Abstract
Purpose
Long-term survival of women with advanced-stage ovarian cancer is relatively rare. Little is known about quality of life (QOL) and survivorship concerns of these women. Here, we describe QOL of women with advanced-stage ovarian cancer surviving for 8.5 years or longer and compare women with 0–1 recurrence to those with multiple recurrences.
Methods
Participants (n=56) recruited from 5 academic medical centers and the Ovarian Cancer Research Fund Alliance completed surveys regarding QOL (FACT-O), mood (CESD), social support (SPS), physical activity (IPAQ-SF), diet, and clinical characteristics. Median survival was 14.0 years (range 8.8–33.3).
Results
QOL and psychological adjustment of long-term survivors was relatively good, with mean FACT-G scores (multiple recurrences: 80.81±13.95; 0−1 recurrence: 89.05 ±10.80) above norms for healthy community samples (80.1±18.1). Survivors with multiple recurrences reported more compromised QOL in domains of physical and emotional well-being (p <.05), and endorsed a variety of physical and emotional concerns compared to survivors with 0−1 recurrence. Difficulties in sexual functioning were common in both groups. Almost half (43%) of the survivors reported low levels of physical activity.
Conclusions
Overall, women with advanced-stage ovarian cancer who have survived at least 8.5 years report good QOL and psychological adjustment. QOL of survivors with multiple recurrences is somewhat impaired compared to those with 0–1 recurrence. Limitations include a possible bias towards participation by healthier survivors, thus under-representing the level of compromise in long-term survivors. Health care practitioners should be alert to psychosocial issues faced by these long-term survivors to provide interventions that enhance QOL.
Keywords: Ovarian cancer, long-term survivor, psychosocial, quality of life, mood, lifestyle
Introduction
Although the majority of advanced-stage ovarian cancer patients have poor survival, a subset of patients live for many years after diagnosis. Women with stage III disease have 5- and 10-year survival rates of 36% and 23%, respectively, with rates of 17% and 8% for stage IV disease [1]. Clinical predictors of long-term survival have recently been described [2]; however, quality of life (QOL) and survivorship concerns of long-term advanced-stage survivors have been minimally characterized, thus little is known about the needs of this population.
Studies of ovarian cancer survivors within the first few years post-diagnosis frequently describe elevated distress, depression, anxiety, and sexual concerns in this population [3]. These negative sequelae are compounded for survivors experiencing physical complications and treatment side effects. In contrast, some survivors report personal growth and strengthened relationships post-diagnosis [3]. Prior studies of long-term adjustment of ovarian cancer survivors predominantly included early-stage survivors, or women surviving for at least three or five years without recurrence [4–6]. In one study of women with advanced stage non-recurrent disease averaging 6 years post-diagnosis, 64% of survivors reported mental health at or above medical outpatient norms, and most (71.4%) reported a strong sense of life purpose [7]. However, a subset of these survivors (28.6%) reported feeling depressed and 45.2% reported substantial anxiety [7].
The 2016 Institute of Medicine report on the state of ovarian cancer research highlighted the need for improved patient care across the continuum of survivorship [8]. Although ovarian cancer patients have been surviving longer [9, 10] there has been minimal characterization of QOL needs of advanced-stage ovarian cancer patients surviving for 8.5 years or longer post-diagnosis. Furthermore, there is substantial heterogeneity in disease course among long-term survivors [2]. Although some live for many years recurrence-free and disease-free, others have multiple recurrences, and may have long-lasting intermittent treatment. From a clinical perspective, long-term survivors with a single recurrence tend to be regarded similarly as those with no recurrence in terms of prognosis and clinical management [2]. This study thus examined QOL, survivorship concerns, and lifestyle factors (e.g. exercise) among long-term (8.5+ years) survivors of advanced-stage epithelial ovarian cancer, and compared women with 0−1 recurrences to those with multiple recurrences. We hypothesized that survivors with multiple recurrences would have poorer QOL, more survivorship concerns, higher levels of distress, poorer well-being and relationships, and be less physically active than those with 0−1 recurrence.
Methods
Participants
Women with epithelial ovarian, peritoneal, or fallopian tube cancer were recruited from Cedars-Sinai Medical Center, Memorial Sloan Kettering Cancer Center, University of Iowa Holden Comprehensive Cancer Center, Stephenson Cancer Center at the University of Oklahoma Health Sciences Center, University of Texas MD Anderson Cancer Center (MDACC), and the Ovarian Cancer Research Fund Alliance (OCRFA). The study was approved by the IRB of each academic site. Potentially eligible participants from medical centers were screened for eligibility and enrolled at a clinic visit if interested in participation. OCRFA recruited participants via online advertisements; these survivors contacted MDACC, and, if eligible, were enrolled by MDACC by phone. Surveys were completed at one time-point by internet or by hard copy if the patient was reluctant to use the internet-based-survey platform. Patients were eligible if they a) spoke English and b) were at least 8.5 years from diagnosis with stage III−IV epithelial ovarian cancer. All participants provided written informed consent.
The precise cut-point for designating a woman as a long-term survivor of ovarian cancer is currently not specifically defined; however, examination of conditional survival rates in the general vicinity of 10 years (e.g., between 8.5 and 10 years) do not show clear differences [11]. We performed a sensitivity analysis to examine cutoffs of 8.5, 9, and 10 years, but changing this cutoff did not alter the pattern of results. In the present study, therefore, a cutoff of 8.5 years was employed to maximize sample size while preserving relative homogeneity of outcomes.
Psychosocial Assessments
Quality of Life
Quality of Life was measured using the Functional Assessment of Cancer Therapy-Ovarian (FACT-O), a validated 38-item questionnaire consisting of physical, social, functional, and emotional well-being subscales (comprising the FACT-G) plus a disease-specific subscale measuring ovarian cancer-specific concerns [12, 13]. An additional FACT-Spiritual subscale was also included. Lower scores on the FACT indicate greater impairment.
Depressed Mood
Depressed Mood was measured using the Center for Epidemiological Studies Depression Scale (CES-D), a well-validated 20-item measure. Scores of 16 and above indicate clinically significant levels of depressed mood [14].
Social Support
Social Support was measured by the Social Provisions Scale, a 24-item self-report scale measuring extent to which social relationships are perceived as supportive. Items are rated on a 4-point scale with higher scores indicating greater support [15). The 7-item Abbreviated Dyadic Adjustment Scale (ADAS) was used to measure marital adjustment and satisfaction [16].
Psychological Well-Being
Psychological Well-Being was assessed using four subscales from the Ryff Psychological Well-Being Scales (PWBS) [17]. Each scale consists of 7 items rated on a 7-point scale, with higher scores indicating greater well-being.
Physical Activity
Physical Activity was measured with the 7-item International Physical Activity Questionnaire Short-Form (IPAQ-SF), which assesses physical activity over the past 7 days [18].
Each survey above has been extensively validated.
Demographic and Clinical Characteristics
Demographic and clinical information was provided by self-report. Upper body obesity was measured using the waist-to-hip ratio [19] with a tape measure and DVD provided for instructions. Body mass index (BMI) was categorized into underweight (< 18.5), normal weight (≥ 18.5 and < 25), overweight (≥ 25 and < 30) and obese/morbidly obese (≥ 30).
Descriptive assessments
Changes in social support and marital relationships after cancer were assessed with 9 descriptive survey items designed for this study asking about changes in quality of relationships with friends and partners and changes in sexual health since diagnosis. Two additional questions addressed change in diet and exercise. Alternative and complementary treatment use was assessed with 6 survey items regarding use of complementary methods since diagnosis. (See Supplement 2)
Statistical Analyses
Stata v14.1 (College Station, TX) was used to analyze data. All distributions were examined for outliers and assumptions of non-normality. Descriptive statistics were used to examine dependent variables. ANOVAs and the Wilcoxon rank-sum test were conducted to compare differences between survivor groups on continuous measures. Fisher’s exact test and Chi squared tests were used for categorical measures. To affirm the validity of dividing survivors into two groups based on number of recurrences, preliminary analyses were performed comparing the primary QOL outcome variable, FACT-O total scores, between survivors with 0 recurrences, one recurrence, and multiple recurrences. A 3-group analysis of variance (ANOVA) confirmed that the groups were significantly different (p=0.045). Post-hoc tests indicated that survivors with no recurrences had significantly higher FACT-O scores (mean=125.18±15.23) than those with multiple recurrences (mean=113.56±17.35, p=0.026), but were not significantly different from survivors with only one recurrence (mean=125.91±13.15, p=0.89). Moreover, FACT-O scores of survivors with one recurrence were lower than means of those with multiple recurrences (p=0.057), but this did not reach significance, likely due to the small sample size. As 3–7 points on the FACT represents a clinically significant difference in QOL [13], these data suggest relative similarity between survivors with 0 and 1 recurrence along with clinically significant differences between these survivors and those with multiple recurrences, thus supporting the use of a two group analytic strategy (i.e., 0–1 recurrence vs. multiple recurrences) for testing the present hypotheses. Thus, analyses compared survivors in two groups: 40 women reporting 0–1 recurrence and 16 women reporting two or more recurrences or persistent disease, categorized as multiple recurrences. A p-value of 0.05 was used for statistical significance.
Results
Participant Characteristics
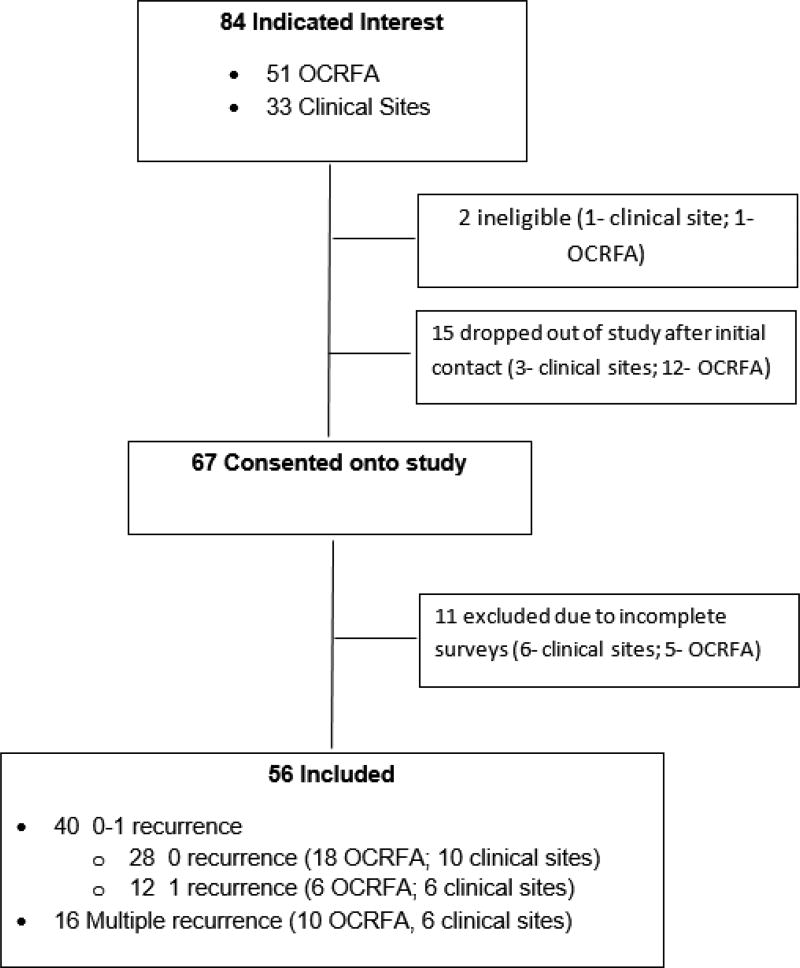
Of the potentially eligible patients at referring sites, 6 refused and 8 were not approached because of health issues. Of the 84 women who indicated interest, 51 were from OCFRA and 33 were from consortium sites. Two were ineligible, 5 withdrew, and 21 did not complete surveys. (Figure 1). The final sample included 56 long-term survivors with advanced-stage disease at diagnosis (34 from OCRFA and 22 from clinical sites). Of these women, 91.1% (n=51) were at least 10 years post-diagnosis (median survival time 14.0 years (range 8.8–33.3).
Figure 1.
Consort (Consolidated Standards of Reporting Trials) Diagram displaying patient inclusion.
Mean age of participants was 65.5 (range: 40.2−85.9) years. Respondents were predominantly white, non-Hispanic, and well-educated; more than half (55.4%) had completed college and/or graduate/professional school. About two thirds (66.1%) were married or living with a partner. Half of the participants (50.0%, n=28) reported no recurrences, 21.4% (n=12) reported one recurrence, 25.0% (n=14) reported multiple recurrences, and 3.6% (n=2) reported persistent disease. Treatment status included 73.2% (n=41) of survivors not receiving treatment, 21.4% (n=12) currently receiving treatment, and 5.4% (n=3) not specified (Table 1–Table 2).
Table 1.
Demographics characteristics of long-term survivors by recurrence status
| 0 – 1 recurrences |
Multiple recurrences |
Total | |||||
|---|---|---|---|---|---|---|---|
| (n = 40) | (n = 16) | (n = 56) | |||||
| Characteristic | N | % | N | % | N | % | p- value |
| Age at diagnosis | 0.394 | ||||||
| N | 40 | 16 | 56 | ||||
| Mean (SD) | 50.5 (9.9) | 48.5 (11.1) | 49.9 (10.2) | ||||
| Min - Max | 22.7 – 68.5 | 21.8 – 65.0 | 21.8 – 68.5 | ||||
| Age at survey | 0.079 | ||||||
| N | 40 | 16 | 56 | ||||
| Mean (SD) | 66.9 (9.2) | 62.2 (10.0) | 65.5 (9.6) | ||||
| Min - Max | 44.1 – 85.9 | 40.2 – 77.1 | 40.2 – 85.9 | ||||
| BMI | 0.816 | ||||||
| Underweight | 1 | 2.5 | 0 | 0 | 1 | 1.8 | |
| Normal | 17 | 42.5 | 9 | 56.3 | 26 | 46.4 | |
| Overweight | 14 | 35.0 | 4 | 25 | 18 | 32.1 | |
| Obese | 8 | 20.0 | 3 | 18.8 | 11 | 19.6 | |
| Waist to hip ratio | 0.926 | ||||||
| Low risk | 10 | 28.6 | 5 | 33.3 | 15 | 30.0 | |
| Moderate risk | 9 | 25.7 | 3 | 20 | 12 | 24.0 | |
| High risk | 13 | 45.7 | 7 | 46.7 | 23 | 46.0 | |
| Racial background | 0.644 | ||||||
| Black | 1 | 2.5 | 0 | 0 | 1 | 1.8 | |
| Asian | 0 | 0.0 | 0 | 0 | 0 | 0.0 | |
| Other | 1 | 2.5 | 0 | 0 | 1 | 1.8 | |
| White | 38 | 95 | 15 | 93.8 | 53 | 94.6 | |
| White/Native American | 0 | 0 | 1 | 6.3 | 1 | 1.8 | |
| White/Other | 0 | 0.0 | 0 | 0 | 0 | 0.0 | |
| Ethnic background | 0.557 | ||||||
| Hispanic | 3 | 7.7 | 0 | 0 | 3 | 5.7 | |
| Not Hispanic | 36 | 92.3 | 14 | 100.0 | 50 | 94.3 | |
| Education completed | 0.021 | ||||||
| Less than high school | 1 | 2.5 | 0 | 0 | 1 | 1.8 | |
| High school/GED | 2 | 5.0 | 0 | 0 | 2 | 3.6 | |
| Some college/Technical school | 18 | 45.0 | 3 | 18.8 | 21 | 37.5 | |
| Four-year college or university | 7 | 17.5 | 9 | 56.3 | 16 | 28.6 | |
| Graduate school/Professional school | 12 | 30.0 | 3 | 18.8 | 15 | 26.8 | |
| Other | 0 | 0.0 | 1 | 6.3 | 1 | 1.8 | |
| Employment status: | 0.276 | ||||||
| Employed outside of the home | 12 | 30.0 | 6 | 37.5 | 18 | 32.1 | |
| Homemaker | 5 | 12.5 | 3 | 18.8 | 8 | 14.3 | |
| Retired | 23 | 57.5 | 6 | 37.5 | 29 | 51.8 | |
| Unemployed - looking for work | 0 | 0.0 | 0 | 0 | 0 | 0.0 | |
| Unemployed - due to disability | 0 | 0.0 | 1 | 6.3 | 1 | 1.8 | |
| Annual total household income | 0.691 | ||||||
| Less than $25,000 | 1 | 2.7 | 0 | 0 | 1 | 1.9 | |
| $25,001 - $50,000 | 14 | 37.8 | 3 | 20 | 17 | 32.7 | |
| $50,001 - $75,000 | 7 | 18.9 | 4 | 26.7 | 11 | 21.2 | |
| $75,001 - $100,000 | 5 | 13.5 | 2 | 13.3 | 7 | 13.5 | |
| Greater than $100,000 | 10 | 27.0 | 6 | 40 | 16 | 30.8 | |
| Current marital status | 0.495 | ||||||
| Never married | 0 | 0.0 | 1 | 6.3 | 1 | 1.8 | |
| Married | 23 | 57.5 | 12 | 75 | 35 | 62.5 | |
| Living with partner | 2 | 5.0 | 0 | 0 | 2 | 3.6 | |
| Separated | 1 | 2.5 | 0 | 0 | 1 | 1.8 | |
| Divorced | 10 | 25.0 | 2 | 12.5 | 12 | 21.4 | |
| Widowed | 4 | 10.0 | 1 | 6.3 | 5 | 8.9 | |
| Given birth to a live infant | >0.999 | ||||||
| Yes | 34 | 85.0 | 14 | 87.5 | 48 | 85.7 | |
| No | 6 | 15.0 | 2 | 12.5 | 8 | 14.3 | |
| Smoking status | >0.999 | ||||||
| Never | 19 | 47.5 | 7 | 46.7 | 26 | 47.3 | |
| Current | 2 | 5.0 | 1 | 6.7 | 3 | 5.5 | |
| Former | 19 | 47.5 | 7 | 46.7 | 26 | 47.3 | |
| Current Smoking | 0.636 | ||||||
| Everyday | 1 | 4.8 | 0 | 0 | 1 | 3.5 | |
| Some days | 1 | 4.8 | 1 | 12.5 | 2 | 6.9 | |
| Not at all | 19 | 90.5 | 7 | 87.5 | 26 | 89.7 | |
Table 2.
Clinical Characteristics by Recurrence Status
| 0–1 | Multiple | Total | |||||
|---|---|---|---|---|---|---|---|
| (n = 40) | (n= 16) | (N = 56) | |||||
| Characteristic | N | % | N | % | N | % | p- value |
| Time from diagnosis to survey | 0.355 | ||||||
| N | 40 | 16 | 56 | ||||
| Mean (SD) | 16.3 (6.2) | 13.7 (2.6) | 15.6 (5.5) | ||||
| Min – Max | 8.8 – 33.3 | 9.2 – 18.3 | 8.8 – 33.3 | ||||
| What stage was your ovarian cancer at the time of diagnosis? | 0.274 | ||||||
| Stage I or II | 0 | 0 | 0 | 0 | |||
| Stage III | 37 | 92.5 | 13 | 81.3 | 50 | 89.3 | |
| Stage IV | 2 | 5.0 | 3 | 18.8 | 5 | 8.9 | |
| not sure | 1 | 2.5 | 0 | 0 | 1 | 1.8 | |
| Grade | 0.889 | ||||||
| High grade | 14 | 35.0 | 4 | 26.7 | 18 | 32.7 | |
| Low grade | 3 | 7.5 | 1 | 6.7 | 4 | 7.3 | |
| not sure | 23 | 57.5 | 10 | 66.7 | 33 | 60.0 | |
| Histology | 0.741 | ||||||
| Serous | 27 | 67.5 | 10 | 66.7 | 37 | 67.3 | |
| Non-Serous | 1 | 2.5 | 1 | 6.7 | 2 | 3.6 | |
| not sure | 12 | 30.0 | 4 | 26.7 | 16 | 29.1 | |
| Current Treatment Status | 0.005 | ||||||
| Currently no treatment | 34 | 85.0 | 7 | 43.8 | 41 | 73.2 | |
| On treatment | 5 | 12.5 | 7 | 43.8 | 12 | 21.4 | |
| Missing | 1 | 2.5 | 2 | 12.5 | 3 | 5.4 | |
| Neoadjuvant chemotherapy | >0.999 | ||||||
| Yes | 2 | 5.0 | 0 | 0 | 2 | 3.6 | |
| No | 38 | 95.0 | 16 | 100 | 54 | 96.4 | |
| Radiation at any point since diagnosis | 0.395 | ||||||
| Yes | 4 | 10.0 | 3 | 18.8 | 7 | 12.5 | |
| No | 36 | 90.0 | 13 | 81.3 | 49 | 87.5 | |
| Second surgery for ovarian cancer | 0.015 | ||||||
| Yes | 12 | 30.0 | 11 | 68.8 | 23 | 41.1 | |
| No | 28 | 70.0 | 5 | 31.3 | 33 | 58.9 | |
| BRCA testing | 0.475 | ||||||
| Yes | 30 | 75.0 | 14 | 87.5 | 44 | 78.6 | |
| No | 10 | 25.0 | 2 | 12.5 | 12 | 21.4 | |
| If yes, do you have a BRCA mutation? | 0.391 | ||||||
| Yes | 13 | 43.3 | 5 | 38.5 | 18 | 41.9 | |
| No | 16 | 53.3 | 6 | 46.2 | 22 | 51.2 | |
| I’m not sure | 1 | 3.3 | 2 | 15.4 | 3 | 7.0 | |
| Recurrence | <0.001 | ||||||
| Yes | 12 | 30.0 | 16 | 100 | 28 | 50.0 | |
| No | 28 | 70.0 | 0 | 0 | 28 | 50.0 | |
| Other cancer | 0.741 | ||||||
| Yes | 10 | 25.0 | 5 | 31.3 | 15 | 26.8 | |
| No | 30 | 75.0 | 11 | 68.8 | 41 | 73.2 | |
| Change in diet since diagnosis? | >0.999 | ||||||
| Yes | 21 | 52.5 | 8 | 50.0 | 29 | 51. 8 | |
| No | 19 | 47.5 | 8 | 50.0 | 27 | 48.2 | |
| Change in exercise habits since diagnosis? | >0.999 | ||||||
| Yes | 14 | 35.0 | 5 | 31.3 | 19 | 33.9 | |
| No | 26 | 65.0 | 11 | 68.8 | 37 | 66.1 | |
Quality of Life
In addition to the group differences in total FACT-O scores described above, survivors with multiple recurrences reported significantly poorer QOL on each of the two major components of the FACT-O score, namely, the total FACT-G score and the ovarian-specific item scale. The FACT-G is useful because it can be compared to population norms. FACT-G scores of survivors with multiple recurrences (80.81±13.95) were significantly poorer than those of survivors with 0−1 recurrence (89.05 ±10.80; p=0.031). (Table 3). Although these data suggest clinical decrements in QOL among survivors with multiple recurrences, mean scores of both survivor groups approximated normative FACT-G scores of the U.S. population (80.1±18.1) [13], suggesting that overall QOL was quite good. Survivors with multiple recurrences had poorer scores on the ovarian concerns subscale (32.75±5.25) than those with 0–1 recurrence (36.30±4.54, p =0.009), indicating greater impairment from ovarian-specific issues.
Table 3.
Fact-O Total and Subscale scores by recurrence status
| Score | Recurrences | N | Mean | SD | Med | Min | Max | p-value |
|---|---|---|---|---|---|---|---|---|
| Physical Well Being Subscale Score | 0 – 1 | 40 | 25.43 | 2.72 | 26 | 15 | 28 | 0.016 |
| Multiple | 16 | 23.25 | 3.49 | 23 | 16 | 28 | ||
| Social Family Well Being Subscale Score | 0 – 1 | 39 | 19.77 | 4.38 | 20 | 7 | 28 | 0.730 |
| Multiple | 16 | 18.88 | 4.84 | 20.5 | 6 | 24 | ||
| Emotional Well Being Subscale Score | 0 – 1 | 39 | 20.69 | 2.92 | 21 | 11 | 24 | 0.019 |
| Multiple | 16 | 17.88 | 4.44 | 20 | 9 | 24 | ||
| Functional Well Being Subscale Score | 0 – 1 | 40 | 23.00 | 4.22 | 24 | 12 | 28 | 0.184 |
| Multiple | 16 | 20.81 | 5.60 | 22.5 | 11 | 28 | ||
| Additional Concerns Subscale Score | 0 – 1 | 40 | 36.30 | 4.54 | 38 | 22 | 44 | 0.009 |
| Multiple | 16 | 32.75 | 5.25 | 33 | 20 | 42 | ||
| Total Fact-G score | 0 – 1 | 39 | 89.05 | 10.80 | 90 | 62 | 104 | 0.031 |
| Multiple | 16 | 80.81 | 13.95 | 85 | 56 | 101 | ||
| Total Fact-O | 0 – 1 | 39 | 125.38 | 14.51 | 129 | 84 | 148 | 0.017 |
| Multiple | 16 | 113.56 | 17.35 | 115.5 | 79 | 143 | ||
| Trial Outcome Index Score | 0 – 1 | 40 | 84.72 | 9.75 | 87.5 | 56 | 99 | 0.013 |
| Multiple | 16 | 76.81 | 11.83 | 77.5 | 52 | 97 | ||
| FACT Spiritual Well-being Peace Scale Score | 0 – 1 | 40 | 26.35 | 4.96 | 26.5 | 15 | 32 | 0.603 |
| Multiple | 16 | 25.31 | 6.06 | 26.5 | 9 | 32 | ||
| FACT Spiritual Well-being Faith Scale Score | 0 – 1 | 40 | 12.03 | 4.40 | 13 | 2 | 16 | 0.941 |
| Multiple | 16 | 12.13 | 3.77 | 12 | 5 | 16 | ||
| FACT Spiritual Well-being Total Score | 0 – 1 | 40 | 38.38 | 8.13 | 39.64 | 19 | 48 | 0.669 |
| Multiple | 16 | 37.44 | 8.80 | 37 | 19 | 48 |
FACT subscale scores of survivors with multiple recurrences were significantly lower than those of survivors with 0–1 recurrence on Physical Well-being (PWB) (0–1: 25.43±2.72 vs. multiple: 23.25±3.49, p< 0.02) and Emotional Well-being (EWB) (0–1: 20.69±2.92 vs. multiple: 17.88±4.44, p< 0.02) but not Functional well-being (FWB) (0–1: 23.00±4.22 vs. multiple: 20.81±5.60, p=0.18) (Table 3). As subscale scores of 2–3 points reflect clinically significant differences [13], these decrements indicate potential clinically significant impairments in each domain. Moreover, mean EWB scores of survivors with multiple recurrences (shown above) were below normative population levels (19.9 ± 4.8) [13], suggesting poorer-than-average emotional well-being in this group. In contrast, PWB and FWB of both groups were above population norms (22.7±5.4 and 18.5±6.8, respectively) [13]. Both survivor groups reported high levels of social well-being, with over 85% of each group reporting substantial emotional support from their families.
Item-specific analyses of the FACT-O were performed to examine specific concerns of long-term survivors. Compared to survivors with 0−1 recurrence, the most salient concerns of survivors with multiple recurrences were physical and emotional problems (Supplemental Table 1). Survivors with multiple recurrences endorsed more physical health symptoms such as lack of energy (p=0.009), having to spend time in bed (p=0.006), abdominal swelling (p=0.002), hair loss (p=0.02), problems with independent mobility (p=0.007), and bowel control (p=0.045). Emotionally, a greater percentage of survivors with multiple recurrences reported losing hope in the fight against their illness (p = 0.001), worry that their condition would get worse (p=0.047), difficulty accepting their illness (p=0.03), and less enjoyment of things usually done for fun (p=0.06).
Both groups of survivors reported relatively high levels of spiritual well-being on the FACT spiritual scale (See [13] for population norms). Survivors with multiple recurrences reported greater trouble feeling peace of mind (p=0.005) and less ability to reach inside for comfort (p=0.07). Nevertheless, a majority of both groups (0–1: 60%; multiple: 56.3%) reported that their illness had strengthened their faith/spiritual beliefs and a majority of survivors (0–1: 75%; multiple: 81.3%) reported finding strength in their faith and/or spiritual beliefs (Table 3).
Psychosocial Adjustment
As seen in Table 4, both groups of long-term survivors reported relatively low levels of depressed mood (CES-D). Moreover, only 10% of survivors had CESD scores within the depressed range (≥ 16) and this did not differ by recurrence group (p=0.54). Survivors with 0–1 recurrence reported significantly higher levels of personal growth on the Psychological Well-being Scales (41.52±6.32) than those with multiple recurrences (38.49±4.6, p=0.018); however, even the latter group approximated the mean of a community sample (38.4±6.8) [20]. Mean levels of other facets of psychological well-being did not differ according to survivor group (p values > 0.22) and averaged across both groups were also within the range of a community sample (Purpose in Life: 39.54±6.48; Environmental Mastery: 39.98±6.46; Self-Acceptance: 37.89±5.88) [20].
Table 4.
Psychosocial Scales by Recurrence Status
| Item | Recurrences | N | Mean | SD | Med | Min | Max | p-value |
|---|---|---|---|---|---|---|---|---|
| Center for Epidemiologic Studies Depression Scale | 0 – 1 | 40 | 7.17 | 6.46 | 5 | 0 | 22 | 0.542 |
| Multiple | 16 | 7.38 | 2.58 | 8.5 | 2 | 10 | ||
| Psychological Well-Being: Environmental Mastery | 0 – 1 | 40 | 40.58 | 6.14 | 40 | 29 | 49 | 0.344 |
| Multiple | 16 | 38.50 | 7.18 | 39 | 21 | 47 | ||
| Psychological Well-Being: Personal Growth | 0 – 1 | 40 | 41.52 | 6.32 | 43 | 23 | 48 | 0.018 |
| Multiple | 16 | 38.49 | 4.6 | 38 | 30 | 44 | ||
| Psychological Well-Being: Purpose in Life | 0 – 1 | 40 | 40.10 | 6.85 | 41.5 | 21 | 49 | 0.217 |
| Multiple | 16 | 38.13 | 5.38 | 38 | 27 | 46 | ||
| Psychological Well-Being: Self-Acceptance | 0 – 1 | 40 | 37.65 | 6.34 | 40 | 22 | 46 | 0.920 |
| Multiple | 16 | 38.50 | 4.68 | 39.5 | 28 | 44 | ||
| Social Provisions Scale: Total Social Support | 0 – 1 | 40 | 84.75 | 9.48 | 87 | 61 | 96 | 0.531 |
| Multiple | 16 | 86.63 | 8.55 | 89.5 | 69 | 96 | ||
| Social Provisions Scale: Attachment subscale | 0 – 1 | 40 | 14.15 | 2.23 | 15 | 9 | 16 | 0.765 |
| Multiple | 16 | 14.44 | 1.90 | 15.5 | 11 | 16 | ||
| ADAS Total Score | 0 – 1 | 27 | 23.96 | 5.91 | 25 | 7 | 33 | 0.820 |
| Multiple | 13 | 23.54 | 4.48 | 23 | 15 | 31 |
Social Relationships
Both survivor groups reported relatively strong perceived social support (SPS) with means of 84.75 (±9.48) for those with 0–1 recurrence and 86.63 (±8.55) for those with multiple recurrences. These compare favorably with norms from other populations which often range between 71.0 and 76.0 (e.g., [21, 22]). The two recurrence groups did not differ in levels of total social support, social attachment, or perceptions of dyadic adjustment (ADAS) (n.s.) (Table 4). A change in marital status since diagnosis was reported by 28.6% of long-term survivors (0–1: 32.5% vs. multiple: 18.75%, n.s.), including 5 widowed, 7 divorced or separated post-diagnosis, and two remarried. A change in relationship quality after diagnosis was reported by 34.0% of survivors (0–1: 38.8% vs. multiple: 21.4%, n.s.), with 9 reporting stronger relationships and 6 reporting poorer relationship quality (including emotional abandonment, spouse had an affair, left them, disease caused them to stay in an unhealthy relationship, etc.). Cancer survivor support group participation was reported by 71.4% of participants at some point after their diagnosis, 65.0% indicated that they lend support to other survivors; these proportions also did not differ by group. (n.s.)
Sexual Functioning
Sexual health changes after diagnosis and treatment were quite prevalent (0–1: 65% vs. multiple: 75%), with predominant concerns being decreased or absent desire, pain during intercourse, and reduced quality of orgasms. The majority of survivors (0–1: 58.9% vs. multiple: 62.5%) reported being in a relationship that could involve sex; however only about half of these women (0−1: 52.2% vs. multiple: 50.0%) reported being sexually active in the last month. Almost two thirds of women in each group (0−1: 65.8% vs. multiple: 66.7%) reported that they were “not at all” or only “somewhat” satisfied with their sex life. Nevertheless, a majority of women (0−1: 81.5% vs. multiple: 75%) reported feeling close to their partners. These proportions did not differ by group (n.s.).
Physical Activity and Diet
With respect to exercise (IPAQ-SF), overall, 42.9% of survivors reported low levels of activity, 37.5% were moderately active, and 19.6% were highly active [18]. Exercise levels of the two survivor groups did not significantly differ, with two exceptions. First, survivors with 0–1 recurrence spent significantly fewer minutes/day (331.8± 209.5) and minutes/week (2322.3 ± 1466.8) sitting than those with multiple recurrences (452.3±205.8 minutes/day, p=0.036; 3166.1±1440.9 minutes/week, p= 0.036), and second, they reported twice as many minutes of daily walking (0–1: 44.85± 51.41 vs. multiple: 22.19 ± 31.36 minutes, p=0.049) (Table 5). The majority of survivors who made changes in exercise (n=12) reported increasing their exercise substantially after diagnosis, including activities such as vigorous daily walking, Tai Chi, Kung Fu, stretching, yoga, and joining an exercise group. Seven survivors reported that they had decreased their exercise due to fatigue, back pain, neuropathy, and incisional hernias.
Table 5.
International Physical Activity Questionnaire by Recurrence status
| IPAQ item | Status | N | Mean | SD | Min | Max | p-value |
|---|---|---|---|---|---|---|---|
| Vigorous Activity Minutes Per Day | 0–1 | 40 | 38.63 | 69.64 | 0 | 300 | 0.338 |
| Multiple | 16 | 23.44 | 61.23 | 0 | 240 | ||
| Vigorous Activity minutes last 7 days | 0–1 | 38 | 114.47 | 187.76 | 0 | 720 | 0.503 |
| Multiple | 14 | 123.21 | 322.48 | 0 | 1200 | ||
| Moderate Activity Minutes Per Day | 0–1 | 40 | 44.00 | 68.73 | 0 | 300 | 0.921 |
| Multiple | 16 | 47.50 | 76.29 | 0 | 240 | ||
| Moderate Activity minutes last 7 days | 0–1 | 38 | 181.45 | 281.95 | 0 | 1200 | 0.965 |
| Multiple | 14 | 213.57 | 351.56 | 0 | 1200 | ||
| Walking Minutes Per Day | 0–1 | 40 | 44.85 | 51.41 | 0 | 240 | 0.049 |
| Multiple | 16 | 22.19 | 31.36 | 0 | 120 | ||
| Walking Minutes Last 7 Days | 0–1 | 38 | 232.45 | 312.59 | 0 | 1680 | 0.153 |
| Multiple | 14 | 133.57 | 216.5 | 0 | 840 | ||
| Sitting Minutes Per Day | 0–1 | 34 | 331.76 | 209.54 | 120 | 1200 | 0.036 |
| Multiple | 13 | 452.31 | 205.84 | 180 | 900 | ||
| Sitting Minutes Last 7 Days | 0–1 | 34 | 2322.35 | 1466.76 | 840 | 8400 | 0.036 |
| Multiple | 13 | 3166.15 | 1440.87 | 1260 | 6300 | ||
| Vigorous and Moderate Minutes combined (last 7 days) | 0–1 | 38 | 295.92 | 383.81 | 0 | 1200 | 0.627 |
| Multiple | 14 | 336.79 | 649.3 | 0 | 2400 | ||
| Total minutes of activity (last 7 days) | 0–1 | 38 | 528.37 | 637.04 | 0 | 2840 | 0.508 |
| Multiple | 14 | 470.36 | 697.24 | 0 | 2580 | ||
| Met Score for walking | 0–1 | 38 | 767.08 | 1031.54 | 0 | 5544 | 0.153 |
| Multiple | 14 | 440.79 | 714.44 | 0 | 2772 | ||
| Met Score for moderate activity | 0–1 | 38 | 725.79 | 1127.82 | 0 | 4800 | 0.965 |
| Multiple | 14 | 854.29 | 1406.24 | 0 | 4800 | ||
| Met Score for vigorous activity | 0–1 | 38 | 915.79 | 1502.05 | 0 | 5760 | 0.503 |
| Multiple | 14 | 985.71 | 2579.81 | 0 | 9600 | ||
| Total Physical Activity Score | 0–1 | 38 | 2408.66 | 2911.29 | 0 | 11944 | 0.402 |
| Met Minutes Per week | Multiple | 14 | 2280.79 | 3957.3 | 0 | 14994 | |
|
| |||||||
| Low Activitya | 0–1 | 15 | 40.48% | 0.391 | |||
| Total both groups 42.9% | Multiple | 9 | 56.25% | ||||
| Moderately Activity | 0–1 | 17 | 40.48% | ||||
| Total both groups 37.5% | Multiple | 4 | 25.00% | ||||
| High Activity | 0–1 | 8 | 19.04% | ||||
| Total both groups 19.6% | Multiple | 3 | 18.75% | ||||
Responses are categorized into three levels of physical activity: low (< moderate); moderate (3 or more days/week of vigorous activity of at least 20 minutes/day or 5 or more days/week of moderate activity, or 30 minutes/day walking) and high (vigorous activity on at least 3 days or 7 days/week of any combination of walking, moderate or vigorous intensity activities.) [14]
Approximately one-third (32.1%) of all participants had BMI in the overweight range and 19.6% had BMI in the obese range. Almost half of the participants had a waist-hip ratio in the high-risk range (46%) and 24% had waist-to-hip ratios in the moderate risk range. However, there were no significant differences in BMI or waist-to-hip ratio between the two groups (n.s.). Among all survivors, 51.8% reported changing their diet and 33.9% reported changing exercise habits since their diagnosis, with no significant differences between groups in the proportions who reported making a change (n.s.). Of those who made diet changes, all but four reported diet choices involving more careful food selection, such as checking food labels, eating fewer processed foods, more organic foods, vegetables, fresh fruits, lower fat, less sugar, and juicing (extraction of juice from fresh fruits and vegetables to maximize consumption of vitamins, minerals and phytonutrients). Three individuals reported persistent digestive difficulties since treatment requiring a more bland or restricted diet and one reported giving up on previous dietary changes after a recurrence.
Mind-Body or Complementary Treatments
A subset of long-term survivors used mind-body or integrative medicine treatments. Meditation was the most popular approach, with 35.2% of survivors (0–1: 40.0% vs. multiple: 21.4%) reporting having tried meditation. Of those who meditated, overall, 42.1% reported meditating 3 or more days/week, 52.6% reported meditating 1–2 days/week, and 42.1% reported meditating for more than 2 years.
Discussion
Key findings from this study indicate that QOL and psychological functioning of long-term advanced-stage ovarian cancer survivors tends to be relatively good, with mean QOL scores commensurate with those of healthy community samples. To our knowledge, this is the first study characterizing QOL in this unique population, including contrasts between survivors with differing disease trajectories. QOL of survivors with multiple recurrences was more compromised for all domains except social and functional well-being; their decrements in emotional and physical well-being were clinically significant and their emotional well-being was below community norms. Survivors with multiple recurrences were more likely to report losing hope and worry that their condition would worsen. Even so, both groups of long-term survivors reported relatively low levels of depressed mood, and only 10% had scores in the depressed range. Both groups of women reported low levels of physical activity; almost half the long-term survivors were only minimally active, and more than half were overweight. Sexual functioning was a major concern of both groups. Social relationships were described as strong.
Ovarian cancer patients commonly report elevations in depressed mood, anxiety, and sleep disorders at the time of diagnosis [3, 23], and sustained elevations in depressed mood and sleep disorders have been observed at one year in a sizeable subset of survivors [23]. The present finding of relatively normal mood in both groups of long-term survivors is consistent with previous reports of good mental health in a majority of advanced-stage ovarian cancer survivors at 6 years post-diagnosis [7] and reported trends towards improving mental health among heterogeneous cancer patients as time since diagnosis increases [25]. The proportion of patients in our sample with clinical levels of depressive symptoms (10%) was similar to levels of depression reported in a meta-analysis of cancer patients 7 years post-diagnosis (11.6%), and was also similar to the rate of depression in healthy controls in that study (10.2%) [26]. Consistent with our finding of poorer emotional well-being among survivors with multiple recurrences, higher levels of depression have been reported in survivors with more advanced cancers, more physical symptoms, and greater loneliness [25]. Fear of recurrence or of worsening disease remains a salient concern in our long-term survivors, particularly in those who have already had a recurrence. A recent systematic review reported that a majority of long-term (≥ 5 years) cancer survivors experience at least moderate intensity fear of recurrence [27]. Our findings are consistent with previous reports that fear of recurrence is a common and often debilitating concern among ovarian cancer survivors [24], and one that may need health care provider attention.
The finding that QOL of long-term survivors of ovarian cancer is similar to that of the general population was surprising, and may reflect a variety of circumstances. The functional abilities of long-term survivors, particularly those with 0–1 recurrence, may be minimally compromised by cancer. These findings may also reflect support that our predominantly white, educated participants may have received from the health care system or from affiliation with resources like OCRFA.
With respect to physical activity, lower-body functional limitations have previously been reported in 52.8% of ovarian survivors [28]. Ovarian cancer survivors have reported greater difficulty performing activities including stooping, crouching, kneeling, lifting 10 pounds, walking one quarter mile, and walking up and down ten steps without rest [29], which may explain the low activity levels of our long-term survivors. Physical exercise is known to enhance physical, cognitive, and emotional functioning [30]; thus, long-term survivors may potentially be helped by low-impact exercise or yoga programs that could address specific deficiencies, as well as by interventions that address obstacles to undertaking exercise training.
The majority of both groups of survivors reported difficulties with sexual functioning and low levels of sexual satisfaction, with sexual changes including minimal desire, pain during intercourse, and reduced quality of orgasms. These findings are consistent with previous reports of sexual health concerns among ovarian cancer survivors [4–7] and highlight the need for interventions to help long-term survivors with sexual functioning.
Both groups of survivors reported strong relationships and relatively high levels of perceived social support. Prospective longitudinal research has indicated that perceived emotional support at the time of diagnosis is related to longer survival in ovarian cancer [31]. Previous work has highlighted biological pathways by which social support may mediate survival in ovarian cancer, including angiogenesis, invasion, inflammation, cellular immunity [32, 33] and transcriptional changes in the tumor genome suggestive of less pro-inflammatory and pro-metastatic signaling [34]. Moreover, ovarian cancer survivors with high levels of psychological well-being and/or social support have lower tumor norepinephrine [35, 36], a stress hormone shown to be linked.to tumor progression. These findings suggest the possibility that along with molecular and clinical determinants of long-term survival in ovarian cancer, biobehavioral factors may influence survival outcomes. Longitudinal research on trajectories of long-term survivors will help to further elucidate these issues.
Limitations
Although only a small proportion of those approached at study sites declined, some long-term survivors were not approached because of poor health. We do not know how many eligible long-term survivors did not respond to postings about the study on the OCRFA website. It is possible that only the healthiest long-term survivors volunteered, thus under-representing the level of compromise in long-term survivors as a whole. Additionally, it is possible that high levels of QOL may reflect the demographics of populations seeking care at academic medical centers or seeking support from OCRFA. We also did not assess health insurance status, which may have been able to shed light on the availability of health care to participants. As the vast majority of patients were white and educated, the demographic homogeneity may limit generalizability of findings. Because there were only 16 individuals in the multiple recurrence group, the inferences that can be drawn from their data are limited. Number of recurrences ranged from 2 to 8. It is also possible that individuals with multiple recurrences may have had additional debility due to being on treatment or maintenance chemotherapy currently or in the recent past; this may have enhanced between group differences. Because we did not collect data on time since last treatment, we were not able to examine this as a potential covariate. Thus, there may be substantial heterogeneity in the multiple recurrence group and the present data may not fully capture the impact of multiple recurrences on QOL. We also did not collect data on use of antidepressants or anti-anxiety medications, and thus do not know how such drugs may have influenced findings. The cross-sectional nature of this study does not shed light on the trajectory of QOL of survivors over time. As we did not collect data on cognitive functioning and neurotoxicity, findings may underestimate these serious treatment side effects. Information on cancer stage, treatment, and recurrences was obtained by self-report and was not objectively verified. Although there has been some indication of concordance of self-reported and medical chart information among cancer patients [37], these studies have not examined self-reports of long-term survivors, and thus this information should be treated with caution. Finally, since long-term survival in advanced stage ovarian cancer is extremely rare, the sample size is relatively small.
Conclusions and Clinical Implications
Overall, advanced-stage ovarian cancer survivors surviving at least 8.5 years report good QOL and psychological adjustment. QOL of survivors with multiple recurrences is somewhat impaired compared to those with 0−1 recurrences. In light of these findings and the recent IOM recommendations, health care providers should be sensitive to psychosocial issues such as distress, sexual health difficulties, physical impairments, and low levels of physical activity among survivors of advanced-stage ovarian cancer from diagnosis through long-term survival. Interventions to improve QOL and to address limitations in physical activity should also be considered to help survivors with these issues.
Supplementary Material
Research Highlights.
QOL of long-term advanced-stage ovarian cancer survivors is relatively good
Survivors with multiple recurrences reported more compromised QOL
Low levels of exercise were reported by 43% of long-term survivors
Among these survivors, 52% were overweight or obese
Sexual health concerns are common in long-term survivors
Acknowledgments
Funding Support: This research was supported in part by Department of Defense CDMRP Grant W81XWH-13-1-0192 (AKS), SIOP-06-258-01-COUN (BK), and National Institutes of Health grants CA109298 (AKS), CA140933 (SL), grant P30CA086862 to the Holden Comprehensive Cancer Center, and grant CA016672 to the Biostatistical Service at MD Anderson Cancer Center.
We would like to express our gratitude to all the ovarian cancer survivors who participated in the study and to Ainslee Johnson for administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflicts of Interest
Dr. Moore reports advisory board honoraria from Astra Zeneca, Clovis, Immunogen, Genentech/Roche, steering committee from Tesaro, steering committee/advisory board from Advaxis, outside the submitted work; other authors declare no competing financial interests.
References
- 1.Baldwin LA, Huang B, Miller RW. Ten year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012;120:612–8. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 2.Dao F, Schlappe BA, Tseng J, Lester J, Nick AM, Lutgendorf SK, McMeekin S, Coleman RL, Moore KN, Karlan BY, Sood A, Levine DA. Characteristics of 10-year survivors of high-grade serous ovarian carcinoma. Gynecol. Oncol. 2016;141:260–3. doi: 10.1016/j.ygyno.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roland KB, Rodriguez JL, Patterson JR, Trivers KF. A literature review of the social and psychological needs of ovarian cancer survivors. Psychooncology. 2013;22:2408–18. doi: 10.1002/pon.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matulonis UA, Kornblith A, Lee H, Bryan J, Gibson C, Wells C, et al. Long-term adjustment of early-stage ovarian cancer survivors. Int. J. Gynecol. Cancer. 2008;18:1183–93. doi: 10.1111/j.1525-1438.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel LB, Donnelly JP, Fowler JM, Habbal R, Taylor TH, Aziz N, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: A gynecologic oncology group study. Psychooncology. 2002;11:142–53. doi: 10.1002/pon.567. [DOI] [PubMed] [Google Scholar]
- 6.Mirabeau-Beale K, Kornblith AB, Penson RT, Lee H, Goodman A, Campos SM, et al. Comparison of the quality of life of early and advanced stage ovarian cancer survivors. Gynecol. Oncol. 2009;114:353–9. doi: 10.1016/j.ygyno.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Kornblith AB, Mirabeau-Beale K, Lee H, Goodman AK, Penson RT, Pereira L, et al. Long-term adjustment of survivors of ovarian cancer treated for advanced-stage disease. J. Psychosoc. Oncol. 2010;28:451–69. doi: 10.1080/07347332.2010.498458. [DOI] [PubMed] [Google Scholar]
- 8.National Academies of Sciences E, and Medicine. Ovarian Cancers: Evolving Paradigmsin Research and Care. National Academies Press; Washington, DC: 2016. [PubMed] [Google Scholar]
- 9.Huang L, Cronin KA, Johnson KA, Mariotto AB, Feuer EJ. Improved survival time: What can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer. 2008;112:2289–300. doi: 10.1002/cncr.23425. [DOI] [PubMed] [Google Scholar]
- 10.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2013;10:211–24. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi M, Fuller CD, Thomas CR, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol. Oncol. 2008;109:203–9. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, Webster K, Cella D, Hu S, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J. Clin. Oncol. 2001;19:1809–17. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 13.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- 15.Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. Adv. Pers. Relat. 1987;1:37–67. [Google Scholar]
- 16.Sharpley CF, Rogers HJ. Preliminary validation of the Abbreviated Spanier Dyadic Adjustment Scale: Some psychometric data regarding a screening test of marital adjustment. Educ. Psychol. Meas. 1984;44:1045–9. [Google Scholar]
- 17.Ryff CD, Keyes CLM. The structure of psychological well-being revisited. J. Pers. Soc. Psychol. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- 18.Sjostram M, Ainsworth B, Bauman A, Bull F, Craig C, Sallis J. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)–Short and Long Forms: Nov 2005. 2009;5(11) Available at wwwipaqkise/scoring Accessed . [Google Scholar]
- 19.American College of Sports Medicine. ACSM’s Guidelines for exercise testing and prescription. 9. Philadelphia: Lipincott Williams & Wilkins; 2014. [Google Scholar]
- 20.Costanzo ES, Ryff CD, Singer BH. Psychosocial adjustment among cancer survivors: findings from a national survey of health and well-being. Health Psychol. 2009;28:147–56. doi: 10.1037/a0013221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron R, Cutrona C, Hicklin D, Russell D, Lubaroff D. Social support and immune function among spouses of cancer patients. J. Pers. Soc. Psychol. 1990;59:344–52. doi: 10.1037//0022-3514.59.2.344. [DOI] [PubMed] [Google Scholar]
- 22.Cutrona CE. Social support and stress in the transition to parenthood. J. Abnormal Psychol. 1984;93:378–90. doi: 10.1037//0021-843x.93.4.378. [DOI] [PubMed] [Google Scholar]
- 23.Clevenger L, Schrepf A, DeGeest K, Bender D, Goodheart M, Ahmed A, et al. Sleep Disturbance, Distress, and Quality of Life in Ovarian Cancer Patients during the First Year Post Diagnosis. Cancer. 2013;119:3234–41. doi: 10.1002/cncr.28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl L, Wittrup I, Væggemose U, Petersen LK, Blaakaer J. Life after gynecologic cancer--a review of patients quality of life, needs, and preferences in regard to follow-up. Int. J. Gynecol. Cancer. 2013;23:227–34. doi: 10.1097/IGC.0b013e31827f37b0. [DOI] [PubMed] [Google Scholar]
- 25.Stanton A, Rowland JH, Ganz PA. Life after diagnosis and treatment of cancer in Adulthood: Contributions from Psychosocial Oncology Research. Am Psychol. 2015;70:159–74. doi: 10.1037/a0037875. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013;14:721–32. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 27.Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (≥5 years) cancer survivors—a systematic review of quantitative studies. Psychooncology. 2013;22:1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- 28.Schootman M, Aft R, Jeffe DB. An evaluation of lower-body functional limitations among long-term survivors of 11 different types of cancers. Cancer. 2009;115:5329–38. doi: 10.1002/cncr.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peel AB, Barlow CE, Leonard D, DeFina LF, Jones LW, Lakoski SG. Cardiorespiratory fitness in survivors of cervical, endometrial, and ovarian cancers: The Cooper Center Longitudinal Study. Gynecol. Oncol. 2015;138:394–7. doi: 10.1016/j.ygyno.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Lowe KA, Andersen MR, Sweet E, Standish L, Drescher CW, Goff BA. The effect of regular exercise and yoga on health-related quality of life among ovarian cancer survivors. Evid. Based Complement. Alternat. Med. 2012;17:155–60. [Google Scholar]
- 31.Lutgendorf SK, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, et al. Social Influences on Clinical Outcomes of Patients with Ovarian Cancer. J. Clin. Oncol. 2012;30:2885–90. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer. 2015;15:563–72. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005;23:7105–13. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Lutgendorf SK, DeGeest K, Sung CY, Arevalo JMG, Penedo F, Lucci JAI, et al. Depression, Social Support, and Beta-Adrenergic Transcription Control in Human Ovarian Cancer. Brain Behav. Immun. 2009;23:176–83. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, et al. Social Isolation is associated with Elevated Tumor Norepinephrine in Ovarian Carcinoma Patients. Brain Behav. Immun. 2010;25:250–5. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis L, Slavich GM, Thaker PH, Goodheart MJ, Bender DP, Dahmoush L, et al. Eudaimonic well-being and tumor norepinephrine in patients with epithelial ovarian cancer. Cancer. 2015;121:3543–50. doi: 10.1002/cncr.29516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta V, Gu K, Chen Z, Lu W, Shu XO, Zheng Y. Concordance of self-reported and medical chart information on cancer diagnosis and treatment BMC Med. Res. Methodol. 2011;11:72. doi: 10.1186/1471-2288-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.