Abstract
Apoptosis is a type of programmed cell death that regulates cellular homeostasis by removing damaged or unnecessary cells. Its importance in host defenses is highlighted by the observation that many viruses evade, obstruct, or subvert apoptosis, thereby blunting the host immune response. Infection with Flaviviruses such as Japanese encephalitis virus (JEV), Dengue virus (DENV) and West Nile virus (WNV) has been shown to activate several signaling pathways such as endoplasmic reticulum (ER)-stress and AKT/PI3K pathway, resulting in activation or suppression of apoptosis in virus-infected cells. On the other hands, expression of some viral proteins induces or protects apoptosis. There is a discrepancy between induction and suppression of apoptosis during flavivirus infection because the experimental situation may be different, and strong links between apoptosis and other types of cell death such as necrosis may make it more difficult. In this paper, we review the effects of apoptosis on viral propagation and pathogenesis during infection with flaviviruses.
Keywords: apoptosis, B-cell lymphoma 2 (BCL2), flavivirus, dengue virus, Japanese encephalitis virus, West Nile virus
1. Introduction
1.1. Overview of Apoptosis Signaling
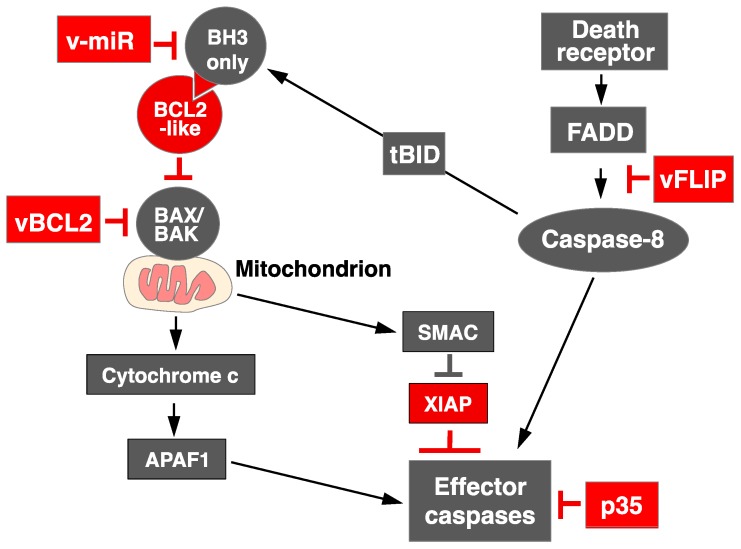
Apoptosis, or programmed cell death, is an evolutionarily conserved process essential for the removal of damaged, infected or excess amounts of cells [1,2]. It is required for normal development, tissue homeostasis, and countering infection. Whether a cell lives or dies in response to diverse developmental cues or cellular stresses is largely determined by the interactions of three members of this protein family, Bcl-2 homology 3 (BH3)-only proteins, BCL2 proteins and Bcl-2-associated X protein (BAX)/Bcl-2 homologous antagonist/killer (BAK) effectors [1]. To promote apoptosis, the BH3-only proteins (e.g., Bcl-2 interacting mediator (BIM), Bcl-2 associated death promoter (BAD), NADPH oxidase activator (NOXA) sense cellular damage, but the critical downstream mediators of apoptosis are BAX and BAK, and their combined absence abolishes most apoptotic responses [1,2]. When activated, BAX and BAK permeabilize the outer mitochondrial membrane, releasing proapoptogenic factors, such as cytochrome c, which then promote the activation of the cysteine proteases (caspases) that mediate cellular destruction [3]. The activation of BAX and BAK is opposed by the prosurvival proteins, including BCL2, B-cell lymphoma-extra -large (BCLXL), BCL2-like 2 protein (BCLW), myeloid cell lymphoma 1 (MCL1), and Bcl-2 related protein A1 (A1). These proteins are inactivated by the insertion of BH3 domains of the BH3-only proteins into a groove on the prosurvival proteins to allow the activation of BAX and BAK [1,4]. Activated BAX and BAK then permeabilize the outer mitochondrial membrane to release proapoptotic molecules, such as cytochrome c and second mitochondria-derived activator of caspases (SMAC). Cytochrome c interacts with apoptotic protease-activating factor 1 (APAF1) to activate the effector caspases, including caspase 9 [5]. At same time, SMAC block the caspase inhibitor, X-linked inhibitor of apoptosis proteins (XIAP). Death receptors, such as FAS/CD95 and tumor necrosis factor receptors (TNFR), are activated by their ligands (FASL and TNF, respectively) and then recruit the FAS-associated death domain protein (FADD) to cytoplasmic domains of receptors, resulting in the activation of caspase 8 and the effector caspases [6]. To link mitochondria-mediated apoptosis and death-receptor-mediated apoptosis, caspase 8 is activated by the death receptors and cleaves BH3 interacting domain death agonist (BID) to generate truncated BID, inducing mitochondria-mediated apoptosis [7,8] (Figure 1).
Figure 1.
Pathways of apoptosis. Prosurvival proteins (BCL2, BCLW, BCLXL, MCL1, and A1) sequester BH3-only proteins to prevent the activation of BAX and BAK under normal conditions. In the presence of apoptotic stimuli, such as the deprivation of cytokines or cellular damage, BH3-only proteins are activated and displace the prosurvival proteins from their interaction with BAX and BAK, disrupting the mitochondrial outer membrane and thus releasing cytochrome c, second mitochondria-derived activator of caspases (SMAC), and so on. The cytochrome c released interacts with apoptotic protease-activating factor 1 (APAF1) to activate effector caspases, including caspase 9. At same time, SMAC blocks the caspase inhibitor X-linked inhibitor of apoptosis proteins (XIAP). Death receptors, such as FAS/CD95 or tumor necrosis factor receptors (TNFR), are activated by their ligands (FASL or TNF, respectively) and then recruit FAS-associated death domain protein (FADD) to cytoplasmic domains of receptors, resulting in the activation of caspase 8 and effector caspases. Activated caspase 8 cleaves BID to generate truncated BID (tBID), inducing mitochondria-mediated apoptosis. Some DNA viruses encode several genes to control apoptosis (shown in red). The black arrows indicated signaling pathways and T bars shows suppression of activity of indicated proteins.
1.2. Application of Mitochondria Mediated Apoptosis to Cancer Therapy
Small-molecule inhibitors ABT-737 [9] (or ABT-263 (navitoclax), an orally available clinical derivative of ABT-737 [10]), ABT-199 (venetoclax) [11], and A-1331852 [12] have been developed as cancer therapeutics. Whereas ABT-737/ABT-263 targets BCL2, BCLW, and BCLXL, ABT-199 and A-1331852 target BCL2 and BCLXL, respectively. The U.S. Food and Drug Administration has approved ABT-199 for the treatment of 17-p-deleted chronic lymphocytic leukemia (CLL) [13]. It should be noted that the induction of cell death by targeting BCL2 proteins is a promising therapeutic strategy based on the removal of unnecessary cells.
1.3. Apoptosis Regulation by RNA Virus: Lessons from DNA Virus
The consequences of effects of apoptosis during infection with RNA virus remain unclear. However, apart from RNA virus, DNA viruses have evolved a capacity to control cell death [14]. For example, adenovirus, Karposi’s sarcoma-associated herpesvirus (KSHV), Epstein-Barr virus (EBV), and murine gamma-herpesvirus 68 (γHV68) encode homologues of mammalian BCL2: ADE1B19K, KSHV vBCL2, BHRF-1, and HV68 M11, respectively. These homologues act as prosurvival proteins by inhibiting the activation of BAX and BAK. Some viruses also encode microRNAs that target proapoptotic host proteins [15,16,17]. For example, EBV encodes a microRNA, miR-BART5, that targets BH3-only proteins, including p53 upregulated modulator of apoptosis (PUMA) [18]. Furthermore, the majority of poxviruses encode a viral BCL2, which has comparable functions to the BCL2 protein family, despite of the lack of sequence similarity [19,20]. Importantly, these viral proteins are also associated with viral virulence. The p35 protein encoded by the baculoviruses is a viral inhibitor of apoptosis that inhibits a broad range of caspases [21]. Furthermore, virus-encoded Fas-associated death domain-like interleukin-1β (IL-1β)-converting enzyme inhibitory proteins (vFLIP) inhibit the activation of caspase 8 by targeting a complex of FADD and caspase 8 [22,23,24]. The CrmB protein encoded by orthopoxviruses is a soluble protein that tightly binds TNF to inhibit TNF/TNFR-mediated apoptosis [25]. These data suggest that DNA viruses have acquired strategies to inhibit cell death in order to prolong infection and enhance the production of progeny viruses.
1.4. Life Cycle of Flavivirus Infection
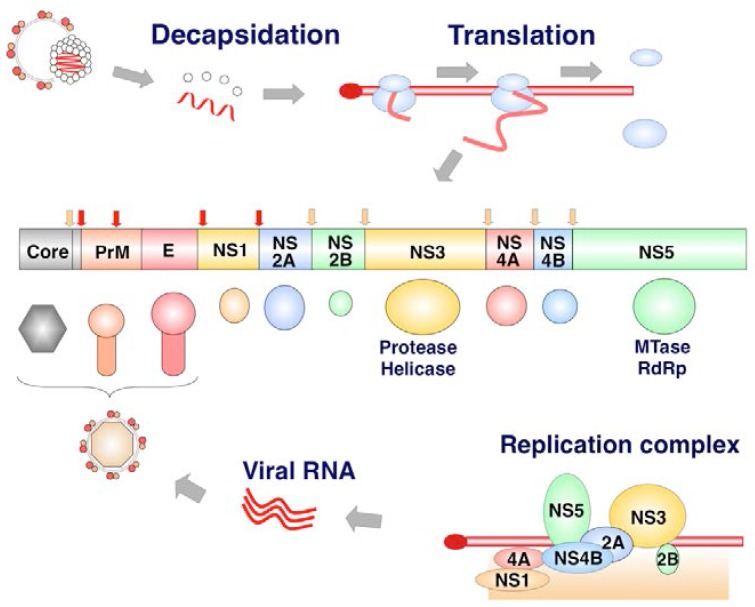
The family Flaviviridae contains viruses that are global human health concerns, including Dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and Zika virus (ZIKV) [26,27]. The viruses of the family Flaviviridae have a positive-sense single-stranded RNA genome. The viral RNA is translated to a single polyprotein (of about 3000 amino acids) that is cleaved by host proteases and specific viral proteases, producing at least 10 viral proteins (Figure 2).
Figure 2.
Structure of the flavivirus polyprotein. After decapsidation, the Flavivirus genome acts as an mRNA and is directly translated to a polyprotein in a Cap-dependent manner. The polyprotein is cleaved by host signal peptidase (SP) and viral protease (NS3). Core, prM, and E are components of the viral particles. Nonstructural (NS) proteins form a complex on the ER membrane and produce viral RNA. NS5 has methyltransferase (MTase) and RNA-dependent RNA polymerase (RdRp) activities. Red arrow shows host proteases cleave viral proteins while yellow arrow shows cleavages of viral proteins by viral proteases.
The core protein is the first protein to be translated from the viral genome and cleaved by the viral NS2B/3 protease and then the host signal peptidase. The core protein forms a dimer and the viral capsid. The precursor membrane (PrM) and envelope (E) are translated into the ER. The PreM assists the proper folding of E, and plays a role in shielding the fusion peptide of E protein. The E protein is a protein representing on the surface of virions, and is important for receptor binding and membrane fusion. Seven nonstructural proteins are components of the viral replication complex (VRC) [28,29]. The NS1 glycoprotein is translated into the ER, and the intracellular dimer form of NS1 plays roles in viral RNA replication, where the NS1 hexamer is secreted from mammalian cells to play a role in evasion of humoral immune responses. The NS2B and NS3 (NS2B-3) is a serine protease. The NS5 possesses activity of methyltransferase (MTase) and RNA-dependent RNA polymerase (RdRp). The life cycle of the flaviviruses is summarized in Figure 3.
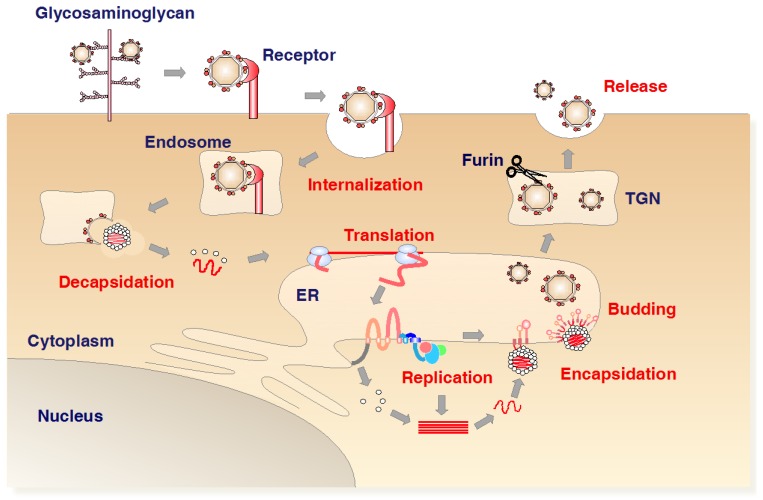
Figure 3.
Life cycle of the flaviviruses. The virus first binds to glycosaminoglycan (GAG) and then to specific receptors, and enters the cell by endocytosis. The Flavivirus genome is a single-stranded positive-sense RNA molecule that acts directly as an mRNA, which is translated into a polyprotein (Figure 1). Nonstructural proteins form a replication complex that replicates the viral RNA on the ER. Capsid protein molecules incorporate the viral RNA, and the immature virus buds from the ER together with prM and E. The immature protein is processed in the trans-Golgi network, with the removal of prM by furin. Mature virus is subsequently released by exocytosis.
Viral particles primarily interact with glycosaminoglycans, then bind to specific receptors, and are internalized into cells via endocytosis. Under the acidic conditions in the endosome, the capsid releases into cytoplasm after fusion of the viral membrane with the cellular membrane, and the viral RNA is directly translated into a precursor polyprotein. The VRC is formed on the endoplasmic reticulum (ER). The immature viral particles bearing prM and E bud into ER rumen after nucleocapsid formation with viral RNA. PrM is cleaved in the trans-Golgi network by the cellular protease furin to form the mature particles. The mature virions are released by exocytosis.
2. Apoptosis during Flavivirus-Infection
2.1. Pro-Survival and Pro Apoptotic Activity of Viral Proteins
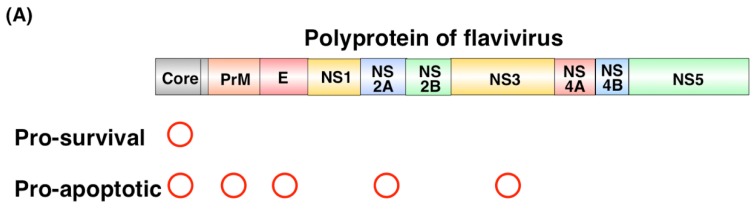
Viral proteins have been shown to regulate apoptosis, as summarized in Table 1. Netsawang et al. showed that nuclear localization of DENV capsid protein is required for its interaction with Fas death domain associated protein xx (DAXX) and the induction of apoptosis [30]. The WNV capsid protein also induces apoptosis through the interaction with importin-α and the phosphorylation by protein kinase C [31]. These results suggest that the nuclear localization of the capsid protein facilitates caspase-9-dependent apoptosis. In contrast, the WNV capsid protein stimulates the phosphorylation of AKT to suppress the activation of caspases 3 and 8 [32]. The ectodomain of the flavivirus M protein also induces apoptosis, but the overexpression of BCL2 suppresses the cell death induced by the M protein [33]. The E protein, but not the NS2B-3, of Langat virus (another flavivirus) also induces apoptosis [34]. Injection of recombinant DENV-E protein domain III suppressed megakaryopoiesis through activation of apoptosis in its progenitors [35]. A mutation in the WNV NS2A protein, converting alanine 30 to proline (A30P), attenuated viral virulence by an unknown mechanism [36]. The propagation of the mutant A30P virus was similar to that of the wild-type (WT) virus, but the A30P virus showed less severe cytopathic effects. The number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells was significantly reduced by the infection with the mutant WNV [37], suggesting that NS2A is involved in WNV-induced apoptosis and pathogenesis. The protease activity of NS3 in JEV, DENV, and WNV induces apoptosis through the activation of caspase 3 or caspase 8. NS2B, a cofactor of NS3, is required for the induction of NS3-induced apoptosis [38,39,40,41] (Figure 4A).
Table 1.
Pro-apoptotic or pro-survival activity of viral proteins.
| Viral Protein | Virus | Tested Cell Lines | Function | Reference |
|---|---|---|---|---|
| Core | DENV | HepG2 | Pro-apoptotic | [30] |
| WNV | BHK | Pro-apoptotic | [31] | |
| A549, HEL/18 | Pro-survival | [32] | ||
| M | DENV, JEV, WNV, Yellow fever virus (YFV) | HeLa, HepG2, COS-7 | Pro-apoptotic | [33] |
| E | Langat virus | Vero, Neuro-2a | Pro-apoptotic | [34] |
| DENV | Progenitor cells of megakaryocytes | Pro-apoptotic | [35] | |
| NS2A | WNV | A549, L929, Vero | Pro-apoptotic | [36,37] |
| NS2B and NS3 | JEV, DENV, WNV | TE671, BHK | Pro-apoptotic | [38] |
Figure 4.
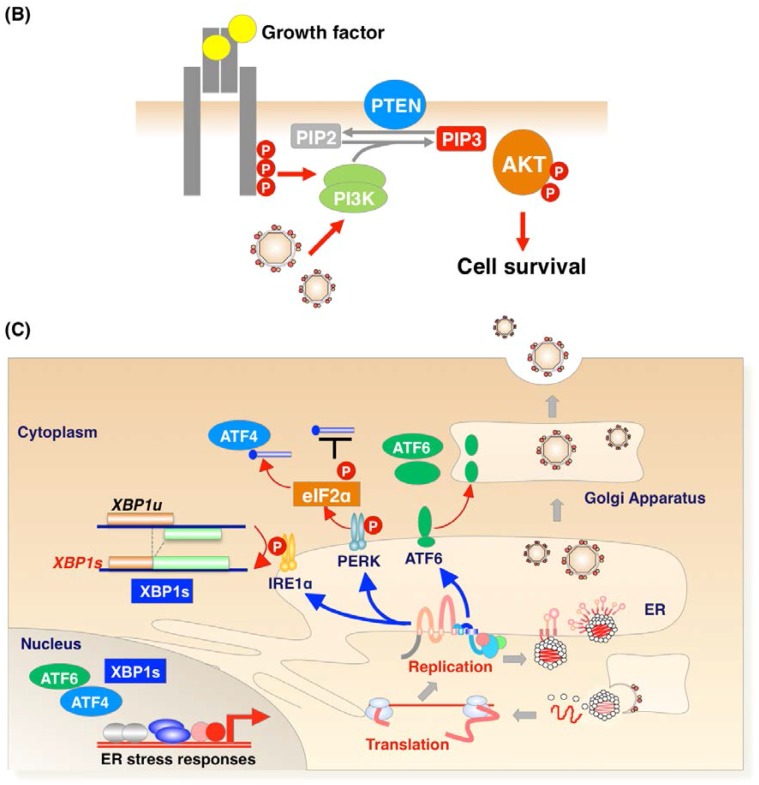
Flavivirus infection regulates apoptosis. (A) Regulation of viral proteins. Several viral proteins function as proapoptotic proteins; (B) AKT/PI3K signaling pathway. In general, growth factors, such as insulin, bind to their receptors to induce receptor phosphorylation, leading to the activation of PI3K, which stimulates the phosphorylation of AKT. Phosphorylation of AKT promotes cellular events, including the suppression of proapoptotic proteins or the promotion of cell survival. Core protein of WNV or infection by JEV or DENV activates the phosphorylation of AKT by activating PI3K to block caspase-dependent apoptosis; (C) ER stress induction. The accumulation of unfolded proteins induces ER stress, which stimulates three molecules, IRE1α, PERK, and ATF6. IRE1α is a serine/threonine protein kinase with endonuclease activity. ER stress induces the autophosphorylation of IRE1α to catalyze the excision of XBP1 mRNA. The phosphorylation of PERK, a protein kinase, is induced by ER stress and p-PERK phosphorylates eIF2α to suppress protein translation and to induce the expression of ATF4. ATF6 is also activated by ER stress, translocated to the Golgi, and activated by cleavage of the site-2 proteinase. XBP1, ATF4, and ATF6 are transcription factors to result in response to ER stress. Thus, Flavivirus infection stimulates ER stress in many ways.
2.2. Pro-Apoptotic or Pro-Survival Activity during Viral Infection
It is important for us to understand the consequences of regulation of apoptosis during viral infection, and not only the viral protein itself. Flavivirus infection has been shown to induce or protect apoptosis as summarized in Table 2. DENV isolated from human patients induced apoptosis in the mouse neuroblastoma cell line, Neuro 2a [42], endothelial cells [43,44], liver cancer cell lines, such as HepG2 [45,46] and Hep3B [47], and human monocyte-derived dendritic cells (Mo-DC) [48,49]. DENV also induced TP53 expression and mitochondrial permeabilization through TP53 [50]. WNV also induced BAX-dependent apoptosis in Neuro 2a cells and K562 cells [51]. Furthermore, apoptosis was induced in neurons derived from embryonic stem (ES) cells during WNV infection [52]. The AKT/PI3K pathway controls cell growth, survival, and energy consumption (Figure 4B). Lee et al. showed that infection with JEV or DENV upregulated the phosphorylation of AKT to activate PI3K/AKT signaling, resulting in the blockage of caspase-mediated cell death [53]. Treatment with LY294002, a PI3K inhibitor, accelerated virus-induced apoptosis [53]. The accumulation of unfolded proteins in the ER triggers the unfolded protein stress response, leading to ER stress [54]. ER stress induces the upregulation of chaperones to refold the unfolded proteins, the ER-associated degradation of any unfolded proteins, and the death of unnecessary cells. Flavivirus infection stimulates the ER stress response. JEV, WNV, and DENV infections activate inositol-requiring protein 1α (IRE1α), protein kinase RNA-like ER kinase (PERK), or activating transcription factor 6 (ATF6) [46,55,56,57,58]. The activation of IRE1α, PERK1, and ATF6 causes the mRNA encoding unspliced X box-binding protein 1 (XBP1u) to be processed, the expression of ATF4, and the cleavage of ATF6 in the Golgi apparatus, respectively. The ER stress induced by JEV and DENV has been shown to activate ER-associated protein degradation to protect the infected cells from cell death [59]. However, other research groups have shown that the induction of ER stress is associated with DENV pathogenesis [56] (Figure 4C). These discrepancies may be attributable to the different extent of ER stress induced in different cell lines or by different viral strains. Further research, using animal models or gene-edited mice, is required to understand the physiological consequences of the induction of ER stress by flaviviruses. WNV infection also upregulates cellular microRNA Hs_154, to induce apoptosis by targeting antiapoptotic proteins, including the coamplified CCCTC-binding factor (CTCF) and epidermal growth factor receptor (EGFR) and the overexpressed ECOP and VOPP1 proteins. The inhibition of Hs_154 inhibited apoptosis during WNV infection [60].
Table 2.
Pro-apoptotic or pro-survival activity of flavivirus infection.
| Virus | Tested Cell | Function | Reference |
|---|---|---|---|
| DENV | Neuro 2a | Pro-apoptotic | [42] |
| DENV | HUVEC EA.hy926 |
Pro-apoptotic | [43] |
| DENV | HMEC-1 | Pro-apoptotic | [44] |
| DENV | HepG2 | Pro-apoptotic | [45,46,47] |
| DENV | Hep3B | Pro-apoptotic | [47] |
| DENV | Monocyte derived dendritic cells (Mo-DC) | Pro-apoptotic | [48,49] |
| DENV | Huh7, BHK, Vero | Pro-apoptotic | [50] |
| WNV | Neuro 2a, K562 | Pro-apoptotic | [51] |
| WNV | ES cells derived neuron | Pro-apoptotic | [52] |
| JEV, DENV | N18, A549, BHK | Pro-survival | [53] |
| WNV | SK-N-MC, MEF, HEK293T Primary rat hippocampal neuron |
Pro-apoptotic | [55] |
| DENV | 2fTGH, MEF | Pro-apoptotic | [56] |
| JEV | BHK | Pro-apoptotic | [57] |
| JEV | Neuro 2a | Pro-apoptotic | [58] |
| WNV | MEF | Pro-apoptotic | [59] |
2.3. Physiological Significance of Apoptosis Signaling during Flavivirus Infection
As described above, many studies have shown that viral infection or the expression of viral proteins induces various types of apoptosis, with the activation of caspases. However, it is important to understand the physiological consequences of apoptotic regulation during viral infection and especially whether the induction or suppression of apoptosis is regulated by host factors to clear infected cells or by the virus to achieve its efficient propagation in vivo. In a mouse model, a deficiency of caspase 3 reduced the disease symptoms and mortality rate during WNV infection, whereas viral propagation was similar in the knockdown and WT mice. This suggests that the lack of caspase 3 activity impaired cell death, suppressing the viral burden and reducing neuronal injury [61]. Not only the extrinsic pathway, but also the mitochondria-mediated apoptotic pathway, leads to the activation of effector caspases, such as caspase 3. A recent study suggested that the blockage of effector caspases caused adverse effects, such as the cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS)/stimulator of interferon gene (STING)-mediated induction of type I interferon (IFN) through mitochondrial DNA (mtDNA) [62,63].
The same research group also reported the functions of the TNF family of ligands, TNF-related apoptosis-inducing ligand (TRAIL) and Fas-ligand (FASL), during WNV infection. The pathogenicity of WNV was enhanced in FASL-deficient (gld) mice or TRAIL−/− mice after their subcutaneous infection [64,65]. There were significantly more viral particles in the brains of the gene-deficient mice than in the WT mice, whereas viral propagation in the brain after intracranial infection with WNV did not differ between the two groups. The CD8+ T cells in both mouse groups showed impaired clearance of the WNV-infected cells. These findings strongly suggest that death-receptor-mediated apoptosis is required for the clearance of WNV-infected cells.
3. Conclusions and Future Directions
The regulation of apoptosis by RNA viruses, including flaviviruses, seems to be more complex than its regulation by DNA viruses. Lessons from the DNA viruses suggest that viruses inhibit apoptosis to achieve efficient propagation in vivo, but apoptosis seems to be induced in virus-infected cells by a host response to clear the infected cells in vitro. Recent advances in gene-editing technologies, such as CRISPR/Cas9, allow us to generate gene-modified mice, including knockout and knockin mice. Therefore, using these technologies, future studies should extend our understanding of the physiological importance of apoptosis during viral infection in vivo. In terms of antiviral therapies, apoptotic cells were detected within 4 h in CLL patients treated with ABT-199 in vitro experiments and the rapid clearance of apoptotic cells was observed at 6–24 h in vivo [66,67]. Therefore, the control of apoptosis by BH3 mimetics can efficiently remove apoptotic cells, including viral-infected cells, from the human body.
Acknowledgments
The authors are grateful to Minako Tomiyama and Junko Higuchi for secretarial work. This work was supported by the Program for Basic and Clinical Research on Hepatitis from Japan Agency for Medical Research and development (AMED) (17fk0210206h0002, 17fk0210305h0003, 17fk0210210h0002, 17fk0210209h0502, 17fk0210304h0003), the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (16H06432, 16H06429, 16K21723, 15H04736, 16K19139).
Author Contributions
T.O. drafted the manuscript. T.S., S.K., M.T., J.H., Y.Mi. and Y.Ma. edited and corrected the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of apoptosis by the BCL-2protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 2.Tait S.W.G., Green D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 3.Youle R.J., Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 4.Willis S.N., Fletcher J.I., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., et al. Apoptosis initiated when BH3 ligands engage multiple BCL-2 homologs, not BAX or BAK. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 5.Adams J.M., Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouillet P., O’Reilly L.A. CD95, BIM and T cell homeostasis. Nat. Rev. Immunol. 2009;9:514–519. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann T., Schlipf S., Sanz J., Neubert K., Stein R., Borner C. Characterization of the signal that directs BCL-x(L), but not BCL-2, to the mitochondrial outer membrane. J. Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schütze S., Tchikov V., Schneider-Brachert W. Regulation of TNFR1 and CD95 signaling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 9.Oltersdorf T., Elmore S.W., Shoemaker A.R., Armstrong R.C., Augeri D.J., Belli B.A., Bruncko M., Deckwerth T.L., Dinges J., Hajduk P.J., et al. An inhibitor of BCL-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A., Fairbrother W.J., Leverson J.D., Souers A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017;16:273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 11.Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 12.Leverson J.D., Phillips D.C., Mitten M.J., Boghaert E.R., Diaz D., Tahir S.K., Belmont L.D., Nimmer P., Xiao Y., Ma X.M., et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015;7:279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 13.Roberts A.W., Huang D. Targeting BCL2 with BH3 mimetics: Basic science and clinical application of venetoclax in chronic lymphocytic leukemia and related B cell malignancies. Clin. Pharmacol. Ther. 2016;101:89–98. doi: 10.1002/cpt.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuconati A. Viral homologs of BCL-2: Role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16:2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- 15.Campion E.M., Hakimjavadi R., Loughran S.T., Phelan S., Smith S.M., D’Souza B.N., Tierney R.J., Bell A.I., Cahill P.A., Walls D. Repression of the proapoptotic cellular BIK/NBK gene by Epstein-Barr virus antagonizes transforming growth factor β1-induced B-cell apoptosis. J. Virol. 2014;88:5001–5013. doi: 10.1128/JVI.03642-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee J., White R.E., Anderton E., Allday M.J. Latent Epstein-Barr virus can inhibit apoptosis in B cells by blocking the induction of NOXA expression. PLoS ONE. 2011;6:28506–28515. doi: 10.1371/journal.pone.0028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinozaki-Ushiku A., Kunita A., Isogai M., Hibiya T., Ushiku T., Takada K., Fukayama M. Profiling of virus-encoded microRNAs in Epstein-Barr virus-associated gastric carcinoma and their roles in gastric carcinogenesis. J. Virol. 2015;89:5581–5591. doi: 10.1128/JVI.03639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choy E.Y.-W., Siu K.-L., Kok K.-H., Lung R.W.-M., Tsang C.M., To K.-F., Kwong D.L.-W., Tsao S.W., Jin D.-Y. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J. Exp. Med. 2008;205:2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvansakul M., van Delft M.F., Lee E.F., Gulbis J.M., Fairlie W.D., Huang D.C.S., Colman P.M. A structural viral mimic of prosurvival Bcl-2: A pivotal role for sequestering proapoptotic BAX and BAK. Mol. Cell. 2007;25:933–942. doi: 10.1016/j.molcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T., Campbell S., Mehta N., Thibault J., Colman P.M., Barry M., Huang D.C.S., Kvansakul M. Sheeppox virus SPPV14 encodes a BCL-2-like cell death inhibitor that counters a distinct set of mammalian proapoptotic proteins. J. Virol. 2012;86:11501–11511. doi: 10.1128/JVI.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q., Krebs J.F., Snipas S.J., Price A., Alnemri E.S., Tomaselli K.J., Salvesen G.S. Interaction of the baculovirus anti-apoptotic protein p35 with caspases. Specificity, kinetics, and characterization of the caspase/p35 complex. Biochemistry. 1998;37:10757–10765. doi: 10.1021/bi980893w. [DOI] [PubMed] [Google Scholar]
- 22.Bertin J., Armstrong R.C., Ottilie S., Martin D.A., Wang Y., Banks S., Wang G.H., Senkevich T.G., Alnemri E.S., Moss B., et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stürzl M., Hohenadl C., Zietz C. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi’s sarcoma spindle cells. J. Natl. Cancer Inst. 1999;91:1725–1733. doi: 10.1093/jnci/91.20.1725. [DOI] [PubMed] [Google Scholar]
- 24.Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J.L., Schröter M., et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 25.Hu F.Q., Smith C.A., Pickup D.J. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology. 1994;204:343–356. doi: 10.1006/viro.1994.1539. [DOI] [PubMed] [Google Scholar]
- 26.Gould E.A., Solomon T. Pathogenic flaviviruses. Lancet. 2008;371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie J.S., Gubler D.J., Petersen L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004;10:98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Garcia M.-D., Mazzon M., Jacobs M., Amara A. Pathogenesis of flavivirus infections: Using and abusing the host cell. Cell Host Microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhyay S., Kuhn R.J., Rossmann M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 30.Netsawang J., Noisakran S., Puttikhunt C., Kasinrerk W., Wongwiwat W., Malasit P., Yenchitsomanus P.-T., Limjindaporn T. Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res. 2010;147:275–283. doi: 10.1016/j.virusres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Bhuvanakantham R., Cheong Y.K., Ng M.-L. West Nile virus capsid protein interaction with importin and HDM2 protein is regulated by protein kinase C-mediated phosphorylation. Microbes Infect. 2010;12:615–625. doi: 10.1016/j.micinf.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Urbanowski M.D., Hobman T.C. The West Nile virus capsid protein blocks apoptosis through a phosphatidylinositol 3-kinase-dependent mechanism. J. Virol. 2012;87:872–881. doi: 10.1128/JVI.02030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catteau A. Dengue virus M protein contains a proapoptotic sequence referred to as ApoptoM. J. Gen. Virol. 2003;84:2781–2793. doi: 10.1099/vir.0.19163-0. [DOI] [PubMed] [Google Scholar]
- 34.Prikhod’ko G.G., Prikhod’ko E.A., Cohen J.I., Pletnev A.G. Infection with Langat flavivirus or expression of the envelope protein induces apoptotic cell death. Virology. 2001;286:328–335. doi: 10.1006/viro.2001.0980. [DOI] [PubMed] [Google Scholar]
- 35.Lin G.-L., Chang H.-H., Lien T.-S., Chen P.-K., Chan H., Su M.-T., Liao C.-Y., Sun D.-S. Suppressive effect of dengue virus envelope protein domain III on megakaryopoiesis. Virulence. 2017;40:1–13. doi: 10.1080/21505594.2017.1343769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W.J., Wang X.J., Clark D.C., Lobigs M., Hall R.A., Khromykh A.A. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit α/β interferon induction and attenuates virus virulence in mice. J. Virol. 2006;80:2396–2404. doi: 10.1128/JVI.80.5.2396-2404.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melian E.B., Edmonds J.H., Nagasaki T.K., Hinzman E., Floden N., Khromykh A.A. West Nile virus NS2A protein facilitates virus-induced apoptosis independently of interferon response. J. Gen. Virol. 2013;94:308–313. doi: 10.1099/vir.0.047076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang T.-C., Shiu S.-L., Chuang P.-H., Lin Y.-J., Wan L., Lan Y.-C., Lin C.-W. Japanese encephalitis virus NS2B-NS3 protease induces caspase 3 activation and mitochondria-mediated apoptosis in human medulloblastoma cells. Virus Res. 2009;143:77–85. doi: 10.1016/j.virusres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Matusan A.E., Kelley P.G., Pryor M.J., Whisstock J.C., Davidson A.D., Wright P.J. Mutagenesis of the dengue virus type 2 NS3 proteinase and the production of growth-restricted virus. J. Gen. Virol. 2001;82:1647–1656. doi: 10.1099/0022-1317-82-7-1647. [DOI] [PubMed] [Google Scholar]
- 40.Shafee N. Dengue virus type 2 NS3 protease and NS2B-NS3 protease precursor induce apoptosis. J. Gen. Virol. 2003;84:2191–2195. doi: 10.1099/vir.0.19022-0. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan M.P., Chambers J.A., Pankhong P., Chattergoon M., Attatippaholkun W., Dang K., Shah N., Weiner D.B. Host cell killing by the West Nile virus NS2B-NS3 proteolytic complex: NS3 alone is sufficient to recruit caspase-8-based apoptotic pathway. Virology. 2006;345:56–72. doi: 10.1016/j.virol.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 42.Despres P., Flamand M., Ceccaldi P.E., Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J. Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J., Li Y., Qi Y., Zhang Y., Zhang L., Wang Z., Zhang X., Gui L. Coordinated regulation of autophagy and apoptosis determines endothelial cell fate during Dengue virus type 2 infection. Mol. Cell. Biochem. 2014;397:157–165. doi: 10.1007/s11010-014-2183-3. [DOI] [PubMed] [Google Scholar]
- 44.Vásquez Ochoa M., García Cordero J., Gutiérrez Castañeda B., Santos Argumedo L., Villegas Sepúlveda N., Cedillo Barrón L. A clinical isolate of dengue virus and its proteins induce apoptosis in HMEC-1 cells: A possible implication in pathogenesis. Arch. Virol. 2009;154:919–928. doi: 10.1007/s00705-009-0396-7. [DOI] [PubMed] [Google Scholar]
- 45.El-Bacha T., Midlej V., Pereira da Silva A.P., Silva da Costa L., Benchimol M., Galina A., Da Poian A.T. Mitochondrial and bioenergetic dysfunction in human hepatic cells infected with dengue 2 virus. Biochim. Biophys. Acta (BBA) 2007;1772:1158–1166. doi: 10.1016/j.bbadis.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Thepparit C., Khakpoor A., Khongwichit S., Wikan N., Fongsaran C., Chingsuwanrote P., Panraksa P., Smith D.R. Dengue 2 infection of HepG2 liver cells results in endoplasmic reticulum stress and induction of multiple pathways of cell death. BMC Res. Notes. 2013;6:372. doi: 10.1186/1756-0500-6-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thongtan T., Panyim S., Smith D.R. Apoptosis in dengue virus infected liver cell lines HepG2 and Hep3B. J. Med. Virol. 2004;72:436–444. doi: 10.1002/jmv.20004. [DOI] [PubMed] [Google Scholar]
- 48.Olagnier D., Peri S., Steel C., van Montfoort N., Chiang C., Beljanski V., Slifker M., He Z., Nichols C.N., Lin R., et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in Dengue virus-infected dendritic cells. PLoS Pathog. 2014;10:e1004566. doi: 10.1371/journal.ppat.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silveira G.F., Meyer F., Delfraro A., Mosimann A.L.P., Coluchi N., Vasquez C., Probst C.M., Bafica A., Bordignon J., Santos C.N.D.D. Dengue virus type 3 isolated from a fatal case with visceral complications induces enhanced proinflammatory responses and apoptosis of human dendritic cells. J. Virol. 2011;85:5374–5383. doi: 10.1128/JVI.01915-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasirudeen A.M.A., Wang L., Liu D.X. Induction of p53-dependent and mitochondria-mediated cell death pathway by Dengue virus infection of human and animal cells. Microbes Infect. 2008;10:1124–1132. doi: 10.1016/j.micinf.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Parquet M.D., Kumatori A., Hasebe F., Morita K., Igarashi A. West Nile virus-induced BAX-dependent apoptosis. FEBS Lett. 2001;500:17–24. doi: 10.1016/S0014-5793(01)02573-X. [DOI] [PubMed] [Google Scholar]
- 52.Shrestha B., Gottlieb D., Diamond M.S. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee C.J., Liao C.L., Lin Y.L. Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection. J. Virol. 2005;79:8388–8399. doi: 10.1128/JVI.79.13.8388-8399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hetz C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 55.Medigeshi G.R., Lancaster A.M., Hirsch A.J., Briese T., Lipkin W.I., Defilippis V., Früh K., Mason P.W., Nikolich-Zugich J., Nelson J.A. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J. Virol. 2007;81:10849–10860. doi: 10.1128/JVI.01151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pena J., Harris E. Dengue virus modulates the unfolded protein response in a time-dependent manner. J. Biol. Chem. 2011;286:14226–14236. doi: 10.1074/jbc.M111.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang M., Xu A., Wu X., Zhang Y., Guo Y., Guo F., Pan Z., Kong L. Japanese encephalitis virus induces apoptosis by the IRE1/JNK pathway of ER stress response in BHK-21 cells. Arch. Virol. 2015;161:699–703. doi: 10.1007/s00705-015-2715-5. [DOI] [PubMed] [Google Scholar]
- 58.Bhattacharyya S., Sen U., Vrati S. Regulated IRE1-dependent decay pathway is activated during Japanese encephalitis virus-induced unfolded protein response and benefits viral replication. J. Gen. Virol. 2013;95:71–79. doi: 10.1099/vir.0.057265-0. [DOI] [PubMed] [Google Scholar]
- 59.Ambrose R.L., Mackenzie J.M. ATF6 signaling is required for efficient West Nile virus replication by promoting cell survival and inhibition of innate immune responses. J. Virol. 2013;87:2206–2214. doi: 10.1128/JVI.02097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith J.L., Grey F.E., Uhrlaub J.L., Nikolich-Zugich J., Hirsch A.J. Induction of the cellular microRNA, Hs_154, by West Nile virus contributes to virus-mediated apoptosis through repression of antiapoptotic factors. J. Virol. 2012;86:5278–5287. doi: 10.1128/JVI.06883-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuel M.A., Morrey J.D., Diamond M.S. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 2007;81:2614–2623. doi: 10.1128/JVI.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White M.J., McArthur K., Metcalf D., Lane R.M., Cambier J.C., Herold M.J., van Delft M.F., Bedoui S., Lessene G., Ritchie M.E., et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rongvaux A., Jackson R., Harman C.C.D., Li T., West A.P., de Zoete M.R., Wu Y., Yordy B., Lakhani S.A., Kuan C.-Y., et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shrestha B., Diamond M.S. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J. Virol. 2007;81:11749–11757. doi: 10.1128/JVI.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shrestha B., Pinto A.K., Green S., Bosch I., Diamond M.S. CD8+ T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons. J. Virol. 2012;86:8937–8948. doi: 10.1128/JVI.00673-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson M.A., Deng J., Seymour J.F., Tam C., Kim S.Y., Fein J., Yu L., Brown J.R., Westerman D., Si E.G., et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood. 2016;127:3215–3224. doi: 10.1182/blood-2016-01-688796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts A.W., Davids M.S., Pagel J.M., Kahl B.S., Puvvada S.D., Gerecitano J.F., Kipps T.J., Anderson M.A., Brown J.R., Gressick L., et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]