Abstract
Background
Although essential tremor (ET) is the most common tremor disorder, its pathogenesis is not fully understood. The traditional model of ET, proposed in the early 1970s, posited that the inferior olivary nucleus (ION) was the prime generator of tremor in ET and that ET is a disorder of electrophysiological derangement, much like epilepsy. This article comprehensively reviews the origin and basis of this model, its merits and problems, and discusses whether it is time to lay this model to rest.
Methods
A PubMed search was performed in March 2017 to identify articles for this review.
Results
The olivary model gains support from the recognition of neurons with pacemaker property in the ION and the harmaline-induced tremor models (as the ION is the prime target of harmaline). However, the olivary model is problematic, as neurons with pacemaker property are not specific to the ION and the harmaline model does not completely represent the human disease ET. In addition, a large number of neuroimaging studies in ET have not detected structural or functional changes in the ION; rather, abnormalities have been reported in structures related to the cerebello-thalamo-cortical network. Moreover, a post-mortem study of microscopic changes in the ION did not detect any differences between ET cases and controls.
Discussion
The olivary model largely remains a physiological construct. Numerous observations have cast considerable doubt as to the validity of this model in ET. Given the limitations of the model, we conclude that it is time now to lay this model to rest.
Keywords: Essential tremor, pathogenesis, inferior olive, cerebellum, harmaline
Introduction
Essential tremor (ET) is the most common tremor disorder.1 Although for a long time ET was regarded as a monosymptomatic illness, in recent years this view has been challenged by studies that have documented the presence in some patients of additional motor signs aside from tremor and a repertoire of non-motor features.2–5 In parallel with this reconceptualization of the clinical features of ET, research over the past several years has also called into question traditional views about the underlying neurobiology of ET. Recent studies, and especially those based on histopathological investigation of brain tissues, have resulted in a new hypothesis regarding the pathogenesis of ET. This hypothesis posits that ET may be a neurodegenerative disease centered in the cerebellum,6 and it contrasts with the traditional olivary hypothesis, which posited that 1) the inferior olivary nucleus (ION) was the prime generator of tremor in ET and 2) ET is a disorder of electrophysiological derangement, much like epilepsy.
This article aims to revisit the olivary hypothesis of ET to discuss 1) the origins and basis for this model, 2) the evidence in support of this model, 3) the considerable limitations of this model, and finally 4) whether it is time to lay this model to rest.
Methodology
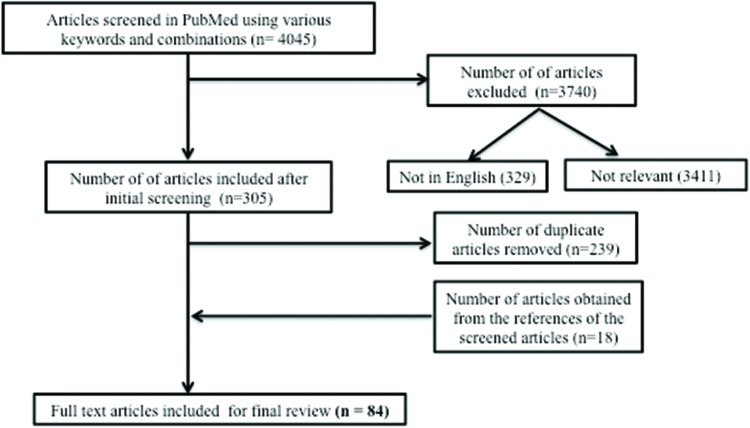
In March 2017, the authors used PubMed to search the relevant literature using the term “essential tremor” with additional search terms being “biology”, “pathology”, “pathophysiology”, “pathogenesis”, “inferior olive”, “thalamus”, “cerebellum”, and “red nucleus”. This yielded 4,045 articles (Table 1, Figure 1). During the initial screening of the abstracts/full texts, the publications that were not relevant to this review, duplicates, and those that were published in languages other than English were removed, leaving 66 articles. The references from these articles were also thoroughly searched for any additional articles, yielding 18 more articles (Table 1, Figure 1). In total, 84 articles pertinent to this topic were included for this review (Table 1, Figure 1).
Table 1. Results of Search for Articles from PubMed Using Various Key Words and their Combinations.
| Key Words and Combinations | Number of Publications | ||
|---|---|---|---|
| Total | Included | Excluded | |
| Essential tremor AND biology | 85 | 11 | 74 (not in English, 7; not relevant, 67) |
| Essential tremor AND pathology | 508 | 77 | 431 (not in English, 28; not relevant, 403) |
| Essential tremor AND pathophysiology 1,181 | 1,241 | 60 | 1,181 (not in English, 101; not relevant, 1,080) |
| Essential tremor AND pathogenesis 1,269 | 1,310 | 41 | 1,269 (not in English, 134; not relevant, 1,135) |
| Essential tremor AND inferior olive | 44 | 16 | 28 (not in English, 3; not relevant, 25) |
| Essential tremor AND thalamus | 528 | 16 | 512 (not in English, 41; not relevant, 471) |
| Essential tremor AND cerebellum | 307 | 79 | 228 (not in English, 12; not relevant, 216) |
| Essential tremor AND red nucleus | 22 | 5 | 17 (not in English, 3; not relevant, 14) |
| Total number of articles included for review after removing the duplicates | 66 | ||
| Total number of articles included from the reference sections of the shortlisted articles | 18 | ||
| Final number of article include for review | 84 | ||
Figure 1. Flow diagram summarizing the steps involved in the literature search.
Origins and evolution of the olivary hypothesis
ION as a central pacemaker
The olivary hypothesis stems in part from the concept of the existence of central oscillators. These central oscillators are neurons with pacemaker properties; in other words, these neurons possess the intrinsic ability to generate rhythmic bursting activity.7 Much of the relevant work relating to the pacemaker properties of neurons in the central nervous system was performed during the past three to four decades and, from this work, we know that numerous structures across the neuroaxis have pacemaker properties: ION,8,9 locus ceruleus,10,11 raphe nucleus,12 thalamus,13 cerebellar nuclei,14 Purkinje cells,15 globus pallidus,16 and sensorimotor cortex17 and, of these structures, the ION has received the greatest attention with regards to pathogenesis of ET. The ION is the largest nucleus in the olivary body and it constitutes the major input to the cerebellum; the olivocerebellar fibers are referred to as climbing fibers.18
Rhythmic oscillatory activity of the olivary neurons has been reported in several animal experiments since the early 1970s. Thus, Lamarre and Mercier19 in 1971 observed a 10 Hz rhythmic activity in the ION, Purkinje cells, neurons in fastigial nucleus, and bulbo-reticular units in decerebrate cats with harmaline-induced tremor. The authors suggested that the tremor induced by harmaline had a central origin, as bursts of activity were persistent in the ventral roots of the spinal cord even after abolishing the tremor by application of paralytic agents. This led to a speculation that tremor most probably was relayed from the brainstem structures such as the ION, reaching the spinal cord through reticulo-spinal and vestibulo-spinal pathways. Two years later, in 1973, Llinás and Volkind20 observed the persistence of rhythmic activity in the ION in cats with harmaline-induced tremor, even after low decerebration, cerebellectomy, and spinal transection. This substantiated the notion that the ION has an intrinsic rhythmic property and it is one of the targets in harmaline-induced tremor in animal models. Later, Llinas et al. also documented oscillatory properties in the neurons of the ION of guinea pigs and their modulation by several pharmacological agents, including harmaline.21
Subsequently, neurophysiological studies of patients with ET also posited a role of a central oscillator in ET. In his experiments in the 1980s aimed at understanding the physiology of tremor, Elble22 observed that the frequency of tremor in ET remains unchanged with inertial loading. Variation of frequency of tremor in response to inertial loading is a common finding in animal models of tremor, and in humans, and when present, it indicates that tremor is secondary to stretch reflex oscillation. This observation on frequency invariance in ET further reinforced the view that central oscillators are crucial in the genesis of tremor in patients with ET.
Evidence from the animal models of tremor
As alluded to above, the role of the ION in the pathogenesis of ET gains support from the observation that tremor secondary to toxicity of certain chemicals shares several features with ET. In a wide range of laboratory animals including mice, cats, and monkeys, intoxication with β-carboline compounds (e.g., harmane, harmine, harmaline, ibogaine) produces action tremor. The resemblance of such tremor with ET has attracted the attention of researchers working on the mechanism of tremor and its pharmacotherapy. The potential mechanisms by which harmaline results in tremor in animals is reviewed comprehensively elsewhere.23,24 In addition to the experiments by Lamarre and Mercier19 and Llinás and colleagues20,21 as described earlier, there are several other lines of evidence from animal studies to support the notion that the ION is one of the major sites of action of harmaline. These include 1) triggering of rhythmic burst activity in the ION on local application/local microinjections of harmaline,25 2) loss of the tremor-generating property of harmaline after damaging the ION neurons with 3-acetylpyridine,26 3) harmaline-induced increase in medial and dorsal ION metabolism in 14C-deoxyglucose uptake studies,27,28 4) increase in c-fos (an immunohistochemical marker of neuronal activation) in the ION after systemic administration of harmaline,29 and 5) harmaline-induced potentiation of the low-threshold voltage-gated calcium channels (CaV3.1) in the ION, which play a crucial role in the genesis of 4–10 Hz tremor-related rhythms.30 The rhythmic burst activity generated in the ION is subsequently transmitted via the climbing fibers to Purkinje cells and to the deep cerebellar nuclei, then to brainstem and spinal cord motor neurons in rodent models. Historically, these observations have been instrumental in setting the foundations for the olivary hypothesis of ET.
Evidence from the neuroimaging studies
In addition to the central pacemaker concept and the harmaline-induced tremor models, results of several functional neuroimaging studies also partially favor the olivary model of ET. Hallett and Dubinsky,31 in a positron emission tomography (PET) study, observed significantly higher regional cerebral metabolism of glucose (rCMRGlc) in the brainstem and thalamus in patients with ET than in the healthy controls. Although not totally clear, it was posited that the ION was the brainstem structure that was responsible for the higher rCMRGlc signal in these studies, although limited resolution of the technique was an issue. It is possible that the higher observed rCMRGlc was the indirect representation of higher intrinsic activity in the ION. Later, another PET study revealed a significant increase in blood flow bilaterally to the IONs in patients with alcohol responsive ET compared with controls after oral administration of ethanol.32 The authors hypothesized that there was a possible increase in the afferent olivary input from the cerebellum after intake of ethanol. This increase in afferent olivary input was speculated to be secondary to the reduction of cerebellar cortex inhibitory input to the central nuclei by ethanol. However, the blood flow to the ION was no different in patients than the controls before taking ethanol. Hence, although there was higher blood flow in the ION after ingestion of ethanol, suggesting putative involvement in tremor pathogenesis, it was not clear how exactly the ION was involved. In a recent resting-state functional magnetic resonance imaging (fMRI) study, Fang et al.33 reported reduced regional homogeneity (ReHo) in the bilateral IONs in patients with ET. As ReHo is a voxel-based measure of brain activity, which evaluates the similarity or synchronization between the time series of a given voxel and its nearest neighbors, reduced ReHo in a small structure like the ION perhaps indicates higher aberrant intrinsic activity than in other structures. Although results of the aforementioned studies have indicated possible involvement of the ION in patients with ET, it is important to note that functional abnormalities in these studies were not limited to the ION, and several other structures including the cerebellum,32,33 motor cortex,33 and thalamus31,33 were also observed to be abnormal in patients with ET.
The other factor that reinforces the potential role of the olivary model of ET is the apparently normal histological examinations in early post-mortem studies on brains of patients with ET.34,35 The results of these initial histopathological studies, which were null studies, served to reinforce the idea that structural or degenerative changes were not associated with the pathogenesis of ET. Lack of pathological changes in ET brains as reported by these studies was an indirect support for the ION model, which posits that ET is a physiological derangement. Taken together, cohesively, the aforementioned observations (pacemaker and harmaline models, functional imaging studies, histopathological studies) supported the notion that ET is an electrical or electrophysiological entity and the ION is the major site of pathogenesis of ET.
Problems with the olivary model
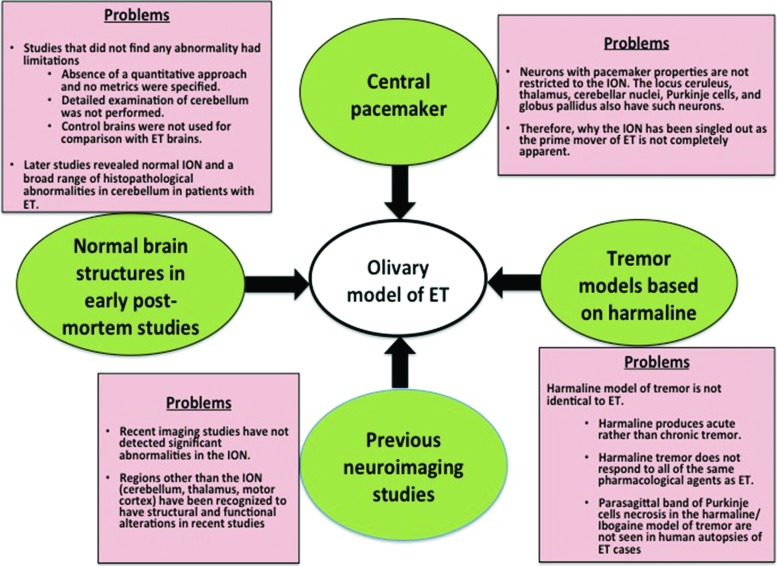
Despite some support for the olivary model, the model is also characterized by a multiplicity of problems, which are discussed below (Figure 2, Table 2). Indeed, the model has faced significant challenges over time. In a workshop organized by the National Institute of Neurological Disorders and Stroke that was aimed to address knowledge gaps in ET and to provide research recommendations, the significance of the olivary model was questioned because of the lack of convincing and conclusive evidence to support it.36
Figure 2. Summary of the observations, which augured the existence of olivary model of ET and their respective problems.
Table 2. Similarities and Differences between Harmaline-induced Tremor and Essential Tremor.
| Similarities | ||
|---|---|---|
| Phenomenology: action tremor | ||
| Phenomenology: action tremor | ||
| Similar response to certain pharmacological agents | ||
| Tremor is suppressed in all cases of harmaline-induced tremor and some cases of essential tremor in response to ethanol, propranolol, primidone, clonazepam, diazepam, gabapentin, zonisamide, gamma hydroxybutyrate, l-octanol | ||
| Tremor is not suppressed by anticholinergic agents, phenoxybenzamine, and leviteracetam | ||
| Tremor is worsened by caffeine, citalopram, and imipramine | ||
| Differences | ||
| Features | Harmaline Model | Essential Tremor |
| Organisms | Cats, monkeys, mouse | Humans |
| Nature of onset | Acute | Insidious |
| Course of tremor | Temporary | Permanent |
| Causative factor (s) | Pharmacologic | Possibly neurodegenerative |
| Role of inferior olivary nucleus | Definite | Uncertain |
| Rest tremor | Absent | May coexist with action tremor in some patients |
| Response to certain pharmacological agents | ||
| Carbamazepine | Suppresses | Doesn’t suppress |
| Valproate | Suppresses | Doesn’t suppress/worsens |
| Lacosamide | Suppresses | Doesn’t suppress |
| Carisbamate | Suppresses | Doesn’t suppress |
| Lithium | Suppresses | Doesn’t suppress/worsens |
| Levodopa | Suppresses | Doesn’t suppress |
| Dopa-agonists | Suppresses | Doesn’t suppress |
| MK-0249 | Suppresses | Doesn’t suppress |
Numerous functional neuroimaging studies have explored the neural correlates of ET, a majority of which reported alterations in the components of the cortico-bulbo-cerebello-thalamo-cortical network. The PET studies by Wills et al.37,38 revealed abnormal activation in the bilateral cerebellum, red nucleus, and thalamus in patients with ET. Despite limited evidence from early studies of activation of the ION,31,32 in these studies,37,38 no activation was observed in the ION. An ever-growing number of recent fMRI studies have also not detected any alterations in the functional connectivity of the ION; rather, an alteration in the cortico-bulbo-cerebello-thalamo-cortical network (and especially the cerebellum) has been the major result of these studies.39–42 Magnetic resonance spectroscopy (MRS) is a neuroimaging technique that allows in vivo quantification of certain chemicals in the brain. Studies using MRS have compared the ratio of N-acetylaspartate (NAA) and creatine (Cr) in patients with ET and healthy controls.43–45 A reduction in NAA or a reduced NAA:Cr, which suggests neuronal dysfunction or neuronal loss, was observed in the cerebellar cortex in the studies by Louis et al.43 and Pagan et al.;44 a negative correlation of the tremor severity with NAA:Cr ratio was documented in the former study.
The structural neuroimaging studies on patients with ET have been largely based on voxel-based morphometry (VBM) and diffusion tensor imaging (DTI). While VBM allows voxel-wise comparison of gray matter density between two groups, DTI allows one to study microstructural white matter changes using several parameters (anisotropy and diffusivity) that are related to the diffusion of water molecules in nerve fibers. Although the results of case–control studies based on VBM are somewhat variable, the majority of these revealed reduced gray matter volume in the cerebellum in patients with ET compared with healthy controls.46–49 Gray matter volume loss in the cerebellum, especially in the vermis was documented in ET patients with head tremor in two of the VBM studies.48,49 A recent volumetry study has revealed significant atrophy of the vermis in patients with ET, especially in those with cerebellar signs.50 However, the structural changes observed in these studies are not limited to the cerebellum, as atrophy in widespread areas in the cerebral cortex was also documented.46,47,51 With the exception of two studies,52,53 most of the DTI studies have documented microstructural white matter changes in patients with ET in several regions of the brain in the form of reduced fractional anisotropy and/or increased mean diffusivity. In addition to white matter changes in multiple areas in the cerebellum and its connections,54–57 the DTI studies also documented changes in the thalamus,55 red nucleus,58 and in the white matter fibers in the frontal, parietal, and temporal cortices.54,55 Neuroimaging studies on ET are comprehensively reviewed elsewhere.59
The apparent absence of functional or structural alterations in the ION in ET, as suggested by the aforementioned neuroimaging studies, gains additional support from a detailed post-mortem study60 that did not detect any differences in the morphological characteristics of the ION in the brains of patients with ET when compared with those of healthy controls. In that report, a detailed post-mortem study was undertaken of the microscopic changes in the ION of ET cases vs. age-matched controls. A series of metrics was used to quantify microscopic neuronal and glial changes in the ION and its input and output tracts. Olivary linear neuronal density was also assessed. ET cases and controls did not differ from one another with respect to any of the assessed metrics. Olivary linear neuronal density was also similar in cases and controls. Thus, a systematic post-mortem study of the microscopic changes in the ION did not detect any differences between ET cases and controls.
A recently published case report similarly suggests that the ION is not the source of tremor in ET.61 In that report, development of hypertrophic olivary degeneration in a patient with longstanding ET of 20 years’ duration did not alter the nature of the tremor. Although the patient developed gait imbalance and palatal tremor, the fact that there was no change in the pattern of upper limb tremor in the presence of a progressively degenerating olivary nucleus, argues against the notion that the ION plays a crucial role in the genesis of tremor in ET. Had the ION played a pivotal role in the pathogenesis of ET, hypertrophic olivary degeneration would presumably have resulted in an apparent reduction in the amplitude and/or frequency of tremor.
More recently, numerous post-mortem studies have documented histopathological changes in patients with ET. These studies have noted a series of distinct changes in the ET cerebellum, and particularly in the Purkinje cell and surrounding neuronal populations, compared with that of control brains.62 These changes are found across several Purkinje cell compartments, including the dendritic arbor (increased numbers of dendritic swellings,63 greater dendritic pruning,64 and loss of dendritic spines64 in ET than controls), the cell body (reduced Purkinje cell linear density in some studies, greater numbers of empty baskets, and more displaced Purkinje cells [heterotopias] in ET than controls),65,66 the axon (increased numbers of axonal thickenings,67 increased axonal branching,67 increased numbers of torpedoes,65 increased numbers of arciform axons,67 increased numbers of recurrent collaterals,67 increased terminal axonal sprouting,67 increased numbers of infraganglionic complexes67 in ET than controls). Other changes include a hypertrophic (“hairy”) appearance,68 and elongated leucine-rich repeat and Ig domain containing 1 (LINGO1) labeled pinceau processes in basket cell axonal processes surrounding Purkinje cells in ET cases when compared with controls.69 In addition, the climbing fiber connections on the Purkinje cells in ET have been reported to be abnormal.70 Other studies using immunohistochemical markers have reported reduction in cerebellar cortical excitatory amino acid transporter type 2 protein levels,71 and reduction in gamma-aminobutyric acid receptors in the dentate nucleus in ET vs. control brains.72 Earlier histopathological studies described above34,35 were characterized by a number of methodological limitations (absence of a quantitative approach and no metrics were specified, detailed examination of the cerebellum was not performed, and absence of control brains for comparison), and this likely contributed to their null findings. More recent studies,63–72 which do not have these limitations, have provided substantial evidence of structural changes in the cerebellum in patients with ET. Hence, aside from suggesting that the origins of ET could lie in the cerebellum rather than the ION, these studies also favor the notion that ET is more than just a physiological derangement.
The pacemaker concept and the animal models of tremor, which have favored the olivary model of ET, are also problematic. Although there is hardly any doubt that the ION has the property to generate rhythmic burst activity, there is no explicit or empiric evidence regarding the role of the ION in the pathogenesis of the human disease ET. In other words, it is a hypothetical construct. Further calling into question a special role of the ION in ET is that pacemaker neurons are not limited to the ION; rather, numerous structures throughout the brain have been recognized to have neurons with intrinsic oscillatory activity. These include the ION,8,9 locus ceruleus,10,11 raphe nucleus,12 thalamus,13 cerebellar nuclei,14 Purkinje cells,15 globus pallidus,16 and sensorimotor cortex17 (Figure 2). These neurons with pacemaker properties are either parts of the cerebellar-thalamic-motor or other motor networks (i.e., thalamus, cerebellar nuclei, Purkinje cells, globus pallidus) or they synapse with components of these motor networks (i.e., red nucleus, raphe nucleus). Why the ION, therefore, has been singled out as the prime mover of ET is not completely apparent.
As noted above, the harmaline model of tremor has augured the existence of the olivary model of ET. There is phenomenological overlap between harmaline-induced tremor in animals and ET as both these conditions are associated with action tremor of similar frequency (8–16 Hz) and have similar responses to several pharmacological agents.23 However, the harmaline model of tremor has several shortcomings as a model for the human disease ET (Table 2, Figure 2). First, this is an artificial, toxin-induced animal model of tremor and not the naturally occurring chronic human disease ET. In fact, it no more represents ET per se than the 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine model recapitulates the precise pathogenesis of Parkinson’s disease73 or the experimental autoimmune encephalomyelitis model recapitulates that of multiple sclerosis.74 Furthermore, the model produces an acute tremor rather than a chronic tremor, as one sees in ET. Thus, although after administering harmaline the tremor may develop within minutes, it then diminishes after several hours. For example, in a videotape documentation of a harmaline-induced tremor in mice, Cheng et al.75 observed tremor 5 minutes after the subcutaneous administration of harmaline but then the tremor resolved after 2 hours. In addition, the majority of rats develop resistance to harmaline within a few days of harmaline administration.76 Lutes et al.76 had demonstrated that repeated administration of harmaline results in rapid tolerance to the tremorogenic property of the drug in rats. Interestingly, tolerance to harmaline was not present in rats that were pretreated with diazepam or morphine in the same study. Although the exact mechanism of tolerance was not clear, it was presumed that altered function of the olivo-cerebellar system is responsible for this phenomenon. After repetitive administration of harmaline, the olivo-cerebellar system could be defective because of loss of Purkinje cell activity secondary to excito-toxicity mediated by excessive glutamate. O’Hearn and Molliver77 have reported degeneration of Purkinje cells in the parasagittal region of the cerebellar vermis in ibogaine- and harmaline-treated rats and later demonstrated that the olivo-cerebellar projections actually mediate the degeneration of Purkinje cells through trans-synaptic excitotoxicity.78 Contrary to the pathological changes observed in the cerebellum of the ibogaine/harmaline-induced animals, these parasagittal bands of necrosis in cerebellum have never been observed in any of the autopsy studies in patients with ET. Hence, although there is a superficial phenomenological similarity with ET, the natural course and pathogenesis of harmaline-induced tremor is significantly different from that of ET. Harmaline-induced tremor and ET also differ in terms of response to various pharmacological agents. For example, treatment with valproate and lithium can suppress harmaline-induced tremor whereas the same agents are well documented to worsen the tremor in patients with ET.23 Similarly, there are numerous other pharmacological agents that suppress the tremor in harmaline models, but they do not have any effect on the tremor in patients with ET (Table 2). In summary, although harmaline may produce a transient action tremor, it is not ET.
Time to look beyond the olivary model?
The ION model for ET originated and first came into favor in the early 1970s. However, it largely remains a physiological construct and there is less and less empiric evidence to support the model over time. Indeed, numerous observations in recent years have cast considerable doubt as to the validity of this model in ET. At the same time, there is growing evidence from clinical, epidemiological, and neuropathological observations that favors the concept that ET is a neurodegenerative disorder.79 Additional evidence of phenotypic and pathological heterogeneity indicate that ET may not even be a single disease entity, further suggesting that a single pathophysiological model is unlikely to encapsulate the entirety of what we now refer to as “ET”.80–84 Given these issues, along with the limitations and problems with the olivary model, it would seem that it is time now to lay the olivary model to rest.
Footnotes
Funding: Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS046436 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University). Dr. Lenka is sponsored by the Indian Council of Medical Research (ICMR, New Delhi) for his MD-PhD (Clinical Neurosciences) fellowship at the National Institute of Mental Health and Neurosciences, Bangalore, India.
Financial Disclosures: None.
Conflicts of interest: The authors report no conflict of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Chandran V, Pal PK, Reddy JYC, Thennarasu K, Yadav R, Shivashankar N. Non-motor features in essential tremor. Acta Neurol Scand. 2012;125:332–337. doi: 10.1111/j.1600-0404.2011.01573.x. doi: 10.1111/j.1600-0404.2011.01573.x. [DOI] [PubMed] [Google Scholar]
- 3.Jhunjhunwala K, Pal PK. The non-motor features of essential tremor: a primary disease feature or just a secondary phenomenon? Tremor Other Hyperkinet Mov. 2014;4 doi: 10.7916/D8D798MZ. doi: 10.7916/D8D798MZ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED, Galecki M, Rao AK. Brief reports four essential tremor cases with moderately impaired gait: how impaired can gait be in this disease? Tremor Other Hyperkinet Mov. 2013;3 doi: 10.7916/D8QV3K7G. doi: 10.7916/D8QV3K7G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis ED. Essential tremor as a neuropsychiatric disorder. J Neurol Sci. 2010;289:144–148. doi: 10.1016/j.jns.2009.08.029. doi: 10.1016/j.jns.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis ED. Re-thinking the biology of essential tremor: From models to morphology. Park Relat Disord. 2014;20((Suppl. 1)) doi: 10.1016/S1353-8020(13)70023-3. doi: 10.1016/S1353-8020(13)70023-3. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez JM, Tryba AK, Peña F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–67. doi: 10.1113/jphysiol.1981.sp013763. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llinás R, Yarom Y. Oscillatory properties of guinea pig olivary neurons and their pharmacological modulation: an study. J Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira RB, Howlett MCH, Gravina FS, Imtiaz MS, Callister RJ, Brichta AM, et al. Pacemaker currents in mouse locus coeruleus neurons. Neuroscience. 2010;170:166–77. doi: 10.1016/j.neuroscience.2010.06.028. doi: 10.1016/j.neuroscience.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 11.De Oliveira RB, Gravina FS, Lim R, Brichta AM, Callister RJ, Van Helden DF. Developmental changes in pacemaker currents in mouse locus coeruleus neurons. Brain Res. 2011;1425:27–36. doi: 10.1016/j.brainres.2011.09.053. doi: 10.1016/j.brainres.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Burlhis TM, Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1:582–588. doi: 10.1002/syn.890010611. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- 13.Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. doi: 10.1002/hbm.20728. [DOI] [PubMed] [Google Scholar]
- 14.Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–16. doi: 10.1523/JNEUROSCI.20-24-09004.2000. doi: 20/24/9004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–95. doi: 10.1113/jphysiol.1980.sp013357. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nambu A, Llinaś R. Electrophysiology of globus pallidus neurons in vitro. J Neurophysiol. 1994;72:1127–39. doi: 10.1152/jn.1994.72.3.1127. [DOI] [PubMed] [Google Scholar]
- 17.Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991;251:432–5. doi: 10.1126/science.1824881. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- 18.Desclin JC. Histological evidence supporting the inferior olive as the major source of cerebellar climbing fibers in the rat. Brain Res. 1974;77:365–384. doi: 10.1016/0006-8993(74)90628-3. doi: 10.1016/0006-8993(74)90628-3. [DOI] [PubMed] [Google Scholar]
- 19.Lamarre Y, Mercier L-A. Neurophysiological Studies of harmaline-induced tremor in the cat. Can J Physiol Pharmacol. 1971;49:1049–1058. doi: 10.1139/y71-149. doi: 10.1139/y71-149. [DOI] [PubMed] [Google Scholar]
- 20.Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87. doi: 10.1007/BF00236557. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- 21.Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–82. doi: 10.1113/jphysiol.1986.sp016147. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elble RJ. Physiologic and essential tremor. Neurology. 1986;36:225–31. doi: 10.1212/wnl.36.2.225. doi: 10.1212/WNL.36.2.225. [DOI] [PubMed] [Google Scholar]
- 23.Handforth A. Harmaline tremor: underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet Mov. 2012;2 doi: 10.7916/D8TD9W2P. doi: 10.7916/d8td9w2p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miwa H. Rodent models of tremor. Cerebellum. 2007;6:66–72. doi: 10.1080/14734220601016080. doi: 10.1080/14734220601016080. [DOI] [PubMed] [Google Scholar]
- 25.De Montigny C, Lamarre Y. Effects produced by local applications of harmaline in the inferior olive. Can J Physiol Pharmacol. 1975;53:845–849. doi: 10.1139/y75-116. doi: 10.1139/y75-116. [DOI] [PubMed] [Google Scholar]
- 26.Simantov R, Snyder SH, Oster-Granite ML. Harmaline-induced tremor in the rat: abolition by 3-acetylpyridine destruction of cerebellar climbing fibers. Brain Res. 1976;114:144–51. doi: 10.1016/0006-8993(76)91016-7. doi: 10.1016/0006-8993(76)91016-7. [DOI] [PubMed] [Google Scholar]
- 27.Batini C, Buisseret-Delmas C, Conrath-Verrier M. Harmaline-induced tremor – I. Regional metabolic activity as revealed by [14C]2-deoxyglucose in cat. Exp Brain Res. 1981;42:371–382. doi: 10.1007/BF00237502. doi: 10.1007/BF00237502. [DOI] [PubMed] [Google Scholar]
- 28.Batini C, Buisseret-Delmas C, Conrath-Verrier M. Olivo-cerebellar activity during harmaline-induced tremor. A 2-[14C]deoxyglucose study. Neurosci Lett. 1979;12:241–6. doi: 10.1016/0304-3940(79)96069-5. doi: 10.1016/0304-3940(79)96069-5. [DOI] [PubMed] [Google Scholar]
- 29.Miwa H, Nishi K, Fuwa T, Mizuno Y. Differential expression of c-fos following administration of two tremorgenic agents: harmaline and oxotremorine. Neuroreport. 2000;11:2385–90. doi: 10.1097/00001756-200008030-00010. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10943690. [DOI] [PubMed] [Google Scholar]
- 30.Park Y-G, Park H-Y, Lee CJ, Choi S, Jo S, Choi H, et al. CaV3.1 is a tremor rhythm pacemaker in the inferior olive. Proc Natl Acad Sci USA. 2010;107:10731–10736. doi: 10.1073/pnas.1002995107. doi: 10.1073/pnas.1002995107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci. 1993;114:45–8. doi: 10.1016/0022-510x(93)90047-3. doi: 10.1016/0022-510X(93)90047-3. [DOI] [PubMed] [Google Scholar]
- 32.Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thompson PD, Findley LJ, et al. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol. 1996;39:650–658. doi: 10.1002/ana.410390515. doi: 10.1002/ana.410390515. [DOI] [PubMed] [Google Scholar]
- 33.Fang W, Lv F, Luo T, Cheng O, Liao W, Sheng Ke, et al. Abnormal regional homogeneity in patients with essential tremor revealed by resting-state functional MRI. PLoS One. 2013;8:e69199. doi: 10.1371/journal.pone.0069199. doi: 10.1371/journal.pone.0069199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: A clinicopathologic study of 20 cases. Neurology. 2004;62:932–936. doi: 10.1212/01.wnl.0000115145.18830.1a. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15037695. [DOI] [PubMed] [Google Scholar]
- 35.Rajput AH, Rozdilsky B, Ang L, Rajput A. Clinicopathologic observations in essential tremor: report of six cases. Neurology. 1991;41:1422–1424. doi: 10.1212/wnl.41.9.1422. doi: 10.1212/WNL.41.9.1422. [DOI] [PubMed] [Google Scholar]
- 36.Hopfner F, Haubenberger D, Galpern WR, Gwinn K, Van’t Veer A, White S, et al. Knowledge gaps and research recommendations for essential tremor. Parkinsonism Relat Disord. 2016;33:27–35. doi: 10.1016/j.parkreldis.2016.10.002. doi: 10.1016/j.parkreldis.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. 1994;36:636–642. doi: 10.1002/ana.410360413. doi: 10.1002/ana.410360413. [DOI] [PubMed] [Google Scholar]
- 38.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of cerebral activation associated with essential and writing tremor. Arch Neurol. 1995;52:299–305. doi: 10.1001/archneur.1995.00540270095025. doi: 10.1001/archneur.1995.00540270095025. [DOI] [PubMed] [Google Scholar]
- 39.Lenka A, Bhalsing KS, Panda R, Jhunjhunwala K, Naduthota RM, Saini J, et al. Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology. 2017;75:657–669. doi: 10.1007/s00234-016-1771-1. doi: 10.1007/s00234-016-1771-1. [DOI] [PubMed] [Google Scholar]
- 40.Benito-León J, Louis ED, Romero JP, Hernández-Tamames JA, Manzanedo E, Álvarez-Linera J, et al. Altered Functional Connectivity in Essential Tremor: A Resting-State fMRI Study. Medicine (Baltimore) 2015;94:e1936. doi: 10.1097/MD.0000000000001936. doi: 10.1097/MD.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin W, Lin W, Li W, Qian S, Mou X. Resting state fMRI demonstrates a disturbance of the cerebello-cortical circuit in essential tremor. Brain Topogr. 2016;29:412–418. doi: 10.1007/s10548-016-0474-6. doi: 10.1007/s10548-016-0474-6. [DOI] [PubMed] [Google Scholar]
- 42.Gallea C, Popa T, García-Lorenzo D, Valabregue R, Legrand AP, Marais L, et al. Intrinsic signature of essential tremor in the cerebello-frontal network. Brain. 2015;138((Pt 10)):2920–33. doi: 10.1093/brain/awv171. doi: 10.1093/brain/awv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333:17–20. doi: 10.1016/s0304-3940(02)00966-7. doi: 10.1016/S0304-3940(02)00966-7. [DOI] [PubMed] [Google Scholar]
- 44.Pagan FL, Butman J A, Dambrosia JM, Hallett M. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. 2003;60:1344–1347. doi: 10.1212/01.wnl.0000065885.15875.0d. doi: 10.1212/01.WNL.0000065885.15875.0D. [DOI] [PubMed] [Google Scholar]
- 45.Kendi ATK, Tan FU, Kendi M, Erdal HH, Tellioglu S. Magnetic resonance spectroscopy of the thalamus in essential tremor patients. J Neuroimaging. 2005;15:362–6. doi: 10.1177/1051228405279039. doi: 10.1111/j.1552-6569.2005.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 46.Benito-León J, Alvarez-Linera J, Hernández-Tamames JA, Alonso-Navarro H, Jiménez-Jiménez FJ, Louis ED. Brain structural changes in essential tremor: Voxel-based morphometry at 3-Tesla. J Neurol Sci. 2009;287:138–142. doi: 10.1016/j.jns.2009.08.037. doi: 10.1016/j.jns.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 47.Bagepally BS, Bhatt MD, Chandran V, Saini J, Bharath RD, Vasudev MK, et al. Decrease in cerebral and cerebellar gray matter in essential tremor: a voxel-based morphometric analysis under 3T MRI. J Neuroimaging. 2012;22:275–8. doi: 10.1111/j.1552-6569.2011.00598.x. doi: 10.1111/j.1552-6569.2011.00598.x. [DOI] [PubMed] [Google Scholar]
- 48.Quattrone A, Cerasa A, Messina D, Nicoletti G, Hagberg GE, Lemieux L, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR Am J Neuroradiol. 2008;29:1692–7. doi: 10.3174/ajnr.A1190. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerasa A, Messina D, Nicoletti G, Novellino F, Lanza P, Condino F, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. Am J Neuroradiol. 2009;30:1240–1243. doi: 10.3174/ajnr.A1544. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin H, Lee DK, Lee JM, Huh YE, Youn J, Louis ED, et al. Atrophy of the cerebellar vermis in essential tremor: segmental volumetric MRI analysis. Cerebellum. 2016;15:174–181. doi: 10.1007/s12311-015-0682-8. doi: 10.1007/s12311-015-0682-8. [DOI] [PubMed] [Google Scholar]
- 51.Lin C-H, Chen C-M, Lu M-K, Tsai C-H, Chiou J-C, Liao J-R, et al. VBM reveals brain volume differences between Parkinson’s disease and essential tremor patients. Front Hum Neurosci. 2013;7:431–435. doi: 10.3389/fnhum.2013.00247. doi: 10.3389/fnhum.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinelli P, Rizzo G, Manners D, Tonon C, Pizza F, Testa C, et al. Diffusion-weighted imaging study of patients with essential tremor. Mov Disord. 2007;22:1182–5. doi: 10.1002/mds.21287. doi: 10.1002/mds.21287. [DOI] [PubMed] [Google Scholar]
- 53.Buijink AWG, Caan MWA, Tijssen MAJ, Hoogduin JM, Maurits NM, van Rootselaar A-F. Decreased cerebellar fiber density in cortical myoclonic tremor but not in essential tremor. Cerebellum. 2013;12:199–204. doi: 10.1007/s12311-012-0414-2. doi: 10.1007/s12311-012-0414-2. [DOI] [PubMed] [Google Scholar]
- 54.Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. AJNR Am J Neuroradiol. 2008;29:151–153. doi: 10.3174/ajnr.A0744. doi: 10.3174/ajnr.A0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saini J, Bagepally BS, Bhatt MD, Chandran V, Bharath RD, Prasad C, et al. Diffusion tensor imaging: Tract based spatial statistics study in essential tremor. Parkinsonism Relat Disord. 2012;18:477–482. doi: 10.1016/j.parkreldis.2012.01.006. doi: 10.1016/j.parkreldis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Nicoletti G, Manners D, Novellino F, Condino F, Malucelli E, Barbiroli B, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74:988–94. doi: 10.1212/WNL.0b013e3181d5a460. doi: 10.1212/WNL.0b013e3181d5a460. [DOI] [PubMed] [Google Scholar]
- 57.Klein JC, Lorenz B, Kang J-S, Baudrexel S, Seifried C, van de Loo S, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp. 2011;32:896–904. doi: 10.1002/hbm.21077. doi: 10.1002/hbm.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia L, Jia-Lin S, Qin D, Qing L, Yan Z. A diffusion tensor imaging study in essential tremor. J Neuroimaging. 2011;21:370–374. doi: 10.1111/j.1552-6569.2010.00535.x. doi: 10.1111/j.1552-6569.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 59.Louis ED, Huang CC, Dyke JP, Long Z, Dydak U. Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov. 2014;4 doi: 10.7916/D8DF6PB8. doi: 10.7916/D8DF6PB8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louis ED, Babij R, Cortés E, Vonsattel J-PG, Faust PL. The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls. Mov Disord. 2013;28:779–86. doi: 10.1002/mds.25400. doi: 10.1002/mds.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elkouzi A, Kattah JC, Elble RJ. Hypertrophic Olivary Degeneration Does Not Reduce Essential Tremor. Mov Disord Clin Pract. 2016;3:209–211. doi: 10.1002/mdc3.12275. doi: 10.1002/mdc3.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Louis ED. Linking Essential tremor to the cerebellum: neuropathological evidence. Cerebellum. 2016;15:235–42. doi: 10.1007/s12311-015-0692-6. doi: 10.1007/s12311-015-0692-6. [DOI] [PubMed] [Google Scholar]
- 63.Yu M, Ma K, Faust PL, Honig LS, Cortés E, Vonsattel JPG, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19:625–30. doi: 10.1111/j.1468-1331.2011.03598.x. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louis ED, Lee M, Babij R, Ma K, Cortés E, Vonsattel JPG, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014;137:3142–3148. doi: 10.1093/brain/awu314. doi: 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louis ED, Faust PL, Vonsattel JPG, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 66.Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–7. doi: 10.1001/archneurol.2007.8. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Babij R, Lee M, Cortés E, Vonsattel JPG, Faust PL, Louis ED. Purkinje cell axonal anatomy: Quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–3061. doi: 10.1093/brain/awt238. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erickson-Davis CR, Faust PL, Vonsattel J-PG, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuo SH, Tang G, Louis ED, Ma K, Babji R, Balatbat M, et al. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 2013;125:879–889. doi: 10.1007/s00401-013-1108-7. doi: 10.1007/s00401-013-1108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JPG, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–3159. doi: 10.1093/brain/awu281. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee M, Cheng MM, Lin C-Y, Louis ED, Faust PL, Kuo S-H. Decreased EAAT2 protein expression in the essential tremor cerebellar cortex. Acta Neuropathol Commun. 2014;2:157. doi: 10.1186/s40478-014-0157-z. doi: 10.1186/s40478-014-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135:105–116. doi: 10.1093/brain/awr301. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 73.Porras G, Li Q, Bezard E. Modeling Parkinson’s disease in primates: The MPTP model. Cold Spring Harb Perspect Med. 2012;2:a009308. doi: 10.1101/cshperspect.a009308. doi: 10.1101/cshperspect.a009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng MM, Tang G, Kuo S. Brief reports harmaline-induced tremor in mice: videotape documentation and open questions about the model. Tremor Other Hyperkinet Mov. 2013;3 doi: 10.7916/D8H993W3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lutes J, Lorden JF, Beales M, Oltmans GA. Tolerance to the tremorogenic effects of harmaline: evidence for altered olivo-cerebellar function. Neuropharmacology. 1988;27:849–855. doi: 10.1016/0028-3908(88)90102-5. doi: 10.1016/0028-3908(88)90102-5. [DOI] [PubMed] [Google Scholar]
- 77.O’Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55((2)):303–10. doi: 10.1016/0306-4522(93)90500-f. doi: 10.1016/0306-4522(93)90500-F. [DOI] [PubMed] [Google Scholar]
- 78.O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17:8828–41. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benito-León J. Essential tremor: a neurodegenerative disease? Tremor Other Hyperkinet Mov. 2014;4 doi: 10.7916/D8765CG0. doi: 10.7916/D8765CG0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lenka A, Bhalsing KS, Jhunjhunwala KR, Chandran V, Pal PK. Are Patients with limb and head tremor a clinically distinct subtype of essential tremor? Can J Neurol Sci. 2015:1–6. doi: 10.1017/cjn.2015.23. doi: 10.1017/cjn.2015.23. [DOI] [PubMed] [Google Scholar]
- 81.Louis ED. Essential tremor with head tremor: trait or state? Can J Neurol Sci. 2016;43:443–4. doi: 10.1017/cjn.2015.352. doi: 10.1017/cjn.2015.352. [DOI] [PubMed] [Google Scholar]
- 82.Teive HAG. Essential tremor: phenotypes. Parkinsonism Relat Disord. 2012;18:S140–S142. doi: 10.1016/S1353-8020(11)70044-X. doi: 10.1016/S1353-8020(11)70044-X. [DOI] [PubMed] [Google Scholar]
- 83.Louis ED. Essential tremors a family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–1208. doi: 10.1001/archneurol.2009.217. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Louis ED. “Essential tremor” or “the essential tremors”: is this one disease or a family of diseases? Neuroepidemiology. 2014;42:81–89. doi: 10.1159/000356351. doi: 10.1159/000356351. [DOI] [PMC free article] [PubMed] [Google Scholar]