Abstract
Non-alcoholic fatty liver disease (NAFLD) is a wide-spread chronic liver condition that places patients at risk of developing cardiovascular diseases and may progress to cirrhosis or hepatocellular carcinoma if untreated. Challenges in clinical and basic research are caused by poor understanding of NAFLD mechanisms. The purpose of current study is to describe molecular changes occurring in human liver during NAFLD progression by defining a reproducible gene expression signature. We conduct a systematic meta-analysis of published human gene expression studies on liver biopsies and bariatric surgery samples of NAFLD patients. We relate gene expression levels with histology scores using regression models and identify a set of genes showing consistent-sign associations with NAFLD progression that are replicated in at least three independent studies. The analysis reveals genes that have not been previously characterized in the context of NAFLD such as HORMAD2 and LINC01554. In addition, we highlight biomarker opportunities for risk stratification and known drugs that could be used as tool compounds to study NAFLD in model systems. We identify gaps in current knowledge of molecular mechanisms of NAFLD progression and discuss ways to address them. Finally, we provide an extensive data supplement containing meta-analysis results in a computer-readable format.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in industrialized countries and a frequent comorbidity of type 2 diabetes and obesity1. NAFLD is often used as an umbrella term for conditions ranging from simple steatosis (SS; accumulation of fat in the liver without inflammation) to advanced cirrhosis. Non-alcoholic steatohepatitis (NASH) is regarded either as an independent disease or as a stage succeeding SS in NAFLD progression. NASH is associated with particularly poor long-term prognosis2. Some patients with SS never develop NASH or cirrhosis. The progression to end-stage liver disease can take decades3, but outcomes for individual patients are tragic. NASH is the second most common and the most rapidly increasing cause of hepatocellular carcinoma (HCC) in patients awaiting liver transplant in the USA4,5. Furthermore, NAFLD is a risk factor for cardiovascular disease, chronic kidney disease, extrahepatic cancers and endocrinal disorders6.
NAFLD can progress without clinical manifestations for many years. The symptoms can be unspecific (for example, fatigue, elevated liver injury markers). The diagnosis is typically established though liver biopsy and exclusion of other causes of liver disease. The treatment is centered on management of comorbidities (life style modification, weight loss, antidiabetic medication)7. Presently, no drugs are approved by the American agency for Food and Drug Administration (FDA) for treatment of NAFLD. Safe and effective medication and noninvasive biomarkers that could distinguish patients at risk of progression to advanced disease are urgently needed8. The challenges in clinical practice are closely related to issues in basic research. The molecular mechanisms of NAFLD progression are poorly understood. The patient population is very heterogeneous. Small numbers of patients in many human studies limit the power to detect associations. As a consequence, basic research on NAFLD progression is plagued by sporadic observations, point-wise hypothesis testing and extensive use of animal models, which may not capture all relevant aspects of disease dynamics in humans9.
A reproducible gene expression (mRNA) signature of NAFLD progression could improve our understanding of the disease and help to identify candidate biomarkers or drug targets. The aim of our study is to obtain such signature in adult human liver. Longitudinal liver biopsies are hard to obtain because of ethical reasons and risk of complications. Histological manifestations such as inflammation or fibrosis reflect the severity of NAFLD. Ordering patients in a cross-sectional study by a given histology score from none to mild to severe results in a pseudo time course of disease progression. Hence, genes associated with severity of histological manifestations build up the signature of NAFLD progression. This is the main idea behind the design of our study. We perform a systematic meta-analysis of microarray experiments on liver tissue of NAFLD patients. We relate mRNA levels to histological features using regression models, identify associations that are replicated in at least 3 independent cohorts and combine genes associated with distinct histology scores into the final signature (presented in heatmaps in the Results section).
Materials
Gene expression data sets
We searched Gene Expression Omnibus (GEO)10, ArrayExpress11 and Sequence Read Archive12 for studies on NAFLD progression in humans and selected GSE4845213, GSE6126014, GSE8963215, GSE5904516, GSE4954117, GSE1565318 and GSE3381419. The studies had at least 15 liver biopsies or samples from bariatric surgery patients. NAFLD was established histologically. Each study either included samples from different stages of NAFLD or contained publicly available individual-patient level information on histology traits, liver injury markers or diabetes-related traits. Histological characteristics for patients in GSE61260 were obtained from the corresponding methylation experiment GSE6125814. We also identified data sets with histologically scored fibrosis in chronic hepatitis C (GSE3325820, GSE3365021 and GSE1153622), chronic hepatitis B (GSE8404423), and fibrosis in chronic mixed viral or parasitic infection (GSE6137624). Three data sets covered progression from normal liver to viral hepatitis-induced HCC (GSE676425, GSE5423826 and GSE1432327) and acted as surrogate material for progression towards NAFLD-induced HCC. Preprocessed gene expression data and sample annotation were obtained from GEO (Series Matrix). Preprocessed data had been quality controlled, background corrected and normalized by the authors of the respective original publications. These data were ready-to-use for downstream analyses. For example, biological samples in GSE48452 were prepared and mRNA extraction was performed according to the standard manufacturers protocols for HuGene 1.1 ST arrays13. Ahrens et al.13 normalized the arrays with RMA method using R package oligo (from sample description on GEO). We mapped internal microarray platform identifiers to NCBI Gene identifiers (Entrez IDs) using annotation included in the data sets and HUGO gene nomenclature committee data28. Entrez IDs were subsequently used to integrate results of regression and co-expression analyses between experiments.
Targets of marketed and clinical trial drugs
Mechanism-of-action human protein targets of marketed and clinical trial drugs were retrieved from ChEMBL version 2229. UniProt identifiers were mapped to Entrez IDs and gene symbols using complete human proteome information downloaded from UniProt website on 28.12.201630.
Data for identification of candidate biomarkers
Genes encoding predicted secreted proteins and proteins with preferential expression in liver (categories ‘Tissue enriched’ and ‘Tissue enhanced’) were identified in Human Protein Atlas data available at www.proteinatlas.org on 29.12.201631.
Genes with genetic evidence for NAFLD
We focused on genes that had been reviewed by Wood et al.32 as well as MBOAT733 and MERTK34 associated with NAFLD severity.
Methods
We provided a detailed explanation of properties of the data and the statistical basis behind the approach in Supplementary Methods. Here, we outlined key features of the analysis and described methods for visualization of results.
Regression analysis
mRNA expression was measured as normalized log2-scale fluorescence on a probe set, i.e., a cluster of sequences targeting a given gene. A gene could be represented by a single or multiple probe sets depending on microarray design. Individual data sets quantified histological features differently (e.g., percent of steatosis in GSE89632 vs steatosis score in GSE61260) and were assayed on unrelated platforms (see Table S7 in Supplementary Methods). Merging data sets and obtaining pooled estimates was inappropriate. Every data set was analysed separately. Each probe set was tested for association with disease severity, histology and biomarker traits using linear or logistic regression as summarized in Table S1 in Supplementary Data. Models were adjusted for the most likely sources of variation (e.g., BMI) when the information was publicly available and sample size permitted estimation of a multivariate model. Two-sided p-values below 0.05 were considered significant (H0: regression coefficient for mRNA level = 0). Regression coefficients and p-values were rough (limited sample size and linear regression as a simplified model for scores) and had different quantitative interpretation (linear vs logistic regression, covariate structure). We extracted information with compatible meaning for all models: presence/absence and sign of association. All models tested null hypotheses of no association between mRNA and a given aspect of NAFLD progression. All outcome variables were encoded so that low values indicated mild disease and high values indicated severe disease. Sign of regression coefficients always showed direction of association, i.e., increase or decrease of mRNA levels with NAFLD progression. Sign error was the least probable error type in our analysis settings (Supplementary Methods, section ‘Robustness of regression analysis with respect to null hypothesis test of no association and estimated direction of regression slope’).
Derivation of NAFLD progression signature
Replication in independent studies was used to control false positive discoveries (Supplementary Methods, section ‘The role of replication in independent studies’) as an alternative to Benjamin-Hochberg correction for multiple testing within a single experiment. Genes, whose mRNA levels showed consistent-sign associations with a trait in at least three independent data sets, were considered high confidence observations and formed the NAFLD progression signature (marked as ‘main’ in Table S1 in Supplementary Data). The criterion for replication in 3 studies was motivated by data availability (state December 2016) but sufficient to fulfill its purpose (see Supplementary Methods, section ‘The role of replication in independent studies’). Associations with other traits, which did not live up to the ‘high confidence’ definition, did not directly contribute to the signature and were not used as selection criteria for the genes. Such associations provided additional supportive evidence and were used for visual examination of results (marked as ‘negative control’ and ‘sanity check’ in Table S1 in Supplementary Data, explained in dedicated sections of Supplementary Methods). If associations between a gene and a trait (e.g., fibrosis) were detected on multiple probe sets, all associations were required to have consistent sign (within-study replication).
Clustering
All associations for a given gene were organized in a fingerprint with 5 possible values: not assayed on microarray platform (either no probe sets targeting a given gene or probe sets with ambiguous mapping to two or more genes), no significant association, association with inconsistent sign for different probe sets, positive or negative association. Complete-linkage hierarchical agglomerative clustering (default hclust implementation in ref.35) was based on modified Hamming distance. Distance (D) between fingerprints was defined as:
where i was the ith position in the fingerprint and N denoted the total number of associations. Weight could take three values. Weight equaled 1 if both genes were not assayed in an experiment or had no associations with trait or inconsistent-sign associations with a trait. Weight equaled 2 if both genes had same-sign associations with a trait. Weight was zero in all other cases.
Meta-network construction
We constructed co-expression networks from NAFLD data sets GSE48452, GSE61260, GSE49541, GSE89632 and GSE33814. GSE59045 and GSE15653 had 15 and 18 samples respectively and were too small to be included in this analysis. In each data set, Spearman correlation coefficients were computed for all pairs of probe sets representing genes in NAFLD progression signature and genes with genetic evidence for NAFLD. Gene level correlations were obtained by averaging correlation coefficients across all pairs of probe set for a pair of genes. For example, gene A was represented by probe sets a1 and a2 and gene B by probe set b in study X. Then, correlation between A and B in X was mean correlation between (a1 and b) and (a2 and b). Threshold >=0.53 on absolute scale was chosen as the smallest cut-off value that resulted in approximate scale-free topology in 4 out of 5 individual networks (model fit for ‘scale-freeness’ with R2 >= 0.8, pickHardThreshold method in WGCNA package36, details in Supplementary Methods, section ‘Notes on the meta-network’). Correlations reproduced in >=3 of 5 individual networks constituted the meta-network. Meta-network construction was motivated by heterogeneity of biological material and suboptimal sample size37.
Software
All analyses were performed in R version 3.2.535. Data sets and sample annotation were retrieved using GEOquery package38. Networks were created using igraph package39. Figures were produced with ggplot240 and ggnetwork41 packages.
Data availability statement
All data sets analysed in the current study are available from Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/). All data generated during this study and data behind the figures are included in this published article and its Supplementary Data.
Results
Genes with genetic evidence for NAFLD
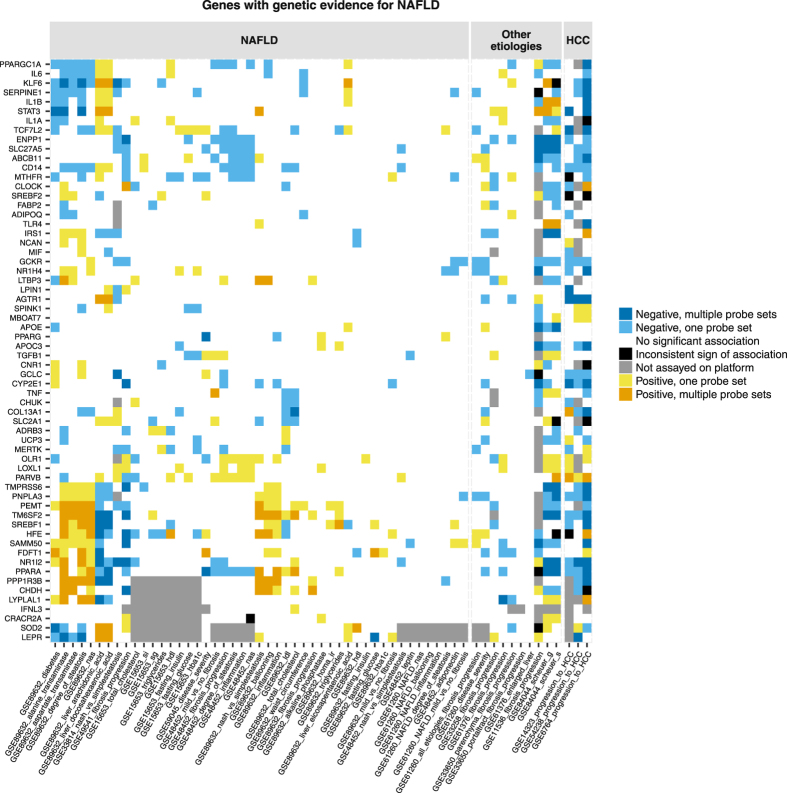
Genetic evidence refers to NAFLD-associated single nucleotide polymorphisms (SNP), i.e., point variants of genomic DNA in immediate vicinity of a given gene or within the gene. Genes with genetic evidence can predispose a patient to develop NAFLD or contribute to disease progression when the patient has at least one copy of risk allele of the SNP. Such genes represent ‘weak spots’ in liver biology and form a special category of interest for our analysis because studying their mRNA expression in NAFLD (regression analysis) and relationships to the signature genes (meta-network) might provide additional insights into NAFLD biology.
The patients in each study represented random samples from the underlying population. Genotypes of patients were unknown. mRNA expression was not allele-specific. Hence, we did not expect a consistent association pattern in all studies. We detected three fairly well defined gene communities by association profile (Fig. 1). Genes related to lipid metabolism FDFT1, PNPLA3, SREBF1 and TM6SF2 clustered together with TMPRSS6, NR1I2 (also known as PXR), SAMM50, HFE and PEMT. Their increasing mRNA levels related to increase in liver injury markers, worsening of steatosis and inflammation as well as decrease in intrahepatic levels of arachidonic and docosahexaenoic acids in GSE89632. An opposite-sign relationship was observed for IL1A, IL1B, IL6, KLF6, PPARGC1A, SERPINE1, STAT3 and TCF7L2. Decreasing expression of ABCB11, CD14, ENPP1, MTHFR and SLC27A5 accompanied fibrosis progression in GSE49541 and/or GSE48452. Decreasing mRNA levels of AGTR1, GCKR, GCLC, CD14, CYP2E1, NR1I2, PPARA, PNPLA3 and TM6SF2 and increasing expression of PARVB were associated with progression from normal liver to HCC (high confidence observations).
Figure 3.
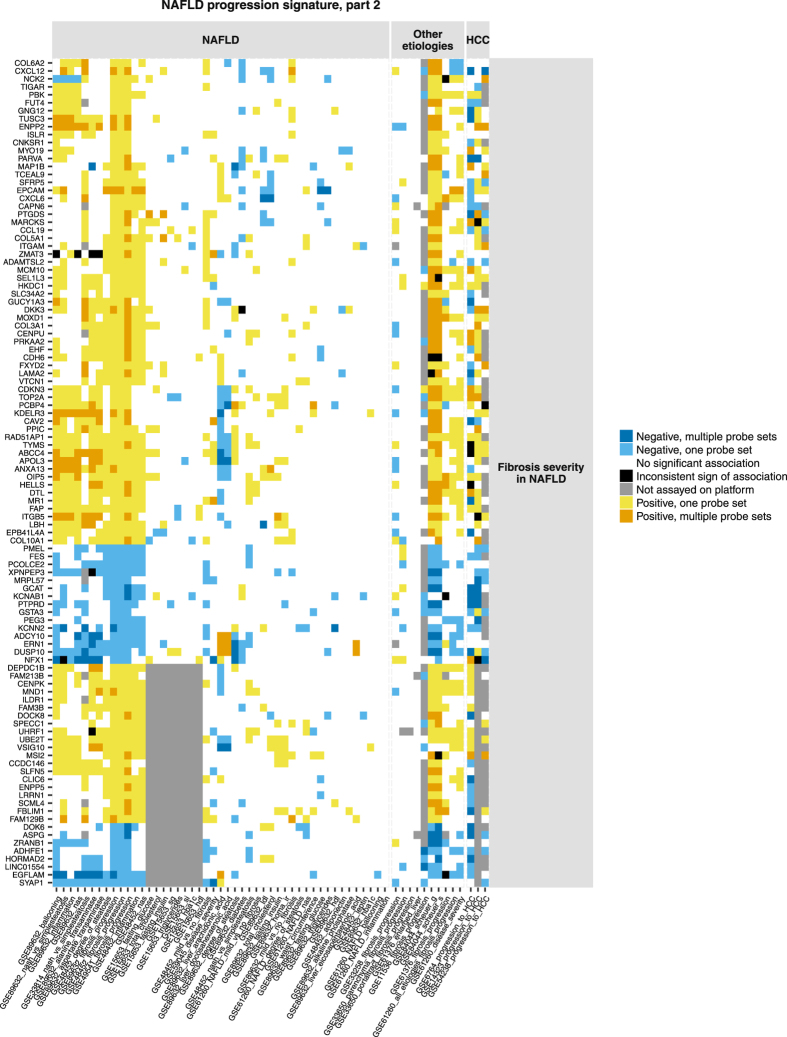
Human NAFLD progression signature (part 2). Figure shows genes with high confidence associations (consistent-sign associations replicated in 3 independent studies) with progression of NAFLD-induced fibrosis. Genes can also display consistent-sign associations with related histological traits. Genes and associations are organized along x and y axes by the same principle as in Fig. 1.
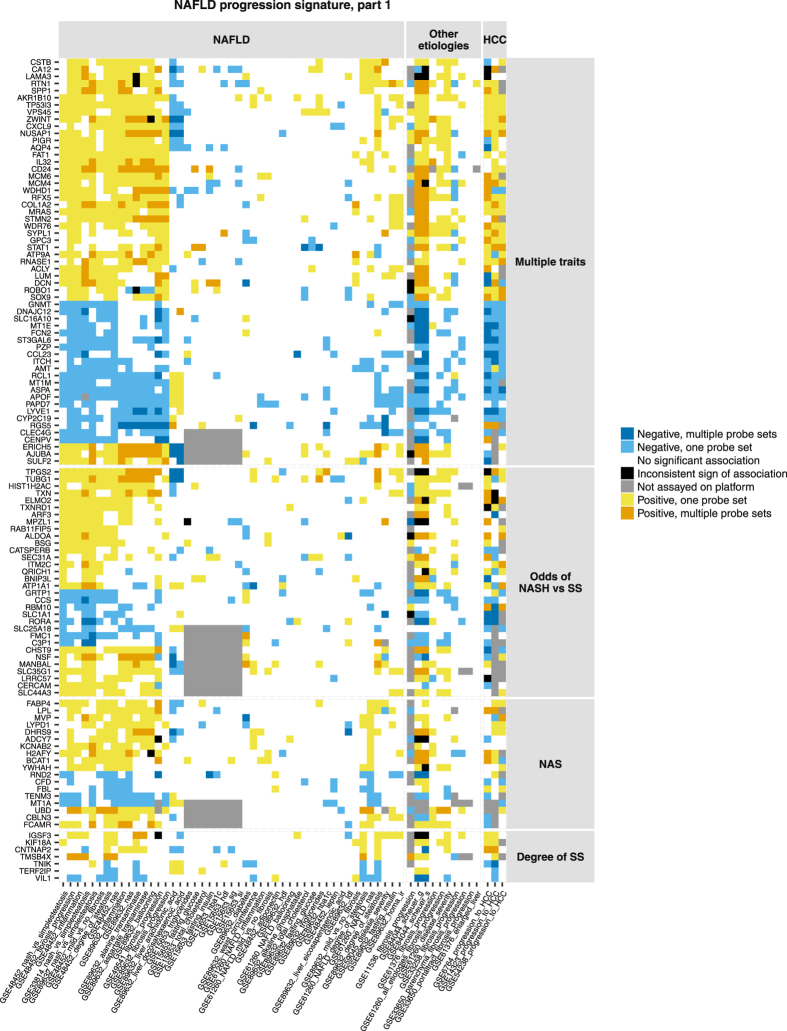
Signature of NAFLD progression
In total, 218 genes showed high confidence associations with at least one histology aspect of NAFLD progression (Figs 2 and 3). The signature genes were unlikely to represent chance findings (Table 1, details of permutation experiment in Supplementary Methods, section ‘The role of replication in independent studies’). Genes associated with fibrosis severity in NAFLD tended to have same-sign associations with fibrosis in hepatitis B but not in hepatitis C (Fig. 3, same-colour columns in GSE84044 and GSE61376 versus predominantly white columns in GSE33650 and GSE33258). We observed no clear separation between gene sets related to distinct aspects of liver histology. The associations were often complemented with supportive same-sign evidence for related traits. For example, elevated mRNA levels of SPP1 (osteopontin) were associated with increasing NAS and inflammation (high confidence observations) as well as with increasing degree of SS in two studies and higher odds of NASH over SS in two studies (Fig. 2). Inflammation and SS were components of NAS score42.
Figure 1.
Association profiles between mRNA levels of genes with genetic evidence with NAFLD and histological and biochemical characteristics of the patients. Associations are clustered along x-axis based on their similarity across genes. Associations are separated into three blocks based on disease etiology: NAFLD, other etiologies (viral, parasitic or mixed etiology liver disease), progression towards HCC (abbreviated as HCC). Genes are clustered along y-axis according to similarity of associations of their mRNA levels with histology scores and biochemical traits in NAFLD. Presence or absence of a significant association and sign of each association are colour-coded.
Figure 2.
Human NAFLD progression signature (part 1). Figure shows genes with high confidence associations (consistent-sign associations replicated in 3 independent studies) with multiple histological traits, odds of NASH versus SS, NAS score or degree of SS. Genes can also display consistent-sign associations with related histological traits. Genes and associations are organized along x and y axes by the same principle as in Fig. 1.
Table 1.
Number of signature genes compared to number of chance findings in random permutations of the data.
| Category | Consistent-sign association in 3 independent studies with … | |||||
|---|---|---|---|---|---|---|
| …at least 1 trait (any) | … multiple traits | … odds of NASH vs SS | … NAS | … degree of SS | ... fibrosis severity in NAFLD | |
| N genes in NAFLD-progression signature | 218 | 57 | 32 | 18 | 7 | 104 |
| N genes in 1,000 random permutations, median (95% CI) | 3 (0–14) | 0 (0–0) | 0 (0–4) | 0 (0–5) | 0 (0–4) | 0 (0–9) |
The analysis confirmed 98 genes that were highlighted in the original publications in the context of NAFLD, hepatitis with other etiologies or progression to HCC (Table S3 in Supplementary Data). Some prominent examples included UBD (ubiquitin D), GPC3 (glypican 3, a gene investigated as diagnostic marker and drug target for HCC43), genes involved in collagen life cycle or associated with risk of cirrhosis44: CD24, COL1A2 and COL3A1, CXCL6, DCN, EHF, FAP, LUM, PCOLCE2 and SOX9. ACLY, a key enzyme responsible for the synthesis of acetyl coenzyme A, has been described by Ahrens et al. as a candidate epigenetic driver of NAFLD13. AKR1B10, an enzyme converting aldehydes to alcohol and whereby carrying out a detoxication function, has been identified through differential expression between steatohepatitis and SS patients by Starmann et al.19 and by Ahrendt et al.15 Starmann et al. extensively studied expression of AKR1B10 in hepatocytes and suggested it as a promising biomarker of NASH and progression to HCC. GNMT has been identified through differential expression between patients with mild and advanced NAFLD-induced fibrosis by Moylan et al.17. The enzyme catalyses synthesis of sarcosine from glycine and plays a role in liver detoxication. Gnmt knock-out mice are experimental hepatitis models45.
By contrast, the signature contained multiple genes with poorly studied biological function in liver such as CBLN3, CERCAM, ERICH5, GRTP1, HORMAD2, LINC01554, MANBAL, MOXD1, MYO19, LRRC57, SEL1L3 and SLC44A3. Increasing expression of SEL1L3 was associated with severity of fibrosis in NAFLD. An intron variant rs959903 in SEL1L3 has been reported in association with ballooning severity46. Decreasing expression of LINC01554 (also known as C5orf27 or FLJ38821) was associated with advancing fibrosis in NAFLD patients. The transcript ENST00000436592.5 corresponding to LINC01554 is polyadenylated according to GENCODE consortium polyadenylation data47. LINC01554 could be reliably measured with microarray and polyA+ protocol. LINC01554 is preferentially expressed in liver according to GTEx consortium data (http://www.gtexportal.org/home/)48. LINC01554 has been previously described in relation to survival of esophageal cancer patients49. HORMAD2 is preferentially expressed in testis and liver31. The gene is related to cancer50 and has genetic associations with immune diseases such as IgA nephropathy51 and inflammatory bowel disease52. These genetic associations were identified through GWAS Catalog53. In our meta-analysis, expression of HORMAD2 decreased with advancing fibrosis in NAFLD.
NAFLD progression meta-network
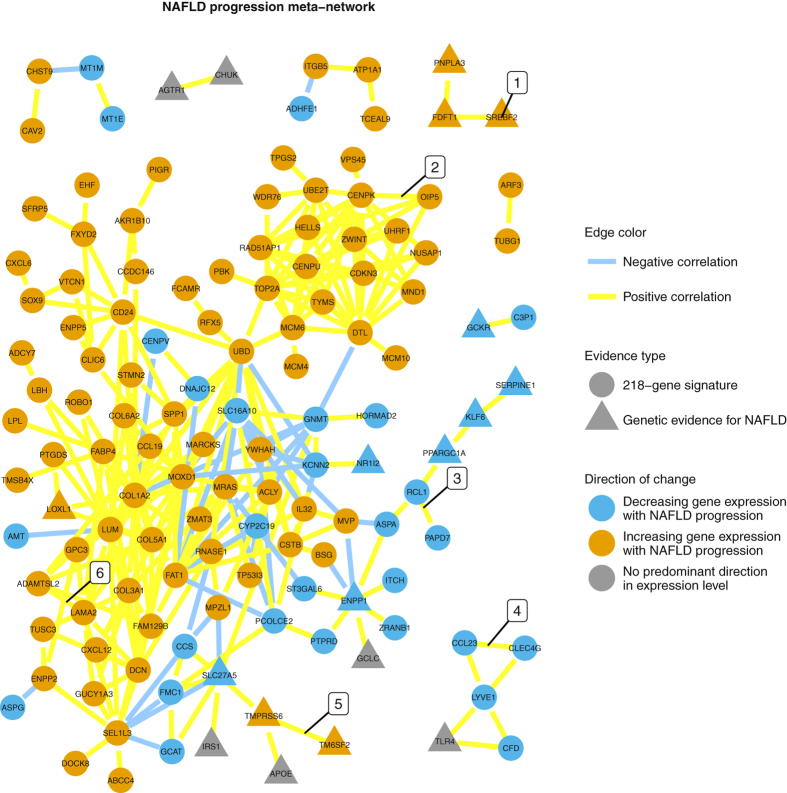
Among 218 genes in the NAFLD progression signature and 62 genes with genetic evidence, 131 (46.8%) genes had reproducible correlations with each other that satisfied criteria for meta-network construction (Table S4 in Supplementary Data). Genes that clustered together based on their associations with histological traits tended to be correlated. Genes with genetic evidence for NAFLD tended to be co-expressed (communities 1, 3 and 5 in Fig. 4). Genes in the NAFLD progression signature were arranged in three well-formed co-expression modules (2, 4 and 6) and a number of smaller disconnected components. Ten genes with highest number of connections were COL1A2 (25 direct network neighbours), LUM, UBD, DTL, FAT1, MOXD1, CENPK, MRAS, SEL1L3 and TOP2A (12 direct neighbours).
Figure 4.
NAFLD progression meta-network. Each node represents a gene. Each edge represents a Spearman correlation between mRNA levels of two genes. An edge is displayed only if the magnitude of correlation coefficient is >=0.53 on absolute scale and the correlation is reproduced in at least three data sets. Lengths of edges vary to improve readability and do not carry mathematical or biological meaning. Six gene communities are highlighted: (1) three genes with genetic associations with NAFLD and role in lipid metabolism, (2) genes involved in cell division, replication and DNA repair, (3) three immune system-related genes with genetic evidence for NAFLD and a ‘bridge’ of genes with enzymatic activity linking them to community 6, (4) putative liver sinusoid endothelium co-expression module65–67, (5) genes with genetic evidence for NAFLD and related to fatty acid metabolism, (6) community of genes formed by a large subset of the 218-gene signature and enriched in genes associated with fibrosis. Functional description is provided according to NCBI Gene summary information unless explicitly indicated otherwise.
Drug targets
Among genes with genetic evidence for NAFLD and genes in the progression signature, 21 genes were targeted by marketed and clinical trial drugs (Table 2). Obeticholic acid54 and angiotensin II antagonists55 are actively investigated in liver disease. Digitoxin has been investigated as anti-inflammatory agent in patients with cystic fibrosis and achieved a noticeable but not statistically significant reduction in inflammation markers56. Acetazoleamide can cause liver injury and is associated with increased death risk in chronic liver disease patients57. Carbonic anhydrase CA12 is one of the targets of acetazoleamide and is expressed at low levels in healthy liver31. In our analysis, elevated expression of CA12 was associated with increased steatosis and NAS (high confidence observations). Sulphonamide diuretics have been used to study the link between activity of carbonic anhydrases and hepatic lipogenesis58. As illustrated by these examples, known drugs could be used to perturb models such as microphysiological systems or liver of animal models, gain mechanistic understanding of NAFLD and identify points of therapeutic intervention.
Table 2.
Marketed and clinical trial drugs targeting protein products of genes in the 218-gene NAFLD progression signature or genes with genetic evidence for NAFLD. The drugs are ordered by their therapeutic application.
| Drug(s) | Phase | Therapeutic application | UniProt ID of target protein(s) | Corresponding gene(s) |
|---|---|---|---|---|
| Obeticholic acid | Marketed | Primary biliary cholangitis | Q96RI1 | NR1H4 |
| Ursodiol | ||||
| Pioglitazone | Marketed | Antidiabetic | P37231 | PPARG |
| Rosiglitazone | ||||
| Troglitazone | ||||
| Metreleptin | Marketed | Dyslipidemia | P48357 | LEPR |
| Clofibrate | Marketed | Dyslipidemia | Q07869 | PPARA |
| Fenofibrate | ||||
| Gemfibrozil | ||||
| Carvedilol | Marketed | Heart failure | P13945 | ADRB3 |
| Epinephrine | ||||
| Labetalol | ||||
| Deslanoside | Marketed | Heart failure | P05023P54710 | ATP1A1FXYD2 |
| Digitoxin | ||||
| Digoxin | ||||
| Irbesartan | Marketed | Hypertension | P30556 | AGTR1 |
| Losartan | ||||
| Valsartan | ||||
| Isosorbide dinitrate | Marketed | Vasodilators | Q02108 | GUCY1A3 |
| Nitroglycerin | ||||
| Riociguat | ||||
| Gavilimomab | Phase 3 | Graft versus host disease | P35613 | BSG |
| RA-18C3 | Phase 2 | Antiinflammatory | P01583 | IL1A |
| Canakinumab | Marketed | Antiinflammatory | P01584 | IL1B |
| Rilonacept | ||||
| Balsalazide | Marketed | Antiinflammatory | P37231 | PPARG |
| Olsalazine | ||||
| Mesalazine | ||||
| Adalimumab | Marketed | Antiinflammatory | P01375 | TNF |
| Etanercept | ||||
| Infliximab | ||||
| Siltuximab | Marketed | Multicentric Castleman’s disease | P05231 | IL6 |
| Capecitabine | Marketed | Cancer | P04818 | TYMS |
| Floxuridine | ||||
| Pemetrexed | ||||
| Daunorubicin | Marketed | Cancer | P11388 | TOP2A |
| Etoposide | ||||
| Arsenic trioxide (TRISENOX) | Marketed | Cancer | Q16881 | TXNRD1 |
| Acetazolamide | Marketed | Diuretic | O43570 | CA12 |
| Ethoxzolamide | ||||
| Nabilone | Marketed | Neuropathic pain | P21554 | CNR1 |
| Ocriplasmin | Marketed | Vitreomacular | P24043 | LAMA2 |
| Adhesion | Q16787 | LAMA3 |
Candidate biomarkers for risk stratification
We identified four genes that could be evaluated as biomarkers to identify patients at risk of progression to severe NAFLD. The genes participated in the 218-gene NAFLD progression signature, encoded secreted plasma proteins and were preferentially expressed in liver (unlikely non-disease-specific and non-source-organ-specific fluctuations in biomarker levels). Decreasing mRNA levels of CYP2C19 and APOF were associated with advancing fibrosis in NAFLD (3 studies out of 3). APOF showed a high confidence association with inflammation and CYP2C19 with NAS score. Lower expression of PZP and FCN2 related to higher odds of NASH over SS in three independent studies. APOF, PZP, FCN2 and CYP2C19 were not highlighted by the authors of original publications13–19 as biomarker opportunities in NAFLD. Plasma APOF concentration has been suggested as fibrosis biomarker in hepatitis C59. An intergenic SNP rs6487679 located near PZP has been reported in association with NAFLD risk as well as elevated alanine aminotransferase60 and aspartate aminotransferase levels in NAFLD patients46.
Signature of NAFLD progression versus clinical outcome
Genes associated with mortality or major complications in NAFLD patients could help to identify pathways for therapeutic intervention and stratify patients in need of close supervision by a physician. We were unable to locate studies investigating relationship between mRNA expression in liver on a genome-wide scale (as opposed to profiling of a small preselected set of genes) and long-term outcome in patients with NAFLD. The 218-gene NAFLD progression signature had little overlap with signatures predicting survival of patients with other liver diseases.
Dominguez et al. assayed hepatic expression of eleven members of CXC chemokine family in patients with severe alcoholic hepatitis and found that CXCL3, CXCL5, CXCL6 and IL18 predicted short-term mortality and related to neutrophil infiltration and portal vein hypertension61. The 218-gene signature contained 3 members of CXC chemokine family (CXCL6, CXCL9 and CXCL12) and transcription activator STAT1 that could modulate recruitment of neutrophils. Increasing expression of these genes was associated with worsening of NAFLD-induced fibrosis (high confidence observation).
Hoshida et al. published a 186-gene signature derived from liver tissue surrounding tumour and predicting mortality and liver decompensation in HCC patients62 and validated a 32-gene subset of this signature in NASH patients undergoing bariatric surgery63. We found only two genes shared between the 218-gene and 186-gene signatures. Increasing expression of CCL19 and RNASE1 was associated with advancing fibrosis in NAFLD. Both genes were linked to bad prognosis in HCC patients (Supplementary Table 2 in ref.62). Among genes with genetic evidence for NAFLD, SREBF2 and GCKR participated in the 186-gene signature and were linked to good outcome (Supplementary Table 2 in ref.62). A 122-gene hepatic stellate cell signature recently reported by the same group in association with multiple clinical outcomes in HCC and cirrhosis patients64 also showed a two-gene overlap: GUCY1A3 and KDELR3.
Discussion
We derived a hepatic gene expression (mRNA) signature of NAFLD progression in adult humans through systematic meta-analysis of publicly available experiments. The signature consisted of genes whose mRNA levels had reproducible consistent-sign associations with histological traits. The main strength of our study is that we put each observation into a broad biological context (from frequently emphasized traits like fibrosis and odds of NASH over SS, to less studied NAS and progression to HCC). The replication-based approach enabled us to identify novel genes, showing potentially subtle but reproducible associations with NAFLD severity that may be non-obvious in conventional case-control design like differential expression. To the best of our knowledge, such high-resolution analysis has not been previously performed in NAFLD.
The limitations of our study were imposed by the scarcity of available data. The signature should be validated in independent studies with true longitudinal design and larger sample size. The signature could be expanded to incorporate genes associated with ballooning, insulin resistance and other comorbidity-related traits as suitable data become available. The statistical analysis was specifically adapted to handle unrelated microarray platforms, limited sample size per data set and distinct quantification systems for histology in individual data sets. Presence/absence and sign of associations represented information with compatible meaning for all models and, combined with criterion for replication, could be extracted with low risk of false positives (demonstrated in Supplementary Methods, Tables S8 and S9). The obvious limitation is lack of quantitative estimates. While we can state that mRNA expression of a given gene increases or decreases with increasing NAFLD severity, it remains an open question whether or not such relationship is strong enough for a specific application. Such questions need to be addressed in follow-up experiments and constitute directions of future work. For example, mRNA expression of APOF decreased with worsening of NAFLD-induced fibrosis. To learn whether APOF can discriminate between e.g., periportal fibrosis and bridging fibrosis, APOF should be measured on protein level with an appropriate assay in blood of NAFLD patients with the corresponding stages of fibrosis.
The 218-gene signature represents a shortlist of genes affected during NAFLD progression. Elucidation of the role of individual genes represents a direction of future work. The signature may incorporate a) potential drivers of NAFLD progression, b) genes affected during NAFLD progression but not actively driving it (down-stream events), and c) genes changing as a compensatory reaction in response to liver damage. Potential driver genes may be identified using other omics data types (e.g., methylation), tool compounds or knock-out experiments. Also, key molecular players in NAFLD may be related to mortality or major complications in NAFLD patients. We anticipate that studies on NAFLD patients, in which omics data are set in the context of phenotype (histological features, blood biomarkers etc.) and survival for the same patients, could improve our understanding of the disease.
The 218-gene NAFLD progression signature could be used to inform the choice of animal models and help to resolve issues in translational research. Hepatic gene expression (mRNA) profile of orthologue genes in a model organism under a given dietary intervention would mirror the human signature. Evaluation of similarities in gene expression profiles would complement assessment of similarities in symptoms and histological manifestations of NAFLD between humans and a given animal model.
In conclusion, NAFLD progression in human liver could be characterized by a small set of genes displaying reproducible consistent-sign associations with histological traits. This gene expression signature could be used a starting point to address current knowledge gaps on NAFLD progression.
Electronic supplementary material
Acknowledgements
We would like to thank the authors of the transcriptomics studies for making their data publicly available and all the patients who contributed to the research. We would like to thank Gerhard Böttcher for providing valuable insights on the histological picture of NAFLD progression and Anna Walentinsson for constructive suggestions for further analysis.
Author Contributions
Conceptualization of the study: M.R. and M.H. Formal statistical analysis, tables and figures: M.R. M.R. and M.H. wrote and reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10930-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):81–84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 2.McPherson S, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Singh, S. et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol13, 643–654 e641–649; quiz e639–640, doi:10.1016/j.cgh.2014.04.014 (2015). [DOI] [PMC free article] [PubMed]
- 4.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174–1197. doi: 10.1002/hep.26717. [DOI] [PubMed] [Google Scholar]
- 7.LaBrecque DR, et al. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467–473. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedtke C, et al. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6:19. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett T, et al. NCBI GEO: archive for functional genomics data sets-update. Nucleic Acids Res. 2013;41:D991–995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolesnikov N, et al. ArrayExpress update-simplifying data submissions. Nucleic Acids Res. 2015;43:D1113–1116. doi: 10.1093/nar/gku1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leinonen R, Sugawara H, Shumway M. The Sequence Read Archive. Nucleic Acids Research. 2010;39:D19–D21. doi: 10.1093/nar/gkq1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahrens M, et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013;18:296–302. doi: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Horvath S, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA. 2014;111:15538–15543. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendt BM, et al. Altered hepatic gene expression in nonalcoholic fatty liver disease is associated with lower hepatic n-3 and n-6 polyunsaturated fatty acids. Hepatology. 2015;61:1565–1578. doi: 10.1002/hep.27695. [DOI] [PubMed] [Google Scholar]
- 16.du Plessis J, et al. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:635–648 e614. doi: 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Moylan CA, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology. 2014;59:471–482. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pihlajamaki J, et al. Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 2009;94:3521–3529. doi: 10.1210/jc.2009-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starmann J, et al. Gene expression profiling unravels cancer-related hepatic molecular signatures in steatohepatitis but not in steatosis. PLoS One. 2012;7:e46584. doi: 10.1371/journal.pone.0046584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad W, Ijaz B, Hassan S. Gene expression profiling of HCV genotype 3a initial liver fibrosis and cirrhosis patients using microarray. J Transl Med. 2012;10:41. doi: 10.1186/1479-5876-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munshaw S, et al. Laser captured hepatocytes show association of butyrylcholinesterase gene loss and fibrosis progression in hepatitis C-infected drug users. Hepatology. 2012;56:544–554. doi: 10.1002/hep.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caillot F, et al. Novel serum markers of fibrosis progression for the follow-up of hepatitis C virus-infected patients. Am J Pathol. 2009;175:46–53. doi: 10.2353/ajpath.2009.080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, et al. Characterization of gene expression profiles in HBV-related liver fibrosis patients and identification of ITGBL1 as a key regulator of fibrogenesis. Sci Rep. 2017;7:43446. doi: 10.1038/srep43446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobert GN, et al. Transcriptional profiling of chronic clinical hepatic schistosomiasis japonica indicates reduced metabolism and immune responses. Parasitology. 2015;142:1453–1468. doi: 10.1017/S0031182015000682. [DOI] [PubMed] [Google Scholar]
- 25.Wurmbach E, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- 26.Yuan SX, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63:499–511. doi: 10.1002/hep.27893. [DOI] [PubMed] [Google Scholar]
- 27.Mas VR, et al. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med. 2009;15:85–94. doi: 10.2119/molmed.2008.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray KA, Yates B, Seal RL, Wright MW, Bruford EA. Genenames.org: the HGNC resources in 2015. Nucleic Acids Res. 2015;43:D1079–1085. doi: 10.1093/nar/gku1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bento AP, et al. The ChEMBL bioactivity database: an update. Nucleic Acids Res. 2014;42:D1083–1090. doi: 10.1093/nar/gkt1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UniProt Consortium. UniProt: a hub for protein information. Nucleic Acids Res43, D204–212, doi:10.1093/nar/gku989 (2015). [DOI] [PMC free article] [PubMed]
- 31.Uhlen M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 32.Wood KL, Miller MH, Dillon JF. Systematic review of genetic association studies involving histologically confirmed non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2015;2:e000019. doi: 10.1136/bmjgast-2014-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krawczyk M, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247–255. doi: 10.1194/jlr.P067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petta S, et al. MERTK rs4374383 polymorphism affects the severity of fibrosis in non-alcoholic fatty liver disease. J Hepatol 64, 682-690. 2016 doi: 10.1016/j.jhep.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 35.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org/ (2013).
- 36.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schönbrodt FD, Perugini M. At what sample size do correlations stabilize? Journal of Research in Personality. 2013;47:609–612. doi: 10.1016/j.jrp.2013.05.009. [DOI] [Google Scholar]
- 38.Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 39.Csardi, G. & Nepusz, T. The igraph software package for complex network research. InterJournal Complex Systems, 1695 (2006).
- 40.Wickham, H. Ggplot2: elegant graphics for data analysis (Springer, 2009).
- 41.Briatte, F. Ggnetwork: geometries to plot networks with 'ggplot2' v. R package version 0.5.1 (2016).
- 42.Kleiner DE, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Liu H, Ding H. GPC-3 in hepatocellular carcinoma: current perspectives. J Hepatocell Carcinoma. 2016;3:63–67. doi: 10.2147/JHC.S116513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu MY, et al. A 6 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis B. Front Biosci (Landmark Ed) 2016;21:479–486. doi: 10.2741/4403. [DOI] [PubMed] [Google Scholar]
- 45.Liu SP, et al. Glycine N-methyltransferase-/- mice develop chronic hepatitis and glycogen storage disease in the liver. Hepatology. 2007;46:1413–1425. doi: 10.1002/hep.21863. [DOI] [PubMed] [Google Scholar]
- 46.Chalasani, N. et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic Fatty liver disease. Gastroenterology139, 1567–1576, 1576 e1561–1566, doi:10.1053/j.gastro.2010.07.057 (2010). [DOI] [PMC free article] [PubMed]
- 47.Harrow J, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Consortium G. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan Q, Liu B. Identification of a RNA-Seq Based 8-Long Non-Coding RNA Signature Predicting Survival in Esophageal Cancer. Med Sci Monit. 2016;22:5163–5172. doi: 10.12659/MSM.902615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu M, et al. HORMAD2/CT46.2, a novel cancer/testis gene, is ectopically expressed in lung cancer tissues. Mol Hum Reprod. 2012;18:599–604. doi: 10.1093/molehr/gas033. [DOI] [PubMed] [Google Scholar]
- 51.Kiryluk K, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu JZ, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welter D, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makri E, Cholongitas E, Tziomalos K. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:9039–9043. doi: 10.3748/wjg.v22.i41.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paschos P, Tziomalos K. Nonalcoholic fatty liver disease and the renin-angiotensin system: Implications for treatment. World J Hepatol. 2012;4:327–331. doi: 10.4254/wjh.v4.i12.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeitlin PL, et al. Digitoxin for Airway Inflammation in Cystic Fibrosis: Preliminary Assessment of Safety, Pharmacokinetics, and Dose Finding. Ann Am Thorac Soc. 2017;14:220–229. doi: 10.1513/AnnalsATS.201608-649OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hug, B. L. et al. Mortality and drug exposure in a 5-year cohort of patients with chronic liver disease. Swiss Med Wkly139, 737–746, doi:smw-12686 (2009). [DOI] [PubMed]
- 58.Lynch CJ, et al. Role of hepatic carbonic anhydrase in de novo lipogenesis. Biochem J. 1995;310(Pt 1):197–202. doi: 10.1042/bj3100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gangadharan B, et al. Discovery of novel biomarker candidates for liver fibrosis in hepatitis C patients: a preliminary study. PLoS One. 2012;7:e39603. doi: 10.1371/journal.pone.0039603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanth VV, et al. Pooled genetic analysis in ultrasound measured non-alcoholic fatty liver disease in Indian subjects: A pilot study. World J Hepatol. 2014;6:435–442. doi: 10.4254/wjh.v6.i6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dominguez M, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–1650. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 62.Hoshida Y, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goossens N, et al. Nonalcoholic Steatohepatitis Is Associated With Increased Mortality in Obese Patients Undergoing Bariatric Surgery. Clin Gastroenterol Hepatol. 2016;14:1619–1628. doi: 10.1016/j.cgh.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang DY, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2016;65:1754–1764. doi: 10.1136/gutjnl-2015-309655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arimoto J, et al. Expression of LYVE-1 in sinusoidal endothelium is reduced in chronically inflamed human livers. J Gastroenterol. 2010;45:317–325. doi: 10.1007/s00535-009-0152-5. [DOI] [PubMed] [Google Scholar]
- 66.Liu W, et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J Biol Chem. 2004;279:18748–18758. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- 67.Han KY, Kim CW, Lee TH, Son Y, Kim J. CCL23 up-regulates expression of KDR/Flk-1 and potentiates VEGF-induced proliferation and migration of human endothelial cells. Biochem Biophys Res Commun. 2009;382:124–128. doi: 10.1016/j.bbrc.2009.02.149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sets analysed in the current study are available from Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/). All data generated during this study and data behind the figures are included in this published article and its Supplementary Data.