Abstract
Kidney and bladder stones (urinary tract stones) and osteoporosis are prevalent, serious conditions for postmenopausal women. Men with kidney stones are at increased risk of osteoporosis; however, the relationship of urinary tract stones to osteoporosis in postmenopausal women has not been established. The purpose of this study was to determine whether urinary tract stones are an independent risk factor for changes in bone mineral density (BMD) and incident fractures in women in the Women’s Health Initiative (WHI). Data were obtained from 150,689 women in the Observational Study and Clinical Trials of the WHI with information on urinary tract stones status: 9856 of these women reported urinary tract stones at baseline and/or incident urinary tract stones during follow-up. Cox regression models were used to determine the association of urinary tract stones with incident fractures and linear mixed models were used to investigate the relationship of urinary tract stones with changes in BMD that occurred during WHI. Follow-up was over an average of 8 years. Models were adjusted for demographic and clinical factors, medication use, and dietary histories. In unadjusted models there was a significant association of urinary tract stones with incident total fractures (HR 1.10; 95% CI, 1.04 to 1.17). However, in covariate adjusted analyses, urinary tract stones were not significantly related to changes in BMD at any skeletal site or to incident fractures. In conclusion, urinary tract stones in postmenopausal women are not an independent risk factor for osteoporosis.
Keywords: DXA, AGING, DXA, OSTEOPOROSIS, GENERAL POPULATION STUDIES, MENOPAUSE
Introduction
Osteoporosis is a significant public health problem, particularly for women.(1) Urinary tract stones are also a major clinical and economic health concern because approximately 7% of women will have symptomatic urinary tract stones by the age of 70 years.(2) Moreover, data from the 3rd National Health and Nutrition Examination Survey (NHANES III) indicate that the prevalence of urinary tract stones has increased 70% in the last 15 years, across all ages and race/ethnic groups, particularly in women.(2) In men, urinary tract stones are not only a common, painful event, they are also associated with osteoporosis.(3–5) In men in the Osteoporotic Fractures in Men (MrOS) study, kidney stones were negatively associated with areal bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA)(6) and were among the strongest negative correlates for femoral neck trabecular volumetric BMD.(7) However, whether there is an association between urinary tract stones and osteoporosis in women remains controversial.(4,8–14) To date, there has not been a longitudinal cohort study of the relationship of urinary tract stones to osteoporosis including a large sample of multiethnic postmenopausal women with BMD measurements at multiple skeletal sites and incident fracture data.
Hypothetically, urinary tract stones may be associated with osteoporosis for a number of reasons. Hypercalciuria and higher sodium excretion, which occur in many patients with urinary tract stones, have in some studies been reported to be a risk factor for osteoporosis.(15–19) Diets higher in potassium intake are inversely associated with both urinary tract stones and osteoporosis.(20,21) Urinary tract stones and vertebral fractures occur frequently in individuals with asymptomatic hyperparathyroidism.(22)
Adequate calcium and vitamin D intakes are beneficial for osteoporosis.(23) Some,(24,25) but not all,(26) studies suggest that higher calcium intakes may increase the risk of urinary tract stones, although this may vary depending on whether the calcium consumed is from diet or supplements, with supplements increasing the risk of urinary tract stones and high dietary calcium intakes decreasing the risk.(27) Theoretically, persons with urinary tract stones might restrict calcium and vitamin D intake to prevent further episodes of symptomatic urinary tract stones, and this may contribute to osteoporosis. Therefore, it is important to consider calcium and vitamin D intake (and type of intake; ie, diet versus supplements) in studies of the relationship of urinary tract stones with osteoporosis.
The purpose of this study was to determine whether urinary tract stones were an independent risk factor for changes in BMD and incident fractures in women in the Women’s Health Initiative (WHI).
Subjects and Methods
Design overview/setting and participants
The WHI included postmenopausal women aged 50 to 79 years recruited between October 1, 1993, and December 31, 1998, at 40 clinical centers in the United States. WHI had both an observational study (OS) and a series of clinical trials (CTs) enrolling 161,808 women. The CTs included the hormone therapy (HT) trial, dietary modification (DM) trial, and calcium and vitamin D trial (CaD, calcium carbonate 1000 mg every day and vitamin D3 400 IU every day).(28)
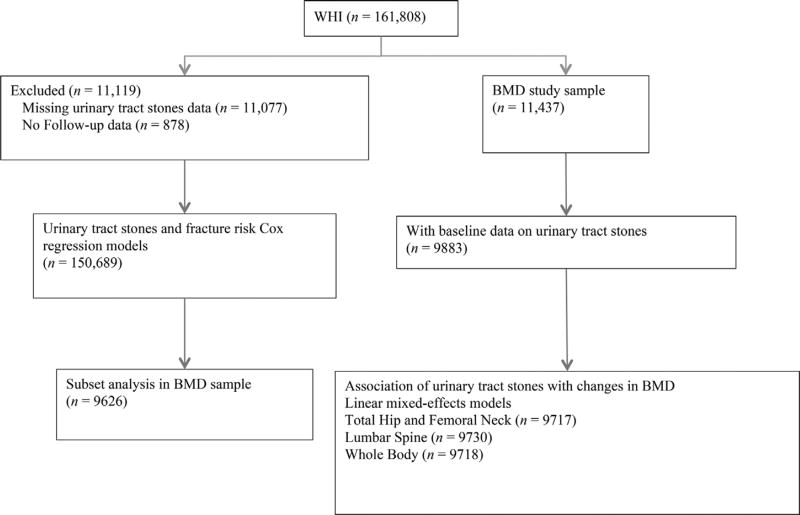
The study population for the association of urinary tract stones with incident fractures included all women in the OS and all arms of the clinical trials (CT) from enrollment to end of the main study and for whom data were not missing regarding urinary tract stones (n = 150,689). A subset analysis was performed of the association of urinary tract stones with incident fractures including those with BMD measurements in the models (n = 9626). The study population for the BMD measurements included all women in the WHI who had a DXA scan performed in the WHI (DXA cohort). BMD measurements of the hip, lumbar spine, or whole body were done on 11,437 women; of these, 9883 responded to the baseline evaluation question as to whether or not they had previously had a kidney or bladder stone. The association of BMD with urinary tract stones thus included 9883 women; 486 (4.9%) of these women reported a history of urinary tract stones at the baseline visit of the WHI (Fig. 1).
Fig. 1.
Flow diagram of study population.
All protocols were approved by the institutional review board (IRB) at each participating center. The project was declared exempt by the IRB at Georgia Regents University. All participants provided written informed consent for their participation in the original WHI study.
Outcome assessments
Outcomes were assessed by questionnaires collected semiannually through the end of the CT and annually in the OS. The original WHI study was conducted between 1993 and 2005; data for this analysis is restricted to outcomes occurring before 2005.
Urinary tract stones
The presence or absence of urinary tract stones included self-report of kidney or bladder stones collected by questionnaires through the end of the main study. A time-dependent variable was used in the modeling to only include stones reported before either fracture or BMD measurement.
Fracture ascertainment
Information regarding self-reported clinical fractures was collected semiannually through the end of the CTs and annually when the trial finished; in the OS, these were reported annually. Specific fracture sites assessed included: hip, upper leg (not hip), pelvis, patella, lower leg or ankle, foot, coccyx, vertebrae, lower arm or wrist (forearm, hand, elbow, upper arm or shoulder, or other site, which was specified). All hip fractures were centrally adjudicated within WHI using radiographs and medical record data. Other fracture outcomes (spine, forearm, any fractures) were centrally adjudicated in the CT trials and for OS participants at the BMD sites during the main study. Total fractures included all reported clinical fractures other than those of the ribs, sternum, skull or face, fingers, toes, and cervical vertebrae.
Validity studies of self-report for fractures within the WHI CT have indicated high agreements for self-report of hip (78%) and forearm/wrist (81%) fractures, but relatively lower agreement for clinical spine fractures (51%).(29)
BMD measurements
BMD of the total hip, femoral neck, anterior-posterior lumbar spine, and whole body was measured at baseline, years 3, 6, and 9 in participants at three of the 40 clinical centers of the WHI (Pittsburgh, PA; Birmingham, AL, and Phoenix/Tucson, AZ) by DXA using a Hologic QDR densitometer Model 2000, 2000+ or 4500 Fan-beam technology (Hologic, Inc., Waltham, MA, USA). Only measurements on the same side (for hip images) and on the same machine (except where calibrated scanner upgrades occurred) were used for this analysis. Technologists were trained and certified by the University of California, San Francisco, Bone Density Coordinating Center. Standard protocols were used for positioning and analysis. Site-specific spine phantoms were used. Calibration phantoms, were scanned across instruments and clinical sites with interscanner variability of <1.5% for the lumbar spine, <4.8% for the hip, and <1.7% for linearity.(25,30)
Covariates
Current medication use was ascertained by having the participants bring all of their medications that they had used for the prior 2 weeks to the baseline visit. Clinic interviewers entered each medication name into the WHI database, which assigned drug codes using Medispan software (First Databank, Inc., San Bruno, CA, USA). Information regarding duration of use, but not dose, of each medication was recorded. In adjusted analyses, use of bisphosphonates, calcitonin, oral corticosteroids, anticonvulsants, proton pump inhibitors, thiazide diuretics, loop diuretics, and thyroid medications were included. Thyroid medications included thyroid replacement therapy for hypothyroidism and medications used to treat hyperthyroidism including methimazole and propylthiouracil.
The use of dietary supplements, including calcium and vitamin D preparations taken at least twice weekly for the prior 2 weeks, were also entered into the database. Dietary intakes of potassium, calcium, vitamin D, oxalate, sodium, animal proteins, fluid/water, and total caloric intake were measured with a semiquantitative food-frequency questionnaire (FFQ). As in previous WHI studies, dietary intake values for women who reported extremes of caloric intakes were not included in the analysis.(31) Dietary energy intake was analyzed categorically as <1800, 1800 to 2199 (referent group), 2200 to 2499, and ≥2500 kilocalories per day. These categories were selected based on the distribution of energy intake in the women in the WHI-OS cohort, and the estimated energy requirements from the Institute of Medicine dietary reference intakes for women aged 50 to 79 years with varying levels of physical activity.(32) Questionnaires obtained at the baseline visit were used to collect information regarding age, race and ethnicity, smoking exposure (current, past, never), alcohol intake, parental history of fractures after age 40 years, history of prevalent fractures on or after age 55 years, age at menopause, menopausal hormone therapy (estrogen therapy or estrogen + progesterone), socioeconomic status including family income (total over prior year), education (less than or equal to high school diploma/some college or vocational school/completed college and higher), and history of diabetes mellitus. Total energy expenditure from self-reported recreational physical activity was used to determine physical activity levels. Physical activity was determined by a questionnaire that addressed the frequency, duration, and intensity of participation in different forms of physical activity. Weekly recreational physical activity and walking per kilogram of body weight was calculated by multiplying an assigned energy expenditure level for each category of activity by the hours exercised per week to calculate total metabolic equivalents per week (METs per week). (33,34) The Short Form 36 Health Survey (SF-36) was used as a measure of physical function construction, with higher scores indicating better function. Height and weight at the baseline visit were measured in WHI as described(28); these were used to calculate body mass index (BMI). Geographic study site was categorized by region (Northeast, South, Midwest, West). (35) Indicators were created for participation in each arm (treatment and control) of each clinical trial.
Statistical analysis
Selected baseline characteristics of the study population according to the presence or absence of urinary tract stones, including prevalent (at baseline visit), incident (developed during WHI), and recurrent (prevalent and incident) urinary tract stones were described. For each characteristic, we present the number of participants, means and standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables.
Baseline covariates were compared across urinary tract stone groups using ANOVA; p values from Tukey-Kramer post hoc pairwise comparisons are reported herein.
Linear mixed-effects models were used to determine the association of urinary tract stones with changes in BMD over time. Repeated observations of BMD and urinary tract stones (yes/no) were used to compute the annual change in BMD as a function of urinary tract stones status. An F-test was used to test equality of slopes.
Cox regression models were used to examine the relationship of urinary tract stones with fractures (total [all fractures] and site-specific fractures [hip, clinical vertebral, and other fractures]) with time to first fracture as the dependent variable and self-report of urinary tract stones as a time-varying independent variable. Women were followed to first fracture; women without fracture were censored at the end of follow-up. This analysis was repeated in the BMD cohort, where adjustment for BMD was possible.
Model covariates were selected a priori based on their associations with osteoporosis (age; ethnicity; education; income; study arm; smoking and alcohol use; fracture history; physical functioning; age at menopause; BMI; diabetes; dietary calcium; vitamin D, sodium, and potassium intakes; calcium and vitamin D supplements; and use of bisphosphonates, oral corticosteroids, calcitonin, estrogen, selective estrogen receptor modulators [SERMs], thiazolidinediones, antiepileptics, proton pump inhibitors, thiazide and loop diuretics, and thyroid medications) and/or urinary tract stones (age; ethnicity; BMI; study arm; dietary calcium; vitamin D, sodium, potassium, oxalic acid, animal protein, energy, and fluid intakes; and use of supplemental calcium and vitamin D supplements, thiazide diuretics, and allopurinol) and all were entered into the models simultaneously. The collinearity of the variables was assessed by variance inflation factors. Covariates were obtained from the baseline visit except for urinary tract stones where we constructed a time-varying indicator Neph(t) = at least one episode of urinary tract stones prior to t. Unadjusted models and models adjusted for all covariates described above are presented. Sensitivity analyses were analyzed including women who had a constant status of urinary tract stones (ie, never or at baseline already) and also using only the portion of longitudinal data for each woman during which urinary tract stones status did not change. In a subset analysis of the association of urinary tract stones with incident fractures who had DXA measurements (n = 9626), baseline lumbar spine and hip BMD were used as covariates in adjusted analyses. Finally, sensitivity analyses of the association of urinary tract stones with changes in BMD and fractures confined to women in the OS were done; this analysis was performed to mitigate any biases from trial assignment and arm assignment (placebo versus treatment) that might have still been a factor, despite statistical adjustment for CT trial and trial assignment in women in the CTs of the WHI included in the primary analyses.
All analyses were done using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Characteristics of women with and without a history of urinary tract stones including those with baseline (prevalent urinary tract stones), incident (during study), recurrent (prevalent and incident urinary tract stones) are shown in Supporting Table 1. A total of 9856 women reported having urinary tract stones at baseline or during follow-up. Overall, 5993 women (4%) had a prevalent stone reported at the baseline visit; of those, 5259 women (3.5%) had stones only at baseline and 734 women (14%) had another stone during WHI follow-up (recurrent). An initial stone during follow-up without prior history occurred in 3863 women (2.6%). Women were followed for an average of 8 years. Differences in demographic and clinical characteristics by history of urinary tract stones were similar to those recently reported from women in the WHI OS.(36) In women with urinary tract stones, in particular for those with recurrent stones, there were higher dietary intakes of energy (p<0.001), animal protein (p = 0.002), and sodium (p<0.001), and relatively lower intakes of potassium (p = 0.042), fluid/water (p = 0.002), oxalic acid (p = 0.009), calcium (p = 0.309), and vitamin D (p = 0.986) reported than in non–stone-formers. There were no significant differences in calcium (p = 0.309) or vitamin D (p = 0.986) in women with urinary tract stones compared to those without.
In unadjusted and adjusted models, respectively, there was no significant association of urinary tract stones with changes in BMD at the total hip (p = 0.57 and p = 0.33), femoral neck (p = 0.50 and p = 0.49), lumbar spine (p = 0.13 and p = 0.38), or whole body (p = 0.15 and p = 0.38). In sensitivity analyses including women who had a constant status of urinary tract stones (never or at baseline already) there was no significant association of urinary tract stones with changes in BMD in unadjusted and adjusted models, respectively, for the skeletal sites of the total hip (p = 0.91 and p = 0.49), femoral neck (p = 0.99 and p = 0.96), lumbar spine (p = 0.13 and p = 0.38), or whole body (p = 0.32 and p = 0.75). In sensitivity analyses including, 1) only women with prevalent urinary tract stones or, 2) those who never had urinary tract stones there was no significant association of urinary tract stones with changes in BMD at the total hip (p = 0.91 and p = 0.49), femoral neck (p = 0.99 and p = 0.96), or whole body (p = 0.32 and p = 0.75). There was a significant positive association of urinary tract stones with increases in BMD at the lumbar spine in unadjusted models (p = 0.03), which disappeared after adjustment for covariates (p = 0.17). In sensitivity analysis using only the portion of longitudinal data for each woman during which urinary tract stones status did not change, there was again no significant association of urinary tract stones with changes in BMD in unadjusted and adjusted models, respectively, for the total hip (p = 0.90 and p = 0.48), femoral neck (p = 1.00 and p = 0.97), or whole body (p = 0.32 and p = 0.75). There was a significant positive association of urinary tract stones with increases in BMD at the lumbar spine in unadjusted models (p = 0.02), which disappeared after adjustment for covariates (p = 0.16) (Table 1).
Table 1.
Association of Urinary Tract Stones With Annual Change in BMD
| Urinary tract stones
|
|||||
|---|---|---|---|---|---|
| na | Yes Annual change (beta ± SE) | No Annual change (beta ± SE) | pa | pb | |
| Total hip | 9717 | −0.0013 ± 0.0003 | −0.00113 ± 0.00006 | 0.57 | 0.33 |
| Sensitivity 1c | 9446 | −0.0012 ± 0.0003 | −0.00114 ± 0.00006 | 0.91 | 0.49 |
| Sensitivity 2d | 9717 | −0.0012 ± 0.0003 | −0.00114 ± 0.00006 | 0.90 | 0.48 |
| Femoral neck | 9717 | −0.0007 ± 0.0003 | −0.00087 ± 0.00007 | 0.50 | 0.49 |
| Sensitivity 1c | 9446 | −0.0009 ± 0.0003 | −0.00087 ± 0.00007 | 0.99 | 0.96 |
| Sensitivity 2d | 9717 | −0.0009 ± 0.0003 | −0.00087 ± 0.00007 | 1.00 | 0.97 |
| Lumbar spine | 9730 | 0.0046 ± 0.0004 | 0.00525 ± 0.00010 | 0.13 | 0.38 |
| Sensitivity 1c | 9457 | 0.0042 ± 0.0005 | 0.00523 ± 0.00010 | 0.03 | 0.17 |
| Sensitivity 2d | 9730 | 0.0042 ± 0.0005 | 0.00525 ± 0.00010 | 0.02 | 0.16 |
| Whole body | 9718 | 0.0030 ± 0.0003 | 0.00251 ± 0.00008 | 0.15 | 0.38 |
| Sensitivity 1c | 9447 | 0.0029 ± 0.0004 | 0.00250 ± 0.00008 | 0.32 | 0.75 |
| Sensitivity 2d | 9718 | 0.0029 ± 0.0004 | 0.00250 ± 0.00007 | 0.32 | 0.75 |
Coefficient of year (g/cm2 per year) in linear mixed model for BMD stratified on time-dependent urinary tract stone status. Linear mixed model was used to estimate beta coefficients and significance level.
Unadjusted; p value is test across slopes for those with kidney stones compared to those without.
Adjusted for age, race, BMI, physical functioning, age at menopause, education, income, region, smoking, fracture at age 55+ years, parent with fracture after 40 years old, general health, alcohol intake, diabetes history, hormone therapy use, physical activity, bisphosphonate, calcitonin, oral corticosteroids, antiepileptic, proton pump inhibitor, thiazide, loop diuretic, thyroid meds, HT arm, DM arm, CaD arm, and diet and supplement intake.
Sensitivity analysis 1: Including only women with baseline urinary tract stones (prevalent urinary tract stones) or never urinary tract stones (not incident).
Sensitivity analysis 2: Including the portion of longitudinal data for which urinary tract stones status did not change; this includes all the women in sensitivity analysis 1 and the initial data for women who developed urinary tract stones during study.
Incident fractures occurred at any site in 21,762 of 150,689 women over an average of 7.6 years of follow-up, for a fracture rate of 19.4 per 1000 person-years. This included 20,591 fractures in 141,326 women without urinary tract stones, for a fracture rate of 19.3 per 1000 person-years and 1171 incident fractures in 9363 women with urinary tract stones preceding the fracture, for a fracture rate of 21.4 per 1000 person-years. In unadjusted models there was a significant association of urinary tract stones with incident total fractures (HR 1.10; 95% CI, 1.04 to 1.17), clinical spine fractures (HR 1.30; 95% CI, 1.10 to 1.53), and other fracture sites (HR 1.08; 95% CI, 1.01 to 1.15); although in a similar direction, these associations were no longer statistically significant after covariate adjustment (HR 1.04 [95% CI, 0.97 to 1.12]; HR 1.17 [95% CI, 0.95 to 1.43]; and HR 1.02 [95% CI, 0.94 to 1.10], respectively) (Table 2). In a subset analysis including 9626 women who had BMD measurements there was no significant association of urinary tract stones after inclusion of lumbar spine BMD (HR 1.08; 95% CI, 0.87 to 1.34) or hip BMD (HR 1.08; 95% CI, 0.87 to 1.34) in the models. Sensitivity analyses of the association of urinary tract stones with changes in BMD and incident fractures limited to only the WHI OS women, revealed similar findings to the whole cohort (data not shown).
Table 2.
Association of Urinary Tract Stones with Incident Fractures
| Urinary tract stones
|
||||
|---|---|---|---|---|
| Total (n) | Events | No | Yes HR (95% CI) | |
| Total fracture | ||||
| Unadjusted (entire cohort) | 150,689 | 21,762 | 1 (ref) | 1.10 (1.04–1.17) |
| Adjusted (entire cohort)a | 110,958 | 15,660 | 1 (ref) | 1.04 (0.97–1.12) |
| Adjusted (BMD cohort)b | 9,626 | 1,385 | 1 (ref) | 1.08(0.87–1.34) |
| Adjusted (BMD cohort)c | 9,626 | 1,385 | 1 (ref) | 1.08(0.87–1.34) |
| Hip fracture | ||||
| Unadjusted | 150,689 | 1,871 | 1 (ref) | 1.05 (0.86–1.29) |
| Adjusteda | 110,958 | 1,281 | 1 (ref) | 0.89 (0.69–1.16) |
| Clinical spine fracture | ||||
| Unadjusted | 150,689 | 2,350 | 1 (ref) | 1.30 (1.10–1.53) |
| Adjusteda | 110,958 | 1,605 | 1 (ref) | 1.17 (0.95–1.43) |
| Other fracture | ||||
| Unadjusted | 150,689 | 18,900 | 1 (ref) | 1.08 (1.01–1.15) |
| Adjusteda | 110,958 | 13,672 | 1 (ref) | 1.02 (0.94–1.10) |
HRs and 95% CIs from Cox regression model for time to fracture on time-dependent urinary tract stones status. Cox proportional hazard model was used including urinary tract stones as time varying variable.
Adjusted for: age, race, BMI, physical functioning, age at menopause, education, income, region, smoking, fracture at age 55+ years, parent with fracture after 40 years old, general health, alcohol intake, diabetes history, hormone therapy use, physical activity, bisphosphonate, calcitonin, oral corticosteroids, antiepileptic, proton pump inhibitor, thiazide, loop diuretic, thyroid meds, HT arm, DM arm, CaD arm, and diet and supplement intake.
Adjusted for: age, race, BMI, and total hip BMD.
Adjusted for: age, race, BMI, and lumbar spine BMD.
Discussion
This is the largest cohort of multiethnic postmenopausal women with urinary tract stones (including approximately 10,000 women with kidney/bladder stones) ever examined for fracture risk and osteoporosis. The major finding of this report is that, after model adjustments, there was no significant association of urinary tract stones with changes in BMD at multiple skeletal sites. Fractures, including total fractures and site -specific fractures of the hip, vertebral fractures, and other fracture sites, were also not significantly associated with urinary tract stones in the multivariable model.
In longitudinal adjusted analyses there was no association of changes in BMD at any site with a history of kidney/bladder stones. These findings are in accord with some other smaller studies, which have reported that urinary tract stones are not associated with hip BMD(4,8,11) or lumbar spine BMD.(8,11) In contrast, a recent case-control study from Taiwan suggested that there was a positive association between a first time diagnosis of osteoporosis by BMD measurements and prior urinary calculus; however, this study included both men and women and 98% of participants were of Hans Chinese ethnicity.(14) To our knowledge, we are the first to report the finding that in postmenopausal women in the WHI, whole-body BMD was not related to urinary tract stones.
The findings in women in WHI that urinary tract stones were not significantly related to incident fractures are in accord with cross-sectional data from women in NHANES III.(4) In contrast, one retrospective cohort study that included 181 women followed over 25 years suggested that there was an increased standard morbidity ratio (compared with population-based expected rates) of vertebral fractures in women with urinary tract stones; although, similar to WHI, they found no relationship of morbidity ratios for other fracture sites, including the hip, humerus, and forearm, with urinary tract stones.(9) More recently, a population-based cohort study using The Health Improvement Network (THIN) reported a slightly higher fracture risk in women with urolithiasis compared to those without; the risk was greatest in women younger than WHI participants, specifically, in those aged 30 to 39 years.(37) In WHI, prior to covariate adjustment, risks for fracture in those with urolithiasis were very similar to what was reported in THIN, with a 10% increased risk in WHI and a 17% increase in older women in THIN.(37) In contrast with THIN, in WHI, the increased risk for fracture was no longer present after covariate adjustment. However, in WHI we were able to adjust for a number of important covariates that were not included in THIN, including diet, smoking and alcohol intake, physical activity, menopausal history, and family history of osteoporosis. Additionally, race/ethnicity was not reported in THIN and only a subset had BMI data.(37)
Calcium and vitamin D supplementation,(26,27) higher salt intakes,(17–19) higher caloric consumption,(38) higher animal protein intake,(39) lower potassium intake,(20,21) and lower water consumption(40) are risk factors for stone formation. In WHI, the association of urinary tract stones with total, clinical spine, and other fractures in unadjusted models disappeared after adjustment for covariates that are associated with osteoporosis and/or urinary tract stones; this was prior to adjustment for dietary or supplement intakes. Additionally, the lack of association of urinary tract stones with longitudinal changes in BMD was independent of when the stone occurred or how many times a kidney/bladder stone occurred.
There are a number of limitations to our analysis. To start, this is an observational cohort (with respect to the outcomes examined) and data is limited for some covariates, in particular for medication use, for which dose was not collected. History of urinary tract stones was obtained from self- report only. However, self-report of urinary tract stones is very reliable and has been reported to be 97% to 98% accurate.(41) Stone composition was not known; however, the authors suspect that only a few of these stones were uric acid stones, because less than 1% of women were on allopurinol, which might be used to treat uric acid–containing stones.(42) Radiographs or ultrasounds to assess for asymptomatic stones or vertebral fractures were not obtained. Biochemical analysis of urine specimens for hypercalciuria were not done, and it is possible that urinary tract stones in the subset of women with hypercalciuria might be associated with osteoporosis.(10,11,43) The questionnaire used in WHI did not distinguish kidney from bladder stones, although in women bladder stones are very rare (<5% of all stones).(44) The three clinical centers that participated in the DXA measurements were selected to provide maximum racial and ethnic diversity, and thus were not representative of the WHI as a whole.(45) The statistical analysis also has limitations. All covariates that were available in WHI and associated with osteoporosis and/or urinary tract stones were included and entered simultaneously in the model. By adjusting for all these covariates, it is possible that we “overadjusted”; ie, adjusted for a potential confounder that could also be a mediator of the association of urinary tract stones with osteoporosis. For example, individuals with urinary tract stones are frequently prescribed hydrochlorothiazide,(46) which in some studies has been associated with higher BMD(47) and fewer fractures.(48)
This study had a number of important strengths. To the authors’ knowledge, this is the largest, most comprehensive analysis of the association of urinary tract stones with incident fractures to date in postmenopausal women. It includes women of all races and ethnicities and includes both BMD measurements from multiple sites and fractures from multiple locations.
In conclusion, urinary tract stones are not an independent risk factor for osteoporosis in postmenopausal women.
Supplementary Material
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services. The WHI publications committee approved the final version of this manuscript. This material is the result of work supported with resources and the use of facilities at the Charlie Norwood Veterans Affairs Medical Center. The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authors’ roles: LDC and CAA had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Other author contributions follow. Study concept and design: LDC, KCJ, and FT. Acquisition of data: KMH, CAA, and FT. Analysis and interpretation of data: KMH, CAA, LDC, and FT. Drafting of the manuscript: LDC. Critical revision of the manuscript for important intellectual content: MDS, CJC, NBW, MB, and KCJ. Statistical analysis: KMH, CAA, and FT. Obtained funding: KCJ. Study supervision: KCJ.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–9. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaeger P, Lippuner K, Casez JP, Hess B, Ackermann D, Hug C. Low bone mass in idiopathic renal stone formers: magnitude and significance. J Bone Miner Res. 1994;9(10):1525–32. doi: 10.1002/jbmr.5650091004. [DOI] [PubMed] [Google Scholar]
- 4.Lauderdale DS, Thisted RA, Wen M, Favus MJ. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res. 2001;16(10):1893–8. doi: 10.1359/jbmr.2001.16.10.1893. [DOI] [PubMed] [Google Scholar]
- 5.Bleicher K, Cumming RG, Naganathan V, et al. Lifestyle factors, medications, and disease influence bone mineral density in older men: findings from the CHAMP study. Osteoporos Int. 2011;22(9):2421–37. doi: 10.1007/s00198-010-1478-9. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Fullman RL, Stone KL, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16(12):1525–37. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 7.Cauley JA, Blackwell T, Zmuda JM, et al. Correlates of trabecular and cortical volumetric bone mineral density at the femoral neck and lumbar spine: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2010;25(9):1958–71. doi: 10.1002/jbmr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sowers MR, Jannausch M, Wood C, Pope SK, Lachance LL, Peterson B. Prevalence of renal stones in a population-based study with dietary calcium, oxalate, and medication exposures. Am J Epidemiol. 1998;147(10):914–20. doi: 10.1093/oxfordjournals.aje.a009381. [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ, 3rd, Crowson CS, Khosla S, Wilson DM, O’Fallon WM. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int. 1998;53(2):459–64. doi: 10.1046/j.1523-1755.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 10.Pietschmann F, Breslau NA, Pak CY. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res. 1992;7(12):1383–8. doi: 10.1002/jbmr.5650071205. [DOI] [PubMed] [Google Scholar]
- 11.Asplin JR, Bauer KA, Kinder J, et al. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int. 2003;63(2):662–9. doi: 10.1046/j.1523-1755.2003.00763.x. [DOI] [PubMed] [Google Scholar]
- 12.Caudarella R, Vescini F, Buffa A, et al. Bone mass loss in calcium stone disease: focus on hypercalciuria and metabolic factors. J Nephrol. 2003;16(2):260–6. [PubMed] [Google Scholar]
- 13.Alhava EM, Juuti M, Karjalainen P. Bone mineral density in patients with urolithiasis. A preliminary report. Scand J Urol Nephrol. 1976;10(2):154–6. doi: 10.3109/00365597609179678. [DOI] [PubMed] [Google Scholar]
- 14.Keller JJ, Lin CC, Kang JH, Lin HC. Association between osteoporosis and urinary calculus: evidence from a population-based study. Osteoporos Int. 2013;24(2):651–7. doi: 10.1007/s00198-012-2019-5. [DOI] [PubMed] [Google Scholar]
- 15.Giannini S, Nobile M, Dalle Carbonare L, et al. Hypercalciuria is a common and important finding in postmenopausal women with osteoporosis. Eur J Endocrinol. 2003;149(3):209–13. doi: 10.1530/eje.0.1490209. [DOI] [PubMed] [Google Scholar]
- 16.Liern M, Bohorquez M, Vallejo G. Treatment of idiopathic hypercalciuria and its impact on associated diseases. Arch Argent Pediatr. 2013;111(2):110–4. doi: 10.5546/aap.2013.eng.110. [DOI] [PubMed] [Google Scholar]
- 17.Aruga S, Honma Y. [Renal calcium excretion and urolithiasis] Clin Calcium. 2011;21(10):1465–72. Japanese. [PubMed] [Google Scholar]
- 18.Caudarella R, Vescini F, Rizzoli E, Francucci CM. Salt intake, hypertension, and osteoporosis. J Endocrinol Invest. 2009;32(4 Suppl):15–20. [PubMed] [Google Scholar]
- 19.Teucher B, Dainty JR, Spinks CA, et al. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008;23(9):1477–85. doi: 10.1359/jbmr.080408. [DOI] [PubMed] [Google Scholar]
- 20.Weaver CM. Potassium and health. Adv Nutr. 2013;4(3):368S–77S. doi: 10.3945/an.112.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69(4):727–36. doi: 10.1093/ajcn/69.4.727. [DOI] [PubMed] [Google Scholar]
- 22.Cipriani C, Biamonte F, Costa AG, et al. Prevalence of kidney stones and vertebral fractures in primary hyperparathyroidism using imaging technology. J Clin Endocrinol Metab. 2015 Apr;100(4):1309–15. doi: 10.1210/jc.2014-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W, Langsetmo L, Berger C, et al. Longitudinal changes in calcium and vitamin D intakes and relationship to bone mineral density in a prospective population-based study: the Canadian Multicentre Osteoporosis Study (CaMos) J Musculoskelet Neuronal Interact. 2013;13(4):470–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Cauley JA. The Women’s Health Initiative: hormone therapy and calcium/vitamin D supplementation trials. Curr Osteoporos Rep. 2013;11(3):171–8. doi: 10.1007/s11914-013-0150-7. [DOI] [PubMed] [Google Scholar]
- 25.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 26.Candelas G, Martinez-Lopez JA, Rosario MP, Carmona L, Loza E. Calcium supplementation and kidney stone risk in osteoporosis: a systematic literature review. Clin Exp Rheumatol. 2012;30(6):954–61. [PubMed] [Google Scholar]
- 27.Favus MJ. The risk of kidney stone formation: the form of calcium matters. Am J Clin Nutr. 2011;94(1):5–6. doi: 10.3945/ajcn.111.018481. [DOI] [PubMed] [Google Scholar]
- 28.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–74. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 30.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 Suppl):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 31.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 32.Trumbo P, Schlicker S, Yates AA, Poos M Food; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 34.McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA. 2003;290(10):1331–6. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 35.Hall WD, Pettinger M, Oberman A, et al. Risk factors for kidney stones in older women in the southern United States. Am J Med Sci. 2001;322(1):12–8. doi: 10.1097/00000441-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen MD, Hsi RS, Chi T, et al. Women’s Health Initiative Writing Group. Dietary intake of fiber, fruit, and vegetables decrease the risk of incident kidney stones in women: a Women’s Health Initiative (WHI) Report. J Urol. 2014 Dec;192(6):1694–9. doi: 10.1016/j.juro.2014.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denburg MR, Leonard MB, Haynes K, et al. Risk of fracture in urolithiasis: a population-based cohort study using the health improvement network. Clin J Am Soc Nephrol. 2014;9(12):2133–40. doi: 10.2215/CJN.04340514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daudon M. [Epidemiology of nephrolithiasis in France] Ann Urol (Paris) 2005;39(6):209–31. doi: 10.1016/j.anuro.2005.09.007. French. [DOI] [PubMed] [Google Scholar]
- 39.Tracy CR, Best S, Bagrodia A, et al. Animal protein and the risk of kidney stones: a comparative metabolic study of animal protein sources. J Urol. 2014;192(1):137–41. doi: 10.1016/j.juro.2014.01.093. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen MD, Kahn AJ, Reiner AP, et al. Impact of nutritional factors on incident kidney stone formation: a report from the WHI OS. J Urol. 2012;187(5):1645–9. doi: 10.1016/j.juro.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328(12):833–8. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 42.Marangella M, Vitale C, Bagnis C, Petrarulo M, Tricerri A. Use of drugs for nephrolithiasis. Clin Cases Miner Bone Metab. 2008;5(2):131–4. [PMC free article] [PubMed] [Google Scholar]
- 43.Lalau JD, Achard JM, Bataille P, et al. [Vertebral density of hypercalciuric lithiasis. Its relation to calcium-protein intake and vitamin D metabolism] Ann Med Interne (Paris) 1992;143(5):293–8. French. [PubMed] [Google Scholar]
- 44.Schwartz BF, Stoller ML. The vesical calculus. Urol Clin North Am. 2000;27(2):333–46. doi: 10.1016/s0094-0143(05)70262-7. [DOI] [PubMed] [Google Scholar]
- 45.Orchard TS, Larson JC, Alghothani N, et al. Magnesium intake, bone mineral density, and fractures: results from the Women’s Health Initiative Observational Study. Am J Clin Nutr. 2014;99(4):926–33. doi: 10.3945/ajcn.113.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vigen R, Weideman RA, Reilly RF. Thiazides diuretics in the treatment of nephrolithiasis: are we using them in an evidence-based fashion? Int Urol Nephrol. 2011;43(3):813–9. doi: 10.1007/s11255-010-9824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolland MJ, Ames RW, Horne AM, Orr-Walker BJ, Gamble GD, Reid IR. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int. 2007;18(4):479–86. doi: 10.1007/s00198-006-0259-y. [DOI] [PubMed] [Google Scholar]
- 48.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. A prospective study of thiazide use and fractures in women. Osteoporos Int. 1997;7(1):79–84. doi: 10.1007/BF01623465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.