Abstract
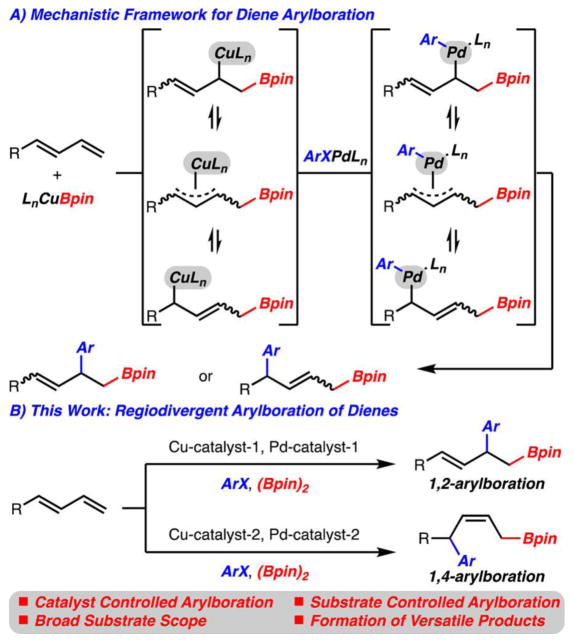
A method for the regiodivergent arylboration of dienes is presented. These reactions allow for the formation of a diverse range of synthetically versatile products from simple precursors. Through mechanistic studies, these reactions likely operate by initial addition of a Cu–Bpin complex across the diene followed by Pd-catalyzed cross coupling with an aryl halide or pseudohalide.
Difunctionalization of alkenes is an important class of reactions because molecular complexity can be rapidly generated from simple precursors.1 Variants that involve conjugated dienes are particularly interesting as the potential for controlled formation of multiple isomeric products becomes possible. Known methods for conjugated diene difunctionalization include (but are not limited to)2 diamination,3 dihydroxylation,4 diarylation(vinylation),5 diboration,6 and cycloadditions.7
An important emerging class of reactions includes carboboration of π-bonds.8 These reactions have been shown to function with alkyne, allene, or alkene derived substrates.9,10 Only recently has the carboboration of dienes (butadiene and 2-substituted dienes) been reported.9a,d A subclass of carboboration reactions involves the arylboration of alkenyl arenes by Pd/Cu-cooperative catalysis that was independently reported by our lab11 and that of Semba and Nakao.12–14 Our lab has recently reported a method for arylboration of isoprene and its derivatives in which an additive effect of DMAP was uncovered.15 Although this method did allow for control of regioselectivity, translation to dienes of varying substitution patterns was unsuccessful. Development of diene arylboration reactions are challenging as based on the proposed mechanism, formation of isomeric Cu–16 and Pd–π-allyl17 complexes can be formed, which may lead to poorly selective reactions (Scheme 1A). Herein, we describe a process for the regio divergent arylboration of a variety of substituted dienes by Pd/Cu-cooperative catalysis (Scheme 1B).
Scheme 1.
Arylboration of Dienes by/Cu-Catalysis
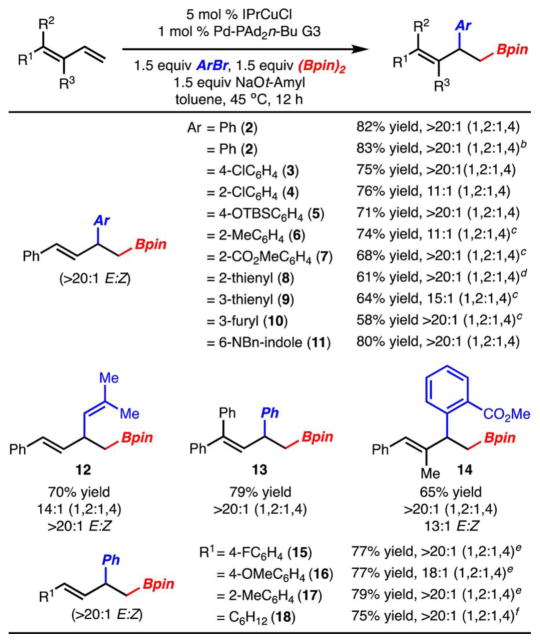
Our initial investigations began with arylboration of 1-phenylbutadiene. Translation of the optimal reaction conditions described for arylboration of styrenes (5 mol % SIMesCuCl, 1 mol % Pd-XPhos G3,18 PhBr, (Bpin)2, NaOt-Bu, toluene, 22 °C)11 to that of 1-phenylbutadiene led to formation of 2 in good yield and 6:1 regioselectivity.19 Standard optimization of this initial result led to the conditions illustrated in Table 1, entry 1. Key to identifying a set of conditions that are highly selective for formation of 2 was the use of the sterically more demanding IPrCuCl vs SIMesCuCl. Although use of Pd-PAd2n-Bu gave rise to product in the highest yield and selectivity,20 it was found that other sterically demanding and electron-rich phosphines also allowed for highly regioselective reaction to occur (Table 1, entries 3 and 4). When PhBr was replaced with PhI or PhOTf the product was generated in good yield and selectivity (Table 1, entries 6–8). In the case of PhOTf, use of XPhos was required, as use of PAd2n-Bu did not lead to product formation.21 Use of PhCl was not tolerated as only recovered starting material was observed (Table 1, entry 9).
Table 1.
Change from Standard Conditions
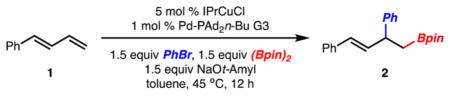
| |||
|---|---|---|---|
|
| |||
| entry | change from standard conditions | yield (%)a | (1,2): (1,4)a |
| 1 | no change | 93 | 23:1 |
| 2 | IMes instead of IPr | 68 | 7:1 |
| 3 | XPhos instead of PAd2n-Bu | 85 | 11:1 |
| 4 | Pt-Bu3 instead of PAd2n-Bu | 67 | 22:1 |
| 5 | PCy3 instead of PAd2n-Bu | 18 | 35:1 |
| 6 | Phl instead of PhBr | 94 | 20:1 |
| 7 | PhOTf instead of PhBr | <2 | ND |
| 8 | PhOTf and XPhos instead of PhBr and PAd2n-Bu | 88 | 14:1 |
| 9 | PhCI instead of PhBr | <2 | ND |
| |||
Yield of 1,2-product and (1,2):(1,4) determined by GC analysis with a calibrated internal standard. ND = Not determined.
The scope of the reaction was evaluated with respect to the aryl bromide and diene component (Scheme 2). A variety of aryl bromides underwent highly regioselective arylboration. Notably, electron rich (products 5 and 6), electron deficient (products 3, 4, 7), and sterically hindered aryl bromides (products 6 and 7) all functioned well. With sterically demanding aryl bromides it was found that use of Pd–Pt-Bu3 allowed for product formation in slightly higher yields and regioselectivities.19 Various heterocyclic aryl bromides could also be used with little effect on the reaction yield or selectivity (products 8–11). 1-Phenylbutadiene could also be substituted for other sterically and electronically modified 1,3-dienes with no significant change in yield or selectivity (products 13–17). It should be noted that in some of these examples (15–17), the starting dienes were used as mixtures of E- and Z-isomers, yet the product was always generated as a single alkene isomer (>20:1 E:Z). An alkyl-substituted diene could also be employed and arylboration product 18 was formed in good yield and selectivity. However, in this case the formation of a 1,1-arylboration product was also generated in ~20% yield.19 Finally, the reactions could be run on a 5 mmol scale with little change in yield or selectivity.
Scheme 2. Reaction with Various Substituted Dienes and ArBra.
aYield of isolated product that represents the average of two or more experiments. All reactions were carried out on a 0.5 mmol scale unless noted otherwise. bReaction run on a 5 mmol scale. cPt-Bu3 was used instead of PAd2n-Bu. dContains ~6% of a product derived from protonation of the allyl Cu intermediate. See the SI for details. eThe starting diene was a mixture of alkene isomers (2:1–1:5:1 E:Z). fYield determined by NMR analysis with an internal standard. The reaction generated ~20% of a rearranged product, see the SI for details.
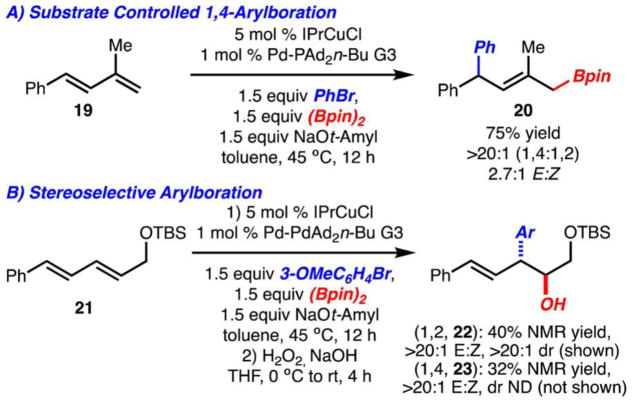
In the case of 1,3-susbtituted diene 19 the product of a 1,4-arylboration (20) was exclusively formed (Scheme 3A). In this reaction, a mixture of alkene isomers was also observed. Arylboration product 20 is likely generated because formation of the corresponding 1,2-arylboration product would require formation of a quaternary carbon. Thus, a strategy is provided such that regioselectivity can be controlled by design of substrate.
Scheme 3.
Arylboration of Substituted Dienes
For reaction involving 1,4-subtituted diene 21, the diastereoselectivty of the process could be explored (Scheme 3B). Under standard reaction conditions, a mixture of 1,2- and 1,4-arylboration products (22 and 23, respectively) were formed with the former being slightly preferred. The (1,2)-arylboration product 22 could be easily purified after oxidation of the Bpin unit to an alcohol.19 Furthermore, 1,2-arylboration product 22 was generated in >20:1 dr with the anti-addition isomer being major.
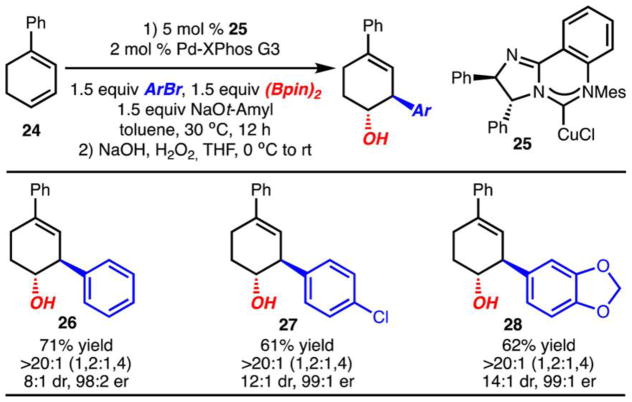
Cyclic diene 24 was also investigated under the optimized conditions and provided 27 in 68% yield as a single regio- and diastereomer (not shown).19 Furthermore, enantioselective reactions could be carried out with chiral catalyst 25 (Scheme 4).11c,22,23 In each case, the products (26–28) were generated with high levels and enantioselectivity and diastereoselectivity. The absolute stereochemistry of 27 was established by X-ray crystallography. Attempts to use this chiral catalyst with the acyclic dienes presented in Schemes 2 and 3 resulted in formation of racemic products.
Scheme 4. Enantioselective Arylboration of a Cyclic Dienea.
aYield of isolated product that represents the average of two or more experiments. All reactions carried out on a 0.3 mmol scale.
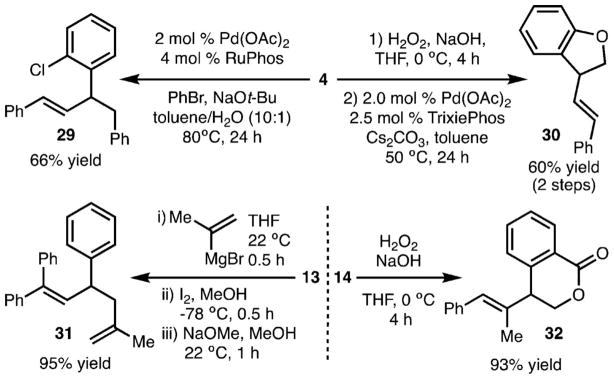
The products generated by these reactions represent useful intermediates for chemical synthesis (Scheme 5). Application of C–C24 and C–O25 cross-coupling reactions with 4 led to synthesis of 29 and 30, respectively. Zweifel-type olefination with 13 led to formation of diene 31, whereas oxidation of 14 allowed for synthesis of 32.26
Scheme 5.
Functionalization of Products
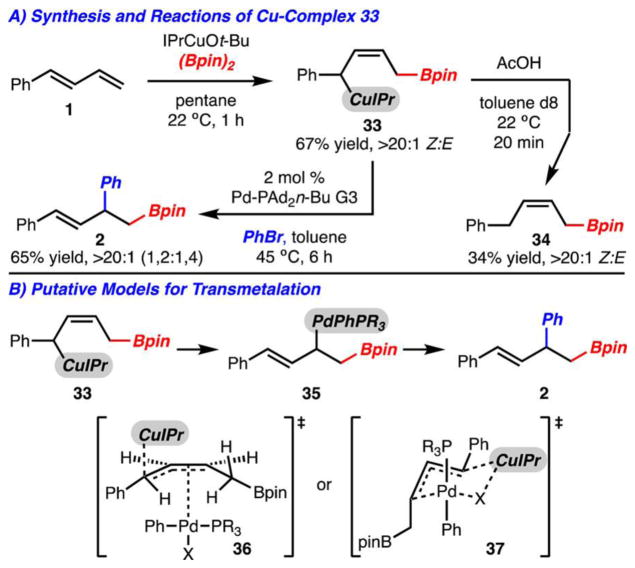
The mechanism of the 1,2-arylboration process was also studied (Scheme 6A). Treatment of 1-phenylbutadiene through in situ prepared IPrCuBpin led to the rapid formation of Cu-complex 33 as determined by 1H NMR analysis. It is important to note that this complex is formed exclusively as the Z-alkene isomer and is the formal 1,4-addition product of the IPrCuBpin across the diene. The formation of the Z-alkene may either be the result of a formation of thermodynamically more stable complex27 or a kinetically favored insertion to an s-cis-diene.28 To provide evidence that this complex is generated under the catalytic reaction conditions, it could be subjected to Pd–PAd2n-Bu G3 and PhBr to yield the expected 1,2-arylboration product 2 with high regioselectivity. In addition, the intermediate could be quenched with AcOH to furnish allylborane 34 as the Z-isomer.10
Scheme 6.
Mechanistic Investigations
Many factors may control the regioselectivity of these reactions. If transmetalation is the selectivity-determining step, two likely possibilities present themselves for the reaction between Cu-complex 33 and ArPdBrLn (Scheme 6B, 36 and 37).29 While at this time it is too early to confirm which transmetalation pathway is major, early evidence points toward model 36. The regioselectivity of the reaction is largely independent of halide (PhBr, PhI) or pseudohalide (PhOTf) employed (Table 1, entries 1, 6, 8). Especially evident is that PhOTf undergoes reaction with high selectivity.21 It is unlikely that the triflate ligand can bridge Cu and Pd as is likely necessary for model 37.
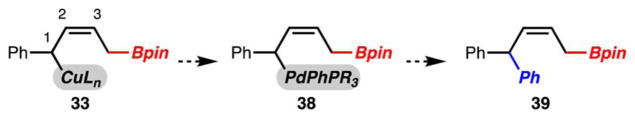
With an effective process for the 1,2-arylboration of a variety of dienes with aryl bromides, the development of a 1,4-arylboration was targeted (Scheme 7). The knowledge gained from the mechanistic studies outlined in Scheme 6 was crucial in this regard. It was reasoned that by proper choice of ligand bound to Cu and Pd, a transmetalation process might be favored at the C(1)–Cu bond rather than at C3 to generate 38. Furthermore, if the process can be tuned to achieve 1,4-selectivity, the desired arylboration product (e.g., 39) should be the Z-alkene isomer.
Scheme 7.
Mechanistic Hypothesis for 1,4-Arylboration
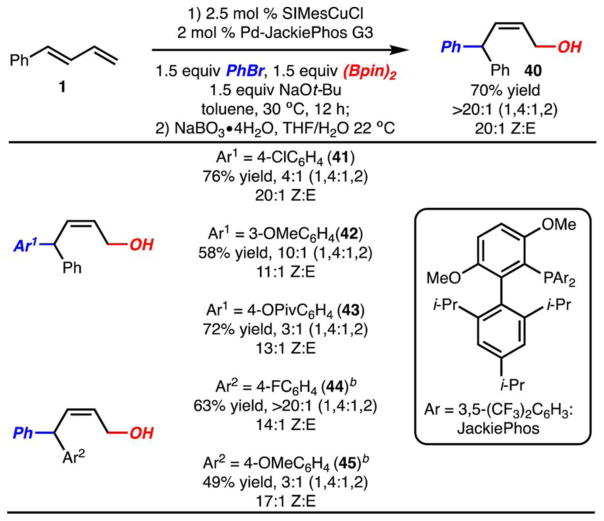
Two guiding principles were used to aid in the identification of a set of conditions that favor 1,4-arylboration. (1) Use of less sterically demanding ligands might open the possibility for direct transmetalation at the sterically encumbered C(1)–Cu bond. (2) Use of a more electrophilic Pd-complex might prefer to react at the C(1)–Cu bond rather than at the C(2)–C(3) double bond. Largely on the basis of these principles, it was identified that use of SIMesCuCl and PdJackiePhos G330 allowed for highly selective formation of 32 as the Z-isomer (Scheme 8). The formation of the Z-isomer is likely due to a rapid reductive elimination with the electron deficient JackiePhos ligand prior to isomerization with Pd–π-allyl complexes.
Scheme 8. 1,4-Arylborationa.
aYield determined by 1H NMR analysis of the crude reaction mixture after oxidation with an internal standard, represents the average of two or more experiments. All reactions carried out on a 0.3 mmol scale. bThe starting diene was a mixture of alkene isomers (2:1–1:5:1 E:Z).
On the basis of the optimized conditions, the scope of the 1,4-arylboration reactions was briefly investigated (Scheme 8). Several points are noteworthy. (1) Electron deficient aryl bromides function well in the reaction; however, mixtures of 1,4- and 1,2-arylboration products are formed (product 41). This may be due to a slower reductive elimination that may allow for isomerization to a Pd-complex analogous 35 (Scheme 6B). (2) Use of electron rich aryl bromides as well as heterocyclic aryl bromides resulted in low yield.19 (3) Other electronically modified dienes work well (products 44 and 45). The regioselectivity of reactions with electron rich dienes was low, perhaps due to a less selective transmetalation process.
In conclusion, a method for the regioselective arylboration of dienes has been developed. This has been achieved by design of substrate and by modification of reaction conditions. Mechanistic studies have also been carried out to elucidate the details of this process and allow for further reaction development. Future efforts will be directed toward expanding the scope of this process, development of enantioselective variants, and application in synthesis.
Supplementary Material
Acknowledgments
We thank Indiana University and the NIH (5R01GM114443) for generous financial support.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b05477.
Crystallographic data (PDB)
Experimental procedures, analytical data for all compounds (PDF)
Crystallographic data (CIF)
References
- 1.Coombs JR, Morken JP. Angew Chem, Int Ed. 2016;55:2636. doi: 10.1002/anie.201507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For recent examples of diene difunctionalization, see: Wu X, Lin HC, Li ML, Li LL, Han ZY, Gong LZ. J Am Chem Soc. 2015;137:13476. doi: 10.1021/jacs.5b08734.Liu Y, Xie Y, Wang H, Huang H. J Am Chem Soc. 2016;138:4314. doi: 10.1021/jacs.6b00976.
- 3.For a representative example, see: Zhao B, Peng X, Cui S, Shi Y. J Am Chem Soc. 2010;132:11009. doi: 10.1021/ja103838d.
- 4.Xu DQ, Crispino GA, Sharpless KB. J Am Chem Soc. 1992;114:7570. [Google Scholar]
- 5.For select examples, see: Zhang X, Larock RC. Tetrahedron. 2010;66:4265. doi: 10.1016/j.tet.2009.12.012.McCammant MS, Sigman MS. Chem Sci. 2015;6:1355. doi: 10.1039/c4sc03074e.
- 6.For select examples, see: Ishiyama T, Yamamoto M, Miyaura N. Chem Commun. 1996;17:2073.Kliman LT, Mlynarski SN, Ferris GE, Morken JP. Angew Chem, Int Ed. 2012;51:521. doi: 10.1002/anie.201105716.
- 7.For a representative review, see: Corey EJ. Angew Chem, Int Ed. 2009;48:2100. doi: 10.1002/anie.200805374.
- 8.(a) Shimizu Y, Kanai M. Tetrahedron Lett. 2014;55(28):3727. [Google Scholar]; (b) Semba K, Fujihara T, Terao J, Tsuji Y. Tetrahedron. 2015;71(15):2183. [Google Scholar]; (c) Lazreg F, Nahra F, Cazin CSJ. Coord Chem Rev. 2015;293–294:48. [Google Scholar]; (d) Neeve EC, Geier SJ, Mkhalid IAI, Westcott SA, Marder TB. Chem Rev. 2016;116:9091. doi: 10.1021/acs.chemrev.6b00193. [DOI] [PubMed] [Google Scholar]
- 9.For selected recent examples, see: Li X, Meng F, Torker S, Shi Y, Hoveyda AH. Angew Chem, Int Ed. 2016;55:9997. doi: 10.1002/anie.201605001.Semba K, Ohtagaki Y, Nakao Y. Org Lett. 2016;18:3956. doi: 10.1021/acs.orglett.6b01675.Yeung K, Ruscoe RE, Rae J, Pulis AP, Procter DJ. Angew Chem, Int Ed. 2016;55:11912. doi: 10.1002/anie.201606710.Zhao W, Montgomery J. J Am Chem Soc. 2016;138:9763. doi: 10.1021/jacs.6b05216.Jiang L, Cao P, Wang M, Chen B, Wang B, Liao J. Angew Chem, Int Ed. 2016;55:13854. doi: 10.1002/anie.201607493.For a 1,1-arylboration, see: Nelson HM, Williams BD, Miro J, Toste FD. J Am Chem Soc. 2015;137:3213. doi: 10.1021/jacs.5b00344.
- 10.For Cu-catalyzed protoboration of 1,3-dienes, see: Semba K, Shinomiya M, Fujihara T, Terao J, Tsuji Y. Chem - Eur J. 2013;19:7125. doi: 10.1002/chem.201300443.For Cu-catalyzed protoboration of other dienes, see: Sasaki Y, Zhong C, Sawamura M, Ito H. J Am Chem Soc. 2010;132(4):1226. doi: 10.1021/ja909640b.
- 11.(a) Smith KB, Logan KM, You W, Brown MK. Chem -Eur J. 2014;20:12032. doi: 10.1002/chem.201404310. [DOI] [PubMed] [Google Scholar]; (b) Logan KM, Smith KB, Brown MK. Angew Chem, Int Ed. 2015;54:5228. doi: 10.1002/anie.201500396. [DOI] [PubMed] [Google Scholar]; (c) Logan KM, Brown MK. Angew Chem, Int Ed. 2017;56:851. doi: 10.1002/anie.201609844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semba K, Nakao Y. J Am Chem Soc. 2014;136:7567. doi: 10.1021/ja5029556. [DOI] [PubMed] [Google Scholar]
- 13.Jia T, Cao P, Wang B, Lou Y, Yin X, Wang M, Liao J. J Am Chem Soc. 2015;137:13760. doi: 10.1021/jacs.5b09146. [DOI] [PubMed] [Google Scholar]
- 14.For related Pd/Cu-catalyzed hydroarylation reactions, see: Semba K, Ariyama K, Zheng H, Kameyama R, Sakaki S, Nakao Y. Angew Chem. 2016;128:6383. doi: 10.1002/anie.201511975.Friis SD, Pirnot MT, Buchwald SL. J Am Chem Soc. 2016;138:8372. doi: 10.1021/jacs.6b04566.
- 15.Smith KB, Brown MK. J Am Chem Soc. 2017;139:7721. doi: 10.1021/jacs.7b04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.For a recent study, see: Russo V, Herron JR, Ball ZT. Org Lett. 2010;12:220. doi: 10.1021/ol902458v.
- 17.Pregosin PS, Salzmann R. Coord Chem Rev. 1996;155:35. [Google Scholar]
- 18.Bruno NC, Tudge MT, Buchwald SL. Chem Sci. 2013;4:916. doi: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.See the Supporting Infromation for details.
- 20.Zapf A, Ehrentraut A, Beller M. Angew Chem, Int Ed. 2000;39:4153. doi: 10.1002/1521-3773(20001117)39:22<4153::aid-anie4153>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.The failure of PhOTf to work with Pd-PAd2n-Bu is likely due to the instability/poor reactivity of the generated Ph(OTf)PdPAd2n-Bu complex under the reaction conditions. See the SI for details.
- 22.Park J, McQuade D. Synthesis. 2012;44:1485. [Google Scholar]
- 23.For enantioselective protoboration of cyclic dienes, see: Sasaki Y, Zhong C, Sawamura M, Ito H. J Am Chem Soc. 2010;132:1226. doi: 10.1021/ja909640b.
- 24.Yang CT, Zhang ZQ, Liu YC, Liu L. Angew Chem, Int Ed. 2011;50:3904. doi: 10.1002/anie.201008007. [DOI] [PubMed] [Google Scholar]
- 25.Torraca KE, Kuwabe SI, Buchwald SL. J Am Chem Soc. 2000;122:12907. doi: 10.1021/ja012046d. [DOI] [PubMed] [Google Scholar]
- 26.Sonawane RP, Jheengut V, Rabalakos C, Larouche-Gauthier R, Scott HK, Aggarwal VK. Angew Chem, Int Ed. 2011;50:3760. doi: 10.1002/anie.201008067. [DOI] [PubMed] [Google Scholar]
- 27.Bates RB, Beavers WA. J Am Chem Soc. 1974;96:5001.Schleyer PV, Kaneti J, Wu YD, Chandrasekhar J. J Organomet Chem. 1992;426:143.Liepins V, Bäckvall JE. Eur J Org Chem. 2002;2002:3527. doi: 10.1021/jo016229u.(d) ref 9e.
- 28.For a seminal report, see: Ojima I, Kumagai M. J Organomet Chem. 1978;157:359.
- 29.Yang Y, Buchwald SL. J Am Chem Soc. 2013;135:10642. doi: 10.1021/ja405950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks JD, Hyde AM, Cuezva AM, Buchwald SL. J Am Chem Soc. 2009;131:16720. doi: 10.1021/ja9044357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.