Abstract
Mango fruit tocopherol levels vary in different varieties during ripening. This study shows that tocopherol accumulation is highly correlated with its p-hydroxyphenyl pyruvate dioxygenase (MiHPPD) gene expression during ripening. MiHPPD transcript is ethylene induced and differentially expressed in four mango varieties used in this study. Higher/lower accumulation of tocopherol (mainly α-tocopherol) was achieved by heterologous expression of MiHPPD in Arabidopsis and tomato. The results suggest that tocopherol accumulation in mango fruit is correlated to MiHPPD gene expression. Over-expression of MiHPPD gene channelizes the flux towards tocophreol biosynthesis and could be used as a potential tool for metabolic engineering.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0991-3) contains supplementary material, which is available to authorized users.
Keywords: Arabidopsis, HPPD, Mango, Tocopherol, Tomato, Transgenic
Introduction
Tocopherols are lipid soluble antioxidant molecules synthesized only by photosynthetic organisms via shikimate pathway. Tocopherols and tocotrienols are collectively known as vitamin E. Vitamin E function as antioxidants with the ability to scavenge free peroxyl radicals providing plants protection against photo-oxidative stress and lipid peroxidation (Serbinova et al. 1991; Traber and Atkinson 2008; Miret and Munné-Bosch 2015). Other than antioxidant properties, tocopherols have been shown to have possible role in seed longevity, seed germination and plant growth etc. (Sattler et al. 2004; Mène-Saffrané et al. 2010). Vitamin E is an essential component of human diet. α-tocopherol is considered to be the most nutritionally beneficial form of vitamin E due to its quick absorption and retention by the body (Kamal-Eldin and Appelqvist 1996). Vitamin E synthesis and accumulation varies in temporal and spatial manner. Tocopherol levels are also influenced by external and internal stimuli such as light, low temperature, salt and drought. (Munné-Bosch 2005; Maeda et al. 2008; Abbasi et al. 2007; Quadrana et al. 2013). Varying levels of vitamin E in different tissues and plant development is a result of tightly regulated biosynthesis process which is mainly controlled by expression of key metabolic genes of its pathway.
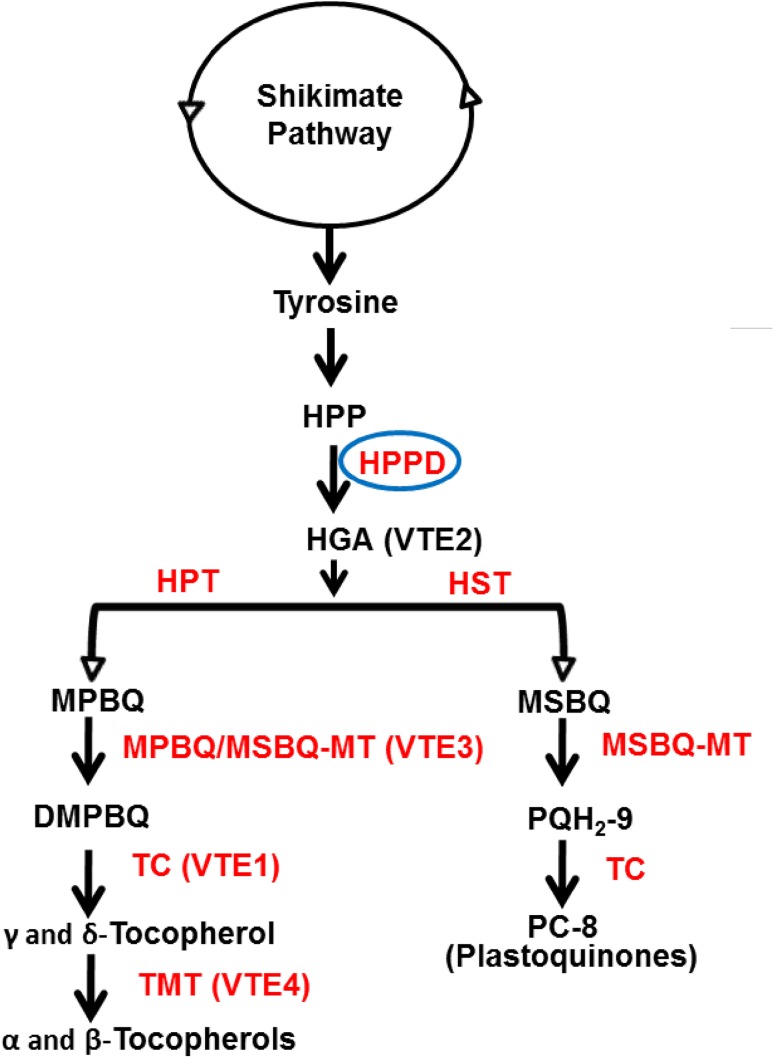
Tocopherol biosynthesis in plants takes place as shown in Fig. 1. Conversion of p-hydroxyphenylpyruvate (HPP) to homogentisic acid (HGA) by HPP dioxygenase is the first committed step in both tocopherol and plastoquinone synthesis in plants. HGA is the common precursor for both tocopherol and plastoquinone and is further catalyzed by methyltransferases and tocopherol cyclase to give desired products. Flux into tocopherol is predominantly controlled by HPPD and HPT (VTE2), while methyltransferases and tocopherol cyclase (also known as VTE1, 3 and 4) determine tocopherol composition and are not limiting (Tsegaye et al. 2002; Collakova and Dellapenna 2003a, b; DellaPenna and Pogson 2006; DellaPenna and Mène-Saffrané 2011).
Fig. 1.
A simplified tocopherol and plastoquinone pathway in plants. DMPBQ 2,3-dimethyl-5-phytyl-1,4-benzoquinone, HGA homogentisic acid, HPP p-hydroxyphenylpyruvate, MPBQ 2-methyl-6-phytyl-1,4-benzoquinone, MSBQ 2-methyl-6-solanyl-1,4-benzoquinone. Enzymes are indicated by red colour: HPP dioxygenase (HPPD); homogentisatephytyltransferase (HPT); homogentisatesolanyltransferase (HST); MPBQ/MSBQ methyltransferase; Tocopherol cyclase (TC); and Tocopherol methyltransferase (TMT). Enzymes are marked with red font while substrates and products in black
Vitamin E biosynthesis pathway is well studied in model plants Arabidopsis with few studies in other plants such as tobacco, tomato, lettuce and soybean. Edible plants (mainly fruits, vegetables and oil producing crops) are the major source of vitamin E in human nutrition and, therefore, understanding mechanism underlying vitamin E synthesis and accumulation in crop species is of great interest (Grusak and DellaPenna 1999; Fitzpatrick et al. 2012). Recently, more insights in mechanism controlling vitamin E levels in tomato have been emerged as tomato being a potential crop for value addition (Almeida et al. 2011, 2016). Despite being major source of vitamin E only compositional data of vitamin E (tocopherols) content is available for fresh fruits. Molecular mechanism underlying accumulation and the synthesis of tocopherols during fruit development and ripening is still under covered.
Mango (Mangifera indica L.) is one of the most important fruit in India with huge varietal differences in every aspect of fruit development, nutritional composition, and flavor (Srivastava et al. 2016). The fruit is an important source of several nutrients and health beneficial compounds and its main antioxidants nutrients are vitamin E, vitamin C, and carotenoids. In mango, β-carotene and α-tocopherol are the major components among the carotenes and tocopherols, respectively (Shah et al. 2010). Different varieties of mango fruits have different levels of carotene and tocopherols. Singh et al. (2011) identified a p-hydroxyphenyl pyruvate dioxygenase (MiHPPD) gene from mango that showed activation by ethylene and correlates with tocopherol levels in during ripening in Dashehari variety. The present study is conducted to check whether this correlation extends to other mango varieties having different ripening patterns. The varieties used in the current study differ in color, fruit firmness, geographical distribution and mainly flavor composition during ripening. Since mango transformation is difficult, functional characterization of MiHPPD was carried out in two heterologous systems Arabidopsis and tomato to check whether the gene functions in two different systems and manipulates tocopherol levels.
Materials and methods
Chemicals and reagents
All chemicals such as Methanol, Acetone, Petroleum ether, Glacial acetic acid, Acetonitrile and anhydrous sodium sulphate were of HPLC grade and procured from reputed Indian suppliers. β Carotene and α-tocopherol standards were purchased from Sigma-Aldrich. Molecular biology grade fine chemicals and kits were purchased from Sigma, MBI-Fermentas and Qiagen.
Plant material
Mature unripe green mangoes of four commercially important Indian varieties namely, Alphonso, Banganpalli, Dashehari and Kesar were harvested from R&D farms of Jain Irrigation Systems Ltd, Jalgaon, Maharashtra, India. Ripening was initiated by exogenous ethylene exposure (100 ppm) to the fruits in ripening chambers with controlled humidity for 24 h. After ethylene treatment fruits were allowed to ripen in air for 6 days. Samples were collected at D0 (-No treatment), D1 (24 h), D3 (72 h) and D6 (144 h) after ethylene exposure. Samples were frozen under liquid nitrogen and kept at −80 °C till further use.
β-Carotene and α-tocopherol separation and estimation by HPLC
10 g of mango pulp tissue was crushed in 25 ml of acetone using mortar and pestle. The homogenate was filtered through a filter paper by vacuum filtration. Solids retained on the filter paper were collected and washed with 25 ml of acetone until solids collected in the filter paper became colorless (This process was repeated 3–4 times with 25 ml of acetone). Petroleum ether (25 ml) was added to the acetone extract and mixture was mixed with vigorous shaking. Phase separation was done in separatory funnel adding 50 ml distilled water and 10 g sodium sulphate was also added to it. The ether extract was evaporated in a rotavapor (Medica make) at 42 °C. Residue after drying was dissolved in 5 ml of mobile phase (comprising of 70% acetonitrile, 30% methanol and 0.01% glacial acetic acid) and filtered through 0.2 micron membrane. 20 μl of prepared sample was injected onto the HPLC system (Waters HPLC with 2998 PDA detector, 515 pump, automatic injector 717 and Empower 2 system data processor). A C-18 reverse phase Novapak column from Waters with a dimension of 3.9 mm × 150 mm and particle size of 4.0 micron was used for tocopherol and carotenoid separation. Samples were separated with modifications in procedure described by Gomez-Coronado and Barbas (2003) with mobile phase comprising of 70% acetonitrile–30% methanol and 0.01% glacial acetic acid ratio and a flow rate of 1.5 ml/min. Sample peak and standard peaks were compared for retention time. Samples were detected at 295 nm and 450 nm for tocopherol and carotene, respectively. After peak area determination the amount was calculated using standards of α-tocopherol and β-carotene (Sigma).
RNA isolation and cDNA preparation for expression analysis by semi-quantitative PCR
RNA was isolated from mango fruit pulp using cetyltrimethyl ammonium bromide (CTAB) as described previously (Chourasia et al. 2008; Singh et al. 2011). Total RNA was treated with DNase I and first strand cDNA was prepared using the Revertaid MuMLV Reverse transcriptase (MBI Fermentas) and 3′adapter primer (3′AP-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT) according to the manufacturer’s instructions. Semi-quantitative PCR reactions were carried out using First strand cDNA and MiHPPD primers (Fwd-GTGTTGCTTCCGTTGAATGAGCCAGTG; Rev-GTGGATCCTCATACAACTGAGGCTGCTC). Miactin (Fwd-GTGGTCGTACAACTGGTATTGTGCT and Rev-TTGCATGTGGGAGTGCATAACCCT) was used as an internal control. For checking endogenous Arabidopsis HPPD expression in Arabidopsis transgenic lines AtHPPD (Fwd-CCGGAGAGATTAAACCGACA and Rev-GGAGAACAACATCGCCGTAT and Actin (Fwd-GAGAGTTTTGATGTCCCTGCCATG and Rev-CAACGTCGCATTTCATGATGGAGT) primers were used. Same Actin primers worked for both Arabidopsis and tomato. MiHPPD primers were specific to mango and did not amplify endogenous AtHPPD or SlHPPD.
Construction of MiHPPD over-expression and suppression cassette and genetic transformation of Arabidopsis and tomato
The full length coding region of MiHPPD (1604 bp, Acc no. HQ244994, Singh et al. 2011) was used to prepare the construct for developing over-expression (sense) and suppression (antisense) lines. MiHPPD CDS was inserted at BamHI site of the binary vector pBI121 under CaMV35S promoter. Positive clone selected through PCR was designated as pBIMiHPPD and used for subsequent transformation of Arabidopsis (Col0) and tomato (Variety Ailsa Craig) through Agro infection. The construct was introduced into Agrobacterium tumefaciens (GV3101) prior to plant transformation. Arabidopsis plants were transformed with recombinant plasmid constructs by floral dip method as described by Clough and Bent (1998). Tomato plants were transformed as protocol mentioned in Singh 2011(PhD thesis). Kanamycin was used as a selection marker. Five independent lines were used for quantification in tomato (T1 generation), while three independent homozygous lines of Arabidopsis for sense and antisense constructs were used for further studies. Tocopherol contents were estimated in wild type control and sense and antisense transgenic Arabidopsis leaves harboring MiHPPD gene using HPLC as mentioned earlier. In tomato, tocopherol content was estimated in fruits and leaves of transgenic as well as control plants. Pool of 2–3 fruits/leaves from 3 plants each was used for tocopherol measurement from both control and transgenic tomato plants.
Results
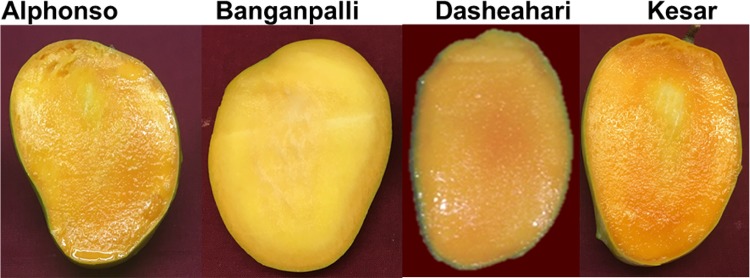
Mango pulp composition varies depending upon cultivar, location and ripening stage. Several genes and enzymes are expressed differentially to provide specific pulp composition to each variety. Mostly the molecular mechanism (genes and enzymes) producing a specific compound is conserved but it is regulated differentially by external (location, temperature, water, etc.) and internal factors (hormones, substrates, etc.). In the present study, we have used four different varieties of mango which are grown in different regions of India, and differ largely in shape, color (Fig. 2) and fruit pulp composition (Pandit et al. 2009). Alphonso and Kesar are primarily grown in western part of India (Maharashtra and Gujarat), Banganpalli in south (Andhra Pradesh) and Dashehari in northern part of India (Uttar Pradesh). These varieties were used to check tocopherol content and its regulation in relation to MiHPPD gene expression.
Fig. 2.
Different mango varieties at ripe stage
α-Tocopherol and β-carotenoid content of Mango pulp
α-Tocopherol is the major constituent of total tocopherol (α, β, γ and δ tocopherols in plants comprising of 90–95%, therefore, only α- tocopherol was quantified. Variable levels of α tocopherol levels were observed during ripening in different varieties. Relatively high tocopherol levels were present in unripe fruit of Alphonso as compared to other varieties. After 24 h of ethylene treatment, levels increased in all the varieties, however, there was a sharp increase in case of Alphonso where levels doubled from 8.32 ± 1.0 to 16.6 µgg−1 fw. Except for Dashehari, tocopherol levels reduced with the progression of ripening in other varieties. High levels of tocopherols (12.13 ± 0.65 µgg−1 fw) were found in D6 stage whereas in other varieties tocopherols were estimated to be in the range of 1.3–2.23 µgg−1 fw (Table 1).
Table 1.
α-Tocopherol and β-Carotenoid content in four mango varieties. 0–6 represents day after ethylene treatment
| Alphonso | Dashehari | Banganpalli | Kesar | |
|---|---|---|---|---|
| α-Tocopherol μgg−1 fw | ||||
| 0D | 8.32 ± 1.0 | 3.51 ± 0.55 | 4.58 ± 0.32 | 6.14 ± 0.33 |
| 1D | 16.6 ± 1.2 | 4.98 ± 0.76 | 6.17 ± 0.66 | 7.32 ± 0.87 |
| 3D | 9.28 ± 0.89 | 11.84 ± 0.67 | 2.98 ± 0.53 | 3.67 ± 0.56 |
| 6D | 2.23 ± 0.33 | 12.13 ± 0.65 | 1.56 ± 0.11 | 1.3 ± 0.22 |
| β-Carotene μgg−1 fw | ||||
| 0D | 0.18 ± 0.001 | 0.25 ± 0.002 | 0.018 ± 0.002 | 0.16 ± 0.001 |
| 1D | 4.73 ± 0.32 | 3.14 ± 0.65 | 0.22 ± 0.11 | 4.38 ± 0.22 |
| 3D | 9.1 ± 0.56 | 5.34 ± 0.33 | 1.39 ± 0.5 | 2.08 ± 0.45 |
| 6D | 20.79 ± 1.0 | 11.36 ± 1.23 | 6.98 ± 0.75 | 12.26 ± 0.89 |
β-Carotene levels were also quantified in four mango varieties. The levels increased in four varieties tested (Table 1) during progression of ripening and it correlated with the fruit pulp color change (Fig. 2). There was 10–20 folds increase in carotene content after 24 h of ethylene treatment in all the varieties. The carotenoid content was the highest at fully ripe stage in all the varieties with Alphonso having highest carotene content followed by Kesar and Dashehari, respectively. Low β-carotene content in Banganpalli (6.98 ± 0.75 µgg−1 fw as compared to 20.79 ± 1.00 µgg−1 fw in Alphonso at D6 stage) might be the reason of its light yellow pulp color compared to other varieties.
Correlation between MiHPPD gene expression and tocopherol levels during ripening
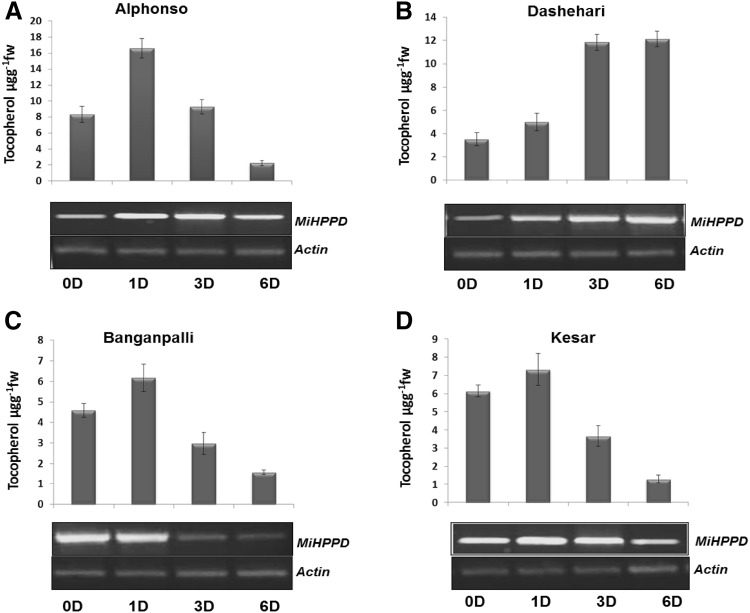
MiHPPD expression and its correlation with tocopherols levels have previously been shown by Singh et al. (2011) in Dashehari variety of mango. To verify whether this correlation extends to other important Indian varieties also, expression of MiHPPD gene was checked during the course of ripening in four different varieties of mango by semi-quantitative PCR. MiHPPD expression varied in different varieties during ripening period (D0–D6) but in each variety expression was induced by ethylene treatment (D1) (Fig. 3). MiHPPD expression gradually decreased in Alphonso, Kesar and Banganpalli (D1–D6) during ripening while kept on increasing in Dashehari mango and peaked at fully ripened stage (D6). Expression of MiHPPD correlated with tocopherol levels in all the varieties during ripening (Fig. 3). Banganpalli has maximum tocopherol levels and highest MiHPPD expression at unripe (D0) or early stage (D1) of ripening while Dashehari behaved vice versa.
Fig. 3.
Tocopherol levels and corresponding MiHPPD expression in 4 different varieties of mango a Alphonso, b Dashehari, c Banganpalli and d Kesar. Upper panel shows tocopherol levels while lower panel shows MiHPPD gene expression during ripening in different varieties (0D–6D, represents days after ethylene treatment). For tocopherol three independent extractions were analyzed from pulp obtained from pool of 4–5 mangoes. Data were analysed and expressed as mean ± standard deviation. Content was measured in μgg−1 fw
Over-expression of MiHPPD gene increases tocopherol content in Arabidopsis and tomato
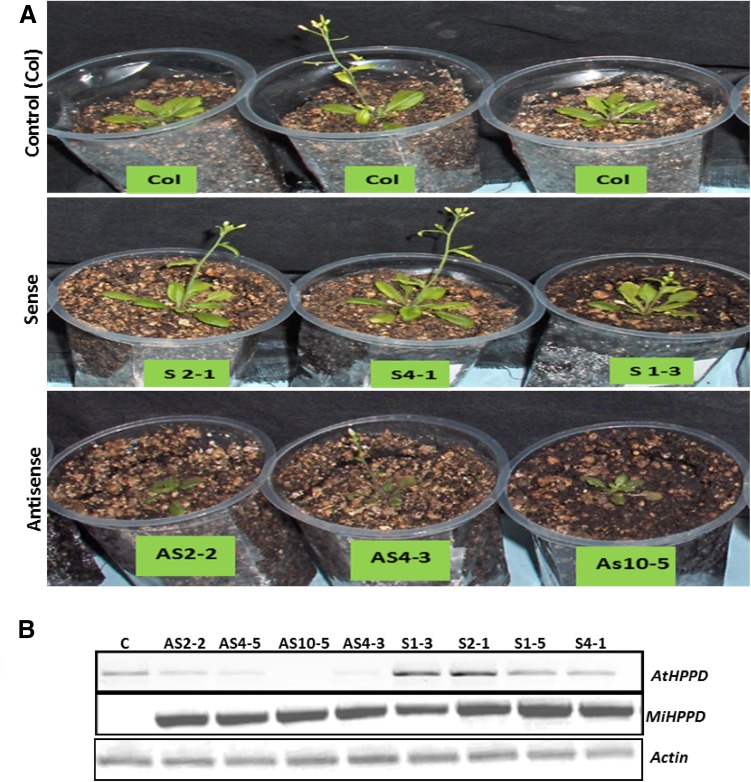
Positive correlation between MiHPPD gene expression and tocopherol levels in different varieties of mango suggested that MiHPPD transcript levels might influence flux and tocopherol accumulation in mango. To functionally characterize MiHPPD’s role, transgenic lines of Arabidopsis and tomato expressing MiHPPD under constitutive promoter were developed. MiHPPD antisense construct was also transformed in Arabidopsis to see its suppression effects. Semi-quantitative PCR carried out using cDNA prepared from transformed plants and MiHPPD primers confirmed that MiHPPD over-expression/suppression cassette introduced in Arabidopsis is expressing.
No significant phenotypic change was observed in MiHPPD over-expressing Arabidopsis plants compared to control. While, transgenic plants harboring MiHPPD in antisense orientation showed slow and retarded growth in comparison to control and sense plants during early stages of life cycle but regained normal growth at later stages (Fig. 4a). No other phenotypic difference was observed in normal growth conditions in these plants. Semi quantitative RT-PCR was carried out to assess the effect on AtHPPD expression due to the over-expression of MiHPPD in sense and antisense direction. Transgenic lines harboring MiHPPD in antisense orientation had reduced expression of endogenous AtHPPD transcript (Fig. 4b).
Fig. 4.
Phenotype of transgenic homozygous Arabidopsis plants transformed with sense and antisense CaMV35S::MiHPPD and wild type as control. a Antisense plants showed slow and retarded growth compared to control and sense plants. b Semi quantitative PCR to assess the transgene MiHPPD and AtHPPD expression in control and antisense plants
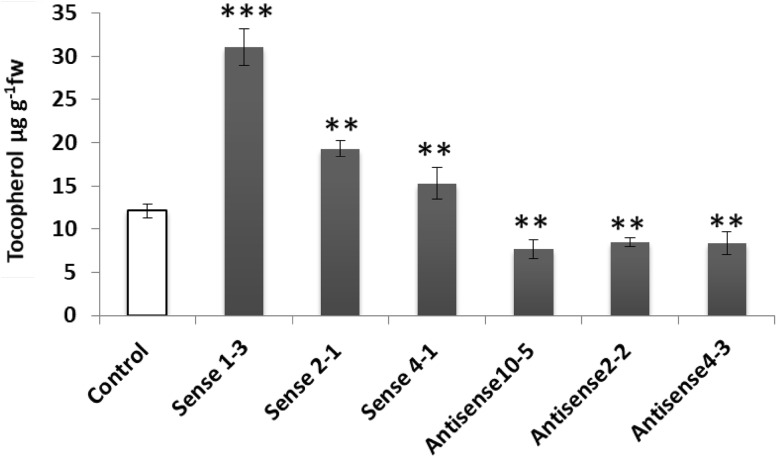
Leaves of antisense plants showing maximum inhibition of AtHPPD transcript and sense plants over-expressing MiHPPD were analyzed for tocopherol content. Tocopherol content in leaves of different Arabidopsis transgenic lines expressing MiHPPD in sense direction were 31 ± 1.5 μgg−1 fw (Line 1–3), 19.2 ± 0.88 μgg−1 fw (Line 2–1) and 15.25 ± 1.02 μgg−1 fw (Line 4–1). These levels were higher than the content present in control leaves (12 ± 0.67 μgg−1 fw). Transgenic plants expressing MiHPPD in antisense direction showed 30–40% decrease in tocopherol content compared to control (Fig. 5).
Fig. 5.
Tocopherol contents in 4 weeks old leaves of control and transgenic Arabidopsis plants expressing MiHPPD in sense and antisense direction. Three independent homozygous lines each of sense and antisense were taken and data from three independent experiments were analysed and expressed as mean ± standard deviation. Content was measured in μgg−1 fw. Asterix (*) indicate a significant differences at P < 0.005, *Significant, **very significant and ***extremely significant, with respect to corresponding controls calculated using students t test
No significant difference was seen in transgenic tomato plants. Fruits of transgenic lines were also similar to control with respect to size, color, seed, shape and shelf life (Fig S1A). The transgenic lines were confirmed for the expression of transgene (MiHPPD) through semi quantitative RT-PCR, carried out using the cDNA prepared from ripe fruit RNA (Fig S1B).
Red ripe tomato fruit was analyzed for change in tocopherol levels in control and transgenic lines. Transgenic lines showed variable increase in fruit tocopherol content with line 2–1 having the maximum increase of up to fivefolds compared to wild type control (Fig. 6) which also correlated with the high level expression of MiHPPD in this line (Fig S1B). Since MiHPPD was expressed in transgenic lines under constitutive CaMV35S promoter, the leaves of transgenic were also analyzed for the levels of α-tocopherol. α-tocopherol levels in different transgenic lines were found to be 5.25 ± 0.20 μgg−1 fw (Line 1–2), 4.96 ± 0.19 μgg−1 fw (Line 1–4), 11.5 ± 0.27 μgg−1 fw (Line 2–1), 8.5 ± 0.2 μgg−1 fw (Line 2–2) and 8.3 ± 0.13 μgg−1 fw (Line 2–4), showing an increase in tocopherol level compared to control (4.3 ± 0.15 μgg−1 fw). There was a maximum increase of 2.5 folds in α-tocopherol levels in line 2–3 compared to control (Fig. 6). These results suggest that heterologous expression of MiHPPD in tomato leads to increase in tocopherol levels in both fruits and leaves.
Fig. 6.
Tocopherol contents in control and tomato transgenic lines expressing MiHPPD under CaMV35S promoter. a Tocopherol content in leaves. b Tocopherol content in ripe tomato fruits. Five independent lines (T1 generation) were analyzed and data consisting of pooled samples of 2–3 fruit/leaves from 3 different plants from each line. Data from three independent experiments were analysed and expressed as mean ± standard deviation. Content was measured in μgg−1 fw. Asterix (*) indicate a significant differences at P < 0.005, *Significant, **very significant and ***extremely significant, with respect to corresponding controls calculated using students t test
Discussion
HPPD has been used as a candidate gene for metabolic engineering to increase tocopherol levels and providing herbicide resistance (Tsegaye et al. 2002; Falk et al. 2003, 2005; Rippert et al. 2004; Karunanandaa et al. 2005; Siehl et al. 2014). In a study carried out in Dashehari, MiHPPD expression correlated with tocopherol levels (Singh et al. 2011) in Dashehari variety. MiHPPD expression and tocopherol levels were analyzed in other commercially important mango varieties to functionally characterize the role of MiHPPD in tocopherol biosynthesis. MiHPPD expression and tocopherol levels positively correlated during ripening in four mango varieties analyzed in this study confirming the role of MiHPPD gene in controlling flux for tocopherol accumulation. Such kind of correlation of HPPD gene expression with increasing tocopherol levels have been studied in plants such as pepper, tomato and olive (Osuna-Garcia et al. 1998; Quadrana et al. 2013; Georgiadou et al. 2015). The increase in HPPD expression in pepper correlated with the level of tocopherols during ripening. From a study carried out in 16 genotypes of tomato with varied tocopherol content in ripened fruits, HPPD and VTE3 were identified as probable major role player in vitamin E accumulation on the basis of expression correlation (Quadrana et al. 2013, 2014). Two homologs of HPPD gene in tomato (SlHPPD1 and SlHPPD2) have different patterns of expression during ripening. SlHPPD1 expresses more in unripe green tomato fruits, while expression of SlHPPD2 increases with the progression of ripening (from green to red ripe stage) and correlates with tocopherol content of fruit. Similarly higher HPPD gene expression was found in the barley cultivars with higher level of vitamin E than in the cultivars with lower vitamin E content (Kosar et al. 2009).
MiHPPD expression was induced by ethylene in all four varieties tested confirming its regulation by ethylene. Induction of MiHPPD in Dashehari by ethylene and its inhibition by ethylene inhibitor has already been shown (Singh et al. 2011). Ethylene activation of HPPD has also been reported for the barley HPPD where it was associated with the senescence related expression of HPPD (Falk et al. 2002). In barley exogenous ethylene treatment also enhanced HPPD levels (Falk et al. 2002). MiHPPD promoter fragment analyzed by Singh et al. (2011) also showed presence of ethylene binding elements. In tomato HPPD gene expression has been shown to increase at and after breaker stage of ripening. Tomato being a climacteric fruit releases ethylene after its climacteric burst at breaker stage of ripening (Alexander and Grierson 2002). Thus, increase in HPPD expression at breaker stage could be correlated with ethylene release (Quadrana et al. 2013).
The Arabidopsis plants expressing MiHPPD sense constructs showed 4–5 folds increase in tocopherol levels compared to control in leaves while tomato plants showed only twofolds increase in fruits and up to threefolds increase in leaves. Similarly, over-expression of the AtHPPD gene in Arabidopsis thaliana has also shown to increase vitamin E levels by 37 and 43% in leaves, of which major increase was in α-tocopherol level (Tsegaye et al. 2003; Li et al. 2010). Tocopherol accumulation in different tissues depends on substrate availability for downstream enzymes. Over-expression of AtHPPD gene in tobacco did not affect tocopherol content in leaves (Rippert et al. 2004), however, higher levels of tocopherols and tocotrienols (vitamin E) were produced in transgenic plants co-expressing AtHPPD and a yeast PDH gene, suggesting that synthesis of p-hydroxyphenylpyruvate is a limiting step for the accumulation of vitamin E in plants. Barley HPPD gene when over-expressed in tobacco (Falk et al. 2003), resulted in increased tocopherol levels in seed but not in leaves. Crowell et al. (2008) expressed AtHPPD in tissue specific manner in tubers of potato and showed 266% increase in α-tocopherol, although the levels were still less than control leaves or seeds, respectively. Transient expression of Lettuce HPPD in lettuce leaves led to 12 folds increase in HPPD expression and fourfold increase in α-tocopherol content (Ren et al. 2011). Thus, over-expression of HPPD is one of the main target genes used for the genetic manipulation of tocopherols in transgenic plants. Several HPPD genes from bacteria, yeast to plants have been used to engineer tocopherol levels successfully but mostly in non-edible plants such as Arabidopsis and tobacco and in edible plants restricted to oil crops like soybean.
Metabolic engineering for enhancing level of tocopherols in fruit has not been reported so far also tocopherol biosynthesis at molecular level is not very well characterized in fruits plants except for tomato. Thus, engineering tomato plants for higher tocopherols by overexpressing MiHPPD not only validates HPPD’s role in tocopherol biosynthesis but also helps in developing an economically important value added fruit crop. This knowledge can be extended to other fruit crops.
Arabidopsis plants harboring MiHPPD in antisense direction showed a slow and retarded growth in comparison to control and MiHPPD sense plants. This reduction in growth of antisense plants was related to reduced level of tocopherols produced in the plants. MiHPPD gene which shows 78% homology with AtHPPD when expressed in antisense reduced the AtHPPD levels in planta leading to reduced tocopherol levels in Arabidopsis plants. Norris et al. (1998) showed that mutation in AtHPPD known as pds1 mutant caused an albino phenotype and plants were not able to survive. Arabidopsis plants homozygous for the pds1 mutation were unable to synthesize both plastoquinone and tocopherol because of an inability to convert HPP–HGA. Arabidopsis plants when grown outdoors accumulate more tocopherols compared to plants grown indoors and plants having reduced levels of tocopherols showed retarded growth compared to wild type plants (Semchuk et al. 2009). Tobacco plants with reduced tocopherol levels achieved by antisense expression of a tocopherol biosynthesis enzyme intermediate geranylgeranyl reductase (CHL P) also showed a delayed growth rate and a pale or variegated phenotype (Tanaka et al. 1999).
Conclusion
MiHPPD expression is induced by ethylene and correlates with tocopherol levels during ripening in different varieties of mango. MiHPPD encodes for a functional protein and its genetic manipulation can lead to enhanced/reduced tocopherol levels in heterologous system, confirming it as a major player in tocopherol accumulation in mango fruit.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thanks Dr. Bal Krishna and Dr. AP Sane for providing different mango samples from orchards and other help and facilities at JISL, Jalgaon. Senior Research Fellowship provided to Rajesh K. Singh by CSIR, India is gratefully acknowledged.
Author’s contribution
RKS and VAS conceptualized the idea. RKS, AKC and RB conducted the experiments. RKS and VAS wrote and edited the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0991-3) contains supplementary material, which is available to authorized users.
References
- Abbasi AR, Hajirezaei M, Hofius D, Sonnewald U, Voll LM. Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007;143:1720–1738. doi: 10.1104/pp.106.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Almeida J, Quadrana L, Asís R, et al. Genetic dissection of vitamin E biosynthesis in tomato. J Exp Bot. 2011;62:3781–3798. doi: 10.1093/jxb/err055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Azevedo MDS, Spicher L, Glauser G, vom Dorp K, Guyer L, Rossi M. Down-regulation of tomato PHYTOL KINASE strongly impairs tocopherol biosynthesis and affects prenyllipid metabolism in an organ-specific manner. J Exp Bot. 2016;67(3):919–934. doi: 10.1093/jxb/erv504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourasia A, Sane VA, Singh RK, Nath P. Isolation and characterization of the MiCel1 gene from mango: ripening related expression and enhanced endoglucanase activity during softening. Plant Growth Regul. 2008;56:117–127. doi: 10.1007/s10725-008-9292-5. [DOI] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 2003;131:632–642. doi: 10.1104/pp.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D. The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol. 2003;133:930–940. doi: 10.1104/pp.103.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell EFJ, McGrath M, Douches DS. Accumulation of vitamin E in potato (Solanum tuberosum) tubers. Transgenic Res. 2008;17(2):205–217. doi: 10.1007/s11248-007-9091-1. [DOI] [PubMed] [Google Scholar]
- DellaPenna D, Mène-Saffrané L. Vitamin E. Adv in Bot Res. 2011;59:179–227. doi: 10.1016/B978-0-12-385853-5.00002-7. [DOI] [Google Scholar]
- DellaPenna D, Pogson BJ. Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol. 2006;57:11–38. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- Falk J, Krauss N, Dahnhardt D, Krupinska K. The senescence associated gene of barley encoding 4-hydroxyphenylpyruvate dioxygenase is expressed during oxidative stress. J Plant Physiol. 2002;159:1245–1253. doi: 10.1078/0176-1617-00804. [DOI] [Google Scholar]
- Falk J, Andersen G, Kernebeck B, Krupinska K. Constitutive overexpression of barley 4-hydroxyphenylpyruvate dioxygenase in tobacco results in elevation of the vitamin E content in seeds but not in leaves. FEBS Lett. 2003;540:35–40. doi: 10.1016/S0014-5793(03)00166-2. [DOI] [PubMed] [Google Scholar]
- Falk J, Brosch M, Schafer A, Braun S, Krupinska K. Characterization of transplastomic tobacco plants with a plastid localized barley 4-hydroxyphenylpyruvate dioxygenase. J Plant Physiol. 2005;162:738–742. doi: 10.1016/j.jplph.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB, Basset GJC, Borel P, et al. Vitamin deficiencies in humans: can plant science help? Plant Cell. 2012;24:395–414. doi: 10.1105/tpc.111.093120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadou EC, NtourouT Goulas V, Manganaris GA, Kalaitzis P, Fotopoulos V. Temporal analysis reveals a key role for VTE5 in vitamin E biosynthesis in olive fruit during on-tree development. Front Plant Sci. 2015;6:871. doi: 10.3389/fpls.2015.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Coronado DJ, Barbas C. Optimized and validated HPLC method for α- and –γ tocopherol measurement in Laurusnobilis leaves. New data on tocopherol content. J Agric Food Chem. 2003;51:5196–5201. doi: 10.1021/jf030143f. [DOI] [PubMed] [Google Scholar]
- Grusak MA, DellaPenna D. Improving the nutrient composition of plants to enhance human nutrition and health. Ann Rev Plant Physiol Plant Mol Biol. 1999;50:133–161. doi: 10.1146/annurev.arplant.50.1.133. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- Karunanandaa B, Qi Q, Hao M, Baszis SR, Jensen PK, Wong Y, Jiang J, Venkatramesh M, Gruys KJ, Moshiri F, et al. Metabolically engineered oilseed crops with enhanced seed tocopherol. Met Eng. 2005;7:384–400. doi: 10.1016/j.ymben.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kosar M, Holkova L, Březinová-Belcredi N, Ehrenbergerová J. Acta univ agric et silvic. Mendel Brun LVII. 2009;4:13–18. [Google Scholar]
- Li Y, Zhou Y, Wang Z, SunX Tang K. Engineering tocopherol biosynthetic pathway in Arabidopsis leaves and its effect on antioxidant metabolism. Plant Sci. 2010;178:312–320. doi: 10.1016/j.plantsci.2010.01.004. [DOI] [Google Scholar]
- Maeda H, Sage TL, Isaac G, Welti R, Dellapenna D. Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell. 2008;20:452–470. doi: 10.1105/tpc.107.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mène-Saffrané L, Jones AD, DellaPenna D. Plastochromanol-8 and tocopherols are essential lipid-soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc Natl Acad Sci USA. 2010;107:17815–17820. doi: 10.1073/pnas.1006971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miret JA, Munné-Bosch S. Redox signaling and stress tolerance in plants: a focus on vitamin E. Ann N Y Acad Sci. 2015;1340:29–38. doi: 10.1111/nyas.12639. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S. The role of a-tocopherol in plant stress tolerance. J Plant Physiol. 2005;162:743–748. doi: 10.1016/j.jplph.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Norris SR, Shen X, DellaPenna D. Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol. 1998;117:1317–1323. doi: 10.1104/pp.117.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna-Garcia JA, Wall MM, Waddell CA. Endogenous levels of tocopherols and ascorbic acid during fruit ripening of New Mexican-type chilli (Capsicum annuum L.) J Agric Food Chem. 1998;46:5093–5096. doi: 10.1021/jf980588h. [DOI] [Google Scholar]
- Pandit SS, Chidley HG, Kulkarni RS, Pujari KH, Giri AP, Gupta VS. Cultivar relationships in mango based on fruit volatile profiles. Food Chem. 2009;114:363–372. doi: 10.1016/j.foodchem.2008.09.107. [DOI] [Google Scholar]
- Quadrana L, Almeida J, Otaiza SN, et al. Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Mol Biol. 2013;81:309–325. doi: 10.1007/s11103-012-0001-4. [DOI] [PubMed] [Google Scholar]
- Quadrana L, Almeida J, Asís R, et al. Natural occurring epialleles determine vitamin E accumulation in tomato fruits. Nat Commun. 2014;5:3027. doi: 10.1038/ncomms5027. [DOI] [PubMed] [Google Scholar]
- Ren W, Zhao L, Zhang L, Wang Y, CuiL Tang Y, et al. Molecular cloning and characterization of 4-hydroxyphenylpyruvate dioxygenase gene from Lactuca sativa. J Plant Physiol. 2011;168:1076–1083. doi: 10.1016/j.jplph.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Rippert P, Scimemi C, Dubald M, Matringe M. Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiol. 2004;134:92–100. doi: 10.1104/pp.103.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, Dellapenna D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell. 2004;16:1419–1432. doi: 10.1105/tpc.021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semchuk NM, Lushchak OV, Falk J, Krupinska K, Lushchak VI. Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol Biochem. 2009;47:384–390. doi: 10.1016/j.plaphy.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radical Biol Med. 1991;10:263–275. doi: 10.1016/0891-5849(91)90033-Y. [DOI] [PubMed] [Google Scholar]
- Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (Mango) Pharmacogn Rev. 2010;4(7):42–48. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl DL, Tao Y, Albert H, Dong Y, Heckert M, Madrigal A, et al. Broad 4-hydroxyphenylpyruvate dioxygenase inhibitor herbicide tolerance in soybean with an optimized enzyme and expression cassette. Plant Physiol. 2014;166:1162–1176. doi: 10.1104/pp.114.247205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK (2011) Identification and characterization of genes involved in aroma volatile and value addition during mango fruit ripening (Mangifera indica Var. Dashehari). Ph.D. Thesis; Department of Biotechnology, Barkatullah University, Bhopal, India
- Singh RK, Ali SA, Nath P, Sane VA. Activation of ethylene responsive p-hydroxyphenylpyruvate dioxygenase leads to increased tocopherol levels during ripening in mango. J Exp Bot. 2011;62:3375–3385. doi: 10.1093/jxb/err006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Singh RK, Pathak G, Goel R, Asif MH, Sane AP, Sane VA. Comparative transcriptome analysis of unripe and mid-ripe fruit of Mangifera indica (var. “Dashehari”) unravels ripening associated genes. Sci Rep. 2016 doi: 10.1038/srep32557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Oster U, KruseE Rudiger W, Grimm B. Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol. 1999;120:695–704. doi: 10.1104/pp.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biol Med. 2008;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegaye J, Shintani D, DellaPenna D. Over-expression of the enzyme p-hydroxyphenylpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol Biochem. 2002;40:913–920. doi: 10.1016/S0981-9428(02)01461-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.