Abstract
Rapid diagnosis is crucial to effectively treating any disease. Biological markers, or biomarkers, have been widely used to diagnose a variety of infectious and non-infectious diseases. The detection of biomarkers in patient samples can also provide valuable information regarding progression and prognosis. Interestingly, many such biomarkers are composed of lipids, and are amphiphilic in biochemistry, which leads them to be often sequestered by host carriers. Such sequestration enhances the difficulty of developing sensitive and accurate sensors for these targets. Many of the physiologically relevant molecules involved in pathogenesis and disease are indeed amphiphilic. This chemical property is likely essential for their biological function, but also makes them challenging to detect and quantify in vitro. In order to understand pathogenesis and disease progression while developing effective diagnostics, it is important to account for the biochemistry of lipid and amphiphilic biomarkers when creating novel techniques for the quantitative measurement of these targets. Here, we review techniques and methods used to detect lipid and amphiphilic biomarkers associated with disease, as well as their feasibility for use as diagnostic targets, highlighting the significance of their biochemical properties in the design and execution of laboratory and diagnostic strategies. The biochemistry of biological molecules is clearly relevant to their physiological function, and calling out the need for consideration of this feature in their study, and use as vaccine, diagnostic and therapeutic targets is the overarching motivation for this review.
Keywords: biomarkers, biosensors, amphiphile, lipid, diagnostics
1. Introduction
The utility of a diagnostic is measured by its ability to provide rapid and reliable information to guide treatment. The past century has seen a rise in molecular diagnostic strategies for measuring disease-specific signatures in patient samples (e.g., blood, urine, tissue and others) using a variety of biosensor technologies. A biosensor utilizes a biological component or biocatalyst to detect the presence of an analyte and a transducer to create a quantifiable signal from this interaction. For a biosensor to be used feasibly in a clinical setting, it must be highly specific for the target analyte, accurate in patient samples, rapid and reliable, and resistant to non-specific interactions in clinical samples. In addition, for some applications, especially in resource-poor conditions, it is also desirable for the sensor to be cost-effective and easy-to-use [1]. In order to avoid false positive (signal in the absence of analyte) and false negative (analyte present without signal) signals, the biocatalyst and transducer must be carefully considered for each target analyte.
Identifying target analytes and their presentation in a host during disease is of crucial importance for the success of such molecular diagnostic technologies. Biomarkers are indicators of biological processes and disease-specific biomarkers are of great clinical value for diagnostics. Understanding the interactions between the biomarker and its host is key to developing assays for the detection of such analytes in clinical samples. Because of the ease of purification and detection, proteins and nucleic acids have been extensively used as diagnostic targets. Traditionally, nucleic acids and proteins have been targeted as biomarkers for the diagnosis and detection of disease, primarily because of the availability of a variety of sensitive and tailored methods for the detection of these biochemical signatures (reference). However, another category of biomolecules that play important roles in a variety of cellular processes, and can therefore serve as indicators of disease and analytes for biosensors is lipids and amphiphiles [2]. Besides membrane formation, lipids also play a crucial role in cell signaling (steroids) and energy storage. The brain is composed of 40%–81% lipid, with the myelin sheath being 78%–81% lipidic in biochemistry [3]. Many of the bacterial virulence factors recognized by the human innate immune response, such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA), are amphiphilic lipoglycans [4,5,6,7,8,9,10]. The structure of lipids is far more diverse than that of proteins, nucleic acids or carbohydrates, allowing for highly specific biomarkers for a variety of diseases. However, all lipids contain at least one hydrocarbon chain that is insoluble in water making them difficult to detect in aqueous solutions such as blood, which has largely limited their application as diagnostic targets. Indeed, currently, rapid detection of lipidated targets is largely achieved via methods that were originally designed for proteins. Here, we review advancements in methods for detection of lipid and amphiphilic biomarkers associated with both infectious and non-infectious diseases describing the advantages and limitations of each approach in clinical diagnostics (Table 1).
Table 1.
Advantages and limitations of sensors as clinical diagnostic tools for detecting lipid and amphiphilic biomarkers.
| Method | Advantages | Limitations |
|---|---|---|
| Mass spectrometry | Sensitive | Expensive |
| Specific | Sample preparation can be extensive | |
| Works in patient samples | Requires highly trained personnel | |
| Requires laboratory infrastructure | ||
| NMR-based sensors | Rapid | Low sensitivity |
| Reproducible | Low specificity | |
| Works in patient samples | Sample preparation can be extensive | |
| Requires highly trained personnel | ||
| Requires laboratory infrastructure | ||
| Optical biosensors | ||
| SPR-based sensors | Rapid | Low sensitivity in patient samples |
| Specific | ||
| Interferometry-based sensors | Low-cost | Low specificity in patient samples |
| Sensitive | ||
| Waveguide-based sensors | Rapid | Short shelf-life of labeled reagents |
| Reproducible | ||
| Sensitive | ||
| Specific | ||
| Works in patient samples | ||
| Electrochemical biosensors | Rapid | Low sensitivity in patient samples |
| Reproducible | Low specificity in patient samples | |
| Mechanical biosensors | Rapid | Low sensitivity in patient samples |
| Low specificity in patient samples | ||
| Reproducibility |
2. Methods for Detecting Lipid and Amphiphilic Biomarkers
2.1. Storage and Processing of Patient Samples
Sample collection and preparation is a critical consideration for the detection any biomarker [11], but lipidic targets warrant further attention [12]. Common lab methods such as freeze/thaw and chemical extractions can have a profound effect on the viability of amphiphilic biomarkers [13,14]. Further, depending on the sample, lipid and amphiphilic biomarkers are often sequestered by host factors, further reducing their availability for direct detection [15]. Therefore, any biosensor designed to detect amphiphilic biomarkers must account for their unique biochemical properties.
2.2. Mass Spectrometry
Lipidomics requires a thorough characterization of the structure and the function of lipids within a living system. The study of amphiphilic biomarkers, as described here, is a composite of lipidomics, but involves the consideration of the hydrophilic components of the biomarker in addition to the hydrophobic lipids. Since its inception in the early 20th century, mass spectrometry (MS) has played a significant role in characterization of lipids [16,17,18], and this has been extensively reviewed. Here, we will focus on the adaptive application of MS to amphiphilic biomarkers.
MS ionizes lipids and sorts ions based on their mass-to-charge ratio. It has been widely used to characterize lipids [19,20,21,22,23], especially with the development of soft ionization techniques such as electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI). Lipid extraction is usually the first step for lipid analysis, and separates the lipidic components (organic phase) from other components such as proteins and nucleic acids (aqueous phase). Most widely used extraction methods have been adapted from Folch [24] or Bligh and Dyer [25], in which a mixture of methanol, chloroform and water are applied for phase separation. However, shotgun lipidomic methods have also been developed which omit the chromatographic separation and sample processing described above, and analyzes all lipid classes together, instead using ionization additives to provide discriminative identification [16]. This method might be more suitable especially for biomarker-discovery, wherein there is no prior art regarding the biomarkers being identified/measured.
Chromatographic methods such as gas chromatography, thin-layer chromatography, high-performance liquid chromatography (HPLC), or ultra-performance liquid chromatography (UPLC) [21,26] are used for separation of lipid mixtures. HPLC and UPLC are have broader applications in lipid analysis [20,26] and can be performed in normal phase (lipid class separation based on their different polarities and dipole moments) or reverse phase (separates lipid according to their hydrophobicity and is based on fatty-acyl compositions). Coupling of separation with LC methods allows lipids to be resolved sequentially with greater ionization yields, decreased ion suppression from major lipid classes, and increased sensitivity [20,27]. However, this LC step can be bypassed, with direct infusion (shotgun lipidomics) of lipid samples, which simultaneously analyzes the lipid species in the crude lipid extract [28,29]. This method does not require a priori decisions on which lipid species to measure, is relatively simple, high-throughput, and fast with short data acquisition times. However, a major limitation of this method is that highly abundant lipids can compete for ionization with the minor species, and mask detection of the latter.
MALDI-MS is based on the utilization of an ultraviolet–absorbing matrix that initially absorbs the energy of the laser and mediates the generation of ions [30,31]. ESI-MS uses a high voltage electrospray to aerosolize the lipid sample and generate ions to charge the lipid molecule [19,32]. MALDI or ESI ionization can be combined with several types of mass analyzers such as triple quadrupole, time-of-flight (TOF), ion trap, and orbitrap to further characterize different lipid classes and species [32,33]. Both quadrupole and TOF mass analyzers are commonly used and their configuration together (e.g., triple quadrupule (QqQ), quadrupole time-of-flight (QTOF)) as tandem mass spectrometric instruments to break down the precursor ions into fragmented product ions, can provide further chemical and structural resolution on the lipid species [34,35]. As an example, ESI-MS has been successfully applied to characterize cardiolipin [35]. Cardiolipin, a biomarker implicated in heart disease and cancer, is a phospholipid present in mitochrondria and is a component of bacterial membrane [36,37]. The diversity of cardiolipin molecular species is found in both the identity and position of its four fatty acyl moieties. Minkler and Hoppel showed that reverse-phase ion pair HPLC coupled with a triple quadrupole MS/MS linear ion trap mass spectrometer to generate MS/MS was highly effective in characterizing cardiolipin from different species (e.g., rat liver, mouse heart, bovine heart, dog heart) [35], with the spectra facilitating deduction of the structure of cardiolipin: the diacylglycerol phosphate region, the monoacylglycerol region, and the fatty acid region [35].
Mass spectrometry has been used by clinical laboratories to rapidly diagnose both infectious and non-infectious diseases, detect drug toxicity, monitor treatment as well as to discover new biomarkers [38]. However, clinical mass spectrometry systems such as VITEK® MS by BioMerieux are expensive to set up and maintain and require trained personnel to perform and analyze the tests limiting its utility as a point-of-care diagnostic tool.
2.3. Nuclear Magnetic Resonance (NMR)
NMR spectroscopy is one of the most powerful analytical techniques for lipid analysis of biological matrices (e.g., cells, tissue, and biological fluids), owing to the natural abundance of hydrogen in such samples [39,40,41,42,43]. This method is nondestructive, nonselective, and provides detailed molecular information, which can be of extensive value in analyzing amphiphilic biomarkers. Specifically, high-field proton nuclear magnetic resonance (1H-NMR) spectroscopy is used for molecular profiling [44,45], allowing for identification of biomarkers associated with diseases such as tuberculosis [46], cancer [47,48], heart disease [49]; and others, identifying underlying causes of disease [50], and identifying diagnostic biomarkers and new therapeutic strategies [51]. High-resolution, one-dimensional (1D) 1H NMR is reproducible and simple [39,49]. It has been applied for metabolomics since the 1980s [39,42], specifically, for plasma [48], serum [52], urine [43], and feces [53]. Tissues are normally examined intact [54] and spectra can be obtained rapidly (<5 min) [39] with detection limits around 1 to 10 µM at > 500 MHz [42,50] MS and NMR are often used in conjunction with one another [55,56]. Although NMR has decreased sensitivity in comparison to MS [50,57], it has other advantages such as being a non-destructive technique with minimal sample preparation, and providing quantitation of molecular structures [18,57]. Coupling the two method yields significantly greater sensitivity (attomole to femtomole) [58].
Lipidomic NMR is associated with many challenges. A substantial number of biomolecules in complex matrices have similar resonances, due to similar proton chemical shift ranges [42,59] resulting in considerable peak overlap [46,60] and a low signal-to-noise ratio [61]. Minimizing peak overlap and increasing sensitivity can be resolved by using high resolution spectrometers (800 to 950 MHz) [62]. Resonance line broadening [63] attributed to the formation of micelles in an aqueous environment or constrained molecular movement as a result of lipid aggregation into bilayers [42] is another challenge that is overcome by chemical or physical treatment of the sample [42].
The use of two-dimensional (2D) NMR [64,65] can also overcome the above challenges of 1D 1H NMR. 2D techniques such as the heteronuclear single quantum coherence (HSQC) method are better suited for lipid profiling because of its ability to detect resonances for both 1H nuclei and 13C nuclei [61], thus allowing for correlation between 1H and 13C chemical shifts and providing elucidation of C-H bonds within a structure [50]. Although detection of 13C is possible with HSQC, the natural abundance of 13C [66] is relatively low, requiring prior enrichment of cells with 13C [46]. 2D NMR is more time consuming (>1 h/spectrum) in comparison to 1D 1H NMR (<10 min) [43,50,67]. Absolute quantitation of lipids using NMR remains a challenge [68].
High resolution NMR has been shown to detect a variety of diseases directly in human tissue and fluid samples [69]. Portable NMR-based biosensors have also been developed for use as medical diagnostics [70]. However, portable NMR sensors suffer from low sensitivity and specificity while high-resolution NMR tools require expensive equipment and trained personnel to analyze results.
2.4. Biosensors
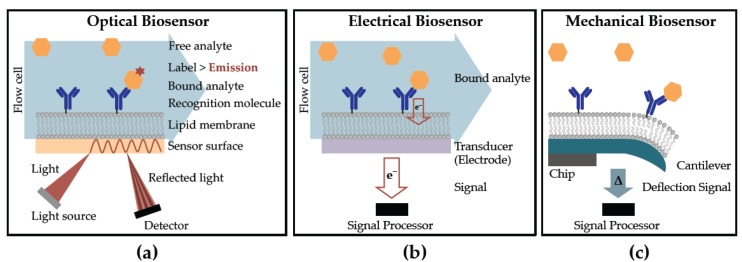
Broadly defined, biosensors recognize target molecules and produce a measurable signal. Optical, electrochemical and mechanical biosensors (Figure 1) have been adapted for the detection of lipidic and amphiphilic biomarkers, using a variety of assay methodologies which can be categorized as (a) labeled and (b) label-free. Labeled assays indirectly measure binding of an analyte to the target molecule, using a reporter molecule (an indicator). Labeled assays have the advantage of readily multiplexing, and are highly desirable in clinical applications. Label-free assays measure signal changes directly associated with either target binding or cellular processes, without the need for an external reporter. The advantages of label-free biosensors include reduced assay complexity, cost and decreasing interference of the label on binding between ligand and target.
Figure 1.
Examples of biosensor techniques incorporating lipids for the detection of analytes include (a) optical (b) electrical and (c) mechanical.
2.4.1. Optical Biosensors Detecting Lipids
Optical biosensors measure binding-induced changes in light from the sensor surface. Surface plasmon resonance (SPR) is a label-free technology that has been used to detect the interaction of amphiphilic molecules with lipid bilayers, for ligand screening and biomarker discovery [71]. In SPR sensing, a ligand is immobilized on a gold-coated surface while an interacting molecule is injected in aqueous solution, and the change in resonance associated with binding is measured optically using a spectrometer. SPR has been optimized for measuring protein-protein affinities using the BIAcore™ (GE Healthcare) system. In original iteration, SPR systems lack resolution, cannot multiplex, and are difficult to miniaturize. However, several researchers are working on overcoming these limitations, which may provide for more robust sensors in the future [72]. SPR is not conducive for detection of amphiphiles in biologically relevant conditions, as required for diagnostic applications.
Interferometry, particularly Backscattering interferometry (BSI), allows for label-free detection of both surface-bound and free-solution molecules [73]. In BSI, excitation of a microfluidic channel containing the sample, as well as the channel surface, usually made of polydimethylsulfoxide or glass, yields interference fringes. Changes in fringes associated with binding of two molecules are measured in the backscatter region. BSI detects interactions of amphiphiles with lipid bilayers [74] directly in human serum [75]. However, in complex samples, non-specific interactions and associated changes in refractive index can prove challenging, but can be limited using suitable surface functionalization chemistry [76]. BSI is inexpensive, sensitive and very versatile, making it a promising candidate for detection of amphiphilic biomarkers in patient samples.
Ellipsometry measures the refractive index of a thin film, and has been utilized in label-free assays where changes in refracted light are measured upon interaction of giant lipid vesicles with a poly-l-lysine coated surface, using a biosensor based on total internal reflection imaging ellipsometry (TIRIE) [77]. This sensor can detect µm size particles such as cells, capsules and liposomes. However, the ability to detect such particles in tissues, as well as the overall clinical utility of this system is yet to be demonstrated.
An optical assay based on UV absorption of lipid-functionalized gold nanorods has been used to detect the lipopeptide myristoyl-Lys-Arg-Thr-Leu-Arg, and a variety of other lipid biomarkers, in serum [78]. However, the low specificity of this label-free technique requires mass spectrometry analysis in order for multiplexing to take place.
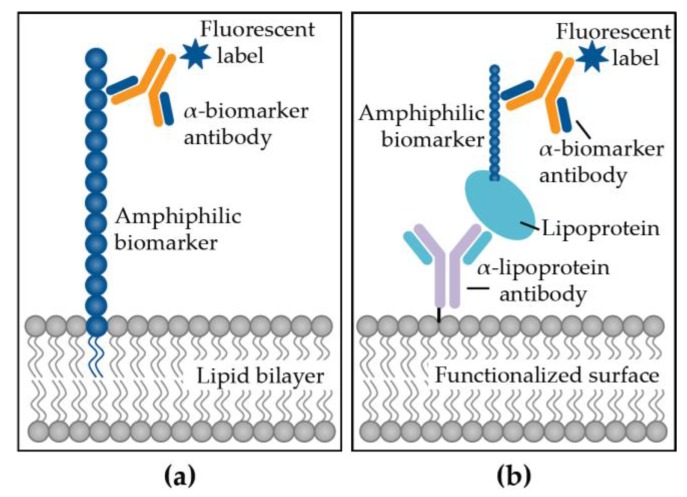
In labeled assays, the target molecule is immobilized on the surface of a biosensor and then probed with an analyte, typically an antibody, coupled to a label (fluorophore, quantum dot, radioisotope, enzyme) [79]. Mukundan et al. have employed an optical waveguide-based biosensor measuring only surface-bound fluorescent signals from labeled antibodies for the sensitive and specific detection of lipid and amphiphilic targets directly in clinical samples [80,81,82]. This system utilizes the interactions of these biomarkers with lipid bilayers and lipoproteins to capture them directly to the waveguide surface, followed by probing with labeled-antibodies excited by waveguide-coupled laser light (Figure 2). This system has been demonstrated to effectively detect amphiphilic biomarkers associated with Mycobacterium tuberculosis [15,83], M. bovis [84], Escherichia coli [85], Salmonella Typhimurium [86], influenza [87], tumor markers [88,89] oftentimes directly in human serum, proving potential utility as a diagnostic tool [80,82]. These assays have been multiplexed for the simultaneous detection of several biomarkers [81]. However, the main disadvantage of using labeled reagents is the time and cost of the labeling process, and shelf life of the labeled ingredients [90].
Figure 2.
Immunoassay strategies to detect lipid and amphiphilic biomarkers using (a) membrane insertion or (b) lipoprotein capture.
2.4.2. Electrochemical Biosensors Detecting Lipids
Electrochemical sensing is a label-free method where an electrode is used to directly detect associated reactions [91]. Electrochemical biosensors have had the greatest commercial success (glucose monitors) due to their low-cost, ease of use and miniaturization properties [92]. Amperometric sensors measure current typically as a result of electron transfer during the binding between a molecule, and chemically functionalized surface, as exemplified in the detection of LPS using redox diacetylenic vesicles on a sol-gel thin-film electrode [93]. Another example is the binding of cholera toxin to a glycosphingolipid ganglioside GM1 coated gold surface, which has been developed into a sensitive, low-cost sensor [94]. Potentiometric sensors measure potential or charge accumulation and have been used to detect lipid antigens [95] such as amphiphilic cholesterol using lipid films [96,97], without interference from ascorbic acid, glucose, urea or other proteins and lipids. In addition, label-free electrochemical biosensors have been developed for the detection of lipids such as low-density lipoprotein (LDL) with high sensitivity and specificity [98,99,100]. These sensors have also displayed long-term stability and high reproducibility with small sample volumes, which make for a promising clinical tool. However, a problem with electrochemical detection of biomarkers is specificity of detection in complex biological samples, as these platforms are more sensitive to even small perturbations in the background, which is always a possibility in clinical measurements.
2.4.3. Mechanical Biosensors Detecting Lipids
Mechanical biosensors provide rapid and sensitive measurements without extensive sample processing, ideal for clinical application. The two main mechanical sensing techniques are based on cantilever and quartz crystal microbalances (QCM), both of which are label-free. QCM detects changes in resonance frequency on the sensor surface from increased mass due to analyte minding [92]. QCM has been used to observe the formation of supported lipid bilayers in real-time [101] and lipid exchange between a bilayer and vesicle [102]. The latter measures mechanical bending of a receptor-functionalized microcantilever upon binding of the target molecule, as demonstrated for LPS [103]. Another example is the measurement of the interaction between amyloid-β and the cell membrane as an early indicator of Alzheimer's disease [104]. A major drawback to cantilever sensors is that they often operate in air, rather than liquid samples, limiting their clinical utility.
3. Lipid Biomarkers for Infectious Diseases
Rapid diagnosis is important to halt the spread of both endemic and emerging pathogens, as well as multi-drug resistant organisms [105]. Infectious diseases are caused by bacterial, viral, fungal and parasitic organisms and are routinely diagnosed using culture, microscopy, serology and genetic tests [106], all of which are time-consuming and performed by trained laboratory personnel. Several biosensors have been developed for diagnosis of infectious diseases [11], however all come with challenges that limit their application in the clinic. Many biomarkers associated with infectious diseases are amphiphiles, which are difficult to detect, especially in aqueous blood (Table 2).
Table 2.
Select lipid and amphiphilic biomarkers used for the diagnosis of infectious diseases.
| Biomarker | Disease | Location | Interacting Molecules | Reference |
|---|---|---|---|---|
| Lipopolysaccharide (LPS) | Sepsis | Blood | LBP, HDL, LDL | [107,108] |
| holotransferrin | [109] | |||
| Urinary tract infection | Urine | n.d. | [110] | |
| Antimicrobial resistance | Any sample | n.d. | [111] | |
| Lipoteichoic acid (LTA) | Sepsis | Blood | HDL, LDL, VLDL | [108,112] |
| LBP, holotransferrin | [109] | |||
| Lipoarabinomannan (LAM) | Tuberculosis | Urine | n.d. | [113] |
| Blood | HDL | [15] | ||
| Lipomannan (LM) | Bovine tuberculosis | Blood | HDL | [84] |
| OmpK36 porin | Antimicrobial resistance | Any sample | n.d. | [111] |
| Hemozoin (HZ) | Malaria | Blood | LBP, HDL, LDL, VLDL, apolipoprotein E, α-1-antitrypin | [114] |
LBP: LPS-binding protein; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low density lipoprotein; n.d.: not determined.
3.1. Sepsis
Sepsis is an infection of the bloodstream resulting in uncontrolled activation of the immune system [115]. Morbidity and mortality results from organ failure, which can be avoided with early and effective treatment, which in turn, requires rapid diagnostics [116]. There are several lipid and amphiphilic biomarkers associated with sepsis. LPS (also known as endotoxin) is a classic pathogen associated molecular pattern (PAMP) shed by Gram-negative bacteria. LPS activates the innate immune receptor, Toll-like Receptor 2, resulting in a cytokine cascade, which is the mechanism of endotoxic shock [117]. The presence of LPS in patient blood is a clear indicator of sepsis. However, detection of LPS in aqueous blood is complicated by the molecule’s amphiphilic biochemistry, which drives it to associate with host carrier lipoproteins [118] and other molecules such as LPS-binding protein (LBP), high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL) and bactericidal/permeability-increasing protein [109]. Similarly, LTA is an amphiphilic cell wall component of Gram-positive bacteria whose presence in blood also indicates sepsis. Staphylococcus aureus LTA in human blood preferentially binds to HDL (68%), then to LDL (28%), and minimally to very low density lipoprotein (4%) [112]. LBP also binds to LTA [109]. These associations complicate the detection of amphiphilic LPS and LTA in blood.
3.2. Mycobacterial Infections
Lipoarabinomannan (LAM) is a biomarker for diagnosis of tuberculosis (TB) [119,120,121,122]. LAM is an amphiphilic heat stable lipoglycan, which forms an integral component of the mycobacterial cell wall [119,120], and is secreted during TB infection. LAM has proved to be an excellent diagnostic target especially in smear negative patients (HIV-positive, pediatric population and others). Some limitations of LAM include assay cross reactivity with common oral flora like Candida and Actinomyces that can result in lower predictive value of detection [123]. LAM assays can be improved using standardized sample processing methods and more sensitive sensor technology, as well as more specific antibodies [124]. LAM is readily detected in urine [88,89], but not in blood, likely because it associates with HDL in blood [15]. To this end, lipoprotein capture assays that utilize antibodies to target HDL have been used to pull down bound LAM in serum samples [15,83,84]. This method targets host pathogen interactions, and can be applied to other amphiphilic biomarkers in blood [83]. Pathogen biomarker based measurements can be used in both human and animal hosts [84,113]. However, the high cost of such technology, and biochemical nature of LAM are current challenges to the development of such assays [124].
3.3. Antimicrobial Resistance
The global spread of antimicrobial resistance underlines the need for rapid and accurate assays for the determination of resistant organisms prior to antibiotic treatment. A common mechanism of antibiotics is inhibition of bacterial cell wall components, which are lipid or amphiphilic molecules [125]. Modifications of membrane lipids are common mechanisms of antimicrobial resistance in bacteria [126,127,128] and the direct detection of such changes would support effective treatment. In fact, targeting PAMPs that are highly conserved, such as membrane lipids, for molecular diagnostics has a tremendous benefit over approaches targeting more rapidly evolving aspects of bacterial physiology (i.e., PCR). MALDI-TOF MS has been used to detect antibiotic resistance caused by lipid alterations [111]. E.g., detection of LPS alterations that reduce the molecules net negative charge resulting in resistance to antimicrobial peptides such as colistin, and OmpK36 porin loss in Klebsiella pneumoniae resulting in resistance to carbapenems. However, the initial cost of setting up a clinical MALDI-TOF system is prohibitive toward application in routine diagnostic settings.
3.4. Malaria
Hemozoin is an insoluble amphiphilic crystalline byproduct formed from the degradation of blood by parasites such as Plasmodium falciparum, the causative agent of malaria [114]. Hemozoin is released into circulation upon erythrocyte lysis, and its presence in patient blood is a direct indication of malarial infection [129]. Detection of hemozoin has mostly been achieved using microscopy and flow cytometry in blood [130]. The amphiphilic nature of hemozoin hampers its direct detection in blood due to sequestration by host immune components [129] such as apolipoprotein E and LBP [114]. Methods like Lipoprotein capture can potentially be adapted for the detection of amphiphilic hemozoin in blood.
4. Lipid Biomarkers for Non-infectious Diseases
Non-infectious diseases including cancer and cardiovascular disorders are a result of a malfunction in normal physiological processes. Therefore, identifying specific biomarkers for diagnosing non-infectious diseases presents a major challenge. Rather than targeting a foreign molecule that is absent in a healthy patient, the diagnosis of non-infectious diseases often measures irregularities in expression of host-derived molecules [131,132]. Many lipid and amphiphilic molecules associated with human metabolism can be used as biomarkers when differentially detected in diseased versus healthy individuals (Table 3). Detection of such biomarkers can potentially be used to determine prognosis, monitor recurrence, evaluate disease progression, and predict a patient’s risk of developing a particular disease [133].
Table 3.
Select lipid and amphiphilic biomarkers used for diagnosing non-infectious diseases.
| Biomarker | Disease | Location | Interacting Lipoproteins | Reference |
|---|---|---|---|---|
| Cholesterol | Cardiovascular disease | Blood | HDL, LDL | [134,135] |
| Cancer | Blood | HDL, LDL | [136,137,138,139,140,141,142,143] | |
| Preeclampsia | Blood | HDL, LDL | [144,145,146,147] | |
| Lipotoxicity | Blood | HDL, LDL | [148] | |
| Triglycerides (TG) | Cardiovascular disease | Blood | LDL, VLDL | [132,149,150] |
| Cancer | Blood | LDL, VLDL | [136,137,138,139,140,141,142,143] | |
| Preeclampsia | Blood | LDL, VLDL | [144,145,146,147] | |
| Lipotoxicity | Blood | LDL, VLDL | [148,151,152] | |
| Cardiolipin (CL) | Cardiovascular disease | Blood | LDL, HDL, VLDL | [153] |
| Cancer | Brain tissue, Prostate tissue | n.d. | [37,154] |
HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low density lipoprotein; n.d.: not determined.
4.1. Cardiovascular Diseases and Disorders
Cardiovascular diseases (CVD) [155] account for 17.5 million deaths, accounting for 31% of all deaths worldwide [155]. Heart attacks and strokes, mainly caused by a blockage that prevents blood from flowing to the heart or brain account for about 80% of all CVD deaths. In many cases, dyslipidemia, or abnormal levels of lipids in the blood, is a major mediator of CVD [156,157]. It is typically characterized by increased levels of total cholesterol, increased levels of low density lipoprotein cholesterol (LDL-C), increased levels of TG, decreased levels of high density lipoprotein cholesterol (HDL-C), modified function of lipid molecules, or a combination of some or all of these factors [156,157,158]. Cholesterol is a major component of cell membranes, and contributes to their structural integrity and fluidity [131]. Cholesterol is also a precursor of vitamin D and important steroid hormones such as progesterones, glucocorticoids (cortisol), androgens (testosterone) and estrogens [131,159]. Cholesterol homeostasis is critical to cardiovascular health. High cholesterol can raise CVD risk [135,160], by creation of sticky plaque deposits along arterial walls, eventually blocking blood flow [125,160], causing heart attacks and strokes. LDL and HDL are important carriers of cholesterol. Increased levels of LDL-C can cause cholesterol buildup in arteries, resulting in their narrowing, raising cardiovascular risk [161]. HDL is a scavenger, carrying cholesterol away from the arteries and back to the liver for breakdown [134]. A high TG level combined with high LDL-C and low HDL-C is linked with fatty buildups in artery walls [132,149,150].
Assessing CVD risk largely involves serum lipid profiling, focused on measuring total cholesterol, HDL-C, LDL-C, and TG levels [162], largely by use of labeled optical immunoassay platforms. For instance, the Alere Cholestech LDX® System combines enzymatic optical detection with solid-phase technology to rapidly and sensitively measure total cholesterol, HDL cholesterol, triglycerides and glucose in a finger prick of blood [163]. Cholesterol is measured enzymatically in serum in a series of coupled reactions that hydrolyze cholesteryl esters and oxidize the 3-OH group of cholesterol, byproducts of which are measured colorimetrically [164,165,166]. TGs are measured enzymatically in serum via a series of coupled reactions that produce glycerol, which is oxidized using glycerol oxidase. H2O2, one of the reaction products, is measured colorimetrically [164]. In clinical practice, the standard measure of HDL is quantification of its cholesterol content after precipitation of apolipoprotein B (coat protein for LDL) [164]. Other techniques to determine HDL-C in serum include ultracentrifugation, electrophoresis, HPLC, precipitation-based method, direct measuring methods, and NMR [164,167].
LDL-C is often determined using the “Friedewald formula” which incorporates measured values for total cholesterol, HDL-C, and TG as: LDL-C = (total cholesterol) – (HDL-C) – (TG/5) [168]. This equation assumes that the ratio of TG to cholesterol is constant, which is not always the case. The Friedewald formula can thus underestimate LDL-C at lower levels of LDL-C, and higher levels of TG (>400 mg/dL) [169]. Modifications to the Friedewald formula have tried to address these shortcomings [169]. Direct LDL-C measurements (e.g., ultracentrifugation, electrophoresis, chemical precipitation, immunoseparation, and homogenous assays) are available, but standardization and extensive validations are needed for use in clinical measurements [169]. For clinical sampling, serum lipid serum profiles are determined using diagnostic test kits, and analyzed with chemical automated continuous flow analyzers that use photometric and colorimetric testing (Hitachi 7180/7020 Hitachi Clinical Analyzers, Randox RX Series Clinical Chemistry Analyzers), and point-of-care devices (Alere Cholestech LDX microfluidic device system, Professional CardioChek PA test strip system, Accutrend Plus test strip system) [170].
Many CVDs cannot be explained by these current standards [171], as many patients have lipid levels within the recommended range. Thus, there is a need for additional biomarkers for early diagnosis and prevention of CVD. Several biomarkers have been identified and are being explored for diagnostic potential. Cardiolipin is one such biomarker, reduced concentrations and altered composition of which have been implicated in cardiomyopathy associated with Barth Syndrome [172,173]. Diabetic cardiomyopathy is characterized by altered lipid composition and mitochondrial dysfunction [173,174] and reduced cardiolipin metabolism has been implicated here as well. Mitochondrial dysfunction due to reactive oxygen species effect has been shown to be associated with cardiac ischemia/reperfusion [21,23]. Mitochondrial cardiolipin have been proposed to undergo lipid peroxidation because of either their high content of unsaturated fatty acids or because of their location in the mitochondria [173,175]. Oxidized cardiolipin, a natural antigen that has pro-inflammatory effects, is also associated with atherosclerosis [173,176]. Current methods to detect the pro-inflammatory effects and thrombosis induced by cardiolipin rely on determination of antibodies against oxidized cardiolipin with commercially available standard enzyme-linked immunosorbent kits (e.g., Orgentec Anti-Cardiolipin Screen, Mainz, Germany) [173,176].
4.2. Cancer
Cancer is the leading cause of death worldwide, with 8.8 million deaths in 2015 [155]. Most cancers are initially diagnosed either because of the appearance of signs or symptoms of the disease, or via screening, which is not always available [177]. Definitive diagnosis is by biopsy of the affected tissue. The main treatments for many cancers are surgery, chemotherapy, and radiation therapy. The effectiveness of these treatments depends on early diagnosis, which results in a higher chance of survivability [177]. Therefore, there is a need for more research on molecular diagnostics based screening and early detection of cancer.
Dyslipidemia has been proposed to have an association with an increased risk of cancer, particularly breast and prostate cancers. Some studies exploring causal associations between serum lipids and breast cancer have observed increased levels of total cholesterol, TG, and LDL-C and decreased levels of HDL-C [136,137,139,142]. However, these trends are not always consistent [178,179]. Similar observations were made for prostate cancer, with some studies showing an abnormal lipid trend, similar to breast cancer [136,138,140,141,143]. Other studies found no correlation or opposite trends [180,181,182]. Thus, we clearly do not have a good understanding of the pathophysiological effects of how lipids levels contribute to cancer. Methods to detect serum lipid levels are similar to those that are currently being applied to assessing cardiovascular disease, described in the CVD section [170].
Nobel laureate Otto Warburg claimed that cancer originated from irreversible injury to mitochondrial respiration, the structural basis for which is still unclear [183,184]. A possible hypothesis is that mitochondrial dysfunction induced by oxidative stress can cause lipid peroxidation that contributes to the development of cancer [37]. One of the culprits implicated as a candidate for lipid peroxidation is cardiolipin [37]. Kiebish et al. found abnormalities in cardiolipin content or composition in several types of mouse brain tumors, compared to normal brain [37]. These abnormalities were closely associated with significant reductions in energy-generating activities [37]. ESI-MS showed that the cardiolipin composition was significantly different in prostate tissue isolated from normal individuals versus cancer patients [154].
More discovery efforts need to be undertaken to understand the roles lipids play in cancer [185,186,187,188]. Most of the findings suggest that there are abnormal levels of lipids- phosphatidylcholines, phosphatidylethanolamines, lysophosphatidylcholine, lysophosphatidic acid, LysoPI, ceramides, cholesteryl oleate- in biological samples from cancer patients than in normal ones [185,186,187,188].
Detection of lipidated biomarkers has been explored for many cancers. For instance, the carcinoembryonic antigen (CEA) has been implicated in a variety of cancers such as breast, colorectal, pancreatic and others [189,190,191]. Detection of CEA is mainly performed using targeted antibodies, and optical detection platform—labeled immunoassays. Studies have found that serial CEA measurements can detect recurrent colorectal cancer with ~80% sensitivity and 70% specificity, 5 months in advance of other diagnostics! CEA has been suggested to be the most frequent indicator of recurrence in asymptomatic patients and detection of this biomarker is the most cost-effective test for the preclinical detection of resectable disease. CEA is most useful for the early detection of liver metastasis in patients with diagnosed colorectal cancer. The biomarker is also valuable as a prognostic indicator for breast cancer [192]. In addition to optical colorimetric measurements, radioimmunoassays have also been used for CEA measurement [193,194,195,196,197,198]. Our team has developed and validated the measurement of CEA in serum and nipple aspirated fluid from patients with abnormal mammograms using a fluorescence sandwich immunoassay on a waveguide-based optical biosensor platform, demonstrating picomolar sensitivity of detection of the antigen [88,89]. The challenges with CEA detection include the fact that the biomarker has been implicated with other non-cancerous conditions such as smoking, and that the presence of the biomarker in blood is not specific indicator of a type of cancer.
Another significant cancer biomarker family, the Carbohydrate antigens, are also amphiphilic and belong to the mucin-class of molecules, which promote cancer cell proliferation and inhibits anti-cancer immune responses [199]. Of these, CA125 is best known as a biomarker to monitor epithelial ovarian cancer and for the differential diagnosis of pelvic masses [200,201]. Serum levels of CA125 are routinely monitored in patients with ovarian cancer as prognostic indicators of cancer recurrence. Genway Biosystems has developed a colorimetric immunoassay for this biomarker, which is just one of several such assays available for this target [202]. CA15-3 (MUC1) is yet another biomarker, which has been extensively implicated in a variety of cancers, and is routinely used as a prognostic indicator, and a measure of cancer severity [203]. Again, immunoassay methods, including radioimmunoassay methods are used for the measurement of these antigens in serum. These assays lack sensitivity and specificity, and are associated with a high false-positive rate, which needs to be addressed to increase their clinical utility. For example, Roche Laboratories has developed an electro-chemiluminescence assay for the detection of CA15-3 in blood [204]. These immunoassays utilize a ruthenium-complex and tripropylamine. The chemiluminescence reaction for the detection of the reaction complex is initiated by applying a voltage to the sample solution resulting in a precisely controlled reaction.
Detection of exosomes has been explored for many cancers. Exosomes are small heterogeneous extracellular vesicles (40–150 nm) released by most cell types in bodily fluids such as urine, plasma, saliva and breast milk [205,206]. While their exact function is still relatively unknown, the current theories are that they are involved in intracellular communication and cellular waste disposal [205,206]. The lipid composition of exosomes includes many different lipid classes as well as DNAs, RNAs and proteins. The exosomes are believed to contain the molecular constituents of the cell that they were derived from. In particular, exosomes have been identified as a potential biomarker for early detection and prognosis of cancer as they promote tumorigenesis, growth, progression and metastasis [205,206,207,208]. Exosome secretion by cancer cells is considerably up regulated compared to non-cancerous cells [1,2,3,4]. RNAs (messenger RNA, microRNA, long non-coding RNA), DNAs (mitochondrial DNA, single stranded DNA, double stranded DNA), and proteins (small Rab family GTPases, annexins, survivin, CD9, CD 24 and CD34) are endosomal payloads that have been found to be elevated or altered in cancer cells compared to non-cancerous cells [207,208,209]. Quantitative real-time polymerase chain reaction, nucleic acid sequencing, antibody-based methods for detection and quantitation (e.g., Western blot, ELISA), nanoparticle tracking, dynamic light scattering, flow cytometry, transmission electron microscopy have all been applied to the detection and quantification of endosomes as well as the characterization of the contents of the endosomes [207,208,209].
4.3. Preeclampsia
Preeclampsia (PE) is a complex pregnancy disorder that is characterized by hypertension and proteinuria [210]. PE affects 2%–8% of pregnancies worldwide [211]. It is the most common pregnancy complication and is associated with high maternal and perinatal mortality. PE may be life-threatening for both mother and child, increasing both fetal and maternal morbidity and mortality [212,213]. In the mother, PE may cause premature cardiovascular disease, such as chronic hypertension, ischemic heart disease, and stroke [212,213]. Children birthed from preeclamptic pregnancies have an increased risk of stroke, coronary heart disease, and metabolic syndrome [212,213]. While the pathophysiology of PE remains unclear, this disorder is mediated abnormal placentation that trigger endothelial dysfunction, resulting in vasoconstriction, thrombosis, and end-organ ischemia [210]. Dyslipidemia has been shown to be associated with an increased risk of preeclampsia [147,214]. Several meta-analysis studies, which involved PE case-control studies as well as prospective cohort studies show that while this abnormal lipid pattern may involve increased TC, TG, LDL-C and decreased HDL-C, the results are not always consistent [144,145,146,147]. However, in many of these studies there seems to be a clear consensus of raised TC levels, resulting in hypertriglyceridemia [144,145,146,147]. It is still unclear whether hypertriglyceridemia is a risk factor for preeclampsia or whether there is any causal association between them [145,146,147]. More studies need to be done to understand the role maternal TG plays the pathophysiology of PE. Because PE display an abnormal serum lipid profile, many of the bioassays and methods developed for CVD are readily applied to test for PE. These bioassays and methods have been discussed in the CVD section. Recent mass spectrometry based lipidomic studies have revealed several potential lipid biomarkers associated with PE [34,215]. These include oxidized cholesterol, cholesteryl ester, oxidized sphingomyelin, ceramide, glycerophosphocholine, and lysophosphatidylcholines.
4.4. Lipotoxicity
Lipotoxicity is a metabolic disorder characterized by excessive accumulation of fatty acids within the cell and has been implicated in the development of heart failure, obesity and diabetes [216,217]. Adipose tissues play a critical role in energy storage [218]. Adipocytes, the primary cells forming adipose tissue, contain cytosolic lipid droplets composed of a core of neutral lipids, including sterol esters and TGs, surrounded by a phospholipid monolayer [218,219,220,221,222,223,224]. Lipotoxicity is the abnormal accumulation of lipid droplets in non-adipose tissues, which leads to mitochondrial dysfunction, inhibition of ATP generation, and ultimately cell death by apoptosis [217,225,226]. Manifestation of lipotoxicity typically occurs in kidney, liver, heart and skeletal muscle [217]. For example, a disease estimated to affect 10 to 24% of the global population [227,228] is the accumulation of fat within the liver termed nonalcoholic fatty liver disease (NAFLD) [229]. NAFLD is characterized by increased hepatocyte accumulation of TGs within the cytosol [230] and can progress from fatty liver accumulation to liver fibrosis and cirrhosis [227,231]. Serum TG and cholesterol have been implicated as potential biomarkers for lipotoxicity and are currently detected using serum lipid profiling as described in the CVD section [148,151,152,170].
5. Discussion and Conclusions
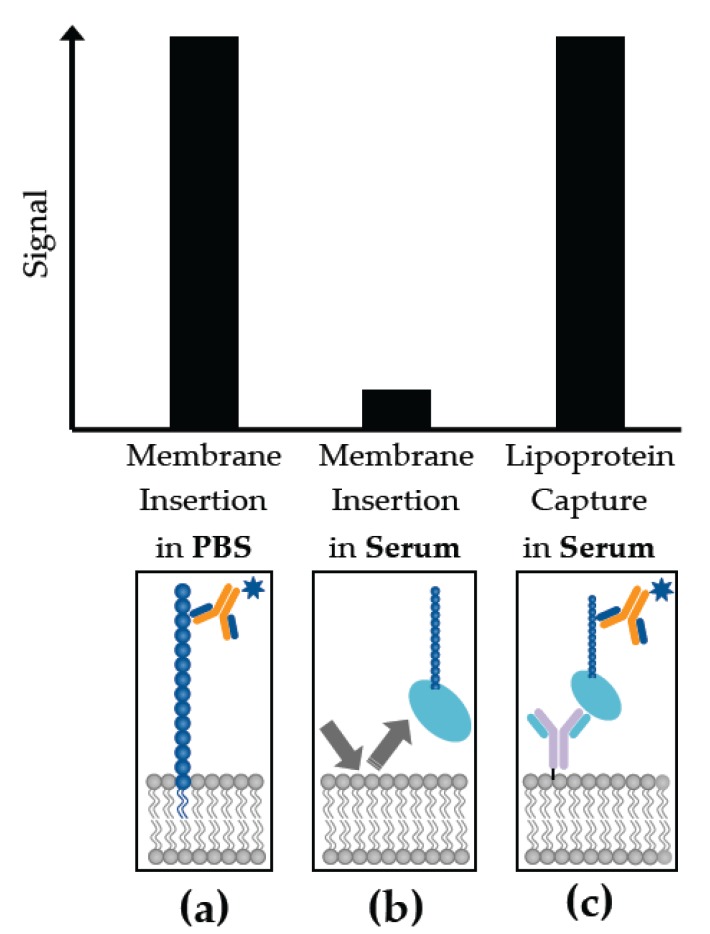
Amphiphilic and lipidic molecules are significant mediators in a variety of biological processes associated with infectious diseases and non-infectious conditions such as cancer and CVD. Many of the PAMPs secreted by bacteria are amphiphilic, as are a significant proportion of biomarkers associated with neurological processes. Many cancer signatures are also lipidated. Thus, ignoring this unique category of molecules will only limit our understanding of host-pathogen biology and disease processes. Because of the challenges in raising antibodies to such targets, and developing sensitive labeled assays, many of these biomarkers are not efficiently used in the diagnosis of disease. The biochemistry of these molecules should be considered in the design and execution of these assays. For instance, the conformation of the amphiphilic biomarkers changes dramatically depending on the milieu in which they are present (Figure 3), and the critical micelle concentrations of these molecules [232]. Lipidated biomarkers adhere to plastic surfaces, decreasing the sensitivity of their detection [233]. These characteristics also manifest themselves in physiological samples such as blood. Amphiphilic molecules, depending on their critical micelle concentration, will either self-aggregate into micelles, or associate with carrier moieties such as HDL and LDL, making their direct and rapid detection more challenging (Figure 3). Understanding the biochemical characteristics of the amphiphilic targets and anticipating the impact of these features in physiological systems can allow for the development of tailored, sensitive assays for their detection.
Figure 3.
A comparison of the sensitivity of membrane insertion and lipoprotein capture technologies for the measurement of amphiphilic biomarkers based on the medium of presentation (serum vs. buffer). Biomarkers that are readily detected in (a) phosphate buffered saline (PBS) can be poorly observed in (b) serum when utilizing a membrane insertion assay strategy, because of the uptake of these signatures by host serum carrier molecules. This signal can be recovered if (c) lipoproteins are incorporated in the assay format, exploiting the host pathogen interaction for maximal sensitivity of detection.
Acknowledgments
The writing and compilation of this manuscript was supported by Los Alamos National Laboratory Directed Research Award (Mukundan and McMahon). Mendez was supported by Agriculture and Food Research Initiative Competitive Grant No. 2012-68003-30155 from the United States Department of Agriculture’s National Institute of Food and Agriculture. The authors thank Aaron Anderson (LANL), Basil Swanson (retired Laboratory Fellow, co-inventor of the Lipoprotein capture methodology), Benjamin McMahon (LDRD PI) and Steven Graves (UNM) for their support.
Author Contributions
J.Z.K.-S., D.M.V., H.M.M., S.J. and H.M. contributed to the writing and editing of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Murugaiyan S.B., Ramasamy R., Gopal N., Kuzhandaivelu V. Biosensors in clinical chemistry: An overview. Adv. Biomed. Res. 2014;3:67. doi: 10.4103/2277-9175.125848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridgway N., McLeod R. Biochemistry of Lipids, Lipoproteins and Membranes. 6th ed. Elsevier; Amsterdam, The Netherlands: 2016. [Google Scholar]
- 3.O’Brien J.S., Sampson E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 4.Akira S., Hemmi H. Recognition of pathogen-associated molecular patterns by tlr family. Immunol. Lett. 2003;85:85–95. doi: 10.1016/S0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T., Akira S. Pathogen recognition with toll-like receptors. Curr. Opin Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Kumar H., Kawai T., Akira S. Pathogen recognition in the innate immune response. Biochem. J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 7.Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 8.Kumagai Y., Takeuchi O., Akira S. Pathogen recognition by innate receptors. J. Infect. Chemother. 2008;14:86–92. doi: 10.1007/s10156-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 9.Akira S. Pathogen recognition by innate immunity and its signaling. Proc. Jpn. Acad. Ser. B. 2009;85:143–156. doi: 10.2183/pjab.85.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Sin M.L., Mach K.E., Wong P.K., Liao J.C. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert. Rev. Mol. Diagn. 2014;14:225–244. doi: 10.1586/14737159.2014.888313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zivkovic A.M., Wiest M.M., Nguyen U.T., Davis R., Watkins S.M., German J.B. Effects of sample handling and storage on quantitative lipid analysis in human serum. Metabolomics. 2009;5:507–516. doi: 10.1007/s11306-009-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudy M.D., Kainz M.J., Graeve M., Colombo S.M., Arts M.T. Handling and storage procedures have variable effects on fatty acid content in fishes with different lipid quantities. PLoS ONE. 2016;11:e0160497. doi: 10.1371/journal.pone.0160497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marion E., Prado S., Cano C., Babonneau J., Ghamrawi S., Marsollier L. Photodegradation of the mycobacterium ulcerans toxin, mycolactones: Considerations for handling and storage. PLoS ONE. 2012;7:e33600. doi: 10.1371/journal.pone.0033600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamuri R.M., Price D.N., Lee M., Cho S.N., Barry C.E., 3rd, Via L.E., Swanson B.I., Mukundan H. Association of lipoarabinomannan with high density lipoprotein in blood: Implications for diagnostics. Tuberculosis (Edinb) 2013;93:301–307. doi: 10.1016/j.tube.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harkewicz R., Dennis E.A. Applications of mass spectrometry to lipids and membranes. Annu. Rev. Biochem. 2011;80:301–325. doi: 10.1146/annurev-biochem-060409-092612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths W.J., Wang Y. Mass spectrometry: From proteomics to metabolomics and lipidomics. Chem. Soc. Rev. 2009;38:1882–1896. doi: 10.1039/b618553n. [DOI] [PubMed] [Google Scholar]
- 18.Gross R.W., Han X. Lipidomics at the interface of structure and function in systems biology. Chem. Biol. 2011;18:284–291. doi: 10.1016/j.chembiol.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugger B. Lipidomics: Analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Ann. Rev. Biochem. 2014;83:79–98. doi: 10.1146/annurev-biochem-060713-035324. [DOI] [PubMed] [Google Scholar]
- 20.Hinterwirth H., Stegemann C., Mayr M. Lipidomics—quest for molecular lipid biomarkers in cardiovascular disease. Circ. Cardiovasc. Genet. 2014;7:941–954. doi: 10.1161/CIRCGENETICS.114.000550. [DOI] [PubMed] [Google Scholar]
- 21.Li L., Han J., Wang Z., Liu J.a., Wei J., Xiong S., Zhao Z. Mass spectrometry methodology in lipid analysis. Int. J. Mol. Sci. 2014;15:10492–10507. doi: 10.3390/ijms150610492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne S., Ivanova P., Forrester J., Alex Brown H. Lipidomics: An analysis of cellular lipids by esi-ms. Methods. 2006;39:92–103. doi: 10.1016/j.ymeth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Murphy R.C., Gaskell S.J. New applications of mass spectrometry in lipid analysis. J. Biol. Chem. 2011;286:25427–25433. doi: 10.1074/jbc.R111.233478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Canadian J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Pati S., Nie B., Arnold R.D., Cummings B.S. Extraction, chromatographic and mass spectrometric methods for lipid analysis. Biomed. Chromatogr. 2016;30:695–709. doi: 10.1002/bmc.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cajka T., Fiehn O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal. Chem. TRAC. 2014;61:192–206. doi: 10.1016/j.trac.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X., Gross R.W. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 29.Han X., Gross R.W. Shotgun lipidomics: Multidimensional ms analysis of cellular lipidomes. Expert Rev. Proteom. 2005;2:253–264. doi: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs B., Süß R., Schiller J. An update of maldi-tof mass spectrometry in lipid research. Prog. Lipid Res. 2010;49:450–475. doi: 10.1016/j.plipres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Schiller J., Süß R., Arnhold J., Fuchs B., Leßig J., Müller M., Petković M., Spalteholz H., Zschörnig O., Arnold K. Matrix-assisted laser desorption and ionization time-of-flight (maldi-tof) mass spectrometry in lipid and phospholipid research. Prog. Lipid Res. 2004;43:449–488. doi: 10.1016/j.plipres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Bugni T.S. Review of mass spectrometry: Instrumentation, interpretation, and applications. J. Nat. Prod. 2017;80:574–575. doi: 10.1021/acs.jnatprod.7b00030. [DOI] [Google Scholar]
- 33.El-Aneed A., Cohen A., Banoub J. Mass spectrometry, review of the basics: Electrospray, maldi, and commonly used mass analyzers. Appl. Spectrosc. Rev. 2009;44:210–230. doi: 10.1080/05704920902717872. [DOI] [Google Scholar]
- 34.Anand S., Young S., Esplin M.S., Peaden B., Tolley H.D., Porter T.F., Varner M.W., D’Alton M.E., Jackson B.J., Graves S.W. Detection and confirmation of serum lipid biomarkers for preeclampsia using direct infusion mass spectrometry. J. Lipid Res. 2016;57:687–696. doi: 10.1194/jlr.P064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minkler P.E., Hoppel C.L. Separation and characterization of cardiolipin molecular species by reverse-phase ion pair high-performance liquid chromatography-mass spectrometry. J. Lipid Res. 2010;51:856–865. doi: 10.1194/jlr.D002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparagna G.C., Chicco A.J., Murphy R.C., Bristow M.R., Johnson C.A., Rees M.L., Maxey M.L., McCune S.A., Moore R.L. Loss of cardiac tetralinoleoyle cardiolipin in human and experimental heart failure. J. Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Kiebish M.A., Han X., Cheng H., Chuang J.H., Seyfried T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the warburg theory of cancer. J. Lipid Res. 2008;49:2545–2556. doi: 10.1194/jlr.M800319-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crutchfield C.A., Thomas S.N., Sokoll L.J., Chan D.W. Advances in mass spectrometry-based clinical biomarker discovery. Clin. Proteom. 2016;13:1. doi: 10.1186/s12014-015-9102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Q.N., Issaq H.J., Jiang Q.J., Li Q.L., Muschik G.M., Waybright T.J., Lou H., Dean M., Uitto J., Veenstra T.D. Comparison of 1d and 2d nmr spectroscopy for metabolic profiling. J. Proteome Res. 2008;7:630–639. doi: 10.1021/pr700594s. [DOI] [PubMed] [Google Scholar]
- 40.Fonville J.M., Maher A.D., Coen M., Holmes E., Lindon J.C., Nicholson J.K. Evaluation of full-resolution j-resolved h-1 nmr projections of biofluids for metabonomics information retrieval and biomarker identification. Anal. Chem. 2010;82:1811–1821. doi: 10.1021/ac902443k. [DOI] [PubMed] [Google Scholar]
- 41.Adosraku R.K., Choi G.T.Y., Constantinoukokotos V., Anderson M.M., Gibbons W.A. Nmr lipid profiles of cells, tissues, and body-fluids—Proton nmr analysis of human erythrocyte lipids. J. Lipid Res. 1994;35:1925–1931. [PubMed] [Google Scholar]
- 42.Nicholson J.K., Wilson I.D. High-resolution proton magnetic-resonance spectroscopy of biological-fluids. Prog. Nucl. Magn. Reson. Spectrosc. 1989;21:449–501. doi: 10.1016/0079-6565(89)80008-1. [DOI] [Google Scholar]
- 43.Beckonert O., Keun H.C., Ebbels T.M.D., Bundy J.G., Holmes E., Lindon J.C., Nicholson J.K. Metabolic profiling, metabolomic and metabonomic procedures for nmr spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 44.Casu M., Anderson G.J., Choi G., Gibbons W.A. Nmr lipid profiles of cells, tissues and body-fluids 1. 1d and 2d proton nmr of lipids from rat-liver. Magn. Reson. Chem. 1991;29:594–602. doi: 10.1002/mrc.1260290610. [DOI] [Google Scholar]
- 45.Kostara C.E., Papathanasiou A., Cung M.T., Elisaf M.S., Goudevenos J., Bairaktari E.T. Evaluation of established coronary heart disease on the basis of hdl and non-hdl nmr lipid profiling. J. Proteome Res. 2010;9:897–911. doi: 10.1021/pr900783x. [DOI] [PubMed] [Google Scholar]
- 46.Mahrous E.A., Lee R.B., Lee R.E. A rapid approach to lipid profiling of mycobacteria using 2d hsqc nmr maps. J. Lipid Res. 2008;49:455–463. doi: 10.1194/jlr.M700440-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Whitehead T.L., Monzavi-Karbassi B., Kieber-Emmons T. H-1-nmr metabonomics analysis of sera differentiates between mammary tumor-bearing mice and healthy controls. Metabolomics. 2005;1:269–278. doi: 10.1007/s11306-005-0006-y. [DOI] [Google Scholar]
- 48.Beger R.D., Schnackenberg L.K., Holland R.D., Li D.H., Dragan Y. Metabonomic models of human pancreatic cancer using 1d proton nmr spectra of lipids in plasma. Metabolomics. 2006;2:125–134. doi: 10.1007/s11306-006-0026-2. [DOI] [Google Scholar]
- 49.Kostara C.E., Papathanasiou A., Psychogios N., Cung M.T., Elisaf M.S., Goudevenos J., Bairaktari E.T. Nmr-based lipidomic analysis of blood lipoproteins differentiates the progression of coronary heart disease. J. Proteome Res. 2014;13:2585–2598. doi: 10.1021/pr500061n. [DOI] [PubMed] [Google Scholar]
- 50.Gebregiworgis T., Powers R. Application of nmr metabolomics to search for human disease biomarkers. Comb. Chem. High Throughput Screen. 2012;15:595–610. doi: 10.2174/138620712802650522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giovane A., Balestrieri A., Napoli C. New insights into cardiovascular and lipid metabolomics. J. Cell. Biochem. 2008;105:648–654. doi: 10.1002/jcb.21875. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X.L., Xu L., Shen J.M., Cao B., Cheng T., Zhao T., Liu X.Y., Zhang H.X. Metabolic signatures of esophageal cancer: Nmr-based metabolomics and uhplc-based focused metabolomics of blood serum. Biochim. Biophys. Acta-Mol. Basis Dis. 2013;1832:1207–1216. doi: 10.1016/j.bbadis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Monleon D., Morales J.M., Barrasa A., Lopez J.A., Vazquez C., Celda B. Metabolite profiling of fecal water extracts from human colorectal cancer. Nmr in Biomed. 2009;22:342–348. doi: 10.1002/nbm.1345. [DOI] [PubMed] [Google Scholar]
- 54.Beckonert O., Coen M., Keun H.C., Wang Y.L., Ebbels T.M.D., Holmes E., Lindon J.C., Nicholson J.K. High-resolution magic-angle-spinning nmr spectroscopy for metabolic profiling of intact tissues. Nat. Protoc. 2010;5:1019–1032. doi: 10.1038/nprot.2010.45. [DOI] [PubMed] [Google Scholar]
- 55.Pan Z.Z., Raftery D. Comparing and combining nmr spectroscopy and mass spectrometry in metabolomics. Anal. Bioanal. Chem. 2007;387:525–527. doi: 10.1007/s00216-006-0687-8. [DOI] [PubMed] [Google Scholar]
- 56.Yu Y., Vidalino L., Anesi A., Macchi P., Guella G. A lipidomics investigation of the induced hypoxia stress on hela cells by using ms and nmr techniques. Mol. Biosyst. 2014;10:878–890. doi: 10.1039/C3MB70540D. [DOI] [PubMed] [Google Scholar]
- 57.Nicholson J.K., Lindon J.C. Systems biology—metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 58.Dettmer K., Aronov P.A., Hammock B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007;26:51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urbina J., Waugh J.S. Proton-enhanced c-13 nuclear magnetic-resonance of lipids and biomembranes. Proc. Natl. Acad. Sci. USA. 1974;71:5062–5067. doi: 10.1073/pnas.71.12.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng C., Zhang S.C., Ragg S., Raftery D., Vitek O. Identification and quantification of metabolites in h-1 nmr spectra by bayesian model selection. Bioinformatics. 2011;27:1637–1644. doi: 10.1093/bioinformatics/btr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bothwell J.H.F., Griffin J.L. An introduction to biological nuclear magnetic resonance spectroscopy. Biol. Rev. 2011;86:493–510. doi: 10.1111/j.1469-185X.2010.00157.x. [DOI] [PubMed] [Google Scholar]
- 62.Bertram H.C., Malmendal A., Petersen B.O., Madsen J.C., Pedersen H., Nielsen N.C., Hoppe C., Molgaard C., Michaelsen K.F., Duus J.O. Effect of magnetic field strength on nmr-based metabonomic human urine data comparative study of 250, 400, 500, and 800 mhz. Anal. Chem. 2007;79:7110–7115. doi: 10.1021/ac070928a. [DOI] [PubMed] [Google Scholar]
- 63.Foster M.P., McElroy C.A., Amero C.D. Solution nmr of large molecules and assemblies. Biochemistry. 2007;46:331–340. doi: 10.1021/bi0621314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis I.A., Schommer S.C., Hodis B., Robb K.A., Tonelli M., Westler W.M., Suissman M.R., Markley J.L. Method for determining molar concentrations of metabolites in complex solutions from two-dimensional h-1-c-13 nmr spectra. Anal. Chem. 2007;79:9385–9390. doi: 10.1021/ac071583z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindon J.C., Nicholson J.K., Holmes E., Everett J.R. Metabonomics: Metabolic processes studied by nmr spectroscopy of biofluids. Concepts Magn. Reson. 2000;12:289–320. doi: 10.1002/1099-0534(2000)12:5<289::AID-CMR3>3.0.CO;2-W. [DOI] [Google Scholar]
- 66.Carrasco-Pancorbo A., Navas-Iglesias N., Cuadros-Rodriguez L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part 1: Modern lipid analysis. Trac-Trends Anal. Chem. 2009;28:263–278. doi: 10.1016/j.trac.2008.12.005. [DOI] [Google Scholar]
- 67.Zhang F., Bruschweiler-Li L., Robinette S.L., Brushweiler R. Self-consistent metabolic mixture analysis by heteronuclear nmr. Application to a human cancer cell line. Anal. Chem. 2008;80:7549–7553. doi: 10.1021/ac801116u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rai R.K., Tripathi P., Sinha N. Quantification of metabolites from two-dimensional nuclear magnetic resonance spectroscopy: Application to human urine samples. Anal. Chem. 2009;81:10232–10238. doi: 10.1021/ac902405z. [DOI] [PubMed] [Google Scholar]
- 69.Smith I.C., Baert R. Medical diagnosis by high resolution nmr of human specimens. IUBMB Life. 2003;55:273–277. doi: 10.1080/1521654031000134833. [DOI] [PubMed] [Google Scholar]
- 70.Ng S.M. Applications of nmr spectroscopy. Volume 2 Elsevier; Amsterdam, The Netherlands: 2015. Portable Nmr-Based Sensors in Medical Diagnosis. [Google Scholar]
- 71.Maynard J.A., Lindquist N.C., Sutherland J.N., Lesuffleur A., Warrington A.E., Rodriguez M., Oh S.-H. Surface plasmon resonance for high-throughput ligand screening of membrane-bound proteins. Biotechnol. J. 2009;4:1542–1558. doi: 10.1002/biot.200900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoa X.D., Kirk A.G., Tabrizian M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007;23:151–160. doi: 10.1016/j.bios.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Kussrow A., Enders C.S., Bornhop D.J. Interferometric methods for label-free molecular interaction studies. Anal. Chem. 2012;84:779–792. doi: 10.1021/ac202812h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baksh M.M., Kussrow A.K., Mileni M., Finn M.G., Bornhop D.J. Label-free quantification of membrane-ligand interactions using backscattering interferometry. Nat. Biotechnol. 2011;29:357–360. doi: 10.1038/nbt.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kussrow A., Enders C.S., Castro A.R., Cox D.L., Ballard R.C., Bornhop D.J. The potential of backscattering interferometry as an in vitro clinical diagnostic tool for the serological diagnosis of infectious disease. Analyst. 2010;135:1535–1537. doi: 10.1039/c0an00098a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson A.S., Dattelbaum A.M., Montano G.A., Price D.N., Schmidt J.G., Martinez J.S., Grace W.K., Grace K.M., Swanson B.I. Functional peg-modified thin films for biological detection. Langmuir. 2008;24:2240–2247. doi: 10.1021/la7033438. [DOI] [PubMed] [Google Scholar]
- 77.Liu L., Viallat A., Jin G. Vesicle adhesion visualized with total internal reflection imaging ellipsometry biosensor. Sens. Actuators B. 2014;190:221–226. doi: 10.1016/j.snb.2013.08.044. [DOI] [Google Scholar]
- 78.Castellana E.T., Gamez R.C., Russell D.H. Label-free biosensing with lipid-functionalized gold nanorods. J. Am. Chem. Soc. 2011;133:4182–4185. doi: 10.1021/ja109936h. [DOI] [PubMed] [Google Scholar]
- 79.Martinez J.S., Grace W.K., Grace K.M., Hartman N., Swanson B.I. Pathogen detection using single mode planar optical waveguides. J. Mater. Chem. 2005;15:4639–4647. doi: 10.1039/b502329g. [DOI] [Google Scholar]
- 80.Sakamuri R.M., Capek P., Dickerson T.J., Barry C.E., 3rd, Mukundan H., Swanson B.I. Detection of stealthy small amphiphilic biomarkers. J. Microbiol. Methods. 2014;103:112–117. doi: 10.1016/j.mimet.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukundan H., Xie H., Price D., Kubicek-Sutherland J.Z., Grace W.K., Anderson A.S., Martinez J.S., Hartman N., Swanson B.I. Quantitative multiplex detection of pathogen biomarkers on multichannel waveguides. Anal. Chem. 2010;82:136–144. doi: 10.1021/ac901497g. [DOI] [PubMed] [Google Scholar]
- 82.Mukundan H., Anderson A.S., Grace W.K., Grace K.M., Hartman N., Martinez J.S., Swanson B.I. Waveguide-based biosensors for pathogen detection. Sensors. 2009;9:5783–5809. doi: 10.3390/s90705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukundan H., Price D.N., Goertz M., Parthasarathi R., Montano G.A., Kumar S., Scholfield M.R., Anderson A.S., Gnanakaran S., Iyer S., et al. Understanding the interaction of lipoarabinomannan with membrane mimetic architectures. Tuberculosis. 2012;92:38–47. doi: 10.1016/j.tube.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Vu D.M., Sakamuri R.M., Waters W.R., Swanson B.I., Mukundan H. Detection of lipomannan in cattle infected with bovine tuberculosis. Anal. Sci. 2017;33:457–460. doi: 10.2116/analsci.33.457. [DOI] [PubMed] [Google Scholar]
- 85.Stromberg L.R., Hengartner N.W., Swingle K.L., Moxley R.A., Graves S.W., Montano G.A., Mukundan H. Membrane insertion for the detection of lipopolysaccharides: Exploring the dynamics of amphiphile-in-lipid assays. PLoS ONE. 2016;11:e0156295. doi: 10.1371/journal.pone.0156295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noormohamed A., Stromberg L.R., Anderson A.S., Karim Z., Dighe P., Kempaiah P., Ong’echa J.M., Perkins D.J., Doggett N., McMahon B., et al. Optical Diagnostics and Sensing XVII: Toward Point-of-Care Diagnostics. Volume 10072 Proc. SPIE; San Francisco, CA, USA: 2017. Detection of Lipopolysaccharides in Serum Using a Waveguide-Based Optical Biosensor. [Google Scholar]
- 87.Kale R.R., Mukundan H., Price D.N., Harris J.F., Lewallen D.M., Swanson B.I., Schmidt J.G., Iyer S.S. Detection of intact influenza viruses using biotinylated biantennary s-sialosides. J. Am. Chem. Soc. 2008;130:8169–8171. doi: 10.1021/ja800842v. [DOI] [PubMed] [Google Scholar]
- 88.Mukundan H., Xie H., Anderson A.S., Grace W.K., Shively J.E., Swanson B.I. Optimizing a waveguide-based sandwich immunoassay for tumor biomarkers: Evaluating fluorescent labels and functional surfaces. Bioconjug. Chem. 2009;20:222–230. doi: 10.1021/bc800283e. [DOI] [PubMed] [Google Scholar]
- 89.Mukundan H., Kubicek J.Z., Holt A., Shively J.E., Martinez J.S., Grace K., Grace W.K., Swanson B.I. Planar optical waveguide-based biosensor for the quantitative detection of tumor markers. Sens. Actuators B. 2009;138:453–460. doi: 10.1016/j.snb.2009.01.073. [DOI] [Google Scholar]
- 90.Goncalves M.S. Fluorescent labeling of biomolecules with organic probes. Chem. Rev. 2009;109:190–212. doi: 10.1021/cr0783840. [DOI] [PubMed] [Google Scholar]
- 91.Grieshaber D., MacKenzie R., Voros J., Reimhult E. Electrochemical biosensors—Sensor principles and architectures. Sensors. 2008;8:1400–1458. doi: 10.3390/s8031400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmed A., Rushworth J.V., Hirst N.A., Millner P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014;27:631–646. doi: 10.1128/CMR.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng T., Cheng Q., Stevens R.C. Amperometric detection of escherichia coli heat-labile enterotoxin by redox diacetylenic vesicles on a sol-gel thin-film electrode. Anal. Chem. 2000;72:1611–1617. doi: 10.1021/ac990406y. [DOI] [PubMed] [Google Scholar]
- 94.Cheng Q., Zhu S., Song J., Zhang N. Functional lipid microstructures immobilized on a gold electrode for voltammetric biosensing of cholera toxin. Analyst. 2004;129:309–314. doi: 10.1039/b315656g. [DOI] [PubMed] [Google Scholar]
- 95.Shiba K., Umezawa Y., Watanabe T., Ogawa S., Fujiwara S. Thin-layer potentiometric analysis of lipid antigen-antibody reaction by tetrapentylammonium (tpa+) ion loaded liposomes and tpa+ ion selective electrode. Anal. Chem. 1980;52:1610–1613. doi: 10.1021/ac50061a018. [DOI] [PubMed] [Google Scholar]
- 96.Psychoyios V.N., Nikoleli G.-P., Tzamtzis N., Nikolelis D.P., Psaroudakis N., Danielsson B., Israr M.Q., Willander M. Potentiometric cholesterol biosensor based on zno nanowalls and stabilized polymerized lipid film. Electroanalysis. 2013;25:367–372. doi: 10.1002/elan.201200591. [DOI] [Google Scholar]
- 97.Nikoleli G.-P., Ibupoto Z.H., Nikolelis D.P., Likodimos V., Psaroudakis N., Tzamtzis N., Willander M., Hianik T. Potentiometric cholesterol biosensing application of graphene electrode with stabilized polymeric lipid membrane. Cent. Eur. J. Chem. 2013;11:1554–1561. doi: 10.2478/s11532-013-0285-5. [DOI] [Google Scholar]
- 98.Ali M.A., Srivastava S., Pandey M.K., Agrawal V.V., John R., Malhotra B.D. Protein-conjugated quantum dots interface: Binding kinetics and label-free lipid detection. Anal. Chem. 2014;86:1710–1718. doi: 10.1021/ac403543g. [DOI] [PubMed] [Google Scholar]
- 99.Ali M.A., Kamil Reza K., Srivastava S., Agrawal V.V., John R., Malhotra B.D. Lipid-lipid interactions in aminated reduced graphene oxide interface for biosensing application. Langmuir. 2014;30:4192–4201. doi: 10.1021/la4049852. [DOI] [PubMed] [Google Scholar]
- 100.Ali M.A., Solanki P.R., Srivastava S., Singh S., Agrawal V.V., John R., Malhotra B.D. Protein functionalized carbon nanotubes-based smart lab-on-a-chip. ACS Appl. Mater. Interfaces. 2015;7:5837–5846. doi: 10.1021/am509002h. [DOI] [PubMed] [Google Scholar]
- 101.Cho N.J., Frank C.W., Kasemo B., Hook F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010;5:1096–1106. doi: 10.1038/nprot.2010.65. [DOI] [PubMed] [Google Scholar]
- 102.Edvardsson M., Svedhem S., Wang G., Richter R., Rodahl M., Kasemo B. Qcm-d and reflectometry instrument: Applications to supported lipid structures and their biomolecular interactions. Anal. Chem. 2009;81:349–361. doi: 10.1021/ac801523w. [DOI] [PubMed] [Google Scholar]
- 103.Nieradkaa K., Kapczyńskab K., Rybkab J., Lipińskib T., Grabiecc P., Skowickid M., Gotszalka T. Microcantilever array biosensors for detection and recognition of gram-negative bacterial endotoxins. Sens. Actuators B. 2014;198:114–124. doi: 10.1016/j.snb.2014.03.023. [DOI] [Google Scholar]
- 104.Zhang Z., Murakami Y., Taniguchi T., Sohgawa M., Yamashita K., Noda M. A cantilever-based biosensor for real-time monitoring of interactions between amyloid-β(1–40) and membranes comprised of phosphatidylcholine lipids with different hydrophobic acyl chains. Electroanalysis. 2017;29:722–729. doi: 10.1002/elan.201600416. [DOI] [Google Scholar]
- 105.O’Neill J. Tackling drug-resistant infections globally: Final report and recommendations. [(accessed on 31 May 2017)]; Available online: https://amr-review.org/sites/default/files/160518_Finalpaper_with cover.pdf.
- 106.Washington J.A. Principles of Diagnosis. In: Baron S., editor. Medical Microbiology. 4th ed. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. [PubMed] [Google Scholar]
- 107.Levin J., Poore T.E., Zauber N.P., Oser R.S. Detection of endotoxin in the blood of patients with sepsis due to gram-negative bacteria. N. Engl. J. Med. 1970;283:1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- 108.Feingold K.R., Grunfeld C. The role of hdl in innate immunity. J. Lipid Res. 2011;52:1–3. doi: 10.1194/jlr.E012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Triantafilou M., Mouratis M.A., Lepper P.M., Haston R.M., Baldwin F., Lowes S., Ahmed M.A., Schumann C., Boyd O., Triantafilou K. Serum proteins modulate lipopolysaccharide and lipoteichoic acid-induced activation and contribute to the clinical outcome of sepsis. Virulence. 2012;3:136–145. doi: 10.4161/viru.19077. [DOI] [PubMed] [Google Scholar]
- 110.Matsumoto T., Tanaka M., Ogata N., Mizunoe Y., Takahashi K., Kumazawa J. Significance of urinary endotoxin concentration in patients with urinary tract infection. Urol. Res. 1991;19:293–295. doi: 10.1007/BF00299061. [DOI] [PubMed] [Google Scholar]
- 111.Hrabak J., Chudackova E., Walkova R. Matrix-assisted laser desorption ionization-time of flight (maldi-tof) mass spectrometry for detection of antibiotic resistance mechanisms: From research to routine diagnosis. Clin. Microbiol. Rev. 2013;26:103–114. doi: 10.1128/CMR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levels J.H., Abraham P.R., van Barreveld E.P., Meijers J.C., van Deventer S.J. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect Immun. 2003;71:3280–3284. doi: 10.1128/IAI.71.6.3280-3284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mukundan H., Kumar S., Price D.N., Ray S.M., Lee Y.J., Min S., Eum S., Kubicek-Sutherland J., Resnick J.M., Grace W.K., et al. Rapid detection of mycobacterium tuberculosis biomarkers in a sandwich immunoassay format using a waveguide-based optical biosensor. Tuberculosis. 2012;92:407–416. doi: 10.1016/j.tube.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kassa F.A., Shio M.T., Bellemare M.J., Faye B., Ndao M., Olivier M. New inflammation-related biomarkers during malaria infection. PLoS ONE. 2011;6:e26495. doi: 10.1371/journal.pone.0026495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biron B.M., Ayala A., Lomas-Neira J.L. Biomarkers for sepsis: What is and what might be? Biomark. Insights. 2015;10:7–17. doi: 10.4137/BMI.S29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faix J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab. Sci. 2013;50:23–36. doi: 10.3109/10408363.2013.764490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aderem A., Ulevitch R.J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 118.Chaby R. Lipopolysaccharide-binding molecules: Transporters, blockers and sensors. Cell. Mol. Life Sci. 2004;61:1697–1713. doi: 10.1007/s00018-004-4020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chatterjee D., Khoo K.H. Mycobacterial lipoarabinomannan: An extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 120.Means T.K., Lien E., Yoshimura A., Wang S., Golenbock D.T., Fenton M.J. The cd14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for toll-like receptors. J. Immunol. 1999;163:6748–6755. [PubMed] [Google Scholar]
- 121.Pathak S.K., Basu S., Bhattacharyya A., Pathak S., Kundu M., Basu J. Mycobacterium tuberculosis lipoarabinomannan-mediated irak-m induction negatively regulates toll-like receptor-dependent interleukin-12 p40 production in macrophages. J. Biol. Chem. 2005;280:42794–42800. doi: 10.1074/jbc.M506471200. [DOI] [PubMed] [Google Scholar]
- 122.Lawn S.D. Point-of-care detection of lipoarabinomannan (lam) in urine for diagnosis of hiv-associated tuberculosis: A state of the art review. BMC Infect Dis. 2012;12:103. doi: 10.1186/1471-2334-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dheda K., Davids V., Lenders L., Roberts T., Meldau R., Ling D., Brunet L., van Zyl Smit R., Peter J., Green C., et al. Clinical utility of a commercial lam-elisa assay for tb diagnosis in hiv-infected patients using urine and sputum samples. PLoS ONE. 2010;5:e9848. doi: 10.1371/journal.pone.0009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakamuri R.M., Moodley P., Yusim K., Fen S.H., Sturm A.W., Korber B.T.M., Mukundan H. Current Methods for Diagnosis of Human Tuberculosis and considerAtions for Global Surveillance. In: Mukundan H., Chambers M.A., Waters W.R., Larsen M.H., editors. Tuberculosis, Leprosy and Mycobacterial Diseases of Man and Animals: The Many Hosts of Mycobacteria. CABI; Oxfordshire, UK: 2015. p. 72. [Google Scholar]
- 125.Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kubicek-Sutherland J.Z., Lofton H., Vestergaard M., Hjort K., Ingmer H., Andersson D.I. Antimicrobial peptide exposure selects for staphylococcus aureus resistance to human defence peptides. J. Antimicrob. Chemother. 2017;72:115–127. doi: 10.1093/jac/dkw381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andersson D.I., Hughes D., Kubicek-Sutherland J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016;26:43–57. doi: 10.1016/j.drup.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 128.Kubicek-Sutherland J.Z., Heithoff D.M., Ersoy S.C., Shimp W.R., House J.K., Marth J.D., Smith J.W., Mahan M.J. Host-dependent induction of transient antibiotic resistance: A prelude to treatment failure. EBioMedicine. 2015;2:1169–1178. doi: 10.1016/j.ebiom.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Olivier M., Van Den Ham K., Shio M.T., Kassa F.A., Fougeray S. Malarial pigment hemozoin and the innate inflammatory response. Front. Immunol. 2014;5:25. doi: 10.3389/fimmu.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frita R., Rebelo M., Pamplona A., Vigario A.M., Mota M.M., Grobusch M.P., Hanscheid T. Simple flow cytometric detection of haemozoin containing leukocytes and erythrocytes for research on diagnosis, immunology and drug sensitivity testing. Malar. J. 2011;10:74. doi: 10.1186/1475-2875-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tabas I. Cholesterol in health and disease. J. Clin. Investig. 2002;110:583–590. doi: 10.1172/JCI0216381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miller M., Stone N.J., Ballantyne C., Bittner V., Criqui M.H., Ginsberg H.N., Goldberg A.C., Howard W.J., Jacobson M.S., Kris-Etheron P.M., et al. Triglycerides and cardiovascular disease: A scientific statement from the american heart association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 133.Henry N.L., Hayes D.F. Cancer biomarkers. Mol. Oncol. 2012;6:140–146. doi: 10.1016/j.molonc.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rader D.J., Hovingh G.K. Hdl and cardiovascular disease. Lancet. 2014;384:618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 135.Wadhera R.K., Steen D.L., Khan I., Giugliano R.P., Foody J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016;10:472–489. doi: 10.1016/j.jacl.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 136.Bielecka-Dąbrowa A., Hannam S., Rysz J., Banach M. Malignancy-associated dyslipidemia. Open Cardiovasc. Med. J. 2011;5:35–40. doi: 10.2174/1874192401105010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.dos Santos C.R., Domingues G., Matias I., Matos J., Fonseca I., de Almeida J.M., Dias S. Ldl-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:9. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Esposito K., Chiodini P., Capuano A., Bellastella G., Maiorino M.I., Parretta E., Lenzi A., Giugliano D. Effect of metabolic syndrome and its components on prostate cancer risk: Meta-analysis. J. Endocrinol. Investig. 2013;36:132–139. doi: 10.1007/BF03346748. [DOI] [PubMed] [Google Scholar]
- 139.Kitahara C.M., Berrington de González A., Freedman N.D., Huxley R., Mok Y., Jee S.H., Samet J.M. Total cholesterol and cancer risk in a large prospective study in korea. J. Clin. Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Magura L., Blanchard R., Hope B., Beal J.R., Schwartz G.G., Sahmoun A.E. Hypercholesterolemia and prostate cancer: A hospital-based case–control study. Cancer Causes Control. 2008;19:1259–1266. doi: 10.1007/s10552-008-9197-7. [DOI] [PubMed] [Google Scholar]
- 141.Pelton K., Freeman M.R., Solomon K.R. Cholesterol and prostate cancer. Curr. Opin. Pharmacol. 2012;12:751–759. doi: 10.1016/j.coph.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ray G., Husain S.A. Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin. Biochem. 2001;34:71–76. doi: 10.1016/S0009-9120(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 143.Solomon K.R., Freeman M.R. The complex interplay between cholesterol and prostate malignancy. Urol. Clin. N. Am. 2011;38:243–259. doi: 10.1016/j.ucl.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bartsch E., Medcalf K.E., Park A.L., Ray J.G. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ. 2016;353 doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gallos I.D., Sivakumar K., Kilby M.D., Coomarasamy A., Thangaratinam S., Vatish M. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: A meta-analysis. Int. J. Obstet. Gynaecol. 2013;120:1321–1332. doi: 10.1111/1471-0528.12375. [DOI] [PubMed] [Google Scholar]
- 146.Ray J.G., Diamond P., Singh G., Bell C.M. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. Int. J. Obstet. Gynaecol. 2006;113:379–386. doi: 10.1111/j.1471-0528.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 147.Spracklen C.N., Smith C.J., Saftlas A.F., Robinson J.G., Ryckman K.K. Maternal hyperlipidemia and the risk of preeclampsia: A meta-analysis. Am. J. Epidemiol. 2014;180:346–358. doi: 10.1093/aje/kwu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., Tiribelli C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 150.Singh A.K., Singh R. Triglyceride and cardiovascular risk: A critical appraisal. Indian J. Endocrinol. Metab. 2016;20:418–428. doi: 10.4103/2230-8210.183460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ibrahim S.H., Kohli R., Gores G.J. Mechanisms of lipotoxicity in nafld and clinical implications. J. Pediatr. Gastroenterol. Nutr. 2011;53:131–140. doi: 10.1097/MPG.0b013e31822578db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wende A.R., Abel E.D. Lipotoxicity in the heart. Biochim. Biophys. Acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deguchi H., Fernandez J.A., Hackeng T.M., Banka C.L., Griffin J.H. Cardiolipin is a normal component of human plasma lipoproteins. Proc. Natl. Acad. Sci. USA. 2000;97:1743–1748. doi: 10.1073/pnas.97.4.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sapandowski A., Stope M., Evert K., Evert M., Zimmermann U., Peter D., Päge I., Burchardt M., Schild L. Cardiolipin composition correlates with prostate cancer cell proliferation. Mol. Cell. Biochem. 2015;410:175–185. doi: 10.1007/s11010-015-2549-1. [DOI] [PubMed] [Google Scholar]
- 155.World Health Organization Cardiovascular diseases (cvds) [(accessed on 31 May 2017)]; Available online: http://www.who.int/mediacentre/factsheets/fs317/en/
- 156.Mearns B.M. Targeting levels and functions of blood lipids in the prevention of cvd. Nat. Rev. Cardiol. 2011;8:179–180. doi: 10.1038/nrcardio.2011.42. [DOI] [PubMed] [Google Scholar]
- 157.Tabatabaei-Malazy O., Qorbani M., Samavat T., Sharifi F., Larijani B., Fakhrzadeh H. Prevalence of dyslipidemia in iran: A systematic review and meta-analysis study. Int. J. Prev. Med. 2014;5:373–393. [PMC free article] [PubMed] [Google Scholar]
- 158.Arsenault B.J., Boekholdt S.M., Kastelein J.J.P. Lipid parameters for measuring risk of cardiovascular disease. Nat. Rev. Cardiol. 2011;8:197–206. doi: 10.1038/nrcardio.2010.223. [DOI] [PubMed] [Google Scholar]
- 159.Hu J., Zhang Z., Shen W.-J., Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhatnagar D., Soran H., Durrington P.N. Hypercholesterolemia and its management. BMJ. 2008;337:a993. doi: 10.1136/bmj.a993. [DOI] [PubMed] [Google Scholar]
- 161.Ridker P.M. Ldl cholesterol: Controversies and future therapeutic directions. Lancet. 2014;384:607–617. doi: 10.1016/S0140-6736(14)61009-6. [DOI] [PubMed] [Google Scholar]
- 162.National Cholesterol Education Program Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3412. [PubMed] [Google Scholar]
- 163.Alere Alere cholestech ldx® analyzer. [(accessed on 31 May 2017)]; Available online: http://www.alere.com/en/home/product-details/cholestech-ldx-system.html.
- 164.Kwiterovich P.O., Jr. National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2004. Laboratory Procedure Manual: Total Cholesterol, Hdl-Cholesterol, Triglycerides, and Ldl-Cholesterol. [Google Scholar]
- 165.Schaefer E.J., Tsunoda F., Diffenderfer M., Polisecki E., Thai N., Asztalos B. The Measurement of Lipids, Lipoproteins, Apolipoproteins, Fatty Acids, and Sterols, and Next Generation Sequencing for the Diagnosis and Treatment of Lipid Disorders. In: De Groot L.J., Chrousos G., Dungan K., Feingold K.R., Grossman A., Hershman J.M., Koch C., Korbonits M., McLachlan R., New M., et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [PubMed] [Google Scholar]
- 166.Evans K., Laker M.F. Intra-individual factors affecting lipid, lipoprotein and apolipoprotein measurement: A review. Ann. Clin. Biochem. 1995;32:261–280. doi: 10.1177/000456329503200303. [DOI] [PubMed] [Google Scholar]
- 167.Hafiane A., Genest J. High density lipoproteins: Measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015;3:175–188. doi: 10.1016/j.bbacli.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 169.Nauck M., Warnick G.R., Rifai N. Methods for measurement of ldl-cholesterol: A critical assessment of direct measurement by homogeneous assays versus calculation. Clin. Chem. 2002;48:236–254. [PubMed] [Google Scholar]
- 170.Global Industry Analysts . Global Cholesterol Testing—Clinical Diagnostics. Global Industry Analysts; San Jose, CA, USA: 2016. p. 219. [Google Scholar]
- 171.Ekroos K., Jänis M., Tarasov K., Hurme R., Laaksonen R. Lipidomics: A tool for studies of atherosclerosis. Curr. Atheroscler. Rep. 2010;12:273–281. doi: 10.1007/s11883-010-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schlame M., Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 173.Shen Z., Ye C., McCain K., Greenberg M.L. The role of cardiolipin in cardiovascular health. BioMed Res. Int. 2015;215:12. doi: 10.1155/2015/891707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Han X., Yang J., Yang K., Zhao Z., Abendschein D.R., Gross R.W. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: A shotgun lipidomics study. Biochemistry. 2007;46:6417–6428. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Petrosillo G., Di Venosa N., Ruggiero F.M., Pistolese M., D’Agostino D., Tiravanti E., Fiore T., Paradies G. Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim. Biophys. Acta. 2005;1710:78–86. doi: 10.1016/j.bbabio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 176.Frostegard A.G., Su J., Hua X., Vikstrom M., Faire U., Frostegard J. Antibodies against native and oxidized cardiolipin and phosphatidylserine and phosphorylcholine in atherosclerosis development. PLoS ONE. 2014;9:3111764. doi: 10.1371/journal.pone.0111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.World Health Organization . World Cancer Report 2014. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 178.Laisupasin P., Thompat W., Sukarayodhin S., Sornprom A., Sudjaroen Y. Comparison of serum lipid profiles between normal controls and breast cancer patients. J. Lab. Physician. 2013;5:38–41. doi: 10.4103/0974-2727.115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ni H., Liu H., Gao R. Serum lipids and breast cancer risk: A meta-analysis of prospective cohort studies. PLoS ONE. 2015;10:e0142669. doi: 10.1371/journal.pone.0142669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gacci M., Russo G.I., De Nunzio C., Sebastianelli A., Salvi M., Vignozzi L., Tubaro A., Morgia G., Serni S. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:146–155. doi: 10.1038/pcan.2017.1. [DOI] [PubMed] [Google Scholar]
- 181.Heir T., Falk R.S., Robsahm T.E., Sandvik L., Erikssen J., Tretli S. Cholesterol and prostate cancer risk: A long-term prospective cohort study. BMC Cancer. 2016;16:643. doi: 10.1186/s12885-016-2691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu Y.P., Zhang Y.X., Li P.F., Cheng C., Zhao Y.S., Li D.P., Du C. Cholesterol levels in blood and the risk of prostate cancer: A meta-analysis of 14 prospective studies. Cancer Epidemiol. Biomark. Prev. 2015 doi: 10.1158/1055-9965.epi-14-1329. [DOI] [PubMed] [Google Scholar]
- 183.Boland M.L., Chourasia A.H., Macleod K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 185.Chen X., Chen H., Dai M., Ai J., Li Y., Mahon B., Dai S., Deng Y. Plasma lipidomics profiling identified lipid biomarkers in distinguishing early-stage breast cancer from benign lesions. Oncotarget. 2016;7:36622–36631. doi: 10.18632/oncotarget.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li J., Ren S., Piao H.-l., Wang F., Yin P., Xu C., Lu X., Ye G., Shao Y., Yan M., et al. Integration of lipidomics and transcriptomics unravels aberrant lipid metabolism and defines cholesteryl oleate as potential biomarker of prostate cancer. Sci. Rep. 2016;6:20984. doi: 10.1038/srep20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mistry D.A.H., French P.W. Circulating phospholipids as biomarkers of breast cancer: A review. Breast Cancer. 2016;10:191–196. doi: 10.4137/BCBCR.S40693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Perrotti F., Rosa C., Cicalini I., Sacchetta P., Del Boccio P., Genovesi D., Pieragostino D. Advances in lipidomics for cancer biomarkers discovery. Int. J. Mol. Sci. 2016;17:1992. doi: 10.3390/ijms17121992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Berinstein N.L. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: A review. J. Clin. Oncol. 2002;20:2197–2207. doi: 10.1200/JCO.2002.08.017. [DOI] [PubMed] [Google Scholar]
- 190.Duffy M.J. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin. Chem. 2001;47:624–630. [PubMed] [Google Scholar]
- 191.Hammarstrom S. The carcinoembryonic antigen (cea) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 192.Beard D.B., Haskell C.M. Carcinoembryonic antigen in breast cancer. Clinical review. Am. J. Med. 1986;80:241–245. doi: 10.1016/0002-9343(86)90015-X. [DOI] [PubMed] [Google Scholar]
- 193.Huyghe J. Cea radioimmunoassay. Clinical applications in colorectal cancer. Acta Chir. Belg. 1983;83:77–88. [PubMed] [Google Scholar]
- 194.Chester S.J., Maimonis P., Vanzuiden P., Finklestein M., Bookout J., Vezeridis M.P. A new radioimmunoassay detecting early stages of colon cancer: A comparison with Cea, Afp, and Ca 19–9. Dis. Markers. 1991;9:265–271. [PubMed] [Google Scholar]
- 195.Lamerz R., Leonhardt A., Ehrhart H., von Lieven H. Serial carcinoembryonic antigen (cea) determinations in the management of metastatic breast cancer. Oncodev Biol. Med. 1980;1:123–135. [PubMed] [Google Scholar]
- 196.Chan D.W., Beveridge R.A., Muss H., Fritsche H.A., Hortobagyi G., Theriault R., Kiang D., Kennedy B.J., Evelegh M. Use of truquant br radioimmunoassay for early detection of breast cancer recurrence in patients with stage ii and stage iii disease. J. Clin. Oncol. 1997;15:2322–2328. doi: 10.1200/JCO.1997.15.6.2322. [DOI] [PubMed] [Google Scholar]
- 197.Borthwick N.M., Wilson D.W., Bell P.A. Carcinoembryonic antigen (cea) in patients with breast cancer. Eur. J. Cancer. 1977;13:171–176. doi: 10.1016/0014-2964(77)90196-7. [DOI] [PubMed] [Google Scholar]
- 198.Petitte B., Bialczak D., Fink L., Golightly D. Radioimmunoassay’s role in patient managemen. J. Nucl. Med. Technol. 1991;19:155–159. [Google Scholar]
- 199.Kufe D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Duffy M.J., Bonfrer J.M., Kulpa J., Rustin G.J., Soletormos G., Torre G.C., Tuxen M.K., Zwirner M. Ca125 in ovarian cancer: European group on tumor markers guidelines for clinical use. Int. J. Gynecol. Cancer. 2005;15:679–691. doi: 10.1111/j.1525-1438.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- 201.Sturgeon C.M., Duffy M.J., Stenman U.H., Lilja H., Brunner N., Chan D.W., Babaian R., Bast R.C., Jr., Dowell B., Esteva F.J., et al. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin. Chem. 2008;54:e11–e79. doi: 10.1373/clinchem.2008.105601. [DOI] [PubMed] [Google Scholar]
- 202.Genway Biotech Inc. Cancer antigen ca125 enzyme immunoassay test kit. [(accessed on 31 May 2017)]; Available online: https://www.genwaybio.com/elisas/tumor-marker-elisa/ovarian-cancer-antigen-ca125.
- 203.Kallioniemi O.P., Oksa H., Aaran R.K., Hietanen T., Lehtinen M., Koivula T. Serum ca 15–3 assay in the diagnosis and follow-up of breast cancer. Br. J. Cancer. 1988;58:213–215. doi: 10.1038/bjc.1988.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Roche Diagnostics Ltd. Elecsys® cancer antigen 15–3 (ca. 15–3) [(accessed on 31 May 2017)]; Available online: http://www.roche-diagnostics.ch/content/dam/corporate/roche-dia_ch/documents/broschueren/professional_diagnostics/serumarbeitsplatz/immunologie/tumor-marker/EN_CA15–3_FactSheet.pdf.
- 205.Kalluri R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mollaei H., Safaralizadeh R., Pouladi N. A brief review of exosomes and their roles in cancer. Meta Gene. 2017;11:70–74. doi: 10.1016/j.mgene.2016.11.010. [DOI] [Google Scholar]
- 207.Soung Y.H., Ford S., Zhang V., Chung J. Exosomes in cancer diagnostics. Cancers. 2017;9:8. doi: 10.3390/cancers9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang X., Yuan X., Shi H., Wu L., Qian H., Xu W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ko J., Carpenter E., Issadore D. Detection and isolation of circulating exosomes and microvesicles for cancer monitoring and diagnostics using micro-/nano-based devices. Anal. 2016;141:450–460. doi: 10.1039/C5AN01610J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Uzan J., Carbonnel M., Piconne O., Asmar R., Ayoubi J.-M. Pre-eclampsia: Pathophysiology, diagnosis, and management. Vasc. Health Risk Manag. 2011;7:467–474. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.World Health Organization . Who Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. World Health Organization; Geneva, Switzerland: 2011. [PubMed] [Google Scholar]
- 212.Backes C.H., Markham K., Moorehead P., Cordero L., Nankervis C.A., Giannone P.J. Maternal preeclampsia and neonatal outcomes. J. Pregnancy. 2011;2011:214365. doi: 10.1155/2011/214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Meads C.A., Cnossen J.S., Meher S., Juarez-Garcia A., ter Riet G., Duley L., Roberts T.E., Mol B.W., van der Post J.A., Leeflang M.M., et al. Methods of prediction and prevention of pre-eclampsia: Systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol. Assess. 2008;12:1–270. doi: 10.3310/hta12060. [DOI] [PubMed] [Google Scholar]
- 214.Siddiqui I.A. Maternal serum lipids in women with pre-eclampsia. Ann. Med. Health Sci. Res. 2014;4:638–641. doi: 10.4103/2141-9248.139358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Brown S.H.J., Eather S.R., Freeman D.J., Meyer B.J., Mitchell T.W. A lipidomic analysis of placenta in preeclampsia: Evidence for lipid storage. PLoS ONE. 2016;11:e0163972. doi: 10.1371/journal.pone.0163972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schweiger M., Eichmann T.O., Taschler U., Zimmermann R., Zechner R., Lass A. Measurement of lipolysis. Methods Adipose Tissue Biol. Pt B. 2014;538:171–193. doi: 10.1016/b978-0-12-800280-3.00010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schaffer J.E. Lipotoxicity: When tissues overeat. Curr. Opin. Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 218.Konige M., Wang H., Sztalryd C. Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim. Biophys. Acta-Mol. Basis Dis. 2014;1842:393–401. doi: 10.1016/j.bbadis.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schaffer J.E. Fatty acid transport: The roads taken. Am. J. Physiol.-Endocrinol. Metab. 2002;282:E239–E246. doi: 10.1152/ajpendo.00462.2001. [DOI] [PubMed] [Google Scholar]
- 220.Brasaemle D.L. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 221.Zechner R., Zimmermann R., Eichmann T.O., Kohlwein S.D., Haemmerle G., Lass A., Madeo F. Fat signals—Lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Farese R.V., Walther T.C. Lipid droplets finally get a little r-e-s-p-e-c-t. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Murphy D.J. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 2001;40:325–438. doi: 10.1016/S0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 224.Walther T.C., Farese R.V. Lipid droplets and cellular lipid metabolism. Ann. Rev. Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Malhi H., Gores G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Maaetoft-Udsen K., Greineisen W.E., Aldan J.T., Magaoay H., Ligohr C., Shimoda L.M.N., Sung C., Turner H. Comparative analysis of lipotoxicity induced by endocrine, pharmacological, and innate immune stimuli in rat basophilic leukemia cells. J. Immunotoxicol. 2015;12:385–394. doi: 10.3109/1547691X.2014.990655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ahmed M.H., Byrne C.D. Modulation of sterol regulatory element binding proteins (srebps) as potential treatments for non-alcoholic fatty liver disease (nafld) Drug Discov. Today. 2007;12:740–747. doi: 10.1016/j.drudis.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 228.Dam-Larsen S., Franzmann M., Andersen I.B., Christoffersen P., Jensen L.B., Sorensen T.I.A., Becker U., Bendtsen F. Long term prognosis of fatty liver: Risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Angulo P., Lindor K.D. Non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2002;17:S186–S190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 230.Kawano Y., Cohen D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mukerjee P., Mysels K.J. Critical Micelle Concentrations of Aqueous Surfactant Systems. NIST National Institute of Standards and Technology; Washington, DC, USA: 1971. [Google Scholar]
- 233.Avanti Polar Lipids Inc. Storage & Handling of Lipids. [(accessed on 31 May 2017)]; Available online: https://avantilipids.com/tech-support/storage-handling-of-lipids/