Abstract
Adipocytes of the marrow adipose tissue (MAT) are distributed throughout the skeleton, are embedded in extracellular matrix, and are surrounded by cells of the hematopoietic and osteogenic lineages. MAT is a persistent component of the skeletal microenvironment and has the potential to impact local processes including bone accrual and hematopoietic function. In this review, we discuss the initial evolution of MAT in vertebrate lineages while emphasizing comparisons to the development of peripheral adipose, hematopoietic, and skeletal tissues. We then apply these evolutionary clues to define putative functions of MAT. Lastly, we explore the regulation of MAT by two major components of its microenvironment, the extracellular matrix and the nerves embedded within. The extracellular matrix and nerves contribute to both rapid and continuous modification of the MAT niche and may help to explain evolutionary conserved mechanisms underlying the coordinated regulation of blood, bone, and MAT within the skeleton.
Keywords: Evolution, Marrow fat, Bone, Adipose, Marrow adipose tissue, Matrix
Introduction
Marrow adipose tissue (MAT) is a collection of adipocytes, distributed throughout the skeleton. These cells are embedded in extracellular matrix and surrounded by cells of the hematopoietic and osteogenic lineages [1–3]. There is evidence that MAT behaves differently depending on where it is located in the skeleton [4]. For example, MAT in the red, hematopoietic marrow is depleted in response to systemic hemolysis in rabbits [5] or with prolonged cold exposure in mice [4]. The terms regulated and constitutive MAT (rMAT and cMAT) have attempted to capture some of this complexity [4]. Regulated MAT adipocytes are defined as single cells that are interspersed with areas of active hematopoiesis [3]. They form gradually throughout life, are located in the proximal-central regions of the skeleton, and their lipid composition and transcription factor expression mimics peripheral white adipose tissue (WAT) [4]. Constitutive MAT develops shortly after birth in the most distal portions of the skeleton, forming sheets of confluent adipocytes that are relatively devoid of hematopoiesis. Relative to rMAT and WAT, cMAT adipocytes have increased lipid unsaturation and higher expression of the transcription factors Cebpa/Cebpb [4]. They are refractory to change. It is unknown if differential responses to cold exposure and hemolysis are driven by cell-autonomous differences between rMAT and cMAT adipocytes, or, are instead dictated by their surrounding microenvironment.
There is substantial evidence that MAT has the potential to impact surrounding cells within its local microenvironment, potentially contributing to bone accrual and hematopoietic function [2, 6]. There are also accumulating reports in rodents and humans that MAT expansion is a common feature of metabolic diseases including anorexia, diabetes, obesity, gonadal dysfunction, and estrogen deficiency [7]. MAT can secrete paracrine and endocrine mediators, capable of modifying the action of surrounding cells and distant tissues (reviewed in [7]). It is also becoming increasingly apparent that MAT can undergo pathologic change with age and disease, potentially contributing to fracture risk and metabolic dysfunction [1, 8]. In humans, MAT persists for years after radiation- or bed rest-induced expansion, even after regaining health or resuming physical activity [9, 10]. Thus, identification of the molecular mechanisms underlying MAT expansion and regulation is clearly of high priority with broad implications.
Previous reviews have discussed the function of MAT as it compares to peripheral white and brown adipose tissues (WAT, BAT) [3, 7]. However, the first appearance of MAT in vertebrate evolution, particularly relative to WAT and BAT, has not yet been established. To gain insight into these relationships, the first part of this review examines the presence of MAT in vertebrate lineages with a discussion of how it relates to evolution of WAT, bone, and hematopoiesis. As a component of the microenvironment, MAT has the potential to receive large quantities of information from its surroundings: mechanical forces and chemical signals from the extracellular matrix, paracrine factors from surrounding cells, and endocrine mediators delivered via the circulation. However, we know very little about which of these signals are assimilated by the MAT adipocytes and which are ignored. In addition to its interactions with cells of the hematopoietic and osteogenic lineages, MAT has evolved alongside two additional components of the microenvironment that, up until this point, have been rarely discussed in the context of MAT function. These include the structural and signaling components of the extracellular matrix and the large variety of neural fibers that are embedded within. Thus, we will follow our overview of MAT evolution with a discussion of the microenvironmental determinants of MAT expansion and turnover, with particular emphasis on the extracellular matrix and nerves.
MAT Adipocytes as an Evolutionarily Conserved Component of the Bone Marrow Microenvironment
Placing MAT within the context of vertebrate evolution can inform hypotheses about its function. Indeed, naturally occurring variation between species can be used to test the relationship of a tissue’s form and function without the need for experimental modifications [11]. In this section, we will discuss the evolution of MAT with comparisons to the development of bone, adipose, and hematopoietic tissues (Fig. 1).
Fig. 1.
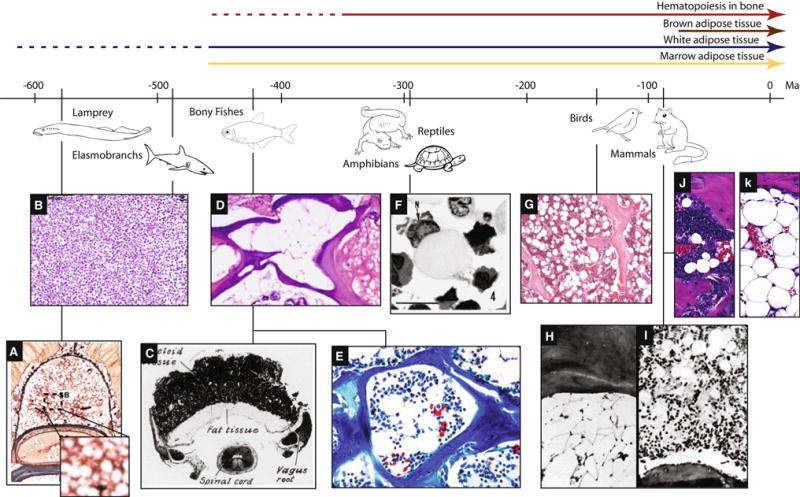
Evolution of MAT in vertebrates relative to bone marrow and peripheral adipose tissue. The first evidence of WAT in vertebrates occurs in the sea lamprey, prior to the evolution of an ossified skeleton. The Elasmobranchs, a group of cartilaginous fishes, have a partially ossified skeleton but no bone marrow, MAT, or WAT. MAT first becomes apparent in the bony fishes, after the evolution of endochondral bone resorption, both in the presence and absence of hematopoietic marrow. By comparison, brown adipose tissue is only present in mammals. a Groups of hematopoietic cells in the adipose tissue of the supraneural body of a postmetamorphic sea lamprey [18]. b Normal epigonal organ composed of a relatively uniform sheet of mature and developing granulocytes without adipocytes [134]. c Cross-section through the myeloid organ of Amia bony fish with adjacent fat tissue [25]. d Cross-section of zebrafish bone showing fatty filling of the skeletal space (middle) and adjacent cartilage (right) [135].e Cancellous bone of the lower jaw in the bony fish Garra congoensis with numerous marrow adipocytes (Image credit: Franck Genten) [26]. f Slimy salamander Plethodon glutinosus. Semi-thin plastic section of a fat cell in marrow. The densely stained nucleus (N) is located eccentrically in the cell, and the cytoplasm is filled with lightly stained lipid material [27]. g Bone marrow of a female Leghorn chicken showing trabeculae, hematopoietic marrow, and abundant adipocytes [36]. h Armadillo dermal plate marrow in December. Few hematopoietic cells are present but fat cells are abundant [41]. i Armadillo dermal plate marrow in October. Active hematopoietic cells interspersed with adipocytes in a typical dermal plate marrow [41]. j Red, hematopoietic bone marrow of a C3H/HeJ mouse femur containing rMAT adipocytes (Image by E.L.S.). k Yellow, fatty bone marrow of a C3H/HeJ mouse caudal vertebrae containing cMAT adipocytes (Image by E.L.S.). Adapted from [11] (Color figure online)
Vertebrae evolution is thought to trace to a common ancestor that lived 500 to 600 million years ago [11]. The jawed vertebrates include the cartilaginous fishes, bony fishes, amphibians, reptiles, birds, and mammals. Excluding the cartilaginous fish, WAT-like structures are present in the majority of vertebrates with varying degrees of specialization [12–14]. By contrast, BAT has a purely mammalian structure [12, 14]. Though birds, reptiles, and fish are capable of thermogenesis, generally via their muscle, true BAT has not been found despite significant effort [12]. In regards to blood cell production, vertebrate evolution is characterized by an increasing complexity of secondary lymphoid tissues— such as the lymph nodes, bone marrow, and spleen [11]. Ossified ‘skeletal’ tissue first appeared in vertebrate fishes as an exoskeleton. This was followed by specialization of endoskeletal elements, such as the jaw in sharks, that undergo perichondrial ossification and lack a defined marrow space [15]. The pairing of perichondral and endochondral ossification, with subsequent development of a cavity within the bone, did not occur until the evolution of the Osteichthyes, also known as bony fishes [15].
Jawless Vertebrates
Jawless vertebrates such as the hagfish lack a defined bone marrow and are supported by a cartilage-like endoskeleton [16, 17]. The sea lamprey, Petromyzon marinus, provides an ancestral example of the merging of fat and hematopoiesis [18]. The sea lamprey has an extraskeletal structure known as the supraneural body, also known as the dorsal fat body, that is organized from adipose progenitors of the dorsal connective tissue sheath around the spinal cord and meningeal tissue (Fig. 1a). At the beginning of metamorphosis, this fat body is colonized by hematopoietically active cells—leading to the development of a mixed hematopoietic/adipose organ [18]. This mixed organ is reminiscent of MAT (Fig. 1a), however, since the adipocytes were present prior to hematopoietic infiltration and exist outside of an ossified skeleton, they seem to be more akin to peripheral WAT. Thus, while this is an early example of functional WAT, it does not yet represent MAT.
Cartilaginous Fish
Cartilaginous fish primarily store triacylglycerols in the liver and/or skeletal muscle and lack a defined adipose tissue [12, 14]. The Elasmobranch fishes, cartilaginous species such as sharks and rays, also lack lymph nodes and bone marrow. They do, however, have a specialized lymphomyeloid tissue, the epigonal organ, which closely resembles a sinusoidal bone marrow (Fig. 1b). In the Elasmobranch species studied to date, the epigonal organ is noted to be devoid of fat [19, 20].
Bony Fish
Bony fish such as zebrafish, rainbow trout, and salmon have defined adipose tissue which contributes to circulating adipokines [12]. Most bony fish do not have a functional bone marrow and instead delegate hematopoiesis to the spleen, kidney, intestinal submucosa, and thymus [21, 22]. In zebrafish, mono- and multi-nucleated osteoclasts contribute to remodeling and allometric growth of the skeleton. Spaces created by resorption of cartilage and/or bone lack hematopoietic activity, but are uniformly filled with adipose tissue [23]. Thus, the MAT in the zebrafish resembles the cMAT found in the distal extremities of mammals [4] (Fig. 1d).
Unlike most bony fishes, some Osteichthyes have bone marrow. There is an intriguing report describing the presence of a mature extraskeletal bone marrow organ in the bowfin fish Amia, which also extends into the bone-lined cavities of the skull [24, 25] (Fig. 1c). This is thought to represent the first evidence of bone marrow in vertebrates. The marrow organ of this boneless fish is bordered by adipose tissue which fills the space between the cartilage of the skull and the brain [25]. The authors note that this tissue is WAT-like and not comparable to yellow fatty bone marrow, particularly because fatty conversion of marrow in older Amia fish or those bred in captivity was not observed [25]. Thus, in these animals, it seems that MAT is not yet present despite evidence of a mature marrow organ, ossified bone, and WAT. By contrast, an image of the jaw of the ray-finned fish Garra congoensis clearly demonstrates hematopoietic bone marrow with associated adipocytes of the ‘rMAT’ type [4], thus, the potential for their formation in fish clearly exists [26] (Fig. 1e).
Amphibians and Reptiles
Both reptiles and amphibians have a well-developed adipose tissue; many species also begin to display the depot-like arrangement of fat tissues that is commonly observed in mammals [12]. Unlike the fish, a functional hematopoietic bone marrow becomes the norm rather than the exception. For example, salamanders of the family Plethodontidae possess actively hemopoietic bone marrow [27], however, this marrow is unique in that it lacks erythropoietic activity. Unilocular fat-containing cells are frequent in the lymphogranulopoietic bone marrow of the northern slimy salamander Plethodon glutinosus and are comparable in number to cells of the granulocyte lineage [27]. These MAT-like cells appear smaller, with a larger rounded nucleus, than traditional mammalian MAT adipocytes [27] (Fig. 1f). In the newt Notophthalmus viridescens, an amphibian that lacks a hematopoietic bone marrow, the space within the skeleton is filled with fat cells and fibroblasts—similar to that of the zebrafish [28].
The leopard frog Rana Pipiens provides one of the first examples of seasonal variation of MAT [29]. Conversion of fatty yellow marrow to red hemopoietic marrow occurs in frogs to provide space for blood cell formation [29], generally in the early summer. The stroma of the red marrow is packed centrally with fat cells and blood vessels, covered peripherally with a layer of differentiating leukocytes. During seasonal fasting in the frog, the red coloration of the bones has been noted to disappear in the distal extremities, presumably due to the expansion of MAT at the expense of blood cells, concurrent with the loss of peripheral adipose tissue [29, 30]. This is comparable to the recent work in mice and humans showing that MAT increases in states of caloric restriction and anorexia, while peripheral WAT is lost [31, 32].
Unlike the amphibians described above, information concerning the bone marrow of reptiles is relatively sparse, however, images demonstrating a marrow cavity containing both adipocytes and hematopoietic marrow have been documented in the gekkonoid lizard Ptyodactylus [33].
Birds
The peripheral WAT in birds closely resembles that of mammals [4]. As in lower vertebrates, erythropoiesis in birds is generally intravascular, meaning that new erythroid cells are formed in the sinusoidal lumen and released directly into circulation [34, 35]. By contrast, mammalian erythropoiesis occurs extravascularly, necessitating trafficking of the new cells into the vascular lumen. Relative to mammals, the bone marrow of lower vertebrates characteristically contains large amounts of lymphatic tissue [35]. The bone marrow of pigeons and chickens, for example, contains numerous centers of lymphatic tissue which blend gradually into the surrounding myeloid tissue [35]. In birds, the lining cells of the vascular sinuses in the bone marrow are frequently closely associated with MAT-like adipocytes [35] (Fig. 1g). As in mammals, chicken MAT progenitors respond to methylprednisone with MAT expansion [36] indicating conserved regulation of MAT between mammalian and avian species [37].
Mammals
The universal presence of MAT in mammals has been reviewed previously [3]. The formation of MAT generally begins shortly after birth in the most distal extremities, such as the fingers, toes, and tail [3]. This early wave of perinatal MAT formation, thought to be cMAT-like, is then followed by a gradual accumulation of rMAT-like adipocytes in areas of hematopoietic marrow with age [3, 4]. Thus far, a consistent finding is that larger species have more MAT that extends farther into their skeleton, displacing the blood-forming bone marrow to central skeletal sites [3].
Based on evidence from rabbits, the distribution of MAT within the marrow is specified embryonically. At birth, prior to MAT formation, sites of fatty marrow are significantly less cellular than central regions of hematopoiesis [38]. The marrow phenotype, fatty versus hematopoietic, is preserved even after transplantation. For example, in rabbits, ectopic implantation of yellow, fatty marrow into the subcutaneous tissue of the abdomen or the capsule of the spleen results in the formation of fatty marrow nodules, while implantation of hematopoietic marrow leads to the formation of hematopoietic nodules that remain active even after 6-months [39, 40]. This occurs despite similar cellular depletion after implantation prior to reconstitution and growth of the nodule [40].
The armadillo provides unique insight into the role of MAT in mammals. The armadillo is a placental mammal that is covered by plates of dermal bone [41]. The dermal plates of the dorsal bands have an irregular marrow cavity, the contents of which fluctuate seasonally. The volume of marrow is greatest and most hematopoietically active in the spring, summer, and fall. In the winter, the dermal plate marrow becomes dull gray-white and filled with fat cells [41]. Bone is deposited simultaneously with MAT expansion, such that the size of the marrow space decreases as it becomes filled with fat. In the spring, fat regression coincides with hematopoietic expansion and osteoclast activation and bone resorption. As in mice, there is a gradient of adiposity with anterior regions of marrow being more hematopoietic and posterior regions more fatty [42]. Interestingly, the armadillo’s endoskeleton also has both hematopoietic and fatty marrow; however, unlike that of the dermal plates, its composition is not acutely regulated by seasonal change [41].
Evolution Summary
From these studies, it appears that in vertebrate species the first evidence of MAT occurs in the bony fishes. This is after the appearance of WAT, which is present in the sea lamprey, and many years earlier than BAT, which is only present in mammals (Fig. 1). In the absence of a hematopoietic marrow, cMAT-like cells fill the space formed by endochondral resorption of the bone. Conversely, in the presence of hematopoiesis within bone, the adipocytes take on the appearance of rMAT-like cells. The presence of both phenotypes in bony fishes suggests that rMAT and cMAT adipocytes evolved at roughly the same time and supports the hypothesis that differences in function are, at least in part, a product of their surrounding microenvironment. Further, the regulation of MAT, for example by glucocorticoids, is conserved between at least some vertebrate species (e.g., chickens and mice).
The Function of Adipose Tissue Within Bone
An assessment of vertebrate evolution establishes that MAT likely appeared ~400 to 500 million years ago in bony fish, both in the presence and absence of hematopoietic bone marrow (Fig. 1). At this point, MAT becomes a ubiquitous, presumably essential, component of the bone marrow. Why? Hypothetically, MAT can respond to its surroundings in several ways. MAT precursors within the marrow can be induced to form new MAT adipocytes [43]. Mature MAT adipocytes can be stimulated to undergo lipid storage through fatty acid uptake or de novo lipogenesis. Conversely, stored lipid can be broken down and released by lipolysis [4]. Lastly, MAT adipocytes can be removed from the bone marrow through apoptosis or necrosis [44]. The common theme being dynamic storage and release of energy and, unique to bone, preservation or loss of space. Before discussing the regulation of these functions at a microscopic scale, we will explore several putative functional relationships between MAT, bone, and blood cell production.
MAT and Bone
The relationship between MAT and bone has been reviewed previously [2, 4]. Currently, there is no evidence to suggest that MAT existed prior to the evolution of endochondral bone resorption and subsequent generation of void space within the skeleton. This is in contrast to WAT, which is readily demonstrated in the dorsal fat body of the cartilaginous sea lamprey [18]. This apparent separation in evolution between MAT and WAT supports the current hypothesis that these cells are derived from distinct mesenchymal progenitor populations [7].
In the absence of a hematopoietic bone marrow, such as in the zebrafish [23] or newt [28], MAT fills the skeletal void space and takes on the appearance of cMAT, as defined in mammals [4]. This indicates that filling the bone with MAT, instead of fluid, offers some evolutionary advantage to the bone itself. This could be in the form of mechanical [45] or biochemical support [46]. For example, MAT adipocytes may protect surrounding cells from the lipotoxic effects of circulating fatty acids and provide a storage reservoir that can be induced to release energy in times of local need [46].
It is also worth noting that more MAT does not always mean less bone. In the dermal plates of the armadillo, increases in bone volume accompany MAT expansion during regression of hematopoietic bone marrow, conversely, MAT loss is accompanied by hematopoietic expansion and bone resorption [41]. The ability of the resulting rMAT-like adipocytes to actively suppress bone formation, perhaps leaving room for blood cell production, remains unknown. However, there have been reports that MAT has the potential to promote osteoclast activity, particularly in the context of cancer metastasis [47].
MAT and Hematopoiesis
With the exception of the bowfin, a basal member of the bony fishes, MAT is easily demonstrated within the hematopoietic bone marrow of most species. One of the evolutionary functions for MAT, as discussed in frogs and armadillos, is the seasonal displacement of hematopoiesis. This likely serves to balance peripheral energy supply with hematopoietic demand and is consistent with work in mice showing that sites of high MAT cause increased quiescence of hematopoietic progenitors, which can be activated in times of need [6].
An intriguing observation in recent years is that MAT is preserved, or even increased, in states of food restriction or anorexia [31]. Conversely, MAT is readily depleted by hematopoietic demand as demonstrated in the context of phenylhydrazine-induced hemolysis [5, 39]. As with cold exposure in rodents [4], loss of MAT with hemolysis occurs primarily in the rMAT-containing regions of the red marrow—not in the yellow, cMAT-enriched areas [5]. This is similar in many ways to the adipose tissue around the lymph nodes, which responds less readily to the lipolytic signals such as norepinephrine than conventional WAT [48]. Perinodal adipocytes are instead stimulated to undergo lipolysis with cytokines, such as TNFα and IL-6, that are released by local immune cells [48, 49]. Most of the lipids in new lymphoid cells are derived from triacylglycerols in perinodal adipocytes [50]. Perinodal adipocytes fuel the local immune response, as needed, without depending on circulating energy reserves. In this way, local paracrine control of adipocyte lipolysis reduces competition with other tissues for essential lipids, enabling immune responses even in times of peripheral energy deficit [51].
There is substantial evidence that the immune system takes priority when it comes to accessing essential lipids. An excellent example of this is illness-induced anorexia. It is well-known that sick animals tend to eat less—despite mounting an aggressive internal defense against disease. This is actually an evolutionarily conserved adaptation that may help to bias physiological pathways to promote immune function. The relationship between illness and anorexia is conserved in species ranging from arthropods [52] to mammals [53, 54]. With the appearance of a functional hematopoietic marrow within the bone, MAT may have become a nutrient source that could be locally conserved, despite peripheral wasting, to support hematopoiesis in times of systemic demand. Thus, like perinodal WAT, MAT may help to emancipate the immune system from illness, malnutrition, and anorexia [12].
The MAT Microenvironment—Beyond Bone and Blood
The evolution of MAT in vertebrate species implies several possible functional relationships between MAT, bone, and blood. However, the ability of MAT to perform these functions locally within its microenvironment is dictated by several additional layers of complexity. The extracellular matrix is the foundation on which the microenvironment is organized. Embedded within the matrix are the numerous axons of the autonomic and sensory nervous system. These under-discussed components of the MAT microenvironment have the capacity to modify the spatial and temporal interactions between bone, fat, and blood within the skeleton.
Extracellular Matrix
The rise of multicellular organisms necessitated the development of the extracellular matrix scaffolds to organize and stabilize cellular interactions. The major ECM components (collagen fibers, fibronectin, basement membrane proteins…etc.) were all present by the time of the metazoan [55]. Thus, the evolution of vertebrate ECM is defined by its innovation. Through gene duplication, diversification of retained gene products, and domain shuffling, the vertebrate ECM evolved into complex structures that enabled the development of an elaborate central nervous system, a closed circulatory system, and an endoskeleton made of bones, cartilage, ligaments, and tendons. Supramolecular structures also allowed the creation of tissue-specific microenvironments in which the ECM components directly regulate cellular processes. Specifically, the ECM interacts with cellular receptors such as integrins to activate intracellular signaling pathways. The ECM establishes local signaling gradients in both time and space by sequestering (or presenting) molecules like growth factors and cytokines to cell surface receptors. The mechanical rigidity of the matrix mediates cell spreading and motility. Lastly, in bone, the ECM also directs the process of mineralization by providing a scaffold for hydroxyapatite deposition.
The exact composition of the ECM associated with marrow adipocytes has not been thoroughly characterized. However, there have been multiple reports on the spatial localization of ECM components throughout the bone [56–60]. These include fibronectin, laminin, and fibers of collagens-I, III, and IV. Large fibrillar matrix molecules like collagen-I and collagen-III concentrate near the endosteal surface, contributing to the mechanical stiffness of this interface (Fig. 2). Collagen-IV and laminin are basement membrane proteins associated with the marrow sinuses and arteries. Interestingly, collagen-IV has also been shown to localize around marrow adipocytes [56]. The space between the vessels and bone is enriched with mesh-like structures of fibronectin, collagen-V, and collagen-VI [56–58]. Though not discussed by Klein et al. [56], collagen-VI may be enriched around marrow adipocytes; this would be expected as collagen-VI is a major component of the peripheral adipose tissue ECM [61]. Although their spatial localization has not been fully characterized, tenascin-C, fibrillin-1, and alpha-2-HS-glycoprotein (aka. hemonectin) have also been identified within the marrow microenvironment [62–64].
Fig. 2.
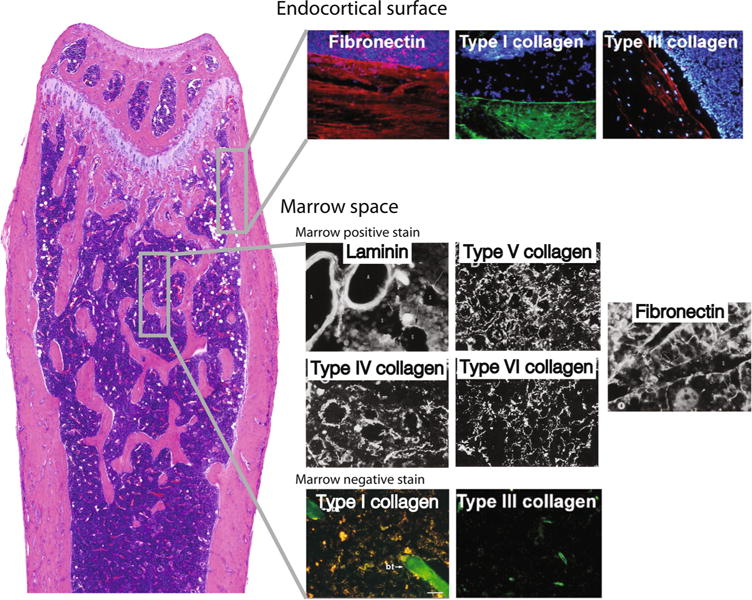
Distribution of extracellular matrix (ECM) structures within the bone. ECM composition varies across the bone; marrow adipocytes likely reside in distinct ECM microenvironments depending on their location in the marrow space. This figure is a compilation of immunofluorescence images from multiple studies, providing a basic overview of skeletal ECM organization. The endocortical surface is enriched with fibronectin and large fiber collagens (collagen-I, collagen-III). Shown is fibronectin in red, collagen-I in green, and collagen-III in red. Blue staining is nuclear staining of marrow cells. Collagen-I and collagen-III staining in the marrow space images (bottom) is limited to trabecular bone (collagen-I, green) or vessels (collagen-III, green). The remaining marrow space pictures are black-and-white immunofluorescence images. Laminin and collagen-IV (white) are basement membrane proteins associated with the marrow sinuses and arteries. Collagen-IV is also shown to localize around marrow adipocytes. The space between the vessels and bone is enriched with collagen-V and collagen-VI fibers, as well as mesh-like structures of fibronectin. Based on these images, collagen-VI may be concentrated around marrow adipocytes, similar to collagen-IV. Images reproduced with permission from the following sources: [56–58, 60] (Color figure online)
Based on a study performed by Mori et al. [65], adipocytes likely reside in at least two distinct ECM microenvironments. Specifically, in adult rats, visceral (gonadal) WAT is enriched for laminin and fibronectin and expresses very little collagen-I [65]. Conversely, subcutaneous adipose tissue is enriched for collagen-I. When compared to the ECM of the bone marrow, this study suggests that central MAT adipocytes may reside in an ECM niche that is more similar to visceral adipocytes, while the ECM around MAT within the endosteal niche may be more similar to subcutaneous adipose tissue.
Though relatively few reports discuss the consequence of ECM mutations on MAT, Smaldone et al. recently demonstrated that loss of fibrillin-1 by PRX-1-expressing cells caused aberrant activation of the TGFβ pathway in the stem cell niche and impeded MAT development [64]. We could not find evidence of collagen’s effect on marrow adipocytes specifically, but in general, fibrillar collagens are thought to restrict adipocyte expansion (reviewed in [66]). It is important to note that the majority of the matricellular proteins found in the marrow interact with the collagens [67]; changes in collagen will have a secondary effect on matricellular proteins. Fibronectin is thought to restrain adipocyte cell shape, furthermore, assembly of a fibronectin rich matrix impedes adipocyte differentiation [68–70]. Lastly, laminin is an adipocyte basement membrane protein which in WAT appears to be expressed not by the adipocyte precursor, but the mature adipocyte itself [71].
Matricellular proteins are a second component of the ECM. Unlike the structural ECM proteins, they are dynamically regulated, facilitate proper ECM assembly, and are largely responsible for regulating the biochemical cues cells receive. While bound to structural ECM components, matricellular proteins can also engage cell surface receptors or growth factors. Matricellular protein families associated with the bone marrow include small leucine rich proteoglycans (SLRPs; i.e., decorin, biglycan), small integrin-binding ligand N-linked glycoproteins (SIBLINGs; i.e., osteopontin and BSP-1), osteonectin/SPARC, CCN (i.e., connective tissue growth factor), thrombospondin, and sulfated proteoglycans (heparin and chondroitin sulfate proteoglycans) (reviewed in [72–74]). SPARC (osteonectin) is critical to proper collagen fiber assembly. In the absence of SPARC, collagen fibers appear disorganized and fragmented, and bone volume is severely reduced (reviewed in [75]). Interestingly, during phenotypic characterization of the SPARC-null mouse bones, it was observed that marrow adipocyte volume, but not number, was increased [76]. It is likely that a weakened collagen fiber network facilitated unrestrained growth of the marrow adipocytes. This would be similar to the finding by Khan et al. that loss of collagen-VI allows stress-free expansion of peripheral adipocytes due to a weakened extracellular scaffold [61]. It has been shown, ex vivo, that thrombospondin-2 (TSP2) is a negative regulator of adipocytes; bone marrow mesenchymal progenitor cells from TSP2-null mice have increased lipid accumulation and a slight propensity for adipogenesis [77]. Finally, Bi et al. [78] demonstrated that the SIBLINGs biglycan and decorin support maturation of bone marrow stromal cells (BMSC) into osteoblasts, and using a BMSC transplant model, they found loss of the two proteins impairs bone formation and appears to be associated with adipocyte accumulation.
The following are examples of matricellular proteins that have been shown to regulate peripheral adipose tissue biology, but have not yet been linked to MAT. Osteopontin facilitates the attachment of cells (monocytes, osteoclasts) to the matrix thereby supporting adhesion and motility. Interestingly, osteopontin-deficient mice are equally susceptible to diet-induced obesity (relative to wild-type mice), but due to impaired macrophage recruitment these obese mice are metabolically healthy [79]. Thrombospondin-1 (TSP-1) promotes adipocyte proliferation, and through its interaction with CD36 facilitates fatty acid uptake. TSP-1′s capacity to activate transforming growth factor beta (TGFβ) would also support excess adiposity. Indeed, TSP-1 deficient mice are protected from fat gain despite normal caloric intake or energy expenditure [80]. Heparin sulfate proteoglycans (HSPGs) have been shown to facilitate lipid accumulation in adipocytes. The proposed mechanism is that HSPGs help anchor apoE-enriched VLDL (apoE-VLDL) and lipoprotein lipase (LPL) near the adipocyte cell surface facilitating fatty acid release from triglycerides and subsequent uptake by fatty acid transporters on the cell surface [81].
Our knowledge of the dynamic relationship between adipocytes and the ECM in peripheral adipose tissue (reviewed in [65, 82, 83]) leads us to predict that marrow ECM will undergo remodeling with MAT expansion or depletion, influence MAT cellular processes, and contribute to the differentiation of MAT adipocytes from progenitor cells. Further defining the ECM-MAT microenvironment will provide insight to the biochemical and biophysical factors influencing the adipocytes.
Innervation of the Skeleton—Overview
The motor neural networks were the first to evolve, followed by the sensory and autonomic [84]. All vertebrates have a well-developed motor network. Sensory systems and their neuropeptides are also highly conserved, though their function has been adapted to suit the needs of each particular species [85]. The autonomic nervous system, consisting of the parasympathetic and sympathetic divisions, has shifted with respect to the balance between adrenergic and cholinergic fibers during vertebrate evolution [86]. The relevance of these findings to nerve function within the skeleton and hematopoietic organs, particularly in an evolutionary context, is poorly defined. Thus, this section will discuss autonomic and sensory nerves as a component of the MAT microenvironment primarily based on what has been reported in the mammalian literature.
Nerves are classified based on their diameter, conduction velocity, and function. The ‘efferent’ nerves are those that send an output from the central nervous system to the periphery—generally motor neurons and autonomics. Conversely, the ‘afferent’ sensory nerves are those that receive an input from the periphery and send it to the central nervous system. Another important distinction is whether the peripheral nerve axons are myelinated or unmyelinated. This is directly related to the conduction velocity, with more myelination leading to faster nerve conduction. Skeletal innervation has been reported in reptiles, birds, and mammals [87–91]. The mammalian skeleton is highly innervated by sympathetic efferent and somatosensory afferent nerves of the thinly myelinated A-δ and unmyelinated C fiber types [92]. Within the bone marrow, unmyelinated fibers greatly outnumber myelinated axons, as much as 24-fold in one study [93]; they have an average diameter of 0.4 and 1.4 μm, respectively [93]. The periosteum contains the highest density of nerve fibers; however, given its small total volume, there are actually more axons in the mineralized bone and marrow [92].
Incorporation of nerve endings within the skeletal microenvironment allows for rapid modulation of surrounding cells by neurotransmitters. These short-acting molecules and peptides can provide selective stimulation to regions of cells, or an entire tissue, depending on upstream signal integration. Neurotransmitters are also unique in that they often invoke a quantitative response that is ‘tunable’ in terms of magnitude.
Autonomic Innervation
In mammals, autonomic fibers within bone are primarily of the sympathetic division, though a recent report also supports the parasympathetic innervation of the skeleton (Fig. 3a) [91]. Before reaching the skeleton, presynaptic autonomic nerves travel from the spinal cord to the sympathetic ganglia, postsynaptic nerves then extend to the bone. In the case of the tibia, sympathetic efferent fibers descend in the sciatic nerve, alongside the sensory and motor neurons, then pass through the medial popliteal nerve to enter the bone alongside nutrient vessels [94]. Within the bone marrow, most sympathetic fibers form dense networks around the arteriolar blood vessels [94], however, sympathetic nerve endings are also present in the mineralized bone and hematopoietic marrow space (Fig. 3b) [92].
Fig. 3.
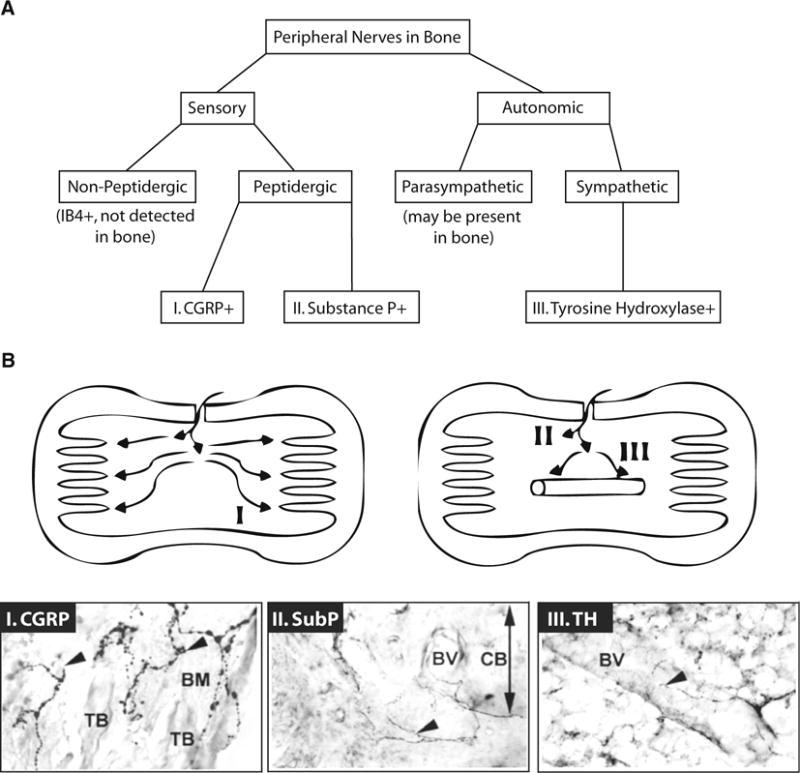
Peripheral nerve distribution in bone. a Sensory and autonomic nerves enter the skeleton with nutrient vessels and are widely distributed around the vasculature, throughout the medullary cavity, and within the ossified matrix. Nonpeptidergic sensory nerve fibers, as evidenced by isolectin B4 (IB4) staining, have not been detected in bone [92]. However, unmyelinated peptidergic sensory fibers containing CGRP, substance P, or both neuropeptides are common [121]. There is limited evidence that the bone may be innervated by parasympathetic fibers [91]. By contrast, tyrosine hydroxylase expressing sympathetic efferent neurons are readily demonstrated [92, 121]. b Within the bone, three main patterns of neuron distribution have been identified. Pattern I, which corresponds to CGRP + sensory fibers, shows axons extending apart from the vessels, subsequently terminating in the vicinity of the osteochondral junction. Pattern II represents the substance P + sensory fibers with terminate shortly after entering through the cortical bone. Pattern III shows the tyrosine hydroxylase-positive (TH +) sympathetic axons which spiral around blood vessels with some extension into the marrow space. Corresponding immunohistochemical stains are also presented. TB: trabecular bone, BM: bone marrow, BV: blood vessel, CB: cortical bone. Diagrams and images reproduced with permission from [121]
Sympathetic postsynaptic nerves release norepinephrine, a potent vasoregulator [94], osteoclast activator [95], and inducer of adipocyte lipolysis [96]. Production of norepinephrine is mediated by tyrosine hydroxylase, the rate-limiting enzyme of catecholamine biosynthesis that catalyzes the conversion of L-tyrosine to L-DOPA. L-DOPA can be sequentially converted to dopamine, norepinephrine, and epinephrine depending on which enzymes are present in the cell. In addition to sympathetic neurons, several types of immune cells can express tyrosine hydroxylase and make catecholamines [97–99]. Norepinephrine acts on receptors of the α1-, α2-, and β-adrenergic families, each with three subtypes. Lymphoid organs including the thymus, spleen, lymph nodes, gut-associated lymphoid tissue (GALT), and bone marrow are innervated by sympathetic fibers [100], and sympathoadrenergic regulation of hematopoiesis is a well-established phenomenon [101]. In healthy mice, β2-adrenergic activation enhances mobilization of hematopoietic progenitor cells [102]. In myeloproliferative neoplasms, sympathetic fibers, schwann cells, and nestin + progenitor cells are reduced; however, treatment with β3-adrenergic agonists can delay progression of disease [103]. Loss of sympathetic innervation can also promote leukemic bone marrow infiltration in a model of acute myeloid leukemia [104]. Thus, the sympathetic efferent fibers have the potential to function within the hematopoietic marrow, presumably releasing neurotransmitters in the vicinity of MAT adipocytes.
Based on evidence in WAT [96], we would predict that norepinephrine can act directly on MAT adipocytes, stimulating breakdown of lipid and release of fatty acids by lipolysis. This hypothesis is supported by rodent models of increased sympathetic tone. For example, in mice, cold exposure for 21 days leads to loss of rMAT adipocytes within the red bone marrow due to decreases in cell size and number [4]. Stimulation of sympathetic outflow with leptin, injected directly into the brain, also results in rapid loss of MAT within the red marrow [105]. Conversely, the ob/ob mouse has low sympathetic tone and high MAT volume [106, 107]. Many genetic rodent models have been used to model the action of norepinephrine on adrenergic receptors (reviewed in [107]). Unfortunately, the MAT phenotypes of these mice have not yet been reported; this includes several adrenergic-receptor mutants [95, 108–112], dopamine β-hydroxylase knock-out [113], and mice with downstream changes in intracellular mediators such as Gs [114], PDE4 [115], or GRK2 [116]. Future work is needed to define the expression of adrenergic receptors on MAT adipocytes and establish the regulation of rMAT and cMAT by norepinephrine.
Sympathetic drive to WAT, as measured by norepinephrine turnover, is not uniform [96]. This may also be true in the skeleton. In WAT adipocytes, norepinephrine-induced lipolysis depends on the number, type, and affinity of adrenergic receptors on the cell membrane [96]. The β1-, β2-, and β3-adrenergic receptors promote lipolysis, while the α2-receptor is inhibitory [96]. Current evidence supports a hypothetical model, whereby release of norepinephrine into the local microenvironment stimulates mobilization of hematopoietic progenitors in addition to lipid breakdown and fatty acid release by MAT adipocytes. In this model, the sympathetic nervous system would help to maintain blood cell production while utilizing MAT as a local fuel source. Though this is an attractive hypothesis, there are likely many surrounding factors, such as the ECM, with the potential to modify these relationships. Clearly, more work is needed to understand the complex interplay between sympathetic efferent neurotransmission and coordination of the MAT microenvironment.
Sensory Innervation
Sensory afferent nerves can be divided into categories based on expression of neuropeptides and are classified as peptidergic or nonpeptidergic (Fig. 3a). The skeleton is highly innervated by peptidergic nerve fibers [92, 117]. Nonpeptidergic fibers, based on expression of isolectin B4, have not been detected [92]. Based on studies in developing rats, the appearance of peptidergic fibers generally occurs within the skeleton around the same time as the initiation of mineralization [87]. Peptidergic sensory fibers release neuropeptides including calcitonin gene-related peptide (CGRP) and substance P which are potent vasodilators [118, 119] and osteoanabolic [120, 121]. There are also reports that CGRP and substance P can stimulate adipocyte lipolysis [122, 123]. There is evidence that vasoactive intestinal peptide (VIP +) and neuropeptide Y positive (NPY +) fibers are also present in bone, though their distribution does not appear to be as extensive and their role remains unclear [120, 124].
Neuropeptides are derived from large precursor proteins, generated through alternative splicing and/or precursor processing. Packaged neuropeptides are stored in secretory granules and released from axon terminals into the local microenvironment. Nonsynaptic neurotransmitter release, as occurs within the bone marrow, is much slower than synaptic transmission (minutes vs seconds) and results in diffusion of neuropeptides through a greater volume of space [121]. Thus, though they are classified as ‘sensory afferent’ nerves, peptidergic fibers can also release signaling mediators that can modulate local cellular functions in an efferent manner.
CGRP is a member of the calcitonin family of peptides and has two isoforms, CGRPα and CGRPβ, of which the α-isoform is released from sensory nerve terminals. CGRP-containing nerve fibers in the bone marrow are unmyelinated [117]. They enter the bone with nutrient vessels, extend away from the vasculature, and terminate near the osteochondral junction (Fig. 3b) [121]. They maintain their extension along (and in contact) with the trabecular bone, despite skeletal growth and remodeling, suggesting that CGRP-containing nerve fibers can adapt to alterations in their environment [117, 121]. CGRP is generally osteoanabolic, thought to promote bone formation by its positive actions on osteoblasts [121, 125] and inhibition of osteoclasts [121]. In vivo, in mice, CGRPα can stimulate fatty acid β-oxidation and mobilization of lipid from muscle, but not epididymal adipose tissue [126]. However, in vitro, CGRPα is a potent inducer of adipocyte lipolysis [126]. CGRPα knock-out mice are osteopenic; the MAT phenotype has not been reported [125]. Thus, the regulation of MAT by CGRP remains unclear.
Substance P is a member of the tachykinin family and has been isolated from all major vertebrate groups [85]. Substance P-containing fibers do not distribute as widely throughout the bone marrow, and terminate as free nerve endings shortly after entering the marrow space (Fig. 3b) [121]. The function of substance P likely evolved throughout vertebrate evolution, as there is only 64 % sequence identity between goldfish and mammals [127]. Even within the fish there are functional differences; it decreases blood flow to the gut in dogfish, but increases blood flow to gut in rainbow trout and lungfish [85]. There is in vitro evidence that substance P can support hematopoiesis [128] and both substance P and CGRP are known to have a variety of pro-inflammatory actions [129]. For example, elimination of capsaicin-sensitive peptidergic sensory fibers can selectively reduce intramedullary inflammation in adjuvant arthritic rats [129]. Interestingly, this also prevents MAT loss in this model [129], providing evidence of a relationship between sensory innervation and MAT regulation. Similarly, knock-out of receptors for substance P or CGRP reduces inflammation and allodynia in a mouse tibia fracture model [130]. In addition to regulating hematopoietic function, there is in vitro evidence that substance P can stimulate lipolysis [122]. However, its actions on MAT adipocytes remain entirely unknown.
Sensory and sympathetic nerves within the bone are tightly coupled. Ablation of sympathetic nerves by chemical treatment with guanethidine increases substance P and CGRP-positive sensory fibers in the bone by 23–54 % [131] and enhances CGRP and substance P expression in the dorsal root ganglion [119]. There is evidence in WAT that sensory neurons respond to leptin, provoking dose-dependent increases in the firing of sensory nerves from the fat pad, and eliciting increases in sympathetic nerve activity in the contralateral fat pad, potentially via a sensory-sympathetic reflex arc [132, 133]. Similar to the sympathetic neurons, peptidergic sensory fibers have the potential to regulate hematopoietic function through neuropeptides such as CGRP and substance P. Peptidergic sensory fibers may also contribute to lipolytic responses of adipocytes, providing another mechanism by which MAT and hematopoiesis could be coupled. Future work is needed to define the actions of sensory neuropeptides on MAT adipocytes and to study their relevance in an in vivo setting.
Conclusions
As demonstrated by the bony fishes—MAT evolved in two settings, both with and without adjacent hematopoiesis. MAT then became a persistent component of the local environment in all higher vertebrate species studied to date, implying that MAT has important, context-specific functions depending on its surrounding microenvironment. For example, MAT within the marrow niche may help to regulate hematopoietic function, whereas bone-lining adipocytes may influence bone formation and remodeling. Central to the balance between MAT, blood, and bone are two understudied components of the MAT microenvironment—the extracellular matrix and the nervous system. These structures are highly conserved throughout vertebrate evolution and there exists a significant basis, particularly in the context of WAT, for their role as essential modifiers of adipose tissue function. They have the ability to build upon and modulate local cellular functions and may explain coordinated changes in MAT, hematopoiesis, and bone. It is important to note that in some pathologic states MAT may become detrimental to bone homeostasis (e.g., cancer metastasis, obesity/diabetes, inflammatory arthritis), however, its evolutionary conservation across vertebrate species suggests that MAT likely has positive effects on skeletal health.
Acknowledgments
This work was supported by the National Institutes of Health (K99/R00-DE024178 to E.L.S.), the American Diabetes Association (7-13-JF-16 to C.S.C.), and Washington University’s Musculoskeletal Research Center (JIT2014_Craft_1 to C.S.C.).
Footnotes
Conflict of Interest Clarissa S. Craft and Erica L. Scheller declare that they have no conflict of interest.
References
- 1.Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10:737–748. doi: 10.1038/nrendo.2014.169. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AV. Marrow fat and bone: review of clinical findings. Front Endocrinol (Lausanne) 2015;6:40. doi: 10.3389/fendo.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheller EL, Rosen CJ. What’s the matter with MAT? marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheller EL, Doucette CR, Learman BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavassoli M. Marrow adipose cells. histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976;100:16–18. [PubMed] [Google Scholar]
- 6.Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheller EL, Cawthorn WP, Burr AA, et al. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. 2016 doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patsch JM, Li X, Baum T, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28:1721–1728. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trudel G, Payne M, Mädler B, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the women international space simulation for exploration study. J Appl Physiol. 2009;107:540–548. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- 10.Casamassima F, Ruggiero C, Caramella D, et al. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood. 1989;73:1677–1681. [PubMed] [Google Scholar]
- 11.Boehm T, Hess I, Swann JB. Evolution of lymphoid tissues. Trends Immunol. 2012;33:315–321. doi: 10.1016/j.it.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Pond CM. The evolution of mammalian adipose tissue. In: ME Symonds., editor. Adipose tissue biology. Springer; New York, New York: 2012. pp. 227–269. [Google Scholar]
- 13.Vague J, Fenasse R. Comparative anatomy of adipose tissue. Compr Physiol. 2010 doi: 10.1002/cphy.cp050105. [DOI] [Google Scholar]
- 14.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa T, Kuratani S. Evolution of the vertebrate skeleton: morphology, embryology, and development. Zool Lett. 2015;1:2. doi: 10.1186/s40851-014-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raison RL, dos Remedios NJ. The Biology of Hagfishes. Springer; Netherlands: 1998. The hagfish immune system; pp. 334–344. [Google Scholar]
- 17.Wagner DO, Aspenberg P. Where did bone come from? Acta Orthop. 2011;82:393–398. doi: 10.3109/17453674.2011.588861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amemiya CT, Saha NR, Zapata A. Evolution and development of immunological structures in the lamprey. Curr Opin Immunol. 2007;19:535–541. doi: 10.1016/j.coi.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Histology and Morphology Of The Epigonal Organ With Special Reference To The Lymphomyeloid System In Rhinobatos rhinobatos. https://www.researchgate.net/publication/257307727_Histology_and_Morphology_Of_The_Epigonal_Organ_With_Special_Reference_To_The_Lymphomyeloid_System_In_Rhinobatos_rhinobatos. Accessed 9 May 2016.
- 20.Walsh CJ, Luer CA. Elasmobranch hematology: identification of cell types and practical applications. In: Smith M, Warmolts D, Thoney D, Hueter R, editors. Elasmobranch husbandry manual. Ohio Biological Survey Inc; Columbus: 2004. pp. 307–323. [Google Scholar]
- 21.Bennett CM, Kanki JP, Rhodes J, et al. Myelopoiesis in the zebrafish, danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 22.Ma D, Zhang J, Lin HF, et al. The identification and characterization of zebrafish hematopoietic stem cells. Blood. 2011;118:289–297. doi: 10.1182/blood-2010-12-327403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witten PE, Hansen A, Hall BK. Features of mono- and multinucleated bone resorbing cells of the zebrafish danio rerio and their contribution to skeletal development, remodeling, and growth. J Morphol. 2001;250:197–207. doi: 10.1002/jmor.1065. [DOI] [PubMed] [Google Scholar]
- 24.Tavassoli M. Bone marrow in boneless fish: lessons of evolution. Med Hypotheses. 1986;20:9–15. doi: 10.1016/0306-9877(86)90081-2. [DOI] [PubMed] [Google Scholar]
- 25.Scharrer E. The histology of the meningeal myeloid tissue in the ganoids amia and lepisosteus. Anat Rec. 1944;88:291–310. doi: 10.1002/ar.1090880307. [DOI] [Google Scholar]
- 26.Genten F, Terwinghe E, Danguy A. Atlas of fish histology, hardcover; 2009-01-01. Science Publishers; Enfield: 2009. [Google Scholar]
- 27.Curtis SK, Cowden RR, Nagel JW. Ultrastructure of the bone marrow of the salamander plethodon glutinosus (Caudata: plethodontidae) J Morphol. 1979;159:151–183. doi: 10.1002/jmor.1051590202. [DOI] [PubMed] [Google Scholar]
- 28.Hightower JA, Pierre RLS. Hemopoietic tissue in the adult newt, Notopthalmus viridescens. J Morphol. 1971;135:299–307. doi: 10.1002/jmor.1051350304. [DOI] [PubMed] [Google Scholar]
- 29.Jordan HE. The histology of the blood and the red bone-marrow of the leopard frog, rana pipiens. Am J Anat. 1919;25:436–480. doi: 10.1002/aja.1000250404. [DOI] [Google Scholar]
- 30.Wells KD. The ecology and behavior of amphibians. University of Chicago Press; Chicago: 2010. [Google Scholar]
- 31.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devlin MJ, Cloutier AM, Thomas NA, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werner YL. The ontogenic development of the vertebrae in some gekkonoid lizards. J Morphol. 1971;133:41–91. doi: 10.1002/jmor.1051330104. [DOI] [PubMed] [Google Scholar]
- 34.Zapata A, Leceta J, Villena A. Reptilian bone marrow. an ultrastructural study in the spanish lizard. Lacerta hispanica. J Morphol. 1981;168:137–149. doi: 10.1002/jmor.1051680203. [DOI] [PubMed] [Google Scholar]
- 35.Campbell F. Fine structure of the bone marrow of the chicken and pigeon. J Morphol. 1967;123:405–439. doi: 10.1002/jmor.1051230407. [DOI] [PubMed] [Google Scholar]
- 36.Li SC, Lin CY, Kuo TF, et al. Chicken model of steroid-induced bone marrow adipogenesis using proteome analysis: a preliminary study. Proteome Sci. 2010;8:47. doi: 10.1186/1477-5956-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawthorn WP, Scheller EL, Parlee SD, et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology. 2016;157:508–521. doi: 10.1210/en.2015-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigelow CL, Tavassoli M. Fatty involution of bone marrow in rabbits. Acta Anat (Basel) 1984;118:60–64. doi: 10.1159/000145823. [DOI] [PubMed] [Google Scholar]
- 39.Bigelow CL, Tavassoli M. Studies on conversion of yellow marrow to red marrow by using ectopic bone marrow implants. Exp Hematol. 1984;12:581–585. [PubMed] [Google Scholar]
- 40.Tavassoli M, Crosby WH. Bone marrow histogenesis: a comparison of fatty and red marrow. Science. 1970;169:291–293. doi: 10.1126/science.169.3942.291. [DOI] [PubMed] [Google Scholar]
- 41.Weiss LP, Wislocki GB. Seasonal variations in hematopoiesis in the dermal bones of the nine-banded armadillo. Anat Rec. 1956;126:143–163. doi: 10.1002/ar.1091260203. [DOI] [PubMed] [Google Scholar]
- 42.Hill RV. Comparative anatomy and histology of xenarthran osteoderms. J Morphol. 2006;267:1441–1460. doi: 10.1002/jmor.10490. [DOI] [PubMed] [Google Scholar]
- 43.Li GW, Xu Z, Chen QW, et al. The temporal characterization of marrow lipids and adipocytes in a rabbit model of glucocorticoid-induced osteoporosis. Skeletal Radiol. 2013;42:1235–1244. doi: 10.1007/s00256-013-1659-7. [DOI] [PubMed] [Google Scholar]
- 44.Hamrick MW, Della Fera MA, Choi YH, et al. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007;327:133–141. doi: 10.1007/s00441-006-0312-3. [DOI] [PubMed] [Google Scholar]
- 45.Ma HT, Ren R, Chen Y, et al. A simulation study of marrow fat effect on bone biomechanics. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:4030–4033. doi: 10.1109/EMBC.2014.6944508. [DOI] [PubMed] [Google Scholar]
- 46.Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155:108–116. doi: 10.1210/en.2013-1712. [DOI] [PubMed] [Google Scholar]
- 47.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis. 2015;32:353–368. doi: 10.1007/s10585-015-9714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattacks CA, Pond CM. Interactions of noradrenalin and tumour necrosis factor alpha, interleukin 4 and interleukin 6 in the control of lipolysis from adipocytes around lymph nodes. Cytokine. 1999;11:334–346. doi: 10.1006/cyto.1998.0442. [DOI] [PubMed] [Google Scholar]
- 49.MacQueen HA, Pond CM. Immunofluorescent localisation of tumour necrosis factor-alpha receptors on the popliteal lymph node and the surrounding adipose tissue following a simulated immune challenge. J Anat. 1998;192(Pt 2):223–231. doi: 10.1046/j.1469-7580.1998.19220223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pond CM, Mattacks CA. The source of fatty acids incorporated into proliferating lymphoid cells in immune-stimulated lymph nodes. Br J Nutr. 2003;89:375–383. doi: 10.1079/BJN2002784. [DOI] [PubMed] [Google Scholar]
- 51.Pond CM. Interactions of adipose and lymphoid tissues. In: Fantuzzi G, Mazzone T, editors. Adipose tissue and adipokines in health and disease. Humana Press; Totowa: 2007. pp. 133–150. [Google Scholar]
- 52.Adamo SA, Bartlett A, Le J, et al. Illness-induced anorexia may reduce trade-offs between digestion and immune function. Anim Behav. 2010;79:3–10. doi: 10.1016/j.anbehav.2009.10.012. [DOI] [Google Scholar]
- 53.Johnson RW. The concept of sickness behavior: a brief chronological account of four key discoveries. Vet Immunol Immunopathol. 2002;87:443–450. doi: 10.1016/s0165-2427(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 54.Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267:543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 55.Adams JC. Extracellular matrix evolution: an overview. In: Keeley FW, Mecham RP, editors. Evolution of extracellular matrix. Springer; New York: 2013. pp. 1–25. [Google Scholar]
- 56.Klein G, Müller CA, Tillet E, et al. Collagen type VI in the human bone marrow microenvironment: a strong cytoadhesive component. Blood. 1995;86:1740–1748. [PubMed] [Google Scholar]
- 57.Nilsson SK, Debatis ME, Dooner MS, et al. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46:371–377. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- 58.Malara A, Currao M, Gruppi C, et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32:926–937. doi: 10.1002/stem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter DH, Sloan P, Aaron JE. Immunolocalization of collagen types I and III, tenascin, and fibronectin in intramembranous bone. J Histochem Cytochem. 1991;39:599–606. doi: 10.1177/39.5.1707904. [DOI] [PubMed] [Google Scholar]
- 60.Hamilton R, Campbell FR. Immunochemical localization of extracellular materials in bone marrow of rats. Anat Rec. 1991;231:218–224. doi: 10.1002/ar.1092310210. [DOI] [PubMed] [Google Scholar]
- 61.Khan T, Muise ES, Iyengar P, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamura-Ishizu A, Okuno Y, Omatsu Y, et al. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119:5429–5437. doi: 10.1182/blood-2011-11-393645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campell AD, Long MW, Wicha MS. Haemonectin, a bone marrow adhesion protein specific for cells of granulocyte lineage. Nature. 1987;329:744–746. doi: 10.1038/329744a0. [DOI] [PubMed] [Google Scholar]
- 64.Smaldone S, Clayton NP, Del Solar M, et al. Fibrillin-1 regulates skeletal stem cell differentiation by modulating TGFβ activity within the marrow niche. J Bone Miner Res. 2016;31:86–97. doi: 10.1002/jbmr.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mori S, Kiuchi S, Ouchi A, et al. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; comparison with visceral adipose tissue. Int J Biol Sci. 2014;10:825–833. doi: 10.7150/ijbs.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 69.Kamiya S, Kato R, Wakabayashi M, et al. Fibronectin peptides derived from two distinct regions stimulate adipocyte differentiation by preventing fibronectin matrix assembly. Biochemistry. 2002;41:3270–3277. doi: 10.1021/bi015660a. [DOI] [PubMed] [Google Scholar]
- 70.Luo W, Shitaye H, Friedman M, et al. Disruption of cell-matrix interactions by heparin enhances mesenchymal progenitor adipocyte differentiation. Exp Cell Res. 2008;314:3382–3391. doi: 10.1016/j.yexcr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noro A, Sillat T, Virtanen I, et al. Laminin production and basement membrane deposition by mesenchymal stem cells upon adipogenic differentiation. J Histochem Cytochem. 2013;61:719–730. doi: 10.1369/0022155413502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein G. The extracellular matrix of the hematopoietic microenvironment. Experientia. 1995;51:914–926. doi: 10.1007/BF01921741. [DOI] [PubMed] [Google Scholar]
- 73.Trotter TN, Yang Y. Matricellular proteins as regulators of cancer metastasis to bone. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oguri K, Okayama E, Caterson B, Okayama M. Isolation, characterization, and localization of glycosaminoglycans in rabbit bone marrow. Blood. 1987;70:501–510. [PubMed] [Google Scholar]
- 75.Delany AM, Hankenson KD. Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal. 2009;3:227–238. doi: 10.1007/s12079-009-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mansergh FC, Wells T, Elford C, et al. Osteopenia in Sparc (osteonectin)-deficient mice: characterization of phenotypic determinants of femoral strength and changes in gene expression. Physiol Genomics. 2007;32:64–73. doi: 10.1152/physiolgenomics.00151.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shitaye HS, Terkhorn SP, Combs JA, Hankenson KD. Thrombospondin-2 is an endogenous adipocyte inhibitor. Matrix Biol. 2010;29:549–556. doi: 10.1016/j.matbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bi Y, Stuelten CH, Kilts T, et al. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 79.Nomiyama T, Perez-Tilve D, Ogawa D, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kong P, Gonzalez-Quesada C, Li N, et al. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab. 2013;305:E439–E450. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilsie LC, Chanchani S, Navaratna D, Orlando RA. Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 2005;4:2. doi: 10.1186/1476-511X-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Catalán V, Gómez-Ambrosi J, Rodríguez A, Frühbeck G. Role of extracellular matrix remodelling in adipose tissue pathophysiology: relevance in the development of obesity. Histol Histopathol. 2012;27:1515–1528. doi: 10.14670/HH-27.1515. [DOI] [PubMed] [Google Scholar]
- 83.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Mongera A, Bonanomi D, et al. A conserved axon type hierarchy governing peripheral nerve assembly. Development. 2014;141:1875–1883. doi: 10.1242/dev.106211. [DOI] [PubMed] [Google Scholar]
- 85.Holmgren S, Jensen J. Evolution of vertebrate neuropeptides. Brain Res Bull. 2001;55:723–735. doi: 10.1016/s0361-9230(01)00556-1. [DOI] [PubMed] [Google Scholar]
- 86.Burnstock G. Evolution of the autonomic innervation of visceral and cardiovascular systems in vertebrates. Pharmacol Rev. 1969;21:247–324. [PubMed] [Google Scholar]
- 87.Sisask G, Bjurholm A, Ahmed M, Kreicbergs A. Ontogeny of sensory nerves in the developing skeleton. Anat Rec. 1995;243:234–240. doi: 10.1002/ar.1092430210. [DOI] [PubMed] [Google Scholar]
- 88.Martini R, Schachner M. Complex expression pattern of tenascin during innervation of the posterior limb buds of the developing chicken. J Neurosci Res. 1991;28:261–279. doi: 10.1002/jnr.490280214. [DOI] [PubMed] [Google Scholar]
- 89.Edoff K, Grenegård M, Hildebrand C. Retrograde tracing and neuropeptide immunohistochemistry of sensory neurones projecting to the cartilaginous distal femoral epiphysis of young rats. Cell Tissue Res. 2000;299:193–200. doi: 10.1007/s004419900142. [DOI] [PubMed] [Google Scholar]
- 90.Dénes A, Boldogkoi Z, Uhereczky G, et al. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005;134:947–963. doi: 10.1016/j.neuroscience.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 91.Bajayo A, Bar A, Denes A, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci U S A. 2012;109:15455–15460. doi: 10.1073/pnas.1206061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 93.Yamazaki K, Allen TD. Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the “neuro-reticular complex”. Am J Anat. 1990;187:261–276. doi: 10.1002/aja.1001870306. [DOI] [PubMed] [Google Scholar]
- 94.Duncan CP, Shim SS. J. Edouard samson address: the autonomic nerve supply of bone. An experimental study of the intraosseous adrenergic nervi vasorum in the rabbit. J Bone Joint Surg Br. 1977;59:323–330. doi: 10.1302/0301-620X.59B3.19482. [DOI] [PubMed] [Google Scholar]
- 95.Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 96.Bartness TJ, Shrestha YB, Vaughan CH, et al. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Musso NR, Brenci S, Setti M, et al. Catecholamine content and in vitro catecholamine synthesis in peripheral human lymphocytes. J Clin Endocrinol Metab. 1996;81:3553–3557. doi: 10.1210/jcem.81.10.8855800. [DOI] [PubMed] [Google Scholar]
- 98.Cosentino M, Marino F, Bombelli R, et al. Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci. 1999;64:975–981. doi: 10.1016/s0024-3205(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 99.Marino F, Cosentino M, Bombelli R, et al. Endogenous catecholamine synthesis, metabolism storage, and uptake in human peripheral blood mononuclear cells. Exp Hematol. 1999;27:489–495. doi: 10.1016/s0301-472x(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 100.Felten DL, Felten SY, Carlson SL, et al. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 101.Cosentino M, Marino F, Maestroni GJ. Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives. Front Cell Neurosci. 2015;9:302. doi: 10.3389/fncel.2015.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 103.Arranz L, Sánchez-Aguilera A, Martín-Pérez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 104.Hanoun M, Zhang D, Mizoguchi T, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15:365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hamrick MW, Della-Fera MA, Choi YH, et al. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 106.Enriori PJ, Sinnayah P, Simonds SE, et al. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elefteriou F, Campbell P, Ma Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int. 2014;94:140–151. doi: 10.1007/s00223-013-9752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mlakar V, Jurkovic Mlakar S, Zupan J, et al. ADRA2A is involved in neuro-endocrine regulation of bone resorption. J Cell Mol Med. 2015;19:1520–1529. doi: 10.1111/jcmm.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kajimura D, Hinoi E, Ferron M, et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208:841–851. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pierroz DD, Bonnet N, Bianchi EN, et al. Deletion of β-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res. 2012;27:1252–1262. doi: 10.1002/jbmr.1594. [DOI] [PubMed] [Google Scholar]
- 111.Bouxsein ML, Devlin MJ, Glatt V, et al. Mice lacking beta-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology. 2009;150:144–152. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fonseca TL, Jorgetti V, Costa CC, et al. Double disruption of α2A- and α2C-adrenoceptors results in sympathetic hyperactivity and high-bone-mass phenotype. J Bone Miner Res. 2011;26:591–603. doi: 10.1002/jbmr.243. [DOI] [PubMed] [Google Scholar]
- 113.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 114.Hsiao EC, Boudignon BM, Chang WC, et al. Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci U S A. 2008;105:1209–1214. doi: 10.1073/pnas.0707457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kinoshita T, Kobayashi S, Ebara S, et al. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000;27:811–817. doi: 10.1016/s8756-3282(00)00395-1. [DOI] [PubMed] [Google Scholar]
- 116.Wang L, Liu S, Quarles LD, Spurney RF. Targeted overexpression of G protein-coupled receptor kinase-2 in osteoblasts promotes bone loss. Am J Physiol Endocrinol Metab. 2005;288:E826–E834. doi: 10.1152/ajpendo.00422.2004. [DOI] [PubMed] [Google Scholar]
- 117.Imai S, Tokunaga Y, Maeda T, et al. Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res. 1997;15:133–140. doi: 10.1002/jor.1100150120. [DOI] [PubMed] [Google Scholar]
- 118.Loaiza LA, Yamaguchi S, Ito M, Ohshima N. Vasodilatation of muscle microvessels induced by somatic afferent stimulation is mediated by calcitonin gene-related peptide release in the rat. Neurosci Lett. 2002;333:136–140. doi: 10.1016/s0304-3940(02)01030-3. [DOI] [PubMed] [Google Scholar]
- 119.Supowit SC, Ethridge RT, Zhao H, et al. Calcitonin gene-related peptide and substance P contribute to reduced blood pressure in sympathectomized rats. Am J Physiol Heart Circ Physiol. 2005;289:H1169–H1175. doi: 10.1152/ajpheart.00973.2004. [DOI] [PubMed] [Google Scholar]
- 120.Norevall LI, Matsson L, Forsgren S. Main sensory neuropeptides, but not VIP and NPY, are involved in bone remodeling during orthodontic tooth movement in the rat. Ann N Y Acad Sci. 1998;865:353–359. doi: 10.1111/j.1749-6632.1998.tb11195.x. [DOI] [PubMed] [Google Scholar]
- 121.Imai S, Matsusue Y. Neuronal regulation of bone metabolism and anabolism: calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc Res Tech. 2002;58:61–69. doi: 10.1002/jemt.10119. [DOI] [PubMed] [Google Scholar]
- 122.Miegueu P, St-Pierre DH, Lapointe M, et al. Substance P decreases fat storage and increases adipocytokine production in 3T3-L1 adipocytes. Am J Physiol Gastrointest Liver Physiol. 2013;304:G420–G427. doi: 10.1152/ajpgi.00162.2012. [DOI] [PubMed] [Google Scholar]
- 123.Chatzipanteli K, Goldbergt RB, Howard G, Roos BA. Calcitonin gene-related peptide is an adipose-tissue neuropeptide with lipolytic actions. Endocrinol Metab. 1996;3:235–242. [Google Scholar]
- 124.Lundberg P, Lerner UH. Expression and regulatory role of receptors for vasoactive intestinal peptide in bone cells. Microsc Res Tech. 2002;58:98–103. doi: 10.1002/jemt.10124. [DOI] [PubMed] [Google Scholar]
- 125.Schinke T, Liese S, Priemel M, et al. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res. 2004;19:2049–2056. doi: 10.1359/JBMR.040915. [DOI] [PubMed] [Google Scholar]
- 126.Danaher RN, Loomes KM, Leonard BL, et al. Evidence that alpha-calcitonin gene-related peptide is a neurohormone that controls systemic lipid availability and utilization. Endocrinology. 2008;149:154–160. doi: 10.1210/en.2007-0583. [DOI] [PubMed] [Google Scholar]
- 127.Conlon JM, O’Harte F, Peter RE, Kah O. Carassin: a tachykinin that is structurally related to neuropeptide-gamma from the brain of the goldfish. J Neurochem. 1991;56:1432–1436. doi: 10.1111/j.1471-4159.1991.tb11442.x. [DOI] [PubMed] [Google Scholar]
- 128.Rameshwar P, Ganea D, Gascón P. In vitro stimulatory effect of substance P on hematopoiesis. Blood. 1993;81:391–398. [PubMed] [Google Scholar]
- 129.Imai S, Rauvala H, Konttinen YT, et al. Efferent targets of osseous CGRP-immunoreactive nerve fiber before and after bone destruction in adjuvant arthritic rat: an ultramorphological study on their terminal-target relations. J Bone Miner Res. 1997;12:1018–1027. doi: 10.1359/jbmr.1997.12.7.1018. [DOI] [PubMed] [Google Scholar]
- 130.Guo TZ, Wei T, Shi X, et al. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain. 2012;8:85. doi: 10.1186/1744-8069-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cherruau M, Facchinetti P, Baroukh B, Saffar JL. Chemical sympathectomy impairs bone resorption in rats: a role for the sympathetic system on bone metabolism. Bone. 1999;25:545–551. doi: 10.1016/s8756-3282(99)00211-2. [DOI] [PubMed] [Google Scholar]
- 132.Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett. 1999;262:125–128. doi: 10.1016/s0304-3940(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 133.Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst. 1998;73:19–25. doi: 10.1016/s0165-1838(98)00109-x. [DOI] [PubMed] [Google Scholar]
- 134.Camus A, Berliner A, Clauss T, et al. Serratia marcescens associated ampullary system infection and septicaemia in a bonnethead shark, sphyrna tiburo (L.) J Fish Dis. 2013;36:891–895. doi: 10.1111/jfd.12107. [DOI] [PubMed] [Google Scholar]
- 135.Kent ML, Spitsbergen JM, Matthews JM, et al. ZIRC Health Services Zebrafish Disease Manual. Diseases of Zebrafish in Research Facilities. 2012 http://zebrafish.org/health/diseaseManual.php. Accessed 16 May 2016.


