Abstract
The development of formulas estimating glomerular filtration rate (eGFR) from serum creatinine and cystatin C and accounting for certain variables affecting the production rate of these biomarkers, including ethnicity, gender and age, has led to the current scheme of diagnosing and staging chronic kidney disease (CKD), which is based on eGFR values and albuminuria. This scheme has been applied extensively in various populations and has led to the current estimates of prevalence of CKD. In addition, this scheme is applied in clinical studies evaluating the risks of CKD and the efficacy of various interventions directed towards improving its course. Disagreements between creatinine-based and cystatin-based eGFR values and between eGFR values and measured GFR have been reported in various cohorts. These disagreements are the consequence of variations in the rate of production and in factors, other than GFR, affecting the rate of removal of creatinine and cystatin C. The disagreements create limitations for all eGFR formulas developed so far. The main limitations are low sensitivity in detecting early CKD in several subjects, e.g., those with hyperfiltration, and poor prediction of the course of CKD. Research efforts in CKD are currently directed towards identification of biomarkers that are better indices of GFR than the current biomarkers and, particularly, biomarkers of early renal tissue injury.
Keywords: Chronic kidney disease, Serum creatinine, Creatinine clearance, Creatinine excretion, Estimated glomerular filtration rate, Cystatin C, Renal imaging, Hyperfiltration, Biomarkers of chronic kidney disease
Core tip: Detection of the presence and severity of chronic kidney disease (CKD) is currently based on estimates of glomerular filtration rate based on serum creatinine and cystatin C concentrations plus factors that affect the rate of production of these two biomarkers, and on albuminuria. This scheme has improved detection of CKD and monitoring its course and the effects of therapeutic interventions. However, the scheme’s performance in detecting early stages of CKD and in predicting its course is poor, in general. Research in this field is directed towards finding better biomarkers of glomerular filtration rate and, particularly, biomarkers indicating early injury of the renal tissues.
INTRODUCTION
Chronic kidney disease (CKD) has been recognized as a major health problem worldwide with rising incidence, pronounced morbidity and mortality, and rising costs[1]. Early diagnosis, prevention and management of CKD, including treatment of its underlying disease and prevention and treatment of medical conditions for which the presence of CKD is a risk factor, has acquired great importance for health providers[1]. The prevalence of CKD in the United States during the years 1999-2004 was estimated to be equal to 13.1%[2]. Reported prevalence of CKD in various regions of the world varies. For example, recent estimates using different approaches computed a CKD prevalence of 32.5% in a small subject sample in Brazil[3], and 6.7% and 5.8% in larger subject cohorts in Romania and Poland respectively[4,5]. The prevalence of CKD is high in populations with conditions predisposing to it. For example, CKD was detected in 38.6% of individuals with hypertension and a high prevalence of advanced age and obesity[6].
The diagnosis of CKD is associated with important risks of disease in other organs. For example, cardiovascular disease has been recognized as a major risk associated with CKD[4,7]. In a cross sectional study of a large number of subjects with low and middle income in 12 countries, the incidence of CKD was 14.3% overall and 36.1% in high risk individuals, while the awareness of CKD was low and the rate of detection of cardiovascular disease in patients with CKD was also low[8]. Adverse effects of CKD on cardiac function have been reported in patients with heart failure, but preserved ejection fraction[9], diabetics with a doubling of their serum creatinine levels[10], and even healthy kidney transplant donors[11]. In a study from Korea, CKD was associated primarily with increased mortality risks from cardiovascular disease, but also with risks for other morbid conditions including malignancies[12].
Despite the universal recognition of the importance of its early detection, CKD is diagnosed late in several parts of the world[13]. Primary care services have a major role in the diagnosis and management of CKD[6]. Guidelines for detection and management of CKD addressed to primary care medical practitioners have been published for adult[14] and pediatric patients[15]. Education of the public is an important step for early management of CKD. Patients with CKD aware of its importance desire to be informed about its risks and management[16]. Information about CKD is provided to the public in the medical press[17]. Finally, methods for evaluation of the economic impact of CKD[18] and for technological developments addressing the detection and prevention of early stages of CKD[19] are studied.
In this report, we address the current methods for diagnosing CKD. The derivation, uses and limitations of these methods will be detailed. Finally, emerging methods for early diagnosis of CKD will be briefly presented.
CURRENT METHOD FOR DIAGNOSING AND STAGING CKD
Establishing the presence and degree of renal dysfunction has been based on measuring glomerular filtration rate (GFR). The rational for this is a rough correlation between GFR levels and clinical manifestations of renal failure. Serum creatinine level was the traditional surrogate index of GFR. Currently, the diagnosis and staging of CKD is based on estimated values of GFR (eGFR) and presence of albuminuria[14]. The first development leading to substitution of eGFR for serum creatinine was the computation of the Cockroft-Gault formula[20], which estimates creatinine clearance from serum creatinine, age, body weight and gender and was used extensively in the past for the diagnosis and management of CKD. The Cockroft-Gault formula estimates renal creatinine clearance, not GFR. The differences between these two clearances will be addressed later in this report.
The next important step in the diagnosis and staging of CKD was the development of carefully developed equations computing eGFR based on serum creatinine levels in large prospective studies in which GFR was measured by standard methods. The Modification of Diet in Renal Disease (MDRD) Study was the first one to be used for this purpose[21]. The MDRD formulas for eGFR were subsequently reexpressed using standardized serum creatinine values[22]. In addition to serum creatinine, the determinants of eGFR in the currently used 4-variable MDRD formula include gender, age and race (black or not black). A second 6-variable MDRD formula, which utilizes serum urea nitrogen and albumin levels in addition to the four determinants of eGFR used in the first formula, has similar performance characteristics with the 4-variable formula[22].
A newer set of formulas, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulas, was developed by combining data from several studies in which GFR was measured by standard methods[23]. The CKD-EPI formulas, which use essentially the same determinants of eGFR as the MDRD formula, were found to be more accurate than the MDRD formula[23]. This higher accuracy, largely, concerned the range of eGFR values greater than 60 mL/kg per 1.73 m2. However, the estimates of eGFR by the two formulas do not differ substantially for patients with moderate and advanced CKD, in general. Figures 1, 2 and 3 show simulated estimates of eGFR by the MDRD and CKD-EPI formulas at different serum creatinine levels and ages in various ethnic groups and genders. Figure 4 shows the comparison of eGFR values obtained from the MDRD and CKD-EPI formulas in subjects enrolled in the NHANES and MDRD studies. In patients with CKD, MDRD and CKD-EPI eGFR values are close in general.
Figure 1.
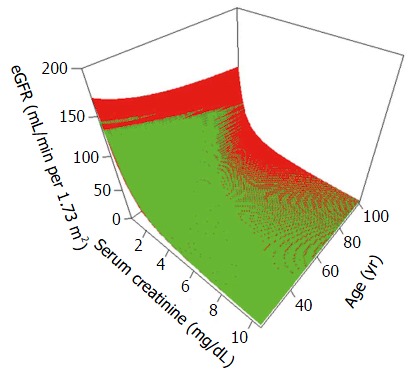
Modification of Diet in Renal Disease[22] and Chronic Kidney Disease Epidemiology Collaboration[23] formulae for estimating glomerular filtration rate fit to variations in serum creatinine (X axis) and age (Y axis) assuming males of Caucasian race. Note that the CKD-EPI formula yields slightly higher eGFR values with higher serum creatinine values and lower age whereas the MDRD formula leads to significantly higher eGFR values at very low serum creatinine values. MDRD: Modification of Diet in Renal Disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; eGFR: Estimating glomerular filtration rate.
Figure 2.
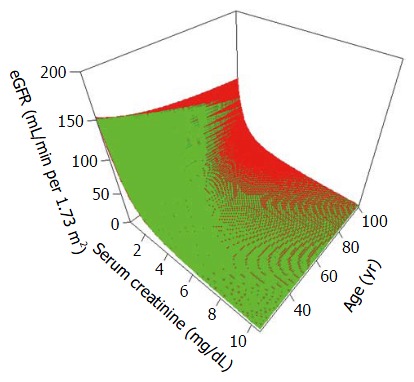
Modification of Diet in Renal Disease[22] and Chronic Kidney Disease Epidemiology Collaboration[23] formulas for estimating glomerular filtration rate fit to variations in serum creatinine (X axis) and age (Y axis) assuming females of Black race. The CKD-EPI formula yields slightly higher eGFR values with higher serum creatinine values and lower age whereas the MDRD formula leads to significantly higher eGFR values at very low serum creatinine values in this population also. MDRD: Modification of Diet in Renal Disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; eGFR: Estimating glomerular filtration rate.
Figure 3.
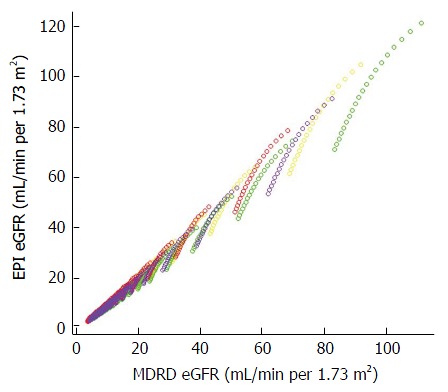
Scatterplot demonstrating close relationships between estimating glomerular filtration rate values calculated by the Chronic Kidney Disease Epidemiology Collaboration formula[23] (Y axis) and the Modification of Diet in Renal Disease formula[22] (X axis). Different colors are used to indicate the races and genders depicted in this figure: Yellow indicates Caucasian males, Green Black males, Red Caucasian females, and Purple Black females. A straight line to fit the data minimizes the least square error with an intercept of -1.03 and a beta coefficient of 1.04 achieving an R2 value of 0.99. MDRD: Modification of Diet in Renal Disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; eGFR: Estimating glomerular filtration rate.
Figure 4.
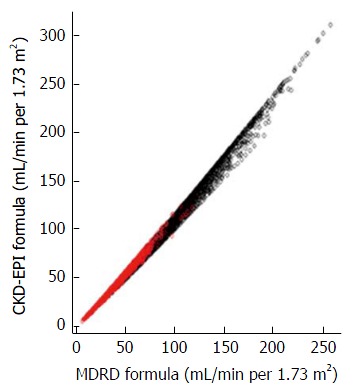
Comparison of estimating glomerular filtration rate values obtained by the Modification of Diet in Renal Disease[22] and Chronic Kidney Disease Epidemiology Collaboration[23] formulas in subjects who were enrolled in the NHANES study (Serum creatinine > 0.4 mg/dL, age ≥ 20 year) and the MDRD study[22]. MDRD: Modification of Diet in Renal Disease; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration.
The next step in the development of eGFR formulas was the introduction of cystatin C, which is a small molecular weight (13.3 kDa) protein produced at a steady rate from all nucleated body cells, filtered in the glomeruli and taken up and metabolized by the proximal tubules. Serum cystatin C levels were reported to be superior to serum creatinine levels as indices of GFR. The Chronic Epidemiology Collaboration developed formulas estimating GFR from serum cystatin C levels (the CKD-EPI cystatin C equations) and from both serum cystatin and creatinine levels (the CKD-EPI creatinine-cystatin C equations)[24]. The determinants of eGFR are gender and the level of cystatin C in the CKD-EPI cystatin C equations, and serum cystatin and creatinine, gender and ethnicity (black or other) in the CKD-EPI creatinine-cystatin C equations. eGFR formulas based on serum creatinine or serum creatinine and cystatin C were developed for specific ethnic or age groups, e.g., Chinese[25-27], Japanese[28,29], pediatric[30-33] and elderly[34] populations. Currently, several eGFR equations have been developed or are being developed[35].
Extensive sets of guidelines base the diagnosing and staging of CKD on combinations of eGFR cut-off values and albuminuria[36-39]. In the next section, we will discuss the applications of these guidelines. The limitations of this approach are of importance. In a population study mean measured GFR was higher in men than women but was not different between blacks and whites[40]. The authors of this study concluded that the different incidences of renal disease between blacks and whites were not due to the baseline renal function. The differences between the various equations computing eGFR are also not due to the baseline renal factors, but are keys to understanding the limitations of these equations as will be examined later in this text.
CLINICAL APPLICATIONS OF THE VARIOUS FORMULAS ESTIMATING GFR
Formula comparisons
A number of studies compared the accuracy of various eGFR formulas in various populations and clinical conditions[41-63]. The great majority of these studies concluded that formulas based on serum cystatin C alone or on combined cystatin-creatinine levels are superior to other formulas[41-47,49,50,52-54,56-58,60-63]. One study found greater accuracy with the use of the average creatinine-based and creatinine/cystatin-based eGFR formulas[51]. Another study found that in a Korean population an eGFR formula developed in a Japanese population was superior to other formulas[55]. Two studies found superiority of different eGFR formulas in different patient groups[42,48]. Finally, one study[59] found that the CKD-EPI formula[23] and a Japanese formula for eGFR based on serum creatinine[64] are superior to measured creatinine clearance in monitoring patients receiving cisplatin in high doses.
Uses of eGFR formulas in clinical studies
The older method for the diagnosis of CKD was to compare the serum level of creatinine of a subject to a normal range of creatinine concentrations. The clear advantage of eGFR formulas over this older method is that the formulas allow earlier detection of CKD and more precise following of its course in the early stages of CKD, when large decreases in GFR lead to small rises in serum creatinine. Formulas computing eGFR have been applied in clinical studies for a variety of purposes[43,45,46,57,58,60,65-74]. The Chronic Renal Insufficiency Cohort (CRIC) study, which is studying several aspects of CKD, is using its own eGFR formula based on serum creatinine and cystatin C levels[65]. Estimating the incidence of CKD in populations is one area where eGFR formulas have been useful. Risk prediction in various patient groups with CKD[43,45,46,48,57,58,60,67-69,71], choice and outcome of surgical and medical interventions in patients with CKD[56,72-74], and association of the CKD stage with specific clinical manifestations in various patient groups[66,70] are conditions for which eGFR equations based on cystatin C or on cystatin-creatinine have been shown to provide accuracy. In clinical studies targeting specific end-points of decline in renal function, use of eGFR instead of serum creatinine has the potential of reducing substantially both the required number of participants[75] and the targeted degree of decline in renal function[76].
LIMITATIONS OF THE FORMULAS COMPUTING eGFR
The introduction of the MDRD formula for eGFR[21] and the subsequent development of the current method for detecting and classifying CKD based on eGFR[34,77,78] has enhanced CKD awareness among clinicians and the public and has created new vigor in the study of prevention and management of CKD. Nevertheless, this approach to CKD has significant limitations. This section will discuss sequentially issues with the accuracy of eGFR formulas in clinical states associated with CKD, the analysis in the literature about these issues, the main cause of inaccuracies of the eGFR formulas, and the steps required for establishing the presence or absence of CKD when an eGFR formula computes a value less than 60 mL/min per 1.73 m2.
Discrepancies of the diagnosis of CKD by eGFR formulas in various clinical states
Discrepancies between various formulas estimating eGFR and between these formulas and measurements of GFR by standard methods have been reported. To illustrate the types of conditions in which eGFR formulas may be inaccurate, we will discuss a few examples of these discrepancies. Table 1 shows clinical conditions in which the accuracy of eGFR formulas has been disputed.
Table 1.
Clinical conditions affecting the accuracy of estimating glomerular filtration rate formulas
| Diabetes mellitus |
| Human immunodeficiency viral infection |
| Chronic liver disease |
| Cardiovascular disease |
| Kidney transplants (recipients and donors) |
| Sarcopenia |
| Critical illness |
| Hereditary disease (e.g., Fabry’s) |
| Obesity |
The increasing incidence of diabetes mellitus in many parts of the world has been the major cause of the increasing incidence of CKD. CKD secondary to diabetic nephropathy, which particularly in its early stages may not be associated with albuminuria, especially in type 2 diabetics, has its own diagnostic difficulties[79-92]. Several studies concluded that some creatinine-based eGFR formulas are not as accurate in detecting early CKD as cystatin-based formulas[80-84,86-88,91]. In addition, cystatin-based eGFR formulas were the only ones found to be independent predictors of diabetic complications[83,86] and creatinine-based eGFR formulas did not detect early declines in renal function[89,90]. One study concluded that the prediction of CKD was similar with eGFR formulas calculated by the Cockroft-Gault formula, the MDRD formula and a cystatin-based eGFR formula[79]. A study using inulin clearance to measure GFR concluded that cystatin-based and creatinine-based eGFR formulas have significant inaccuracies in the diagnosis and staging of diabetic CKD[92]. Based on the discrepancies of eGFR formulas, one report proposed the use of one of the standard techniques, iohexol clearance, for evaluation of renal function in type 1 diabetics[85].
Infection with human immunodeficiency virus (HIV) is an important cause of CKD in several parts of the world, for example in South Africa[93]. Proper management of patients infected with HIV requires repeated screening for CKD[94]. Screening for CKD is of great importance for patients treated with nephrotoxic antiretroviral medications[95]. Discrepancies between various eGFR formulas in HIV-infected patients have been reported[96-102]. Extrarenal influences on cystatin C and creatinine metabolism may cause eGFR formula computations differing from the actual renal function[97]. For example, serum cystatin C levels may be elevated in patients with active HIV-infection causing a large underestimation of GFR by cystatin-based eGFR formulas[96,102]. Several eGFR formulas, based on either cystatin C or creatinine, were found to underestimate GFR in one study[98]. Other studies in HIV-infected subjects found superiority of either cystatin-based eGFR formulas[98,100] or creatinine-based formulas[99] in detecting CKD and determining the risks associated with it.
Chronic liver disease is associated with inaccuracy of the eGFR formulas[103-105]. Creatinine-based eGFR formulas systematically overestimate measured GFR in this patient group and the degree of overestimation increases with the severity of liver disease[104]. Cystatin-based eGFR equations are more accurate in these patients[103], but cystatin-based formulas derived in populations with liver disease may prove to have the greater usefulness[105].
Limitations of various eGFR formulas have been reported in subjects with cardiovascular diseases[106-109]. One study calculated similar assessment of cardiovascular risks by the Cockroft-Gault formula and by serum cystatin C level[106]. However, a larger study found substantial differences between eGFR values calculated by creatinine-based and cystatin-based formulas in patients with varying severity of cardiac disease, with creatinine-based eGFR values exceeding the cystatin-based values in most patient categories[107]. Another study concluded that measured GFR, not eGFR formulas, should be used for evaluating the relationship between retinal vasculopathy and renal disease[108]. Differences in the association of creatinine-based and cystatin-based eGFR formulas with non-traditional cardiovascular risk factors (asymmetric and symmetric dimethylarginine blood levels, insulin resistance) in subjects without diagnosed cardiovascular disease, diabetes or CKD was reported in another study[109]. Finally, eGFR formulas were found to be inaccurate in heart transplant recipients[110]. The authors of this last study proposed the use of measured GFR for assessing kidney function in this patient group.
Discrepancies between eGFR formulas have been found in recipients and donors of kidney transplants. A recent study concluded that creatinine-based and creatinine/cystatin-based eGFR formulas are more accurate than cystatin-based formulas in renal transplant recipients[111]. One study concluded that the MDRD eGFR formula was more accurate in detecting GFR values lower than 60 mL/min per 1.73 m2 than the CKD-EPI creatinine/cystatin C formula after kidney donation[112], while a second study concluded that creatinine-based eGFR formulas have low accuracy in evaluating renal function in prospective kidney donors[113]. Of note is that the eGFR formulas used in this last study were derived in different ethnic groups. Various problems posed by creatinine-based and cystatin-based eGFR formulas in renal transplant recipients were reviewed by Santos and Martins[114]. Based on these and other studies, United Networks for Organ Sharing (UNOS) require a measured creatinine clearance or GFR for evaluating the renal function of prospective kidney donors. Prospective renal transplant donors illustrate the limitations of eGFR formulas in subjects in whom the need of accuracy in establishing absence of CKD is critical.
Issues with the eGFR formulas were reported in patients with neurological diseases causing sarcopenia[115,116], critically ill patients[117], and patients with hereditary disease[118]. Sarcopenia in subjects with neuromuscular disease is the source of systematic overestimation of GFR by creatinine-based eGFR formulas. Studies have found differences between various eGFR formulas in obese subjects[119-122]. One large study concluded that cystatin-based eGFR formulas are deficient in detecting CKD stage 3 or 4 in obese subjects[119]. In contrast, two smaller studies concluded that creatinine-based equations produce higher eGFR values than cystatin-based formulas and may lead to underestimation of the presence and degree of CKD[120,122]. The finding that sarcopenia is highly prevalent in CKD patients leading to underestimation of the degree of obesity in this patient group[121] provides an explanation for the discrepancies between cystatin-based and creatinine-based eGFR in obese subjects with CKD.
Applications of eGFR formulas in population studies
Several reports have analyzed the performance of various eGFR formulas in different populations[123-133]. Several studies compared various eGFR formulas. A Scandinavian study found a substantially different prevalence of CKD with the use of the MDRD formula than with the use of the Cockroft-Gault formula or of two cystatin-based equations[123]. Similar findings were reported in a study from Uruguay, in which the lowest values of eGFR were found when using the CKD-EPI cystatin C equation, while the CKD-EPI creatinine-cystatin formula computed intermediate eGFR values and the MDRD formula computed the highest values of eGFR[129]. A study in Asian Indians, which also found lower overall eGFR values when using cystatin-based equations, noted that these equations resulted in widely varying eGFR values which affected the classification of CKD[130]. A large study analyzing United States subjects with eGFR determination by the MDRD formula reported that only eGFR values lower than 45 mL/min per 1.73 m2 yielded a high probability of CKD[132].
In addition, studies in smaller numbers of subjects from various parts of the World compared eGFR formulas and GFR measurements by standard research methods[124-128,131]. Discrepancies between eGFR computed by cystatin-based equations and measured GFR were found in a pediatric Canadian group[124]. Differences between eGFR and measured GFR were also noted in an elderly Chinese group regardless of whether the formula used to compute eGFR was based on cystatin C or not[125]. In a study of Japanese subjects, the creatinine-based Japanese eGFR formula, overestimated GFR in subjects who had poor renal function or were malnourished[126]. In another study in elderly Chinese subjects, there were differences between several eGFR formulas and GFR, with some cystatin-based eGFR equations performing better than other equations[127]. In a study at Mayo Clinic, combined cystatin- and creatinine-based eGFR correlated better with measured GFR than creatinine-based or cystatin-based eGFR values, but creatinine-based eGFR was found to have a better association with most risk factors than the other eGFR values[128]. Another Mayo Clinic study comparing GFR with the CKD-EPI eGFR formulas based on creatinine and cystatin in recipients of organ transplants, patients with known CKD and prospective kidney donors concluded that the performance of various eGFR formulas based on creatinine or cystatin C was affected significantly by the clinical characteristics of the subjects[131]. GFR in these studies was measured by technetium-99m-diethylene-triamine penta-acetic acid [(99m)DTPA][124,125,127], inulin[126], or iothalamate[128,131] clearance.
Formulas computing eGFR, in conjunction with other factors, have been found to be of use in assessing risks associated with CKD. The risk of progression of CKD was recently evaluated in a metaanalysis of studies in large numbers of subjects in North America and other parts of the World performed by the CKD Prognosis Cohort (CKDPC)[133]. This metaanalysis concluded that formulas predicting the risk of progression developed in Canada and including eGFR, age, gender, and albuminuria, plus four serum biochemical values (calcium, phosphate, bicarbonate, and albumin) were accurate in predicting progression, with the proviso that calibration may be needed in certain parts of the World.
Commentaries on creatinine, cystatin C, and eGFR
The issues raised by various eGFR formulas as well as reference methods for measuring GFR have been addressed in several reports[134-142]. Conditions that may cause false values of creatinine-based or cystatin-based eGFR formulas were addressed in two reviews[134,135]. One study analyzed factors leading to agreement or disagreement between measured creatinine clearance and creatinine-based formulas estimating renal function[136]. The issues faced with the development of risk prediction formulas based on eGFR plus various other factors and with the applications of these formulas were explored in another review[137].
One study found a systematic difference between iothalamate and iohexol, two standard markers of GFR in research studies: The average iothalamate clearance was 15% higher than the average iohexol clearance, while the average creatinine clearance exceeded the corresponding iohexol value by 42%[138]. A related editorial discussed the potential effects of differences in the clearances of standard markers of GFR on the derivation of eGFR formulas[139]. Two reports discussed the use of various indicators of GFR in pediatric[140] and adult[141] populations. Finally, one report presented a complex computer-based program for the diagnosis of CKD based on eGFR and pertinent clinical information[142].
THE MAIN LIMITATION COMMON TO OF ALL eGFR FORMULAS
The section will start with the presentation of three subjects under the care of nephrologists in two hospitals in Albuquerque. These subjects illustrate issues created by eGFR formulas and suggest the proper way to address these issues. Creatinine-based eGFR values were computed by the MDRD[22] and CKD-EPI[23] formulas in all three subjects. In the third subject, cystatin-based and creatinine/cystatin-based eGFR were computed by the CKD-EPI formulas[24]. All eGFR values in these patients are in mL/min per 1.73 m2.
Illustrative cases
Case 1: A 61-year-old white man with quadriplegia for 25 years following a motor vehicle accident and on hemodialysis for two years transferred to an inpatient spinal cord injury unit in New Mexico from another state. Immediately prior to the first hemodialysis session, his serum creatinine level was 1.27 mg/dL, with eGFR values of 58 by the MDRD formula and 61 by the CKD-EPI formula. Serum creatinine levels ranged between 1.12 and 1.32 mg/dL throughout the dialysis period. The agency overseeing chronic dialysis facilities in New Mexico requested definitive proof of end-stage kidney disease (ESKD). A 48-h urine collection through the permanent indwelling urinary bladder catheter carried by the patient revealed the following values: Volume of collected urine 320 mL; total creatinine content in this urine specimen 62 mg.
Case 2: A 31-year-old white woman had one year after donating a kidney a stable serum creatinine concentration of 1.32 mg/dL, with eGFR values of 47 by the MDRD formula and 53 by the CKD-EPI formula. Urinalysis was repeatedly clean and urine albumin was undetectable. She is dedicated to exercise. Creatinine excretion in a 24-h urine specimen was 1672 mg and serum creatinine collected at the end of the urine collection remained at 1.32 mg/dL. Her body surface area is 1.81 m2. Calculated renal creatinine clearance was 84 mL/min per 1.73 m2.
Case 3: A 71-year-old white man with hypertension under excellent control had on repeated testing a serum creatinine level of 1.60 mg/dL, no albuminuria, and ultrasound showing no abnormalities in the renal texture and no post-void residual urinary bladder volume. He is exercising intensely. Serum cystatin C level was 1.0 mg/L. Calculated eGFR was 43 by both the MDRD and CKD-EPI creatinine equations, 57 by the CKD-EPI creatinine-cystatin equation, and 74 by the MDRD-EPI cystatin C equation.
The main limitation of eGFR formulas
The main limitation of the eGFR formulas is significant lack of accuracy in individuals as compared to groups. This causes problems in establishing the presence or absence of early CKD. The inaccuracy is rooted in the method for developing these formulas and the nature of the biomarkers used in them. eGFR formulas are multiple regression equations estimating GFR values using the serum concentration of one or more biomarkers eliminated by glomerular filtration and of other factors that affect the production of the biomarkers. Factors entered in these formulas so far are ethnicity, gender and age. A group of subjects with the same GFR, ethnicity, age and gender will not have the same serum concentration of the biomarker. Instead, they will have a range of concentrations around the mean value for this group, which is the value computed by the eGFR formula. Conversely, the values of GFR will have a range around the mean value provided by the eGFR value. The width of the range determines the standard error of the eGFR estimate provided by the regression equation.
The errors of eGFR formulas derived by regression have been quantified by P30 statistics, i.e., the probability that the measured GFR will differ from eGFR by 30% or less in various cohorts including the cohort that was used for the CKD-EPI formula[143], a cohort of elderly subjects[144] and a cohort of transplant recipients[145]. GFR and eGFR values differ by more than 30% in approximately 20% of the individuals. The origin of this difference lies in the fact that eGFR formulas do not include all the factors that affect the serum concentration of a biomarker or account for changes in the quantitative effects of factors with varying intensity (e.g., degree of sarcopenia). Indeed, development of an eGFR formula accounting for all the influences on the steady state serum concentration of a biomarker and for quantitative variations of these influences would be an exceedingly difficult task.
The nature of the biomarkers used in eGFR formulas is also a source of differences between eGFR and GFR values. eGFR formulas are applicable only to steady states of biomarkers. In the steady state, the rates of production and removal (the total clearance) of a biomarker are equal and its serum concentration is the fraction production/clearance. An ideal biomarker for GFR would also be exceedingly difficult to find. Such a biomarker should be eliminated exclusively by glomerular filtration and have the same exactly production rate in all subjects with the same age, gender, ethnicity and whatever other factors may be entered in eGFR formulas in the future. In examining whether a biomarker is suitable as an indicator of GFR one should investigate both its production and routes of elimination. Creatinine and cystatin C fail to fulfill the criteria for ideal biomarkers of GFR.
The production of creatinine receives influences from a host of factors other than those entered in the eGFR formulas and its elimination is not only through glomerular filtration. Factors affecting the production of creatinine and not included in the eGFR formulas include level of exercise, diet, particularly red meat ingestion or intake of dietary supplements containing creatine[146-148], neuromuscular diseases leading to loss of muscle mass, and disease states affecting the rate of conversion of creatine to creatinine[149]. Not accounting in the eGFR formulas for factors affecting creatinine production can affect the accuracy of eGFR estimates in individuals as well as cohorts. For example, the reported larger degree of underestimation of GFR by the MDRD formula in healthy individuals than in CKD patients[150] was probably the consequence of higher production rates of creatinine in the healthy individuals.
Creatinine production differences between subjects with and without diabetes mellitus, which is a major cause of CKD, are the reason for the inaccuracies of creatinine-based eGFR formulas discussed earlier. There is evidence suggesting that diabetes affects creatinine production. Serum creatinine levels tend to be low in diabetic individuals[151], reflecting low rate of creatinine production, hyperfiltration (see below) or a combination of the two. Lean body mass, a large part of which is muscle mass, decreases with age rapidly in diabetic subjects[152]. The loss of muscle mass in diabetic subjects is the source of discrepancies between GFR and creatinine-based eGFR formulas[153]. Creatinine excretion is systematically lower in diabetic than non-diabetic subjects with ESKD treated by peritoneal dialysis[154]. A formula developed in a peritoneal dialysis cohort includes diabetes among the predictors of creatinine excretion[155]. Future developments in creatinine-based eGFR formulas should study, and most probably include, diabetes among the factors affecting serum creatinine concentration.
Factors other than GFR affect creatinine excretion. Renal creatinine excretion is not exclusively through glomerular filtration. Tubular secretion contributes a small part, around 15%, of the urinary creatinine at normal GFR values. In glomerulopathic CKD, the fraction of urinary creatinine excreted through tubular excretion increases progressively as GFR decreases and serum creatinine rises[156]. Removal of creatinine through tubular secretion is the source of significant overestimation of GFR by creatinine-based eGFR formulas in CKD. In addition to tubular secretion, creatinine is removed from the body through extrarenal routes, mainly through the gastrointestinal tract. An indirect approach computed an average extrarenal creatinine clearance of 0.042 L/kg per 24-h in males and 0.041 L/kg per 34-h in females with advanced CKD[157]. This approach suggests that progressively larger amounts of creatinine are removed through the extrarenal pathway as serum creatinine rises progressively in worsening CKD. This would cause progressive overestimation of GFR by creatinine-based eGFR formulas.
Sickle cell disease is one condition leading to large differences between renal creatinine clearance and GFR. Creatinine-based eGFR formulas have greatly overestimated true GFR in patients with sickle cell nephropathy. This is related to the supranormal proximal tubular function in subjects with sickle cell disease resulting in enhanced creatinine secretion and various electrolyte disturbances[158,159]. It has been suggested that serum cystatin C and possibly cystatin-based eGFR formulas can be better indicators of renal function in such patients[160] Conditions that affect the extrarenal removal of creatinine need further study.
Several medications affect the production or tubular excretion of creatinine. Table 2 shows medications that affect creatinine production[161,162] or block tubular creatinine secretion[163-168]. In the past, several research studies measured GFR by creatinine clearance with the use of medications blocking tubular creatinine excretion[163]. A great number of medications may induce myopathy leading to varying rates of creatinine production. Drugs induce myopathy by direct myotoxicity (e.g., alcohol, cocaine, glucocorticoids, statins, antimalarial compounds, colchicine, zidovudine), immunological mechanisms causing inflammation (e.g., D-penicillamine), or various indirect mechanisms (e.g., drug-induced coma causing muscle ischemia from compression, diuretic-induced hypokalemia, and drug-induced hyperkinetic syndromes, dystonic states, hyperthermia or neuroleptic malignant syndrome)[169].
Table 2.
Drugs raising serum creatinine concentration
Acute drug-induced rhabdomyolysis is manifested by elevation in the serum concentration of muscle enzymes (e.g., creatinine phosphokinase) and leads to acute kidney injury in some instances, in addition to a rise in serum creatinine due to overproduction. In chronic drug-induced myopathy causing increased creatinine production, however, serum levels of muscle enzymes may not be elevated[169]. In subjects with advanced CKD, for example ESKD patients treated by peritoneal dialysis, increased creatinine production secondary to drugs can cause large rises in serum creatinine concentration and excretion without a change in creatinine clearance or in the serum levels of muscle enzymes[170]. Finally, errors in serum creatinine values are caused by interference of various substances, endogenous or exogenous, with the creatinine assay. This issue was more common with the older method of measuring creatinine concentration in biological fluids by the non-specific Jaffe colorimetric method[171]. However, even the newer specific enzymatic methods receive interference from other substances, including dopamine, ascorbate[171], the analgesic dipyrone (metamizol), N-acetylcysteine and other substances.
Like creatinine levels, serum cystatin C levels receive influences independent of GFR[172-183]. Changes in renal and extrarenal function and in the production of cystatin C may affect its serum level. As noted cystatin C is filtered in the glomeruli and then reabsorbed and catabolized in the proximal tubules. Its urinary excretion is a small fraction of its filtered load. It has been postulated that tubulointerstitial disease damaging the integrity of the tubular barrier may lead to back leak of cystatin C into the peritubular blood capillaries and increase in serum cystatin C levels[172]. A change in tubular handling of cystatin C was reported in children with nephrotic syndrome who exhibited significant rises in the urinary excretion of the compound at times of heavy proteinuria[175]. Potential influences of renal tubular dysfunction on cystatin C serum levels will require further study.
Factors affecting the extrarenal clearance and production of cystatin C have not been studied adequately. Indirect methods to quantitate these influences have been proposed[177]. Increases in the rate of production and the serum levels of cystatin C have been reported with the use of corticosteroids[173], and in subjects with obesity[180], large lean body mass[178], hyperthyroidism[178,183], elevated serum triglyceride levels[181], and after coffee consumption[182]. Two reports studied by multivariable statistical analysis factors affecting serum cystatin C levels independently of GFR[174,179]. Older age, male gender, large weight and height, current cigarette smoking and higher C-reactive protein levels were associated with higher serum cystatin C levels in a study from the Netherlands[174]. A study pooling data from three large research studies identified younger age, male gender, diabetes mellitus, high levels of C-reactive protein, high white blood cell count and low levels of serum albumin as independent factors associated with high levels of cystatin C[179]. Race had also a small independent effect on cystatin C in this last study. Finally, potential interferences with the measurement of cystatin C have not been adequately studied.
How to proceed when a low eGFR value suggest CKD in an individual
Serum creatinine is routinely measured for surveillance of the renal function, while cystatin C is not a routine blood test. Currently, the initial step for diagnosing and staging CKD is based on determination of serum creatinine and albuminuria. Creatinine-based eGFR values are of paramount importance in this process[39]. The rate of creatinine production is the cause of questioning the accuracy of eGFR in the great majority of subjects. The suggested next step in the evaluation of individuals for whom there are questions about the accuracy of creatinine-based eGFR is to measure serum cystatin C and compute cystatin-based and creatinine/cystatin- based eGFR values[39]. This approach, which was based on the finding that the effect of muscle mass on serum cystatin C levels is small[179], may confirm the presence of CKD in subjects in whom the cystatin-based eGFR values agree with the creatinine-based values, but will create new problems if the various eGFR values disagree. The subject reported in case 3 above illustrates this problem.
A different approach for confirming or rejecting a questionable creatinine-based eGFR value is required. The hypothesis that an unusual rate of creatinine production led to an unusual serum creatinine level is addressed directly by measuring creatinine excretion rate[184]. Cases 1 and 2 of this report illustrate this point. As noted, candidates for living kidney donation are a group of subjects who should also have their creatinine clearance determined. Differences between estimates of eGFR and estimates of creatinine clearance by the Cockroft-Gault formula affecting significantly the dosing of potentially toxic drugs have been reported[185]. Dosing of drugs may require measurement of creatinine clearance in selected cases. In addition to clarifying the information provided by creatinine-based eGFR, determination of creatinine excretion has prognostic significance. Low urinary creatinine excretion levels in CKD patients are associated with adverse outcomes[186].
Establishing the presence or absence of CKD from creatinine clearance also has drawbacks. Urine collection and storage for 24 h is a demanding task. Errors in the timing of urine specimens and presence of obstructive urinary tract disease are sources of inaccuracy of urine collection. Detailed explanation of the importance of accurate urine collection and detailed instruction about the timing of this procedure are imperative and minimize collection errors. Motivation of individuals with questionable eGFR values is important. Subjects with low creatinine-based eGFR values and suggestion of large muscle mass are usually very motivated to know whether they have CKD or not. Evaluation for urinary obstruction should be considered a necessary step of the diagnosis of CKD.
A third drawback of the measured creatinine clearance is the systematic overestimation of GFR because of tubular secretion of creatinine. This may become significant when the value of the measured creatinine clearance is above, but close to 60 mL/min per 1.73 m2. In glomerulopathic subjects with normal GFR values, creatinine clearance exceeded by 16%, on the average, GFR measured by inulin clearance[156]. We suggest that creatinine clearance values less than 72 mL/min per 1.73 m2, that is values exceeding 60 mL/min per 1.73 m2 by less than 20%, should call for further investigation. Cystatin C measurements may improve the accuracy of diagnosis of CKD in this last group of subjects, but will require further studying.
As noted, careful history taking about conditions predisposing to CKD is very important in establishing the diagnosis of CKD[142]. Imaging techniques are also of help. Size and texture of the kidneys and features of obstructive disease of the urinary tract are routinely investigated by traditional ultrasonographic techniques. Addition of color Doppler and spectral Doppler to conventional ultrasonography allows detailed investigation of the renal circulation. Circulatory indices derived from these newer techniques, including the resistivity index and the strain index, as well as evaluation of renal fibrosis by elastography, can provide valuable assistance in the diagnosis of CKD[187-189]. Measurement of GFR by nuclear scanning[190,191] is another imaging technique assisting the early detection of CKD in vulnerable patient groups, e.g., renal transplant recipients. Other imaging techniques applied in the diagnosis of renal diseases (e.g., radiography, computed tomography, nuclear magnetic resonance methods) have limited value in the detection of CKD. Despite the proliferation of studies evaluating various biomarkers as indicators of specific renal histology, renal biopsy remains the gold standard of the histologic diagnosis of renal disease[192]. The role of renal biopsy in establishing the presence of CKD, however, is questionable.
FUTURE DEVELOPMENTS
Two types of findings have generated questions about the use of either measured GFR or eGFR formulas for the diagnosis and classification of CKD. The first issue was the varying association between the serum levels of several small molecular weight substances classified as uremic toxins and eGFR values calculated by several formulas in subjects with CKD stages 2 to 5[193]. This finding, which was attributed to varying effects on the serum concentration of uremic solutes of factors other than GFR, including tubular handling, extrarenal removal and production[194] provides a stimulus for searching for new biomarkers of eGFR better associated with various uremic toxins, but does not eliminate the current principle of diagnosis and staging CKD based on GFR. Based on the conclusions of the last report[194], it is possible that other factors have significant effects on the serum concentration of uremic toxins independently or in addition to the presence and stage of CKD. Findings of elevated serum levels of “uremic” toxins in subjects without clinical features of CKD and normal levels of GFR measured by standard methods would provide support to this last postulate.
Hyperfiltration is the second category of findings creating questions about using eGFR or GFR to diagnose and stage CKD. Hyperfiltration has been extensively investigated in subjects with type 1 or type 2 diabetes mellitus in whom it is considered an important factor in the initiation and progression of diabetic nephropathy[195]. Subsequently, hyperfiltration was reported in a variety of clinical conditions including obesity, hypertension, metabolic syndrome, smoking, sickle cell disease, thalassemia, IgA nephropathy, reflux nephropathy, kidney donors, transplanted kidneys, cirrhosis, pregnancy, lead poisoning, autosomal dominant polycystic kidney disease, primary aldosteronism, nephropathy from Puumala hantavirus, and apparently healthy subjects to whom it confers a risk of CKD and hypertension[196]. It is of interest that intense exercise can decrease the prevalence of hyperfiltration in the general population[197].
Establishing the presence of hyperfiltration is of great importance. A review of studies on hyperfiltration disclosed the use of a variety of methods for measuring or estimating GFR and a variety of GFR or eGFR cut-off values defining hyperfiltration[198]. Hyperfiltration is defined as either supernormal GFR or increased filtration fraction (GFR over renal plasma flow), which is normally around 0.20. Elevated glomerular capillary hydrostatic pressure, with or without an elevation in the renal plasma flow, leads to hyperfiltration[195]. Subjects with hyperfiltration on the setting of increased renal plasma flow, for example a subset of patients with early diabetic nephropathy, have supranormal GFR. A few studies of hyperfiltration have measured both GFR and renal plasma flow. In addition to its accuracy in establishing presence or absence of hyperfiltration, this method allows detection of hyperfiltration in subjects with established CKD and low GFR values.
Hyperfiltration creates two major problems with the diagnosis of CKD. The first problem is a documented lack of accuracy of various eGFR formulas in subjects with hyperfiltration[199,200]. The second problem is even more serious and cannot be corrected by finding new biomarkers providing accurate eGFR formulas in subjects with hyperfiltration. Even if such biomarkers are found in the future, subjects with hyperfiltration, early stages of CKD, absence of albuminuria and GFR in the normal range will be misclassified as not having CKD by the current scheme. Elderly subjects with apparently normal renal function, but with loss of nephrons and hypertrophy of the remaining nephrons[201-203] are one such group. Detection of CKD in these subjects requires an approach other than measurement or estimation of GFR.
The limitations of the available tools for diagnosing and staging CKD have complicated the development of accurate models for early detection of CKD and prediction of its progression. One approach that has been investigated is the measurement of the renal functional reserve (RFR)[204]. RFR, the temporary increase in renal blood flow and GFR after a standardized heavy protein meal, is a homeostatic mechanism prominent in carnivores. Healthy humans exhibit a less pronounced, but quite large RFR after a protein meal[204]. Certain studies suggested that measurement of renal functional reserve may be a useful method in detecting early CKD. In one study, vasculopathic patients with normal GFR and absent RFR by GFR measurement developed within two years a significant decrease in GFR[205]. In another study, the magnitude of RFR decreased progressively with higher stages of CKD[206]. Doppler ultrasonography can assist in the evaluation of RFR[207,208]. However, conflicting RFR findings have been reported in several studies of patients with CKD. Whether RFR measurement can provide a useful method for establishing the presence or absence of early CKD is not clear currently.
Another approach to the early detection of CKD is acquiring strength. Recent reports have stressed the need for new biomarkers that can enhance the accuracy of CKD detection[137,209,210]. Table 3 shows biomarkers evaluated in CKD. Current practices utilize two categories of biomarkers for CKD, those that estimate GFR and those that indicate damage to a specific renal function (albuminuria). Similarly, the search for new biomarkers has two directions, indicators of GFR which are routinely assayed in the serum[211-219] and indicators of specific types of kidney injury which are assayed in the urine or serum[220-227].
Table 3.
New biomarkers for chronic kidney disease
| Biomarker | Ref. |
| Biomarkers for GFR | |
| Symmetrical dimethylarginine | [212,213] |
| Beta-trace protein | [214,216,217] |
| β2-microglobulin | [215-218] |
| Galectin-3 | [219] |
| Biomarkers for injury of renal tissue | |
| MicroRNA | [220,221] |
| Soluble urokinase-type plasminogen activator receptor | [209,222,223] |
| Proteomics | [224,225] |
| Gelatinase-associated lipocalin | [226,227] |
GFR: Glomerular filtration rate.
Demographic and clinical associations of creatinine and newer GFR biomarkers, including β-trace protein, β2-microglobulin, and cystatin C, differ[216]. In specific patient groups, newer GFR biomarkers may offer specific advantages. For example, cystatin C and β2-microglobulin are more sensitive indicators of decreased GFR than creatinine in critically ill children[215], while β-trace protein and β2-microglobulin serum levels may identify additional risk factors in patients with CKD[218]. However, the role of these newer biomarkers in establishing the diagnosis of CKD will need further investigation. For example, eGFR estimates from equations based on β-trace protein or β2-microglobulin were found to be less accurate that the creatinine-based or creatinine/cystatin-based CKD EPI equations[217].
In patients with ESKD, serum levels of biomarkers eliminated poorly by dialytic techniques can provide more accurate estimates of residual GFR than the estimation as the average of renal creatinine and urea clearances[228]. Residual renal clearance may be estimated by pre-dialysis β2-microglobulin or β-trace protein concentrations in patients receiving high flux hemodialysis or hemodiafiltration[229,230]. A preliminary report identified four metabolites, including acetyl-threonine, pseudouridine, acetyl-alanine, and myo-inositol with higher correlation values with measured GFR than serum creatinine levels[231]. eGFR formulas containing multiple metabolites appear to be more accurate than creatinine-based formulas in estimating GFR[232-234]. Despite the promise that this strategy holds, it should be noted that even system-biology combined markers derived from a rational process only moderately improve performance relative to clinical and standard laboratory evaluations. Also, the cost of measuring these biomarkers vs measuring GFR by a standard method is an issue that will be raised.
Regardless of the potential advantages of eGFR estimates from new biomarkers, these estimates will have some of the limitations discussed above. The main progress in the diagnosis of CKD and the prediction of its course is expected to result from introduction of biomarkers of renal tissue damage in clinical practice[221]. Note that biomarkers of renal tissue injury assayed in serum may also provide estimates of GFR and have the potential of providing clues about the histologic diagnosis of CKD[235] and combination of clinical predictors and biomarkers can predict progression of CKD in specific patient groups, e.g., subjects with type 2 diabetes mellitus[236].
CONCLUSION
The diagnosis and staging of CKD based on estimates of GFR from serum creatinine and cystatin C concentrations represents a major step in the diagnosis of CKD and in following its course, but has significant limitations. The main limitations of this methodology are its low discriminatory power in establishing the presence or absence of early CKD in individuals and its unsatisfactory performance in predicting the course of CKD. The direction of Research in this field is currently towards identifying new biomarkers that either are superior indicators of GFR, or indicate early injury of the renal tissue. The last group of biomarkers has the potential of leading to improved early detection of CKD.
ACKNOWLEDGMENTS
The authors express their appreciation to the Research Service of the Raymond G. Murphy VA Medical Center for its support of this work.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Dominic S C Raj is supported by RO1 DK073665-01A1, 1U01DK099914-01 and IU01DK09924-01 from the National Institutes of Health. Joseph I Shapiro is supported by HL105649, HL071556 and HL109015 from the National Institutes of Health. The rest of the authors declare no conflicts of interest. This report was not supported by a grant.
Peer-review started: February 6, 2017
First decision: March 6, 2017
Article in press: May 31, 2017
P- Reviewer: Bellomo G, Kiykim A, Raikou VD, Zuo L S- Editor: Ji FF L- Editor: A E- Editor: Li D
Contributor Information
Ahmed Alaini, Division of Nephrology, Department of Medicine, University of New Mexico School of Medicine, Albuquerque, NM 87131, United States.
Deepak Malhotra, Division of Nephrology, Department of Medicine, University of Toledo School of Medicine, Toledo, OH 43614-5809, United States.
Helbert Rondon-Berrios, Renal and Electrolyte Division, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15260, United States.
Christos P Argyropoulos, Division of Nephrology, Department of Medicine, University of New Mexico School of Medicine, Albuquerque, NM 87131, United States.
Zeid J Khitan, Division of Nephrology, Department of Medicine, Joan C. Edwards School of Medicine, Huntington, WV 25701, United States.
Dominic S C Raj, Division of Nephrology, Department of Medicine, George Washington University, Washington, DC 20037, United States.
Mark Rohrscheib, Division of Nephrology, Department of Medicine, University of New Mexico School of Medicine, Albuquerque, NM 87131, United States.
Joseph I Shapiro, Marshall University Joan C. Edwards School of Medicine, Huntington, WV 25701, United States.
Antonios H Tzamaloukas, Nephrology Section, Medicine Service, Raymond G. Murphy VA Medical Center, Albuquerque, NM 87108, United States; Department of Medicine, University of New Mexico School of Medicine, Albuquerque, NM 87108, United States. antonios.tzamaloukas@va.gov.
References
- 1.Said A, Desai C, Lerma EV. Chronic kidney disease. Dis Mon. 2015;61:374–377. doi: 10.1016/j.disamonth.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Pereira ER, Pereira Ade C, Andrade GB, Naghettini AV, Pinto FK, Batista SR, Marques SM. Prevalence of chronic renal disease in adults attended by the family health strategy. J Bras Nefrol. 2016;38:22–30. doi: 10.5935/0101-2800.20160005. [DOI] [PubMed] [Google Scholar]
- 4.Moţa E, Popa SG, Moţa M, Mitrea A, Penescu M, Tuţă L, Serafinceanu C, Hâncu N, Gârneaţă L, Verzan C, et al. Prevalence of chronic kidney disease and its association with cardio-metabolic risk factors in the adult Romanian population: the PREDATORR study. Int Urol Nephrol. 2015;47:1831–1838. doi: 10.1007/s11255-015-1109-7. [DOI] [PubMed] [Google Scholar]
- 5.Zdrojewski Ł, Zdrojewski T, Rutkowski M, Bandosz P, Król E, Wyrzykowski B, Rutkowski B. Prevalence of chronic kidney disease in a representative sample of the Polish population: results of the NATPOL 2011 survey. Nephrol Dial Transplant. 2016;31:433–439. doi: 10.1093/ndt/gfv369. [DOI] [PubMed] [Google Scholar]
- 6.da Silva LS, Cotta RM, Moreira TR, da Silva RG, Rosa CO, Machado JC, da Silva LS, Bastos MA. Hidden prevalence of chronic kidney disease in hypertensive patients: the strategic role of primary health care. Public Health. 2016;140:250–257. doi: 10.1016/j.puhe.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Gargiulo R, Suhail F, Lerma EV. Cardiovascular disease and chronic kidney disease. Dis Mon. 2015;61:403–413. doi: 10.1016/j.disamonth.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, Islam N, Bravo RF, Aleckovic-Halilovic M, Zou H, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4:e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 9.Unger ED, Dubin RF, Deo R, Daruwalla V, Friedman JL, Medina C, Beussink L, Freed BH, Shah SJ. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2016;18:103–112. doi: 10.1002/ejhf.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider C, Coll B, Jick SS, Meier CR. Doubling of serum creatinine and the risk of cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes mellitus: a cohort study. Clin Epidemiol. 2016;8:177–184. doi: 10.2147/CLEP.S107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moody WE, Ferro CJ, Edwards NC, Chue CD, Lin EL, Taylor RJ, Cockwell P, Steeds RP, Townend JN; CRIB-Donor Study Investigators. Cardiovascular Effects of Unilateral Nephrectomy in Living Kidney Donors. Hypertension. 2016;67:368–377. doi: 10.1161/HYPERTENSIONAHA.115.06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mok Y, Matsushita K, Sang Y, Ballew SH, Grams M, Shin SY, Jee SH, Coresh J. Association of Kidney Disease Measures with Cause-Specific Mortality: The Korean Heart Study. PLoS One. 2016;11:e0153429. doi: 10.1371/journal.pone.0153429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adejumo OA, Akinbodewa AA, Okaka EI, Alli OE, Ibukun IF. Chronic kidney disease in Nigeria: Late presentation is still the norm. Niger Med J. 2016;57:185–189. doi: 10.4103/0300-1652.184072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M, Sequist TD; National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Practical Approach to Detection and Management of Chronic Kidney Disease for the Primary Care Clinician. Am J Med. 2016;129:153–162.e7. doi: 10.1016/j.amjmed.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Pietrement C, Allain-Launay E, Bacchetta J, Bertholet-Thomas A, Dubourg L, Harambat J, Vieux R, Deschênes G; Groupe maladie rénale chronique de la Société de néphrologie pédiatrique, membre de la filière de santé ORKID. [Diagnosis and management of chronic kidney disease in children: Guidelines of the French Society of Pediatric Nephrology] Arch Pediatr. 2016;23:1191–1200. doi: 10.1016/j.arcped.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Wright Nunes J, Roney M, Kerr E, Ojo A, Fagerlin A. A diagnosis of chronic kidney disease: despite fears patients want to know early. Clin Nephrol. 2016;86:78–86. doi: 10.5414/CN108831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razmaria AA. JAMA PATIENT PAGE. Chronic Kidney Disease. JAMA. 2016;315:2248. doi: 10.1001/jama.2016.1426. [DOI] [PubMed] [Google Scholar]
- 18.Sutton AJ, Breheny K, Deeks J, Khunti K, Sharpe C, Ottridge RS, Stevens PE, Cockwell P, Kalra PA, Lamb EJ; eGFR-C study group. Methods Used in Economic Evaluations of Chronic Kidney Disease Testing - A Systematic Review. PLoS One. 2015;10:e0140063. doi: 10.1371/journal.pone.0140063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsides N, Keane DF, Lindley E, Mitra S. Technology innovation for patients with kidney disease. J Med Eng Technol. 2014;39:424–433. doi: 10.3109/03091902.2015.1088089. [DOI] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, et al. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72:1535–1542. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
- 26.Feng JF, Qiu L, Zhang L, Li XM, Yang YW, Zeng P, Guo XZ, Qin Y, Liu HC, Han XM, et al. Multicenter study of creatinine- and/or cystatin C-based equations for estimation of glomerular filtration rates in Chinese patients with chronic kidney disease. PLoS One. 2013;8:e57240. doi: 10.1371/journal.pone.0057240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Xie P, Huang JM, Qu Y, Zhang F, Wei LG, Fu P, Huang XJ. The new Asian modified CKD-EPI equation leads to more accurate GFR estimation in Chinese patients with CKD. Int Urol Nephrol. 2016;48:2077–2081. doi: 10.1007/s11255-016-1386-9. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo S, Yasuda Y, Imai E, Horio M. Current status of estimated glomerular filtration rate (eGFR) equations for Asians and an approach to create a common eGFR equation. Nephrology (Carlton) 2010;15 Suppl 2:45–48. doi: 10.1111/j.1440-1797.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 29.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S; Collaborators Developing the Japanese Equation for Estimated GFR. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61:197–203. doi: 10.1053/j.ajkd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chehade H, Cachat F, Jannot AS, Meyrat BJ, Mosig D, Bardy D, Parvex P, Girardin E. New combined serum creatinine and cystatin C quadratic formula for GFR assessment in children. Clin J Am Soc Nephrol. 2014;9:54–63. doi: 10.2215/CJN.00940113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, Fujita N, Akioka Y, Kaneko T, Honda M. Cystatin C-based equation for estimating glomerular filtration rate in Japanese children and adolescents. Clin Exp Nephrol. 2014;18:718–725. doi: 10.1007/s10157-013-0910-9. [DOI] [PubMed] [Google Scholar]
- 34.Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, Kuhlmann MK, Schuchardt M, Tölle M, Ziebig R, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 35.Maple-Brown LJ, Lawton PD, Hughes JT, Sharma SK, Jones GR, Ellis AG, Hoy W, Cass A, Macisaac RJ, Sinha AK, et al. Study Protocol--accurate assessment of kidney function in Indigenous Australians: aims and methods of the eGFR study. BMC Public Health. 2010;10:80. doi: 10.1186/1471-2458-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 37.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 38.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inker LA, Shafi T, Okparavero A, Tighiouart H, Eckfeldt JH, Katz R, Johnson WC, Dermond N, Tariq Z, Benayache I, et al. Effects of Race and Sex on Measured GFR: The Multi-Ethnic Study of Atherosclerosis. Am J Kidney Dis. 2016;68:743–751. doi: 10.1053/j.ajkd.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 41.van Deventer HE, Paiker JE, Katz IJ, George JA. A comparison of cystatin C- and creatinine-based prediction equations for the estimation of glomerular filtration rate in black South Africans. Nephrol Dial Transplant. 2011;26:1553–1558. doi: 10.1093/ndt/gfq621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grubb A, Nyman U, Björk J. Improved estimation of glomerular filtration rate (GFR) by comparison of eGFRcystatin C and eGFRcreatinine. Scand J Clin Lab Invest. 2012;72:73–77. doi: 10.3109/00365513.2011.634023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manzano-Fernández S, Flores-Blanco PJ, Pérez-Calvo JI, Ruiz-Ruiz FJ, Carrasco-Sánchez FJ, Morales-Rull JL, Galisteo-Almeda L, Pascual-Figal D, Valdes M, Januzzi JL. Comparison of risk prediction with the CKD-EPI and MDRD equations in acute decompensated heart failure. J Card Fail. 2013;19:583–591. doi: 10.1016/j.cardfail.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Masson I, Maillard N, Tack I, Thibaudin L, Dubourg L, Delanaye P, Cavalier E, Bonneau C, Kamar N, Morelon E, et al. GFR estimation using standardized cystatin C in kidney transplant recipients. Am J Kidney Dis. 2013;61:279–284. doi: 10.1053/j.ajkd.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Peralta CA, Lee A, Odden MC, Lopez L, Zeki Al Hazzouri A, Neuhaus J, Haan MN. Association between chronic kidney disease detected using creatinine and cystatin C and death and cardiovascular events in elderly Mexican Americans: the Sacramento Area Latino Study on Aging. J Am Geriatr Soc. 2013;61:90–95. doi: 10.1111/jgs.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westland R, Abraham Y, Bökenkamp A, Stoffel-Wagner B, Schreuder MF, van Wijk JA. Precision of estimating equations for GFR in children with a solitary functioning kidney: the KIMONO study. Clin J Am Soc Nephrol. 2013;8:764–772. doi: 10.2215/CJN.07870812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogacev KS, Pickering JW, Seiler S, Zawada AM, Emrich I, Fliser D, Heine GH. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation incorporating both cystatin C and creatinine best predicts individual risk: a cohort study in 444 patients with chronic kidney disease. Nephrol Dial Transplant. 2014;29:348–355. doi: 10.1093/ndt/gft422. [DOI] [PubMed] [Google Scholar]
- 49.Fan L, Inker LA, Rossert J, Froissart M, Rossing P, Mauer M, Levey AS. Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrol Dial Transplant. 2014;29:1195–1203. doi: 10.1093/ndt/gft509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horio M, Yasuda Y, Kaimori J, Ichimaru N, Kakuta Y, Isaka Y, Matsuo S, Takahara S. Performance of the Japanese glomerular filtration rate equation based on standardized serum cystatin C in potential kidney donors. Transplant Proc. 2014;46:314–317. doi: 10.1016/j.transproceed.2013.11.151. [DOI] [PubMed] [Google Scholar]
- 51.Tsujita M, Goto N, Yamamoto T, Hiramitsu T, Nanmoku K, Inaguma D, Takeda A, Kobayashi T, Tominaga Y, Morozumi K, et al. How to estimate kidney function in kidney transplant recipients with mild to moderate kidney impairment: comparison of estimated glomerular filtration (eGFR) values between creatinine-based GFR equations and cystatin C-based GFR equations for Japanese population. Clin Exp Nephrol. 2014;18:130–134. doi: 10.1007/s10157-013-0811-y. [DOI] [PubMed] [Google Scholar]
- 52.Ye X, Wei L, Pei X, Zhu B, Wu J, Zhao W. Application of creatinine- and/or cystatin C-based glomerular filtration rate estimation equations in elderly Chinese. Clin Interv Aging. 2014;9:1539–1549. doi: 10.2147/CIA.S68801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doğaner YÇ, Aydoğan Ü, Rohrer JE, Aydoğdu A, Çaycı T, Barçın C, Sağlam K. Comparison of estimated GFR equations based on serum cystatin C alone and in combination with serum creatinine in patients with coronary artery disease. Anatol J Cardiol. 2015;15:571–576. doi: 10.5152/akd.2014.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan L, Levey AS, Gudnason V, Eiriksdottir G, Andresdottir MB, Gudmundsdottir H, Indridason OS, Palsson R, Mitchell G, Inker LA. Comparing GFR Estimating Equations Using Cystatin C and Creatinine in Elderly Individuals. J Am Soc Nephrol. 2015;26:1982–1989. doi: 10.1681/ASN.2014060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim BS, Lee YK, Choi HY, Choi SO, Shin SK, Ha SK, Lee KW, Kim YW, Kim YL, Yasuda Y, et al. Is the new GFR equation using inulin clearance a more accurate method for Asian patients? Clin Nephrol. 2015;84:331–338. doi: 10.5414/CN108496. [DOI] [PubMed] [Google Scholar]
- 56.Otaki Y, Takahashi H, Watanabe T, Yamaura G, Funayama A, Arimoto T, Shishido T, Miyamoto T, Kubota I. Cystatin C-based eGFR is a superior prognostic parameter to creatinine-based eGFR in post-endovascular therapy peripheral artery disease patients. Circ J. 2015;79:2480–2486. doi: 10.1253/circj.CJ-15-0762. [DOI] [PubMed] [Google Scholar]
- 57.Almeida I, Caetano F, Barra S, Madeira M, Mota P, Leitão-Marques A. Estimating glomerular filtration rate in acute coronary syndromes: Different equations, different mortality risk prediction. Eur Heart J Acute Cardiovasc Care. 2016;5:223–230. doi: 10.1177/2048872615576219. [DOI] [PubMed] [Google Scholar]
- 58.Canales MT, Blackwell T, Ishani A, Taylor BC, Hart A, Barrett-Connor E, Lewis C, Beyth RJ, Stone K, Ensrud KE; Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. Estimated GFR and Mortality in Older Men: Are All eGFR Formulae Equal. Am J Nephrol. 2016;43:325–333. doi: 10.1159/000445757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Funakoshi Y, Fujiwara Y, Kiyota N, Mukohara T, Shimada T, Toyoda M, Imamura Y, Chayahara N, Tomioka H, Umezu M, et al. Validity of new methods to evaluate renal function in cancer patients treated with cisplatin. Cancer Chemother Pharmacol. 2016;77:281–288. doi: 10.1007/s00280-016-2966-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helmersson-Karlqvist J, Ärnlöv J, Larsson A. Cystatin C-based glomerular filtration rate associates more closely with mortality than creatinine-based or combined glomerular filtration rate equations in unselected patients. Eur J Prev Cardiol. 2016;23:1649–1657. doi: 10.1177/2047487316642086. [DOI] [PubMed] [Google Scholar]
- 61.Selistre L, Rabilloud M, Cochat P, de Souza V, Iwaz J, Lemoine S, Beyerle F, Poli-de-Figueiredo CE, Dubourg L. Comparison of the Schwartz and CKD-EPI Equations for Estimating Glomerular Filtration Rate in Children, Adolescents, and Adults: A Retrospective Cross-Sectional Study. PLoS Med. 2016;13:e1001979. doi: 10.1371/journal.pmed.1001979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M, Xu G, Ling L, Niu J, Lu T, Du X, Gu Y. Performance of the creatinine and cystatin C-based equations for estimation of GFR in Chinese patients with chronic kidney disease. Clin Exp Nephrol. 2017;21:236–246. doi: 10.1007/s10157-016-1273-9. [DOI] [PubMed] [Google Scholar]
- 63.Ye X, Liu X, Song D, Zhang X, Zhu B, Wei L, Pei X, Wu J, Lou T, Zhao W. Estimating glomerular filtration rate by serum creatinine or/and cystatin C equations: An analysis of multi-centre Chinese subjects. Nephrology (Carlton) 2016;21:372–378. doi: 10.1111/nep.12636. [DOI] [PubMed] [Google Scholar]
- 64.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 65.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG; Chronic Renal Insufficiency Cohort (CRIC) Study Group. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ensrud KE, Parimi N, Fink HA, Ishani A, Taylor BC, Steffes M, Cauley JA, Lewis CE, Orwoll ES; Osteoporotic Fractures in Men Study Group. Estimated GFR and risk of hip fracture in older men: comparison of associations using cystatin C and creatinine. Am J Kidney Dis. 2014;63:31–39. doi: 10.1053/j.ajkd.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, et al. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63:236–243. doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yaffe K, Kurella-Tamura M, Ackerson L, Hoang TD, Anderson AH, Duckworth M, Go AS, Krousel-Wood M, Kusek JW, Lash JP, et al. Higher levels of cystatin C are associated with worse cognitive function in older adults with chronic kidney disease: the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2014;62:1623–1629. doi: 10.1111/jgs.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman M, Yang W, Akkina S, Alper A, Anderson AH, Appel LJ, He J, Raj DS, Schelling J, Strauss L, et al. Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin J Am Soc Nephrol. 2014;9:1190–1198. doi: 10.2215/CJN.09320913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parsh J, Seth M, Aronow H, Dixon S, Heung M, Mehran R, Gurm HS. Choice of Estimated Glomerular Filtration Rate Equation Impacts Drug-Dosing Recommendations and Risk Stratification in Patients With Chronic Kidney Disease Undergoing Percutaneous Coronary Interventions. J Am Coll Cardiol. 2015;65:2714–2723. doi: 10.1016/j.jacc.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 73.Hebert SA, Molony DA. ACP Journal Club: the CKD-EPI equation for eGFR predicted adverse outcomes after PCI better than other equations. Ann Intern Med. 2015;163:JC12. doi: 10.7326/ACPJC-2015-163-10-012. [DOI] [PubMed] [Google Scholar]
- 74.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J Am Soc Nephrol. 2017;28:368–375. doi: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greene T, Teng CC, Inker LA, Redd A, Ying J, Woodward M, Coresh J, Levey AS. Utility and validity of estimated GFR-based surrogate time-to-event end points in CKD: a simulation study. Am J Kidney Dis. 2014;64:867–879. doi: 10.1053/j.ajkd.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 76.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–835. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 77.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 78.Zhang JJ, Yu GZ, Zheng ZH, Liu YF, Du YY, Quan SX, Liu YJ, Lv JC, Zhang H. Dividing CKD stage 3 into G3a and G3b could better predict the prognosis of IgA nephropathy. PLoS One. 2017;12:e0175828. doi: 10.1371/journal.pone.0175828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, Poon A, Jenkins MA, Ratnaike SI, Power DA, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49:1686–1689. doi: 10.1007/s00125-006-0275-7. [DOI] [PubMed] [Google Scholar]
- 80.Borges RL, Hirota AH, Quinto BM, Ribeiro AB, Zanella MT, Batista MC. Is cystatin C a useful marker in the detection of diabetic kidney disease? Nephron Clin Pract. 2010;114:c127–c134. doi: 10.1159/000254385. [DOI] [PubMed] [Google Scholar]
- 81.Iliadis F, Didangelos T, Ntemka A, Makedou A, Moralidis E, Gotzamani-Psarakou A, Kouloukourgiotou T, Grekas D. Glomerular filtration rate estimation in patients with type 2 diabetes: creatinine- or cystatin C-based equations? Diabetologia. 2011;54:2987–2994. doi: 10.1007/s00125-011-2307-1. [DOI] [PubMed] [Google Scholar]
- 82.Oh SJ, Lee JI, Ha WC, Jeong SH, Yim HW, Son HS, Sohn TS. Comparison of cystatin C- and creatinine-based estimation of glomerular filtration rate according to glycaemic status in Type 2 diabetes. Diabet Med. 2012;29:e121–e125. doi: 10.1111/j.1464-5491.2012.03628.x. [DOI] [PubMed] [Google Scholar]
- 83.Schöttker B, Herder C, Müller H, Brenner H, Rothenbacher D. Clinical utility of creatinine- and cystatin C-based definition of renal function for risk prediction of primary cardiovascular events in patients with diabetes. Diabetes Care. 2012;35:879–886. doi: 10.2337/dc11-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lamb EJ, Brettell EA, Cockwell P, Dalton N, Deeks JJ, Harris K, Higgins T, Kalra PA, Khunti K, Loud F, et al. The eGFR-C study: accuracy of glomerular filtration rate (GFR) estimation using creatinine and cystatin C and albuminuria for monitoring disease progression in patients with stage 3 chronic kidney disease--prospective longitudinal study in a multiethnic population. BMC Nephrol. 2014;15:13. doi: 10.1186/1471-2369-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maahs DM, Bushman L, Kerr B, Ellis SL, Pyle L, McFann K, Bouffard A, Bishop FK, Nguyen N, Anderson PL. A practical method to measure GFR in people with type 1 diabetes. J Diabetes Complications. 2014;28:667–673. doi: 10.1016/j.jdiacomp.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Tsai CW, Grams ME, Inker LA, Coresh J, Selvin E. Cystatin C- and creatinine-based estimated glomerular filtration rate, vascular disease, and mortality in persons with diabetes in the U.S. Diabetes Care. 2014;37:1002–1008. doi: 10.2337/dc13-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Avinash S, Singh VP, Agarwal AK, Chatterjee S, Arya V. Identification and Stratification of Diabetic Kidney Disease Using Serum Cystatin C and Serum Creatinine Based Estimating Equations in Type 2 Diabetes: A Comparative Analysis. J Assoc Physicians India. 2015;63:28–35. [PubMed] [Google Scholar]
- 88.Einhorn D, Mende CW. Combining creatinine-based egfr with cystatin c-based egfr to better assess renal function in patients with diabetes and chronic kidney disease 3a: implications for drug selection and dosage in type 2 diabetes. Endocr Pract. 2015;21:1301–1302. doi: 10.4158/EP15821.ED. [DOI] [PubMed] [Google Scholar]
- 89.MacIsaac RJ, Ekinci EI, Premaratne E, Lu ZX, Seah JM, Li Y, Boston R, Ward GM, Jerums G. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation does not improve the underestimation of Glomerular Filtration Rate (GFR) in people with diabetes and preserved renal function. BMC Nephrol. 2015;16:198. doi: 10.1186/s12882-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin CH, Chang YC, Chuang LM. Early detection of diabetic kidney disease: Present limitations and future perspectives. World J Diabetes. 2016;7:290–301. doi: 10.4239/wjd.v7.i14.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mende C, Katz A. Cystatin C- and Creatinine-Based Estimates of Glomerular Filtration Rate in Dapagliflozin Phase 3 Clinical Trials. Diabetes Ther. 2016;7:139–151. doi: 10.1007/s13300-016-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsuda A, Ishimura E, Uedono H, Yasumoto M, Ichii M, Nakatani S, Mori K, Uchida J, Emoto M, Nakatani T, et al. Comparison of the Estimated Glomerular Filtration Rate (eGFR) in Diabetic Patients, Non-Diabetic Patients and Living Kidney Donors. Kidney Blood Press Res. 2016;41:40–47. doi: 10.1159/000368545. [DOI] [PubMed] [Google Scholar]
- 93.Wearne N, Okpechi IG. HIV-associated renal disease - an overview. Clin Nephrol. 2016;86:41–47. doi: 10.5414/CNP86S117. [DOI] [PubMed] [Google Scholar]
- 94.Diana NE, Naicker S. Update on current management of chronic kidney disease in patients with HIV infection. Int J Nephrol Renovasc Dis. 2016;9:223–234. doi: 10.2147/IJNRD.S93887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Achhra AC, Nugent M, Mocroft A, Ryom L, Wyatt CM. Chronic Kidney Disease and Antiretroviral Therapy in HIV-Positive Individuals: Recent Developments. Curr HIV/AIDS Rep. 2016;13:149–157. doi: 10.1007/s11904-016-0315-y. [DOI] [PubMed] [Google Scholar]
- 96.Mauss S, Berger F, Kuschak D, Henke J, Hegener P, Wolf E, Schauseil S, Schmutz G. Cystatin C as a marker of renal function is affected by HIV replication leading to an underestimation of kidney function in HIV patients. Antivir Ther. 2008;13:1091–1095. [PubMed] [Google Scholar]
- 97.Estrella MM, Parekh RS, Astor BC, Bolan R, Evans RW, Palella FJ, Jacobson LP. Chronic kidney disease and estimates of kidney function in HIV infection: a cross-sectional study in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2011;57:380–386. doi: 10.1097/QAI.0b013e318222f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Praditpornsilpa K, Avihingsanon A, Chaiwatanarat T, Chaiyahong P, Wongsabut J, Ubolyam S, Chulakadabba A, Avihingsanon Y, Ruxrungtham K, Tunsanga K, et al. Comparisons between validated estimated glomerular filtration rate equations and isotopic glomerular filtration rate in HIV patients. AIDS. 2012;26:1781–1788. doi: 10.1097/QAD.0b013e328356480d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vrouenraets SM, Fux CA, Wit FW, Garcia EF, Brinkman K, Hoek FJ, van Straalen JP, Furrer H, Krediet RT, Reiss P; Prepare Study Group. A comparison of measured and estimated glomerular filtration rate in successfully treated HIV-patients with preserved renal function. Clin Nephrol. 2012;77:311–320. doi: 10.5414/cn107214. [DOI] [PubMed] [Google Scholar]
- 100.Wyatt CM, Schwartz GJ, Owino Ong’or W, Abuya J, Abraham AG, Mboku C, M’mene LB, Koima WJ, Hotta M, Maier P, et al. Estimating kidney function in HIV-infected adults in Kenya: comparison to a direct measure of glomerular filtration rate by iohexol clearance. PLoS One. 2013;8:e69601. doi: 10.1371/journal.pone.0069601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanagisawa N, Sasaki S, Suganuma A, Imamura A, Ajisawa A, Ando M. Comparison of cystatin C and creatinine to determine the incidence of composite adverse outcomes in HIV-infected individuals. J Infect Chemother. 2015;21:84–89. doi: 10.1016/j.jiac.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 102.Brown CS, Kashani KB, Clain JM, Frazee EN. Cystatin C Falsely Underestimated GFR in a Critically Ill Patient with a New Diagnosis of AIDS. Case Rep Nephrol. 2016;2016:9349280. doi: 10.1155/2016/9349280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adachi M, Tanaka A, Aiso M, Takamori Y, Takikawa H. Benefit of cystatin C in evaluation of renal function and prediction of survival in patients with cirrhosis. Hepatol Res. 2015;45:1299–1306. doi: 10.1111/hepr.12508. [DOI] [PubMed] [Google Scholar]
- 104.Beben T, Rifkin DE. GFR Estimating Equations and Liver Disease. Adv Chronic Kidney Dis. 2015;22:337–342. doi: 10.1053/j.ackd.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cholongitas E, Ioannidou M, Goulis I, Chalevas P, Ntogramatzi F, Athanasiadou Z, Notopoulos A, Alevroudis M, Sinakos E, Akriviadis E. Comparison of creatinine and cystatin formulae with (51) Chromium-ethylenediaminetetraacetic acid glomerular filtration rate in patients with decompensated cirrhosis. J Gastroenterol Hepatol. 2017;32:191–198. doi: 10.1111/jgh.13446. [DOI] [PubMed] [Google Scholar]
- 106.Zamora E, Lupón J, de Antonio M, Vila J, Galán A, Gastelurrutia P, Urrutia A, Bayes-Genis A. Limited value of cystatin-C over estimated glomerular filtration rate for heart failure risk stratification. PLoS One. 2012;7:e51234. doi: 10.1371/journal.pone.0051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Åkerblom A, Helmersson-Karlqvist J, Flodin M, Larsson A. Comparison between Cystatin C- and Creatinine-Estimated Glomerular Filtration Rate in Cardiology Patients. Cardiorenal Med. 2015;5:289–296. doi: 10.1159/000437273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eriksen BO, Løchen ML, Arntzen KA, Bertelsen G, Winther Eilertsen BA, von Hanno T, Herder M, Jenssen TG, Mathisen UD, Melsom T, et al. Estimated and Measured GFR Associate Differently with Retinal Vasculopathy in the General Population. Nephron. 2015;131:175–184. doi: 10.1159/000441092. [DOI] [PubMed] [Google Scholar]
- 109.Melsom T, Fuskevåg OM, Mathisen UD, Strand H, Schei J, Jenssen T, Solbu M, Eriksen BO. Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol. 2015;41:7–15. doi: 10.1159/000371557. [DOI] [PubMed] [Google Scholar]
- 110.Kolsrud O, Ricksten SE, Holmberg E, Felldin M, Karason K, Hammarsten O, Samuelsson O, Dellgren G. Measured and not estimated glomerular filtration rate should be used to assess renal function in heart transplant recipients. Nephrol Dial Transplant. 2016;31:1182–1189. doi: 10.1093/ndt/gfv338. [DOI] [PubMed] [Google Scholar]
- 111.Keddis MT, Amer H, Voskoboev N, Kremers WK, Rule AD, Lieske JC. Creatinine-Based and Cystatin C-Based GFR Estimating Equations and Their Non-GFR Determinants in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2016;11:1640–1649. doi: 10.2215/CJN.11741115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Issa N, Kukla A, Jackson S, Riad SM, Foster MC, Matas AJ, Eckfeldt JH, Ibrahim HN. Comparison of cystatin C and creatinine-based equations for GFR estimation after living kidney donation. Transplantation. 2014;98:871–877. doi: 10.1097/TP.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 113.Hafeez AR, Idrees MK, Akhtar SF. Accuracy of GFR estimation formula in determination of glomerular filtration rate in kidney donors: Comparison with 24 h urine creatinine clearance. Saudi J Kidney Dis Transpl. 2016;27:320–325. doi: 10.4103/1319-2442.178551. [DOI] [PubMed] [Google Scholar]
- 114.Santos J, Martins LS. Estimating glomerular filtration rate in kidney transplantation: Still searching for the best marker. World J Nephrol. 2015;4:345–353. doi: 10.5527/wjn.v4.i3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Erlandsen EJ, Hansen RM, Randers E, Petersen LE, Abrahamsen J, Johannesen IL. Estimating the glomerular filtration rate using serum cystatin C levels in patients with spinal cord injuries. Spinal Cord. 2012;50:778–783. doi: 10.1038/sc.2012.52. [DOI] [PubMed] [Google Scholar]
- 116.Tetsuka S, Morita M, Ikeguchi K, Nakano I. Utility of cystatin C for renal function in amyotrophic lateral sclerosis. Acta Neurol Scand. 2013;128:386–390. doi: 10.1111/ane.12134. [DOI] [PubMed] [Google Scholar]
- 117.Carlier M, Dumoulin A, Janssen A, Picavet S, Vanthuyne S, Van Eynde R, Vanholder R, Delanghe J, De Schoenmakere G, De Waele JJ, et al. Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med. 2015;41:427–435. doi: 10.1007/s00134-014-3641-9. [DOI] [PubMed] [Google Scholar]
- 118.Rombach SM, Baas MC, ten Berge IJ, Krediet RT, Bemelman FJ, Hollak CE. The value of estimated GFR in comparison to measured GFR for the assessment of renal function in adult patients with Fabry disease. Nephrol Dial Transplant. 2010;25:2549–2556. doi: 10.1093/ndt/gfq108. [DOI] [PubMed] [Google Scholar]
- 119.Vupputuri S, Fox CS, Coresh J, Woodward M, Muntner P. Differential estimation of CKD using creatinine- versus cystatin C-based estimating equations by category of body mass index. Am J Kidney Dis. 2009;53:993–1001. doi: 10.1053/j.ajkd.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mysliwiec P, Jasiewicz P, Hady HR, Choromanska B, Mroczko B, Mysliwiec H, Siemiatkowski A, Dadan J, Szmitkowski M. Creatinine or cystatin C - which is a better index of renal function in morbid obesity? Adv Med Sci. 2013;58:376–381. doi: 10.2478/ams-2013-0020. [DOI] [PubMed] [Google Scholar]
- 121.Sharma D, Hawkins M, Abramowitz MK. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clin J Am Soc Nephrol. 2014;9:2079–2088. doi: 10.2215/CJN.02140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lemoine S, Panaye M, Pelletier C, Bon C, Juillard L, Dubourg L, Guebre-Egziabher F. Cystatin C-Creatinine Based Glomerular Filtration Rate Equation in Obese Chronic Kidney Disease Patients: Impact of Deindexation and Gender. Am J Nephrol. 2016;44:63–70. doi: 10.1159/000447365. [DOI] [PubMed] [Google Scholar]
- 123.Wetmore JB, Palsson R, Belmont JM, Sigurdsson G, Franzson L, Indridason OS. Discrepancies between creatinine- and cystatin C-based equations: implications for identification of chronic kidney disease in the general population. Scand J Urol Nephrol. 2010;44:242–250. doi: 10.3109/00365591003709450. [DOI] [PubMed] [Google Scholar]
- 124.Sharma AP, Yasin A, Garg AX, Filler G. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol. 2011;6:1599–1608. doi: 10.2215/CJN.10161110. [DOI] [PubMed] [Google Scholar]
- 125.DU X, Liu L, Hu B, Wang F, Wan X, Jiang L, Zhang R, Cao C. Is the Chronic Kidney Disease Epidemiology Collaboration four-level race equation better than the cystatin C equation? Nephrology (Carlton) 2012;17:407–414. doi: 10.1111/j.1440-1797.2012.01568.x. [DOI] [PubMed] [Google Scholar]
- 126.Nakata J, Ohsawa I, Onda K, Tanimoto M, Kusaba G, Takeda Y, Kobayashi N, Asanuma K, Tanaka Y, Sato M, et al. Risk of overestimation of kidney function using GFR-estimating equations in patients with low inulin clearance. J Clin Lab Anal. 2012;26:248–253. doi: 10.1002/jcla.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pei X, Liu Q, He J, Bao L, Yan C, Wu J, Zhao W. Are cystatin C-based equations superior to creatinine-based equations for estimating GFR in Chinese elderly population? Int Urol Nephrol. 2012;44:1877–1884. doi: 10.1007/s11255-012-0278-x. [DOI] [PubMed] [Google Scholar]
- 128.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int. 2013;83:1169–1176. doi: 10.1038/ki.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lujambio I, Sottolano M, Luzardo L, Robaina S, Krul N, Thijs L, Carusso F, da Rosa A, Ríos AC, Olascoaga A, et al. Estimation of Glomerular Filtration Rate Based on Serum Cystatin C versus Creatinine in a Uruguayan Population. Int J Nephrol. 2014;2014:837106. doi: 10.1155/2014/837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teo BW, Sabanayagam C, Liao J, Toh QC, Saw S, Wong TY, Sethi S. Comparison of CKD-EPI Cystatin C and Creatinine Glomerular Filtration Rate Estimation Equations in Asian Indians. Int J Nephrol. 2014;2014:746497. doi: 10.1155/2014/746497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meeusen JW, Rule AD, Voskoboev N, Baumann NA, Lieske JC. Performance of cystatin C- and creatinine-based estimated glomerular filtration rate equations depends on patient characteristics. Clin Chem. 2015;61:1265–1272. doi: 10.1373/clinchem.2015.243030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Satchidanand N, Withiam-Leitch M, Dickinson M, Pace W, Bublitz-Emsermann C, Allen GM, Yang M, Vassalotti J, Arora P, Glasgow P, et al. Positive Predictive Value of a Single Assessment of Estimated GFR in the Diagnosis of Chronic Kidney Disease. South Med J. 2016;109:351–355. doi: 10.14423/SMJ.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, Chodick G, Collins AJ, Djurdjev O, Elley CR, et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 135.Rule AD, Glassock RJ. GFR estimating equations: getting closer to the truth? Clin J Am Soc Nephrol. 2013;8:1414–1420. doi: 10.2215/CJN.01240213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simetić L, Zibar L, Drmić S, Begić I, Serić V. Creatinine Clearance and Estimated Glomerular Filtration Rate--When are they Interchangeable. Coll Antropol. 2015;39:735–743. [PubMed] [Google Scholar]
- 137.Kadatz MJ, Lee ES, Levin A. Predicting Progression in CKD: Perspectives and Precautions. Am J Kidney Dis. 2016;67:779–786. doi: 10.1053/j.ajkd.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 138.Seegmiller JC, Burns BE, Schinstock CA, Lieske JC, Larson TS. Discordance Between Iothalamate and Iohexol Urinary Clearances. Am J Kidney Dis. 2016;67:49–55. doi: 10.1053/j.ajkd.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 139.Levey AS, Inker LA. GFR as the “Gold Standard”: Estimated, Measured, and True. Am J Kidney Dis. 2016;67:9–12. doi: 10.1053/j.ajkd.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 140.Pasala S, Carmody JB. How to use… serum creatinine, cystatin C and GFR. Arch Dis Child Educ Pract Ed. 2017;102:37–43. doi: 10.1136/archdischild-2016-311062. [DOI] [PubMed] [Google Scholar]
- 141.Rule AD, Kremers WK. What Is the Correct Approach for Comparing GFR by Different Methods across Levels of GFR? Clin J Am Soc Nephrol. 2016;11:1518–1521. doi: 10.2215/CJN.07530716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neves J, Martins MR, Vilhena J, Neves J, Gomes S, Abelha A, Machado J, Vicente H. A Soft Computing Approach to Kidney Diseases Evaluation. J Med Syst. 2015;39:131. doi: 10.1007/s10916-015-0313-4. [DOI] [PubMed] [Google Scholar]
- 143.Kilbride HS, Stevens PE, Eaglestone G, Knight S, Carter JL, Delaney MP, Farmer CK, Irving J, O’Riordan SE, Dalton RN, et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61:57–66. doi: 10.1053/j.ajkd.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 144.Shaffi K, Uhlig K, Perrone RD, Ruthazer R, Rule A, Lieske JC, Navis G, Poggio ED, Inker LA, Levey AS. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis. 2014;63:1007–1018. doi: 10.1053/j.ajkd.2014.01.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rocco MV, Chapman A, Chertow GM, Cohen D, Chen J, Cutler JA, Diamond MJ, Freedman BI, Hawfield A, Judd E, et al. Chronic Kidney Disease Classification in Systolic Blood Pressure Intervention Trial: Comparison Using Modification of Diet in Renal Disease and CKD-Epidemiology Collaboration Definitions. Am J Nephrol. 2016;44:130–140. doi: 10.1159/000448722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bleiler RE, Schedl HP. Creatinine excretion: variability and relationships to diet and body size. J Lab Clin Med. 1962;59:945–955. [PubMed] [Google Scholar]
- 147.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 148.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fitch CD, Sinton DW. A study of creatine metabolism in diseases causing muscle wasting. J Clin Invest. 1964;43:444–452. doi: 10.1172/JCI104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 151.Aviram A, Ben-Ishay D, Chowers I, Czaczkes JW. Low plasma creatinine in diabetes mellitus. J Lab Clin Med. 1966;67:473–476. [PubMed] [Google Scholar]
- 152.Lee JS, Auyeung TW, Leung J, Kwok T, Leung PC, Woo J. The effect of diabetes mellitus on age-associated lean mass loss in 3153 older adults. Diabet Med. 2010;27:1366–1371. doi: 10.1111/j.1464-5491.2010.03118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rigalleau V, Beauvieux MC, Gonzalez C, Raffaitin C, Lasseur C, Combe C, Chauveau P, De la Faille R, Rigothier C, Barthe N, et al. Estimation of renal function in patients with diabetes. Diabetes Metab. 2011;37:359–366. doi: 10.1016/j.diabet.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 154.Tzamaloukas AH, Oreopoulos DG, Murata GH, Servilla K, Rao P, Din S, Malhotra D. The relation between nutrition indices and age in patients on continuous ambulatory peritoneal dialysis receiving similar small solute clearances. Int Urol Nephrol. 2001;32:449–458. doi: 10.1023/a:1017579105158. [DOI] [PubMed] [Google Scholar]
- 155.Tzamaloukas AH, Murata GH. A population-specific formula predicting creatinine excretion in continuous peritoneal dialysis. Perit Dial Int. 2002;22:67–72. [PubMed] [Google Scholar]
- 156.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 157.Mitch WE, Walser M. A proposed mechanism for reduced creatinine excretion in severe chronic renal failure. Nephron. 1978;21:248–254. doi: 10.1159/000181400. [DOI] [PubMed] [Google Scholar]
- 158.De Jong PE, de Jong-van Den Berg LT, Statius van Eps LW. The tubular reabsorption of phosphate in sickle-cell nephropathy. Clin Sci Mol Med. 1978;55:429–434. doi: 10.1042/cs0550429. [DOI] [PubMed] [Google Scholar]
- 159.Allon M. Renal abnormalities in sickle cell disease. Arch Intern Med. 1990;150:501–504. [PubMed] [Google Scholar]
- 160.Alvarez O, Lopez-Mitnik G, Zilleruelo G. Short-term follow-up of patients with sickle cell disease and albuminuria. Pediatr Blood Cancer. 2008;50:1236–1239. doi: 10.1002/pbc.21520. [DOI] [PubMed] [Google Scholar]
- 161.Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002;92:536–541. doi: 10.1159/000064083. [DOI] [PubMed] [Google Scholar]
- 162.Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD. Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int. 2011;80:1073–1079. doi: 10.1038/ki.2011.207. [DOI] [PubMed] [Google Scholar]
- 163.Hilbrands LB, Artz MA, Wetzels JF, Koene RA. Cimetidine improves the reliability of creatinine as a marker of glomerular filtration. Kidney Int. 1991;40:1171–1176. doi: 10.1038/ki.1991.331. [DOI] [PubMed] [Google Scholar]
- 164.German P, Liu HC, Szwarcberg J, Hepner M, Andrews J, Kearney BP, Mathias A. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr. 2012;61:32–40. doi: 10.1097/QAI.0b013e3182645648. [DOI] [PubMed] [Google Scholar]
- 165.Duncker D, Oswald H, Gardiwal A, Lüsebrink U, König T, Schreyer H, Klein G. Stable cystatin C serum levels confirm normal renal function in patients with dronedarone-associated increase in serum creatinine. J Cardiovasc Pharmacol Ther. 2013;18:109–112. doi: 10.1177/1074248412453873. [DOI] [PubMed] [Google Scholar]
- 166.Opravil M, Keusch G, Lüthy R. Pyrimethamine inhibits renal secretion of creatinine. Antimicrob Agents Chemother. 1993;37:1056–1060. doi: 10.1128/aac.37.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Burry HC, Dieppe PA. Apparent reduction of endogenous creatinine clearance by salicylate treatment. Br Med J. 1976;2:16–17. doi: 10.1136/bmj.2.6026.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Berg KJ, Gjellestad A, Nordby G, Rootwelt K, Djøseland O, Fauchald P, Mehl A, Narverud J, Talseth T. Renal effects of trimethoprim in ciclosporin- and azathioprine-treated kidney-allografted patients. Nephron. 1989;53:218–222. doi: 10.1159/000185747. [DOI] [PubMed] [Google Scholar]
- 169.Miller ML. 2016. Drug-induced myopathies; p. UpToDate. Available from: https://www.uptodate.com. [Google Scholar]
- 170.Xu Z, Gabaldon D, Wiggins B, Rondon-Berrios H, VanderJagt DJ, Tzamaloukas AH. Increase in serum creatinine in a patient on continuous peritoneal dialysis: potential mechanisms and management. Adv Perit Dial. 2012;28:32–36. [PubMed] [Google Scholar]
- 171.Peake M, Whiting M. Measurement of serum creatinine--current status and future goals. Clin Biochem Rev. 2006;27:173–184. [PMC free article] [PubMed] [Google Scholar]
- 172.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C serum concentrations underestimate glomerular filtration rate in renal transplant recipients. Clin Chem. 1999;45:1866–1868. [PubMed] [Google Scholar]
- 173.Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–2059. [PubMed] [Google Scholar]
- 174.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 175.Tkaczyk M, Nowicki M, Lukamowicz J. Increased cystatin C concentration in urine of nephrotic children. Pediatr Nephrol. 2004;19:1278–1280. doi: 10.1007/s00467-004-1566-1. [DOI] [PubMed] [Google Scholar]
- 176.Manetti L, Pardini E, Genovesi M, Campomori A, Grasso L, Morselli LL, Lupi I, Pellegrini G, Bartalena L, Bogazzi F, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28:346–349. doi: 10.1007/BF03347201. [DOI] [PubMed] [Google Scholar]
- 177.Sjöström P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005;65:111–124. doi: 10.1080/00365510510013523. [DOI] [PubMed] [Google Scholar]
- 178.Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, Barron J, Lemmey A. GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis. 2006;48:712–719. doi: 10.1053/j.ajkd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 179.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Naour N, Fellahi S, Renucci JF, Poitou C, Rouault C, Basdevant A, Dutour A, Alessi MC, Bastard JP, Clément K, et al. Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity (Silver Spring) 2009;17:2121–2126. doi: 10.1038/oby.2009.96. [DOI] [PubMed] [Google Scholar]
- 181.Witzel SH, Butts K, Filler G. Elevated triglycerides may affect cystatin C recovery. Clin Biochem. 2014;47:676–678. doi: 10.1016/j.clinbiochem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 182.Saito M, Nemoto T, Tobimatsu S, Ebata M, Le Y, Nakajima K. Coffee consumption and cystatin-C-based estimated glomerular filtration rates in healthy young adults: results of a clinical trial. J Nutr Metab. 2011;2011:146865. doi: 10.1155/2011/146865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ye Y, Gai X, Xie H, Jiao L, Zhang S. Impact of thyroid function on serum cystatin C and estimated glomerular filtration rate: a cross-sectional study. Endocr Pract. 2013;19:397–403. doi: 10.4158/EP12282.OR. [DOI] [PubMed] [Google Scholar]
- 184.Tzamaloukas AH, Malhotra D. Measuring creatinine excretion and clearance for diagnosing and staging chronic kidney disease. Int Urol Nephrol. 2017;49:551–552. doi: 10.1007/s11255-016-1468-8. [DOI] [PubMed] [Google Scholar]
- 185.Schwartz JB. Potential Effect of Substituting Estimated Glomerular Filtration Rate for Estimated Creatinine Clearance for Dosing of Direct Oral Anticoagulants. J Am Geriatr Soc. 2016;64:1996–2002. doi: 10.1111/jgs.14288. [DOI] [PubMed] [Google Scholar]
- 186.Wilson FP, Xie D, Anderson AH, Leonard MB, Reese PP, Delafontaine P, Horwitz E, Kallem R, Navaneethan S, Ojo A, et al. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC study. Clin J Am Soc Nephrol. 2014;9:2095–2103. doi: 10.2215/CJN.03790414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Correas JM, Anglicheau D, Joly D, Gennisson JL, Tanter M, Hélénon O. Ultrasound-based imaging methods of the kidney-recent developments. Kidney Int. 2016;90:1199–1210. doi: 10.1016/j.kint.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 188.Menzilcioglu MS, Duymus M, Citil S, Gungor G, Saglam M, Gungor O, Boysan SN, Sarıca A, Avcu S. The comparison of resistivity index and strain index values in the ultrasonographic evaluation of chronic kidney disease. Radiol Med. 2016;121:681–687. doi: 10.1007/s11547-016-0652-3. [DOI] [PubMed] [Google Scholar]
- 189.Meola M, Samoni S, Petrucci I. Imaging in Chronic Kidney Disease. Contrib Nephrol. 2016;188:69–80. doi: 10.1159/000445469. [DOI] [PubMed] [Google Scholar]
- 190.Cerino M, Lette J, Eybalin MC, Levasseur A. In vivo glomerular filtration rate measurement based solely on image processing. Clin Nucl Med. 1991;16:79–83. doi: 10.1097/00003072-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 191.Carlsen O. The gamma camera as an absolute measurement device: determination of glomerular filtration rate in 99mTc-DTPA renography using a dual head gamma camera. Nucl Med Commun. 2004;25:1021–1029. doi: 10.1097/00006231-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 192.Visconti L, Cernaro V, Ricciardi CA, Lacava V, Pellicanò V, Lacquaniti A, Buemi M, Santoro D. Renal biopsy: Still a landmark for the nephrologist. World J Nephrol. 2016;5:321–327. doi: 10.5527/wjn.v5.i4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Eloot S, Schepers E, Barreto DV, Barreto FC, Liabeuf S, Van Biesen W, Verbeke F, Glorieux G, Choukroun G, Massy Z, et al. Estimated glomerular filtration rate is a poor predictor of concentration for a broad range of uremic toxins. Clin J Am Soc Nephrol. 2011;6:1266–1273. doi: 10.2215/CJN.09981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vanholder R, Eloot S, Schepers E, Neirynck N, Glorieux G, Massy Z. an Obituary for GFR as the main marker for kidney function? Semin Dial. 2012;25:9–14. doi: 10.1111/j.1525-139X.2011.01003.x. [DOI] [PubMed] [Google Scholar]
- 195.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 196.Vrdoljak A, Ivkovic V, Karanovic S, Dika Z, Domislovic V, Dapic K, Gallineo L, Ivandic E, Josipovic J, Vucovic I, et al. Glomerular hyperfiltration as a risk factor for renal impairment and hypertension in apparently healthy subjects. J Hypertens. 2016;34 Suppl 2:319. [Google Scholar]
- 197.Melsom T, Mathisen UD, Eilertsen BA, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO. Physical exercise, fasting glucose, and renal hyperfiltration in the general population: the Renal Iohexol Clearance Survey in Tromsø 6 (RENIS-T6) Clin J Am Soc Nephrol. 2012;7:1801–1810. doi: 10.2215/CJN.02980312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol. 2015;10:382–389. doi: 10.2215/CJN.03080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang SH, Sharma AP, Yasin A, Lindsay RM, Clark WF, Filler G. Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol. 2011;6:274–280. doi: 10.2215/CJN.02760310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Perrin N, Berg UB. Estimated glomerular filtration rates cannot replace measured GFR in type 1 diabetes patients with hyperfiltration. Acta Paediatr. 2015;104:730–737. doi: 10.1111/apa.12993. [DOI] [PubMed] [Google Scholar]
- 201.Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD, Larson JJ, Kremers WK, Vrtiska TJ, Chakkera HA, et al. Detection and Clinical Patterns of Nephron Hypertrophy and Nephrosclerosis Among Apparently Healthy Adults. Am J Kidney Dis. 2016;68:58–67. doi: 10.1053/j.ajkd.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J Am Soc Nephrol. 2017;28:313–320. doi: 10.1681/ASN.2016020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Denic A, Glassock RJ, Rule AD. Structural and Functional Changes With the Aging Kidney. Adv Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bosch JP, Saccaggi A, Lauer A, Ronco C, Belledonne M, Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983;75:943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- 205.Fuiano G, Mancuso D, Indolfi C, Mongiardo A, Sabbatini M, Conte G, De Nicola L, Minutolo R, Mazza G, Cianfrone P, et al. Early detection of progressive renal dysfunction in patients with coronary artery disease. Kidney Int. 2005;68:2773–2780. doi: 10.1111/j.1523-1755.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- 206.Barai S, Gambhir S, Prasad N, Sharma RK, Ora M. Functional renal reserve capacity in different stages of chronic kidney disease. Nephrology (Carlton) 2010;15:350–353. doi: 10.1111/j.1440-1797.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- 207.Greene ER, Avasthi PS. Effect of a high-protein meal on blood flow to transplanted human kidneys. Transplantation. 1989;48:584–587. [PubMed] [Google Scholar]
- 208.Samoni S, Nalesso F, Meola M, Villa G, De Cal M, De Rosa S, Petrucci I, Brendolan A, Rosner MH, Ronco C. Intra-Parenchymal Renal Resistive Index Variation (IRRIV) Describes Renal Functional Reserve (RFR): Pilot Study in Healthy Volunteers. Front Physiol. 2016;7:286. doi: 10.3389/fphys.2016.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Skorecki KL, Freedman BI. A suPAR Biomarker for Chronic Kidney Disease. N Engl J Med. 2015;373:1971–1972. doi: 10.1056/NEJMe1512997. [DOI] [PubMed] [Google Scholar]
- 210.Sanchez-Niño MD, Sanz AB, Ramos AM, Fernandez-Fernandez B, Ortiz A. Clinical proteomics in kidney disease as an exponential technology: heading towards the disruptive phase. Clin Kidney J. 2017;10:188–191. doi: 10.1093/ckj/sfx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Filler G, Huang SH, Lindsay RM. The Search for More Reliable Estimated GFR Biomarkers. Am J Kidney Dis. 2016;67:5–8. doi: 10.1053/j.ajkd.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 212.Tutarel O, Denecke A, Bode-Böger SM, Martens-Lobenhoffer J, Schieffer B, Westhoff-Bleck M, Kielstein JT. Symmetrical dimethylarginine outperforms CKD-EPI and MDRD-derived eGFR for the assessment of renal function in patients with adult congenital heart disease. Kidney Blood Press Res. 2011;34:41–45. doi: 10.1159/000322614. [DOI] [PubMed] [Google Scholar]
- 213.El-Khoury JM, Bunch DR, Hu B, Payto D, Reineks EZ, Wang S. Comparison of symmetric dimethylarginine with creatinine, cystatin C and their eGFR equations as markers of kidney function. Clin Biochem. 2016;49:1140–1143. doi: 10.1016/j.clinbiochem.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 214.Bhavsar NA, Appel LJ, Kusek JW, Contreras G, Bakris G, Coresh J, Astor BC; AASK Study Group. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis. 2011;58:886–893. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Herrero-Morín JD, Málaga S, Fernández N, Rey C, Diéguez MA, Solís G, Concha A, Medina A. Cystatin C and beta2-microglobulin: markers of glomerular filtration in critically ill children. Crit Care. 2007;11:R59. doi: 10.1186/cc5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Juraschek SP, Coresh J, Inker LA, Levey AS, Köttgen A, Foster MC, Astor BC, Eckfeldt JH, Selvin E. Comparison of serum concentrations of β-trace protein, β2-microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol. 2013;8:584–592. doi: 10.2215/CJN.08700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Foster MC, Coresh J, Hsu CY, Xie D, Levey AS, Nelson RG, Eckfeldt JH, Vasan RS, Kimmel PL, Schelling J, et al. Serum β-Trace Protein and β2-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2016;68:68–76. doi: 10.1053/j.ajkd.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Inker LA, Tighiouart H, Coresh J, Foster MC, Anderson AH, Beck GJ, Contreras G, Greene T, Karger AB, Kusek JW, et al. GFR Estimation Using β-Trace Protein and β2-Microglobulin in CKD. Am J Kidney Dis. 2016;67:40–48. doi: 10.1053/j.ajkd.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ji F, Zhang S, Jiang X, Xu Y, Chen Z, Fan Y, Wang W. Diagnostic and prognostic value of galectin-3, serum creatinine, and cystatin C in chronic kidney diseases. J Clin Lab Anal. 2016 doi: 10.1002/jcla.22074. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Argyropoulos C, Wang K, McClarty S, Huang D, Bernardo J, Ellis D, Orchard T, Galas D, Johnson J. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One. 2013;8:e54662. doi: 10.1371/journal.pone.0054662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nassirpour R, Raj D, Townsend R, Argyropoulos C. MicroRNA biomarkers in clinical renal disease: from diabetic nephropathy renal transplantation and beyond. Food Chem Toxicol. 2016;98:73–88. doi: 10.1016/j.fct.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 222.Spinale JM, Mariani LH, Kapoor S, Zhang J, Weyant R, Song PX, Wong HN, Troost JP, Gadegbeku CA, Gipson DS, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87:564–574. doi: 10.1038/ki.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Joshi MS, Montgomery KA, Giannone PJ, Bauer JA, Hanna MH. Renal injury in neonates: use of “omics” for developing precision medicine in neonatology. Pediatr Res. 2017;81:271–276. doi: 10.1038/pr.2016.206. [DOI] [PubMed] [Google Scholar]
- 225.Mihai S, Codrici E, Popescu ID, Enciu AM, Rusu E, Zilisteanu D, Albulescu R, Anton G, Tanase C. Proteomic Biomarkers Panel: New Insights in Chronic Kidney Disease. Dis Markers. 2016;2016:3185232. doi: 10.1155/2016/3185232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu KD, Yang W, Go AS, Anderson AH, Feldman HI, Fischer MJ, He J, Kallem RR, Kusek JW, Master SR, et al. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65:267–274. doi: 10.1053/j.ajkd.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Papadopoulou-Marketou N, Skevaki C, Kosteria I, Peppa M, Chrousos GP, Papassotiriou I, Kanaka-Gantenbein C. NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up. Hormones (Athens) 2015;14:232–240. doi: 10.14310/horm.2002.1520. [DOI] [PubMed] [Google Scholar]
- 228.Shafi T, Michels WM, Levey AS, Inker LA, Dekker FW, Krediet RT, Hoekstra T, Schwartz GJ, Eckfeldt JH, Coresh J. Estimating residual kidney function in dialysis patients without urine collection. Kidney Int. 2016;89:1099–1110. doi: 10.1016/j.kint.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wong J, Sridharan S, Berdeprado J, Vilar E, Viljoen A, Wellsted D, Farrington K. Predicting residual kidney function in hemodialysis patients using serum β-trace protein and β2-microglobulin. Kidney Int. 2016;89:1090–1098. doi: 10.1016/j.kint.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 230.Vilar E, Boltiador C, Wong J, Viljoen A, Machado A, Uthayakumar A, Farrington K. Plasma Levels of Middle Molecules to Estimate Residual Kidney Function in Haemodialysis without Urine Collection. PLoS One. 2015;10:e0143813. doi: 10.1371/journal.pone.0143813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Coresh J, Inker L, Sang Y, Chen J, Shafi T, Post WS, Shlipak M, Perichon R, Greene T, Levey AS. Multiple metabolites correlate more strongly with measured glomerular filtration rate than creatinine: A verification study Abstract FR-OR021. ASN Abstracts. 2016:39A. [Google Scholar]
- 232.Critselis E, Lambers Heerspink H. Utility of the CKD273 peptide classifier in predicting chronic kidney disease progression. Nephrol Dial Transplant. 2016;31:249–254. doi: 10.1093/ndt/gfv062. [DOI] [PubMed] [Google Scholar]
- 233.Pontillo C, Jacobs L, Staessen JA, Schanstra JP, Rossing P, Heerspink HJ, Siwy J, Mullen W, Vlahou A, Mischak H, et al. A urinary proteome-based classifier for the early detection of decline in glomerular filtration. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw239. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 234.Perichon R, Lindtröm V, Freed T, Ford L, Nyman U, Björk J, Wulff J, Grubb AO. 2016. Equations based on a set of novel metabolites markers provide a more precise determination of the glomerular filtration rate (GFR) than the standard equations in a Swedish population with measured GFR. Abstract FR-PR020. ASN Abstracts; p. 39A. [Google Scholar]
- 235.Siwy J, Zürbig P, Argiles A, Beige J, Haubitz M, Jankowski J, Julian BA, Linde PG, Marx D, Mischak H, et al. Noninvasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw337. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mayer G, Heerspink HJ, Aschauer C, Heinzel A, Heinze G, Kainz A, Sunzenauer J, Perco P, de Zeeuw D, Rossing P, et al. Systems Biology-Derived Biomarkers to Predict Progression of Renal Function Decline in Type 2 Diabetes. Diabetes Care. 2017;40:391–397. doi: 10.2337/dc16-2202. [DOI] [PubMed] [Google Scholar]


