Abstract
Staphylococcus aureus is a major pathogen causing bone infections that can become chronic and difficult to treat. Recently, we described the mechanism employed by S. aureus to switch to small colony variants (SCVs) and trigger intracellular bacterial persistence through the global stress regulator SigB. Here, we studied the role of SigB in the formation of chronic osteomyelitis. We used a murine hematogenous osteomyelitis model, where the mice were infected via the tail vein and subsequently developed chronic osteomyelitis. Mice were infected with S. aureus LS1, LS1ΔsigB and LS1ΔsigB complemented and kidney and bone tissues were analyzed six weeks after infection. S. aureus LS1ΔsigB formed a high rate of abscesses in kidneys, but the bacterial loads and the weight loss of the animals were lower in comparison with animals infected with the wild type and the complemented strain, indicating a more rapid and efficient bacterial clearing by the host immune system. Moreover, the sigB-mutant was not able to form SCV phenotypes either in kidney or in bone tissue. Our results demonstrate that staphylococcal SigB is important to avoid bacterial elimination by the host immune response, establish a bone infection and mediate bacterial adaptation (SCV-formation) for persistent infections
Keywords: sigB, osteomyelitis, abscess, SCVs
1. Introduction
Staphylococcus aureus is an opportunistic pathogen that is able to trigger a variety of diseases including osteomyelitis, endocarditis and indwelling medical devices. These infections can develop to chronic courses, where they become highly refractory to antibiotic treatment [1,2]. The reason is most likely the complex adaption strategies of S. aureus to host tissue. Staphylococcal persistence is associated with a sub-population of phenotypic variants called small colony variants (SCVs) [3]. SCVs grow slowly due to a reduced metabolism and form only very small colonies on agar plates. Recently, we found that SCVs develop in a highly dynamic manner during the infection course and their formation is dependent on the alternative transcription factor sigma B (SigB) [4,5,6]. SigB is known as a stress-induced staphylococcal transcription factor that alters the expression of several genes related with virulence and metabolism. SigB is expressed during the early stationary phase of growth and is involved in the expression of some genes, like adhesins (e.g., fnbA induced during the logarithmic phase and clfA induced during the transition to stationary phase), but has a negative effect on the expression of genes encoding exoproteins in the late stationary growth (e.g., alpha toxin, hla) [4]. In our recent work, we found that after infection of primary human osteoblasts, the ΔsigB mutants were rapidly cleared by the host cells and failed to form SCVs. Moreover, proteomic analysis showed high expression of toxins (such as alpha toxin, hla) by ΔsigB [4]. During the pathogenesis of osteomyelitis, many staphylococcal virulence factors define the course of infection [5]. The expression of toxins allows S. aureus to establish an acute infection, destroy host tissue, invade deep tissue structures and fight against host immune cells [6,7,8]. Moreover, S. aureus develops strategies to hide and persist within host cells/tissue to establish a chronic infection. In this study, we analyzed the role of SigB during an acute hematogenous infection that develops to a chronic osteomyelitis in mice. We demonstrate that the lack of sigB induced high numbers of abscesses which contributed to the clearance of the bacteria. Consequently, SigB represents a potential target to eliminate S. aureus and prevent the development of SCVs associated with chronic osteomyelitis.
2. Materials and Methods
2.1. Strains for Infection Models
S. aureus wild-type LS1, ΔsigB and the complemented sigB strain were used in our study. The characterization of these strains was described recently [4]. For the infection experiments, overnight cultures of the S. aureus strains LS1, ΔsigB and the complemented sigB (ΔsigB complemented) were prepared in 30 mL of brain heart infusion (BHI) broth and incubated at 37 °C and 160 rpm (for each strain). After two washing steps with PBS and centrifugation at 5000 rpm, the concentration of each strain was adjusted to an OD of 1 at 578 nm. Cell pellets were resuspended in PBS. To control the concentration of the inoculum, the number of CFU/mL was determined by plating in 10-fold serial dilutions on blood agar. All strains presented only wild type and not SCV phenotype at the time of administration.
2.2. Haematogenous S. aureus Osteomyelitis Model in Mice
Groups of 6-10 C57bl-6 mice 10 weeks old female were infected with S. aureus LS1, LS1ΔsigB and the complemented mutant for sigB (ΔsigB complemented), which have been described and characterized previously [4]. The model has already been described by Horst et al. [9]. Briefly, the mice were infected intravenously with 106 CFUs of S. aureus in 150 μL of PBS. Mice were sacrificed after 6 weeks post infection by CO2 asphyxiation at the indicated time of infection. The animals were maintained in individually ventilated cages and were given food and water ad libitum. All experiments were approved by the North Rhine-Westphalia Agency for Nature, Environment, and Consumer Protection (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen-LANUV; permit number 84-02.04.2012.A293). For enumeration of bacteria in bones (femur and tibia) and kidneys of infected mice were transferred to one tube with 2 mL PBS and homogenized mechanically on ice (polytron pt2500, Fisher Scientific, Schwerte, Germany). The homogenized tissues were plated in 10-fold serial dilutions on blood agar and the plates were incubated at 37 °C. The percentages of big WT and SCV-like colonies of the intracellular surviving bacteria were determined by a colony counter (Biocount 5000, BioSys GmbH, Karben, Germany). All colonies with a diameter <0.6 mm were considered as SCVs (<5 and <10-fold smaller than those of the wild-type phenotypes, respectively). Due to the slow formation of SCVs, the final values of the amount of SCVs on agar were determined after 72 h of incubation. The animals were weighted daily during the first 22 days. The weight changes of infected mice were assessed by measuring the mice pre-infection and 7 days post-infection because after this point the animals died or recovered the weight.
2.3. Statistical Analysis
Data were analyzed using GraphPad Prism software version 5 (GraphPad Software, La Jolla, CA, USA). Data are expressed as means ± SD. Comparison between groups was performed by use of a one-way analysis of variance test followed by Turkey’s multiple comparisons test. The p value was interpreted as: ns p > 0.05; * p > 0.05; ** p > 0.01; *** p > 0.001 and **** p > 0.0001.
3. Results and Discussion
3.1. SigB Is Required for S. aureus Persistence in Bone and Kidney Tissue
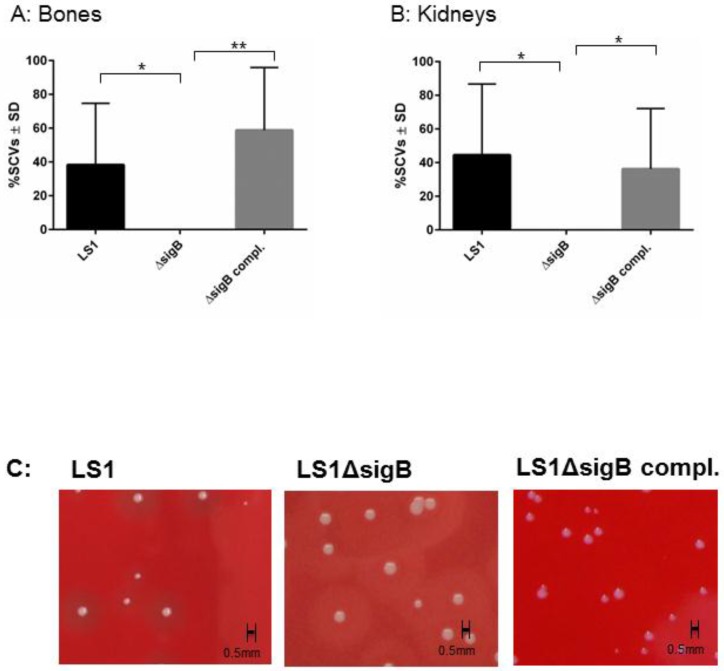
To study the function of SigB in the course from acute to chronic infection, we performed a hematogenous murine model of chronic S. aureus infection. After 6 weeks post infection, we recovered disseminated bacteria from kidneys and bones and the enumeration of CFU/mL was analyzed on blood agar (Figure 1). In both tissues LS1 persisted in significant numbers after 6 weeks. By contrast, LS1ΔsigB was almost cleared whereas this effect could be fully reversed by the complementation of sigB (Figure 1A,B, Table 1). Furthermore, in both tissues a high percentage of SCVs was found indicating S. aureus adaptation (Figure 2A–C). However, after infection with LS1ΔsigB the formation of SCVs was completely absent. These results are in line with previously published cell culture models [4,10] (Figure 2C). The recovery of CFUs was higher in bone than in kidneys, indicating a preference of S. aureus for bone tissue during long-term persistence (Figure 1A,B). Furthermore, the SCV formation was observed in both tissues showing the ability of S. aureus to adapt to different types of host cells/tissue (Figure 2A,B). By using our rat local osteomyelitis model, where S. aureus was directly inoculated in bone[4], we demonstrated that the mutants were not able to establish a local bone infection. In our hematogenous murine model we could analyze the role of sigB during the switch from acute to chronic infection. Taken together, our results demonstrate a crucial role of SigB in establishing a chronic infection, particularly in bone tissue.
Figure 1.
SigB is necessary for persistence of S. aureus in bone and kidney tissues. Bacterial persistence of LS1, ΔsigB and ΔsigB complemented was analyzed in a murine chronic osteomyelitis model 6 weeks p.i. Bones (A) and kidneys (B) were homogenized and plated on agar plates for counting the CFUs on the following day. The results represent the means ± SD and were analyzed by ANOVA test and Turkey’s as multicomparison test.
Table 1.
Survival rate for each strain. The survival rate was calculated in percentage taking in consideration that the initial bacterial inoculum was in log = 6.67 CFU/mL (this amount was taken as 100%).
| Strain | Bone | Kidney |
|---|---|---|
| LS1 | 54.3% | 43.6% |
| LS1ΔsigB | 7.26% | 0% |
| LS1ΔsigB complemented | 55.7% | 46.4% |
Figure 2.
The ΔsigB mutant does not form SCV phenotypes. The phenotypic diversity was analyzed on blood agar plate after 48 and 72 h of incubation. The percentage of small and very small (SCV) phenotypes (all colonies with a diameter <0.6 mm with size <5 and <10-fold smaller than those of the wild-type phenotypes, respectively) recovered (between 200 and 500 colonies examined in each sample) were determined after 6 weeks p.i. from homogenized bones (A) and kidneys (B) infected with LS1, ΔsigB and ΔsigB complemented strains. The values represent the means ± SD and were analyzed by ANOVA and Turkey’s test for multiple comparison. C) Photographs of recovered colonies were performed after infection of C57/Bl6 mice with strains LS1, LS1∆sigB or LS1∆sigB compl.
3.2. Mutation of sigB Induced Several Abscess in Kidneys and Enhanced Bacterial Clearing
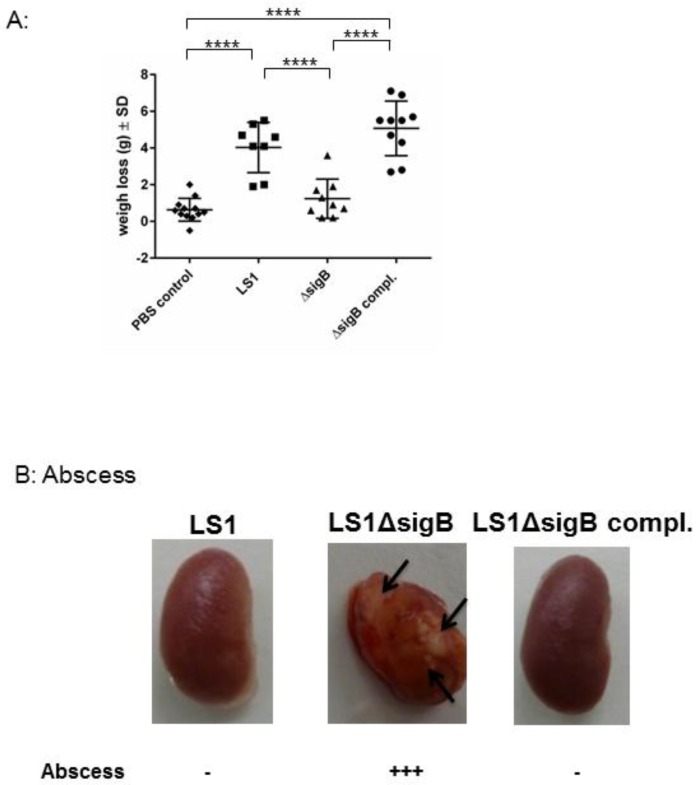
To understand the role of sigB during the whole course of infection, we analyzed the host response, such as abscess formation and the weight changes of all infected animals. S. aureus WT induced a systemic infection reflected by a high weight loss (Figure 3). By contrast, the mice infected with S. aureus ΔsigB showed only a moderate weight change in comparison with mice infected with the WT strain. S. aureus ΔsigB complemented induced a similar effect as the WT strain LS1. Moreover, the kidneys from mice infected with ΔsigB mutant showed several abscesses after 6 weeks compared with mice infected with WT or complemented ΔsigB strain. It is well known that the absence of sigB has an enhancing effect on agr that results in increased expression of proinflammatory virulence factors [11]. Bischoff et al. described that sigB reduces agr expression in a growth phase-dependent manner [11]. Thus, the absence of sigB induces upregulation of staphylococcal toxins and downregulation of adhesins [10,11,12]. Consequently, LS1ΔsigB can cause a high inflammatory response resulting in massive abscess formation and in complete elimination of bacteria directly in kidneys [5,7,11]. Abscess formation is the result of immune host cells recruited by staphylococcal virulence factors [13]. Consequently, the high virulence expression due to the mutation of sigB enhances the elimination of S. aureus during the blood passage in kidneys which is reflected by the formation of abscess. Accordingly, sigB enhances the formation of abscess that apparently promotes the elimination of S. aureus.
Figure 3.
The absence of sigB in S. aureus induced abscess formation in kidneys and early recovery of the infected animals. Mice were infected via tail vein with LS1, ΔsigB and ΔsigB complemented strains. (A) The body weights of surviving mice were monitored for 21 days and recorded at each time point. Here we represent the difference between the day 0 immediately after infection and 7 days post infection. The PBS group showed almost no change in weight. In contrast, mice in the LS1 and ΔsigB complemented strains group showed a significant decrease in body weight during the 7-day period in comparison with PBS and ΔsigB groups (p < 0.05). There was no significant difference in body weight between the ΔsigB and PBS groups (p > 0.05). One-way analyses of variance (ANOVA) followed by the Tukey test were used to compare multiple groups. (B) The photographs of recovered kidney after 6 weeks post infection are shown. The arrows indicate the localization of abscess. The amount of abscess was estimated by eye observation.
4. Conclusions
The staphylococcal global stress regulator SigB is a central factor for establishing chronic staphylococcal bone infections. The lack of SigB induces a high expression of secreted virulence factors[5], which contribute to abscess formation where most of the bacteria are efficiently eliminated. S. aureus is a multifactorial pathogen and it has different strategies to survive in the host. The therapy designed to target one virulence factor will fail to eliminate or treat staphylococcal infections. Recently, it was described that sigB mutants were more susceptible to different antimicrobials [14,15] ]. A possible combination between a vaccine where SigB is included as target in combination with an antimicrobial treatment might be a good alternative for treating chronic staphylococcal infections. Consequently, SigB is a potential target for novel antimicrobial strategies against invasive and persisting infections. However, further investigation is necessary in this field.
Author Contributions
L.T. Designed the experiments, analyzed the data, prepared the figures and wrote the manuscript. J.G. performed the experiments. B.T. contributed to writing the manuscript and corrected the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gordon R.J., Lowy F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008;46(Suppl. 5):S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 3.Proctor R.A., von Eiff C., Kahl B.C., Becker K., McNamara P., Herrmann M., Peters G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006;4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 4.Tuchscherr L., Bischoff M., Lattar S.M., Noto Llana M., Pfortner H., Niemann S., Geraci J., Van de Vyver H., Fraunholz M.J., Cheung A.L., et al. Sigma factor sigb is crucial to mediate staphylococcus aureus adaptation during chronic infections. PLoS Pathog. 2015;11:e1004870. doi: 10.1371/journal.ppat.1004870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright J.A., Nair S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010;300:193–204. doi: 10.1016/j.ijmm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin E.A., Carman R.J., Krakauer T., Stiles B.G. Staphylococcus aureus: The toxic presence of a pathogen extraordinaire. Curr. Med. Chem. 2009;16:4003–4019. doi: 10.2174/092986709789352321. [DOI] [PubMed] [Google Scholar]
- 7.Grundmeier M., Tuchscherr L., Bruck M., Viemann D., Roth J., Willscher E., Becker K., Peters G., Loffler B. Staphylococcal strains vary greatly in their ability to induce an inflammatory response in endothelial cells. J. Infect. Dis. 2010;201:871–880. doi: 10.1086/651023. [DOI] [PubMed] [Google Scholar]
- 8.Haslinger-Loffler B., Kahl B.C., Grundmeier M., Strangfeld K., Wagner B., Fischer U., Cheung A.L., Peters G., Schulze-Osthoff K., Sinha B. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol. 2005;7:1087–1097. doi: 10.1111/j.1462-5822.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 9.Horst S.A., Hoerr V., Beineke A., Kreis C., Tuchscherr L., Kalinka J., Lehne S., Schleicher I., Kohler G., Fuchs T., et al. A novel mouse model of Staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: An integrated view of disease pathogenesis. Am. J. Pathol. 2012;181:1206–1214. doi: 10.1016/j.ajpath.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Tuchscherr L., Loffler B. Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr. Genet. 2016;62:15–17. doi: 10.1007/s00294-015-0503-0. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff M., Entenza J.M., Giachino P. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 2001;183:5171–5179. doi: 10.1128/JB.183.17.5171-5179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell G., Fugere A., Pepin Gaudreau K., Brouillette E., Frost E.H., Cantin A.M., Malouin F. SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants. PLoS ONE. 2013;8:e65018. doi: 10.1371/journal.pone.0065018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi S.D., Malachowa N., DeLeo F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015;185:1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atwood D.N., Beenken K.E., Lantz T.L., Meeker D.G., Lynn W.B., Mills W.B., Spencer H.J., Smeltzer M.S. Regulatory mutations impacting antibiotic susceptibility in an established staphylococcus aureus biofilm. Antimicrob. Agents. Chemother. 2016;60:1826–1829. doi: 10.1128/AAC.02750-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riordan J.T., O’Leary J.O., Gustafson J.E. Contributions of sigb and sara to distinct multiple antimicrobial resistance mechanisms of staphylococcus aureus. Int. J. Antimicrob. Agents. 2006;28:54–61. doi: 10.1016/j.ijantimicag.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]