Abstract
We present a case of a 34-year-old right-handed Caucasian male with chronic occipital neuralgia refractory to medical therapies and minimally invasive pain procedures who underwent surgical cervical dorsal root ganglionectomy which completely relieved his headaches. The histopathological and immunohistochemical findings of the resected cervical dorsal root ganglia were consistent with active herpes simplex virus type 1 (HSV-1) infection causing ganglionitis. To the best of our knowledge, this case represents the first histopathologically proven HSV-1 cervical dorsal root ganglionitis in humans. This case provides an insight into a possible etiology of occipital neuralgia.
Keywords: Herpes simplex virus, Occipital neuralgia, Dorsal root ganglion, Ganglionitis, Histology, Ganglionectomy
Introduction
Herpes simplex virus (HSV) belongs to the alpha herpes virus group of the herpesvirus family [reviewed in 1]. This virus family is known for their special ability to cause latent infections in neurons. After the first exposure, HSV replicates in local epithelial cells causing productive infection, following which it travels in retrograde fashion along the axons of peripheral nerves to sensory ganglion neurons to establish latency. Latent HSV can get reactivated to cause infection again [1, 2]. The pathogenesis of HSV, especially pertaining to its latency and reactivation, has been under investigation since the 1900s [3], but still remains an enigma.
Early studies discovered that HSV-1 established latency in trigeminal ganglion and HSV-2 in sacral ganglion [4, 5]. Subsequently, it was reported that HSV-1 and HSV-2 infections are also distributed in spinal ganglia, evenly from cervical to the sacral ganglia, without any site predilection [6]. Although HSV has been demonstrated in ganglia, there have been no reported cases of human HSV ganglionitis in the scientific literature. We present a rare case of HSV-1 cervical dorsal root ganglionitis in a patient who underwent surgical resection of bilateral C2–C3 dorsal root ganglia (DRG) for occipital neuralgia.
Case Report
A 34-year-old right-handed Caucasian HIV-negative homosexual male with a past medical history of fibromyalgia, chronic headaches, post-traumatic stress disorder, multiple herpes-like blisters episodes in early childhood, syphilis and gonorrhea a year ago, and recent sore throat treated with antibiotics, presented to the emergency room of University of Illinois Medical Center (UIMC) with chronic bilateral occipital headaches that started 15 years ago after a motor vehicle accident and worsened over 5 weeks prior to presentation. He described his headaches as a constant, sharp, and burning pain (“feels like my head is on fire”), extending from the occipital to neck region, affecting the left more than the right side, and tender to touch. The pain worsened on sunlight exposure, exertion, neck motion, bending, and standing, and was relieved with light avoidance, lying down, and opiates. He denied any double vision, weakness, or numbness. He also denied any recent head and neck trauma, sickness, or overseas travel. He used to smoke 2 packs of cigarettes a day for 27 years and quit recently, used to smoke marijuana and quit 5 years ago but denied using alcohol.
On presentation, his vitals and routine labs were within normal limits. He graded his pain as 8/10. General and neurological exam was unremarkable except for tenderness to palpation in the left parieto-occipital and neck region and that he walked using a cane due to fibromyalgia. Cerebrospinal fluid exam was negative for infection. The CT of the head without contrast and MRI of the cervical spine were unremarkable (Fig. 1).
Fig. 1.
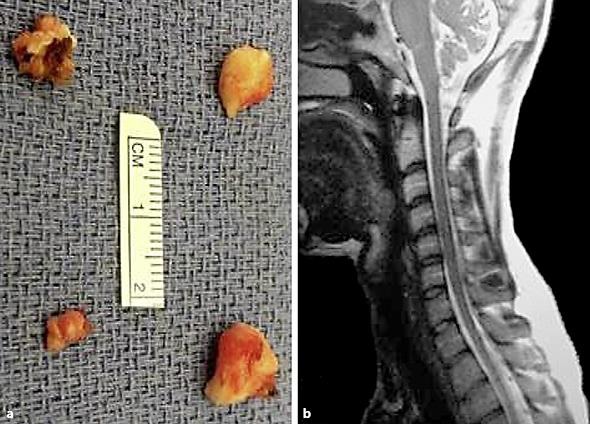
a Gross appearance of the four resected bilateral C2 and C3 cervical dorsal root ganglia. b MRI of the cervical spine and head CT without contrast (not shown here) were unremarkable and did not identify any local pathology responsible for the occipital pain.
His headache was refractory to medical treatment. There was no relief despite trying adequate doses of gabapentin, amitriptyline, baclofen, and naproxen. A combination of methylprednisolone, ondansetron, diphenhydramine, and normal saline IV bolus did not help either. Gabapentin was replaced with carbamazepine, and topiramate was tried as well but to no avail. Bilateral greater occipital nerve blocks were performed on two different occasions with transient and partial relief, and his headache came back 4–6 h later and worsened. Bilateral occipital nerve radiofrequency ablation (RFA) was tried on three different occasions with temporary relief with rebound headaches. Finally, neurosurgical options of occipital neurectomy, occipital nerve stimulation, and cervical ganglionectomy were considered.
The patient elected ganglionectomy and underwent open bilateral C2–C3 cervical dorsal root ganglionectomy (Fig. 1). He tolerated the procedure well with no complications. At 2 weeks' follow-up in clinic, he reported immediate and complete resolution of the headaches and photophobia, which was maintained at 14 months' follow-up. As an expected adverse effect of the procedure, he had local regional numbness which gradually improved to some extent. Subsequently, he reported mild dysesthesia in the C2–C3 sensory dermatome consistent with deafferentation pain which has been reported in a significant number of patients following dorsal root ganglionectomy [7].
Histopathological examination of the removed ganglia revealed chronic inflammation, neuronophagia, and immunohistochemical evidence of HSV-1 protein expression in neurons, consistent with ganglionitis due to active productive HSV-1 infection (Fig. 2).
Fig. 2.
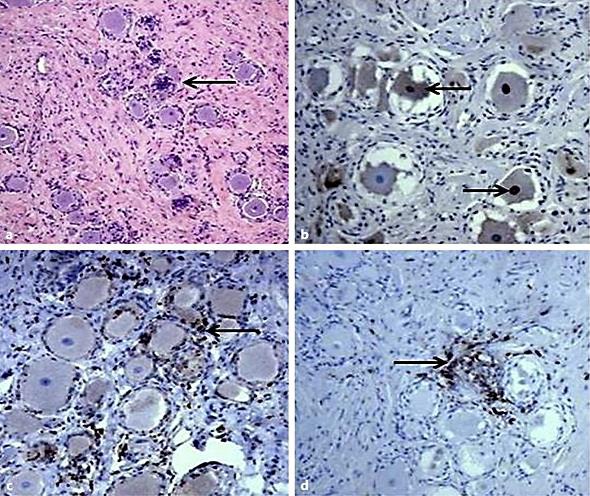
Histopathological and immunohistochemical documentation of HSV-1 cervical dorsal root ganglionitis. a Hematoxylin and eosin-stained section demonstrating multifocal chronic inflammation and focal neuronophagia (leftward arrow). Magnification ×100. b Detection of HSV-1 proteins by immunohistochemistry using a polyclonal anti-HSV-1 antibody. Brown color indicates expression of HSV-1 proteins. Lower rightward arrow points to HSV-1 protein expression in a neuronal nucleus; upper leftward arrow points to HSV-1 protein expression in the cytoplasm of a neuron. Magnification ×200. c Detection of macrophages by CD68 immunostaining in ganglia (brown color highlights macrophages; leftward arrow points to a CD68-positive cell). Magnification ×200. d Detection of lymphocytes by CD45 (lymphocyte common antigen) immunostaining in ganglia (brown color highlights lymphocytes; rightward arrow points to a CD45-positive cell). Magnification ×200.
Discussion
HSV is a DNA-enveloped neurotropic virus. It has subtypes 1 and 2 which are distinguished mainly by their antigenicity and site of lesions [1, 8]. HSV-1 is primarily transmitted in the saliva and so most often results in orolabial lesions. Primary infection usually occurs during childhood. After transmission, the virus replicates in local epithelial cells causing productive infection, which is thought to be cytolytic in nature. Eventually, it travels in retrograde fashion along axons of peripheral nerves and establishes latency in the sensory neurons of peripheral nervous system ganglia including DRG [1, 2, 8]. The main site of latency of HSV-1 is the sensory neurons of the trigeminal ganglion, although latency has also been studied in other peripheral nervous system ganglia like facial, vestibular, and geniculate ganglia [9, 10, 11]. Also, previous autopsy studies indicate that HSV may establish latent infection in spinal ganglia, evenly from cervical to sacral ganglia [6]. In situ hybridization studies have found that even though during latent infection no viral protein or infectious particles are produced, latency-associated HSV transcripts continue to be produced [reviewed in 1, 2].
This latent virus may reactivate either spontaneously or due to a variety of inducers, and cause reactivated infection in the peripheral tissues which are innervated by that nerve or even in the central nervous tissue. In humans, HSV reactivation is typically observed after either systemic stimuli such as physical or emotional stress, hyperthermia, exposure to UV light, menstruation, or after local stimuli to tissues such as trauma to tissues innervated by the latently infected neurons [1, 2]. Similar to these observations, in experimental animal models of HSV pathogenesis, a variety of systemic and local stimuli can induce HSV reactivation including hyperthermia, immunosuppression, physical injury to tissues innervated by neurons harboring virus [reviewed in 1, 2, 8]. Interestingly, some case reports have suggested a role of certain surgical interventions in HSV reactivation including ENT and ophthalmic surgeries, cesarean sections, brain epilepsy surgeries, etc. [12, 13, 14]. Viral reactivation from latency in peripheral ganglia may lead to disease depending on the location of the ganglion involved, with herpes labialis and keratitis associated with virus reactivation in the trigeminal ganglia being prominent examples.
This patient had paroxysmal lancinating pain (which the patient often described as sharp, shooting, or “shock-like”) in the distribution of the greater occipital nerves, which is typical for occipital neuralgia. The exact etiology of occipital neuralgia is unknown. There are no fixed criteria for treatment, and a wide variety of treatments exists [15]. Our patient's occipital neuralgia was refractory to medical treatment, and occipital nerve blockade provided only temporary relief. Before considering surgical options, minimally invasive therapy such as RFA was considered. Patient underwent bilateral occipital nerve RFA but had only short-term relief. Finally, total relief was achieved with surgical bilateral C2–C3 cervical dorsal root ganglionectomy.
The histopathological and immunohistochemical findings of the removed cervical DRG were consistent with active HSV-1 infection. The clinical presentation combined with the pathological and immunohistochemical findings in this case suggests that the detected active HSV-1 infection and ganglionitis in the DRG represents HSV-1 reactivation rather than primary or latent HSV-1 infection as detection of viral proteins is not a feature of HSV-1 latency and epithelial HSV-1 disease typically associated with primary HSV-1 infection was not detected. Our study used a polyclonal anti-HSV-1 antibody that reacts with all HSV-1 proteins. Detection of neuronal destruction (neuronophagia) in the resected ganglion tissues suggest that the HSV infection was lytic and was associated with the expression of the whole range of proteins associated with productive HSV-1 infection. Expression of these proteins likely played a key role in the induction of the detected inflammatory response.
The contribution of the documented HSV reactivation to the patient's symptoms is unclear. Pain got worse after occipital nerve blocks and RFA treatment raising the possibility that HSV reactivation was induced by these treatment modalities and contributed to increased pain. As latent infection is a chronic process interrupted by possible reactivations, the possibility that HSV infection also contributed to the patient's headache in a more chronic fashion cannot be excluded. Occipital neuralgia is considered idiopathic in most cases; however, entrapment, compression, and irritation of occipital nerves by vascular or space-occupying lesions or traumatic injury all have been hypothesized [15, 16]. To our knowledge, there are no case reports in the literature of occipital neuralgia secondary to HSV cervical dorsal root ganglionitis. This could provide a novel insight into the etiology of occipital neuralgia and possibly other neuralgias.
The histopathological presentation of HSV infection of a variety of human tissue types is well understood as surgical pathology specimens of HSV-induced encephalitis, keratitis, hepatitis, etc. are reported in the literature [1, 8]. Tissues derived from autopsies are also readily available to study HSV latent infection in peripheral ganglia. However, human peripheral ganglion tissues with active HSV infection are unlikely to be collected from patients and indeed a thorough literature search did not reveal any report describing the pathology of HSV ganglionitis in humans. In studies of experimental HSV infection of laboratory animals, HSV ganglionitis presents as predominantly lymphocytic inflammation, neuronophagia. Ganglionitis in animal tissues is associated with detection of viral proteins by immunohistochemistry in neurons [reviewed in 1, 2, 8]. Our study indicates that HSV ganglionitis in human tissues presents with a similar pathology.
In conclusion, this case report provides interesting new insight about HSV-1 pathogenesis as well as about at least one possible cause of occipital neuralgia. Observations reported here indicate that active HSV-1 infection can cause ganglionitis in human cervical DRG and suggests that HSV-1 infection may have played a role in the pathogenesis of the occipital headaches in this patient. The exact pathogenesis of HSV in humans is not well understood, and most of our knowledge comes from using animal models. Therefore, case reports documenting HSV-induced pathology not yet described in humans are extremely valuable to enrich the medical literature and help understand this prevalent, complex, and incompletely understood medical problem.
Statement of Ethics
The patient gave written informed consent for his participation in the study and publication.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B, editors. Fields Virology. ed 6. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 1823–1897. [Google Scholar]
- 2.Bloom DC. Alphaherpesvirus latency: a dynamic state of transcription and reactivation. Adv Virus Res. 2016;94:53–80. doi: 10.1016/bs.aivir.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Cushing H. Surgical aspects of major neuralgia of trigeminal nerve: report of 20 cases of operation upon the Gasserian ganglion with anatomic and physiologic notes on the consequence of its removal. JAMA. 1905;44:1002–1008. [Google Scholar]
- 4.Baringer JR, Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973;288:648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- 5.Baringer JR. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974;291:828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- 6.Obara Y, et al. Distribution of herpes simplex virus types 1 and 2 genomes in human spinal ganglia studied by PCR and in situ hybridization. J Med Virol. 1997;52:136–142. [PubMed] [Google Scholar]
- 7.Sweet WH. Deafferentation pain after posterior rhizotomy, trauma to a limb, and herpes zoster. Neurosurgery. 1984;15:928–932. [PubMed] [Google Scholar]
- 8.Valyi-Nagy T, et al. Latency strategies of alphaherpesviruses: herpes simplex virus and varicella-zoster virus latency in neurons. In: Minarovits J, Gonczol E, Valyi-Nagy T, editors. Latency Strategies of Herpesviruses. New York: Springer; 2017. pp. 1–36. chapter 1. [Google Scholar]
- 9.Furuta Y, Takasu T, Fukuda S, Inuyama Y, Sato KC, Nagashima K. Latent herpes simplex virus type 1 in human vestibular ganglia. Acta Otolaryngol Suppl. 1993;503:85–89. doi: 10.3109/00016489309128081. [DOI] [PubMed] [Google Scholar]
- 10.Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, Brandt T. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol. 2001;11:408–413. doi: 10.1111/j.1750-3639.2001.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hüfner K, Arbusow V, Himmelein S, Derfuss T, Sinicina I, Strupp M, Brandt T, Theil D. The prevalence of human herpesvirus 6 in human sensory ganglia and its co-occurrence with alpha-herpesviruses. J Neurovirol. 2007;13:462–467. doi: 10.1080/13550280701447059. [DOI] [PubMed] [Google Scholar]
- 12.Uda T, Koide R, Ito H, Hosono A, Sunaga S, Morino M. Relapse of herpes simplex virus encephalitis after surgical treatment for temporal lobe epilepsy: rare complication of epilepsy surgery. J Neurol. 2013;260:318–320. doi: 10.1007/s00415-012-6735-8. [DOI] [PubMed] [Google Scholar]
- 13.De Stefano A, Neri G, Kulamarva G. Delayed facial nerve paralysis post middle ear surgery: herpes simplex virus activation. B-ENT. 2009;5:47–50. [PubMed] [Google Scholar]
- 14.Crone LA, Conly JM, Storgard C, Zbitnew A, Cronk SL, Rea LM, Greer K, Berenbaum E, Tan LK, To T. Herpes labialis in parturiens receiving morphine following cesarean section. Anesthesiology. 1990;73:208–213. doi: 10.1097/00000542-199008000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty C. Occipital neuralgia. Curr Pain Headache Rep. 2014;18:411. doi: 10.1007/s11916-014-0411-x. [DOI] [PubMed] [Google Scholar]
- 16.Gadient PM, Smith J. The neuralgias: diagnosis and management. Curr Neurol Neurosci Rep. 2014;14:459. doi: 10.1007/s11910-014-0459-3. [DOI] [PubMed] [Google Scholar]


