 26 and epimerized 27 methyl protected hexaketides starting from 28.
Evaluation of Pik TE with native and epi-hexaketides
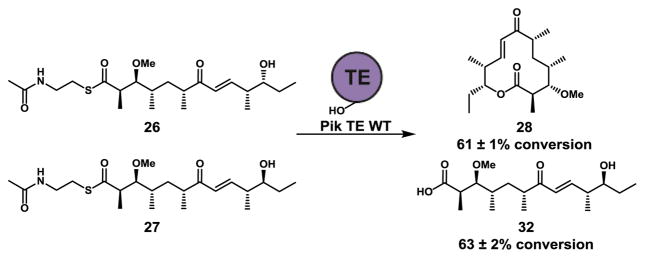
With C-3 methyl-ether hexaketides 26 and 27 in hand, we next evaluated the ability of Pik TE to catalyze the macrolactonization of 26 and 27. Consistent with our previous report26, Pik TE is able to effectively generate methyl protected 10-dml 28 from the N-acetylcysteamine thioester of Pik methyl hexaketide 26. However, reactions containing the epimerized 27 resulted in complete hydrolysis to 32, and failed to produce any macrocylic product (Scheme 4). The exclusive hydrolysis of epimerized 27 confirms the TE domain as the catalytic bottleneck in the processing of diastereomeric pentaketides 10–12.
Scheme 4.
Evaluation of Pik TE with methyl protected hexaketides 26 and 27. Enzymatic reaction conditions: sodium phosphate buffer (400 mM, pH 7.2), 1 mM hexaketide, 8 mM 2-vinylpyridine, purified Pik TE (10 μM), 4 h, stationary, RT. Conversion of 26 to 28 and 27 to 32 was monitored (HPLC) with data represented as the mean ± standard deviation where n = 3.
DISCUSSION
Engineering of Modular PKSs
Direct fermentation of bacterial modular PKS derived polyketide analogs has been pursued by many research groups.8,12,30 Three prominent examples were reported by Kosan Biosciences,13–15 highlighting the ability to generate libraries of polyketide analogs through modular PKS engineering. In these studies, AT and KR domain substitutions were engineered into modules 2, 5, and 6 of the 6-deoxyerythronolide B (DEBS) biosynthetic pathway and the engineered PKSs were expressed in heterologous Streptomyces or E. coli hosts to generate a suite of 6-dEB analogs. While these results provided a glimpse of the macrolactones that were accessible through modular type I PKS combinatorial biosynthesis, they also served to highlight the serious limitations associated with this approach as the yields of the 6-dEB analogs dropped precipitously (highest yield 3.5% relative to 6-dEB production)13
Mutasynthesis has provided further insight into type I PKS substrate flexibility and engineering. Briefly, this strategy chemically complements early pathway knockouts in native or heterologous producers as a means to generate natural product analogs31,32. DEBS mutasynthesis strategies leveraging KS1 knockouts or module 1 truncations have been pursued with a range of synthetic diketide analogs. In this approach, synthetic diketides activated as N-acetylcysteamine thioesters must diffuse across the cell membrane of either the native producer or a heterologous host and acylate a targeted KS domain. Thus, the targeted module must not only accept and process these analogs, but all subsequent modules must tolerate the growing, unnatural polyketide chain elongation intermediate in order to generate a 6-dEB analog. The results from these experiments have shown that the downstream modules of the DEBS pathway display a modest level of substrate flexibility for a multitude of alterations at the 12- and 13-position of the 6-dEB macrolactone16–18,33,34. Significantly, attempts to incorporate starter units containing (S)-configured β-hydroxyl groups failed to result in epimerized macrolactones19.
Direct comparison between analogs in mutasynthesis studies is hindered by variations in the solubility, cellular uptake, and stability of the synthetic starter units in the fermentation broth and cytosol. Accordingly, we pursued a targeted substrate-centric strategy to probe PikAIII (Pik module 5) in vitro to identify the catalytically ineffective domain(s) for processing of unnatural substrates. This biochemical approach with readily controlled variables led to facile isolation and identification of prematurely off-loaded intermediates that failed to be cyclized by the Pik TE domain. As the shunt metabolites 17–22 were recovered in low quantities from the in vitro reactions, isolation of the same compounds from the comparably more complex in vivo mutasynthesis experiments would have presented an even greater challenge, likely precluding the mechanistic insights obtained from this in vitro approach.
TE bottleneck
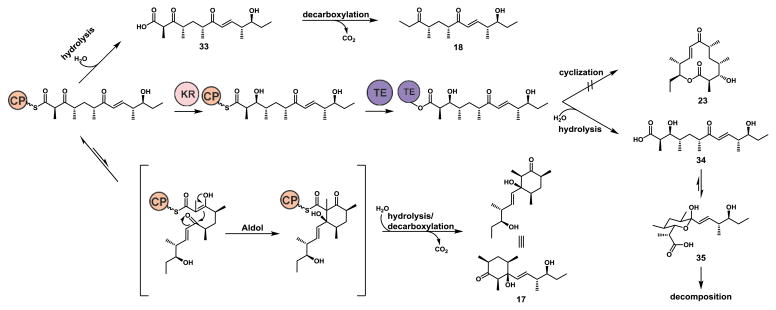
As the only change from the natural Pik pen-taketide intermediate to substrate 10 is epimerization of the C-9 hydroxyl group that serves as the nucleophile during macrolactonization, we reasoned, a priori, that the substrate would detrimentally affect the TE domain responsible for ring formation. TE stereoselectivity has been observed previously from in vitro reactions of DEBS TE with a series of unnatural DEBS heptaketide substrate mimics. The DEBS TE displayed strict stereoselectivity for the natural (R)-configuration of the substrate nucleophilic alcohol and exclusively hydrolyzed the unnatural (S)-stereoisomer35. NMR and MS characterization of products from the panel of stereoisomeric derivatives of Pik pentaketides showed that the products had been extended; therefore the KS domain was at least partially competent with analogs 10–12. Additionally, as the Pik TE is able to cyclize β-keto analogs of the natural hexaketide to yield 3-keto-10-dml21,36 we expected to observe 3-keto macrolactones if the KR was compromised23–25, but KS and TE domains remained functional. We hypothesized that 17–22 resulted from substrate stalling and premature offloading from the PKS via hydrolysis and subsequent decarboxylation (Figure 3). Analysis of these extended but linear off-loaded intermediates suggests that the TE is acting as the dominant catalytic bottleneck to the formation of macrocylic products. The inability to attain a total mass balance for the stereoisomer substrate panel and the low recovery of products 17–22 from each reaction is attributed to the additional facile degradative pathway possible through the TE domain. PKS TE domains serve as flexible hydrolases when cyclization is impaired37,38. Thus, we propose that a portion of the pentaketides were extended and off-loaded as the unstable β-hydroxy hexaketide 34, which subsequently degrades through intramolecular hemiketalization and dehydration pathways14,26 (Figure 3). Indeed, synthesis of the corresponding C-11-epimerized 27 hexaketide and subsequent probing of the Pik TE domain provides direct evidence for the strict stereoselectivity of Pik TE. This stereoselectivity confirms the suspected TE bottleneck for macrolactone formation and explains the loss of macrolactone formation in PikAIII-TE reactions containing the diasteromeric pentaketides 10–12.
Figure 3.
Proposed shunt pathways when macrocyclization is compromised. Release mechanisms of unnatural Pik chain elongation intermediates from PikAIII-TE and the subsequent degradative pathways when TE catalyzed cyclization is impaired.
CONCLUSION
Use of Pik pentaketide and hexaketide analogs in this study has enabled the identification of the TE domain as a key catalytic bottleneck in the processing of unnatural substrates. These results add to the recent studies performed on the KR and DH domains of modules from the pikromycin and tylosin (Tyl) pathways which uncovered a surprising level of stereochemical discrimination inherent in the β-keto and β-hydroxyl processing domains23–25. Probing a KR and DH didomain from Tyl module 3 with tetraketide intermediates identified strict stereochemical requirements for the distal methyl and hydroxyl stereocenters23, with epimerizations at these distal stereocenters having dramatic consequences on the processing of the proximal β-hydroxyl groups. Taken together, the available in vitro results utilizing full-length substrates have shown that an unnatural substrate must overcome multiple points of strict substrate specificity in order to be processed efficiently to yield a final offloaded polyketide analog. Additionally, shunt products 17–22 resemble the known natural product pacificanone A, a byproduct of the rosamicin A PKS, suggesting that similar premature offloading occurs in wild-type pathways39,40.
The results reported within this study serve to highlight the critical importance of maintaining a terminal TE domain that is catalytically competent for processing and off-loading the fully elongated linear intermediate as the final macrolactone analog. Indeed, in an accompanying manuscript41 we report the engineering of the Pik TE domain which results in the ability to catalyze the macrolactonization of an unnatural epimerized substrate. This engineered TE domain alleviates the catalytic bottleneck identified in this study and generates a PKS module capable of processing the C-9-epimerized 10 to the corresponding epimeric macrolactone 23.
Future application of the synthetic pentaketide probes utilized in this report in conjunction with cryo-EM, and computational studies should provide further insights into the structural and mechanistic parameters that govern elongation and processing by allowing visualization of the PKS machinery and unnatural intermediates during the catalytic cycle.
Supplementary Material
Acknowledgments
This research was supported by Rackham Merit Predoctoral Fellowships (D.A.H. and A.A.K.), American Foundation for Pharmaceutical Education Predoctoral Fellowship (D.A.H.), and NIH T32-CA009676 (A.A.K.). NIH grants GM076477, R35 GM118101, and the Hans W. Vahlteich Professorship (D.H.S.) are gratefully acknowledged.
Footnotes
Notes
The authors declare no competing financial interest.
Full experimental details and the spectroscopic data is available free of charge via the Internet at http://pubs.acs.org.”
References
- 1.Newman DJ, Cragg GM. J Nat Prod. 2016;79:629. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 2.Hertweck C. Angew Chem Int Ed. 2009;48:4688. doi: 10.1002/anie.200806121. [DOI] [PubMed] [Google Scholar]
- 3.Du L, Lou L. Nat Prod Rep. 2010;27:255. doi: 10.1039/b912037h. [DOI] [PubMed] [Google Scholar]
- 4.Xue Y, Zhao L, Liu H-w, Sherman DH. Proc Natl Acad Sci U S A. 1998;95:12111. doi: 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kittendorf JD, Sherman DH. Bioorg Med Chem. 2009;17:2137. doi: 10.1016/j.bmc.2008.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen DA, Rath CM, Eisman EB, Narayan AR, Kittendorf JD, Mortison JD, Yoon YJ, Sherman DH. J Am Chem Soc. 2013;135:11232. doi: 10.1021/ja404134f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan AR, Jimenez-Oses G, Liu P, Negretti S, Zhao W, Gilbert MM, Ramabhadran RO, Yang YF, Furan LR, Li Z, Podust LM, Montgomery J, Houk KN, Sherman DH. Nat Chem. 2015;7:653. doi: 10.1038/nchem.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissman KJ, Leadlay PF. Nat Rev Microbiol. 2005;3:925. doi: 10.1038/nrmicro1287. [DOI] [PubMed] [Google Scholar]
- 9.Kittendorf JD, Sherman DH. Curr Opin Biotechnol. 2006;17:597. doi: 10.1016/j.copbio.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Reeves CD. Crit Rev Biotechnol. 2003;23:95. doi: 10.1080/713609311. [DOI] [PubMed] [Google Scholar]
- 11.Wong FT, Khosla C. Curr Opin Chem Biol. 2012;16:117. doi: 10.1016/j.cbpa.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman KJ. Nat Prod Rep. 2016;33:203. doi: 10.1039/c5np00109a. [DOI] [PubMed] [Google Scholar]
- 13.McDaniel R, Thamchaipenet A, Gustafsson C, Fu H, Betlach M, Betlach M, Ashley G. Proc Natl Acad Sci U S A. 1999;96:1846. doi: 10.1073/pnas.96.5.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue Q, Ashley G, Hutchinson CR, Santi DV. Proc Natl Acad Sci U S A. 1999;96:11740. doi: 10.1073/pnas.96.21.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menzella HG, Reid R, Carney JR, Chandran SS, Reisinger SJ, Patel KG, Hopwood DA, Santi DV. Nat Biotechnol. 2005;23:1171. doi: 10.1038/nbt1128. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen JR, Keatinge-Clay AT, Cane DE, Khosla C. Bioorg Med Chem. 1998;6:1171. doi: 10.1016/s0968-0896(98)00081-9. [DOI] [PubMed] [Google Scholar]
- 17.Leaf T, Cadapan L, Carreras C, Regentin R, Ou S, Woo E, Ashley G, Licari P. Biotechnol Prog. 2000;16:553. doi: 10.1021/bp000063l. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita KG, Williard P, Khosla C, Cane DE. J Am Chem Soc. 2001;123:2495. doi: 10.1021/ja004139l. [DOI] [PubMed] [Google Scholar]
- 19.Harvey CJ, Puglisi JD, Pande VS, Cane DE, Khosla C. J Am Chem Soc. 2012;134:12259. doi: 10.1021/ja304682q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Y, Lu H, Khosla C, Cane DE. J Am Chem Soc. 2003;125:5671. doi: 10.1021/ja034574q. [DOI] [PubMed] [Google Scholar]
- 21.Aldrich CC, Beck BJ, Fecik RA, Sherman DH. J Am Chem Soc. 2005;127:8441. doi: 10.1021/ja042592h. [DOI] [PubMed] [Google Scholar]
- 22.Mortison JD, Kittendorf JD, Sherman DH. J Am Chem Soc. 2009;131:15784. doi: 10.1021/ja9060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiers WD, Dodge GJ, Li Y, Smith JL, Fecik RA, Aldrich CC. Chem Sci. 2015;6:5027. doi: 10.1039/c5sc01505g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Dodge GJ, Fiers WD, Fecik RA, Smith JL, Aldrich CC. J Am Chem Soc. 2015;137:7003. doi: 10.1021/jacs.5b02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Fiers WD, Bernard SM, Smith JL, Aldrich CC, Fecik RA. ACS Chem Biol. 2014;9:2914. doi: 10.1021/cb5006883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen DA, Koch AA, Sherman DH. J Am Chem Soc. 2015;137:3735. doi: 10.1021/ja511743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MM-CoA is exorbitantly expensive ($1280/25 mg, Sigma Aldrich, accessed 8/17). As such, a standard 0.2 millimole scale reaction using 2 equivalents of MM-CoA would cost in excess of $15,000. CoA recycling systems are problematic as free thiols react with pentaketide substrates via conjugate addition, requiring the use of a 2-vinylpyridine thiol scavenger. MM-NAC provides similar performance compared to MM-CoA when used in a 10–20 fold excess and can be made in two steps at decagram scale6.
- 28.Parenty A, Moreau X, Niel G, Campagne JM. Chem Rev. 2013;113:PR1. doi: 10.1021/cr300129n. [DOI] [PubMed] [Google Scholar]
- 29.Kopp F, Marahiel MA. Nat Prod Rep. 2007;24:735. doi: 10.1039/b613652b. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Liu Z, Zhao H, Ang EL. Drug Des Devel Ther. 2015;9:823. doi: 10.2147/DDDT.S63023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirschning A, Taft F, Knobloch T. Org Biomol Chem. 2007;5:3245. doi: 10.1039/b709549j. [DOI] [PubMed] [Google Scholar]
- 32.Weist S, Sussmuth RD. Appl Microbiol Biotechnol. 2005;68:141. doi: 10.1007/s00253-005-1891-8. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen JR, Hutchinson CR, Cane DE, Khosla C. Science. 1997;277:367. doi: 10.1126/science.277.5324.367. [DOI] [PubMed] [Google Scholar]
- 34.Marsden AFA, Wilkinson B, Cortés J, Dunster NJ, Staunton J, Leadlay PF. Science. 1998;279:199. doi: 10.1126/science.279.5348.199. [DOI] [PubMed] [Google Scholar]
- 35.Pinto A, Wang M, Horsman M, Boddy CN. Org Lett. 2012;14:2278. doi: 10.1021/ol300707j. [DOI] [PubMed] [Google Scholar]
- 36.Chemler JA, Tripathi A, Hansen DA, O’Neil-Johnson M, Williams RB, Starks C, Park SR, Sherman DH. J Am Chem Soc. 2015;137:10603. doi: 10.1021/jacs.5b04842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aldrich CC, Venkatraman L, Sherman DH, Fecik RA. J Am Chem Soc. 2005;127:8910. doi: 10.1021/ja0504340. [DOI] [PubMed] [Google Scholar]
- 38.Gokhale RS, Hunziker D, Cane DE, Khosla C. Chem Biol. 1999;6:117. doi: 10.1016/S1074-5521(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 39.Oh DC, Gontang EA, Kauffman CA, Jensen PR, Fenical W. J Nat Prod. 2008;71:570. doi: 10.1021/np0705155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awakawa T, Crusemann M, Munguia J, Ziemert N, Nizet V, Fenical W, Moore BS. Chembiochem. 2015;16:1443. doi: 10.1002/cbic.201500177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.In an accompanying manuscript, we report the engineering of the Pik TE domain for expanded substrate scope and improved reaction kinetics which results in the ability to catalyze the macrolactonization of an unnatural epimerized substrate.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.