Abstract
Telomere length, a marker of biological aging, has been associated with cardiovascular disease (CVD) and its risk factors. Ideal cardiovascular health (CVH), defined by the American Heart Association (AHA), has also been associated with a reduced risk of CVD, but the relationship between telomere length and ideal CVH is unclear. We measured leukocyte telomere length (LTL) by qPCR in 2568 American Indians in the Strong Heart Family Study (SHFS). All participants were free of overt CVD at enrollment (2001–2003). CVH indices included four behavioral factors (smoking, physical activity, diet, BMI) and three health factors (blood pressure, cholesterol, fasting glucose). Each index was categorized as poor, intermediate, or ideal according to the AHA’s guideline. CVH was further categorized into below average (0–1), average (2–3) and above average (≥4) based on the total number of ideal indices. Results showed that, 29, 50 and 21 % of study participants had below average, average, and above average CVH, respectively. Participants with above average CVH had significantly longer LTL than those with below average CVH (β = 0.034, P = 0.042) after adjusting for age, sex, education level, marital status, processed meat consumption, alcohol consumption, and study site. Compared to the U.S. general population, American Indians achieved lower rates for five out of the seven ideal CVH metrics, including smoking, BMI, physical activity, diet, and blood pressure. Achieving four or more ideal CVH metrics was significantly associated with longer LTL. This finding suggests that achieving an ideal CVH may prevent or delay CVD, probably through promoting healthy aging.
Keywords: American Indians, Biological aging, Ideal cardiovascular health, Leukocyte telomere length, Strong heart family study
Introduction
Telomeres are repetitive DNA sequences and their associated proteins on the distal ends of the chromosomes [1]. They are critical in maintaining chromosomal stability during mitotic cell proliferation. Telomere length shortens during each round of cell division and when it reaches a critical length, cells stop division and become senescent. Thus, telomere length has been considered a marker of biological aging. Telomere length is heritable [2] and shorter telomere length has been associated with a wide range of age-related disorders including cardiovascular disease (CVD), [3] type 2 diabetes, [4] as well as cancer [5]. A better understanding of the relationship between these disorders and telomeric aging will shed light on the mechanism underlying premature aging and is likely to promote healthy aging and prevent or delay the onset of age-related diseases.
The 2020 strategic goal for the American Heart Association (AHA) has identified Life’s Simple 7 as the key risk factors aimed at achieving ideal cardiovascular health (CVH) [6]. It includes 4 behavioral factors (smoking, diet, physical activity, and body mass index [BMI]) and 3 health factors (blood pressure, total cholesterol, and fasting glucose). Ideal CVH has been associated with a reduced risk for age-related conditions, including CVD, [7–9] cognitive impairment, [10, 11] diabetes, [12] atherosclerosis, [13–16] and total mortality [17, 18]. Moreover, previous studies have demonstrated that behavioral factors (e.g. smoking, [19, 20] alcohol abuse, [21] sedentary lifestyle, [22] obesity [19, 23]) can accelerate telomere shortening, and shorter telomere length has been associated with several health disorders such as hypertension, [24, 25] hypercholesterolemia, [26] and diabetes [4, 27]. Among American Indians in the Strong Heart Family Study (SHFS), we have recently demonstrated that shorter leukocyte telomere length (LTL) significantly and independently predicts future onset of diabetes [4] and carotid atherosclerosis [28] beyond traditional risk factors. Shorter LTL has also been associated with obesity [23] and a higher dietary intake of processed meat in this high risk population [Amanda Fretts, Ph.D., unpublished data, 2016]. However, no study has examined the potential influence of ideal CVH indices on biological aging assessed by LTL. This study is to investigate the association between ideal CVH indices and LTL among American Indians participating in the SHFS (2001–2003).
Methods
Study population
The SHFS is a family-based longitudinal study designed to identify metabolic, genetic and behavioral factors for CVD, diabetes and their risk factors in American Indians in tribes and communities residing in Arizona, North/South Dakota, and Oklahoma. The SHFS recruited 3665 tribal members from 94 multigenerational families (14–93 years) between 2001 and 2003. All participants underwent a clinical examination including a personal interview and physical examination. Information on socio-demographic factors, medical history, medication use, and lifestyle factors was collected by personal interview using standard questionnaires. A physical examination was conducted, and fasting blood samples were collected for laboratory tests, including fasting glucose and lipids. The SHFS protocol was approved by the Institutional Reviews Boards from the Indian Health Service and the participating institutions. Study design and methods of the SHFS have been described elsewhere [29]. In the current study, 874 participants from one community were removed due to withdraw of consent. However, the subsample of 2791 participants did not differ significantly from the total sample of 3665 participants in the distribution of demographics. After excluding participants with overt CVD (n = 135) or missing LTL data (n = 88), a total of 2568 participants were included in the final data analysis.
Assessments of behavioral factors
Cigarette smoking was classified as current smoking, past smoking and never smoking. Current smoking was defined as having smoked at least 100 cigarettes in the subject’s entire life, having smoked cigarettes regularly, and smoking currently. Past smoking was defined as having smoked at least 100 cigarettes in the subject’s entire life, having smoked cigarettes regularly in the past, and not smoking currently. Never smoking was defined as never smoked or having smoked fewer than 100 cigarettes in their life time. Each participant received a pedometer, instructions for wearing the pedometer, and an activity diary at their clinical examination. They were asked to wear the pedometer for seven consecutive days and to record the number of the steps taken daily in the activity diary. Physical activity was assessed by the mean number of steps per day calculated by averaging the total number of steps recorded each day during the 7-day period. Dietary intake was assessed using a Block food frequency questionnaire and an American Indian supplemental foods questionnaire which collected data regarding reported “usual” intake of 119 foods over the past year. Body weight (kg) and height (cm) were measured when participants wore light clothes and no shoes by trained research staff. BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2).
Assessments of health factors
Systolic and diastolic blood pressure were measured three times by trained staff using a standard mercury sphygmomanometer after the participants had been resting for at least 5 min and the mean of the last two measurements was used in statistical analysis. Fasting glucose and blood lipids were measured by standard laboratory methods [30]. Medical history and medications for hypertension, dyslipidemia, and diabetes were obtained by standard questionnaires.
Indices of ideal cardiovascular health
Methods for the calculation of ideal CVH indices in the SHFS have been described previously [12] and were listed in Table 1. Briefly, each of the 7 indices was categorized as poor, intermediate, or ideal in accordance to the AHA’s definitions [6]. A composite score (ranging from 0 to 7) was calculated by summing the total number of ideal indices. This score was further categorized as below average (0–1), average (2–3) and above average (≥ 4) and used in statistical analysis.
Table 1.
Definition of ideal, intermediate, and poor levels of ideal cardiovascular health indices
| Metrics | Poor | Intermediate | Ideal |
|---|---|---|---|
| Smoking | Current smoker | Former smoker | Nonsmoker |
| Physical activitya | <3500 steps/day | 3500–10,000 steps/day | >10,000 steps/day |
| Healthy dietb | 0–1 component | 2–3 components | 4–5 components |
| Body mass index | ≥30 kg/m2 | 25–29.9 kg/m2 | <25 kg/m2 |
| Blood pressure | SBP ≥ 140 or DBP ≥ 90 mmHg | SBP 120–139 or DBP 80–89 mmHg or treated to ideal | SBP<120 and DBP<80 mmHg |
| Total cholesterol | ≥240 mg/dL | 200–239 mg/dL or treated to ideal | <200 mg/dL |
| Fasting glucose | ≥126 mg/dL | 100–125 mg/dL or treated to ideal | <100 mg/dL |
The AHA’s definitions [6] classify physical activity levels based on total activity time (minutes per day), but in the SHFS, physical activity was assessed with pedometers (steps per day). As several studies suggest that individuals who accumulate at least 10,000 steps per day have a decreased risk of obesity and hypertension compared with individuals who accumulate fewer steps per day, [48]>10,000 steps per day was considered an ideal level of activity
Includes (1) fruits and vegetables: ≥4.5 servings per day; (2) fish: ≥200 g per week; (3) fiber-to-carbohydrate ratio:>1 g of fiber per 10 g of carbohydrate; (4) sodium:<1500 mg per day; and (5) sugar-sweetened foods and beverages: ≤450 kcal per week. The 5 healthy diet components here were adapted from the AHA’s definitions of the 5 components, which were “(1) fruits and vegetables: ≥4.5 cups per day; (2) fish: ≥two 3.5-oz servings per week (preferably oily fish); (3) fiber-rich whole grains (≥1.1 g of fiber per 10 g of carbohydrate): ≥three 1-oz-equivalent servings per day; (4) sodium:<1500 mg per day; (5) sugar-sweetened beverages: ≤450 kcal (36 oz) per week.”
Measurement of leukocyte telomere length
Detailed methods for LTL measurement in the SHFS have been described previously [4]. Briefly, genomic DNA from peripheral blood was isolated according to standard protocols. LTL was measured using quantitative PCR at Dr. Blackburn’s lab at the University of California, San Francisco using a high-throughput telomere length assay system. The telomere length assay determines the ratio of telomeric product/single copy gene (T/S) obtained using quantitative PCR according to protocols reported previously [31]. T/S ratio was calculated by taking the difference between the mean of two T values and two S values attained for each of the three replicates. Three of these T/S ratios were averaged, and standard deviation and percent for coefficient of variation (% CV, standard deviation/mean) were calculated. T/S ratios were normalized to the mean of all samples. For quality control, we included seven control DNA samples from various cancer cell lines in each assay plate. These control samples allowed us to create standard curves, which were then integrated into a composite standard curve, used for T and S concentration calculations. In addition, about 20 % of the samples selected randomly were measured twice. Intra- and interassay % CV was 4.6 and 6.9 %, respectively.
Statistical analyses
To examine the association between each individual ideal CVH index and LTL, we constructed generalized estimating equation (GEE) model with independent correlation structure, in which LTL was the dependent variable and each individual ideal CVH index (ideal vs. intermediate vs. poor, using poor level as reference) was the independent variable, adjusting for age and sex. GEE model was used here to account for the family relatedness among study participants. To examine whether socioeconomic information, lifestyle and study site influence the associations between LTL and ideal CVH indices, we further adjusted for education level, marital status, processed meat consumption, alcohol consumption, and study site in the GEE model described above. The association between the composite score of ideal CVH and LTL was similarly examined. To examine whether sex influences the association between ideal CVH and LTL, we conducted a separate analysis by stratifying by sex. Further, we generate an interaction term “ideal CVH 9 sex” to examine the interaction between ideal CVH and sex. A two-tailed P value <0.05 was considered statistically significant. All statistical analysis was conducted using SAS statistical software (version 9.4, Cary, North Carolina).
Results
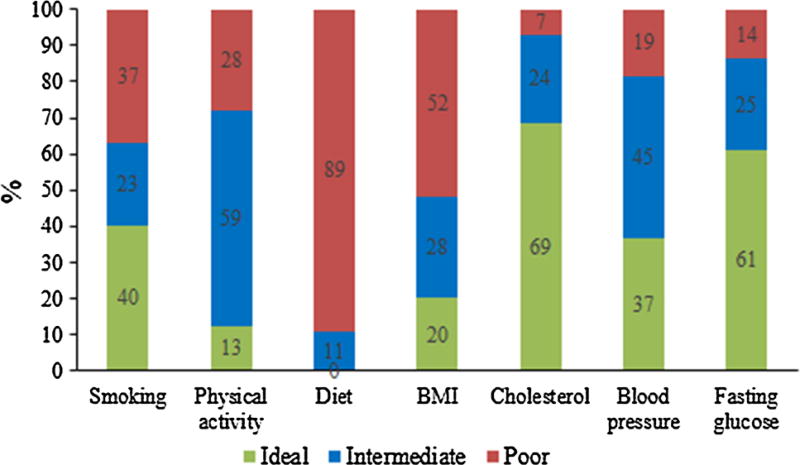
The current analysis included 2568 American Indians (1549 women and 1019 men) free of overt CVD at enrollment. The mean age was 39 ± 16 years (14–93 years). Figure 1 shows the distribution of the 7 ideal CVH metrics. About 40 % participants never smoked, 13 % walked >10,000 steps/day, and 20 % achieved ideal BMI. No participant achieved ideal diet. In contrast, ideal health factors were better achieved. 69 and 61 % participants achieved ideal cholesterol and fasting glucose, respectively, but only 37 % achieved ideal blood pressure. Collectively, only 21 % participants achieved 4 or more ideal CVH components and no one achieved all 7 ideal CVH metrics. About 29–50 % participants achieved 0–1 and 2–3 ideal CVH indices, respectively.
Fig. 1.
Distribution of ideal cardiovascular health indices among American Indians in the strong heart family study
Table 2 shows the clinical characteristics of study participants according to LTL quartiles. Compared to participants with longer LTL, those with shorter LTL had significantly higher BMI and fasting glucose and were more likely to be men (all P < 0.05). No significant difference in other listed characteristics was observed.
Table 2.
Baseline characteristics of SHFS participants according to LTL quartiles (N = 2568)
| Characteristics | LTL quartiles | P for trenda | |||
|---|---|---|---|---|---|
|
|
|||||
| Q1 | Q2 | Q3 | Q4 | ||
| No. of participants | 633 | 626 | 678 | 631 | – |
| Age, years (mean ± SD) | 47.5 ± 16.0 | 42.9 ± 15.9 | 36.8 ± 15.4 | 31.7 ± 14.7 | <0.001 |
| Male, n (%) | 253 (39.97) | 244 (38.98) | 281 (41.45) | 241 (38.19) | 0.026 |
| Current drinking, n (%) | 343 (54.19) | 350 (55.91) | 407 (60.03) | 417 (66.09) | 0.486 |
| Education level, years (mean ± SD) | 12.3 ± 2.3 | 12.3 ± 2.3 | 12.1 ± 2.3 | 11.9 ± 2.2 | 0.960 |
| Processed meat consumption (g/day, mean ± SD)b | 38.6 ± 41.5 | 37.1 ± 39.7 | 47.4 ± 65.0 | 41.2 ± 48.1 | 0.761 |
| Ideal cardiovascular health indices | |||||
| Current smoking, n (%) | 217 (34.34) | 230 (36.86) | 261 (38.50) | 239 (37.88) | 0.722 |
| BMI (kg/m2, mean ± SD) | 32.18 ± 7.62 | 31.92 ± 7.82 | 31.26 ± 7.41 | 29.50 ± 7.15 | <0.001 |
| Physical activity (steps/day, mean ± SD) | 5275.2 ± 3859.5 | 5701.3 ± 3809.2 | 6235.4 ± 3905.2 | 6555.1 ± 4023.0 | 0.079 |
| Diet, healthy score (mean ± SD) | 0.7 ± 0.7 | 0.6 ± 0.7 | 0.5 ± 0.6 | 0.5 ± 0.7 | 0.983 |
| SBP (mmHg, mean ± SD) | 124.5 ± 17.5 | 124.1 ± 17.1 | 122.9 ± 16.5 | 118.8 ± 15.0 | 0.053 |
| DBP (mmHg, mean ± SD) | 76.5 ± 10.6 | 76.6 ± 11.3 | 77.0 ± 11.4 | 74.8 ± 10.5 | 0.803 |
| TC (mg/dL, mean ± SD) | 187.3 ± 38.6 | 185.7 ± 36.2 | 183.0 ± 37.1 | 174.9 ± 37.8 | 0.561 |
| FPG (mg/dL, mean ± SD) | 116.5 ± 52.9 | 112.7 ± 47.8 | 105.3 ± 44.1 | 100.1 ± 33.5 | 0.003 |
LTL leukocyte telomere length, Q quartile, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, FPG fasting plasma glucose
Adjust for age and sex using GEE regression model to account for family relatedness of participants
Processed meat included breakfast sausage, spam, hotdogs, and lunchmeat
Association between LTL and ideal cardiovascular health
Table 3 shows the associations between individual ideal CVH indices and LTL. Among the 7 ideal CVH indices, longer LTL was significantly associated with ideal BMI (β = 0.027, P = 0.0.026) and ideal fasting glucose (β = 0.0.031, P = 0.030) but not the other 5 ideal CVH metrics after adjusting for age, sex, study site, education level, marital status, processed meat consumption, alcohol consumption and other CVH metrics. Table 4 shows the association between LTL and a composite score of ideal CVH metrics. Participants who achieved above average CVH had significantly longer LTL than those with below average CVH after adjusting for age and sex (β = 0.038, P = 0.021). After further adjusting for education level, marital status, processed meat consumption, alcohol consumption, and study site, an achievement of above average ideal CVH remained significantly associated with longer LTL (β = 0.034, P = 0.042). We further examined the associations of LTL with the composite CVH score, health factor score, and behavior factor score, separately. The results showed a significant association of LTL with health factor score (β = 0.013, P = 0.006) but not behavior factor score (P = 0.637) and CVH score (P = 0.107).
Table 3.
Association between cardiovascular health indices and LTL among SHFS participants
| Cardiovascular health indices | Age- and sex-adjusted | Multivariate-adjusteda | ||
|---|---|---|---|---|
|
|
|
|||
| β (SE) | P-value | β (SE) | P-value | |
| Smoking | ||||
| Poor (ref) | – | – | – | – |
| Intermediate | −0.016 (0.012) | 0.189 | −0.009 (0.013) | 0.489 |
| Ideal | −0.010 (0.011) | 0.352 | −0.014 (0.010) | 0.161 |
| Physical activity | ||||
| Continuous (steps/day) | 0.000 (0.000) | 0.049 | 0.000 (0.000) | 0.267 |
| Categorical | ||||
| Poor (ref) | – | – | – | – |
| Intermediate | 0.025 (0.012) | 0.033 | 0.013 (0.011) | 0.242 |
| Ideal | 0.023 (0.017) | 0.172 | 0.007 (0.017) | 0.684 |
| Healthy diet | ||||
| Poor (ref) | – | – | – | – |
| Intermediate | 0 (0.013) | 0.984 | -0.007 (0.013) | 0.610 |
| Ideal | – | – | – | – |
| Body mass index | ||||
| Continuous (kg/m2) | −0.003 (0.001) | <0.001 | −0.002 (0.001) | 0.001 |
| Categorical | ||||
| Poor (ref) | – | – | – | – |
| Intermediate | 0.046 (0.011) | <0.001 | 0.029 (0.011) | 0.005 |
| Ideal | 0.041 (0.011) | <0.001 | 0.027 (0.012) | 0.026 |
| Blood pressure | ||||
| Continuous (mmHg) | ||||
| Systolic blood pressure | 0.001 (0.000) | 0.169 | 0.001 (0.000) | 0.087 |
| Diastolic blood pressure | 0.000 (0.001) | 0.513 | 0.001 (0.000) | 0.085 |
| Categorical | ||||
| Poor (ref) | – | – | – | – |
| Intermediate | −0.027 (0.013) | 0.029 | −0.032 (0.013) | 0.013 |
| Ideal | −0.006 (0.016) | 0.687 | −0.021 (0.017) | 0.223 |
| Total cholesterol | ||||
| Continuous (mg/dL) | −0.000 (0.000) | 0.291 | −0.000 (0.000) | 0.548 |
| Categorical | ||||
| Poor (ref) | – | – | – | – |
| Intermediate | −0.006 (0.022) | 0.796 | −0.015 (0.023) | 0.527 |
| Ideal | −0.004 (0.022) | 0.872 | −0.007 (0.025) | 0.763 |
| Fasting glucose | ||||
| Continuous (mg/dL) | −0.000 (0.000) | <0.001 | −0.000 (0.000) | 0.007 |
| Categorical | ||||
| Poor (ref) | – | – | – | – |
| Intermediate | 0.037 (0.016) | 0.020 | 0.021 (0.015) | 0.149 |
| Ideal | 0.048 (0.015) | 0.001 | 0.031 (0.014) | 0.030 |
Adjust for age, sex, education level, marital status, processed meat consumption, alcohol consumption, study site, and other CVH indices
Table 4.
Association between ideal cardiovascular health and LTL among SHFS participants
| Ideal cardiovascular health |
Age (years) | Mean LTL (T/S ratio) |
Age- and sex-adjusted | Multivariate-adjusteda | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| β (SE) | P-value | β (SE) | P-value | |||
| Categorical | ||||||
| Below average (ref) | 47.5 ± 13.9 | 0.95 ± 0.22 | – | – | – | – |
| Average | 40.3 ± 16.0 | 0.99 ± 0.23 | 0.010 (0.012) | 0.419 | 0.009 (0.012) | 0.450 |
| Above average | 27.2 ± 11.9 | 1.08 ± 0.23 | 0.038 (0.017) | 0.021 | 0.034 (0.016) | 0.042 |
| P for trend | 0.028 | 0.051 | ||||
| Continuous | ||||||
| Composite CVH score | 0.008 (0.004) | 0.057 | 0.007 (0.004) | 0.107 | ||
| Behavior factor score | 0.004 (0.005) | 0.417 | 0.003 (0.005) | 0.637 | ||
| Health factor score | 0.011 (0.006) | 0.041 | 0.013 (0.006) | 0.032 | ||
CVH cardiovascular health
Adjust for age, sex, education level, marital status, processed meat consumption, alcohol consumption, and study site
Sex-specific association between LTL and ideal cardiovascular health
Stratification analysis by sex showed that the association between ideal CVH and LTL was still significant in women (P = 0.044) but not in men (Table 5). However, the effect size of the association was similar in men and women (0.032 vs. 0.039). We did not find a significant interaction between ideal CVH and sex on LTL (P = 0.762). These results indicated that the association between ideal CVH and LTL may not be influenced by sex.
Table 5.
Sex-specific association between ideal cardiovascular health and LTL among SHFS participants
| Ideal cardiovascular health | Mean LTL (T/S ratio) |
Age-adjusted | Multivariate-adjusteda | ||
|---|---|---|---|---|---|
|
|
|
||||
| β (SE) | P-value | β (SE) | P-value | ||
| Men | |||||
| Below average (ref) | 0.95 ± 0.22 | – | – | – | – |
| Average | 0.98 ± 0.23 | 0.000 (0.019) | 0.994 | −0.001 (0.019) | 0.943 |
| Above average | 1.07 ± 0.23 | 0.038 (0.023) | 0.129 | 0.032 (0.024) | 0.280 |
| P for trend | 0.201 | 0.371 | |||
| Women | |||||
| Below average (ref) | 0.95 ± 0.23 | – | – | – | – |
| Average | 1.00 ± 0.24 | 0.017 (0.015) | 0.252 | 0.017 (0.015) | 0.255 |
| Above average | 1.08 ± 0.24 | 0.042 (0.020) | 0.033 | 0.039 (0.019) | 0.044 |
| P for trend | 0.034 | 0.045 | |||
Adjust for age, education level, marital status, processed meat consumption, alcohol consumption, and study site
Discussion
In a large, family-based population cohort of American Indians, we studied the effects of ideal CVH on leukocyte telomere length, independent of traditional cardiovascular risk factors. We found that an achievement of four or more ideal CVH indices was significantly associated with longer leukocyte telomere length, independent of established cardiovascular risk factors.
The AHA’s 2020 goal to improve CVH by 20 % [6] is bold and forward thinking, but achievement of this goal could be challenging for some populations including American Indians. Our analysis in American Indians and those from other ethnic groups indicated that very few adults achieved ideal CVH [7, 9–12, 17, 18]. Compared to the U.S. general population as reported in the NHANES 2005–2006, [6] American Indians appeared to have poorer ideal CVH. For example, lower proportions of American Indians in the SHFS achieved ideal levels of smoking (40 vs. 73 %), physical activity (13 vs. 45 %), diet (none vs. <0.5 %), BMI (20 vs. 33 %), and blood pressure (37 vs. 42 %). Although direct comparison may bring some concerns due to the different distribution of demographics between these two samples of population, the data may indicate a high challenge in promotion of ideal CVH for American Indians. None of the SHFS participants achieved all seven ideal indices. About 40 % of the SHFS participants achieved ideal health factors, but less than 20 % achieved ideal behavioral factors, indicating that ideal health factors could be more achievable than ideal behavioral factors in American Indians. It is noteworthy that, similar to U.S. general population, none of the American Indian participants achieved healthy diet. Developing optimal strategies for healthy eating, smoking cessation, and physical activity is the key to promote healthy aging and prevent or delay CVD.
Previous studies have reported associations of shorter telomere length with behavioral factors (e.g. smoking, [19, 20] alcohol consumption, [21] inactive lifestyle, [22] obesity [19, 23]) and health factors (e.g. hypertension, [24, 25] hypercholesterolemia, [26] and diabetes [4, 27]). In the SHFS, we have demonstrated that shorter telomere length significantly predicted the risk of diabetes [4] and carotid atherosclerosis, [28] independent of traditional risk factors. We have also reported significant associations of LTL with obesity [23] and processed meat and unprocessed red meat [Amanda Fretts, Ph.D., unpublished data, 2016] in this high risk population. However, we failed to find significant associations of telomere length with smoking, physical activity, and diet in our study. The reasons of the discrepant findings are unclear. While these findings provide valuable information for the relationship between biological aging and cardiovascular health factors, previous studies have largely focused on the association of telomere length with each individual behavioral or health factor. However, because many of these factors often coexist and are interwoven in multiple biologically related pathways, modeling their joint effects should reflect true biological processes and thus provide more accurate information compared to studying each factor individually. To our knowledge, no study has investigated the combined effects of all seven behavioral and health factors on biological aging assessed by LTL in American Indians or other ethnic groups as well.
Since the release of AHA’s definition for ideal CVH, several epidemiological studies have consistently reported associations of achieving ideal CVH with reduced risk of age-related disorders such as CVD, [7–9, 32] cognitive impairment, [10, 11], disability, [33] cancer, [32, 34] and all-cause mortality [17, 18]. For example, in the CARDIA study [35] including participants aged 18–30 years, an achievement of ideal CVH predicted a decreased risk of cardiac dysfunction after 25 years of follow-up (OR 0.52, 95 % CI 0.37–0.73). In the NHANES in 1988–1994 (with mortality assessment through 2006), achieving a higher degree of ideal CVH metrics reduced the risk of CVD and total mortality among adults (≥20 years) [18]. In the Women’s Health Initiative study [32] including postmenopausal women aged over 50 years, participants who achieved 6–7 ideal CVH metrics had significantly lower risk for CVD and cancer compared to those who achieved 0–1 ideal CVH metrics. Among participants aged 45–64 years in the ARIC study, [36, 37] ideal CVH attainment or improvement through mid- to later-life was associated with lower CVD prevalence and better cardiac structure and function in later-life. Similar beneficial effects of achieving an above-average ideal CVH were also observed among participants in the REGARDS study [38]. Moreover, ideal CVH has been associated with various measures of subclinical CVD, including arterial stiffness, [39–41] carotid atherosclerosis, [14–16] and coronary artery calcium, [42–44] as well as cardiovascular risk factors such as diabetes, [12] hypertension, [45] and depression [46]. While these results provide strong evidence for a protective effect of achieving ideal CVH on CVD and related conditions, previous investigations did not include American Indians, a minority group suffering from high rates of CVD, diabetes, and other risk factors. In addition, to the best of our knowledge, no study has examined the impact of ideal CVH on biological aging in American Indians or other ethnic groups. Here we identified that achieving four or more ideal CVH metrics was associated with longer telomere length. This finding may improve our understanding of the relationship between healthy aging and CVD.
Recently, a sex difference was found in the association between sex hormones and ideal CVH, [47] indicating a probable effect of sex on the association between ideal CVH and telomere length. Therefore, we conducted a sex-specific analysis to examine whether sex influences the association between ideal CVH and telomere length. We found that the effect size of the association between ideal CVH and LTL was similar in men and women, although the association was not significant in men. Further, we did not find a significant interaction between ideal CVH and sex in our study. These results indicated that sex may not influence the association between ideal CVH and telomere length.
Strengths of this study include the high quality telomere data, the detailed clinical phenotypes on CVH metrics for all study participants, and the extensive adjustments of known cardiovascular risk factors. However, several limitations should also be mentioned. First, our dietary data were obtained from a self-reported food frequency questionnaire, which may be subject to measurement error. Second, the ideal CVH was assessed by a crude additive scoring method based on achievement of the key behavioral and health factor goals; however, the magnitude of the association of each individual index is not the same, and thus this scoring method may oversimplify the association of ideal CVH with telomere length. Third, our findings were derived from American Indians whose cardiovascular health profiles could be different from those with other ethnic backgrounds. Thus, the generalizability of our results to other populations is unknown. Lastly, the cross-sectional nature of our analysis precludes any causal inference for the relationship between telomere length and cardiovascular health factors.
In summary, compared to the U.S. general population, American Indians achieved lower rates for five out of the seven ideal CVH metrics including smoking, BMI, physical activity, diet, and blood pressure. Achieving four or more ideal CVH metrics is associated with longer telomere length, a marker of biological aging and age-related disorders. This finding may deepen our understanding of the biological mechanisms behind aging and cardiovascular disease.
Acknowledgments
The authors would also like to thank the Strong Heart Study participants, Indian Health Service facilities, and participating tribal communities for their extraordinary cooperation and involvement, which has contributed to the success of the Strong Heart Study. The views expressed in this article are those of the authors and do not necessarily reflect those of the Indian Health Service. This study was supported by NIH Grants R01DK091369, K01AG034259, R21HL092363 and cooperative agreement Grants U01HL65520, U01HL41642, U01HL41652, U01HL41654, and U01HL65521.
Footnotes
Compliance with ethical standards
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 2.Broer L, Codd V, Nyholt DR, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21(10):1163–8. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J, Zhu Y, Lin J, et al. Short leukocyte telomere length predicts risk of diabetes in american Indians: the strong heart family study. Diabetes. 2014;63(1):354–62. doi: 10.2337/db13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013:djt016. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/circulationaha.109.192703. [DOI] [PubMed] [Google Scholar]
- 7.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–6. doi: 10.1016/jj.acc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125(24):2975–84. doi: 10.1161/circulationaha.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulshreshtha A, Vaccarino V, Judd SE, et al. Life’s simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44(7):1909–14. doi: 10.1161/strokeaha.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73(2):170–9. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thacker EL, Gillett SR, Wadley VG, et al. The American Heart Association life’s simple 7 and incident cognitive impairment: the reasons for geographic and racial differences in stroke (REGARDS) study. J Am Heart Assoc. 2014;3(3):e000635. doi: 10.1161/jaha.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fretts AM, Howard BV, McKnight B, et al. Life’s simple 7 and incidence of diabetes among American Indians: the strong heart family study. Diabetes Care. 2014;37(8):2240–5. doi: 10.2337/dc13-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laitinen TT, Pahkala K, Magnussen CG, et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood the cardiovascular risk in Young Finns study. Circulation. 2012;125(16):1971–8. doi: 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 14.Sturlaugsdottir R, Aspelund T, Bjornsdottir G, et al. Carotid atherosclerosis and cardiovascular health metrics in old subjects from the AGES-Reykjavik study. Atherosclerosis. 2015;242(1):65–70. doi: 10.1016/j.atherosclerosis.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulshreshtha A, Goyal A, Veledar E, et al. Association between ideal cardiovascular health and carotid intima-media thickness: a twin study. J Am Heart Assoc. 2014;3(1):e000282. doi: 10.1161/jaha.113.000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oikonen M, Laitinen TT, Magnussen CG, et al. Ideal cardiovascular health in young adult populations from the United States, Finland, and Australia and its association with cIMT: the International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc. 2013;2(3):e000244. doi: 10.1161/jaha.113.000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125(8):987–95. doi: 10.1161/circulationaha.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307(12):1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–4. doi: 10.1016/s0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 20.Strandberg TE, Saijonmaa O, Tilvis RS, et al. Association of telomere length in older men with mortality and midlife body mass index and smoking. J Gerontol A Biol Sci Med Sci. 2011;66(7):815–20. doi: 10.1093/gerona/glr064. [DOI] [PubMed] [Google Scholar]
- 21.Strandberg TE, Strandberg AY, Saijonmaa O, Tilvis RS, Pitkala KH, Fyhrquist F. Association between alcohol consumption in healthy midlife and telomere length in older men. The Helsinki businessmen study. Eur J Epidemiol. 2012;27(10):815–22. doi: 10.1007/s10654-012-9728-0. [DOI] [PubMed] [Google Scholar]
- 22.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–8. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Yeh F, Lin J, et al. Short leukocyte telomere length is associated with obesity in American Indians: the strong heart family study. Aging (Albany, NY) 2014;6(5):380–9. doi: 10.18632/aging.100664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrag W, Eid M, El-Shazly S, Abdallah M. Angiotensin II type 1 receptor gene polymorphism and telomere shortening in essential hypertension. Mol Cell Biochem. 2011;351(1–2):13–8. doi: 10.1007/s11010-010-0706-0. [DOI] [PubMed] [Google Scholar]
- 25.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 26.Harte AL, da Silva NF, Miller MA, et al. Telomere length attrition, a marker of biological senescence, is inversely correlated with triglycerides and cholesterol in South Asian males with type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:895185. doi: 10.1155/2012/895185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zee RY, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res. 2010;155(4):166–9. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Lin J, Matsuguchi T, et al. Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: the strong heart family study. Aging (Albany, NY) 2014;6(5):414–27. doi: 10.18632/aging.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North KE, Howard BV, Welty TK, et al. Genetic and environmental contributions to cardiovascular disease risk in American Indians the strong heart family study. Am J Epidemiol. 2003;157(4):303–14. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 30.Lee ET, Welty TK, Fabsitz R, et al. The strong heart study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 31.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foraker RE, Abdel-Rasoul M, Kuller LH, et al. Cardiovascular health and incident cardiovascular disease and cancer: the women’s health initiative. Am J Prev Med. 2015 doi: 10.1016/j.amepre.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhamoon MS, Dong C, Elkind MS, Sacco RL. Ideal cardiovascular health predicts functional status independently of vascular events: the Northern Manhattan study. J Am Heart Assoc. 2015;4(2):e001322. doi: 10.1161/JAHA.114.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen-Torvik LJ, Shay CM, Abramson JG, et al. Ideal cardiovascular health is inversely associated with incident cancer the atherosclerosis risk in communities study. Circulation. 2013;127(12):1270–5. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai CS, Ning H, Liu K, et al. Cardiovascular health in young adulthood and association with left ventricular structure and function later in life: the coronary artery risk development in young adults study. J Am Soc Echocardiogr. 2015;28:1452–61. doi: 10.1016/j.echo.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah AM, Claggett B, Folsom AR, et al. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015 doi: 10.1161/circulationaha.115.017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folsom AR, Shah AM, Lutsey PL, et al. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128(9):970976e2. doi: 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson NC, Cushman M, Judd SE, et al. American Heart Association’s Life’s Simple 7 and risk of venous thromboembolism: the reasons for geographic and racial differences in stroke (REGARDS) study. J Am Heart Assoc. 2015;4(3):e001494. doi: 10.1161/jaha.114.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan N, Zhou Y, Wang Y, et al. Association of ideal cardiovascular health and brachial-ankle pulse wave velocity: a cross-sectional study in Northern China. J Stroke Cerebrovasc Dis. 2015 doi: 10.1016/j.jstrokecerebrovasdis.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Aatola H, Hutri-Kahonen N, Juonala M, et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the cardiovascular risk in Young Finns study. J Am Heart Assoc. 2014;3(2):e000532. doi: 10.1161/jaha.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crichton GE, Elias MF, Robbins MA. Cardiovascular health and arterial stiffness: the Maine-Syracuse longitudinal study. J Hum Hypertens. 2014;28(7):444–9. doi: 10.1038/jhh.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins JM, Petrone AB, Carr JJ, et al. Association of ideal cardiovascular health and calcified atherosclerotic plaque in the coronary arteries: The National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2015;169(3):371378e1. doi: 10.1016/j.ahj.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saleem Y, DeFina LF, Radford NB, et al. Association of a favorable cardiovascular health profile with the presence of coronary artery calcification. Circ Cardiovasc Imaging. 2015;8(1):e001851. doi: 10.1161/CIRCIMAGING.114.001851. [DOI] [PubMed] [Google Scholar]
- 44.Alman AC, Maahs DM, Rewers MJ, Snell-Bergeon JK. Ideal cardiovascular health and the prevalence and progression of coronary artery calcification in adults with and without type 1 diabetes. Diabetes Care. 2014;37(2):521–8. doi: 10.2337/dc13-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao J, Sun H, Liang X, et al. Ideal cardiovascular health behaviors and factors prevent the development of hypertension in prehypertensive subjects. Clin Exp Hypertens. 2015;37(8):650–5. doi: 10.3109/10641963.2015.1047938. [DOI] [PubMed] [Google Scholar]
- 46.Espana-Romero V, Artero EG, Lee DC, et al. A prospective study of ideal cardiovascular health and depressive symptoms. Psychosomatics. 2013;54(6):525–35. doi: 10.1016/j.psym.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Jaspers L, Dhana K, Muka T, et al. Sex steroids, sex hormonebinding globulin and cardiovascular health in men and postmenopausal women: the Rotterdam study. J Clin Endocrinol Metab. 2016;101(7):2844–52. doi: 10.1210/jc.2016-1435. [DOI] [PubMed] [Google Scholar]
- 48.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]