Abstract
Cleft lip with or without cleft palate (CP) is one of the most common congenital malformations. Ultrasonographers involved in the routine 20-wk ultrasound screening could encounter these malformations. The face and palate develop in a very characteristic way. For ultrasonographers involved in screening these patients it is crucial to have a thorough understanding of the embryology of the face. This could help them to make a more accurate diagnosis and save time during the ultrasound. Subsequently, the current postnatal classification will be discussed to facilitate the communication with the CP teams.
Keywords: Cleft lip, Cleft palate, Embryology face, Orofacial clefts, Ultrasound
Core tip: Cleft lip/palate is a very common craniofacial malformation. Currently a thorough ultrasound examination during the 20-wk ultrasound is performed to exclude an oral cleft of the face. This study provides important embryological information to facilitate the ultrasonographer in making an accurate diagnosis and safe time during the ultrasound. Subsequently, the current postnatal classification will be discussed to facilitate the communication with the cleft palate teams.
INTRODUCTION
Orofacial clefts (OFCs) are common craniofacial malformations. Cleft lip (CL) with or without cleft palate (CL/P) occur more commonly in males, while 1:1000 Caucasians, 2:1000 Asians, and 0.3:1000 Africans are affected[1]. However, isolated cleft palate (CP) is more common in females and an equal incidence of 0.4:1000 live born is encountered in all races[1]. Although the distribution for clefts differs per region it is estimated to be 20%-25% CL, 40%-50% CLP and 30%-35% CP. Clefts occur in a ratio of 6:3:1 unilateral left, unilateral right, and bilateral[2]. The etiology of OFCs is complex and believed to be multifactorial, representing an interaction between genetics and environment during a critical stage of development[3]. Recently several genes causing CL and palate have been discovered. The nature and function of these genes vary widely, illustrating high complexity within the craniofacial developmental pathways[4-6]. The interested reader is referred to comprehensive studies that focus specifically on these genes.
In different countries routine ultrasound screening in pregnancy does not consistently include screening for facial clefts. However, the increased use of transabdominal ultrasound (3D) certainly leads to an increased frequency of oral clefts being diagnosed antenatally[7]. There are few articles that focus on different ultrasound approaches to visualize the palate and lips both 2D and 3D[8-10].
As oral clefts typically occur in facial areas where the normal embryological fusion of structures did not occur, knowledge of the embryological background could aid the ultrasonographer to understand and more accurately diagnose these clefts.
The aim of this review is to familiarize the ultrasonographer with the embryology of the face, which will subsequently aid in more accurate diagnosis of the extent of the facial cleft. For a more extensive overview the reader is referred to textbooks[11-13]. The different classifications systems of clefts are also summarized. This might facilitate communication between the ultrasonographer and the CP team after birth.
DEVELOPMENT OF THE LIP
The basic morphology of the face is established between the 4th and 10th week after conception. Upper lip formation commences at 24 d postconception and is completed by 37 d[11-13]. At five weeks’ gestation, when the embryo is 3 mm long, the ectoderm in the vicinity of the neural plate folds on itself to form the neural tube. Special neural crest cells of ectodermal origin differentiate to form a special ectomesenchyme. The ectomesenchyme migrates over and around the head and participates in the formation of five facial prominences that surround the primitive oral cavity: The frontonasal prominence, the paired maxillary prominences and the paired mandibular prominences.
The frontonasal prominence develops in the midline over the brain. During the 5th week of embryogenesis the nasal component of the frontonasal prominence forms bilateral two ectodermal thickenings, the nasal placodes (Figure 1)[13]. Each nasal placode invaginates to form an oval nasal pit and divides the frontonasal prominence into a medial and lateral nasal process. During the 6th week, the two medial nasal processes fuse and gives rise to the midline of the nose, medial part of the upper lip, philtrum, incisor teeth and the primary palate. The primary palate is the part of the palate that is located ventrally to the foramen incisivum, while the secondary palate is the part located dorsally to the foramen incisivum. The lateral nasal process subsequently forms the nasal alae and alar base.
Figure 1.
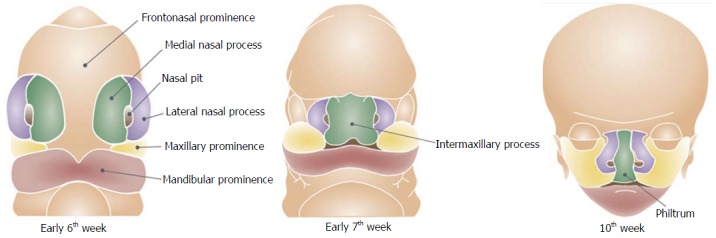
Development of the lip.
During the 6th week the maxillary processes on each side of the mouth grow forward and merge with the medial nasal processes that lead to the formation of the lateral upper lip, the majority of the maxilla and the secondary palate. The mandibular prominences give rise to the mandible and lower lip. The fusion of the facial swellings occurs between the 4th-6th weeks postconception. Failure of fusion between any of the facial swellings results in facial clefts and can occur either unilaterally or bilaterally and typically happens at the junction of the lateral incisor and the first premolar teeth.
In patients with mild CL defects the cleft could be limited to a notch in the vermillion border of the lip that probably represents a failure of localized growth of the medial nasal process. In more severe defects, the cleft runs through all the lip structures and completely separates the lateral lip from the philtrum and nasal cavity. These clefts are caused by failure of fusion between the medial nasal process and the maxillary prominence. The depth of the cleft may vary from the soft tissue of the lip to a complete cleft of the maxillary bone. The normal palate fusion process starts at the foramen incisivum and subsequently closes in a posterior direction. The actual lip fusion starts cranially and subsequently closes in a caudal direction.
Oblique OFCs can also involve the side of the face and even involve the orbit. These clefts comprise less than 1% of facial clefts and can be classified according to Tessier’s anatomical classification[14]. Midline clefting syndromes can be divided into two groups: The premaxilla agenesis-holoproencephaly syndrome and frontonasal-median cleft syndrome[15]. Midline clefts arise due to incomplete merging of the median nasal prominences that form the inter-maxillary segment. The premaxilla agenesis-holoproencephaly syndrome (Demyer sequence) has a frontonasal deformity associated with hypotelorism, holoprosencephaly and facial deformity ranging from cyclopia to midline facial cleft with pre-maxillary ageneses. The median cleft face syndrome is often associated with a nasal deformity and hypertelorism usually either with no or little brain deformity (corpus callosum agenesis). In these cases surgical reconstruction is feasible due to the probability of normal life expectancy.
DEVELOPMENT OF THE PALATE
Palatogenesis begins at the end of the 5th week and the development of the palate is not completed until the 12th week postconception[11-13]. The palate develops from two primordia: The primary and the secondary palate. The most important cell types in palate development are the neural crest-derived palatal mesenchyme, ectoderm-derived epithelial lining and the most apical layer composed of periderm cells[16]. The soft palate also includes the cranial paraxial mesoderm derived myogenic cells.
The primary palate is formed by merging of the medial nasal prominences during the 6th week and gives rise to the four central incisors and extends to the foramen incisivum.
The secondary palate that separates the nasal cavity from the oral cavity is the primordium of the hard and the soft palate and is formed by the fusion of neural crest mesenchyme that lies within the maxillary primordia. The development of the secondary palate starts with the outgrowth of two palatine shelves from the maxillary process that extend vertically on either side of the tongue. As the mandible grows downward and forward, the tongue’s position descends. The palatal shelves subsequently rotate to a horizontal position dorsal to the tongue and then undergo intramembranous ossification to form the palatine process of the maxilla and the palatine bone. The transition from vertical to horizontal position happens in the eight week postconception and is completed, incredibly, in only some hours. There is considerable sex difference in the timing of palatal closure. Shelf elevation and fusion begin a few days earlier in males than in females[13]. Just as the formation of the lip, the subsequent fusion process is an incredibly complex process. Before fusion the palatal shelves are two cell layers thick. The outer layer is sloughed off (by apoptosis), leaving only a basal epithelial layer which composes the medial edge of each palatal shelf. The shelves grow towards each other in the midline and approximate to form the midline epithelial seam. The seam subsequently degenerates, leading to mesenchymal confluence between the two palatal shelves. The fusion process of the shelves starts immediately behind the foramen incisivum and extends dorsally to close the palate like a “zip” (Figure 2). At the same time fusion with the nasal septum and the primary palate occurs. Gradually, bone extends from the palatine process of the maxilla and the palatine bone into the palatal shelves to form the hard palate. The posterior parts do not become ossified and extend posteriorly and fuse to form the soft palate, including the uvula[13].
Figure 2.
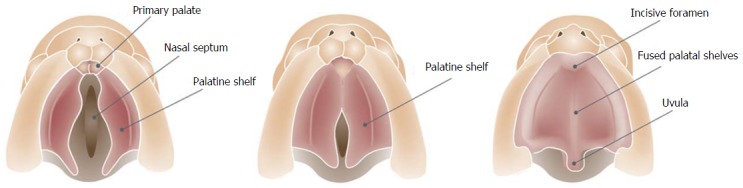
Caudal view of the fusion process of the palatal shelves.
If the fusion process of the palate which occurs between the 9th and 12th week of gestation, is disrupted by either genetic, mechanical or teratogenic factors, a cleft of secondary palate results. Because the secondary palate closes from the foramen incisivum in a posterior(dorsal) direction, it is not possible for the palate to be open just posterior to the foramen incisivum in the hard palate and then subsequently fuse again in the soft palate part. If the initial process of fusion is defect the rest of the fusion process will not take place. This means that an intact soft palate implies the presence of an intact hard palate. Only a handful of cases have been described where “fenestrations” have been found in the midline of the palate seam[17]. These cases have been attributed to trauma and not due to a defective fusion process. Especially the submucous cleft could be vulnerable[17]. However, some mouse models do suggest that initial contact is made in the middle-anterior region with fusion proceeding in both anterior and posterior directions[17].
Abnormality of the mandible appears to have a related cause with CP. Hypoplasia of the mandible (micrognathia) interferes with descent of the tongue and positions the tongue superiorly between the two palatal shelves. This causes mechanical disruption of palatal closure and could result in a CP. Micrognathia could be associated with Robin sequence. This sequence or phenomenon consisting of a triad of micrognathia, glossoptosis and breathing problems often involves an associated CP[18]. This condition could be associated with life threatening breathing problems postnatal and is often associated with other anomalies such as heart defects[19].
IMPLICATIONS FOR DIAGNOSING AN OROFACIAL CLEFT
Fusion between the two maxillary processes differs on a molecular level from the fusion between the medial nasal process and the maxillary process. Together with the epidemiological differences this supports the view that CL/P (CL with or without CP) and isolated CP are two different entities[6-13].
Postnatal the prevalence of associated anomalies is lowest in CL and ranges from 7.6%-41.4%[6-13]. Data from postnatal studies show that concerning CLP the frequency is higher and ranges from 21.1%-61.2%. CP is the category most frequent associated with additional congenital anomalies and the prevalence of associated malformations with CP ranges from 22.2%-78.3%. Antenatally isolated CP is usually not diagnosed and associated abnormalities are reported in 39.1%-66.0% of foetuses with CLP[20,21]. This high percentage probably reflects many different factors: The intra-uterine lethality of associated syndromes such as trisomy 13 or 18, pregnancy termination for severe malformations and the greater likelihood of diagnosing CL/P in the case of more associate abnormalities, especially if evaluation of the lip does not constitute part of routine screening.
It is becoming more frequent to use the transabdominal ultrasound screening during the second trimester of pregnancy to evaluate the face. Evaluation of the upper lip for possible CL/P is an optional element and has a sensitivity of 88% for detecting CL/P[21]. However the overall sensitivity for OFCs is lower because the prenatal detection rate of CP is only 0%-1.4%[21]. The very low detection rate of CP demonstrates that there are no satisfactory sonographic indicators of an isolated CP that might be one of the reasons why the palate is often not visualized during the ultrasound screening. Yet, the ultrasonographer should be aware that micrognathia could be associated with a CP. Subsequently, when the ultrasonographer encounters other malformations (such as heart defects) with the micrognathia, evaluation of the palate is mandatory.
SPECTRUM OF OFCS
Some forms of CL only have a small indentation in the vermillion. This “forme fruste” with a small notch within the borders of the vermillion and a band of fibrous tissue running from the edge of the red lip to the nostril floor or a deformity of the nasal ala on the side of the notch, is unlikely be diagnosed by ultrasound. When CL is seen on 2D or 3D ultrasound, the position of the alar base (nostrils) can help to determine whether the alveolar ridge or palate is involved as well. An isolated incomplete CL without a maxillary or palatal defect will appear as a linear defect running from the lip towards the nasal floor (Figure 3A). In complete CL the lip defect could be well visualized, although the nose distortion is likely to be minimal (Figure 3B). In complete bilateral CL the maxilla is intact (there is no maxillary protrusion) and the alar bases or nostrils are symmetrical. Recently we have demonstrated that with a normal maxilla-nasion-maxilla angle, it was unlikely to find a cleft that included the alveolus[22]. A complete CL with alveolar ridge involvement but without involvement of the primary palate is called an incomplete CL. Incomplete involvement of the alveolar ridge usually does not substantially change the position of the alar base. A complete CL is diagnosed as involvement of the complete lip, alveolar ridge and primary palate. A complete CLP includes the lip, alveolus, primary palate and whole secondary palate from foramen incisivum to include the uvula. In a complete CLP the lip involvement is relatively easy to detect by ultrasound, while the alar base is characteristically lateralized away from the cleft (Figures 3C and 4). In cases with bilateral CLP there is a protrusion of the maxillary process that is visible as an echogenic structure below the alar base (Figure 3D and E). This protrusion is not commonly seen in cases without a secondary CP and could help the ultrasonographer when confronted with a bilateral CL deformity. An important message for sonographers is that cleft alveolus does not necessarily equal CP. Lateralization of the alar base could help the ultrasonographer to diagnose complete CLP patients where the visualisation of the CP is difficult. Different techniques such as the oblique-face or flipped-face view make better 3-D visualization of the alar base[8,9,10,23].
Figure 3.
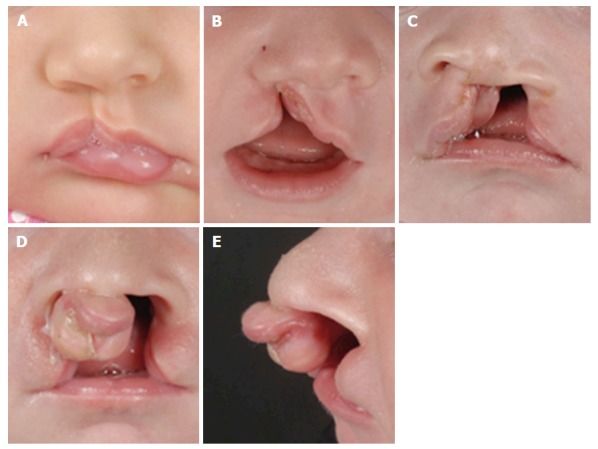
Different types of cleft lips. A: Unilateral microform cleft lip; B: Unilateral cleft lip and alveolus; C: Unilateral cleft lip and palate; D: Bilateral cleft lip and palate with protrusion of the intermaxillary process; E: Lateral view of bilateral cleft lip and palate.
Figure 4.
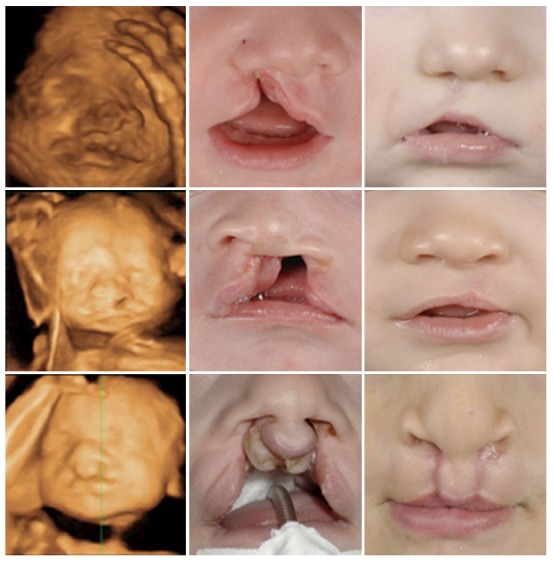
Prenatal, postnatal and post-surgery images of three different patients with cleft lip and alveolus, cleft lip and palate and bilateral cleft lip and palate respectively.
Unless other anomalies alert the ultrasonographer to the possibility, isolated CP is usually not diagnosed prenatally whereas the palate has a dome shaped structure and is surrounded by osseous structures making it difficult to visualize. The fact that the embryological fusion of the secondary palate starts from the foramen incisivum and proceeds dorsally is of importance during ultrasonography. An unremarkable uvula implies the presence of an intact palate. If the uvula is deformed or absent this might indicate a defect in the palate. The uvula can be visualized by ultrasound and the echo pattern of a normal uvula is typical and strongly resembles an “equals sign” (Figure 5)[16]. It is important to realize that the vocal cords also have “two lines” and could resemble the “equal sign of the palate”. However, the vocal cords are located lower than the soft palate. If the equals sign cannot be seen, CP cannot be ruled out and should be further examined by imaging the soft palate in a median sagittal section to visualize the palate structure in more detail. Larger prospective studies are needed to confirm this hypothesis. Implementation of the equals sign technique may improve the prenatal detection rate of isolated CP[16,24]. Moreover if the ultrasonographer cannot visualise the middle part of the secondary palate (e.g., obstruction of the tongue) but the “equals sign” is visible, it is suggestive of an intact palate. This could subsequently save the ultrasonographer time. However important techniques such as the “3D-reverse face” view technique[8] and improved ultrasonography equipment and probes will also lead to better visualization of the palate. Martinez-Ten et al[9,23] described the importance of incorporating the “reverse-face view, with the flipped-face views and subsequently the oblique-face view into the algorithm of analysis a possible CP in the neonate. They also demonstrated that an accurate visualization of the palate required good initially acquired volume, fluid between the fetal tongue and palate, and curving of the plane to follow the structure of the palate[23]. Subsequently, the oblique-face or flipped-face view makes better visualization in selected cases.
Figure 5.
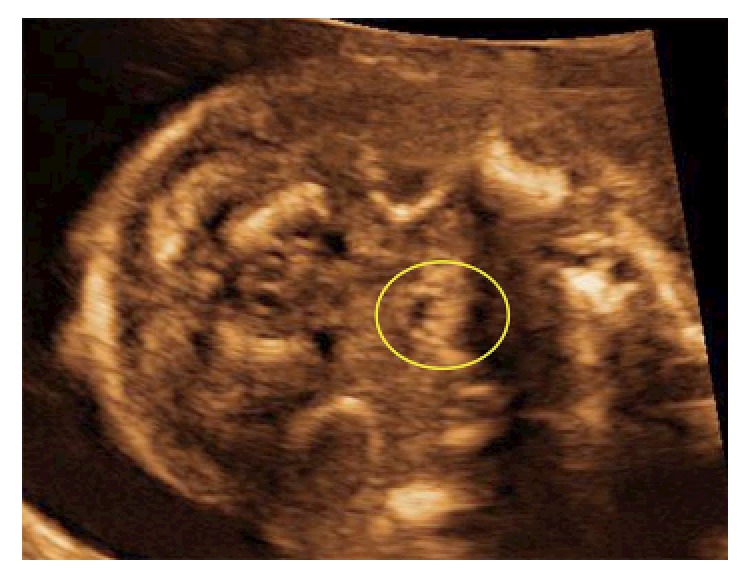
Echo pattern of a normal uvula visualized by ultrasound is typical and strongly resembles an “equals sign”.
CLASSIFICATION
Orofacial clefting is multifactorial and etiologically heterogeneous. Therefore, proper classification is essential as different types of clefts may be variably associated with additional anomalies and chromosomal disorders. Over the years many different classification systems based on morphological, anatomical or etiological features of OFCs have been proposed. However, postnatal classification may not be applicable to prenatal findings.
An antenatal sonographic classification system has been proposed by Nyberg et al[25,26]. This classification shows four types of clefts and their relationship with the primary and secondary palate. Type 1: Isolated CL alone, type 2: Unilateral CL and palate, type 3: Bilateral CL and palate and type 4: Median CL and palate. The so called type 5 clefts is another group of facial clefts associated with the amniotic band syndrome or the limb-body-wall complex and does not follow embryologic patterns but rather shows random types of often large and devastating defects[25]. They further suggest that type 1 clefts are associated with a low rate of anomalies and type 2 and 3 clefts with intermediate prognosis. Type 4 and 5 clefts are universally associated with concurrent anomalies and with fatal outcome[26]. The Nyberg classification has several shortcomings; CP for instance is not mentioned at all, while not all midline clefts have a fatal outcome.
A myriad of classification systems has been proposed and utilized over the years, however only a few have found clinical application. The most generally accepted classification was developed by Kernahan in 1971 (Figure 6), who proposed a striped Y-classification with the incisive foramen as the reference[27]. This system is based on the resemblance of an intra-oral view of a CL and palate to the letter “Y”. The area affected by the cleft is labeled from 1-9, each of which represents a different anatomical structure.
Figure 6.
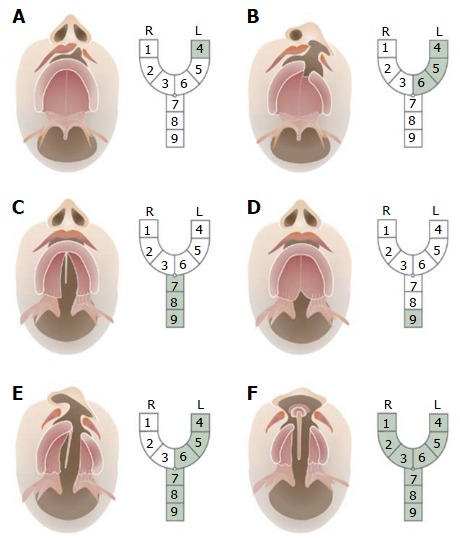
Kernahan’s classification. The area affected by the cleft is labeled from 1-9, each of which represents a different anatomical structure: 1: Right lip; 2: Right alveolus; 3: Right premaxilla; 4: Left lip; 5: Left alveolus; 6: Left premaxilla; 7: Hard palate; 8: Soft palate; 9: Submucous cleft.
There has been a general move to adopt a simple classification system for clefts. The LAHSHAL code is often used as the preferred classification system by cleft surgeons (“L” = lip, “A” alveolus, “H” hard palate, “Soft palate”). It is compatible with ICD10 and allows clefts to be coded for computer use. The LAHSHAL codes split the relevant parts of the mouth in six parts and is written from the perspective of someone looking at the patient (i.e., the first letter is for the patients right lip and the last letter for the patients left lip). The LAHSAL code indicates for each patient whether there is a complete cleft (upper case letter, e.g., “L”) or an incomplete cleft (lower case, e.g., “l”) or no cleft. LAs would subsequently connote a complete cleft of the right lip/alveolus and incomplete cleft of the soft palate. SHAL would connote a complete left-sided unilateral CL, alveolus and palate. The midline Tessier clefts occur very infrequently, and are not included in these classifications.
CONCLUSION
Knowledge of the embryology of the face should add to the understanding and correctly diagnosing OFCs. Failure of fusion between any of the facial swellings results in facial clefts and can occur either unilaterally or bilaterally and typically happens at the junction of the lateral incisor and the first premolar teeth. The depth of the cleft may vary from the soft tissue of the lip to a complete cleft of the maxillary bone. Lateralization of the alar base could help the ultrasonographer to diagnose a complete CLP patient where the visualisation of the CP is difficult. Maxillary protrusion seen with a bilateral CL is highly suggestive of a bilateral complete CL/palate. The embryological fusion of the palatal shelves starts anteriorly and proceeds posteriorly like a zip. An unremarkable uvula, visualised by ultrasound as an “equals sign”, suggests an intact normal palate. If the ultrasonographer cannot visualise the middle part of the secondary palate, but the “equals sign” is visible, it is suggestive of an intact palate. This could safe the ultrasonographer time.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Peer-review started: December 19, 2016
First decision: March 28, 2017
Article in press: May 31, 2017
P- Reviewer: Mattos BSC, Ni Y S- Editor: Ji FF L- Editor: A E- Editor: Li D
Contributor Information
Bram Smarius, Division of Pediatric Plastic Surgery, Cleft Palate Team, Wilhelmina Children’s Hospital, 3584 EA Utrecht, The Netherlands. b.j.a.smarius@umcutrecht.nl.
Charlotte Loozen, Division of Pediatric Plastic Surgery, Cleft Palate Team, Wilhelmina Children’s Hospital, 3584 EA Utrecht, The Netherlands.
Wendy Manten, Division of Gynecology and Obstetrics, Wilhelmina Children’s Hospital, 3584 EA Utrecht, The Netherlands.
Mireille Bekker, Division of Gynecology and Obstetrics, Wilhelmina Children’s Hospital, 3584 EA Utrecht, The Netherlands.
Lou Pistorius, Division of Gynecology and Obstetrics, Stellenbosch University and Tygerberg Hospital, Cape Town 7500, South Africa.
Corstiaan Breugem, Division of Pediatric Plastic Surgery, Cleft Palate Team, Wilhelmina Children’s Hospital, 3584 EA Utrecht, The Netherlands.
References
- 1.Mulliken JB. The changing faces of children with cleft lip and palate. N Engl J Med. 2004;351:745–747. doi: 10.1056/NEJMp048157. [DOI] [PubMed] [Google Scholar]
- 2.Fraser FC. The genetics of cleft lip and cleft palate. Am J Hum Genet. 1970;22:336–352. [PMC free article] [PubMed] [Google Scholar]
- 3.Merritt L. Part 1. Understanding the embryology and genetics of cleft lip and palate. Adv Neonatal Care. 2005;5:64–71. doi: 10.1016/j.adnc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Wong FK, Hagg U. An update on the aetiology of orofacial clefts. Hong Kong Med J. 2004;10:331–336. [PubMed] [Google Scholar]
- 5.Böhmer AC, Mangold E, Tessmann P, Mossey PA, Steegers-Theunissen RP, Lindemans J, Bouwman-Both M, Rubini M, Franceschelli P, Aiello V, et al. Analysis of susceptibility loci for nonsyndromic orofacial clefting in a European trio sample. Am J Med Genet A. 2013;161A:2545–2549. doi: 10.1002/ajmg.a.36141. [DOI] [PubMed] [Google Scholar]
- 6.Shaw GM, Yang W, Perloff S, Shaw NM, Carmichael SL, Zhu H, Lammer EJ. Thymidylate synthase polymorphisms and risks of human orofacial clefts. Birth Defects Res A Clin Mol Teratol. 2013;97:95–100. doi: 10.1002/bdra.23114. [DOI] [PubMed] [Google Scholar]
- 7.Maarse W, Bergé SJ, Pistorius L, van Barneveld T, Kon M, Breugem C, Mink van der Molen AB. Diagnostic accuracy of transabdominal ultrasound in detecting prenatal cleft lip and palate: a systematic review. Ultrasound Obstet Gynecol. 2010;35:495–502. doi: 10.1002/uog.7472. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S, Lees C, Moscoso G, Hall P. Ultrasound antenatal diagnosis of cleft palate by a new technique: the 3D “reverse face” view. Ultrasound Obstet Gynecol. 2005;25:12–18. doi: 10.1002/uog.1819. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Ten P, Adiego B, Illescas T, Bermejo C, Wong AE, Sepulveda W. First-trimester diagnosis of cleft lip and palate using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;40:40–46. doi: 10.1002/uog.10139. [DOI] [PubMed] [Google Scholar]
- 10.Sommerlad M, Patel N, Vijayalakshmi B, Morris P, Hall P, Ahmad T, Campbell S, Lees C. Detection of lip, alveolar ridge and hard palate abnormalities using two-dimensional ultrasound enhanced with the three-dimensional reverse-face view. Ultrasound Obstet Gynecol. 2010;36:596–600. doi: 10.1002/uog.7739. [DOI] [PubMed] [Google Scholar]
- 11.Moore KL, Persaud TVN. The developing Human, clinical oriented embryology. 9th ed. Philadephia (PA): Elsevier/Saunders, 2013 [Google Scholar]
- 12.Larsen WJ. Larsen’s human embryology 4th ed. Philadelphia (PA): Elsevier/Churchill Livingstone, 2009 [Google Scholar]
- 13.Sperber GH, Sperber SM, Guttmann GD. 2nd ed. Shelton (CT): People Medical Publishing House; 2010. Craniofacial embryogenetics and development; pp. 37–60 and 131-144. [Google Scholar]
- 14.Tessier P. Anatomical classification facial, cranio-facial and latero-facial clefts. J Maxillofac Surg. 1976;4:69–92. doi: 10.1016/s0301-0503(76)80013-6. [DOI] [PubMed] [Google Scholar]
- 15.Demyer W, Zeman W, Palmer CG. The face depicts the brain: Diagnosis and significance of median facial anomalies for holoprosencephaly with median cleft lip and palate. Pediatrics. 1964;11:256–263. [PubMed] [Google Scholar]
- 16.Lane J, Kaartinen V. Signaling networks in palate development. Wiley Interdiscip Rev Syst Biol Med. 2014;6:271–278. doi: 10.1002/wsbm.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers GF, Murthy A, Mulliken JB. Congenital fenestration of the palate: A case of embryologic syzygy. Cleft Palate Craniofac J. 2006;43:363–366. doi: 10.1597/05-013.1. [DOI] [PubMed] [Google Scholar]
- 18.Breugem CC, Courtemanche DJ. Robin sequence: clearing nosologic confusion. Cleft Palate Craniofac J. 2010;47:197–200. doi: 10.1597/08-061_1. [DOI] [PubMed] [Google Scholar]
- 19.Izumi K, Konczal LL, Mitchell AL, Jones MC. Underlying genetic diagnosis of Pierre Robin sequence: retrospective chart review at two children’s hospitals and a systematic literature review. J Pediatr. 2012;160:645–650.e2. doi: 10.1016/j.jpeds.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Maarse W, Rozendaal AM, Pajkrt E, Vermeij-Keers C, Mink van der Molen AB, van den Boogaard MJ. A systematic review of associated structural and chromosomal defects in oral clefts: when is prenatal genetic analysis indicated? J Med Genet. 2012;49:490–498. doi: 10.1136/jmedgenet-2012-101013. [DOI] [PubMed] [Google Scholar]
- 21.Maarse W, Pistorius LR, Van Eeten WK, Breugem CC, Kon M, Van den Boogaard MJ, Mink van Der Molen AB. Prenatal ultrasound screening for orofacial clefts. Ultrasound Obstet Gynecol. 2011;38:434–439. doi: 10.1002/uog.8895. [DOI] [PubMed] [Google Scholar]
- 22.de Jong-Pleij EA, Pistorius LR, Ribbert LS, Breugem CC, Bakker M, Tromp E, Bilardo CM. Premaxillary protrusion assessment by the maxilla-nasion-mandible angle in fetuses with facial clefts. Prenat Diagn. 2013;33:354–359. doi: 10.1002/pd.4062. [DOI] [PubMed] [Google Scholar]
- 23.Martínez Ten P, Pérez Pedregosa J, Santacruz B, Adiego B, Barrón E, Sepúlveda W. Three-dimensional ultrasound diagnosis of cleft palate: ‘reverse face’, ‘flipped face’ or ‘oblique face’--which method is best? Ultrasound Obstet Gynecol. 2009;33:399–406. doi: 10.1002/uog.6257. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm L, Borgers H. The ‘equals sign’: a novel marker in the diagnosis of fetal isolated cleft palate. Ultrasound Obstet Gynecol. 2010;36:439–444. doi: 10.1002/uog.7704. [DOI] [PubMed] [Google Scholar]
- 25.Nyberg DA, McGahan JP, Pretorius DH, Pilu G. Diagnostic imaging of fetal anomalies. Philadelphia (PA): Lippincott Williams & Wilkins, 2003 [Google Scholar]
- 26.Nyberg DA, Sickler GK, Hegge FN, Kramer DJ, Kropp RJ. Fetal cleft lip with and without cleft palate: US classification and correlation with outcome. Radiology. 1995;195:677–684. doi: 10.1148/radiology.195.3.7753993. [DOI] [PubMed] [Google Scholar]
- 27.Kernahan DA. The striped Y--a symbolic classification for cleft lip and palate. Plast Reconstr Surg. 1971;47:469–470. doi: 10.1097/00006534-197105000-00010. [DOI] [PubMed] [Google Scholar]


