Abstract
In tissue culture, the formation of callus from detached explants is a key step in plant regeneration; however, the regenerative abilities in different species are variable. While nearly all parts of organs of the dicot Arabidopsis thaliana are ready for callus formation, mature regions of organs in monocot rice (Oryza sativa) and other cereals are extremely unresponsive to tissue culture. Whether there is a common molecular mechanism beyond these different regenerative phenomena is unclear. Here we show that the Arabidopsis and rice use different regeneration‐competent cells to initiate callus, whereas the cells all adopt WUSCHEL‐RELATED HOMEOBOX 11 (WOX11) and WOX5 during cell fate transition. Different from Arabidopsis which maintains regeneration‐competent cells in mature organs, rice exhausts those cells during organ maturation, resulting in regenerative inability in mature organs. Our study not only explains this old perplexity in agricultural biotechnology, but also provides common molecular markers for tissue culture of different angiosperm species.
Keywords: angiosperm, callus, plant regeneration, rice, WOX11, WOX5
1. SUMMARY STATEMENT
WOX11−WOX5 is a conserved molecular pathway adopted by different types of regeneration‐competent cells for callus initiation in angiosperms, and depletion of these cells results in regenerative inability in cereals.
1.1. Significance
An amazing feature of plant cells is their plasticity, which endows plants with remarkable regeneration abilities (Ikeuchi, Ogawa, Iwase, & Sugimoto, 2016; Sugimoto, Gordon, & Meyerowitz, 2011; Vogel, 2005; Xu & Huang, 2014) and has been widely exploited in agricultural technologies (Sussex, 2008). Detached or wounded plant organs usually form a group of fast dividing cell mass, termed callus, in different regenerative systems (Ikeuchi, Sugimoto, & Iwase, 2013). In tissue culture, de novo organogenesis could occur on a type of callus which has a high pluripotency for adventitious root and shoot regeneration (Ikeuchi et al., 2013; Kareem et al., 2016; Sugimoto et al., 2011; Xu & Huang, 2014).
Studies of Arabidopsis thaliana suggested that the callus formation on callus‐inducing medium (CIM) in tissue culture follows the rooting developmental pathway (Atta et al., 2009; Che, Lall, & Howell, 2007; He, Chen, Huang, & Xu, 2012; Liu et al., 2014; Sugimoto, Jiao, & Meyerowitz, 2010). Two steps of cell fate transition occurred in callus formation. Arabidopsis thaliana WUSCHEL‐RELATED HOMEOBOX 11 (AtWOX11) is activated in the first step of cell fate transition from regeneration‐competent cells to founder cells; and cell division occurs in the second step of cell fate transition from founder cells to callus cells which are marked by AtWOX5 (Liu et al., 2014).
The application of plant regeneration in tissue culture has occurred for more than half a century; however, a key obstacle in this biotechnology is that the ability for callus initiation upon hormone induction is highly diverse in different species. For example, while almost all organs of Arabidopsis, the typical dicot plant, have the ability to produce callus during their whole life (He et al., 2012; Sugimoto et al., 2010), mature regions of organs in many monocot cereal species are extremely unresponsive to in vitro culture techniques (Bhojwani, Evans, & Cocking, 1977; Cutler, Saleem, & Wang, 1991), perplexing the agricultural applications of tissue culture in cereal species for a long time. Whether there is a common molecular discipline for callus formation of different species is unclear, and thus how to explain the cereal problem in tissue culture is so far unanswered. In this study, we revealed this common discipline at the molecular level in angiosperms and this might be a marker to monitor tissue culture in future agricultural technologies. In addition, we discuss the regenerative inability in mature organs of cereals.
2. RESULTS
2.1. Identification of regeneration‐competent cells for callus initiation in rice
Dicots and monocots are two major branches of angiosperms, but the cellular and molecular mechanisms of regeneration in monocots are largely unclear. To analyze the cell lineage of callus formation in the monocot model plant rice (Oryza sativa), we cultured leaf and root explants on CIM. The mature rice leaf is unable to form callus in tissue culture (Bhojwani et al., 1977; Cutler et al., 1991). However, the base region of young leaves formed callus in our culture conditions (Fig. 1A) (Cutler et al., 1991), and this region is at the immature stage (Zeng et al., 2016). At the basal part of a young leaf explant about 7 mm in length, the vascular bundle was surrounded by two bundle sheath layers, i.e., the outer sheath and the inner sheath (Zeng et al., 2016) (Fig. 1B). Callus initiated primarily from the outer sheath at 5 days after culture (DAC) (Fig. 1C). Cell division could also be occasionally observed from the inner sheath (Fig. 1C). In the root, callus can be formed at the root tip region and the lateral root formation region (Fig. 1D). Callus initiated from the phloem‐pole pericycle (Fig. 1E, F), where lateral roots usually initiate during root development (Zeng et al., 2016). Therefore, bundle sheath cells in leaves and phloem‐pole pericycle cells in roots serve as regeneration‐competent cells for callus initiation in rice. It is possible that other immature cells in the vasculature may also be competent for callus initiation (see the analysis for maize, below).
Figure 1.
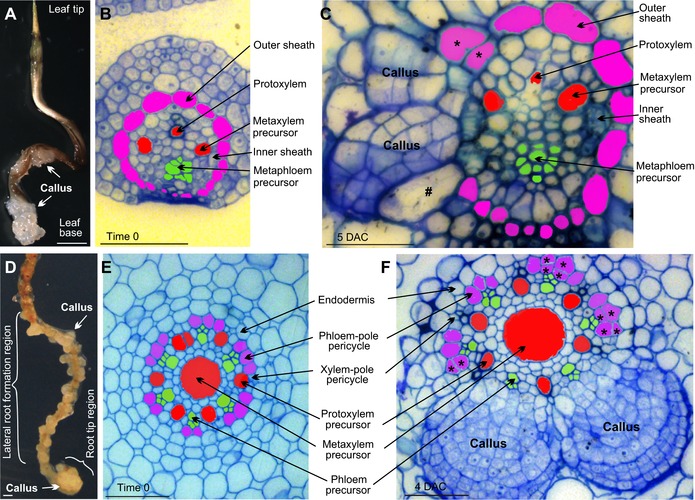
Cellular analysis of callus formation in rice. (A) Wild‐type rice leaf (7‐mm long) used as a source of explants for tissue culture on CIM for 2 weeks. (B), (C) Thin sections from time‐0 (B) and 5‐DAC (C) rice leaf explants cultured on CIM at leaf base. (C) Callus formed primarily from the outer sheath: *, the outer sheath cell that underwent division to form two callus cells; #, elongated outer sheath cell before cell division to form callus cells. Note that some inner sheath cells also underwent division. (D) Wild‐type rice root explants from 5‐day‐old seedling cultured on CIM for 2 weeks. Callus formed from the root tip region and the lateral root formation region. (E), (F) Thin sectioning of rice root explants cultured on CIM at time 0 (E) and 4 DAC (F). Note that callus formed from the phloem‐pole pericycle in (F); asterisks indicate phloem‐pole pericycle cells that underwent cell division to form two callus cells. Cell lineage in rice leaf and root tissue formation was described previously (Zeng et al., 2016). Scale bars: (A), (D) 1 mm; (B), (C), (E), (F) 50 μm
2.2. OsWOX11/12B and OsWOX5 are involved in callus formation in rice
To explore the molecular mechanism that confers the ability to regenerate on certain tissues in rice, we performed an RNA sequence experiment. We identified candidate genes that were highly upregulated during callus formation from the basal part of young leaf explants about 7 mm in length (Fig. S1; Table S1). Oryza sativa WOX11 (OsWOX11) and OsWOX12B, which are intermediate‐clade WOX genes, and OsWOX5 belonging to the WUS clade were among the highly upregulated genes (Fig. S1; Table S1) (van der Graaff, Laux, & Rensing, 2009; Haecker et al., 2004; Lian, Ding, Wang, Zhang, & Xu, 2014; Zeng et al., 2016).
To test whether OsWOX11 is involved in rice regeneration, we analyzed the callus formation ability of the rice Oswox11‐1 mutant (Zhao, Hu, Dai, Huang, & Zhou, 2009). We dissected the 7‐mm young leaf explant into three segments: two 1‐mm segments (segments 1 and 2) at the leaf base and the remaining 5 mm as the third segment (segment 3) (Fig. 2A). In the wild type, callus formed in segments 1 and 2 but not in segment 3 (Fig. 2B). In the Oswox11‐1 mutant, both segments 1 and 2 showed decreased callus formation rates compared with those of wild type (Fig. 2C, D). These data suggested that OsWOX11 could be involved in callus formation in rice, and the partially reduced regenerative ability of the Oswox11‐1 mutant may be due to the redundant function of OsWOX12B.
Figure 2.
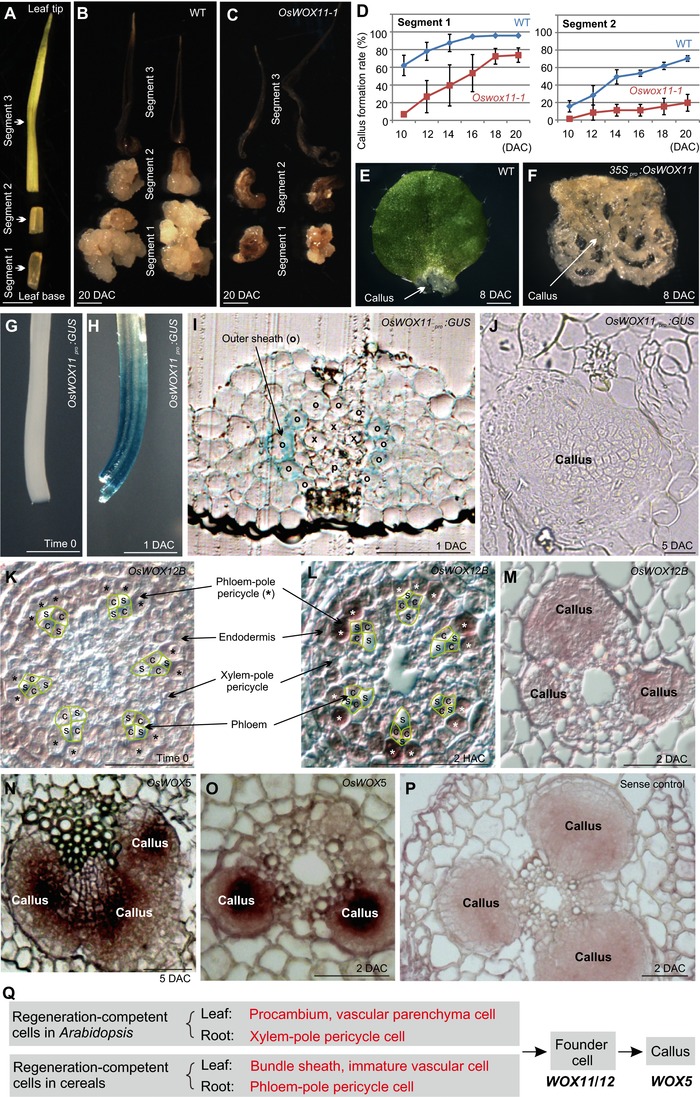
OsWOX11/12B and OsWOX5 are involved in callus formation in rice. (A) Rice leaf (7 mm) as dissected at time 0 into three segments (1, 2, and 3). (B), (C) Dissected rice leaf explants (segments 1, 2, and 3) from wild type (Hwayoung) (B) and Oswox11‐1 (C) cultured on CIM at 20 DAC. Note that the regenerative ability was weaker in Oswox11‐1 than in wild type. (D) Callus formation rate analyses of cultured rice leaf segment explants. The rate was evaluated by counting the ratio of explants that formed callus. Bars show the SD of three biological repeats (n = 30 for each repeat). (E), (F) Callus formation in wild‐type Arabidopsis leaf explants (E) and 35Spro:OsWOX11 Arabidopsis leaf explants (F) at 8 DAC. (G), (H) GUS staining at time 0 (G) and 1 DAC (H) in 7‐mm leaf explants from OsWOX11pro:GUS rice on CIM. (I), (J) Transverse section of leaf explants from OsWOX11pro:GUS rice on CIM at 1 DAC (I) and 5 DAC (J). Note that the GUS signal was present primarily in the outer sheath at 1 DAC (c), and occasionally could also be observed in some parenchyma cells and vascular cells: o, outer sheath; x, xylem; p, phloem. (K)–(M) Transverse sections at the tip region of the rice root explant at time 0 (K), 2 HAC (L), and 2 DAC (M), showing in situ hybridization of OsWOX12B. Note that OsWOX12B was not detected at time 0 (K), was located primarily in the phloem‐pole pericycle and occasionally in the endodermis at 2 HAC (L), and was absent from the callus at 2 DAC (M). The green lines indicate the four‐cell structure of phloem (Zeng et al., 2016); c, companion cell in phloem; s, sieve‐tube element in phloem; asterisks indicate the phloem‐pole pericycle. (N), (O) Transverse sections of rice leaf explants at 5 DAC (N) and rice root explants at 2 DAC (O) showing in situ hybridization of OsWOX5 in callus. (P) Sense control. (Q) Model of cell fate transition during callus formation in Arabidopsis and cereals. Scale bars: (A)–(C), (E)–(H) 1 mm; (I)–(P) 50 μm
In addition, overexpression of OsWOX11 in Arabidopsis resulted in dramatically rapid callus formation. In the Arabidopsis wild‐type Columbia‐0 (Col‐0) leaf explant, a small piece of callus was produced at the wounded region at 8 DAC (Fig. 2E), whereas callus formed everywhere on the Arabidopsis leaf explant from the 35Spro:OsWOX11 line (Fig. 2F).
Next, we analyzed the spatial expression pattern of OsWOX genes during rice callus formation. We constructed the transgenic rice line carrying OsWOX11pro:GUS. The GUS signal was not observed in time‐0 leaf explants (Fig. 2G), but was clearly present in the vasculature of leaf explants at 1 DAC (Figs. 2H and S2). A sectioning experiment showed that the GUS signal was primarily localized in the outer sheath at 1 DAC (Fig. 2I). The GUS signal disappeared when callus was undergoing rapid cell division at 5 DAC (Fig. 2J). The results of in situ hybridization analyses showed that, in the root tip region, the expression of OsWOX12B and OsWOX11 was induced primarily in the phloem‐pole pericycle cells at 2 h after culture (HAC) and disappeared from the dividing callus at 2 DAC (Figs. 2K–M and S3). OsWOX5 was detected in callus cells in both leaf and root explants (Fig. 2N–P). These spatial expression patterns indicated that the expression of OsWOX11/12B marks the appearance of founder cells for callus initiation while OsWOX5 marks callus undergoing rapid cell division in rice. Therefore, OsWOX11/12B and OsWOX5 may serve as molecular markers in cell fate transition during callus formation.
2.3. Angiosperms may have a common mechanism for callus initiation
The diversification of monocots and dicots was estimated to occur in the Jurassic (Zeng et al., 2014). In the dicot Arabidopsis, procambium and vascular parenchyma cells in leaves and xylem‐pole pericycle cells in roots serve as regeneration‐competent cells for callus initiation (Atta et al., 2009; Che et al., 2007; Liu et al., 2014; Yu et al., 2010). This suggests that regeneration‐competent cells differ between the dicot Arabidopsis and the monocot rice (see the model in Fig. 2Q). Whether the diverse regeneration‐competent cells in angiosperms share the same evolutionary lineage is not yet clear. However, the divergent regeneration‐competent cells act similarly at the molecular level during regeneration. OsWOX11/12B and their Arabidopsis homologs AtWOX11/12 mark fate transition from regeneration‐competent cells to founder cells, and OsWOX5 and its Arabidopsis homolog AtWOX5 mark fate transition from founder cells to callus cells (Liu et al., 2014) (see the analysis of Arabidopsis callus formation in Fig. S4). In addition, WOX11 can activate WOX5 expression in both Arabidopsis and rice (Hu & Xu, 2016) (see the activation of OsWOX5 by OsWOX11 in Fig. S5).
To test whether this molecular pathway is generally involved in regeneration, we carried out quantitative reverse transcription polymerase chain reaction (qRT‐PCR) analyses to quantify the transcript levels of WOX11 and WOX5 homologs in the dicot poplar and the monocot maize during callus formation in leaf explants. Our data showed that expression levels of WOX11 and WOX5 homologs were dramatically induced on CIM in poplar and maize (Fig. S6). Therefore, it is possible that the molecular mechanism in regeneration could be conserved among angiosperms.
Overall, activation of WOX5 is the marker of callus cell formation. We do not exclude the possibility that some non‐WOX11/12‐mediated pathways may also be able to activate WOX5 for callus formation (Liu et al., 2014; Sheng et al., 2017).
2.4. Rice and Arabidopsis have different strategies for maintenance of regeneration‐competent cells during organ maturation
It is well known that the dicot Arabidopsis and monocot cereals have different regenerative abilities. Almost all organs of Arabidopsis are able to produce callus during the entire life of the plant (He et al., 2012; Sugimoto et al., 2010). We dissected mature Arabidopsis leaves into four segments (segments 1 to 4, from the base to the tip), and all dissected segments of leaf explants were able to form callus (Fig. 3A). In contrast, mature organs of many monocot cereal species are extremely unresponsive to in vitro culture techniques (Bhojwani et al., 1977; Cutler et al., 1991). We also dissected mature leaves from rice and maize into four segments from the base to the tip, and only the base region in segment 1 (immature region) was able to form callus on CIM (Figs. 3C, D and S7A). This is consistent with the results of previous studies showing that only the very base region of leaves from many monocot cereals, including barley, rice, wheat, oat, and maize, can form callus (Ahmadabadi, Ruf, & Bock, 2007; Becher, Haberland, & Koop, 1992; Chen, Xu, Loschke, Tomaska, & Rolfe, 1995; Chen, Zhuge, & Sundqvist, 1995; Cutler et al., 1991; Wernicke & Milkovits, 1984; Wernicke, Brettell, Wakizuka, & Potrykus, 1981; Zamora & Scott, 1983).
Figure 3.
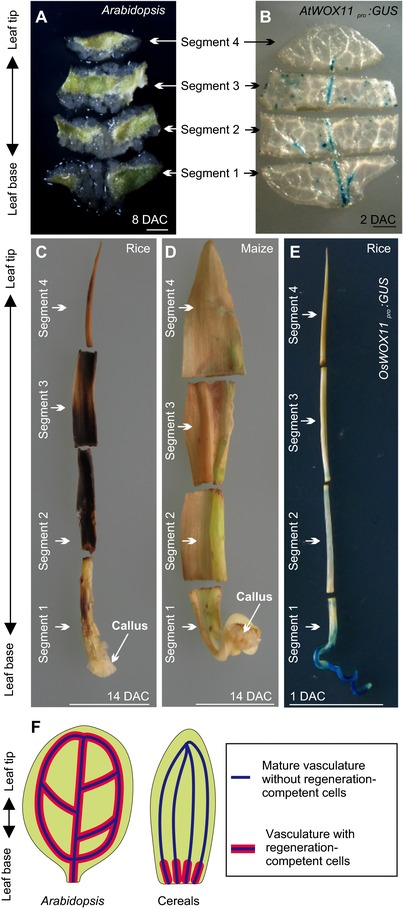
Different regenerative responses between the dicot Arabidopsis and monocot cereals. (A) Dissected mature Arabidopsis leaf explant cultured on CIM. All four segments were able to form callus. (B) GUS staining of mature leaf explants from AtWOX11pro:GUS Arabidopsis on CIM at 1 DAC. GUS signal was present in all four segments. (C), (D) Dissected rice leaf explant (5 cm) (C) and maize leaf explant (5 cm) (D) cultured on CIM, showing that only the very base segment (segment 1) was able to form callus. (E) GUS staining of 5‐cm leaf explants from OsWOX11pro:GUS rice on CIM at 1 DAC. Note that the GUS signal was strongly induced in leaf base segment (segment 1). (F) Model of regenerative abilities in leaves of Arabidopsis and cereals. Scale bars: (A), (B) 1 mm; (C)−(E) 1 cm
We hypothesize that the diversification of regenerative abilities in Arabidopsis and cereals is due to different strategies of maintenance of regeneration‐competent cells in mature organs. Regeneration‐competent cells, such as procambium cells, were maintained in Arabidopsis leaves as they matured. However, regeneration‐competent cells have differentiated into specific cell types (Zeng et al., 2016) and thus have lost their competence upon maturation from the tip region to the base region of the leaf in cereals. For example, the outer sheath in the rice mature leaf differentiated into large parenchyma cells (Zeng et al., 2016) (Fig. S7B). In maize, callus formation could be observed from many cells of the immature vasculature at the base region of the leaf explant when cultured on CIM (Figs. S7C, D and 2Q). During the development of the vasculature, callus formation could be observed primarily from the bundle sheath (Figs. S7C, E and 2Q). In mature maize leaves, the bundle sheath differentiated into Kranz anatomy (Fig. S7F) and therefore lost the ability to form callus.
To test this hypothesis at the molecular level, we analyzed expression patterns of WOX11 in mature leaves of Arabidopsis and rice in tissue culture. We observed that AtWOX11 was induced in the vasculature of all dissected segments of the mature Arabidopsis leaf on CIM (Fig. 3B); this may have rendered the whole Arabidopsis leaf competent to form callus at leaf maturity (see the model in Fig. 3F). In contrast, the GUS signal from the OsWOX11pro:GUS line was present at the base part of segment 1 (immature region), but was barely detected in segments 2−4 (mature region) of the dissected rice leaf explant on CIM (Fig. 3E). Therefore, as the rice leaves matured, the differentiation of regeneration‐competent cells resulted in the loss of their molecular competence for callus initiation (see the model in Fig. 3F).
Next, we tested the callus formation ability in rice leaf explants overexpressing OsWOX11. The data showed that overexpression of OsWOX11 in rice cannot reverse the fate of mature and differentiated vascular cells to be competent for callus formation (Fig. S8). Callus formation requires not only WOX11 but also many other molecular pathways such as LATERAL ORGAN BOUNDARIES DOMAIN genes (Fan, Xu, Xu, & Hu, 2012), WOUND INDUCED DEDIFFERENTIATION 1 (Iwase et al., 2011; Iwase et al., 2017), and some epigenetic factors (He et al., 2012; Li et al., 2011) in Arabidopsis. PLETHORAs contribute to the pluripotency of callus (Kareem et al., 2015). It will be interesting to analyze these pathways during callus formation in rice and to test whether it is possible to endow differentiated cells with competence for callus formation in the future.
3. CONCLUSION AND PERSPECTIVE
In this study, we have provided cellular and molecular frameworks of callus formation in angiosperms. Regeneration‐competent cells differ between the dicot Arabidopsis and the monocot rice, whereas those diverse cells adopt a common mechanism involving WOX11 and WOX5 during cell fate transition for callus initiation. Depletion of regeneration‐competent cells during organ maturation may result in loss of regenerative ability in cereals.
Previous studies indicate that callus formation follows the rooting pathway (He et al., 2012; Liu et al., 2014; Sugimoto et al., 2010) and callus is a group of root primordium‐like cells (Liu et al., 2014). In addition, the regeneration‐competent cells for callus initiation could also initiate roots (Liu et al., 2014). During adventitious rooting in Arabidopsis, AtWOX11 controls root founder cell establishment (Liu et al., 2014) and AtWOX5 is required for root primordium formation (Hu & Xu, 2016). Based on these studies, we hypothesize that the WOX11−WOX5‐mediated root initiation mechanism in the common ancestor of angiosperms was borrowed and developed for callus initiation in regeneration‐competent cells of dicots and monocots, although the morphology of these cells has changed during evolution. Understanding of the regeneration‐competent cell behaviour in different plant species is the basis to utilize and improve the regenerative abilities in tissue culture.
4. MATERIALS AND METHODS
4.1. Plant materials
Oryza sativa L. japonica. cv. Nipponbare, Arabidopsis thaliana Col‐0, maize (Zea mays) B73, and poplar (Populus davidiana XP. bollena cv. Shan‐Xin) were used as wild types in this study unless otherwise noted. The Oswox11‐1 mutant (PFG_2A‐00597, Hwayoung background) was described previously (Jeon et al., 2000; Jeong et al., 2006; Zhao et al., 2009).
To produce OsWOX11pro:GUS transgenic plants, the 4‐kb promoter of OsWOX11 was PCR amplified and inserted into pBImUB (modified from pBI101). AtWOX11pro:GUS and AtWOX5pro:GUS transgenic plants were produced as described previously (Liu et al., 2014). For OsWOX11 overexpression, cDNA fragments encoding the full‐length OsWOX11 protein were PCR amplified and inserted into pCAMBIA1300‐35S (modified from pCAMBIA1300) for overexpression in Arabidopsis or inserted into pCAMBIA1301‐UBiN for overexpression in rice. Transgenic plants were obtained by Agrobacterium tumefaciens‐mediated transformation into rice (Biorun, Wuhan, China) or Arabidopsis. The primers used for plasmid construction are listed in Table S2.
4.2. Tissue culture
Rice seeds were sterilized and placed on half‐strength Murashige and Skoog basal medium with 1% sucrose, 1% agar, and 0.5 g/L 2‐(N‐morpholino)ethanesulfonic acid (MES), pH 5.7 (Murashige & Skoog, 1962), for germination. For tissue cultures, sterilized explants were cultured at 29°C in darkness on CIM (N6 basal medium with 3% w/v sucrose, 0.3% w/v Phytagel, 0.5 g/L MES, pH 5.8, and 2,4‐dichlorophenoxyacetic acid) (Chu et al., 1975). The CIM was supplemented with 2,4‐dichlorophenoxyacetic acid at 2 mg/L for explants from rice, poplar, and maize. Tissue culture conditions for Arabidopsis were described previously (Liu et al., 2014).
4.3. Thin sectioning, in situ hybridization and dual luciferase assay
Thin sectioning was performed as previously described (Zeng et al., 2016). For in situ hybridization, gene fragments used to prepare probes were subcloned into pGEM‐T Easy. In situ hybridization analyses were performed as reported previously (Zeng et al., 2016). To construct OsWOX5pro:LUC, the promoter of OsWOX5 was PCR amplified and inserted into the pGreenII‐0800 vector (Hellens et al., 2005). The dual luciferase assay was performed using the Dual‐Luciferase Reporter Assay System (Promega, Madison, WI). The primers used for plasmid construction are listed in Table S2.
4.4. qRT‐PCR and RNA‐sequencing analyses
RNA extraction and qRT‐PCR were performed as previously described (He et al., 2012), using gene‐specific primers. The qRT‐PCR results are shown as relative transcript levels, which were normalized against that of ACTIN. The primers used for real‐time PCR are listed in Table S2.
For RNA‐sequencing analyses, RNA was isolated from the base region of time‐0, 2‐DAC and 5‐DAC rice leaf explants (Fig. S1). Deep sequencing was carried out using the Illumina HiSeq3000 platform following the manufacturer's instructions (Illumina, San Diego, CA). Library construction and deep sequencing were performed by Genergy Biotechnology Co. Ltd (Shanghai, China). Raw data comprised 100‐bp paired‐end sequences. Raw sequences were aligned to the rice genome with TopHat software (Trapnell et al., 2012), and differential expressed gene analysis was performed using DESeq (Anders & Huber, 2010). Highly upregulated genes were defined as fold change >10.0 and p value <0.05. The analyzed data are shown in Table S1.
4.5. Accession numbers
The RNA‐sequencing data have been deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) under the accession number GSE86869. Sequence data can be obtained using the following accession numbers: Rice Genome Annotation Project, OsWOX11 (LOC_Os07g48560), OsWOX12B (LOC_Os03g20910), and OsWOX5 (LOC_Os01g63510); Genbank, ZmWOX11A (AM234774) and ZmWOX5A (AM234769); Phytozome, PdPbWOX11 (Potri.013G066900) and PdPbWOX5 (Potri.008G065400); and Arabidopsis Genome Initiative, AtWOX11 (At3g03660) and AtWOX5 (At3g11260).
Supporting information
Figure S1. Identification of OsWOX11, OsWOX12B, and OsWOX5 in rice callus formation.
Figure S2. OsWOX11 expression during callus formation from leaf explant.
Figure S3. OsWOX11 expression during callus formation from root explant.
Figure S4. AtWOX11 and AtWOX5 in Arabidopsis callus formation.
Figure S5. OsWOX11 activates OsWOX5.
Figure S6. WOX11 and WOX5 expression during callus formation in poplar and maize.
Figure S7. Leaf explants of rice and maize in tissue culture.
Figure S8. Overexpression of OsWOX11 in rice.
Table S1. RNA‐seq data.
Table S2. List of primers used in this study.
ACKNOWLEDGMENTS
We thank Gynheung An (Kyung Hee University), Hongxia Zhang and Hongtao Liu (Institute of Plant Physiology and Ecology) for seeds of rice mutant, wild‐type poplar and wild‐type maize, respectively. This work was supported by grants from the National Natural Science Foundation of China (31630007), National Basic Research Program of China (973 Program, 2014CB943500), the Key Research Program of CAS (QYZDB‐SSW‐SMC010), the Strategic Priority Research Program “Molecular Mechanism of Plant Growth and Development” of CAS (XDPB0403), and Youth Innovation Promotion Association CAS (2014241).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
B.H. and L.X. designed the research, G.Z., W.L., J.S., M.Q., P.Q., and Y.Z. performed the RNA‐seq experiment and RNA‐seq data analysis. B.H., G.Z., J.S., and J.L. performed thin sectioning. B.H. and H.W. performed in situ hybridization. B.H., G.Z., W.L., and J.S. performed other experiments. All authors analyzed data. H.H. and L.X. wrote the article.
Hu B, Zhang G, Liu W, Shi J, Wang H, Qi M, Li J, Qin P, Ruan Y, Huang H, Zhang Y, Xu L. Divergent regeneration‐competent cells adopt a common mechanism for callus initiation in angiosperms. Regeneration. 2017;4:132–139. https://doi.org/10.1002/reg2.82
REFERENCES
- Ahmadabadi, M. , Ruf, S. , & Bock, R. (2007). A leaf‐based regeneration and transformation system for maize (Zea mays L.). Transgenic Research, 16(4), 437–448. [DOI] [PubMed] [Google Scholar]
- Anders, S. , & Huber, W. (2010). Differential expression analysis for sequence count data. Genome Biology, 11(10), R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta, R. , Laurens, L. , Boucheron‐Dubuisson, E. , Guivarc'h, A. , Carnero, E. , Giraudat‐Pautot, V. , … Chriqui, D. (2009). Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant Journal, 57(4), 626–644. [DOI] [PubMed] [Google Scholar]
- Becher, T. , Haberland, G. , & Koop, H. U. (1992). Callus formation and plant regeneration in standard and microexplants from seedlings of barley (Hordeum vulgare L.). Plant Cell Reports, 11(1), 39–43. [DOI] [PubMed] [Google Scholar]
- Bhojwani, S. S. , Evans, P. K. , & Cocking, E. C. (1977). Protoplast technology in relation to crop plants: progress and problems. Euphytica, 26, 343–360. [Google Scholar]
- Che, P. , Lall, S. , & Howell, S. H. (2007). Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta, 226(5), 1183–1194. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Xu, G. , Loschke, D. C. , Tomaska, L. , & Rolfe, B. G. (1995). Efficient callus formation and plant regeneration from leaves of oats (Avena sativa L.). Plant Cell Reports, 14(6), 393–397. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Zhuge, Q. , & Sundqvist, C. (1995). Oat leaf base: tissue with an efficient regeneration capacity. Plant Cell Reports, 14(6), 354–358. [DOI] [PubMed] [Google Scholar]
- Chu, C. C. , Wang, C. C. , Sun, C. S. , Hsu, C. , Yin, K. C. , & Chu, C. Y. (1975). Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Scientia Sinica, 16, 659–688. [Google Scholar]
- Cutler, A. J. , Saleem, M. , & Wang, H. (1991). Cereal protoplast recalcitrance. In Vitro Cell Dev Biol‐Plant, 27, 104–111. [Google Scholar]
- Fan, M. , Xu, C. , Xu, K. , & Hu, Y. (2012). Lateral organ boundaries domain transcription factors direct callus formation in Arabidopsis regeneration. Cell Research, 22(7), 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff, E. , Laux, T. , & Rensing, S. A. (2009). The WUS homeobox‐containing (WOX) protein family. Genome Biology, 10(12), 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker, A. , Gross‐Hardt, R. , Geiges, B. , Sarkar, A. , Breuninger, H. , Herrmann, M. , & Laux, T. (2004). Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana . Development (Cambridge, England), 131(3), 657–668. [DOI] [PubMed] [Google Scholar]
- He, C. , Chen, X. , Huang, H. , & Xu, L. (2012). Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. Public Library of Science GENET 8(8), e1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens, R. P. , Allan, A. C. , Friel, E. N. , Bolitho, K. , Grafton, K. , Templeton, M. D. , … Laing, W. A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant methods, 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , & Xu, L. (2016). Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiology, 172(4), 2363–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi, M. , Ogawa, Y. , Iwase, A. , & Sugimoto, K. (2016). Plant regeneration: cellular origins and molecular mechanisms. Development (Cambridge, England), 143(9), 1442–1451. [DOI] [PubMed] [Google Scholar]
- Ikeuchi, M. , Sugimoto, K. , & Iwase, A. (2013). Plant callus: mechanisms of induction and repression. Plant Cell, 25(9), 3159–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase, A. , Harashima, H. , Ikeuchi, M. , Rymen, B. , Ohnuma, M. , Komaki, S. , … Sugimoto, K. (2017). WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis . Plant Cell, 29(1), 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase, A. , Mitsuda, N. , Koyama, T. , Hiratsu, K. , Kojima, M. , Arai, T. , … Ohme‐Takagi, M. (2011). The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis . Current Biology, 21(6), 508–514. [DOI] [PubMed] [Google Scholar]
- Jeon, J. S. , Lee, S. , Jung, K. H. , Jun, S. H. , Jeong, D. H. , Lee, J. , … An, G. (2000). T‐DNA insertional mutagenesis for functional genomics in rice. Plant Journal, 22(6), 561–570. [DOI] [PubMed] [Google Scholar]
- Jeong, D. H. , An, S. , Park, S. , Kang, H. G. , Park, G. G. , Kim, S. R. , … An, G. (2006). Generation of a flanking sequence‐tag database for activation‐tagging lines in japonica rice. Plant Journal, 45(1), 123–132. [DOI] [PubMed] [Google Scholar]
- Kareem, A. , Durgaprasad, K. , Sugimoto, K. , Du, Y. , Pulianmackal, A. J. , Trivedi, Z. B. , … Prasad, K. (2015). PLETHORA genes control regeneration by a two‐step mechanism. Current biology: CB, 25(8), 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem, A. , Radhakrishnan, D. , Sondhi, Y. , Aiyaz, M. , Roy, M. V. , Sugimoto, K. , & Prasad, K. (2016). De novo assembly of plant body plan: a step ahead of Deadpool. Regeneration (Oxf), 3(4), 182–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Liu, H. , Cheng, Z. J. , Su, Y. H. , Han, H. N. , Zhang, Y. et al. (2011). DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. Public Library of Science Genet, 7(8), e1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian, G. , Ding, Z. , Wang, Q. , Zhang, D. , & Xu, J. (2014). Origins and evolution of WUSCHEL‐related homeobox protein family in plant kingdom. Thescientificworldjournal [Electronic Resource], 2014, 534140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Sheng, L. , Xu, Y. , Li, J. , Yang, Z. , Huang, H. , & Xu, L. (2014). WOX11 and 12 are involved in the first‐step cell fate transition during de novo root organogenesis in Arabidopsis . Plant Cell, 26(3), 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T. , & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologie Plant, 80, 662–668. [Google Scholar]
- Sheng, L. , Hu, X. , Du, Y. , Zhang, G. , Huang, H. , Scheres, B. , & Xu, L. (2017). Non‐canonical WOX11‐mediated root branching contributes to plasticity in Arabidopsis root system architecture. Development, https://doi.org/10.1242/dev.152132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, K. , Gordon, S. P. , & Meyerowitz, E. M. (2011). Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends in Cell Biology, 21(4), 212–218. [DOI] [PubMed] [Google Scholar]
- Sugimoto, K. , Jiao, Y. , & Meyerowitz, E. M. (2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Developmental Cell, 18(3), 463–471. [DOI] [PubMed] [Google Scholar]
- Sussex, I. M. (2008). The scientific roots of modern plant biotechnology. Plant Cell, 20(5), 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, C. , Roberts, A. , Goff, L. , Pertea, G. , Kim, D. , Kelley, D. R. , … Pachter, L. (2012). Differential gene and transcript expression analysis of RNA‐seq experiments with TopHat and Cufflinks. Nature protocols, 7(3), 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, G. (2005). How does a single somatic cell become a whole plant. Science, 309, 86. [DOI] [PubMed] [Google Scholar]
- Wernicke, W. , Brettell, R. , Wakizuka, T. , & Potrykus, I. (1981). Adventitious embryoid and root formation from rice leaves. Zeitschrift fur Pflanzenphysiologie, 103, 361–365. [Google Scholar]
- Wernicke, W. , & Milkovits, L. (1984). Developmental gradients in wheat leaves—response of leaf segments in different genotypes cultured in vitro. Journal of Plant Physiology, 115(1), 49–58. [DOI] [PubMed] [Google Scholar]
- Xu, L. , & Huang, H. (2014). Genetic and epigenetic controls of plant regeneration. Current Topics in Developmental Biology, 108, 1–33. [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Feng, Z. , Wang, G. , Li, F. , Du, X. , & Zhu, J. (2010). Initiation of dedifferentiation and structural changes in in vitro cultured petiole of Arabidopsis thaliana . Protoplasma, 241(1‐4), 75–81. [DOI] [PubMed] [Google Scholar]
- Zamora, A. B. , & Scott, K. J. (1983). Callus formation and plant regeneration from wheat leaves. Plant Science Letters, 29, 183–189. [Google Scholar]
- Zeng, L. , Zhang, Q. , Sun, R. , Kong, H. , Zhang, N. , & Ma, H. (2014). Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times. Nature Communications, 5, 4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, M. , Hu, B. , Li, J. , Zhang, G. , Ruan, Y. , Huang, H. , … Xu, L. (2016). Stem cell lineage in body layer specialization and vascular patterning of rice root and leaf. Science Bulletin, 61(11), 847–858. [Google Scholar]
- Zhao, Y. , Hu, Y. , Dai, M. , Huang, L. , & Zhou, D. X. (2009). The WUSCHEL‐related homeobox gene WOX11 is required to activate shoot‐borne crown root development in rice. Plant Cell, 21(3), 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Identification of OsWOX11, OsWOX12B, and OsWOX5 in rice callus formation.
Figure S2. OsWOX11 expression during callus formation from leaf explant.
Figure S3. OsWOX11 expression during callus formation from root explant.
Figure S4. AtWOX11 and AtWOX5 in Arabidopsis callus formation.
Figure S5. OsWOX11 activates OsWOX5.
Figure S6. WOX11 and WOX5 expression during callus formation in poplar and maize.
Figure S7. Leaf explants of rice and maize in tissue culture.
Figure S8. Overexpression of OsWOX11 in rice.
Table S1. RNA‐seq data.
Table S2. List of primers used in this study.


