Abstract
Antimicrobial peptides (AMPs) are a group of small peptides, which are secreted by almost all creatures in nature. They have been explored in therapeutic and agricultural aspects as they are toxic to many bacteria. A considerable amount of work has been conducted in analyzing 16S and metagenomics of the gastrointestinal (GI) microbiome of grass carp (Ctenopharyngodon idellus). However, these datasets are still untapped resources. In this present study, a homologous search was performed to predict AMPs from our newly generated metagenome of grass carp. We identified five AMPs with high similarities to previously reported bacterial toxins, such as lantibiotic and class II bacteriocins. In addition, we observed that the top abundant genus in the GI microbiota of the grass carp was generally consistent with the putative AMP-producing strains, which are mainly from Lactobacillales. Furthermore, we constructed the phylogenetic relationship of these putative AMP-producing bacteria existing in the GI of grass carp and some popular commercial probiotics (commonly used for microecologics), demonstrating that they are closely related. Thus, these strains have the potential to be developed into novel microecologics. In a word, we provide a high-throughput way to discover AMPs from fish GI microbiota, which can be developed as alternative pathogen antagonists (toxins) for microecologics or probiotic supplements.
Keywords: antimicrobial peptide (AMP), fish gastrointestinal microbiota, high throughput identification, AMP-producing bacteria
1. Introduction
Antimicrobial peptides (AMPs) are a group of small peptides, which are secreted by almost all creatures in nature. They are being explored with regards to their potential therapeutic and agricultural uses as they are toxic to many bacteria. These AMPs synthesized in the ribosomes of bacteria are also called bacteriocins, which normally present antibacterial activity towards closely-related strains, although it has been reported that a broad range of antimicrobial activity occurs in some bacteriocins [1]. Those bacteriocins of lactic acid bacteria (LAB) have raised a considerable amount of attention nowadays [2]. Nisin, the first bacteriocin applied to therapeutics without any side effects, can effectively inhibit the growth of Gram-positive (G+) bacteria, including many members of Lactobacillus, Pediococcus, Leuconostoc, Micrococcus, Staphylococcus, Listeria, and Clostridium [3].
It is well known that the majority of G+ bacteria are beneficial to intestinal health. Lactobacillus is the largest genus of the LAB group (with over 50 species in total), contributing important metabolic reactions to the production of cheese, yogurt, and other dairy products [4]. They are commonly found in the oral, vaginal, and intestinal regions of many animals. These organisms have also been shown to stimulate the immune system and have antibacterial activity against intestinal pathogens, which indicates that they may be useful as probiotics. Pediococcus acidilactici is usually used to regulate gastrointestinal (GI) floras as they balance microecology by acid production [5]. Bacillus subtilis is a commonly applied microecologic in agriculture feed additives and food products [6]. Streptococcus contains many commensal floras located at the epidermis, respiratory tract, and gut, such as beneficial S. thermophilus and pathogenic S. pyogenes [7].These probiotics are active against microorganisms by antagonism in vivo, particularly against those closely-related competing bacteria. Some of them have a broader inhibitory spectrum in certain conditions, while some of them have a rather narrow spectrum. Additionally, their metabolites improve the activity of acidic proteases and prevent the production of harmful molecules.
The grass carp, Ctenopharyngodon idellus, is an herbivorous freshwater fish of the Cyprinidae family and a commercial species widely cultivated in China. Many previous works have focused on employing the 16S rRNA method in analyzing the GI microbiota of grass carp [8]. However, in order to obtain a more accurate phylogenetic composition, the whole metagenomic data should be put to use. Meanwhile, since antibiotic abuse in aquaculture has caused many problems, such as pathogen resistance and food contamination, we are trying to screen new bacterial species with a high efficacy of AMP production as potential probiotics to combat aquatic pathogens.
In this research, we sequenced the whole metagenome of the GI microbiota in grass carp and a high-throughput homologous search was performed to predict AMPs. In addition, the phylogenetic relationship of some genera in grass carp GI microbiota, putative AMP-producing strains and commonly used probiotics for microecologics were described. Interestingly, these newly identified AMP-producing strains have the potential to be developed into novel microecologics or probiotic supplements.
2. Results
2.1. Summary of Achieved Metagenome Datasets
In total, 11 gigabase (Gb) of raw data were generated from a pool of sequencing data of eight GI samples (see more details in Section 4.1). After filtering the host contamination, 218 megabase (Mb) of metagenome data were assembled. Finally, from these acquired data, a total of 4966 species, 1453 genera, 378 families, 178 orders, 76 classes, and 54 phyla were annotated (Table S1). The GI microbiome of grass carp are dominated by members of phyla Proteobacteria (36.12%), Firmicutes (7.14%), Bacteroidetes (5.16%), Fusobacteria (3.82%), and Actinobacteria (1.31%; Figure 1). The well represented genera are Aeromonas (19.02%), Shewanella (3.79%), Cetobacterium (2.96%), Bacteroides (1.60%), and Clostridium (0.81%), respectively (Figure 1, Table S1).
Figure 1.
Relative abundance of phyla annotated in the metagenome of grass carp.
2.2. AMPs Identified in the Metagenome
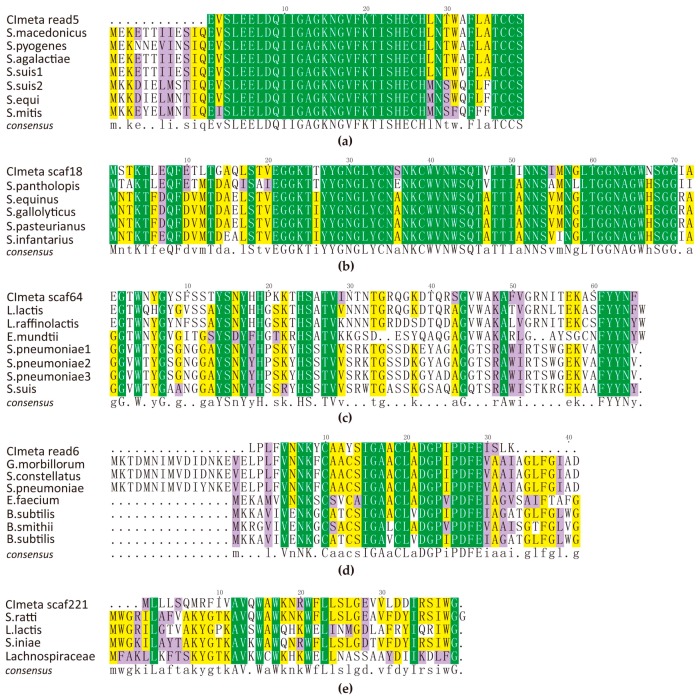
Based on sequence similarity, we identified five AMPs by homologous alignment (Figure 2). However, we could not assert the exact strains for producing them, although four of them seem to be incomplete. Meanwhile, we employed BLASTP to search against public databases in NCBI and found that they are novel bacteriocins except for lantibiotic (Table 1). These putative AMP-producing bacteria, summarized in Table 1, will be useful for further screening and validation of potential probiotic supplements.
Figure 2.
Multiple sequence alignment of identified AMPs with public bacteriocins in the NCBI. (a) lantibiotic; (b) Pediocin-like (Class IIa); (c) Lactococcin 972 (Class IIc); (d) SubtilosinA (Class IIc); (e) Aureocin-like (Class IId). Please note that with the exception of (b), the sequences seem to be incomplete, which may be due to missing reads in the assembly procedure. Similar amino acids of at least 50% were shaded in purple, 80% were shaded in yellow, and those with same characteristics were shaded in green.
Table 1.
Summary of known bacteriocins in the NCBI with the highest ranked hits.
| Category | Identified AMPs 1 | NCBI AMPs | GenBank Accession No. | NCBI AMP-Producing Bacterial Species |
|---|---|---|---|---|
| Class I (Lantibiotic) | a | McdA | ABI30227.1 | Streptococcus macedonicus |
| Streptococcin A-FF22 | P36501.1 | Streptococcus pyogenes | ||
| Nukacin | CZT39525.1 | Streptococcus agalactiae | ||
| Macedocin | CZA89639.1 | Streptococcus suis | ||
| type-A lantibiotic | EEF65507.1 | Streptococcus suis 89/1591 | ||
| type A2 lantipeptide | WP_041790396.1 | Streptococcus equi | ||
| type A2 lantipeptide | WP_033685037.1 | Streptococcus mitis | ||
| lantibiotic nukacin | KEO43205.1 | Streptococcus salivarius | ||
| Class IIa (Pediocin-like) | b | Hypothetical | AND78905.1 | Streptococcus pantholopis |
| piscicolin-126 | EFM26697.1 | Streptococcus equinus | ||
| Bacteriocin | KUE92317.1 | Streptococcus gallolyticus | ||
| putative piscicolin-126 | KXI11412.1 | Streptococcus pasteurianus | ||
| infantaricin E | AHW46171.1 | Streptococcus infantarius | ||
| MundKS | ACI25616.1 | Enterococcus mundtii | ||
| leucocin C | AEY55410.1 | Leuconostoc carnosum | ||
| SakX | AAP44569.1 | Lactobacillus sakei | ||
| Class IIc | c | lactococcin 972 | CAA05247.1 | Lactococcus lactis |
| lactococcin 972 family | WP_061775386.1 | Lactococcus raffinolactis | ||
| lactococcin 972 family | WP_065096983.1 | Enterococcus mundtii | ||
| lactococcin 972 | CTL98394.1 | Streptococcus pneumoniae | ||
| lactococcin 972 family | SNP59245.1 | Streptococcus pneumoniae | ||
| lactococcin 972 family | CWJ26067.1 | Streptococcus pneumoniae | ||
| lactococcin 972 family | CYW17154.1 | Streptococcus suis | ||
| d | sboA protein | EFV34710.1 | Gemella morbillorum M424 | |
| subtilosin A | EGV07582.1 | Streptococcus constellatus | ||
| putative subtilosin A | CVX48913.1 | Streptococcus pneumoniae | ||
| Hypothetical | ELB10075.1 | Enterococcus faecium | ||
| subtilosin A | CAD23198.1 | Bacillus subtilis | ||
| subtilosin A | AKP46487.1 | Bacillus smithii | ||
| subtilosin A | WP_087992738.1 | Bacillus subtilis | ||
| Class IId | e | Mutacin BhtB | AAZ76605.1 | Streptococcus ratti |
| lactolisterin BU | SDR48784.1 | Lactococcus lactis | ||
| Hypothetical | WP_081348647.1 | Streptococcus iniae | ||
| Aureocin-like | SFG15527.1 | Lachnospiraceae C7 |
1 See more details in Figure 2.
A 39 amino acid (aa) fragments identified from filtered reads of current metagenome are exactly the same as the lantibiotics of Streptococcus spp., including S. macedonicus, S. pyogenes, and S. agalactiae. This post-translationally modified peptide with characteristic thioether amino acids represents a large group of promising candidates for future applications as it can be a good template for improving lanthipeptide analogs or introducing thioether cross-links into reported therapeutics [9]. The mature peptide GKNGVFKTISHECHLNTWAFLATCCS belongs to lacticin 481-like peptides of class I of lantibiotics, which are LanM modified globular peptides with a classification into class I type B lantibiotic [10]. However, their structures require further confirmation.
The predicted complete sequence of a pediocin-like bacteriocin belongs to class IIa as it has a highly conserved consensus sequence YYGNGX2CX4CXVXn [11] within its N-terminal domain. This 49-aa mature peptide seems to be produced primarily by strains of genus Streptococcus [12]. Lactococcin 972 (lcn972) was first isolated from Lactococcus lactis subsp. lactis IPLA 972 [13]. Our predicted lcn972-like peptide also has a high similarity to lcn972 and thus, we speculate that it may come from strains of the genus Lactococcus. Furthermore, members of this family tend to be associated with transmembrane putative immunity proteins related to cellular processes, toxin production, and resistance. It has been proven that lcn972 was the first non-lantibiotic bacteriocin that specifically interacts with the cell wall precursor lipid II [14], which was categorized into subclass IIc [11].
The predicted subtilosin A-like bacteriocin is another class IIc member identified by us. Subtilosin A is a group of antilisterial bacteriocins previously reported only produced by Bacillus subtilis, before being found in many other species [15]. Aureocin-like type II bacteriocin is a small family usually encoded on a plasmid. Characteristically, the members are small, cationic, rich in Lys and Trp in addition of bringing about a generalized membrane permeabilization leading to leakage of ions. The family includes aureocin A, lacticins Q and Z, BhtB, as well as an archaeal member [16,17]. However, our predicted BhtB-like seems to be very special compared with others.
2.3. Phylogenetic Analysis
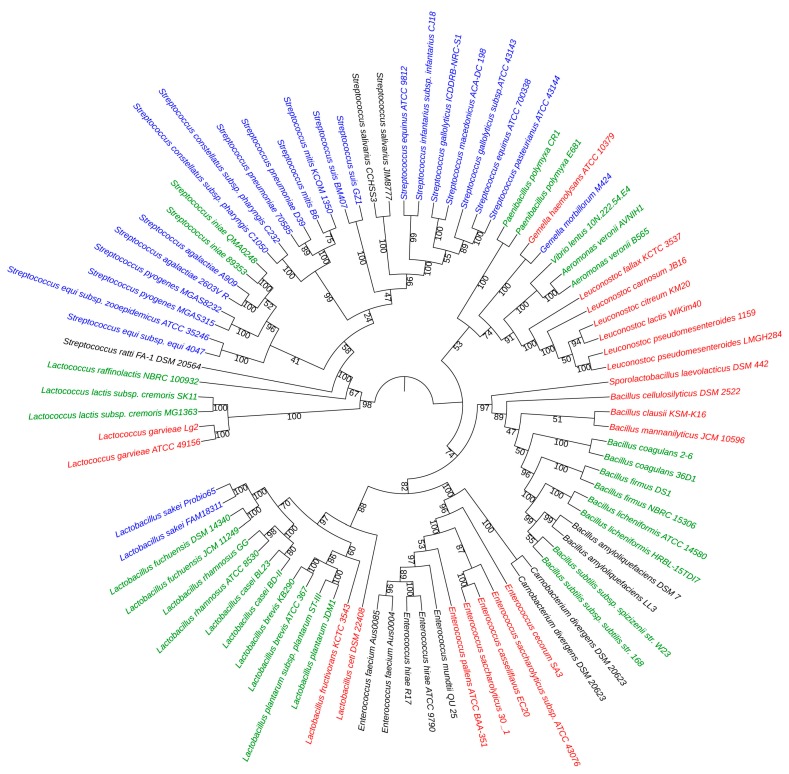
We could not determine the unique strains for these predicted AMP sequences. However, in order to evaluate the utility for potential probiotics in grass carp according to close relations between known probiotics and these putative AMP-producing bacteria, we constructed a phylogenetic tree (Figure 3) using microbiota from annotated top abundant species in class Bacilli, top bacterial hits in the NCBI by searching from our predicted AMP sequences, and those commonly-used commercial probiotics in microecologics.
Figure 3.
Phylogenetic tree of AMP-producing microbiota. Commercial probiotics are green, top abundant bacteria of class Bacilli in grass carp GI are red, and the putative strains producing our predicted AMPs are blue.
Three Lactococcus spp. exist in the GI of grass carp, with both L. raffinolactis and L. lactis being putative AMP-producing probiotics. However, several Lactococcus strains are reported as pathogens. Thus, only some strains have the potential to become probiotics in aquaculture as agonists of S. aureus, while L. garvieae SYP-B-301 has been employed as a feed additive [18,19,20,21]. In Streptococcus spp., S. iniae I1 was selected as predominant LAB in the intestines of young common carp, which is consistent with what we observed. Streptococcaceae was the most plentiful family under the order Lactobacillales, followed by Lactobacillaceae and Enterococcaceae families. Streptococcus was the 7th most abundant genus in the identified 1453 genera, while Bacillus was the 11th, Lactobacillus was the 42nd, and Enterococcus was the 46th. It seems that a large amount of Streptococcus spp. in the GI are predicted to be AMP-producing species (Figure 3) and S. agalactiae with a closest relationship to S. iniae (a previously applied probiotics) can be a probiotic candidate.
Meanwhile, Gemella morbillorum M424, a subtilosin A-producing strain closely related to some probiotics, may be beneficial to the intestine. In addition, while probiotics Bacillus coagulans, B. firmus, B. licheniformis, and B. subtilis, were found at relatively low levels, the predominant species B. cellulosilyticus, B. clausii, and B. mannanilyticus (Table 1) may play an important alternative role in the GI. A similar situation occurred for Lactobacillus spp. as L. sakei, L. fructivorans, and L. ceti may be helpful. Enterococcus spp. are another superior resource, with their functions waiting to be explored.
In summary, based on our phylogenetic tree (Figure 3), we speculate that these bacteria closely related to some known probiotics are promising candidates for novel AMPs or applicable probiotics in the grass carp, such as L. garvieae, S. agalactiae, G. morbillorum, and L. sakei. Furthermore, these predominant species of Lactobacillus and Bacillus are also valuable genetic resources for further investigation.
3. Discussion
In commercial aquaculture, the stressful conditions fish are exposed to usually result in a decrease in production and increase the risk of infectious diseases. However, resistance from antibiotic abuse has encouraged the scientific community to search for alternatives to antibiotics. Our present strategy to identify the potential AMPs in fish GI microbiota is an alternative way to overcome the deficiency of antibiotics.
In this study, we identified five AMPs (pediocin, lactococcin 972, subtilosin A, and aureocin-like bacteriocins) in the GI of grass carp. However, it is still necessary to confirm the exact strains producing these AMPs. Our analysis suggested that these bacteriocins were encoded by rare members of the GI microbiota or those that have not been previously identified as important bacteria. Furthermore, we predicted the microbiota that may produce our five identified AMPs from the GI metagenomics of C. idellus and constructed an interesting phylogenetic tree for them. It revealed that many commensal organisms in grass carp, including three species of Lactococcus (L. raffinolactis, L. lactis, and L. garvieae) as well as many members of Streptococcus, Bacillus, Lactobacillus, and Enterococcus, are potential probiotics in grass carp, as they are closely related to the known strains used in microecologics and consistent with other characterized probiotics [22,23].
Interestingly, all the predicted bacterial species belong to the class Bacilli. In fact, antimicrobial compounds produced by Bacilli and isolated from GI of Japanese costal fish [24] and Indian major carp [25] have been suggested as bio-control agents, while antimicrobial peptides or bacteriocins have received attention as an alternative tool to control colonization of pathogenic bacteria in fish intestine [26]. It can be inferred that these Bacilli are dominant strains in many animals and other metabolites certainly have irreplaceable functions in GI. Actually, apart from G+ bacteria derived bacteriocins, antimicrobial metabolites from G− bacteria have a rather high concentration in antimicrobial providers to protect their host. In Pseudoalteromonas, bacteriocins are almost large proteins (>100 kDa) and their strains are of great ecological significance as part of the resident microbiota or antimicrobial metabolite producers [27]. That may explain why class γ-Proteobacteria is the largest group in the GI of grass carp (Table S1).
GI bacteria can be variably changed by components of food or cultural media, interaction with enterocytes, water temperatures, or seasons [28,29,30]. A healthy gut is always kept in a dynamic equilibrium, which requires the coordination of many factors [29]. It seems that each bacterial species has its own natural enemies in GI and it restricts other competitors in a reasonable condition by secreting toxins such as AMPs. Their abundance in GI flora is surely in accord with their functions in this complex bacteria community. Once their balance is disrupted, diseases will occur in the host. Antibiotics are commonly abused in animal agriculture, thus causing the emergence of antibiotic-resistant bacteria. An alternative way for applying bacteriocins is to deliver some AMP-producing microbiota directly through prophylactic administration of probiotics, as long as they can stably survive in the intestine and maintain physiological homeostasis.
The rapid advancement of next-generation sequencing (NGS) has accelerated our pace of identifying novel probiotics in fish GI. Previously reported bacteriocins are usually purified by traditional isolation [31]. There are more and more approaches being developed to dig out more bacteriocins in nature, including Bacteriocin Operon and gene block Associator (BOA; [32]), Hidden Markov Model (HMM) [33], and BAGEL2 [34]. These genome mining bioinformatic tools facilitate our work in discovering more useful functional genes by fully utilizing the big data in the post-genomics era [35]. They have a great potential to look into the indicators of many diseases or the prediction of novel AMPs in metagenomes of animals. Thus, this improves the digestive conditions of fish and feed efficiency. This also provides opportunities to better determine the specific types of bacteriocins and bacterial species can be found in different environmental niches through the investigation of metagenomic data. Exploiting antimicrobial-producing resources to shape the host-associated microbiota along with high-throughput sequencing may elucidate the roles of different strains for host defense.
This is the first big-data-based study looking for AMPs and AMP-producing bacteria in the GI metagenome of grass carp. There is a great temptation to connect related microbiota that may produce bacteriocins and abundant bacteria groups with commonly acknowledged probiotics. Future studies should focus on AMPs in fish GI and bacterial strains producing them, thus offering efficient probiotic supplements in agriculture and feed industry. Their basic characteristics are worthy of further investigation and their physiological roles in interacting microbiota remain to be elucidated. Finally, in vivo experiments are required to determine the ecological significance of the association between predicted potential probiotics and their host macro-organisms. The major issue is to determine whether AMP-producing microbiota provide a real benefit to their host, especially in the context of pathogenic events. In such a case, they may stand out as the next generation of probiotics of economic importance in aquaculture.
4. Materials and Methods
4.1. Sample Collection
Pond-cultured grass carps were fed twice a day with a commercial feed (crude protein ≥ 30.0%; crude fiber ≥ 12.0%; crude ash ≤ 15.0%; calcium 0.4–2.5%; phosphate ≥ 0.7%; salt 0.3–1.2%; moisture ≤ 12.5%; and lysine ≥ 1.2%) from Shenzhen Alphafeed Co. Ltd., Shenzhen, China. They were originally collected from a local hatchery in Shenzhen, Guangdong, China by trawl netting. These cultured fish, with an approximate size of 1–2 kg, were selected. The body surface of these fish was rinsed with sterile distilled water and subsequently, 70% ethanol was used to reduce contamination. The GI was dissected aseptically from their abdominal cavity, while the GI content and the epithelial GI mucosa were squeezed out for a separate harvest. The GI contents from eight individuals were obtained individually and stored at −80 °C before use.
All experiments were performed in accordance with the guidelines of the Animal Ethics Committee and were approved by the Institutional Review Board on Bioethics and Biosafety of BGI (No. FT15103) on 6 July 2016.
4.2. Assembly and Annotation of the Metagenome
Eight samples were separately sequenced by an Illumina Hiseq PE150 platform at BGI, Shenzhen, China. The pool of metagenomic data were assembled by MegaHIT software [36]. Analysis was implemented by Guangdong Magigene Biotechnology Co., Ltd, Guangzhou, China. Annotation of deduced amino acid sequences was performed through BLASTP against the public Nr database with E ≤ 1 × 10−3 by KAIJU [37]. To determine the accurate phylogenetic composition of GI microbiota, we assigned all the metagenomic reads to prokaryotic reference genomes that have been submitted to the genome database of NCBI using BLASTN with default parameters. The aligned reads with sequence similarity ≥75% were filtered by BLAST against the metagenome of grass carp.
4.3. Prediction and Identification of AMPs
We applied a homology search with the reported AMPs from the newly established C. idellus metagenome datasets. These previously validated AMPs were retrieved from online Antimicrobial Peptides Database (APD3) [38] and were used as queries. Assembled metagenome and raw reads of grass carp GI microbiota were used as a local index database. Subsequently, we applied TBLASTN with the threshold of E-value ≤10−5 to run the queries against the database. Following this, the hits of nucleotides were translated into peptide sequences and submitted to NCBI using BLASTP tool for further verification. However, those AMPs only exist in eukaryotes were removed.
4.4. Alignment and Homology of AMPs
The obtained peptide sequences and downloaded proteins from NCBI (Table 1) were aligned by ClustalW of BioEdit [39]. Amino acid residues with conservation among sequences were shaded by TexShade [40].
4.5. Construction of the Phylogenetic Tree
We downloaded the metagenomes of 84 strains and applied their 16S rRNA sequences to construct a phylogenetic tree. First of all, MUSCLE v3.8.31 [41] was employed for multiple alignment, in which all positions containing gaps and missing data were eliminated. Subsequently, an UPGMA tree was constructed using MEGA7 [42]. Bootstrapping was carried out using 100 replicates and values are indicated on the nodes of the phylogeny. The rate variation among sites was modeled with a gamma distribution (shape parameter = 1). Finally, the phylogenetic tree was shown in iTOL [43].
Acknowledgments
This work was supported by Shenzhen Special Program for Development of Emerging Strategic Industries (No. JSGG20170412153411369), and Zhenjiang Leading Talent Program for Innovation and Entrepreneurship.
Supplementary Materials
The following is available online at www.mdpi.com/2072-6651/9/9/266/s1. Table S1: Bacterial components in the gastrointestinal samples of grass carp.
Author Contributions
Q.S. conceived and designed the project; B.D. and Y.Y. analyzed the data; B.D., Y.Y. and Q.S. wrote the paper; L.L. participated in the data analysis and figure preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rodney H.P., Takeshi Z., Kenji S. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Fact. 2014;13(Suppl. 1):S3. doi: 10.1186/1475-2859-13-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zacharof M.P., Lovitt R.W. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia. 2012;2:50–56. doi: 10.1016/j.apcbee.2012.06.010. [DOI] [Google Scholar]
- 3.Field D., Cotter P.D., Ross R.P., Hill C. Bioengineering of the model lantibiotic nisin. Bioengineered. 2015;6:187–192. doi: 10.1080/21655979.2015.1049781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Z., Harris H.M., McCann A., Guo C., Argimon S., Zhang W., Yang X., Jeffery I.B., Cooney J.C., Kagawa T.F., et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015;6:8322. doi: 10.1038/ncomms9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papagianni M., Anastasiadou S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microb. Cell Fact. 2009;8:3. doi: 10.1186/1475-2859-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Z., Zhang X.H., Boon N., Bossier P. Probiotics in aquaculture of China—Current state, problems and prospect. Aquaculture. 2009;290:15–21. doi: 10.1016/j.aquaculture.2009.02.012. [DOI] [Google Scholar]
- 7.Bassis C.M., Erb-Downward J.R., Dickson R.P., Freeman C.M., Schmidt T.M., Young V.B., Beck J.M., Curtis J.L., Huffnagle G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. 2015;6:e00037-15. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S., Wang G., Angert E.R., Wang W., Li W., Zou H. Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS ONE. 2012;7:e30440. doi: 10.1371/journal.pone.0030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dischinger J., Basi C.S., Bierbaum G. Lantibiotics: Promising candidates for future applications in health care. Int. J. Med. Microbiol. 2014;304:51–62. doi: 10.1016/j.ijmm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Vaillancourt K., LeBel G., Frenette M., Fittipaldi N., Gottschalk M., Grenier D. Purification and characterization of Suicin 65, a novel class I type B lantibiotic produced by Streptococcus suis. PLoS ONE. 2015;10:e0145854. doi: 10.1371/journal.pone.0145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drider D., Fimland G., Hechard Y., McMullen L.M., Prevost H. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 2006;70:564–582. doi: 10.1128/MMBR.00016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y., Zhang C., Wang Y., Shi J., Zhang L., Ding Z., Qu X., Cui H. Class IIa bacteriocins: Diversity and new developments. Int. J. Mol. Sci. 2012;13:16668–16707. doi: 10.3390/ijms131216668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez B., Suárez J.E., Rodríguez A. Lactococcin 972: A hornodimeric lactococcal bacteriocin whose primary target is not the plasma membrane. Microbiology. 1996;142:2393–2398. doi: 10.1099/00221287-142-9-2393. [DOI] [PubMed] [Google Scholar]
- 14.Martinez B., Bottiger T., Schneider T., Rodriguez A., Sahl H.G., Wiedemann I. Specific interaction of the unmodified bacteriocin Lactococcin 972 with the cell wall precursor lipid II. Appl. Environ. Microbiol. 2008;74:4666–4670. doi: 10.1128/AEM.00092-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parveen R.R., Anandharaj M., Hema S., Deepika R., David R.A. Purification of antilisterial peptide (Subtilosin A) from novel Bacillus tequilensis FR9 and demonstrate their pathogen invasion protection ability using human carcinoma cell line. Front. Microbiol. 2016;7:1910. doi: 10.3389/fmicb.2016.01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netz D.A., Pohl R., Beck-Sickinger A.G., Selmer T., Pierik A.J., Bastos M.F., Sahl H.G. Biochemical characterisation and genetic analysis of aureocin A53, a new, a typical bacteriocin from Staphylococcus aureus. J. Mol. Biol. 2002;319:745–756. doi: 10.1016/S0022-2836(02)00368-6. [DOI] [PubMed] [Google Scholar]
- 17.Netz D.A., Bastos M.C., Sahl H.G. Mode of action of the antimicrobial peptide aureocin A53 from Staphylococcus aureus. Appl. Environ. Microbiol. 2002;68:5274–5280. doi: 10.1128/AEM.68.11.5274-5280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vendrell D., Balcázar J.L., Ruiz Z.I., De B.I., Gironés O., Múzquiz J.L. Lactococcus garvieae in fish: A review. Comp. Immunol. Microbiol. Infect. Dis. 2006;29:177–198. doi: 10.1016/j.cimid.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Delpech P., Rifa E., Ball G., Nidelet S., Dubois E., Gagne G., Montel M.C., Delbes C., Bornes S. New insights into the anti-pathogenic potential of Lactococcus garvieae against Staphylococcus aureus based on RNA sequencing profiling. Front. Microbiol. 2017;8:359. doi: 10.3389/fmicb.2017.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugimura Y., Hagi T., Hoshino T. Correlation between in vitro mucus adhesion and the in vivo colonization ability of lactic acid bacteria: Screening of new candidate carp probiotics. Biosci. Biotechnol. Biochem. 2011;75:511–515. doi: 10.1271/bbb.100732. [DOI] [PubMed] [Google Scholar]
- 21.Hagi T., Hoshino T. Screening and characterization of potential probiotic lactic acid bacteria from cultured common carp intestine. Biosci. Biotechnol. Biochem. 2009;73:1479–1483. doi: 10.1271/bbb.80746. [DOI] [PubMed] [Google Scholar]
- 22.Pandiyan P., Balaraman D., Thirunavukkarasu R., George E.J., Subaramaniyan K., Manikkam S., Sadayappan B. Probiotics in aquaculture. Drug Invent. Today. 2013;5:55–59. doi: 10.1016/j.dit.2013.03.003. [DOI] [Google Scholar]
- 23.Merrifield D.L., Dimitroglou A., Foey A., Davies S.J., Baker R.M., Bøgwald J., Castex M., Ringø E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture. 2010;302:1–18. doi: 10.1016/j.aquaculture.2010.02.007. [DOI] [Google Scholar]
- 24.Sugita H., Hirose Y., Matsuo N., Deguchi Y. Production of the antibacterial substance by Bacillus sp. strain NM 12, an intestinal bacterium of Japanese coastal fish. Aquaculture. 1998;165:269–280. doi: 10.1016/S0044-8486(98)00267-1. [DOI] [Google Scholar]
- 25.Giri S.S., Sukumaran V., Sen S.S., Vinumonia J., Banu B.N., Jena P.K. Antagonistic activity of cellular components of potential probiotic bacteria, isolated from the gut of Labeo rohita, against Aeromonas hydrophila. Probiotics Antimicrob. Proteins. 2011;3:214–222. doi: 10.1007/s12602-011-9078-3. [DOI] [PubMed] [Google Scholar]
- 26.Ghanbari M., Jami M., Domig K.J., Kneifel W. Seafood biopreservation by lactic acid bacteria—A review. LWT Food Sci. Technol. 2013;54:315–324. doi: 10.1016/j.lwt.2013.05.039. [DOI] [Google Scholar]
- 27.Offret C., Desriac F., Le C.P., Mounier J., Jegou C., Fleury Y. Spotlight on antimicrobial metabolites from the marine bacteria Pseudoalteromonas: Chemodiversity and ecological significance. Mar. Drugs. 2016;14:129. doi: 10.3390/md14070129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harikrishnan R., Balasundaram C., Heo M.S. Potential use of probiotic- and triherbal extract-enriched diets to control Aeromonas hydrophila infection in carp. Dis. Aquat. Org. 2010;92:41–49. doi: 10.3354/dao02240. [DOI] [PubMed] [Google Scholar]
- 29.Ni J., Yan Q., Yu Y., Zhang T. Factors influencing the grass carp gut microbiome and its effect on metabolism. FEMS Microbiol. Ecol. 2014;87:704–714. doi: 10.1111/1574-6941.12256. [DOI] [PubMed] [Google Scholar]
- 30.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zendo T., Nakayama J., Fujita K., Sonomoto K. Bacteriocin detection by liquid chromatography/mass spectrometry for rapid identification. J. Appl. Environ. Microbiol. 2008;104:499–507. doi: 10.1111/j.1365-2672.2007.03575.x. [DOI] [PubMed] [Google Scholar]
- 32.Morton J.T., Freed S.D., Lee S.W., Friedberg I. A large scale prediction of bacteriocin gene blocks suggests a wide functional spectrum for bacteriocins. BMC Bioinform. 2015;16:381. doi: 10.1186/s12859-015-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh C.J., Guinane C.M., O’Toole P.W., Cotter P.D. A Profile Hidden Markov Model to investigate the distribution and frequency of LanB-encoding lantibiotic modification genes in the human oral and gut microbiome. PeerJ. 2017;5:e3254. doi: 10.7717/peerj.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jong A., van Heel A.J., Kok J., Kuipers O.P. BAGEL2: Mining for bacteriocins in genomic data. Nucleic Acids Res. 2010;38:W647–W651. doi: 10.1093/nar/gkq365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheffler R.J., Colmer S., Tynan H., Demain A.L., Gullo V.P. Antimicrobials, drug discovery, and genome mining. Appl. Microbiol. Biotechnol. 2013;97:969–978. doi: 10.1007/s00253-012-4609-8. [DOI] [PubMed] [Google Scholar]
- 36.Li D., Luo R., Liu C.M., Leung C.M., Ting H.F., Sadakane K., Yamashita H., Lam T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016;102:3–11. doi: 10.1016/j.ymeth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Menzel P., Ng K.L., Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 2016;7:11257. doi: 10.1038/ncomms11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G., Li X., Wang Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 40.Beitz E. TeXshade: Shading and labeling of multiple sequence alignments using LaTeX2e. Bioinformatics. 2000;16:135–139. doi: 10.1093/bioinformatics/16.2.135. [DOI] [PubMed] [Google Scholar]
- 41.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letunic I., Bork P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.