Abstract
Staphylococcal food poisoning (SFP) is a common cause of foodborne illness worldwide, and enterotoxin D (SED) is one of the most frequent Staphylococcus aureus enterotoxins associated with it. It has been reported that the expression and formation of SED in S. aureus is regulated by the quorum sensing Agr system. In this study, the effect of agr deletion on sed expression in S. aureus grown on boiled ham was investigated. Growth, sed mRNA and SED protein levels in an S. aureus wild type strain and its isogenic Δagr mutant were monitored for 14 days at 22 °C. The results showed that although deletion of the agr gene did not affect the growth rate or maximum cell density of S. aureus on boiled ham, it had a pronounced effect on SED formation during the first 5 days of incubation. The SED concentration was not reflected in the amount of preceding sed transcripts, suggesting that sed transcription levels may not always reflect SED formation. The expression of RNAIII transcript, the regulatory signal of the Agr system, was also monitored. Similar transcription patterns were observed for RNAIII and sed. Surprisingly, in the Δagr mutant, sed expression was comparable to that in the wild type strain, and was thus unaffected by deletion of the Agr system. These results demonstrate that the Agr system appears to only partially affect SED formation, even in a real food environment.
Keywords: Staphylococcus aureus, staphylococcal food poisoning, SED formation, Agr quorum sensing system, RNAIII, boiled ham
1. Introduction
New consumption habits and demands for minimally processed food and ready-to-eat meals have provided new and often more favorable conditions for the survival and growth of foodborne pathogens. This may lead to an increased risk of foodborne illness. Deli meats such as ham are often included in ready-to-eat salads and sandwiches, and are interesting food systems for studies on pathogen behavior and food safety. These products are often subjected to slicing and manual handling after initial processing such as boiling or smoking. As they are then consumed without further treatment, they may be subject to post-processing contamination. It is important to study and characterize pathogenic contamination in real food matrices to increase our knowledge and preparedness regarding the expression of virulence factors in ready-to-eat foods.
Staphylococcal food poisoning (SFP) is a foodborne intoxication caused by the consumption of one or several preformed enterotoxins produced by Staphylococcus aureus. SFP symptoms include nausea, vomiting, and abdominal pain, followed by diarrhea [1]. The latest report from the European Food Safety Authority (EFSA) stated that in 2014 alone, 13 countries in the European Union reported a total of 393 SFP outbreaks (both strong and weak evidence outbreaks). The most commonly reported single food category associated with SFP in strong evidence outbreaks was mixed food (29%), followed by pork and related products (9.7%) [2]. Moreover, in the United States, the Centers for Disease Control and Prevention (CDC) reported 17 SFP outbreaks and 566 cases in 2014. The most common contributing factors in these outbreaks were improper maintenance of the cold chain and inadequate food preparation practices, leading to the proliferation of pathogens [3].
In 1993, Wieneke and coworkers presented a comprehensive summary of the United Kingdom’s SFP reports from 1969–1990, showing that S. aureus has the capability to grow and produce enterotoxins in many different food items, mainly processed meat, poultry, and dairy products. Notably, ham was the most common food item involved in SFP, and accounted for 18% of the 359 cases reported [4]. A number of SFP cases have been reported to be linked to SED [4,5,6,7]. In 2014, an SFP outbreak was caused by soft cheese made from raw milk, where both low levels of SEA and high levels of SED (>200 ng of SED × g−1 of cheese) were detected together with >106 CFU × g−1 S. aureus cells [7].
To date, 24 different staphylococcal enterotoxin and enterotoxin-like proteins have been identified in S. aureus [8,9,10,11,12]. Among the five classical staphylococcal toxins, SEA and SED are the two most common enterotoxins associated with food poisoning [4,13]. Unlike SEA, which is encoded in a prophage, SED is located on the plasmid pIB485 and is regulated differently [14].
Previous studies have shown that the expression and formation of SED in S. aureus are partially regulated by the two-component quorum sensing system known as the accessory gene regulator (agr) [14,15,16]. However, high concentrations of SED may be produced independently of agr [16]. The agr operon contains three promoters, P1, P2, and P3, initiating the three transcripts, RNAI, RNAII, and RNAIII, respectively [17]. The RNAII transcript encodes AgrA, AgrB, AgrC, and AgrD, which constitute the quorum sensing system, while the RNAIII transcript encodes δ-hemolysin. RNAIII also serves as a regulatory signal of the Agr system [17,18]. Towards the end of the exponential growth phase, RNAIII levels increase in response to high cell density. This leads to induction of the transcription of many exotoxin genes (e.g., α-toxin, β-hemolysin, δ-hemolysin, toxic shock syndrome toxin-1, and SED), and reduction in the transcription of several genes encoding cell wall protein determinants (e.g., protein A, coagulase, and fibronectin binding protein) [19,20]. In an in vitro study, the level of SED of the S. aureus lab strains ISP546 (agr−) was found to be significantly reduced compared to RN4220 strain (agr+) [14]. RNAIII indirectly induces sed expression by repressing the repressor of toxins (Rot) [21]. The influence of the global Agr regulatory system and numerous food-related stresses including NaCl, nitrite, glucose, and lactic acid on sed expression has so far been studied exclusively using planktonic growth in cultivation broth [14,16,17,18,19,21,22,23,24,25].
The aim of this study was to investigate the influence of the Agr system on sed expression and SED formation in a real food environment. The strain S. aureus RKI1 used in this study was originally isolated from food and patient of an SFP outbreak, and pre-sliced boiled ham was chosen as the food matrix. Cell growth, sed expression, SED formation, and RNAIII (hld) expression in the RKI1 wild type and the corresponding isogenic RKI1Δagr mutant strain were compared during immobilized growth on the surface of slices of boiled ham.
2. Results and Discussions
2.1. Growth Pattern and Visual Changes in Ham
The growth patterns of the RKI1 wild type and RKI1Δagr strains were compared by viable counts on Baird-Parker agar (BPA) and plate count agar (PCA). No differences in growth patterns on boiled ham were found (Figure 1).
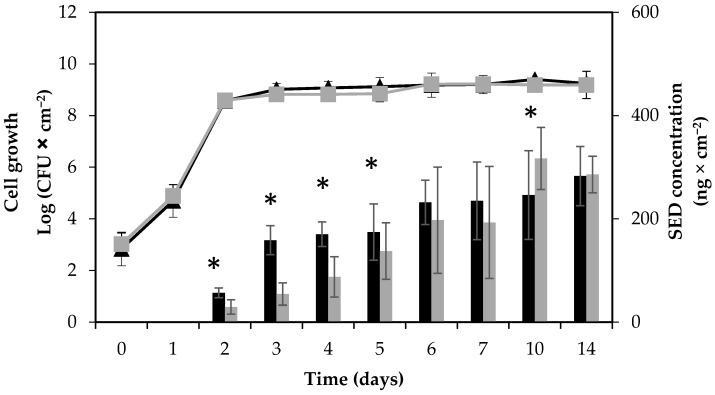
Figure 1.
Growth and enterotoxin D (SED) formation by S. aureus RKI1 and RKI1Δagr when grown on boiled ham at 22 °C for 14 days. Symbols indicate the strain of RKI1 (▲) and RKI1Δagr (■). Bars indicate SED concentration produced by RKI1 (black) and RKI1Δagr (grey). Average values and standard deviations were based on three independent experiments (except for day 10 and day 14, for which two independent experiments were performed), with three technical replicates each. Asterisks indicate statistically significant differences in the SED levels measured.
With a starting inoculum aimed at 103 CFU × cm−2 on the surface of boiled ham, both strains reached the stationary phase and a cell concentration of approximately 109 CFU × cm−2 on the second day of incubation. No decrease in viability was observed until the end of the experiment, and cell counts remained stable until day 14. The background flora, such as lactic acid bacteria (LAB), on the ham slices was monitored simultaneously using de Man–Rogosa–Sharpe (MRS) agar. No LAB was detected in the beginning of the experiment. While over time, the amount of LAB present varied between batches of boiled ham, the background microflora did not cause significant changes in S. aureus growth and SED formation. The absence of pre-existing S. aureus contamination of the boiled ham at the start of each experiment was also verified. Initial characterization of the RKI1 wild type and RKI1Δagr strains at optimal growth conditions 37 °C, Luria Bertani (LB) medium [26] for 24 h has been performed previously by Sihto and coworkers. They established that the strains grew similarly; both reaching a maximum cell density of approximately 108–109 CFU × mL−1 in the stationary phase after 10 h of growth with an initial inoculum of 5 × 103 CFU × mL−1 [23].
During growth of the wild type RKI1 strain on boiled ham, gradual decomposition of the ham was observed from day 3–4 and onwards. In the case of the RKI1Δagr strain, decomposition was delayed and appeared two days later (day 5–6) (data not shown). A clear reduction in secreted protease and lipase activity has been described previously in an agr mutant of S. epidermidis [27]. Both enzymes are known to contribute to the breakdown of tissue protein [28]. As there is a strong similarity in sequence and expression pattern between the Agr systems in both S. epidermidis and S. aureus [29], it is possible that there is also a reduction in the activities of both enzymes in RKI1Δagr, thus leading to delayed decomposition of the ham.
2.2. pH Changes
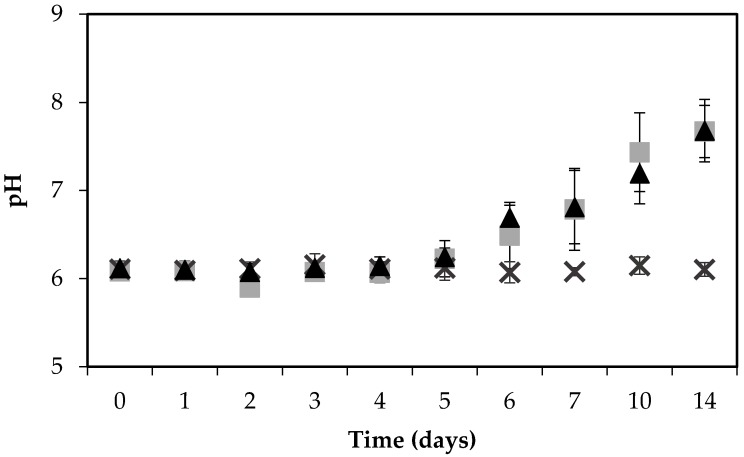
The pH of the ham increased noticeably, and similarly, when inoculated with either strain, from 6 to 7.7 during the 14 days’ incubation, in contrast to the control ham, the pH of which remained stable at pH 6 throughout the experiment (Figure 2).
Figure 2.
pH of boiled ham during the growth of S. aureus RKI1 (▲) and RKI1Δagr (■) at 22 °C for 14 days, compared with the control ham (×). Average values and standard deviations were based on three independent experiments (except for day 10 and day 14, for which two independent experiments were performed), with three technical replicates each.
The increase in pH may be the result of S. aureus-facilitated amino acid deamination releasing ammonia into the food matrix [30]. The change in pH could potentially influence the growth of bacteria, and although S. aureus can grow in a wide pH range from pH 4 to 10, the optimum is a pH of 6 to 7 [8]. In contrast to these findings, another study on a food matrix, investigating the expression and formation of SEA by three different S. aureus strains on pork sausages for 14 days, found that the pH remained stable at pH 6.2 throughout the experiment [31].
2.3. SED Protein Levels
The SED concentration was monitored using quantitative ELISA. A steady increase in SED formation was observed throughout the experiment for both strains; however, the SED concentrations produced by the RKI1Δagr strain were significantly lower than those produced by the wild type from day 2 to day 5 of incubation. In the later stages of growth (10 and 14 days), the SED concentrations were comparable between the two strains (Figure 1). The accumulation of SED was clearly noticeable in the early to mid-stationary phase, from day 2 to day 6. This observation also corroborates previous reports of higher SED production during the post-exponential growth phase, a typical characteristic of toxins regulated by the Agr system [19,23]. The SED concentration formed by RKI1 increased to above 150 ng × cm−2 (>8.9 µg per ham slice) from day 3 and onwards, and that produced by RKI1Δagr from day 5. Assuming that the whole slice of ham is consumed, the concentration of SED formed by day 3 is more than 400 times higher than the amount of 20–100 ng of enterotoxin needed to cause SFP [32]. This observation strengthens the claim that SED can still be formed at high concentrations in the absence of the Agr system [16]. Furthermore, the results suggest that the Agr system has a more pronounced effect during initial SED formation, but not in the late stationary phase.
In a previous study on S. aureus strain Sa45 grown on boiled ham (not pre-sliced), a decrease in SED levels was observed after 3 days of incubation [33]. This was not observed in the present study. This could be explained by different factors, such as strain-specific variation in enterotoxin production or the type of boiled ham supporting different levels or types of background flora.
2.4. sed and RNAIII (hld) mRNA Levels
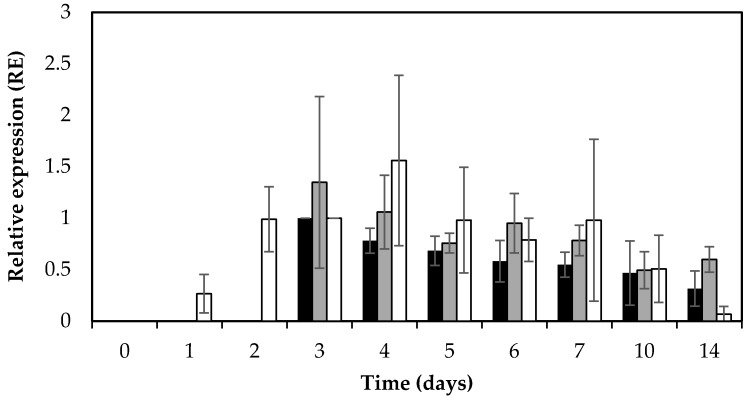
In addition to SED concentration, sed transcription was monitored by qPCR throughout the 14 days of the experiment. Active and extended sed transcription was measurable in both RKI1 and RKI1Δagr, and was observed from day 3, remaining relatively stable until day 14 (Figure 3). This extended sed transcription of both strains translates into the corresponding accumulation of SED measured by ELISA (Figure 1). This is also in agreement with previous findings that extended sed expression occurred on both smoked and boiled ham during a period of seven days when incubated at 23 °C [33]. The sed mRNA levels did not reflect the respective SED concentrations; RKI1Δagr producing significantly lower amounts of SED than RKI1 from day 2 to day 5. This finding corresponds to a previous report suggesting that sed expression does not always correspond to the amount of SED formed [23]. Similar findings have also recently been observed for other enterotoxins including SEA and SEC [31,34]. Previous studies have reported that Rot is affecting the transcription of sed and also SarA and SigB are indirectly affecting the transcription of sed via the Agr system [35,36,37]. Altogether it is most likely that there are other and/or additional possible mechanisms affecting the translation of enterotoxins after the transcripts have been made that are yet to be discovered.
Figure 3.
Average relative expression (RE) levels of sed and RNAIII (hld) in S. aureus RKI1 and RKI1Δagr when grown on boiled ham at 22 °C for 14 days. Bars indicate relative sed expression in RKI1 (black) and RKI1Δagr (grey) and relative RNAIII (hld) expression of RKI1 (white). Average values and standard deviations were based on three independent experiments for sed (except for day 10 and day 14 where two independent experiments were performed), and two independent experiments for RNAIII (hld).
In order to monitor the activity of the Agr system in relation to sed expression, the RNAIII (hld) expression level was also monitored using qPCR. As expected, the RNAIII (hld) transcripts were absent in RKI1Δagr. The RKI1 strain showed increased expression of RNAIII (hld) transcripts from day 1 to day 4 (early exponential–early stationary phase) and a decrease in later stages, from day 5 to day 14 (mid–late stationary phase) of incubation (Figure 3).
The observed increase in RNAIII (hld) transcripts in the exponential to early stationary phase is in line with previous reports that the Agr system is a quorum sensing system, where P2 and P3 promoters (for the RNAII and RNAIII transcripts, respectively) are weakly active in the early exponential phase, becoming more induced later in the early stationary phase [17,20,25]. The pattern of RNAIII (hld) expression in RKI1 was similar to the pattern of sed transcription. Interestingly, in RKI1Δagr, where the RNAIII (hld) transcript was absent, the pattern and amount of sed transcripts were also similar to those in RKI1, suggesting that the absence of RNAIII (hld) does not significantly affect the transcription of sed.
3. Conclusions
Deletion of the agr gene in RKI1 had a more pronounced effect on SED formation during immobilized growth on boiled ham than when the strain was grown planktonically in liquid LB medium, as previously reported [23]. In LB medium there was an observable but non-significant reduction in SED formation by the RKI1Δagr strain compared to the RKI1 wild type strain, from mid-exponential to early stationary phase, while SED formation in the late stationary growth phase was comparable in the two strains [23]. This emphasizes the importance of investigating the influence of the Agr system on toxin formation in real food environments, where the increased complexity, including, for example, growth on solid surfaces, and the presence of background flora and preservatives, is also taken into account. The results of this study and several others have demonstrated that the expression, formation and regulation of S. aureus enterotoxins are dependent not only on the strain, but also on the growth matrix [23,33,38,39]. Further studies are required including several strains and foods of varying composition to elucidate the influence of the complex regulatory network behind SED formation.
In conclusion, deletion of the agr locus in S. aureus RKI1 strain did not affect cell growth on boiled ham, but SED formation was significantly lower in the early to mid-stationary phase. The results also showed that sed expression levels do not necessarily reflect SED protein levels, and that deletion of the Agr system did not significantly affect the transcription of sed in S. aureus RKI1 grown on boiled ham. This is the first study presenting data on the influence of the Agr system on SED formation during immobilized growth directly on a food matrix.
4. Materials and Methods
4.1. Bacterial Strains and Pre-Culture Conditions
S. aureus strain RKI1 was used in this study. This is a CC8/t648 strain isolated from food and the feces of an SFP patient in Germany in 2007. An isogenic agr deletion mutant RKI1Δagr was constructed by transduction using phage 80α, as described previously [22,40]. Strains were stored as glycerol stocks at −80 °C and were resuscitated by streaking on brain heart infusion (BHI) agar (Difco Laboratories; BD Diagnostic Systems, Le Pont de Claix, France) or BHI agar supplemented with erythromycin (10 µg × mL−1, Sigma-Aldrich, Stockholm, Sweden). To prepare pre-cultures, a single colony was transferred to 25 mL BHI broth in a 250 mL baffled Erlenmeyer flask and incubated at 37 °C, 200 rpm, for 16–18 h. These pre-cultures were then centrifuged at 3220× g for 10 min at 4 °C (5810R, Eppendorf AG, Hamburg, Germany) and the cell pellets were washed with sterile 0.9% NaCl solution (Merck Millipore, Darmstadt, Germany) twice to remove preformed toxins and metabolites.
4.2. Experimental Setup
Commercial sliced, boiled ham, containing 95% ham pieces, salt, dextrose, glucose syrup, antioxidant E301 (sodium ascorbate), stabilizer E451 (sodium triphosphate) and preservative E250 (sodium nitrite), packed in a modified atmosphere, was bought from a local supermarket prior to each experiment. The boiled ham was used for the experiments approximately 13–15 days after the production date. Three independent experiments (#1–3) were performed using RKI1, RKI1Δagr, and a non-inoculated control. Washed S. aureus cell suspensions were diluted in sterile 0.9% NaCl solution in 10-fold dilution series to reach a concentration of 105 CFU × mL−1. An aliquot of 100 µL of the 105 CFU × mL−1 cell suspension was inoculated evenly onto the surface of the ham slices. Before inoculation, each ham slice was placed aseptically in a Petri dish, with an internal diameter of 8.7 cm (Sarstedt, Nümbrecht, Germany), resulting in an exposed surface area of 59.45 cm2. The 100 µL of cell suspension resulted in a starting cell concentration of about 103 CFU × cm−2 over the surface of the ham. The mean weight of each ham slice was 8.37 ± 0.46 g (n = 84). One ham slice was prepared for each strain and time point for sample collection. Control ham slices without S. aureus inoculum were prepared in the same way. Immediately after inoculation, the ham slices were incubated in Petri dishes with a lid at 4 °C for 20 min to allow attachment of the cells to the surface. The ham slices were then transferred to a 22 °C incubator (Termaks, B8000S series, Bergen, Norway) and stored aerobically for 7 days for experiment #1 and for 14 days for experiments #2 and #3. Control ham slices were treated and incubated in the same way. Samples were collected daily from day 0 to 7, as well as on day 10 and day 14 for viable cell count determination, ELISA and RNA analyses.
To extract bacterial cells from the ham, the ham slice was aseptically transferred to a stomacher bag with a filter (BagLight 400 PolySilk, Interscience, Saint Nom, France), weighed, and six volumes of 0.9% NaCl were added to detach and resuspend the cells. The ham and 0.9% NaCl solution were homogenized by hand for 30 s followed by filtration through the filter of the stomacher bag. The filtered ham suspension was retrieved from the stomacher bag for viable cell count analysis and pH measurement (FE20 FiveEasy pH, Mettler Toledo, Stockholm, Sweden). The cell suspension was 10-fold serially diluted using sterile 0.9% NaCl and plated on PCA, BPA (both from Fluka analytical, Sigma-Aldrich, Stockholm, Sweden), and MRS agar (Difco Laboratories; BD Diagnostic Systems, Le Point de Claix, France). MRS agar was used to detect the possible presence of LAB. When necessary, fat and meat particles were removed from the filtered homogenate by pre-centrifugation for 3 min at 100× g using a swinging bucket rotor (5810R, Eppendorf AG).
The supernatant from the pre-centrifugation step was further processed to retrieve the bacterial cells for RNA analysis by centrifugation for 10 min at 3220× g at 4 °C (5810R, Eppendorf AG). The pelleted cells and supernatants were separated and stored at −80 °C and −20 °C, respectively, until RNA and ELISA analyses were performed.
4.3. RNA Extraction and cDNA Synthesis
To extract total RNA, each cell pellet was resuspended in 500 µL ice-cold TES buffer, pH 7.5 [50 mM Tris (BDH Prolabo, VWR International, Stockholm, Sweden), 5 mM EDTA and 50 mM NaCl (Merck Millipore, Darmstadt, Germany)]. Cell suspensions were transferred to Precellys lysing kit tubes (VK 0.1) (Bertin Technologies, Montigny-le-Bretonneux, France) and lysed using Precellys 24-homogenizer unit and liquid nitrogen, in a three-cycle run of 60 s at 6500 rpm. Total RNA was extracted using a phenol (Aqua-Phenol, water-saturated, MP Biomedicals, Santa Ana, CA, USA) and chloroform (Merck Millipore, Darmstadt, Germany) extraction method, as described previously [41]. Total RNA was dissolved and kept in RNA storage solution (Applied Biosystems, Foster City, CA, USA). The total RNA concentration and purity were measured using a BioDrop spectrophotometer (BioDrop TOUCH UV/Visible Spectrophotometer, Integrated Scientific Solutions Inc., Walnut Creek, CA, USA), and the RNA was stored at −80 °C. RNA samples were treated with RQ1 RNase-free DNase (Promega Co., Madison, WI, USA) according to the manufacturer’s recommendations, and verified using qPCR prior to the reverse transcription (RT) reaction.
cDNA synthesis was performed using a Gene Amp 9700 thermal cycler (Perkin-Elmer Cetus, Norwalk, CT, USA). RT reactions were performed separately for sed, rrn (used as a reference), and hld. Each first-strand synthesis reaction in a total volume of 20 µL contained RNA template (sed assay: 0.25 µg RNA for sed and 0.1 µg RNA for rrn; RNAIII (hld) assay: 0.25 µg of RNA for both RNAIII (hld) and rrn), 0.5 mM GSEDR-2/rRNA reverse primers (sed assay) or RT primer mix (RNAIII (hld) assay) (Qiagen, Hilden, Germany), 5 mM of dNTPs, 20 U of RNasin RNase inhibitor (Promega Co., Madison, WI, USA), 5 mM dithiothreitol and 1 × first-strand buffer. The mixture was heated to 65 °C for 5 min and chilled on ice before adding 200 U Superscript II RNase reverse transcriptase (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). After the addition of RT enzyme, the mixture was incubated at 42 °C for 50 min followed by incubation at 70 °C for 15 min. The primers and probes [33,42] were purchased from TIB Molbiol GmbH, Berlin, Germany and are presented in Table 1.
Table 1.
Sequence of primers and hybridization probes used for cDNA synthesis and qPCR.
| Target | Primer/Probe | Nucleotide Sequence (5′→3′) | Reference |
|---|---|---|---|
| sed | SED-1 | CTAGTTTGGTAATATCTCCT | [33] |
| GSEDR-2 | ATTGGTATTTTTTTTCGTTC | ||
| entD-FL | TACCCTATAAGATATAGCATTAATTGTT–FL | ||
| entD-LC | LC-Red640–TGGTGGTGAAATAGATAGGACTGCTTG–p | ||
| rrn | rRNA forward | TGTCGTGAGATGTTGGG | [33] |
| rRNA reverse | ACTAGCGATTCCAGCTT | ||
| Probe 1 | GGACAATACAAAGGGCAGCG–FL | ||
| Probe 2 | LC-R705–ACCGCGAGGTCAAGCA–p | ||
| RNAIII (hld) | Forward | TAAGGAAGGAGTGATTTCAATGG | [42] |
| Reverse | GTGAATTTGTTCACTGTGTCGAT |
4.4. qPCR Assay
All qPCR reactions were performed in a 20 µL reaction volume containing a 4 µL cDNA sample using a LightCycler™ 2.0 instrument (Roche Diagnostics GmbH, Mannheim, Germany) with the following program: denaturation at 95 °C for 1 min, followed by 45 cycles of denaturation at 95 °C for 0 s, annealing at 48 °C for 5 s and extension at 72 °C for 25 s with a single fluorescence measurement at the end of the extension step.
For the sed assay, the qPCR mixture consisted of: cDNA template, 1 × PCR Tth buffer, MgCl2 (2.75 mM for sed, 4.6 mM for rrn), 0.2 mM of the dNTP mixture, 0.5 µM of both forward and reverse primers, 0.15 µM of each hybridization probe and 0.05 U of Tth polymerase. The qPCR mixture for the RNAIII (hld) assay consisted of: cDNA template, 1 × PCR Tth buffer, 2.5 mM MgCl2, 0.2 mM of the dNTP mixture, 0.5 µM of both forward and reverse primers, 1 × EvaGreen® dye (Biotium, Fremont, CA, USA) and 0.05 U of Tth polymerase. All qPCR reagents, with the exception of primers, probes, and EvaGreen dye, were purchased from Roche Diagnostics GmbH, Mannheim, Germany.
Relative expression (RE) levels of both sed and RNAIII (hld) were calculated by comparing the expression of the gene of interest to the expression of the reference housekeeping gene (16S rrn gene) from the same qPCR reaction [43]. Expression data for sed and RNAIII (hld) on day 3 were chosen as the calibrator samples. The amplification efficiency and the log-linear range of amplification of each qPCR reaction were determined by including a standard curve, constructed by serial dilutions of total RNA for targets (sed, hld) and the reference gene (rrn). The dilutions were reverse transcribed into cDNA and analyzed in three technical replicates. Analyses of samples and the reference gene for the sed assays were performed in three independent experiments (#1–3) using three technical replicates in each. RNAIII (hld) expression was analyzed in two independent experiments (#2–3). The expression of hld in RKI1 and the effect of agr deletion on sed expression were evaluated by comparing relative expression levels at each sampling point. The absence of genomic DNA in the RNA samples was assured by including a no-reverse transcriptase control.
4.5. ELISA
ELISA analysis was performed according to a modified protocol originally designed for the detection of SEA, as described previously [44]. In this study, affinity-purified polyclonal SED antibodies from sheep (Toxin Technology, Inc., Sarasota, FL, USA) were used to measure the SED concentration. A standard curve for quantification was obtained using purified SED (Toxin Technology, Inc.). Absorbance was measured at 405 nm with a Multiskan Ascent spectrophotometer (Electron Corporation, Thermo Fisher Scientific, Waltham, MA, USA). The detection limit of SED in the ELISA assay was calculated to be 0.31 ng × mL−1. In each independent experiment, ELISA measurements were performed on three technical replicates.
4.6. Statistical Analysis
SED concentrations and sed relative expression data of the wild type and Δagr mutant were confirmed to be normally distributed and compared using Student’s t-test with a two-tailed distribution and equal variance in Microsoft Office Excel (2013). Differences were considered significant at p < 0.05.
Acknowledgments
We would like to acknowledge the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), the Swiss National Research Program 69 (40690_145211/1), the Forschungskredit of the University of Zurich (FK15-055), and the Herrmann Herzer Foundation for financial support.
Author Contributions
Yusak Budi Susilo designed the study together with the co-authors, performed the experiments, analyzed the data and wrote this paper. Henna-Maria Sihto participated in the experiments, data analysis and manuscript preparation. Peter Rådström co-supervised the research work and assisted data interpretation. Roger Stephan and Sophia Johler provided the RKI1Δagr strain, co-supervised the research work and contributed in manuscript preparation. Jenny Schelin was the principal investigator and responsible for manuscript preparation, data interpretation and all other aspects of the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Balaban N., Rasooly A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000;61:1–10. doi: 10.1016/S0168-1605(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 2.EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015;13:4329. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services . Surveillance for Foodborne Disease Outbreaks, United States, 2014, Annual Report. CDC; Atlanta, GA, USA: 2016. [Google Scholar]
- 4.Wieneke A.A., Roberts D., Gilbert R.J. Staphylococcal food poisoning in the United Kingdom, 1969–1990. Epidemiol. Infect. 1993;110:519–531. doi: 10.1017/S0950268800050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerouanton A., Hennekinne J.A., Letertre C., Petit L., Chesneau O., Brisabois A., De Buyser M.L. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int. J. Food Microbiol. 2007;115:369–375. doi: 10.1016/j.ijfoodmicro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Schmid D., Fretz R., Winter P., Mann M., Hoger G., Stoger A., Ruppitsch W., Ladstatter J., Mayer N., de Martin A., et al. Outbreak of staphylococcal food intoxication after consumption of pasteurized milk products, June 2007, Austria. Wien. Klin. Wochenschr. 2009;121:125–131. doi: 10.1007/s00508-008-1132-0. [DOI] [PubMed] [Google Scholar]
- 7.Johler S., Weder D., Bridy C., Huguenin M.C., Robert L., Hummerjohann J., Stephan R. Outbreak of staphylococcal food poisoning among children and staff at a Swiss boarding school due to soft cheese made from raw milk. J. Dairy Sci. 2015;98:2944–2948. doi: 10.3168/jds.2014-9123. [DOI] [PubMed] [Google Scholar]
- 8.Schelin J., Wallin-Carlquist N., Cohn M.T., Lindqvist R., Barker G.C., Rådström P. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence. 2011;2:580–592. doi: 10.4161/viru.2.6.18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennekinne J.A., De Buyser M.L., Dragacci S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012;36:815–836. doi: 10.1111/j.1574-6976.2011.00311.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilson G.J., Seo K.S., Cartwright R.A., Connelley T., Chuang-Smith O.N., Merriman J.A., Guinane C.M., Park J.Y., Bohach G.A., Schlievert P.M., et al. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog. 2011;7:e1002271. doi: 10.1371/journal.ppat.1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono H.K., Sato’o Y., Narita K., Naito I., Hirose S., Hisatsune J., Asano K., Hu D.L., Omoe K., Sugai M., et al. Identification and characterization of a novel staphylococcal emetic toxin. Appl. Environ. Microbiol. 2015;81:7034–7040. doi: 10.1128/AEM.01873-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okumura K., Shimomura Y., Murayama S.Y., Yagi J., Ubukata K., Kirikae T., Miyoshi-Akiyama T. Evolutionary paths of streptococcal and staphylococcal superantigens. BMC Genom. 2012;13:404. doi: 10.1186/1471-2164-13-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergdoll M.S., Czop J.K., Gould S.S. Enterotoxin synthesis by the staphylococci. Ann. N. Y. Acad. Sci. 1974;236:307–316. doi: 10.1111/j.1749-6632.1974.tb41500.x. [DOI] [PubMed] [Google Scholar]
- 14.Bayles K.W., Iandolo J.J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J. Bacteriol. 1989;171:4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoendel M., Kavanaugh J.S., Flack C.E., Horswill A.R. Peptide signaling in the staphylococci. Chem. Rev. 2011;111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarwood J.M., Schlievert P.M. Quorum sensing in Staphylococcus infections. J. Clin. Investig. 2003;112:1620–1625. doi: 10.1172/JCI200320442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novick R.P., Ross H.F., Projan S.J., Kornblum J., Kreiswirth B., Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janzon L., Arvidson S. The role of the delta-lysin gene (Hld) in the regulation of virulence genes by the accessory gene regulator (Agr) in Staphylococcus aureus. EMBO J. 1990;9:1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronner S., Monteil H., Prevost G. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol. Rev. 2004;28:183–200. doi: 10.1016/j.femsre.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Vandenesch F., Kornblum J., Novick R.P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J. Bacteriol. 1991;173:6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Said-Salim B., Dunman P.M., McAleese F.M., Macapagal D., Murphy E., McNamara P.J., Arvidson S., Foster T.J., Projan S.J., Kreiswirth B.N. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 2003;185:610–619. doi: 10.1128/JB.185.2.610-619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sihto H.M., Tasara T., Stephan R., Johler S. Temporal expression of the staphylococcal enterotoxin D gene under NaCl stress conditions. FEMS Microbiol. Lett. 2015;362:6. doi: 10.1093/femsle/fnv024. [DOI] [PubMed] [Google Scholar]
- 23.Sihto H.M., Budi Susilo Y., Tasara T., Rådström P., Stephan R., Schelin J., Johler S. Effect of sodium nitrite and regulatory mutations Δagr, ΔsarA, and ΔsigB on the mRNA and protein levels of staphylococcal enterotoxin D. Food Control. 2016;65:37–45. doi: 10.1016/j.foodcont.2016.01.007. [DOI] [Google Scholar]
- 24.Sihto H.M., Tasara T., Stephan R., Johler S. Growth behavior and temporal enterotoxin D expression of Staphylococcus aureus strains under glucose and lactic acid stress. Food Control. 2016;62:69–73. doi: 10.1016/j.foodcont.2015.10.008. [DOI] [Google Scholar]
- 25.Janzon L., Lofdahl S., Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol. Gen. Genet. MGG. 1989;219:480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- 26.Bertani G. Studies on lysogenesis I.: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuong C., Gotz F., Otto M. Construction and characterization of an agr deletion mutant of Staphylococcus epidermidis. Infect. Immun. 2000;68:1048–1053. doi: 10.1128/IAI.68.3.1048-1053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goguen J.D., Hoe N.P., Subrahmanyam Y.V. Proteases and bacterial virulence: A view from the trenches. Infect. Agents Dis. 1995;4:47–54. [PubMed] [Google Scholar]
- 29.Otto M., Sussmuth R., Jung G., Gotz F. Structure of the pheromone peptide of the Staphylococcus epidermidis agr system. FEBS Lett. 1998;424:89–94. doi: 10.1016/S0014-5793(98)00145-8. [DOI] [PubMed] [Google Scholar]
- 30.Crossley K.B. Staphylococci in Human Disease. 2nd ed. Wiley-Blackwell; Hoboken, NJ, USA: 2010. [Google Scholar]
- 31.Zeaki N., Cao R., Skandamis P.N., Rådström P., Schelin J. Assessment of high and low enterotoxin A producing Staphylococcus aureus strains on pork sausage. Int. J. Food Microbiol. 2014;182:44–50. doi: 10.1016/j.ijfoodmicro.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Asao T., Kumeda Y., Kawai T., Shibata T., Oda H., Haruki K., Nakazawa H., Kozaki S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: Estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect. 2003;130:33–40. doi: 10.1017/S0950268802007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dora M., Wallin-Carlquist N., Schelin J., Borch E., Rådström P. Extended staphylococcal enterotoxin D expression in ham products. Food Microbiol. 2011;28:617–620. doi: 10.1016/j.fm.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Valihrach L., Alibayov B., Zdenkova K., Demnerova K. Expression and production of staphylococcal enterotoxin C is substantially reduced in milk. Food Microbiol. 2014;44:54–59. doi: 10.1016/j.fm.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Tseng C.W., Zhang S., Stewart G.C. Accessory gene regulator control of staphylococcal enterotoxin d gene expression. J. Bacteriol. 2004;186:1793–1801. doi: 10.1128/JB.186.6.1793-1801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bischoff M., Entenza J.M., Giachino P. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 2001;183:5171–5179. doi: 10.1128/JB.183.17.5171-5179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chien Y.T., Cheung A.L. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J. Biol. Chem. 1998;273:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 38.Zeaki N., Susilo Y.B., Pregiel A., Rådström P., Schelin J. Prophage-encoded staphylococcal enterotoxin A: Regulation of production in Staphylococcus aureus strains representing different Sea regions. Toxins. 2015;7:5359–5376. doi: 10.3390/toxins7124889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkovic A. Incidence, growth and enterotoxin production of Staphylococcus aureus in insufficiently dried traditional beef ham “govedja prsuta” under different storage conditions. Food Control. 2012;27:369–373. doi: 10.1016/j.foodcont.2012.03.028. [DOI] [Google Scholar]
- 40.Charpentier E., Anton A.I., Barry P., Alfonso B., Fang Y., Novick R.P. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl. Environ. Microbiol. 2004;70:6076–6085. doi: 10.1128/AEM.70.10.6076-6085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovenklev M., Holst E., Borch E., Rådström P. Relative neurotoxin gene expression in Clostridium botulinum type B, determined using quantitative reverse transcription-PCR. Appl. Environ. Microbiol. 2004;70:2919–2927. doi: 10.1128/AEM.70.5.2919-2927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Even S., Charlier C., Nouaille S., Zakour N.L.B., Cretenet M., Cousin F.J., Gautier M., Cocaign-Bousquet M., Loubiere P., Le Loir Y. Staphylococcus aureus Virulence Expression Is Impaired by Lactococcus lactis in Mixed Cultures. Appl. Environ. Microbiol. 2009;75:4459–4472. doi: 10.1128/AEM.02388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallin-Carlquist N., Cao R., Marta D., da Silva A.S., Schelin J., Rådström P. Acetic acid increases the phage-encoded enterotoxin A expression in Staphylococcus aureus. BMC Microbiol. 2010;10:147. doi: 10.1186/1471-2180-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]