Abstract
Semantic language skills are an integral part of early childhood language development. The semantic association between verbs and nouns constitutes an important building block for the construction of sentences. In this large-scale functional magnetic resonance imaging (fMRI) study, involving 336 subjects between the ages of 5 and 18 years, we investigated the neural correlates of covert verb generation in children. Using group Independent Component Analysis (ICA), seven task-related components were identified including the mid-superior temporal gyrus, the most posterior aspect of the superior temporal gyrus, the parahippocampal gyrus, the inferior frontal gyrus, the angular gyrus and medial aspect of the parietal lobule (precuneus/posterior cingulate). A highly left-lateralized component was found including the medial temporal gyrus, the frontal gyrus, the inferior frontal gyrus and the angular gyrus. The associated independent component (IC) time courses were analyzed to investigate developmental changes in the neural elements supporting covert verb generation. Observed age effects may either reflect specific local neuroplastic changes in the neural substrates supporting language or a more global transformation of neuroplasticity in the developing brain. The results are analyzed and presented in the framework of two theoretical models for neurocognitive brain development. In this context, group ICA of fMRI data from our large sample of children age 5–18, provides strong evidence in support of the regionally-weighted model for cognitive neurodevelopment of language networks.
Introduction
The first scientific study of anatomical correlates of language is now over a century old (Broca 1861; Wernicke 1911). However, most of the past studies and models have been based on adult data for which the language circuitry is assumed to be fully developed and mostly stable. These models fail to consider dynamic changes in the neural circuitry supporting language throughout the life span. Language skills continue to develop rapidly during the school-age years, and the static view of the neural substrate of language provided by adult studies may not be ideal to tease out the dynamic changes inherent in the developing brain. This is particularly true in language domains, like semantics, that show a protracted time course for development.
Verbal fluency tasks place significant and changing demands on the semantic system throughout development. The verb generation task is one example of a verbal fluency task that can be performed readily by children, at a level that improves with the expansion of the child’s lexicon as well as development of phonological and semantic processing skills and other neurocognitive functions. The auditory version of the verb generation task requires attention to auditorily-presented words, processing of their phonological form, and attachment of meaning to that form (Hickok and Poeppel 2004). The act of accessing a word’s meaning is thought to activate a broader network of words that are associated in meaning (Levelt, Roelofs et al. 1999; Pulvermuller 2001). While the noun is held in memory, the child must select words from their mental lexicon that are related to the noun in terms of plausible actions and attach the appropriate phonological forms to these words that are subsequently retrieved and rehearsed sub-vocally (Thompson-Schill, D’Esposito et al. 1997). In addition to these linguistic tasks, children must also recruit attentional resources as nouns are presented, and must hold these nouns in memory as they search for appropriate verbs. In the covert verb generation task the speech motor network is still engaged but must be inhibited so that words are not spoken overtly (Skipper, Nusbaum et al. 2005).
The complexity of semantic verbal fluency tasks make them an important tool for exploring the entire network of semanitc circuitry and associated cognitive functions (Lotsof 1953; Rogers 1953; Monsch, Bondi et al. 1992; Frith, Friston et al. 1995; Gaillard, Hertz-Pannier et al. 2000). Word fluency tasks specifically designed for pediatric populations have also been used with success (Hertz-Pannier, Gaillard et al. 1997; Gaillard, Hertz-Pannier et al. 2000; Gaillard, Sachs et al. 2003; Szaflarski, Holland et al. 2006). The fMRI task used in the present study is a variant of the “child friendly” verb generation task first introduced by Petersen et al. (Petersen, Fox et al. 1988). The plethora of studies that have used verbal fluency tasks have yielded a fairly consistent picture of the resulting activation. Most consistent across studies is activation in the left inferior frontal gyrus. Additional regions that appear routinely include additional dorsolateral prefrontal regions, anterior cingulate, activations in the superior and middle temporal gyrus, and in the parietal and occipital cortex.
The view of semantic processing presented by these prior studies is limited by the method of analysis that has been employed. Each of these studies has employed a model-based approach predicated upon a priori knowledge of the brain’s response to a stimulus evoking a semantically associated response. We suggest that this statistical method may present an oversimplified picture of a complex language task. Specifically, the number of cognitive components suggested for a verb generation task often exceeds the number of distinct regions of activation revealed by model-based analysis, even when co-variates and the hemodynamic response function (HRF) of the brain are incorporated in the statistical model, as in the General Linear Model (GLM). Moreover, certain areas of activation revealed by the GLM analysis might be associated with more than one of the cognitive components of the task. For example, inferior frontal activation should be associated with semantic processing, verbal working memory, and subvocal word production. Likewise, the previously described temporal activations may be linked with auditory perceptual and phonological decoding of the nouns as well as semantic processing of the lexical forms. However, the GLM approach does not permit a view of multiple distinct activations within the same region since it homogeneously identifies all areas that are significantly correlated with the experimental design matrix derived from the stimulus model function. As such, we hypothesize that the network model of semantic processing described by verbal fluency studies to date has been underspecified.
We suggest that an alternate statistical approach, independent component analysis (ICA), may be more suitable for understanding the network involved in complex verbal language tasks in general, and verbal fluency tasks in particular. Independent Component Analysis (ICA) is fast gaining in popularity as a method of fMRI data analysis. One appealing feature of Independent component analysis is the fact that it does not require defining the hemodynamic response function (HRF) or the design matrix a priori. Several studies have raised doubts concerning the assumption of an invariant HRF for the whole brain (Handwerker, Ollinger et al. 2004). Importantly, assuming a fixed HRF may not be ideal in studies investigating developmental changes associated with cognitive functions in general and language in particular. The HRF may exhibit considerable variance across subjects making ICA an ideally suited data analysis technique for group analysis (Schmithorst, Holland et al. 2006). Because ICA does not need to specify an HRF a priori, ICA makes no assumptions regarding either the stimulus or the brain response, rather it is driven by the observed data itself providing an additional advantage over GLM in the case where the HRF may not be known precisely or varies regionally or across individual subjects.
We expect that ICA will reveal that areas previously described as active during verb generation are, in fact, active over multiple time courses during the task period. As described above, multiple activations associated with individual regions (specifically frontal and temporal cortex) will confirm that these areas are involved in multiple cognitive aspects of the task. When anatomical areas are repeatedly activated, these areas will be seen in the context of a unique network of areas that co-activate over a common time course, further supporting the idea that these areas differentially integrate with the larger overall network to support separate cognitive components of the task. This has also been seen in previous studies that have used the ICA methods investigating math processing (Schmithorst and Brown 2004), alcohol intoxication effects on simulated driving (Calhoun, Pekar et al. 2004), music perception (Schmithorst 2005), story comprehension in children (Schmithorst, Holland et al. 2006), and a word-picture matching task (Schmithorst, Holland et al. 2007). We expect that some regions and associated time-courses of the fMRI BOLD response during the Verb Generation task will be highly correlated with the stimulus time-course, while other components may reflect higher-order temporal responses or time delays that might not correlate strongly with an a priori design matrix and would therefore be rejected as insignificant by a GLM analysis.
Here we use ICA as a means of exploring developmental changes in brain activation patterns associated with the verb generation task. To place our analysis in a developmental-theoretical framework, we will explore two current interpretations of neuroimaging findings in brain development. Brain maturation is thought to occur through two primary processes, synaptic pruning and consolidation (Lenneberg 1967; Muller, Rothermel et al. 1998). The subsequent influence of brain maturation on BOLD signal detection given a common threshold value has been discussed elsewhere (Berl, Vaidya et al. 2006). Pruning occurs when less utilized neuronal connections are lost; consolidation occurs when neuronal connections are continually reinforced. Therefore, pruning and consolidation are paramount for mature networks resulting in strong, concentrated signal changes that the MRI system can detect. This is a widely accepted hypothesis for normal brain development changes in children and is sometimes referred to as the focal network model (Lenneberg 1967; Muller, Rothermel et al. 1998). This model predicts the same cortical regions are activated for adults and children for a given task but the extent of activation is greater and the signal change is less for children (Thomas, King et al. 1999; Gaillard, Hertz-Pannier et al. 2000; Nelson, Monk et al. 2000; Balsamo, Xu et al. 2002; Casey, Thomas et al. 2002; Ahmad, Balsamo et al. 2003).
Indirectly, the focal theory is supported by the fact that the underlying neural network structure for language processing is generally established by the age of five (Ahmad, Balsamo et al. 2003). However, it should be noted that although these factors may influence the extent and strength of activation, they may not account for significant differences in activation patterns as shown by a recent study (Turkeltaub, Flowers et al. 2004).
In contrast to the focal theory, some studies show increases in activation with age for some cortical regions as well as decreases in activation with age for other cortical regions (Booth, MacWhinney et al. 2000; Schlaggar, Brown et al. 2002; Schapiro, Holland et al. 2004; Brown, Lugar et al. 2005). In particular, verbal fluency and working memory show increases in signal change with age, but also show increases in extent with age (Thomas, King et al. 19990; Rubia, Overmeyer et al. 2000; Klingberg, Forssberg et al. 2002; Kwon, Reiss et al. 2002; Gaillard, Sachs et al. 2003). The results of these studies support a “regional weighting hypothesis” based on the distribution of activation within a network (Rubia, Overmeyer et al. 2000; Casey, Thomas et al. 2002; Berl, Vaidya et al. 2006). The regionally weighted model proposes that the same areas of a distributed network are involved but the degree of engagement of each region systematically changes with development. According to the regionally weighted model, differences in weights may account for normal variation of cognitive skill level, use of different cognitive strategies, or changes in the biological substrate for a function (Berl, Vaidya et al. 2006).
As children mature, there is evidence of change in BOLD signal in frontal and other areas of the brain (Gaillard, Hertz-Pannier et al. 2000; Schlaggar, Brown et al. 2002; Booth, Burman et al. 2003; Schapiro, Holland et al. 2004; Brown, Lugar et al. 2005). Positive changes in brain connectivity are also observed (Klingberg, Vaidya et al. 1999; Schmithorst, Wilke et al. 2002). These later aspects of development may shape the system so that distributed networks are used with increasing efficiency.
Development of semantic and lexical knowledge may be associated with the development of a specific network of brain regions. Semantic tasks have been shown to rely on a predominately left-hemisphere network of frontal and temporal regions in both children (Balsamo, Xu et al. 2002; Blumenfeld, Booth et al. 2006) and adults (Bookheimer 2002). Previous cross-sectional and longitudinal functional imaging studies have demonstrated developmental changes in these networks. These changes in brain networks supporting semantic skills are attributed to the emergence of more extensive networks of semantic information as children learn. Behavioral data shows that the lexicon continues to develop through the school years and adolescence (McDonald 1997; McGregor, Friedman et al. 2002; Beitchman, Jiang et al. 2008; Nippold and Sun 2008).
These functional imaging findings in child language development support the “regional weighting hypothesis” and correspond closely with patterns of neuroanatomical development. The protracted period of development of the frontal lobe (Giedd, Blumenthal et al. 1999; Schmithorst, Wilke et al. 2002; Giedd 2004; Schneider, Il’yasov et al. 2004) may make the functions supported by this region particularly dynamic through the course of childhood. White matter changes within the temporal and parietal lobes may likewise affect contributions of posterior regions during development (Schmithorst, Wilke et al. 2002).
The approach we introduce here uses independent component analysis as a complementary method to investigate developmental trajectories associated with language sub-networks and explores an alternative means of characterizing brain-behavior relationship based on fMRI data. Brain regions represented in a single independent component image can be thought of as functionally connected and linked to a specific cognitive function. By constructing a mathematical method for investigating changes in the use of these networks (sets of brain regions) with subject age, we are able to directly observe age-related changes in components of the language processing network that support lexical and semantic processing. In relation to verb generation, we expect that activations in brain regions associated with cognitive components that develop with age (i.e., auditory attention, phonological processing, semantic association, verbal memory) will show age-related changes in activation in associated independent component maps computed across the age span 5 to 18 years. Below we outline a new approach with ICA that circumvents some of the methodological and interpretational difficulties inherent in conventional analysis of developmental neuroimaging data (Berl, Vaidya et al. 2006). By addressing the new challenges of group ICA for developmental neuroimaging studies using sophisticated numerical and mathematical approaches that are sensitive to age related changes in IC time courses we find new evidence in support of the regionally weighted model for cognitive neurodevelopment of language networks in children over the age span of 5 to 18 years.
Methods and Material
Participants
Three hundred thirty six children (165 boys, 171 girls) were included in the study following Cincinnati Children’s Hospital Institutional Review Board approval. A total of 481 children, who took part in the study, completed the Verb Generation Task. Of this number data from 336 subjects survived the acceptable motion metric specified below. Informed consent by the child’s parent or guardian was obtained. In addition, assent was also obtained from subjects 8 years and older. Exclusion criteria were: previous neurological illness; learning disability; head trauma with loss of consciousness; current or past use of psychostimulant medication; pregnancy; birth at 37 weeks gestational age or earlier; or abnormal findings at a routine neurological examination performed by an experienced pediatric neurologist.
All subjects were part of a parent study investigating normal language development in children and were considered “healthy” based on neurological, psychological, and structural measures. The parent fMRI study consisted of four fMRI paradigms – silent verb generation, story processing, syntactic prosody, and word-picture matching. These tasks were designed to investigate early- and late-developing, semantic as well as syntactic aspects of language (Holland, Vannest et al. 2007).
The covert verb generation taps skills that continue to develop throughout childhood and it was specifically designed to investigate semantic language development in school-age children. The present group ICA analysis investigates the cognitive modules utilized in silent verb generation.
A complete age and sex breakdown of the subjects is detailed in Table 1. All subjects were native English speakers. Three hundred and eleven of the subjects were right-handed, 24 were left-handed, and 1 was ambidextrous according to the Edinburgh Handedness Inventory (Oldfield 1971). The racial/ethnic background of the subjects was: 302 Caucasian, 21 African–American, 2 Asian, 3 Hispanic, 1 Native American, 2 Asian/European and 5 Multi-Ethnic. All subjects were prescreened for any conditions (such as the presence of orthodontic braces) which would prevent an MRI scan from being acquired. Intelligence was tested in 331 out of the out of 336 subjects enrolled in the study using one of the following instruments: the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-R, ages below 6) (Wechsler 2002), Wechsler Intelligence Scale for Children (Third Edition (WISC-III, ages 6 to 16) (Wechsler 1991), Wechsler Adult Intelligence Scale, Third Edition (WAIS-III, ages 17 and 18) (Wechsler 1997)). Similarly, 330 subjects received the Oral and Written Language Scales (Carrow-Woolfolk 1996). The age range for all subjects was 4.92–18.92 years; Mean Wechsler Full-scale IQ = 111.6 ± 13.84 (range = 70–147); Mean OWLS=107.7 ± 14.3 (range = 66 – 151). A Bruker 3T Medspec (Bruker Medizintechnik, Karlsruhe, Germany) imaging system was used to obtain all MRI scans.
Table 1.
Age and gender breakdown of the study population (165 boys and 171 girls).
| Age in years | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEX | M | 9 | 8 | 9 | 17 | 14 | 12 | 17 | 18 | 17 | 9 | 10 | 9 | 13 | 3 |
| F | 7* | 12 | 17 | 10 | 11 | 12 | 11 | 15 | 21 | 11 | 11 | 10 | 12 | 11 | |
Includes one girl 4 years 11 months.
fMRI paradigm
The fMRI paradigm consisted of a silent verb generation task as previously detailed (Holland, Plante et al. 2001; Holland, Vannest et al. 2007). A 30-s on–off block design was used. All stimuli were presented using MacStim (White Ant Software, Melbourne, Australia). Stimuli were presented at a rate of one every 5 s, for 6 stimuli during each 30 sec. epoch. During the active epochs, the subjects silently generated appropriate verbs such as “throw” or “kick”, to aurally presented nouns such as “ball”. During the control epochs, subjects were asked to tap their fingers when they heard a modulated tone. The control task was specifically designed to control for sublexical auditory processing. Furthermore, it also provided subjects with a task to stop generating verbs into the control epoch. The other useful application of this control task is to provide an indirect measure of subject compliance inside the scanner. The difficulty level of the fMRI task was selected such that children as young as 5 years old would be readily able to perform the task without any difficulty.
Imaging
An MRI-compatible audiovisual system was used for presentation of the stimuli. Details of the techniques used to obtain fMRI data from younger children, as well as the success rates, are given in (Byars, Holland et al. 2002). Success rates range from approximately 50% for 5 year olds to near 100% for 10 year olds and older. EPI-fMRI scan parameters were: TR/TE = 3000/38 ms; 125 kHz; FOV = 25.6 × 25.6 cm; matrix = 64 × 64; slice thickness = 5 mm. Twenty-four slices were acquired, covering the entire cerebrum. One hundred and ten whole brain volumes were acquired (the first 10 were discarded during post-processing to insure image contrast at relaxation equilibrium) for a total scan time of 5 min 30 s. Techniques detailed elsewhere (Byars, Holland et al. 2002) were used to acclimatize the subjects to the MRI procedure and render them comfortable inside the scanner. Soft head restraints were used to minimize head motion. In addition to the fMRI scans, whole-brain T1 weighted MP-RAGE scans were acquired for anatomical co-registration.
Image processing
Data were processed using software written at our institution in IDL (Research Systems Inc., Boulder, CO). A multi-echo reference scan (Schmithorst, Dardzinski et al. 2001) was used to correct for Nyquist ghosts and geometric distortion due to B0 field inhomogeneity during image reconstruction. Data were corrected for subject motion using a pyramid iterative co-registration algorithm (Thevenaz, Ruttimann et al. 1998); all datasets included in this study met the criterion of median voxel displacement at the center of the brain < 2 mm. A detailed analysis of motion (including task related movement) related to this task is discussed elsewhere (Yuan, Altaye et al. 2008). Note that one of the main advantages of ICA over standard statistical methods is that if there is considerable motion associated with the task, ICA will detect this as a separate IC component. This facilitates the process of rejecting motion artifacts in the analysis. The fMRI data were subsequently transformed into stereotaxic space (Talairach 1988) using a linear affine transformation, previously validated for the age range in our study (Muzik, Chugani et al. 2000; Wilke, Schmithorst et al. 2002).
Methods and data analysis
The group ICA was performed according to methods outlined in a previous study by Schmithorst et al. (Schmithorst, Holland et al. 2006). However, the methods and procedures for group ICA are briefly discussed here for the purpose of completeness.
As an initial pre-processing step, each voxel time course was normalized to a percent signal change from the mean. This was followed by a two step data reduction procedure (subject wise and group wise). The first step reduced each subject data set to 40 time points using the principal component analysis (PCA). The reduced subject data were then concatenated (across subjects) and a second data reduction PCA was performed to further reduce the total number of sources to 50. The subject-wise concatenation approach (Calhoun, Adali et al. 2001) used for the group ICA analysis has been shown to outperform all other methods (Schmithorst and Holland 2004).
After PCA reductions, the FastICA (Hyvarinen 1999) algorithm was used for the ICA decomposition. FastICA is a stochastic algorithm, possibly yielding different results at every run of the algorithm. The reason for this is that the algorithm may converge to different local optima depending on the initial conditions (Himberg, Hyvarinen et al. 2004). Therefore, the FastICA algorithm was run 25 times, with hierarchical agglomerative clustering (Himberg, Hyvarinen et al. 2004) to estimate the most reliable components. Group-averaged link was chosen as the choice of agglomeration strategy (Himberg, Hyvarinen et al. 2004). Clusters with less than 6 elements were then discarded as “unreliable” as the given component appeared in very few of the FastICA runs (MacCallum, Roznowski et al. 1992).
The preceding criteria resulted in 51 retained clusters (and thus 51 components), greater than the dimensionality of the reduced data set. Prior to further analysis the components were quasi-decorrelated in a symmetric fashion using a procedure analogous to symmetric decorrelation (Hyvarinen, Karhunen et al. 2001). Additional details regarding current group ICA analysis are available in our previous publication (Schmithorst, Holland et al. 2006).
For each map and for each subject, the corresponding time course was Fourier transformed (FT) and the component at the on–off task frequency was subjected to further analysis. In the following step, each of the FT components for a single map was averaged across subjects and tested for a significant difference (i.e. two-dimensional distance) from zero using a one-sample t-test based on a threshold of t = 3.58 corresponding to a p = 0.01 (Bonferroni-corrected for the 51 components).
Since we were only interested in the components which had greater activation during the active epochs, i.e., verbs, rather than the control task, a further criterion was employed on the average Fourier component at the task frequency. According to this criterion, each average Fourier component had to have an absolute phase difference of less than 60 deg. from the phase of the on–off task reference function (shifted by 3 s relative to the task itself to account for the hemodynamic delay).
As an additional test (and validation of the Fourier analysis) the time courses were evaluated for greater intensities during the active versus the control phases. Using this test we retained all components deemed significant using the Fourier criteria (paired t test; Bonferroni-corrected p < 0.01).
There were 14 components deemed to be “task-related” using the above mentioned criteria. However, 7 components were rejected as artifacts based upon spatial and temporal characteristics following visual inspection by the investigators (data not shown). For the 7 remaining components, a one-sample t-test was performed on the individual IC maps on a voxel-wise basis to determine if each voxel value was significantly different from zero across the group. For this comparison a stringent criterion of t > 15 was used, corresponding to p < 1e−10, Bonferroni-corrected for multiple voxel comparisons. IC maps do not look much different from those shown here (Figure 1) even when a threshold several orders of magnitude lower than 10−9 (e.g. t = 8) is used; however some bleeding of activation into white matter areas can result from the smoothing kernels used in preprocessing. The strict threshold we have selected provides greater confidence in our results as they propagate through the second level analysis described below and allows us to more concisely interpret our ICA results in a theoretical framework. For reference, a standard GLM analysis (Worsley and Friston 1995) was also performed using the on–off task reference function as the regressor of interest; a random-effects analysis was performed.
Figure 1.
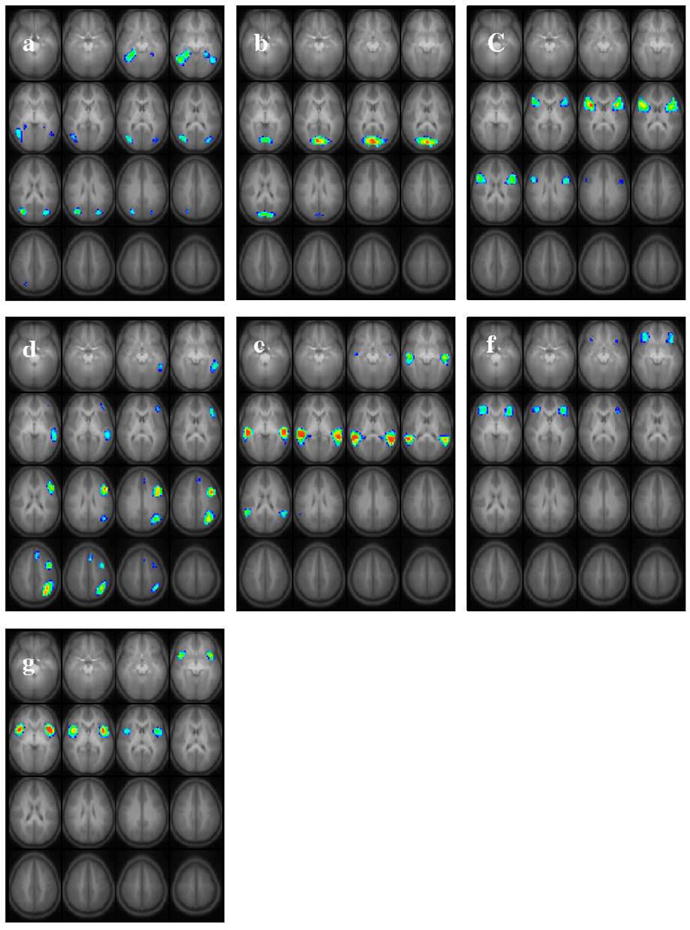
Seven task-related independent components found from group ICA analysis of 313 children ages 5 – 18 performing the task of covert verb generation. Slice range: Z = −25 to +50 mm (Talairach coordinates). All images in radiologic orientation.
In order to investigate developmental changes in IC maps, the following procedure, analogous to a standard GLM approach, was used on the time courses associated with the 7 task-related ICs retained in the previous steps. Here the reader is cautioned that IC time courses do not represent absolute BOLD signal intensities. Furthermore, they can only be determined up to a scaling factor (McKeown, Makeig et al. 1998; Hyvarinen, Karhunen et al. 2001). Thus, the scaling ambiguity associated with standard ICA precludes one from directly testing for any age-related effects on the individual IC maps. However, a method (Calhoun, Adali et al. 2001) has been proposed to scale IC maps to percent signal change from the mean. In this study, the age effects were analyzed and tested using methods described previously (Schmithorst, Holland et al. 2006).
In the first approach, each individual time course (for a given component) was fit to the on–off task reference function. The t scores from the fit were then incorporated into a second-level analysis, and correlated with subject age (in months). A significant second-level correlation coefficient implies an effect of age on the task relatedness of the component. However, most of the time courses associated with task-related components (except for the one shown in Figure 1d) did not show a strong overall fit to the task reference time course; which in this approach is simply the binary on-off function with a fixed phase and period. Poor overall fit may be due to hemodynamic response differences over the age span of the children included in our sample. In other words, lack of fit in this method could simply represent a measurable change in BOLD responsiveness between subjects due to age, brain region, and other physiological and attentional differences. Therefore, to accurately separate age effects in the correlations with language-related independent components from age-related changes in the hemodynamic response function we used an alternative (more flexible) data driven approach to test for any age effects in the second level analysis.
In this approach we allow the hemodynamic reference function to vary during the first level analysis. The second level analysis is still based on the correlation between age of the subjects and the t score derived from the first level analysis. However, in this approach the HRF is modified iteratively between the first and second level analyses in order to maximize the correlation between subject age and the t score for each of the seven independent components tested. This optimization was performed using the downhill simplex method (Press, Teukolsky et al. 1992). To regularize the optimization (e.g., to prevent the algorithm from converging to an exact match to the time course from the subject with the largest age, resulting in a huge t score for that subject), an upper bound was set of t = 25 for the fit with each individual time course. The regularization corresponded to setting a lower bound on the variance of each associated time course, or equivalently, to incorporating a Bayesian prior (e.g., zero prior probability of zero variance). The null distribution for the slope was found via Monte Carlo simulation, repeatedly performing the algorithm on random Gaussian noise of the same dimensionality. A significant difference in the slope from the null distribution would indicate a significant age effect. Finally, the data-driven optimal reference time course was also tested for task relatedness by correlating with the on–off task reference.
The difference between the two approaches can be summarized as follows. In the first method, the significant associations between subject age and shape of the time courses are tested as determined by an a priori criterion (e.g., correlation with the task reference). In contrast, the second method finds a maximum likelihood descriptor of any age effect present, and then analyzes it a posteriori.
Results
Significant effects of subject age on Full-scale IQ were found (Spearman’s r = −0.18, p < 0.0008). This small but significant negative correlation is mainly attributed to our youngest subjects having higher than average IQ (IQ = 113.9 + 13.9 vs. IQ = 110.5 + 14.3 for subjects 5–8 years old vs.9–18). When the children between the ages 5 to 8 years were excluded, the correlation between age and IQ does not reach significance (Spearman‘s r = −0.09, p > 0.1).
The seven task-related group ICA components are displayed in Figure 1. Components were found with bilateral activation in the parahippocampal gyrus, inferior temporal gyrus and the medial temporal gyrus (Figure 1a); cuneus (Figure 1b); inferior frontal gyrus (Figure 1c); a highly left-lateralized component comprising the medial temporal, the inferior/medial frontal, middle frontal and the angular gyri (Figure 1d); a bilateral component comprising the most posterior aspect of the temporal gyrus (Figure 1e); anterior/inferior frontal gyri bilaterally (Figure 1f); and the insula bilaterally (Figure 1g). Table 2 contains a summary of the respective activation foci for each of the components. Coordinates listed for each IC correspond to the center of mass of each separate spatial element of the IC. Figure 2 shows the corresponding average time courses for each IC map. The progression from leading to lagging the task reference time course is clearly visualized in Figure 2. Six out of the seven components were detected in all 25 IC runs. The component shown in Figure 1a was detected in 17 IC runs, thus assuring a high reliability. There is general agreement between the GLM (Szaflarski, Holland et al. 2006) and ICA results reported here (in terms of the brain regions detected by the respective methods). However it is expected that ICA will detect activation that may not be seen in the GLM results because it is not dependent on a pre-defined HRF, as discussed in detail elsewhere (Schmithorst and Brown 2004). All seven averaged IC time courses were correlated with the on–off task reference function and had correlation coefficient of r = 0.43 or greater (Table 3 column 2).
Table 2.
Activation foci (Talairach coordinates) for the ICA components displayed in Figure 1.
| Anatomical Region | BA | Talairach X, Y, Z | |
|---|---|---|---|
|
| |||
| a | R. Parahippocampal Gyrus | 30/35 | 22, −41, −5 |
| L. Parahippocampal Gyrus | 30/35 | −26, −41, −5 | |
| R. Inferior Temporal Gyrus | 19/37 | 42, −57, −5 | |
| L. Inferior Temporal Gyrus | 19/37 | −46, −57, −5 | |
| R. Medial Temporal Gyrus | 19/39 | 34, −69, 20 | |
| L. Medial Temporal Gyrus | 19/39 | −26, −73, 25 | |
|
| |||
| b | Cuneus | 17 | 2, −77, 10 |
|
| |||
| c | R. Inferior Frontal Gyrus | 44 | 34, 11, 10 |
| L. Inferior Frontal Gyrus | 44 | −34, 11, 10 | |
|
| |||
| d | L. Medial Temporal Gyrus | 21 | −54, −41, −5 |
| L. Inferior Frontal Gyrus | 45/46 | −46, 27, 15 | |
| L. Inferior/Medial Frontal Gyrus | 44/9 | −42, 7, 35 | |
| L. Middle Frontal Gyrus | 6/8 | −6, 23, 45 | |
| L. Angular Gyrus/Inferior Parietal Lobule | 39/40 | −30, −65, 40 | |
|
| |||
| e | R. Superior Temporal Gyrus | 22 | 50, −29, 5 |
| L. Superior Temporal Gyrus | 22 | −54, −45, 10 | |
|
| |||
| f | R. Inferior Frontal Gyrus | 45/47 | 30, 31, 0 |
| L. Inferior Frontal Gyrus | 45/47 | −38, 23, 0 | |
|
| |||
| g | R. Insula | Insula | 38, 11, 0 |
| L. Insula | −38, 11, 0 | ||
Figure 2.
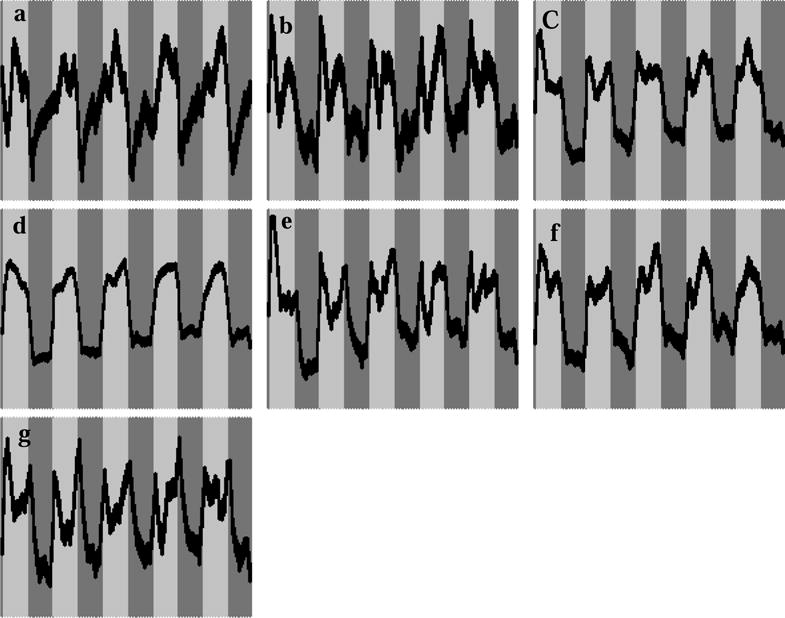
Associated time courses for the independent components shown in Figure 1. Horizontal axis is time and the vertical axis is intensity (pseudo).
Table 3.
Column two shows the correlation coefficient of the average associated time courses (Figure 2) of each component listed in Table 2 with the on-off task reference function. Column three shows the inter-subject variability for each of the ICA time courses shown in Figure 2 for all subjects. Column four shows the Lateralization Indices (LIs) computed for each of the components in Figure 1. Column five shows the correlation coefficient for age-effects using the a priori criterion of fit to the on–off task reference function.
| Component | Correlation with Task | Variability | LI | Correlation with Age |
|---|---|---|---|---|
|
| ||||
| Figure 1a | 0.73 | 0.959891 | −0.40 | 0.055 |
| Figure 1b | 0.77 | 0.952851 | −0.01 | 0.12 |
| Figure 1c | 0.84 | 0.870476 | 0.002 | 0.35 |
| Figure 1d | 0.92 | 0.741831 | 1.0 | 0.42 |
| Figure 1e | 0.63 | 0.913922 | −0.08 | 0.16 |
| Figure 1f | 0.86 | 0.930687 | 0.12 | 0.18 |
| Figure 1g | 0.43 | 0.918818 | 0.09 | 0.25 |
The inter-subject variances of the ICA time courses are listed in Table 3 (column 3). The inter-subject variability is calculated by fitting each IC time course to the respective optimal time course and then calculating the mean of residue squared (Schmithorst, Holland et al. 2006; Schmithorst, Holland et al. 2007). The least inter-subject variability is seen in the component shown in Figure 1d. Similarly, the component shown in Figure 1a had the most variability.
The laterality indices (LIs) for each component were also computed using the formula: LI = ((L − R)/(L + R)) where L = the number of voxels in the left hemisphere, and R = number voxels in the right hemisphere (All IC maps were scaled to unity variance). This approach has been described in detail elsewhere (Binder, Rao et al. 1995; Holland, Plante et al. 2001; Szaflarski, Holland et al. 2006). A threshold value of Z > 5 was used in both the hemispheres and voxels with a z-score greater than this value were counted in the Lateralization Index. Table 3 (column 4) shows the respective LIs for all seven IC maps. A criterion of |LI| > 0.1 was used to define the laterality. Accordingly, the components in Figures 1b, 1c, 1e and 1g are bilateral; the component in Figure 1f is somewhat left lateralized; the component in Figure 1d is strongly left lateralized; and the component in Figure 1a is right lateralized.
The results of a random effects GLM analysis (nominal z = 20, cluster size = 200, corrected p < 0.001) of the same cohort (Figure 3) resulted only in the detection of the regions shown in Figure 1d, in addition to left posterior superior temporal gyrus (shown in Figure 1e) and bilateral superior frontal gyrus.
Figure 3.
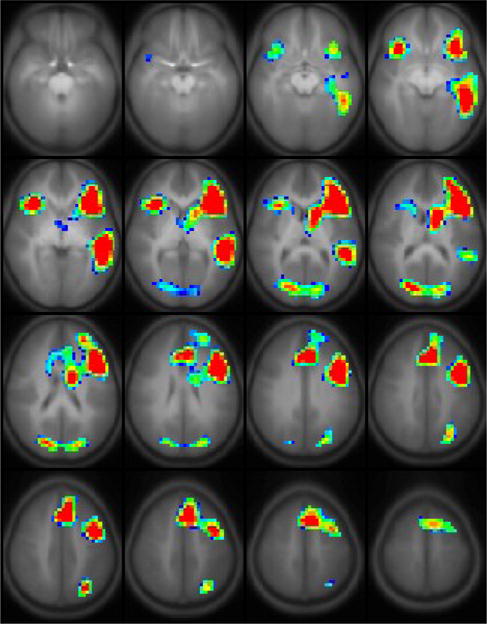
Results from a random-effects GLM analysis of 336 children ages 5 – 18 years performing the task of covert-verb generation (nominal z=20, cluster size = 200, corrected p < 0.001). Slice range: Z = −25 to +50 mm (Talairach coordinates). All images are in radiologic orientation.
For the analysis of developmental trends using the a priori criterion of fit to the on–off task reference function, several ICs showed a significant increase of task-relatedness with age (Table 3 and column 5), after Bonferroni-correcting for the multiple comparisons across the seven components, as follows: Figure 1c (r = 0.35, corrected p < 1e−9), Figure 1d (r = 0.42, corrected p < 1e−14), Figure 1e (r = 0.16, corrected p < 0.03), Figure 1f (r = 0.18, corrected p < 0.008) and Figure 1g (r = 0.25, corrected p < 1e−4). Figure 1a (r = −0.055, p > 0.1) showed no significant increase with age. Figure 1b (r = 0.12, uncorrected p < 0.03) showed a marginally significant increase with age but did not survive correction for multiple comparisons across the seven components. Co-varying for IQ and motion parameters were found not to confound the age-related trends associated with these time courses.
Using the data-driven approach of finding a maximum-likelihood reference time course, the null distribution was found via Monte Carlo simulation as described in Schmithorst et al. (Schmithorst, Holland et al. 2006) (e.g. substituting random numbers for the independent variables). The confidence interval for the slope was 0.0123061±0.000082. All components shown in Figure 1 displayed significant age-effect when compared with the null distribution (Figure 1a, slope = 0.0168610, corrected p < 0.001; Figure 1b, slope = 0.0164591, corrected p < 0.001; Figure 1c, slope = 0.0273920, corrected p < 0.001; Figure 1d, slope =0.0403496, corrected p < 0.001; Figure 1e, slope =0.0220974, corrected p < 0.001; Figure 1f, slope = 0.0182314, corrected p < 0.001), Figure 1g, slope = 0.0232303, corrected p < 0.001) approximating the t distribution (due to the very large number of degrees of freedom) as Gaussian.
The analysis of age-effects displays an overall greater sensitivity of the data-driven approach (over the hypothesis-driven approach) and confirms the overall complementary character of the two methods (Schmithorst, Holland et al. 2007). However, the hypothesis driven approach may display greater sensitivity for an individual component. Correlating the optimal age-related task reference function with the on–off task reference function, except for the component shown in Figure 1a (r = −0.254790, uncorrected p < 0.15), all other components (Figure 1b (r = 0.478964, corrected p < 1e−5), Figure 1c (r = 0.829907, corrected p < 1e−23), Figure 1d (r = 0.939302, corrected p < 1e−42), Figure 1e (r = 0.468820, corrected p < 1e−5), Figure 1f (r = 0.612739, corrected p < 1e−10), and Figure 1g (r = 0.600855, corrected p < 1e−9) showed significant task-related and age-related effects, agreeing with the results of the previous method with greater sensitivity.
Discussion
Spatial ICA reveals sets of “chronoarchitectonically identified areas” (Bartels and Zeki 2004) or functionally connected regions. The presence of different cortical regions in the same ICA component implies that they are active at the same time. The existence of anatomical connections between two cortical regions is a necessary but not a sufficient condition for them to appear in the same task-related ICA component. If a given cognitive task recruits only one of those regions then there will be a component separated out by ICA containing only that region. However, information provided by DTI could be used as complementary to the ICA analysis to corroborate or to strengthen the interpretation of its findings.
Covert verb generation has been shown to produce a highly left lateralized pattern of activation (Hertz-Pannier, Gaillard et al. 1997; Xiong, Rao et al. 1998; Benson, FitzGerald et al. 1999). This is also true for the task analyzed in this study, which likewise shows left lateralization when examined using a traditional general linear model analysis as shown in Figure 3 (Holland, Plante et al. 2001; Szaflarski, Schmithorst et al. 2006): exhibiting left-lateralization that depends on age (Szaflarski, Holland et al. 2006) and handedness (Szaflarski, Binder et al. 2002). ICA uniquely qualifies this picture of strong left hemisphere lateralization. Of the seven components identified, only one (Figure 1d) showed a pattern of strong left hemisphere lateralization. Thus, the overall left-hemisphere lateralization previously described for word fluency tasks appears to mask a more complicated picture of lateralization that is revealed through ICA analysis.
Age-related changes in task activation are consistent with acquisition of new words and continued refinement of the semantic associations between words over the age-span tested. Five of the ICA components (Figure 1c–1g) demonstrated some degree of age-related change, even when using the on-off task reference function as the correlate. Each of these components contained at least one area that included classic language cortex (i.e., Broca’s or Wernicke’s area in the left hemisphere). In contrast, the two components that failed to demonstrate age related changes using the on-off task reference function as a correlate were dominated by regions inferior and posterior to classic language cortex. This suggests that the age related changes found in our analysis are likely to be language-related; providing further evidence supporting the regionally weighted model for neurocognitive brain development.
Previous developmental studies emphasize regional individual differences in children arising from variable rates of biological maturation. Our approach allows investigation of multiregional differences in activation patterns associated with development. The overall, multiregional pattern of activation may depict a functional network and be more informative of different cognitive processes than what is provided by active isolated cortical regions. The goal of the present investigation is to identify task related functional networks (spatially distinct cerebral regions) whose activity is correlated and common across subjects (Calhoun, Adali et al. 2001; Schmithorst, Holland et al. 2006) and to investigate any age-effects associated with these networks. As mentioned earlier, the notion of functional connectivity in ICA is slightly different from the current definition in neuroimaging. Within a given IC map the intra-component functional connectivity (between brain regions comprising the IC) is implicit. Although there are several brain regions in a network (IC map) the activity is represented by a single time course according to ICA formalism. Thus, any associated age-effects (of these networks) are investigated only based on the IC time courses using data driven techniques.
Under certain minimal assumptions (Duann, Jung et al. 2002; Calhoun, Pekar et al. 2004), the spatial independence of the ICA components may be equated with their modularity. This allows one to link each independent component (map) to a specific component of a cognitive task. It is worth mentioning that ICA, by itself, is not capable of revealing the precise cognitive correlates of the components found. Instead, ICA should be considered as a data driven method capable of revealing the spatial distributions of independent components from fMRI data. As with GLM-based analyses, the function of regions must be inferred and should be constrained by a priori knowledge of the functional neuroanatomy. Thus, ICA should be viewed as a tool for either corroboration of prior hypotheses or generation of new ones.
The seven task-related independent components for verb generation identified in this report and shown in Figure 1 are hypothesized to either support word processing or word generation as shown in Figure 4. Figure 4a is a cartoon showing the distribution of these IC modules relative to brain anatomy and cognitive functions. Figure 4b shows the hypothesized connections between the IC modules forming the theoretical cognitive basis for the covert verb generation task. Accordingly, IC maps shown in Figures 1 e, f and d constitute a word processing module. The IC maps shown in Figure 1 a, b, c and g constitute the word generation module. In line with the proposed model for this task (and discussed in the introduction, the above mentioned modules handle linguistic as well as attentional demands deemed necessary for verb generation. Consistent with the regionally-weighted network model for cognitive development, the IC modules incorporate more than one brain region and experience common developmental changes as reflected in the age correlations for each IC time-course of the module.
Figure 4.
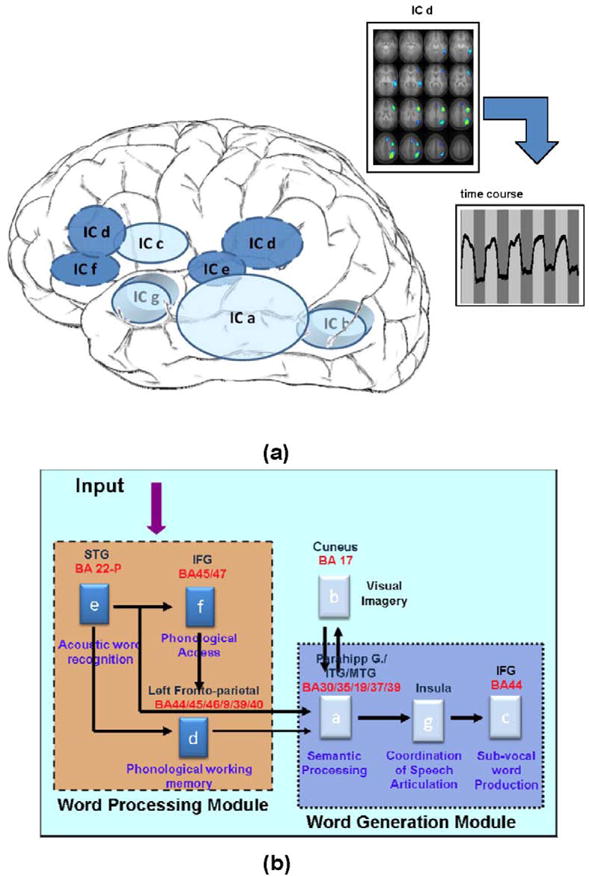
The covert verb generation circuit based on group ICA maps shown in Figure 1. The circuit is divided into word processing and word generation modules. Figure 4a shows how these IC modules are hypothesized to be related to brain regions/cognitive functions. Figure 4b shows how these cognitive modules are graphically connected forming the theoretical cognitive basis for the covert verb generation fMRI task. The connection strengths have not been evaluated in the present manuscript.
Traditional models of language such as the Wernike-Geschwind model that once posited left hemisphere network (semantic fluency) language functions are visibly challenged by our ICA fMRI data. Consistent with neuroimaging results in adults, the ICA results in Figures 1 and 2 demonstrate that language in children comprises left lateralized subnets but also requires bilateral and right-sided subnetworks as well. Therefore, extended models of language development must be qualified with reference to the type of language task employed and the region of interest examined. With this in mind our hypotheses in relation to the functional modularity of each IC module are summarized below.
Associating real physiological changes with IC time courses is not as straight forward as is the case with a BOLD signal time-course from a single functionally related anatomical area. Given the unique challenges posed by developmental studies (Berl, Vaidya et al. 2006), the proposed method (when combined with other statistical techniques) may lay the foundation for testing hypothesis based on proposed developmental models. Though, as with other methods for functional neuroimage analysis, individual variability must be characterized properly for correct interpretation of results from the proposed data driven method.
Figure 1 a: Audio/Visual association
The IC module shown in Figure 1a (parahippocampal and inferior & middle temporal gyri) is thought to be involved in audio/visual association and the related semantic processing; there also is some evidence that it may take part in semantic memory encoding that is affected by pathological processes (Bellgowan, Binder et al. 1998). This module may also have access to amodal semantic information and may also be capable of handling cross-modal associations. In general, parahippocampal gyrus is assumed to play an important role in memory encoding and retrieval. A study by (Epstein, Harris et al. 1999) has demonstrated its involvement in encoding new perceptual information about the appearance and layout of scenes. Similarly, an MEG study has shown that the parahippocampal cortices (including hippocampus) exhibit theta oscillations during spatial navigation in animals and humans, and in the former are thought to mediate spatial memory formation (Cornwell, Johnson et al. 2008). The fusiform gyrus (bilateral), in general, is thought to be concerned with local feature processing (Fink, Halligan et al. 1996; Price 2000). The inferior temporal gyrus, namely the middle fusiform area (Brodmann’s area 20 which is cytoarchitecturally bounded (caudally) by the occipitotemporal area 37) around the collateral sulcus (which separates the fusiform and parahippocampal gyri) has been found to activate when words and pictures are viewed (Bookheimer, Zeffiro et al. 1995; Damasio, Grabowski et al. 1996; Martin, Wiggs et al. 1996; Herbster, Mintun et al. 1997; Moore and Price 1999). It is also more active when people listen to object names and imagine the stimulus relative to when abstract words are listened to passively (D’Esposito, Detre et al. 1997). Thus, it appears that this brain region responds irrespective of the stimulus input modality. Further, one study associated this brain region with the visual attributes of semantic memory (D’Esposito, Detre et al. 1997). However, there is tangible evidence to suggest that this area is not specific to visual memory alone since the same area is activated when congenitally blind subjects read words with abstract meaning using tactile input (Buchel, Price et al. 1998; Moore and Price 1999). In addition, another study found activation in the parahippocampal gyrus during visual perception (Ganis, Thompson et al. 2004). However, they also found activation in a subset of this region during an imagery condition as well. In the imagery condition, participants had to generate a visual mental image of the object they heard. These results are consistent with findings from several other studies that have compared activation in the ventral stream during imagery and perception (O’Craven and Kanwisher 2000). Although parahippocampal gyrus has been reported to respond more strongly to scenes depicting places than to objects, it nevertheless also responds to visual objects (Epstein, Harris et al. 1999). This fact, responding more strongly to scenes depicting places may also be relevant in the context of covert-verb generation in young children. Therefore, it is not unrealistic to assume that young children may visualize actions (or verbs) in relation to places or situations they are familiar with.
The presence of medial temporal regions in IC a suggests that it may play a role in encoding memories of visual objects and events for verbal recall (Brewer, Zhao et al. 1998; Wagner, Schacter et al. 1998). However, in this module, the activation in the angular gyrus may also indicate involvement in semantic processing, a notion supported by several other studies (Hart and Gordon 1990; Nakai, Matsuo et al. 1999; Newman, Pancheva et al. 2001). These studies found activation in the fusiform and inferior temporal gyri. However, only a subset of voxels was activated during visual perception whereas another subset was activated both during visual perception and visual mental imagery. These findings are consistent with previous studies (Ishai, Ungerleider et al. 2000; Ishai, Haxby et al. 2002), and suggest that these regions may be involved in storing memories of visual objects that can be re-activated both by signals from lower-level visual cortex and by signals from higher-level cortices involved in cognitive control processes.
It has been shown that children are not as selective or modality specific, thus activating both auditory and visual areas regardless of presentation modality (Booth, Burman et al. 2001). The audio/visual association and semantic processing required by covert verb generation is most likely fully developed by the age of 5 (Ahmad, Balsamo et al. 2003). The indirect support for this comes from the relatively low inter-subject variability associated with the IC (a) time course found in our study. This possibly explains the failure to detect any age-related trends in this component.
Note that ICa is moderately right lateralized (LI = −0.4) highlighting the importance of right hemisphere contributions to language functions (Mitchell and Crow 2005), although its specific involvement in covert verb generation is still a matter of speculation. It should be noted that with conventional GLM analysis, the right lateralization of this posterior temporal-parietal component has not been observed because the bold signal level is much weaker than in other components of language network, so that thresholding effects in random effects group analysis tend to obscure activation in this region (Holland, Vannest et al. 2007).
Figure 1 b: visual imagery
The module shown in Figure 1b is thought to be engaged in Visual Imagery. Given the nature of covert verb generation, it is somewhat surprising that ICA was able to separate out a component consisting of a low level visual structure. However, these brain regions (cuneus and precuneus) have been consistently activated in fMRI experiments of visual mental imagery. In the context of covert verb generation, this primary visual area (V2) is hypothesized to be involved with visualization of nouns and actions of associated verbs. In support of this idea, several previous studies have demonstrated right precuneus and cuneus activation in visual search (Corbetta, Shulman et al. 1995; Nobre, Sebestyen et al. 2002) and memory search (Moscovitch and Winocur 1995; Kohler, McIntosh et al. 1998; Cabeza, Dolcos et al. 2002) (for review see (Cabeza and Nyberg 2000)). However, some studies have shown that the right precuneus is associated with spatial shifts of attention (Corbetta, Miezin et al. 1993; Culham, Brandt et al. 1998) and nonspatial shifts of attention, such as attentional shifts between target defining dimensions (shape, color, and motion) in target detection tasks (Pollmann and von Cramon 2000; Weidner, Pollmann et al. 2002). Taking the complexity of the current fMRI task into account (task involved generating verbs corresponding to simple nouns), the demand on visual imagery as part of language processing may be very low; this aspect of cognitive processing may be fully developed by the age of 5. A trend for age effect was detected for this IC time course. However, it is reasonable to assume that with increasing age children may be able to generate more verbs for a given noun and the effect of older subjects utilizing visual imagery at a greater level can not be ruled out. This may explain why we did not find strong associations between age and IC map shown in Figure 1b.
Figure 1 c: Sub vocal word production
The IC shown in Figure 1c is the provenance of BA 44 classically referred to as Broca’s Area in the left hemisphere (and contralateral homologue). Based on tractography evidence Catani et al. postulated a functional separation of the classical Broca’s area (Catani, Jones et al. 2005). These authors observed that the distribution of arcuate fibers extended beyond the classical limits of Brocas’s area and included parts of the middle frontal and inferior precentral gyri. This extended area, which they called “Broca’s territory” is in line with several other studies that suggested the existence of projections from classical Broca’s area to nearby cortical regions that are thought to be involved in higher order language processing (Aboitiz and Garcia 1987; Damasio 1992; Matsumoto, Nair et al. 2004). Similarly, as is the case for Broca’s territory, the distribution of tractography-defined fibers in the posterior temporal region turned out to be wider than what the classical model suggested (Catani, Jones et al. 2005). Thus, a similar segregation of function was suggested for Wernicke’s area and the extended region (including the posterior part of both the superior and middle temporal gyrus) was termed “Wernicke’s territory” by Catani et al. These findings argue against the concept of language processing as a one-way serial transfer of information between highly localized cortical regions which are specialized for specific functions. Instead, these findings are more in line with a recently proposed dynamic model for language which supports continuous interaction between semantic and syntactic processing via direct and indirect connections between Broca’s and Wernicke’s territories. The direct pathway, which runs medially, corresponds to the classical description of the arcuate fasciculus. The indirect lateral pathway is composed of an anterior segment connecting the inferior parietal cortex (Geschwind’s territory) with Broca’s territory and a posterior segment connecting Geschwind’s and Wernicke’s territories. Tractography evidence appears to suggest that long segment direct fibers project to anterior Broca’s territory, whereas the anterior segment of the indirect pathway projects more posteriorly (Catani, Jones et al. 2005).
In support of this hypothesis another study investigating intraoperative electrocorticography of perisylvian language networks in humans has shown bidirectional connectivity from Broca’s area to both superior temporal and inferior parietal regions. In general, the inferior parietal lobule is implicated in semantic processing (Price 2000). In further support of the indirect pathway, an unexplained delay was found in the response evoked in superior temporal cortex by stimulation of Broca’s area and vice versa. Although this delay can be explained by a Broca–subcortical–Wernicke pathway (Matsumoto, Nair et al. 2004) it can also be equally explained by routing of the information via the indirect Broca–parietal–Wernicke pathway (Catani, Jones et al. 2005).
Our ICA results not only support such anatomical separation, but also show a clear distinction in functional specialization by separating out the anterior and posterior aspects of Broca’s area into two distinct independent components that support covert verb generation. Furthermore, our results, taken together with ICA group analysis of story comprehension in children (Schmithorst, Holland et al. 2006) reaffirm the proposed two-route model for language processing. A lesion study by McCarthy and Warrington (McCarthy and Warrington 1984) has proposed a similar two-route model for language processing and postulated functional segregation in language areas with different routes for different aspects of language processing and speech production. It is proposed that there are direct and indirect paths. The direct path is utilized in processing of the phonologically based language functions (such as fast automatic word production) while the indirect path is involved in semantically based language functions (auditory comprehension and vocalization of semantic content). This classification does not in any way refer to localization of language-related functions but rather to the ability of the two-route model to differentiate between these two functions.
The IC map shown in Figure 1c encompasses the posterior aspect of BA 44. We suspect that this structure is involved in sub-vocal word production as part of covert verb generation. In support of this, a study by Friederici et al. had implicated the posterior-inferior portion of BA 44, i.e. the inferior tip of the pars opercularis and deep frontal operculum on the border to ventral premotor cortex, in online syntactic structure building processes (Friederici, Opitz et al. 2000; Friederici, Wang et al. 2000). A subsequent study by the same author has further analyzed the involvement of IFG in online syntactic structure building (Friederici, Ruschemeyer et al. 2003). In contrast to the original study these authors propose that the activation in Broca’s area may reflect a greater involvement of language-related working memory rather than online language processing. Accordingly, it appears that the pars opercularis of the left IFG (i.e. BA 44) may not be a necessary part of the network supporting online, sentence-level semantic and syntactic processes, but this region may only come into play under particular task demands. Although the exact role played by BA 44 in covert verb-generation is debatable (Hirshorn and Thompson-Schill 2006) a study by Badre et al. (Badre, Poldrack et al. 2005) has argued for a theoretical distinction between mechanisms they refer to as “post retrieval selection” and “controlled retrieval”. These authors hypothesize that the former is subserved by the mid-LIFG (pars triangularis or BA 45) while the latter is subserved by the anterior-LIFG (pars orbitalis) which is in line with our hypothesis for this particular IC map. A strong age-related effect was observed for this module. This increase may reflect sub-vocal word production becoming more automatic in the covert verb generation task. Performance differences attributed to older children may also contribute to the observed increase in task relatedness.
Figure 1 d: Phonological/Semantic working memory
The module shown in Figure 1d is the only highly left-lateralized component likely associated with generation of semantic representations of the nouns that are heard. This would amount to accessing the semantic store directly from the phonological input stream, rather than activation of an amodal semantic store. This module may also permit the maintenance of the noun in working memory for reference facilitating the sequential generation of verbs. In particular, BA 9/46 has been implicated in manipulating information in the working memory (Dronkers, Wilkins et al. 2004). The activation pattern seen in this IC is typical of fMRI paradigms that involve verbal semantic processing as well as fluency tasks (Binder, Rao et al. 1995; Holland, Plante et al. 2001; Gaillard, Sachs et al. 2003). A common feature of these tasks is the utilization of the left-hemispheric classical Wernicke-Geschwind network of Broca’s and Wernicke’s areas, including the inferior parietal lobule recently shown to be connected to these areas via the indirect pathway (Catani, Jones et al. 2005). Furthermore, the network consisting of Broca’s area and the inferior parietal lobule has previously been implicated in semantic decision tasks (Bullmore, Horwitz et al. 2000; Seghier, Lazeyras et al. 2004; Shivde and Thompson-Schill 2004). This network shows activation in the anterior aspect of Broca’s area that may also support phonological working memory (D’Esposito, Postle et al. 1999; Cabeza and Nyberg 2000; Dronkers, Wilkins et al. 2004). The notion that this activation corresponds to some maintenance demands of phonological processing can not be completely ruled out (Nixon, Lazarova et al. 2004).
However, it is well known that these areas are differentially active to phonological vs. semantic (LoCasto, Krebs-Noble et al. 2004; Seghier, Lazeyras et al. 2004; Shivde and Thompson-Schill 2004) and phonological vs. acoustic demands (LoCasto, Krebs-Noble et al. 2004; Dehaene-Lambertz, Pallier et al. 2005). Therefore, according to our hypothesis, the contribution of this region appears to be independent from earlier and later acoustic demands but may subserve certain semantic demands based on the model we propose for covert verb generation. In other words the presence of the brain regions in this IC component may involve the storing of speech-based information (Awh, Jonides et al. 1996; Schumacher, Lauber et al. 1996; Smith, Jonides et al. 1996) using a covert articulatory mechanism (Baddeley 1992) during the time the heard noun is held in working memory while corresponding verbs are generated sequentially. The frontal component and inferior parietal lobule may recruit the phonological loop as described earlier. The same studies that have identified activation in inferior frontal regions associated with phonological rehearsal also typically identify left lateralized activation in parietal cortex (Gold and Buckner 2002; Jacquemot, Pallier et al. 2003; Logie, Venneri et al. 2003; Ravizza, Delgado et al. 2004; Dehaene-Lambertz, Pallier et al. 2005). These activations are often hypothesized to be related to the involvement of the phonological loop within the working memory system (Baddeley 1986), although it has recently been suggested that this area might be more effectively attributed to increased attentional focus on phonological information (Chein, Ravizza et al. 2003).
A very strong age-related increase was seen for this component corresponding to increasing participation (or becoming more efficient) of this left lateralized network in support of the phonological and semantic expressive functions described above in older children. The age-effect may account for some of the intersubject variability (0.74 in Table 3) found with this otherwise highly task correlated component. The strong correlation between the time-course of component in Figure 1d and the task reference function (0.92 in Table 3), coupled with its relatively low intersubject variability suggests that this component is the most reliably task-related providing further support for the key role for the classical left hemisphere Wernicke-Geschwind network in the execution of semantic association and verbal fluency tasks. The strong positive age-related changes associated with this network provide evidence for the emergence of more extensive/efficient networks of semantic information as children learn; lending further support to the regional-weighting model for neurocognitive brain development. Holland et al. have shown that the largest changes in lateralization occurred for skills that are acquired over a longer period of development (such as semantic skills drawn upon in verb generation) (Holland, Vannest et al. 2007). Correspondingly, this highly left lateralized network also showed the greatest age-effects, implying a relationship between efficient/mature network and the left lateralization for this task.
Figure 1 e: Acoustic word recognition
The superior temporal gyrus (STG) is assumed to be involved in initial acoustic processing, word recognition (Figure 1e). This brain region mainly includes the posterior portion of BA22, classically referred to as Wernicke’s area in the left hemisphere. In general, activation of the STG has been previously reported in studies of processing of semantic anomalies (Kuperberg, McGuire et al. 2000; Ni, Constable et al. 2000; Friederici, Ruschemeyer et al. 2003). However, the notion that access to word meaning is contained in the Wernicke’s area has already been challenged (Scott and Johnsrude 2003). The alternative hypothesis implicates the STG primarily in spectral and temporal processing of auditory input, with the processed information then projected to amodal higher-order association cortices. In support of this view, STG activation was not found for semantic violations when subjects read, rather than listened to, sentences with semantic anomalies (Newman, Pancheva et al. 2001). In addition, differential activation was not found in the STG when subjects listened to words versus pseudowords, or words versus reverse speech (Binder, Frost et al. 2000). The posterior STG (Figure 1e) has previously been proposed as being recruited for non-domain-specific integrative processes, with increased effort being involved in incorporating anomalous structures into sentences (Friederici, Ruschemeyer et al. 2003). However, the same region (posterior portion of the superior temporal lobe), was proposed to be involved in transient representation of phonetic sequences (Wise, Scott et al. 2001), independent of whether or not these sequences constitute intelligible speech. Based on this assumption, one can expect the posterior superior temporal lobe to be activated whenever words or sentences are processed. We propose a model for verb generation that makes a distinction between processes related to the phonological encoding of incoming acoustic stimuli and the subsequent recognition of the string of phonological units as a real word, prior to accessing the meaning of that word.
The rise in the hemodynamic response of this IC (Figure 2e) supports the notion that the posterior STG may be involved in transient and higher-order integrative processes, as the process of integrating the meaning of the noun, the associated verbs and the visual imagery aspect of the covert-verb generation into a more cohesive picture will not begin until a certain period of time after hearing the noun. This is in agreement with the hypothesis that the posterior STG maps different types of information (semantic, syntactic, and pragmatic) onto each other for final interpretation (Friederici, Ruschemeyer et al. 2003). However, in the context of present fMRI task, we hypothesize that the role of the region corresponding to IC e is in line with initial acoustic processing and transient representation of phonetic sequences related to the given noun. However, the notion that this structure is supporting access to word meaning and higher order integration processes can not be completely ruled out.
A significant age-related increase was found for this component. Since the nouns used were at a vocabulary level appropriate for children as young as 5 years, our data seem to be more consistent with the hypothesis of the STG being involved with spectral and temporal processing (since the proficiency of this modality would be expected to increase with age), rather than in higher order integrative processes associated with word meaning.
Figure 1 f: phonological access
The independent component in Figure 1f is hypothesized to be involved in phonological access and semantic working memory. As suggested previously (Thompson-Schill, Swick et al. 1998), we hypothesize that this module is involved in context-sensitive responses perhaps by the weighting of information in working memory. It may also play a prominent role in lexical retrieval and word selection. In other words, the correspondence between codes recognized and the phonological forms of the word may be established in this network. We propose that the area of anterior activation in Figure 1f (inferior frontal) may relate to cognitive processes associated with phonological processing. In support of this hypothesis several studies have reported detecting activation in the inferior frontal region (inferior frontal gyrus and insula) when subjects were asked to remember and make decisions about both words and nonmeaningful phonological stimuli (Gelfand and Bookheimer 2003; Jacquemot, Pallier et al. 2003; Chee, Soon et al. 2004; LoCasto, Krebs-Noble et al. 2004; Seghier, Lazeyras et al. 2004; Shivde and Thompson-Schill 2004; Dehaene-Lambertz, Pallier et al. 2005). In general, the anterior portion of the IFG [i.e. BA 44 on the border to BA 45 (Fiebach, Schlesewsky et al. 2001) and BA 47 (Cooke, Zurif et al. 2002)] seems to support aspects of syntactic memory as necessary in the processing of long antecedent-gap dependencies. However, a review of semantic retrieval studies has concluded that activation related to semantic processing is clustered at or near left BA 44 and 45 (Buckner, Petersen et al. 1995). More recently BA 47 has also been implicated in semantic processing (Poldrack, Wagner et al. 1999). Although it has been previously suggested that the anterior aspects of left IFG play a differential role in semantic retrieval and the posterior portion in phonological processing (Price 1997; Poldrack, Wagner et al. 1999), later studies have demonstrated comparable activation patterns in left IFG whether it is engaged in phonological or semantic processing (Barde and Thompson-Schill 2002; Gold and Buckner 2002). Furthermore, several imaging studies of working memory have implicated BA 45/47 in selection or comparing information (Petrides 1994; D’Esposito, Postle et al. 1999; Cabeza and Nyberg 2000). The idea that the activation in IFG corresponds to maintenance demands of phonetic processing is also tenable. In support of this hypothesis disruption of this area with TMS (Transcranial Magnetic Stimulation) showed impairment only when subjects were instructed to remember stimuli for later use vs. when they made judgments concerning those stimuli (Nixon, Lazarova et al. 2004). However, another study (Thompson-Schill, Swick et al. 1998) suggested that the involvement of BA 45/47 in verb generation is not due to the demands of semantic retrieval but rather due to the non-semantic selection processes involved when dealing with competing information. A strong age-related increase was also seen for this component. It is expected that older children become more proficient in phonological access, lexical retrieval and word selection. In terms of BOLD activation, this result is consistent with the observation that a smaller region in BA 45/7 showed greater activation in adults in a lexical phonologic and semantic fluency task analysis (Berl, Vaidya et al. 2006).
Figure 1 g: Coordination of articulation
The IC map shown in Figure 1g shows areas hypothesized to be involved in coordination of speech articulation. In general, it has been noted that speech input activates bilateral superior and middle temporal cortices while speech output enhances activation in the left posterior superior temporal sulcus and engages the left anterior insula and bilateral sensorimotor cortices (Price 2000). The left posterior inferior temporal cortex may also be involved. We also hypothesize that the area activated in this module takes part in facilitating sub-vocal word production, often assumed to be the function of module c – (BA44). In support of this hypothesis, lesional (Dronkers 1996) and neuroimaging (Wise, Greene et al. 1999) studies have emphasized and identified the critical speech production region to be the anterior insula. Furthermore, it has been noted that more lateral and anterior lesions typically cause deficits in sentence comprehension and production, in particular the ability to generate word lists and assemble phonemes into words and words into sentences (Rubens 1976; Damasio and Geschwind 1984; Costello and Warrington 1989). However, some studies have implicated the left insula (within the left inferior frontal region) in phonological working memory (Paulesu, Frith et al. 1993), mainly in subvocal rehearsal (Fiez, Raife et al. 1996; Smith, Jonides et al. 1998). These studies hypothesize that phonological working memory as it relates to the ability to repeat unfamiliar phonotactic constructs correlates with vocabulary development (Gathercole 1995). Furthermore, a study that investigated the neural correlates of phonological working memory in young bilingual adults observed that equal bilinguals activated left insula region more readily with increased working memory demands when compared to unequal bilinguals. They ascribed this discrepancy to more superficial engagement of the neural circuitry required to incorporate novel speech-like sounds into long-term phonological representations. Somewhat surprisingly, this component also exhibits an age dependence, possibly corresponding to improved speech articulation in older children or an increased number of verbs generated for each noun. Unfortunately, we did not monitor the number of responses made by subjects in this covert verb generation task. Newer, overt speech paradigms will allow this hypothesis to be tested in the future. However there is evidence that increased motor response activity does result in increasing activation in other types of overt motor tasks (Gyngell, Bock et al. 1996; Wexler, Fulbright et al. 1997).
Analysis of trends
In this large pediatric study, we utilized two different but complementary approaches to analyze the developmental trajectories associated with cognitive modules associated with language processing. The data driven approach appears to be more sensitive to the age effects in associated IC time courses. However, the hypothesis-driven approach, where the a priori time course is incorporated, will yield greater sensitivity if the said time course can be specified with sufficient accuracy. The advantage of the data-driven approach becomes apparent when there is insufficient prior knowledge to accurately specify the time-course of activation in a given voxel, considering HRF uncertainties. Consequently the data driven ICA method is capable of yielding information, otherwise inaccessible via a hypothesis-driven approach.
The above mentioned two methods entail investigating any increased use of these task related networks (set of brain regions) with subject age. Our findings of strong correlations with age for many of the task-related ICs points to changing relative weights placed on the corresponding neural elements during development of language skills.
Based on the regionally weighted model it is possible that the adjustment of weights may account for the immediate and efficient use of networks with age. According to ICA results, a given brain region can be part of several distinct networks. Since these networks show relatively different age-effects, conventional methods where one looks at age-effects of single brain regions oversimplifies the problem at hand. Our results suggest a more complex developmental course than proposed by the focal network model although its implications can not be conclusively excluded based on current results. A detailed inter/intra network connectivity analysis based on ICA results may address some of these issues and will be a topic for future research. It is expected that different combinations of weights in a network might be able to capture normal variation in cognitive skill level, cognitive strategies or adaptive strategies utilized to compensate for any impairment (Berl, Vaidya et al. 2006).
The trend analysis suggest that the observed developmental differences may be a consequence of the protracted period of development of the frontal lobe as reflected in regional myelination (Giedd, Blumenthal et al. 1999; Schmithorst, Wilke et al. 2002; Giedd 2004). However, since the data driven approach appears to be more sensitive to the age-effects analysis, it is reasonable to assume that the age-effects capture age-related as well as regional variability inherent in fMRI data (Gaillard, Sachs et al. 2003; Berl, Vaidya et al. 2006). The parameters that can control the sensitivity of the trend analysis are chosen to be very liberal in the current investigation. However, given the large number of subjects involved in the present analysis, the effects of individual variability may be at a minimum compared to investigations with fewer number of subjects.
Limitations
A limitation of this study was the inability to acquire in-scanner performance data, due to the task design and scanner noise. Therefore performance effects on the present analysis cannot be completely discounted even though the task was designed in such a way that the youngest children in our study could easily perform it. Performance effects may be correlated with subject age which in turn has been shown to be correlated with fMRI BOLD signal for this task. While no age-related changes in task performance were found in our previous study measuring noun recall after completion of the fMRI task (Chiu, Schmithorst et al. 2006), confounding effects of age on performance may not be entirely excluded in the current analysis. The present verb generation task does not set a ceiling to the number of nouns a subject can generate for a given verb. Evidence suggests that this design is successful in minimizing the amount of variance attributable to performance (Gaillard, Sachs et al. 2003). In future studies we plan to employ a recently developed version of the verb generation task (Schmithorst and Holland 2004; Chiu, Coen-Cummings et al. 2005) that incorporates a completely silent scanner interval, during which an overt response can be made and recorded to control for in-scanner task performance.
The group ICA method as employed in this analysis identifies common task related spatial modes (networks) within the population. However, we had a surfeit of statistical power available by the large sample. Therefore, we considered examining brain regions identified within each spatial mode that did not achieve significance at such a stringent threshold in our random-effects group-level analyses. These regions would be those that either did not fit each derived IC model as strongly, or whose engagement was not consistently conserved over participants. The latter possibility represents the particularly interesting potential to quantify regional functional connectivity differences with age. However, this was beyond the scope of the current inquiry and will be dealt with in future work. To partly address this issue, the age-effects were investigated entirely based on individual IC time courses, thus capturing some of the individual variability.
It should be noted that ICA is not completely immune to movement related effects. We used a heuristically defined metric to discard bad data sets (subjects) from the ICA analysis. A previous study utilizing the same dataset showed that younger children moved more compared to older children (Yuan, Altaye et al. 2008). This is a limitation of the study as it would be of any study that compares younger and older subjects. As mentioned earlier, some of the motion artifacts can be eliminated in ICA as they will be captured in separate spatial maps. Thresholding can also be used to eliminate motion artifacts within a given map. As noted above, we applied objective criteria to reject data sets that were corrupted by excessive motion in order to minimize bias introduced by age-dependent motion. However, individual variability (including motion) must be fully characterized when using ICA methods to investigate developing cognition.
The trend analysis method (data driven method) can be easily extended to directly associate each component with individual differences in measurable language ability by replacing age with other language related measurements (or reading ability etc.). However, this is a difficult task when one considers the number of cognitive functions involved in covert verb generation. In addition, with the current data set age is highly correlated with the language measures, making it difficult to separate one from the other. This can be considered a limitation of the study.
Finally, we would like to mention that the emphasis of the current study is to investigate overall development trends associated with language circuitry. The study did not take into account developmental milestones such as puberty where the overall effect of structural and functional development shifts might be significant (Booth and Burman 2001). However, in previous investigations using group random effects analysis with the general linear model we have examined lateralization growth curves for specific brain regions (Broca’s Area and Wernicke’s Area) associated with the Verb Generation task and have not found any evidence of nonlinear growth trajectories over the age range covered in this study (5–18 years) (Holland, Vannest et al. 2007). Further, we have not seen evidence of a nonlinear influence of puberty in the lateralization growth curves in this same population. However, addressing nonlinear growth trends with a linear method such as ICA as implemented may not be ideal.
Newer, more sophisticated fMRI analysis techniques such as structural equation modeling (SEM) (Solodkin, Hlustik et al. 2004; Karunanayaka, Holland et al. 2007) or path analysis (Bullmore, Horwitz et al. 2000) may help resolve unanswered questions regarding information flow in the brain. The insight provided by group ICA may help in generating theoretical (predictive) models for subsequent path analysis. In turn, the validity of these theoretical models can then be tested with the procedure. We also note that the control condition, designed to cancel out cortical regions related to primary auditory-related processing, is not strictly necessary for an ICA analysis, as the algorithm will separate out the cortical regions related to different cognitive components. However, failure to include the control period limits the interpretability of results obtained using hypothesis-driven techniques such as GLM analysis (Worsley and Friston 1995), which may be desirable as a complementary analysis strategy to data-driven approaches such as ICA.
Conclusions
A large-scale fMRI study of covert verb generation was conducted on a group of over 300 children ages 5–18. Using group ICA analysis, spatially independent and task-related networks were identified, including bilateral activation in the inferior temporal gyrus, cuneus, insula and the posterior aspect of superior temporal gyrus. A highly left-lateralized network encompasing Broca’s area and the left inferior parietal lobule was also identified. In addition, a slightly right-lateralized network involving the inferior frontal gyrus was detected. Each module was examined in relation to the covert verb generation task. The results show the advantage of ICA that can detect additional task-related spatial modes showing brain activity correlated across distributed brain regions that are undetected in GLM methods. Task-related activity was shown to increase with age in the networks involving the superior temporal gyrus and the inferior frontal gyrus. The age-effects support the idea that these specialized cognitive modules will be used increasingly as children get older; further endorsing the regionally weighted model for neurocognitive development that deals with interactions between brain regions within a network. Finally, the results corroborate the recent hypotheses regarding the functional parcellation of Wernicke’s area and Broca’s area. Furthermore, the bilaterality of cognitive modules (IC maps) deemed task-related in covert verb generation support the notion of parallel right and left language networks reemphasizing the importance of right hemisphere in language processing. The current study lays the foundation for a possible inferential statistical method capable of testing hypothesis based upon existing theoretical models for normal cognitive development. This is a very challenging task as the current results reveal a more complex developmental trajectory than proposed by existing models.
Acknowledgments
The study was supported by a grant from the U.S. National Institute of Child Health and Development, R01-HD38578. The authors acknowledge the assistance of Dr. Anna Byars, Ph.D., in the administration of the Wechsler IQ tests, and Drs. Richard Strawsburg, M.D. and Mark Schapiro, M.D., for performing the neurological examinations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Garcia R. The evolutionary origin of the laguage areas in the human brain. A neuroanatomical perspective. Brain Res Rev. 1987;25:381–396. doi: 10.1016/s0165-0173(97)00053-2. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, et al. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60(10):1598–605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, et al. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychological Science. 1996;7(1):25–31. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford: Oxford university; 1986. [Google Scholar]
- Badre D, Poldrack RA, et al. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–18. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, et al. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol. 2002;59(7):1168–74. doi: 10.1001/archneur.59.7.1168. [DOI] [PubMed] [Google Scholar]
- Barde LH, Thompson-Schill SL. Models of functional organization of the lateral prefrontal cortex in verbal working memory: evidence in favor of the process model. J Cogn Neurosci. 2002;14(7):1054–63. doi: 10.1162/089892902320474508. [DOI] [PubMed] [Google Scholar]
- Beitchman JH, Jiang H, et al. Models and determinants of vocabulary growth from kindergarten to adulthood. J Child Psychol Psychiatry. 2008;49(6):626–34. doi: 10.1111/j.1469-7610.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- Bellgowan PS, Binder JR, et al. Side of seizure focus predicts left medial temporal lobe activation during verbal encoding. Neurology. 1998;51(2):479–84. doi: 10.1212/wnl.51.2.479. [DOI] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52(4):798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, et al. Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage. 2006;30(3):679–91. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10(5):512–28. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, et al. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol. 1995;52(6):593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, et al. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain Lang. 2006;99(3):226–35. doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, et al. Regional cerebral blood flow during object naming and word reading. Human Brain Mapping. 1995;3:93–106. [Google Scholar]
- Booth JR, Burman DD, et al. Development and Disorders of Neurocognitive Systems for Oral Language and Reading. Learning Disability Quarterly. 2001;24:2005–215. [Google Scholar]
- Booth JR, Burman DD, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–51. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, et al. The development of specialized brain systems in reading and oral-language. Child Neuropsychol. 2001;7(3):119–41. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Booth JR, MacWhinney B, et al. Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Dev Neuropsychol. 2000;18(2):139–69. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, et al. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281(5380):1185–7. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siege de la faculte du langage articule; suivies d’une observation d’aphemie. Bull Soc Anat Paris. 1861;6:398–407. [Google Scholar]
- Brown TT, Lugar HM, et al. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15(3):275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Buchel C, Price C, et al. A multimodal language region in the ventral visual pathway. Nature. 1998;394(6690):274–7. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE, et al. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci. 1995;15(1 Pt 1):12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Horwitz B, et al. How good is good enough in path analysis of fMRI data? Neuroimage. 2000;11(4):289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- Byars AW, Holland SK, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17(12):885–90. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, et al. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–30. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, et al. Group ICA of functional MRI data:seperability, stationarity, and inference. Proc Int Conf on ICA and BSS; Sandiago, CA. 2001. [Google Scholar]
- Calhoun VD, Adali T, et al. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, et al. Alcohol intoxication effects on simulated driving: exploring alcohol-dose effects on brain activation using functional MRI. Neuropsychopharmacology. 2004;29(11):2097–17. doi: 10.1038/sj.npp.1300543. [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Oral and Written Language Scales. Circle Pines, MN: American Guidance Service; 1996. [Google Scholar]
- Casey BJ, Thomas KM, et al. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci. 2002;22(19):8647–52. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, et al. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chee MW, Soon CS, et al. Left insula activation: a marker for language attainment in bilinguals. Proc Natl Acad Sci U S A. 2004;101(42):15265–70. doi: 10.1073/pnas.0403703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, et al. Using neuroimaging to evaluate models of working memory and their implications for language processing. J Neurolinguistics. 2003;16:315–339. [Google Scholar]
- Chiu C-YP, Schmithorst VJ, et al. Making memories: A cross-sectional investigation of episodic memory encoding in children using fMRI. Developmental Neuropsychology. 2006;29:321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- Chiu CY, Coen-Cummings M, et al. Sound blending in the brain: a functional magnetic resonance imaging investigation. Neuroreport. 2005;16(9):883–886. doi: 10.1097/00001756-200506210-00002. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, et al. Neural basis for sentence comprehension: grammatical and short-term memory components. Hum Brain Mapp. 2002;15(2):80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, et al. A PET study of visuospatial attention. J Neurosci. 1993;13(3):1202–26. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, et al. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270(5237):802–5. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, et al. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28(23):5983–90. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello AL, Warrington EK, et al. Dynamic aphasia: the selective impairment of verbal planning. Cortex. 1989;25(1):103–14. doi: 10.1016/s0010-9452(89)80010-3. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, et al. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80(5):2657–70. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, et al. A functional MRI study of mental image generation. Neuropsychologia. 1997;35(5):725–30. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, et al. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Damasio A. N Engl J Med. 1992;(326):531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Geschwind N. The neural basis of language. Annu Rev Neurosci. 1984;7:127–47. doi: 10.1146/annurev.ne.07.030184.001015. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, et al. A neural basis for lexical retrieval. Nature. 1996;380(6574):499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Pallier C, et al. Neural correlates of switching from auditory to speech perception. Neuroimage. 2005;24(1):21–33. doi: 10.1016/j.neuroimage.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–61. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, et al. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duann JR, Jung TP, et al. Single-trial variability in event-related BOLD signals. Neuroimage. 2002;15(4):823–35. doi: 10.1006/nimg.2001.1049. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, et al. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23(1):115–25. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, et al. Syntactic working memory and the establishment of filler-gap dependencies: insights from ERPs and fMRI. J Psycholinguist Res. 2001;30(3):321–38. doi: 10.1023/a:1010447102554. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, et al. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 1996;16(2):808–22. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, et al. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382(6592):626–8. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, et al. Segregating semantic and syntactic aspects of processing in the human brain: an fMRI investigation of different word types. Cereb Cortex. 2000;10(7):698–705. doi: 10.1093/cercor/10.7.698. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, et al. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13(2):170–7. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Wang Y, et al. Localization of early syntactic processes in frontal and temporal cortical areas: a magnetoencephalographic study. Hum Brain Mapp. 2000;11(1):1–11. doi: 10.1002/1097-0193(200009)11:1<1::AID-HBM10>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, et al. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry. 1995;167(3):343–9. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, et al. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18(3):176–85. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, et al. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res. 2004;20(2):226–41. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Is nonword repetition a test of phonological memory or long-term knowledge? It all depends on the nonwords. Mem Cognit. 1995;23(1):83–94. doi: 10.3758/bf03210559. [DOI] [PubMed] [Google Scholar]
- Gelfand JR, Bookheimer SY, et al. Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron. 2003;38(5):831–42. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL, et al. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35(4):803–12. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gyngell ML, Bock C, et al. Variation of functional MRI signal in response to frequency of somatosensory stimulation in alpha-chloralose anesthetized rats. Magn Reson Med. 1996;36(1):13–5. doi: 10.1002/mrm.1910360104. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, et al. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21(4):1639–51. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27(3):226–31. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, et al. Regional cerebral blood flow during word and nonword reading. Hum Brain Mapp. 1997;5(2):84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard WD, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48(4):1003–12. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A, et al. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214–22. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–57. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, et al. Normal fMRI Brain Activation Patterns in Children Performing a Verb Generation Task. Neuroimage. 2001;14(4):837–43. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, et al. Functional MRI of language lateralization during development in children. International Journal of Audiology. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10(3):626–34. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Hyvarinen A, Karhunen J, et al. Independent Component Analysis. New York: John Wiley & Sons; 2001. [Google Scholar]
- Ishai A, Haxby JV, et al. Visual imagery of famous faces: effects of memory and attention revealed by fMRI. Neuroimage. 2002;17(4):1729–41. doi: 10.1006/nimg.2002.1330. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, et al. Distributed neural systems for the generation of visual images. Neuron. 2000;28(3):979–90. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Jacquemot C, Pallier C, et al. Phonological grammar shapes the auditory cortex: a functional magnetic resonance imaging study. J Neurosci. 2003;23(29):9541–6. doi: 10.1523/JNEUROSCI.23-29-09541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanayaka PR, Holland SK, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34(1):349–60. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, et al. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10(13):2817–21. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kohler S, McIntosh AR, et al. Functional interactions between the medial temporal lobes and posterior neocortex related to episodic memory retrieval. Cereb Cortex. 1998;8(5):451–61. doi: 10.1093/cercor/8.5.451. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, et al. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci. 2000;12(2):321–41. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, et al. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci U S A. 2002;99(20):13336–41. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenneberg EH. Biological Foundations of Language. New York: Wiley; 1967. [Google Scholar]
- Levelt WJ, Roelofs A, et al. A theory of lexical access in speech production. Behav Brain Sci. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. discussion 38–75. [DOI] [PubMed] [Google Scholar]
- LoCasto PC, Krebs-Noble D, et al. An fMRI investigation of speech and tone segmentation. J Cogn Neurosci. 2004;16(9):1612–24. doi: 10.1162/0898929042568433. [DOI] [PubMed] [Google Scholar]
- Logie RH, Venneri A, et al. Brain activation and the phonological loop: The impact of rehearsal. Brain Cogn. 2003;53:293–296. doi: 10.1016/s0278-2626(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Lotsof EJ. Intelligence, verbal fluency, and the Rorschach test. J Consult Psychol. 1953;17(1):21–4. doi: 10.1037/h0062524. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, et al. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Roznowski M, et al. Model modifications in covariance structure analysis: the problem of capitalization on chance. Psychol Bull. 1992;111:490–504. doi: 10.1037/0033-2909.111.3.490. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, et al. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–52. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127(Pt 10):2316–30. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK, et al. A two-route model of speech production. Evidence from aphasia. Brain. 1984;107(Pt 2):463–85. doi: 10.1093/brain/107.2.463. [DOI] [PubMed] [Google Scholar]
- McDonald JL. Language acquisition: the acquisition of linguistic structure in normal and special populations. Annu Rev Psychol. 1997;48:215–41. doi: 10.1146/annurev.psych.48.1.215. [DOI] [PubMed] [Google Scholar]
- McGregor KK, Friedman RM, et al. Semantic representation and naming in young children. J Speech Lang Hear Res. 2002;45(2):332–46. doi: 10.1044/1092-4388(2002/026). [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6(3):160–88. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, et al. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49(12):1253–8. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ, et al. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10(2):181–92. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Ann N Y Acad Sci. 1995;769:119–50. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Muller RA, Rothermel RD, et al. Developmental changes of cortical and cerebellar motor control: a clinical positron emission tomography study with children and adults. J Child Neurol. 1998;13(11):550–6. doi: 10.1177/088307389801301105. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, et al. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12(5):538–49. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Nakai T, Matsuo K, et al. A functional magnetic resonance imaging study of listening comprehension of languages in human at 3 tesla-comprehension level and activation of the language areas. Neurosci Lett. 1999;263(1):33–6. doi: 10.1016/s0304-3940(99)00103-2. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, et al. Functional neuroanatomy of spatial working memory in children. Dev Psychol. 2000;36(1):109–16. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Newman AJ, Pancheva R, et al. An event-related fMRI study of syntactic and semantic violations. J Psycholinguist Res. 2001;30(3):339–64. doi: 10.1023/a:1010499119393. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, et al. An event-related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci. 2000;12(1):120–33. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Nippold MA, Sun L. Knowledge of morphologically complex words: a developmental study of older children and young adolescents. Lang Speech Hear Serv Sch. 2008;39(3):365–73. doi: 10.1044/0161-1461(2008/034). [DOI] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, et al. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16(2):289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, et al. Filtering of distractors during visual search studied by positron emission tomography. Neuroimage. 2002;16(4):968–76. doi: 10.1006/nimg.2002.1137. [DOI] [PubMed] [Google Scholar]
- O’Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J Cogn Neurosci. 2000;12(6):1013–23. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, et al. The neural correlates of the verbal component of working memory. Nature. 1993;362(6418):342–5. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, et al. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157):585–9. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Curr Opin Neurobiol. 1994;4(2):207–11. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, et al. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pollmann S, von Cramon DY. Object working memory and visuospatial processing: functional neuroanatomy analyzed by event-related fMRI. Exp Brain Res. 2000;133(1):12–22. doi: 10.1007/s002210000396. [DOI] [PubMed] [Google Scholar]
- Press WH, Teukolsky SA, et al. Numerical Recipes in C: The Art of Scientific Computing Cambridge. England: Cambridge University Press; 1992. [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–59. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphrey GW, Wise RSJ. Segregating semantic from phono;ogical processes during reading. Journal of Cognitive Neuroscience. 1997;9:727–33. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F. Brain reflections of words and their meaning. Trends Cogn Sci. 2001;5(12):517–524. doi: 10.1016/s1364-6613(00)01803-9. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, et al. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–73. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Rogers CA. The structure of verbal fluency. Br J Psychol. 1953;44(4):368–80. doi: 10.1111/j.2044-8295.1953.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Rubens AB. Transcortical motor aphasia. New York: Academic Press; 1976. [Google Scholar]
- Rubia K, Overmeyer S, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24(1):13–9. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Holland SK, et al. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15(17):2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, et al. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296(5572):1476–9. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ. Separate Cortical Networks Involved in Music Perception: Preliminary Functional MRI Evidence for Modularity of Music Processing. Neuroimage. 2005;25:444–451. doi: 10.1016/j.neuroimage.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Brown RD, et al. Empirical Validation of the Triple-Code Model of Numerical Processing for Complex Math Operations Using Functional MRI and Group Independent Component Analysis of the Mental Addition and Subtraction of Fractions. Neuroimage. 2004;22(3):1414–1420. doi: 10.1016/j.neuroimage.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, et al. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20(6):535–9. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, et al. Comparison of three methods for generating group statistical inferences from independent component analysis of functional magnetic resonance imaging data. J Magn Reson Imaging. 2004;19(3):365–8. doi: 10.1002/jmri.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, et al. Event-related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magn Reson Med. 2004;51(2):399–402. doi: 10.1002/mrm.10706. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, et al. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2006;29(1):254–66. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, et al. Object identification and lexical/semantic access in children: a functional magnetic resonance imaging study of word-picture matching. Hum Brain Mapp. 2007;28(10):1060–74. doi: 10.1002/hbm.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, et al. Correlation of White Matter Diffusivity and Anisotropy Changes with Age During Childhood: A Cross-Sectional Diffusion Tensor Imaging Study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, et al. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46(4):258–66. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, et al. PET evidence for an amodal verbal working memory system. Neuroimage. 1996;3(2):79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Scott SK, Johnsrude IS, et al. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26(2):100–7. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23(3):140–55. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivde G, Thompson-Schill SL. Dissociating semantic and phonological maintenance using fMRI. Cogn Affect Behav Neurosci. 2004;4(1):10–9. doi: 10.3758/cabn.4.1.10. [DOI] [PubMed] [Google Scholar]
- Skipper JI, Nusbaum HC, et al. Listening to talking faces: motor cortical activation during speech perception. NeuroImage. 2005;25:76–89. doi: 10.1016/j.neuroimage.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, et al. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6(1):11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, et al. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci U S A. 1998;95(3):876–82. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, et al. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14(11):1246–55. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, et al. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27(3):202–12. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59(5):796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thevenaz P, Ruttimann UE, et al. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7(1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10(3 Pt 1):327–38. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, et al. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94(26):14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, et al. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95(26):15855–60. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Flowers DL, et al. The neural basis of hyperlexic reading: an FMRI case study. Neuron. 2004;41(1):11–25. doi: 10.1016/s0896-6273(03)00803-1. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Third. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intlelligence Scale. Third. San Antonio, Tx: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence (WPPSI) San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- Weidner R, Pollmann S, et al. Top-down controlled visual dimension weighting: an event-related fMRI study. Cereb Cortex. 2002;12(3):318–28. doi: 10.1093/cercor/12.3.318. [DOI] [PubMed] [Google Scholar]
- Wernicke C. The symptom of complex aphasia. In: Church AE, editor. Diseases of the nervous system. New York: Appleton; 1911. [Google Scholar]
- Wexler BE, Fulbright RK, et al. An fMRI study of the human cortical motor system response to increasing functional demands. Magn Reson Imaging. 1997;15(4):385–96. doi: 10.1016/s0730-725x(96)00232-9. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, et al. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17(1):48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RJ, Greene J, et al. Brain regions involved in articulation. Lancet. 1999;353(9158):1057–61. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, et al. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124(Pt 1):83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ, et al. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2(3):173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Xiong J, Rao S, et al. Evaluation of hemispheric dominance for language using functional MRI: a comparison with positron emission tomography. Hum Brain Mapp. 1998;6(1):42–58. doi: 10.1002/(SICI)1097-0193(1998)6:1<42::AID-HBM4>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Altaye M, et al. Quantification of head motion in children during various fMRI language tasks. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]


