Abstract
Neuronal lactate uptake supports energy metabolism associated with synaptic signaling and recovery of extracellular ion gradients following neuronal activation. Altered expression of the monocarboxylate transporters (MCT) in temporal lobe epilepsy (TLE) hampers lactate removal into the bloodstream. The resulting increase in parenchymal lactate levels might exert both, anti- and pro-ictogen effects, by causing acidosis and by supplementing energy metabolism, respectively. Hence, we assessed the contribution of lactate to the maintenance of transmembrane potassium gradients, synaptic signaling and pathological network activity in chronic epileptic human tissue. Stimulus induced and spontaneous field potentials and extracellular potassium concentration changes (∆[K+]O) were recorded in parallel with tissue pO2 and pH in slices from TLE patients while blocking MCTs by α-cyano-4-hydroxycinnamic acid (4-CIN) or d-lactate. Intrinsic lactate contributed to the oxidative energy metabolism in chronic epileptic tissue as revealed by the changes in pO2 following blockade of lactate uptake. However, unlike the results in rat hippocampus, ∆[K+]O recovery kinetics and field potential amplitude did not depend on the presence of lactate. Remarkably, inhibition of lactate uptake exerted pH-independent anti-seizure effects both in healthy rat and chronic epileptic tissue and this effect was partly mediated via adenosine 1 receptor activation following decreased oxidative metabolism.
Keywords: lactate, monocarboxylate transporter inhibitors, seizure, interictal activity, mesial temporal lobe epilepsy, adenosine
1. Introduction
Under physiological conditions, brain function predominantly relies on oxidative metabolism of glucose [1]. During extensive neuronal activation, glial glycogen stores are mobilized and surplus lactate from the glycolysis is released via monocarboxylate transporters (MCT), hemichannels or ion channels into the extracellular space [2,3,4] generating a lactate gradient between astrocytes, neurons and blood vessels [5]. Due to the delicate distribution pattern of MCTs on different cell types and subcellular compartments, individual components of synaptic signaling might differentially rely on ATP derived from the oxidative metabolism of lactate [6]. Although the general validity of astrocytic neuronal lactate shuttle (ANLS) [7] has been a matter of debate [8], a subtle but important role for lactate can be found in the presence of glucose in neurovascular signaling [9], synaptic transmission and plasticity [10,11,12,13,14]. The low affinity isoform MCT1 is expressed in astrocytes, endothelial cells of microvessels and on oligodendrocytes [6]. Lactate release mediated by oligodendricytic MCT1 was shown to be critical for maintenance of axonal functions [15]. MCT4 is the major MCT isoform present on astrocytes in several brain structures, including the hippocampus. The density of MCT4 appears to be highest in the area of the astrocytic endfeet enwrapping the cerebrovasculature [16,17], suggesting that MCT4 facilitates the removal of lactate into the circulation in order to prevent lactic acidosis. Although MCT2 immunoreactivity has also been found on perivascular endfeet [18,19,20], its high affinity for lactate (Km: 0.74 mM) and its preferential localization on dendritic post-synaptic densities [16,21] implicates that this isoform might be responsible for the neuronal uptake and for the metabolic support of synaptic signaling [13].
We have recently shown that intrinsic lactate supports synaptic signaling in rat hippocampal slices and is critical for extracellular ion equilibration time course following neuronal activation. Inhibition of the MCT2 mediated lactate transport by α-cyano-4-hydroxycynamate (4-CIN) led to a decrease in postsynaptic action potential generation, and this effect was dependent on the activation of KATP channels. A significant contribution of lactate to oxidative energy metabolism was evidenced by the decrease in the baseline and stimulus induced changes in pO2 as well as by the drift in the ratio of reduced and oxidized flavin adenine dinucleotide (FADH2/FAD+) [22].
Acute provoked epileptic seizures are associated with massive increases of glucose metabolism in order to meet the energy demand for restoration of pathologically altered transmembrane ion gradients [23]. The concomitant increases in parenchymal lactate levels could be both proconvulsive by supporting energy metabolism or anticonvulsive due to the acidotic drift in tissue pH. Interictal extracellular lactate concentration was found to be elevated in mesial temporal lobe epilepsy (mTLE) patients when compared between the epileptogenic and the non-epileptogenic hippocampus [24] indicating serious alterations in the lactate removal mechanisms. Indeed, the expression pattern of several MCTs is disturbed, MCT1 was lost on microvessels and upregulated on astrocytes in the neuropil of the hippocampal formation both in human mTLE samples and in animal models of mTLE [25,26], while cortical MCT4 expression levels were significantly lowered in mTLE patients and also in pilocarpine model of epilepsy [27].
Here, we tested the hypothesis that the contribution of intrinsic lactate to energy metabolism associated with induced stimulus and epileptiform network activity are altered in chronic epileptic tissue. In subsequent experiments, we investigated whether the effect of lactate uptake inhibitors on network activity is exclusively mediated by the change in the extracellular pH or the activation of adenosine 1 (A1) receptors following metabolic restriction.
2. Results
2.1. α-cyano-4-hydroxycinnamic acid (4-CIN) Decreased Stimulus Induced pO2 in Neocortical Slices from Patients with mesial Temporal Lobe Epilepsy (mTLE)
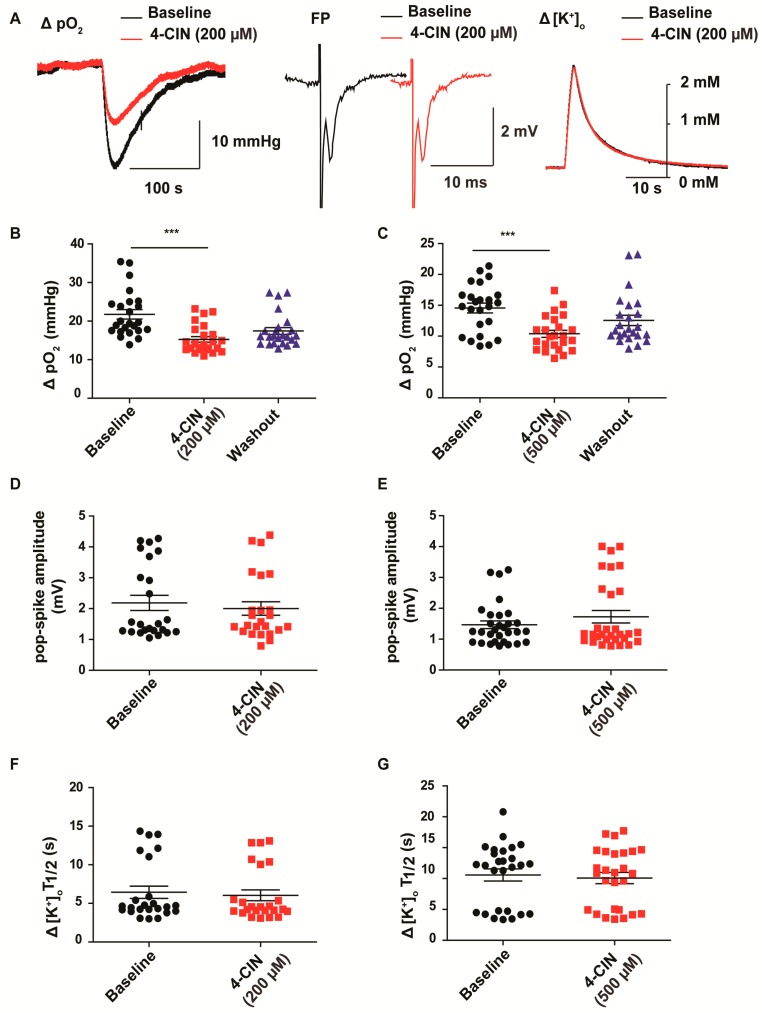
We have previously shown that neuronal lactate uptake supports synaptic transmission and recovery of stimulus induced transmembrane ionic gradients in the Cornu Ammonis 3 (CA3) region of the rat hippocampus [22]. Expression pattern of monocarboxylate transporters (MCTs) is altered in chronic epileptic tissue from mTLE patients [25,28], but it is not known, how these alterations would affect the role of lactate as metabolic substrate. In the first set of experiments, we tried to reproduce the findings from healthy rat hippocampus in neocortical slices from patients with mTLE. Neuronal activation was induced by application of stimulus trains (20 Hz, 2 s) onto the white matter while recording changes in extracellular K+ (∆[K+]O) and oxygen tension (∆pO2) in the deep neocortical layers V/VI. Application of 4-CIN at a concentration which preferentially blocks lactate uptake via MCT2 (200 µM) significantly reduced stimulus induced ∆pO2 from 21.8 ± 1.2 to 15.3 ± 0.7 mmHg (Figure 1A,B; paired t-test, p < 0.001, n = 9, 5 patients) in line with the findings in rat brain slices. Baseline pO2 of 504.4 ± 102.7 mmHg was measured in interface chamber under 95% O2 and 5% CO2. Despite the decrease in ∆pO2, there was no obvious change in baseline pO2 suggesting that lactate contribution to the basal oxidative metabolism is negligible.
Figure 1.
Effects of monocarboxylate transporter (MCT) inhibition by α-cyano-4-hydroxycinnamic acid (4-CIN) on stimulus induced extracellular tissue oxygen changes (ΔpO2), field potential responses (FP), amplitude and recovery kinetics of extracellular K+ concentration changes (Δ[K+]O). Despite the clear effect on the stimulus induced pO2 changes neither recovery kinetics of Δ[K+]O nor field potential amplitude were affected by 4-CIN. (A) Sample traces of ΔpO2 (left), field potential transients (middle) and Δ[K+]O (right) in the presence and the absence of 4-CIN. Inhibition of the MCTs decreased ΔpO2 both at (B) 200 µM and (C) 500 µM 4-CIN concentration, (D,E) whereas it did not affect field potential amplitude and (F,G) first half recovery time of Δ[K+]O for both concentrations, respectively; (B–G) Variables are given on the Y-axis, categories of treatment on the X-axis. *** p < 0.001.
Unlike in the rat hippocampus slices, the population spike components of the field potential responses were not affected by inhibition of lactate uptake (2.2 ± 0.2 mV vs. 2.0 ± 0.2 mV in control condition and during 4-CIN application respectively; Figure 1A,D; Wilcoxon signed rank test, p > 0.5, n = 8, 5 patients). In line with the missing effect on the synaptic signaling, 4-CIN did not change the recovery kinetics of stimulus induced ∆[K+]O (peak ∆[K+]O: 1.7 ± 0.1 mM; n = 10, 5 patients). Neither the peak amplitude nor the 1st half decay time, 6.4 ± 0.8 vs. 6.0 ± 0.7 s, were altered (Figure 1A,F; n = 10, 5 patients). Taking into account the potentially different equilibration times in rat and human brain slices, we have increased the concentration of 4-CIN to 500 µM. Although this concentration readily decreased stimulus induced ∆pO2 from 14.6 ± 0.8 to 10.4 ± 0.6 mmHg (Figure 1C; Wilcoxon signed rank test, p < 0.001, n = 9, 5 patients), the amplitude of the evoked field potential response and the recovery kinetics of ∆[K+]O remained unaltered (Figure 1E,G), thereby suggesting a difference in lactate use between chronic epileptic human and rat brain slices.
2.2. 4-CIN Reduced Incidence of Seizure-Like Events (SLEs) in Neocortical Slices from Patients with mTLE
Ictal increases in extracellular lactate levels have been described during acute provoked seizures in otherwise healthy brain slices and also in chronic epileptic tissue [29]. Surplus lactate could serve as a substrate to cover enhanced oxidative metabolism during seizure activity, whereas the acidotic shift associated with lactate accumulation have been speculated to contribute to the termination of seizures. Epileptiform activity, resembling seizure like events (SLEs), interictal and recurrent discharges can be readily induced in resected human neocortical slices by applying a combination of the GABAA receptor inhibitor bicuculline methiodide (50 µM) along with an increased K+ (8 mM) containing aCSF [30].
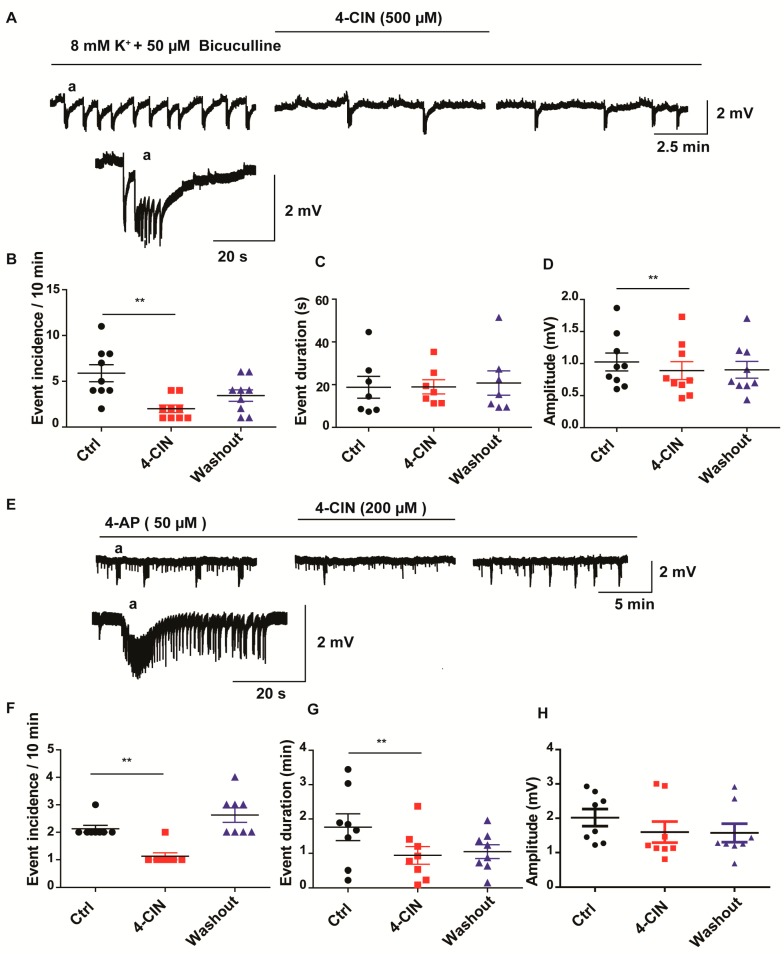
After attaining stable recurrent SLEs (first SLE appeared with a latency of 8.4 ± 4.0 min), 500 µM 4-CIN was applied for 30 min and the incidence, amplitude and duration of SLEs were evaluated. Inhibition of MCTs significantly reduced SLE incidence from 5.9 ± 0.9 to 2 ± 0.4 (Figure 2A,B; paired t-test, p < 0.01, n = 9 slices, 4 patients) and amplitude from 1.0 ± 0.1 to 0.9 ± 0.1 mV (Figure 2A,D; paired t-test, p <0.01, n = 9 slices, 4 patients) while it did not change SLE duration of the last 10 min recordings for each condition (Figure 2A,C). Notably, the effect of 4-CIN developed slowly reaching its maximum at the end of 4-CIN application. The decrease in incidence and amplitude was not due to a time dependent general decline in the propensity of the slices to generate SLEs as these parameters (incidence and amplitude) partially recovered following washout of 4-CIN to 3.4 ± 1.9 (Figure 2A,B; paired t-test, p < 0.05, n = 9 slices, 4 patients).
Figure 2.
Effects of 4-CIN on spontaneous recurrent epileptiform activity induced by elevated potassium plus bicuculline in cortical slices from mesial temporal lobe epilepsy patients (mTLE) or by 4-AP in rat medial entorhinal cortex (MEC) slices. (A) Sample field potential trace representing recurrent SLEs during induction (left) 4-CIN application (middle) and wash out (right) in neocortical slices from mTLE patients, the (a) excerpt showing a single seizure like event on different time scale; (B) Application of 4-CIN significantly decreased incidence of SLEs (C) without affecting event duration (D) but also decreased FP amplitude; (E) Sample field potential trace during seizure induction (left) 4-CIN application (middle) and wash out (right) in MEC slices from rat, (a) the excerpt showing a single seizure like event on different time scale; (F) Application of 4-CIN decreased incidence and (G) duration of SLEs without affecting (H) amplitude. (B–D,F–H) Variables are given on the Y-axis, categories of treatment on the X-axis. ** p < 0.01. Each dot represents a single data point, black dots represent control condition (seizure induction), red dots represent 4-CIN application on top of seizure inducing drugs, and blue dots represent washout phase.
2.3. Lactate Uptake Inhibitors Decreased the Incidence of Pharmacologically Induced Burst Discharges and SLEs in Rat Entorhinal Cortex-Hippocampus Slices
In the next set of experiments, we determined whether the anti-seizure effect of 4-CIN was specific to chronic epileptic tissue or it represents a general consequence of MCT inhibition. Likewise, we induced SLEs in rat medial entorhinal cortex by applying the voltage gated potassium channel blocker, 4-aminopyridine (4-AP, 50 µM). Application of 4-CIN (200 µM) after establishing regular SLEs (with a latency of 21.8 ± 8.6 min) completely stopped SLEs in 2 slices out of 10 and in the rest it decreased SLE incidence from 2.1 ± 0.1 to 1.1 ± 0.1 during the last 10 min of the control and drug exposure phases (Figure 2E,F; Wilcoxon signed rank test, p < 0.01, p = 8, 4 rats) and seizure duration from 1.8 ± 0.4 to 0.9 ± 0.3 s (Figure 2E,G; paired t-test, p < 0.01, p = 8, 4 rats), whereas it did not affect the amplitude of SLEs (Figure 2E,H). Increasing the concentration of 4-CIN to 500 µM, as applied to human brain slices, ceased the SLEs in all slices. Notably, there was a complete recovery upon washout (n = 8, 4 rats).
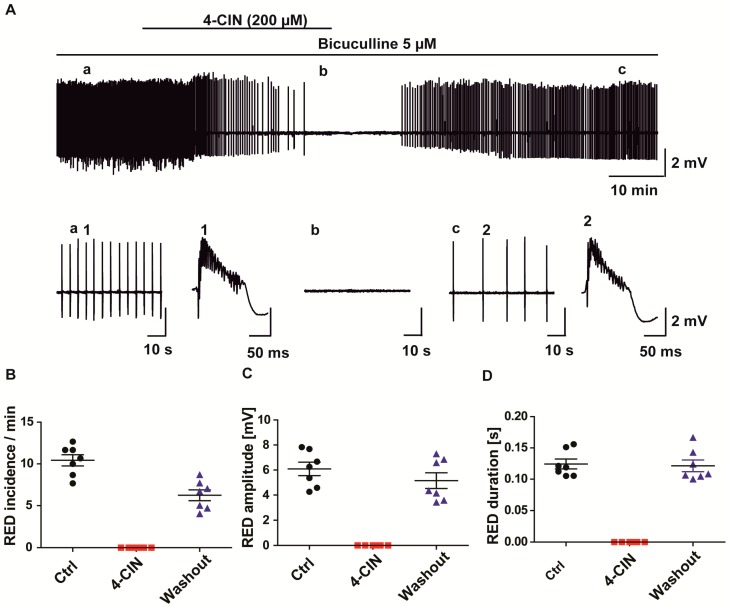
In order to exclude that the observed anti-seizure effect would depend on the type of pro-convulsive treatment (bicuculline in human, 4-AP in rat), we also extended the study to bicuculline induced epileptiform activity in rat hippocampus slices. Inhibition of GABAA receptors have been shown to induce recurrent epileptiform discharges (REDs) of shorter duration rather resembling interictal burst activity than of the SLEs induced by 4-AP [31]. By applying 5 µM bicuculline, we were able to induce REDs in the area CA3 with an amplitude of 6.1 ± 1.4 mV (Figure 3A,C), duration of 124 ± 7 ms (Figure 3A,D) and incidence of 10.4 ± 1.8/min (Figure 3B; n = 7, 3 rats). Application of 200 µM 4-CIN completely ceased REDs. During washout the incidence partially recovered while the amplitude and duration undertook complete recovery (Figure 3A–D). Thus blocking lactate uptake exerts an anti-seizure effect irrespective of the proconvulsive treatment (4-AP, bicuculline, bicuculline plus elevated potassium), species and anatomical structure (human neocortex vs. rat entorhinal cortex—hippocampus slices) as well as the type of the tissue (chronic epileptic vs. healthy control).
Figure 3.
Effect of MCT inhibition by 4-CIN on recurrent epileptiform discharges (REDs) induced by pharmacological blockade of GABAA receptors. (A) Sample traces of bicuculline induced REDs before (left, a), during (middle, b) and after 4-CIN application (right, c). Single RED trace during baseline (1) and washout (2). 4-CIN completely stopped REDs and the effect on (B) incidence (C) amplitude and (D) frequency was partly reversed during washout; (B–D) Variables are given on the Y-axis, categories of treatment on the X-axis. Each dot represents a single data point, black dots represent 5 µM bicuculline application, red dots represent 200 µM 4-CIN and 5 µM bicuculline application, blue dots represent washout in 5 µM bicuculline.
2.4. Anti-Epileptic Effect of 4-CIN Is Mediated by Adenosine through A1 Receptor But Not by Acidosis
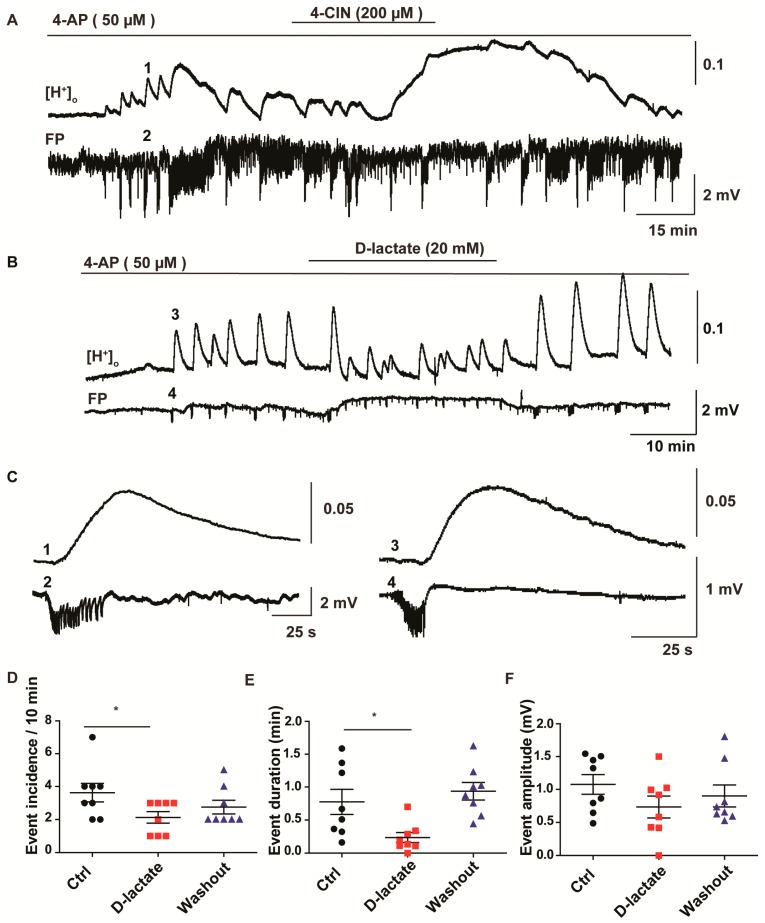
In our previous paper, we have shown that 4-CIN induces extracellular acidosis due to parenchymal lactate accumulation (Figure 4A) [22]. Whether or not this acidosis could contribute to the observed anti-seizure effect was tested by using d-lactate, the non-metabolisable isoform, to inhibit lactate transport. As it competes with l-lactate on all types of MCTs, both for release and uptake, no net lactate accumulation occurs in the parenchyma and consequently no extracellular acidosis is expected (Figure 4B). In line with our previous findings, application of 4-CIN led to an acidotic shift of 0.17 ± 0.01 pH unit also in the presence of 4-AP induced epileptiform activity in rat entorhinal cortex slices [22]. Each seizure like event (SLE) was associated with a corresponding acidotic shift (Figure 4C). Although the amplitude of individual SLE-associated pH transients decreased during the 4-CIN induced pH shift, this might be simply a consequence of the simultaneous decrease of SLE duration (Figure 4A) rather than a consequence of a genuine change in metabolism. In the subsequent set of experiments d-lactate (20 mM) application following establishment of recurrent SLEs reduced SLE incidence/10 min from 3.6 ± 0.6 to 2.1 ± 0.4 (Figure 4D; paired t-test, p < 0.05, n = 8, 4 rats) and SLE duration from 0.78 ± 0.2 to 0.24 ± 0.1 min (Figure 4E; Wilcoxon signed rank test, p < 0.05, n = 8, 4 rats) without affecting the baseline pH. SLE amplitude was slightly decreased as well but it did not reach significance within the timeframe of the application (Figure 4F). The decrease in SLE amplitude and duration was also reflected in the decrease of the SLE-associated acidotic pH transients, despite the absence of a baseline pH change (n = 6, 3 rats). Similar to the anti-seizure effect of 4-CIN, the decrease in incidence and duration was reversible upon washout of d-lactate. Thus, acidosis induced by lactate accumulation cannot be a sole cause of the negative impact of MCT inhibition on seizure incidence.
Figure 4.
Effect of MCT inhibitors—4-CIN and d-lactate—on baseline and SLE-associated changes in extracellular pH. Extracellular H+ ion concentration was measured using ion sensitive electrodes; hence the displayed traces show change in H+ ion concentration which got converted to pH units. (A) Application of 4-CIN resulted in a late onset baseline acidotic shift; (B) In contrast, application of sodium d-lactate (20 mM, osmolality and pH set as in normal aCSF) did not induce changes in baseline pH while exerting similar inhibitory effects on 4-AP induced epileptic form activity; (C) Sample trace of single SLE with corresponding pH change in different time scale. Each individual SLE was associated with small acidotic shifts in both 4-CIN (1,2) and d-lactate (3,4) experiments, traces taken from the recordings in (A,B) as shown by the corresponding number; (D) SLE incidence and (E) duration (F) but not the amplitude were significantly decreased; (D–F) Variables are given on the Y-axis, categories of treatment on the X-axis. * p < 0.05. Each dot represents a single data point, black dots represent 50 µM 4-aminopyridine (4-AP) application, red dots represent 20 mM d-lactate and 50 µM 4-AP application, blue dots represent washout in 50 µM 4-AP.
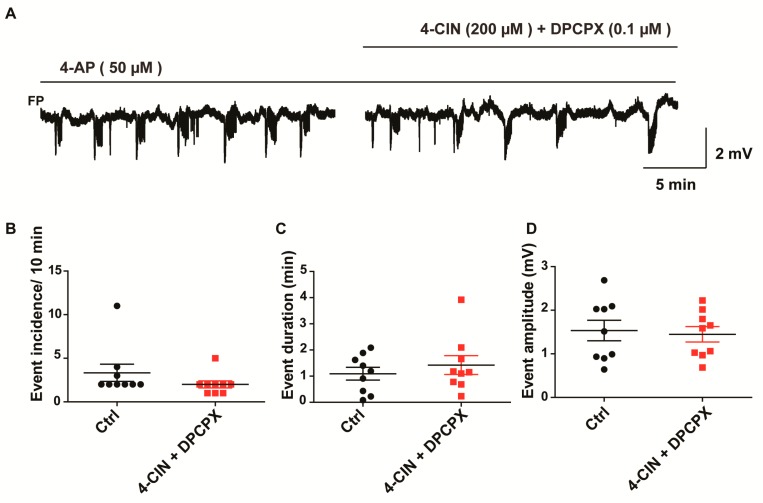
The decrease in stimulus-induced ∆pO2 upon MCT inhibition evidenced a significant contribution of lactate to oxidative metabolism both in chronic epileptic tissue and in hippocampal slices from the rat. Conditions leading to net hydrolysis of ATP increase adenosine level which plays a neuroprotective role by suppressing excitatory neurotransmission through A1 receptors [32,33]. Seizures represent a metabolic burden leading to activation of oxidative metabolism [34,35] and increase in extracellular adenosine concentration [36], which exerts antiepileptic effects even in otherwise pharmacoresistant mTLE tissue [36]. Lactate uptake inhibitors with an effect on oxidative metabolism will presumably augment changes in extracellular adenosine. Hence, we tested whether the anti-seizure effect of 4-CIN and d-lactate is mediated by activation of the A1 receptor. The specific A1 antagonist, 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, 0.1 µM), was co-applied with 4-CIN following the establishment of recurrent SLEs in the presence of 4-AP. Although the SLE incidence was still slightly reduced from 3.3 ± 0.98 to 2.0 ± 0.4 during the concomitant application of 4-CIN and DPCPX, this change did not reach significance (Figure 5A,B; Wilcoxon signed rank test, p = 0.06, n = 9, 4 rats). Neither SLE duration (1.1 ± 0.2 vs. 1.4 ± 0.4 min; Figure 5C; Wilcoxon signed rank test, p = 0.2, n = 9, 4 rats) nor the SLE amplitude (1.5 ± 0.2 vs. 1.4 ± 0.2 mV; Figure 5D; paired t-test, p = 0.3, n = 9, 4 rats) were different from the control if 4-CIN and DPCPX were applied simultaneously to the aCSF. Notably, DPCPX application in the absence of 4-CIN exacerbated 4-AP induced epileptiform activity, suggesting that A1 receptor activation contributes to normal SLE termination and this effect might be augmented following inhibition of lactate transport. Thus, the partial reversal of the antiepileptic effect of 4-CIN by DPCPX proved that the contribution of lactate to the oxidative energy metabolism exerts a pro-seizure effect.
Figure 5.
Dependence of the anti-seizure effect of MCT inhibition on the activation of A1 receptors. (A) Application of the A1 antagonist 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) in parallel with 4-CIN partially reversed the effect of MCT inhibitor on SLE (B) incidence (C) duration (D) amplitude. Variables are given on the Y-axis, categories of treatment on the X-axis. Each dot represents a single data point, black dots represent 50 µM 4-aminopyridine (4-AP) application, red dots represent 5 µM DPCPX and 50 µM 4-AP application.
3. Discussion
The main finding of the study was that inhibition of MCT mediated lactate transport exerts an anti-seizure effect via restriction of the oxidative energy metabolism and subsequent activation of A1 receptors. In contrast, acidotic shift of the tissue pH was dispensable for the effect as it could be repeated by application of d-lactate, which did not result in acidosis. Remarkably, the anti-seizure effect could be observed both in chronic epileptic brain slices from TLE patients and in entorhinal cortex-hippocampus slices from rats irrespective of the type of the pro-convulsive treatment. Despite the negative effect of the inhibition of MCTs on the oxidative energy metabolism, the recovery of the stimulus induced ion transients did not depend on neuronal lactate uptake in epileptic human cortex tissue, which is in contrast to the findings in rat hippocampus slices [22].
Application of the lactate uptake inhibitor in the hippocampus decreased oxygen consumption and induced an over-oxidation of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) pools, indicating that lactate is being used as oxidative energy substrate even in the presence of ample glucose [11,13,22]. Lactate derived ATP was used by ion transport mechanisms and for maintenance of postsynaptic signaling [22]. The changes in the MCT expression pattern observed in epilepsy patients as well as in animal models of epilepsy might hint to an altered use of lactate in chronic epileptic tissue [25,26,27,28]. While the increased expression of MCT1 and MCT2 in the neuropil may be the consequence of enhanced astrocytic anaerobic metabolism, the loss of MCT4 on astrocyte endfeet and of MCT1 on endothelial cells might indicate an impaired clearance into the bloodstream. These changes would favor the elevated lactate levels under conditions of enhanced neuronal activity when the brain is a net producer of lactate [37]. Taking into account the mitochondrial dysfunction in chronic epileptic tissue [38,39,40], whether this lactate is used as a substrate for oxidative metabolism in neurons is not known [24]. In our hands, MCT inhibition had a clear effect on stimulus induced pO2 changes in chronic epileptic tissue, pointing the relevance of lactate for oxidative energy metabolism. However, neither the amplitude of the field potentials nor the recovery kinetics of stimulus induced ion transients were altered, suggesting that these processes are not dependent on lactate derived ATP. Unfortunately, the effect of 4-CIN on the postsynaptic action potential generation might be underestimated in our model as the field potentials in the neocortical layers V/VI did not allow the clear discrimination of anti- and orthodromic population spikes and antidromic action potentials were insensitive to 4-CIN also in the rat hippocampus [22].
The most striking effect of the MCT inhibition was a decrease in the incidence of epileptiform activity up to a complete block of SLEs, irrespective of the type of the proconvulsant, i.e., 4AP, bicuculline and elevated potassium. In addition to its potential role in oxidative metabolism, an anti-seizure effect of ictal lactic acidosis has been suggested in numerous studies [41,42]. The mechanisms by which low pH may exert an anti-seizure effect might include (i) negative modulation of the N-methyl-d-aspartate (NMDA) receptor currents, (ii) inhibition of presynaptic voltage gated Ca2+ channels as well as (iii) facilitation of ecto-ATPases or increased adenosine release [41,42,43,44]. Indeed, the acidosis following 4-CIN application was associated with decreased incidence and duration of SLEs both in chronic epileptic and healthy rat brain slices. However, the anti-seizure effect was preserved also in the presence of d-lactate, which did not induce acidosis, likely due to the fact that d-lactate non-selectively inhibits both, uptake and release of lactate [13]. Thus, while acidosis is not responsible for the observed anti-seizure effect of d-lactate, it might still contribute to seizure suppression in the case of the 4-CIN treatment.
Elevated lactate by itself might alter neuronal excitability irrespective of the change in pH by acting on its G-protein coupled receptor HCA1, which reduces cellular cAMP levels [45] and inhibits action potential generation [46]. However, in our previous study the HCA1 agonist 3,5,DHBA increased orthodromic responses and stimulus associated pO2 changes which is not compatible with the expected inhibitory effect [22]. Therefore we would expect that—at least in rat brain slices—HCA1 activation is not likely to contribute to the inhibitory effect of parenchymal lactate accumulation.
Another possibility for an anti-seizure effect could be an increase in extracellular adenosine concentration, as a consequence of enhanced neuronal energy consumption in the presence of a partial metabolic restriction and pH dependent activation of ecto-ATPases. It has been reported previously that activity dependent basal adenosine tone modulates seizure activity [47,48] in a pH dependent manner [49] and individual seizures are associated with increased extracellular adenosine concentrations [36,50]. In general, increased adenosine levels contributed to the suppression of synaptic activity following events associated with metabolic stress such spreading depression [51], hypoxia [52], inhibition of glycolysis by 2-deoxy-d-glucose [53]. Indeed, application of the A1 receptor antagonist DPCPX was able to recover the effect of MCT inhibition on SLE duration and incidence. DPCPX alone exacerbated 4AP induced recurrent SLEs, suggesting that activity dependent adenosine increases might mediate seizure termination and this mechanism is augmented by restricted lactate availability. With respect to chronic epileptic tissue the relevance of A1 receptor modulation might be even larger as the enzyme adenosine kinase, which contributes to the termination of adenosine effects, is upregulated in astrocytes [54,55]. Indeed, application of a non-metabolisable A1 agonist could reverse recurrent epileptiform activity even in otherwise pharmacoresistant tissue [56].
Alternatively, substrate deprivation by MCT inhibition might also led to the activation of KATP channels, which sense the absence of lactate derived ATP, leading to hyperpolarization and suppression of postsynaptic action potential generation [22]. Whether, in our model inhibiting activation of KATP channels would be able to reverse the inhibitory effect of 4-CIN remains to be determined.
Restriction of oxidative energy metabolism by 2-deoxy-d-glucose, which also affects the lactate synthesis pathways, has been shown to have anti-epileptic effect in acute provoked seizures both in vivo and in vitro [57,58] but led to epileptogenesis in others [59]. Sada et al. 2015 has shown that inhibition of lactate dehydrogenase hyperpolarizes neurons and suppresses seizure in vivo which prompts the importance of lactate in modulating neuronal excitability [60]. Taken together these findings suggest that ictal increases in parenchymal lactate concentrations in chronic epileptic tissue are rather pro- than antiepileptic by supporting seizure associated energy metabolism.
Dietary modifications, like ketogenic diet, has been considered as an alternate treatment for certain types of drug resistant epilepsy but the mechanism of anti-epileptic action remain largely unknown [61]. One of the effects of ketogenic diet has been attributed to inhibition of glycolysis [62] which might result in a similar effect as MCT inhibition in our study. Another documented cellular adaptation in rats is an increase in the transport capacity of ketone bodies across the blood brain barrier [63], via upregulation of MCT1 expression on the brain endothelium [64]. Based on our findings, one could argue that the ketogenic diet likely restores and perhaps even increases the clearance of lactate under conditions when brain is a net producer, thereby limiting the availability of a potential energy resource.
4. Material and Methods
4.1. Slice Preparation
Animal experiments were performed on slices from 25 Wistar rats (180–250 g) in accordance with the Helsinki declaration and institutional guidelines (as approved by the State Office of Health and Social Affairs, Berlin, Germany, Lageso, T0096/02) and the animal welfare regulations of Charité. Animals were decapitated under deep anesthesia with isoflurane (3% vol/vol) and laughing gas (70% N2O, 30% O2). Horizontal hippocampal slices (400 µm thick) were prepared and transferred to an interface chamber perfused with artificial cerebrospinal fluid (aCSF) carbogenated with 95% O2, 5% CO2, at a rate of 2 mL/min. The aCSF solution is composed of NaCl (129), NaHCO3 (21 mM), glucose (10 mM), KCl (3 mM), NaH2PO4 (1.25 mM), CaCl2 (1.6 mM) and MgCl2 (1.8 mM) with an osmolarity of 295–305 mOsm.
The study in resected human tissue was performed after receiving written informed consent from epilepsy patients. All experiments were approved by the Ethics Committee of Charité-Universitätsmedizin Berlin on 01.11.2014 (EA2/111/14) and were in agreement with the Declaration of Helsinki. Transport, slicing and maintenance of the human tissue samples was carried out as described previously [30,56]. Immediately after surgery, the resected tissue was transferred to cold carbogenated transport solution (95% O2, 5% CO2) composed of (in mM) KCl 3, NaH2PO4 1.25, glucose 10, sucrose 200, MgSO4 2, MgCl2 1.6, CaCl2 1.6, and α-tocopherol 0.1 (pH 7.4, osmolality, 304 mOsmol/kg). 500 µM thick slices were prepared in transport solution and transferred to interface chamber perfused with carbogenated aCSF as specified above at a rate of 2 mL/min. Slices were left to recover for 4 h before starting an experiment.
4.2. Electrophysiology and Oxygen Recordings
DC coupled field potential, extracellular K+ ([K+]O) and pH measurements were done in layer V/VI of human neocortex and rat medial entorhinal cortex as well as in the CA3 area of the hippocampus using double barreled ion sensitive microelectrodes prepared as described in Angamo et al., 2016 [22]. For pH electrodes, the H+ sensitive side was filled with a solution consisting of (mM) 500 KCl, 64.7 NaH2PO4, and 85.3 Na2HPO4 (pH 7), while the reference side was filled with 500 mM KCl [22]. For K+ sensitive electrodes, the ion sensitive and the reference barrel were filled with 100 mM KCl and 154 NaCl solutions respectively. Then, the ion sensitive sides of K+ and H+ sensitive microelectrodes were tip filled with Potassium Ionophore I. 60031 and Hydrogen Ionophore II., Cocktail A (Fluka, Buchs, Switzerland) respectively. The sensitivity of the electrodes was tested with a tenfold calibration solution before use. Extracellular changes in K+ and pH were calculated using the modified Nernst equation [22]. Oxygen tension (pO2) was measured using the Clark-style oxygen sensor microelectrodes (tip: 10 μm; Unisense, Aarhus, Denmark) which were polarized overnight and calibrated in aCSF solution saturated with 20 and 95% O2. For stimulus induced responses, a bipolar platinum wire electrode was positioned in the white matter and a 20 Hz stimulus train was applied for 2 s every 4 min with an intensity giving rise of 80% of the maximal response. Data were recorded by using either a home-built differential amplifier for the ion-sensitive electrode or a polarographic amplifier (Chemical Microsensor II; Diamond General Development). Signals were digitized and recorded with a CED-1401 interface and the software Spike2 (Cambridge Electronic Design, Cambridge, UK) at 10 kHz for field potential and 1 kHz for potassium, pH and oxygen concentration.
4.3. Pharmacology
To inhibit the monocarboxylate transporters we used either 4-CIN (200 µM, 500 µM) or the non-metabolisable isomer sodium d-lactate (20 mM). Sodium d-lactate solution was prepared in a special aCSF with a NaCl concentration adjusted to compensate for the osmolarity change. pH of the NaHCO3/CO2 buffer system was set to 7.3 in bubbled aCSF prior to the recording. Epileptiform activity in human neocortex slices was induced by using elevated K+ (8 mM) aCSF solution and 50 µM bicuculline methiodide in the perfusion while in rat brain slices either 50 µM 4-aminopyridine (4-AP) or 5 µM bicuculline methiodide was added to the aCSF. The A1 receptor antagonist DPCPX was used at a concentration of 0.1 µM. All chemicals were bought from Sigma-Aldrich (Taufkirchen, Germany).
4.4. Data Analysis
The last 10 min recordings for each experimental condition (control, MCT inhibitor, and washout) were used to compare incidence, maximum amplitude including fast transients and duration of seizure like events in rat and human neocortex. For stimulus induced responses, we assessed the effect of 4-CIN on extracellular K+ concentration, K+ first half decay time, field potential responses and pO2. The first half decay time represents the time needed for K+ to reach half maximum concentration. To assess amplitude of field potential recordings in-built spike script is used; peak to point of maximum deflection is measured. The last 3 recordings (data points) during baseline and 4-CIN application were used for analysis. Data are presented as mean ± SEM, with a scatter plot where all values are displayed. Analysis was done using spike script and Graphipad Prism. The distribution of the data was tested for normality using D’Agostino and Pearson omnibus normality test; subsequently, t-test and Wilcoxon signed rank test were used for statistical comparison for data with normal and non-normal distribution, respectively.
Acknowledgment
This work was supported by European Union grant Framework Program 7, Development and Epilepsy—Strategies for Innovative Research to improve diagnosis (EU FP7 Desire; Grant Agreement No. 602531-1) and by DFG grant He1128/18-1 to Uwe Heinemann and by the DFG grant Ko3814/1-1 to Richard Kovács. Uwe Heinemann and his lab is supported by EXC Neurocure (EXC 257). We thank Dipl Biol Tanja Specowius for technical assistance. The authors are indebted to Jörg Rolf Paul Geiger and Zin-Juan Klaft for critical reading of the manuscript.
Author Contributions
Conception and design of research: Eskedar Ayele Angamo, Richard Kovács, Uwe Heinemann; performed experiments: Eskedar Ayele Angamo, Rizwan ul Haq, Jörg Rösner; prepared figures: Eskedar Ayele Angamo, Rizwan ul Haq; manuscript writing: Eskedar Ayele Angamo, Siegrun Gabriel, Zoltán Gerevich, Richard Kovács.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Bröer S., Rahman B., Pellegri G., Pellerin L., Martin J.L., Verleysdonk S., Hamprecht B., Magistretti P.J. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- 3.Karagiannis A., Sylantyev S., Hadjihambi A., Hosford P.S., Kasparov S., Gourine A.V. Hemichannel-mediated release of lactate. J. Cereb. Blood Flow Metab. 2015;36:1202–1211. doi: 10.1177/0271678X15611912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotelo-Hitschfeld T., Niemeyer M.I., Machler P., Ruminot I., Lerchundi R., Wyss M.T., Stobart J., Fernández-Moncada I., Valdebenito R., Garrido-Gerter P., et al. Channel-Mediated Lactate Release by K+-Stimulated Astrocytes. J. Neurosci. 2015;35:4168–41478. doi: 10.1523/JNEUROSCI.5036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mächler P., Wyss M.T., Elsayed M., Stobart J., Gutierrez R., von Faber-Castell A., Kaelin V., Zuend M., San Martín A., Romero-Gómez I., et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016;23:94–102. doi: 10.1016/j.cmet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Pellerin L., Bergersen L.H., Halestrap A.P., Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J. Neurosci. Res. 2005;79:55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- 7.Pellerin L., Bouzier-Sore A.K., Aubert A., Serres S., Merle M., Costalat R., Magistretti P.J. Activity-dependent regulation of energy metabolism by astrocytes: An update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 8.Chih C.P., He J., Sly T.S., Roberts E.L., Jr. Comparison of glucose and lactate as substrates during NMDA-induced activation of hippocampal slices. Brain Res. 2001;893:143–154. doi: 10.1016/S0006-8993(00)03306-0. [DOI] [PubMed] [Google Scholar]
- 9.Yamanishi S., Katsumura K., Kobayashi T., Puro D.G. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2005;290:H925–H934. doi: 10.1152/ajpheart.01012.2005. [DOI] [PubMed] [Google Scholar]
- 10.Galow L.V., Schneider J., Lewen A., Ta T.T., Papageorgiou I.E., Kann O. Energy substrates that fuel fast neuronal network oscillations. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galeffi F., Foster K.A., Sadgrove M.P., Beaver C.J., Turner D.A. Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J. Neurochem. 2007;103:2449–6241. doi: 10.1111/j.1471-4159.2007.04939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov A., Mukhtarov M., Bregestovski P., Mukhtarov M., Bregestovski P., Zilberter Y. Lactate Effectively Covers Energy Demands during Neuronal Network Activity in Neonatal Hippocampal Slices. Front. Neuroenerg. 2011;3 doi: 10.3389/fnene.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagase M., Takahashi Y., Watabe A.M., Kubo Y., Kato F. On-site energy supply at synapses through monocarboxylate transporters maintains excitatory synaptic transmission. J. Neurosci. 2014;34:2605–2617. doi: 10.1523/JNEUROSCI.4687-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki A., Stern S.A., Bozdagi O., Huntley G.W., Walker R.H., Magistretti P.J., Alberini C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N., Liu Y., Tsingalia A., Jin L., Zhang P.W., et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergersen L.H., Magistretti P.J., Pellerin L. Selective Postsynaptic Co-localization of MCT2 with AMPA Receptor GluR2/3 Subunits at Excitatory Synapses Exhibiting AMPA Receptor Trafficking. Cereb. Cortex. 2005;15:361–370. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- 17.Pierre K., Pellerin L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 18.Cornford E.M., Hyman S. Blood-brain barrier permeability to small and large molecules. Adv. Drug Deliv. Rev. 1999;36:145–163. doi: 10.1016/S0169-409X(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 19.Gerhart D.Z., Enerson B.E., Zhdankina O.Y., Leino R.L., Drewes L.R. Expression of the monocarboxylate transporter MCT2 by rat brain glia. Glia. 1998;22:272–281. doi: 10.1002/(SICI)1098-1136(199803)22:3<272::AID-GLIA6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Hanu R., McKenna M., O’Neill A., Resneck W.G., Bloch R.J. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Physiol. Cell Physiol. 2000;278:C921–C930. doi: 10.1152/ajpcell.2000.278.5.C921. [DOI] [PubMed] [Google Scholar]
- 21.Bergersen L., Waerhaug O., Helm J., Thomas M., Laake P., Davies A.J., Wilson M.C., Halestrap A.P., Ottersen O.P. A novel postsynaptic density protein: The monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp. Brain Res. 2001;136:523–534. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- 22.Angamo E.A., Rösner J., Liotta A., Kovács R., Heinemann U. A neuronal lactate uptake inhibitor slows recovery of extracellular ion concentration changes in the hippocampal CA3 region by affecting energy metabolism. J. Neurophysiol. 2016;116:2420–2430. doi: 10.1152/jn.00327.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel J., Kuhl D.E., Phelps M.E. Patterns of human local cerebral glucose metabolism during epileptic seizures. Science. 1982;218:64–66. doi: 10.1126/science.6981843. [DOI] [PubMed] [Google Scholar]
- 24.Cavus I., Kasoff W.S., Cassaday M.P., Jacob R., Gueorguieva R., Sherwin R.S., Krystal J.H., Spencer D.D., Abi-Saab W.M. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann. Neurol. 2005;57:226–235. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- 25.Lauritzen F., de Lanerolle N.C., Lee T.S., Spencer D.D., Kim J.H., Bergersen L.H., Eid T. Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol. Dis. 2011;41:577–584. doi: 10.1016/j.nbd.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauritzen F., Perez E.L., Melillo E.R., Roh J.M., Zaveri H.P., Lee T.S., Wang Y., Bergersen L.H., Eid T. Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol. Dis. 2012;45:165–176. doi: 10.1016/j.nbd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B., Niu L., Shen M.Z., Gao L., Wang C., Li J., Song L.J., Tao Y., Meng Q., Yang Q.L., Gao G.D., Zhang H. Decreased Astroglial Monocarboxylate Transporter 4 Expression in Temporal Lobe Epilepsy. Mol. Neurobiol. 2014;50:327–338. doi: 10.1007/s12035-013-8619-z. [DOI] [PubMed] [Google Scholar]
- 28.Lauritzen F., Heuser K., de Lanerolle N.C., Lee T.S., Spencer D.D., Kim J.H., Gjedde A., Eid T., Bergersen L.H. Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia. 2012;60:1172–1181. doi: 10.1002/glia.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.During M.J., Fried I., Leone P., Katz A., Spencer D.D. Direct measurement of extracellular lactate in the human hippocampus during spontaneous seizures. J. Neurochem. 1994;62:2356–2361. doi: 10.1046/j.1471-4159.1994.62062356.x. [DOI] [PubMed] [Google Scholar]
- 30.Antonio L.L., Anderson M.L., Angamo E.A., Gabriel S., Klaft Z.J., Liotta A., Salar S., Sandow N., Heinemann U. In vitro seizure like events and changes in ionic concentration. J. Neurosci. Methods. 2016;260:33–44. doi: 10.1016/j.jneumeth.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Liotta A., Caliskan G., ul Haq R., Hollnagel J.O., Rösler A., Heinemann U., Behrens C.J. Partial Disinhibition Is Required for Transition of Stimulus-Induced Sharp Wave-Ripple Complexes Into Recurrent Epileptiform Discharges in Rat Hippocampal Slices. J. Neurophysiol. 2011;105:172–187. doi: 10.1152/jn.00186.2010. [DOI] [PubMed] [Google Scholar]
- 32.Cunha R.A., Sebastião A.M., Ribeiro J.A. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J. Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunha R.A. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacs R., Schuchmann S., Gabriel S., Kardos J., Heinemann U. Ca2+ signalling and changes of mitochondrial function during low-Mg2+-induced epileptiform activity in organotypic hippocampal slice cultures. Eur. J. Neurosci. 2001;13:1311–1319. doi: 10.1046/j.0953-816x.2001.01505.x. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs R., Schuchmann S., Gabriel S., Kann O., Kardos J., Heinemann U. Free Radical-Mediated Cell Damage after Experimental Status Epilepticus in Hippocampal Slice Cultures. J. Neurophysiol. 2002;88:2909–2918. doi: 10.1152/jn.00149.2002. [DOI] [PubMed] [Google Scholar]
- 36.Van Gompel J.J., Bower M.R., Worrell G.A., Stead M., Chang S.Y., Goerss S.J., Kim I., Bennet K.E., Meyer F.B., Marsh W.R., et al. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia. 2014;55:233–244. doi: 10.1111/epi.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandhi G.K., Cruz N.F., Ball K.K., Dienel G.A. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J. Neurochem. 2009;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kann O., Kovács R., Njunting M., Behrens C.J., Otáhal J., Lehmann T.N., Gabriel S., Heinemann U. Metabolic dysfunction during neuronal activation in the ex vivo hippocampus from chronic epileptic rats and humans. Brain. 2005;128:2396–2407. doi: 10.1093/brain/awh568. [DOI] [PubMed] [Google Scholar]
- 39.Kunz W.S., Kudin A.P., Vielhaber S., Blümcke I., Zuschratter W., Schramm J., Beck H., Elger C.E. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann. Neurol. 2000;48:766–773. doi: 10.1002/1531-8249(200011)48:5<766::AID-ANA10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 40.Zsurka G., Kunz W.S. Mitochondrial dysfunction and seizures: The neuronal energy crisis. Lancet Neurol. 2015;14:956–966. doi: 10.1016/S1474-4422(15)00148-9. [DOI] [PubMed] [Google Scholar]
- 41.Ziemann A.E., Schnizler M.K., Albert G.W., Severson M.A., Howard M.A., Welsh M.J., Wemmie J.A. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velísek L., Dreier J.P., Stanton P.K., Heinemann U., Moshé S.L. Lowering of extracellular pH suppresses low-Mg(2+)-induces seizures in combined entorhinal cortex-hippocampal slices. Exp. Brain Res. 1994;101:44–52. doi: 10.1007/BF00243215. [DOI] [PubMed] [Google Scholar]
- 43.Tang C.M., Dichter M., Morad M. Modulation of the N-methyl-d-aspartate channel by extracellular H+ Proc. Natl. Acad. Sci. USA. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dulla C.G., Dobelis P., Pearson T., Frenguelli B.G., Staley K.J., Masino S.A. Adenosine and ATP Link PCO2 to Cortical Excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauritzen K.H., Morland C., Puchades M., Holm-Hansen S., Hagelin E.M., Lauritzen F., Attramadal H., Storm-Mathisen J., Gjedde A., Bergersen L.H. Lactate Receptor Sites Link Neurotransmission, Neurovascular Coupling, and Brain Energy Metabolism. Cereb. Cortex. 2014;24:2784–2795. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- 46.Bozzo L., Puyal J., Chatton J.Y. Lactate Modulates the Activity of Primary Cortical Neurons through a Receptor-Mediated Pathway. PLoS ONE. 2013;8:e71721. doi: 10.1371/journal.pone.0071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.During M.J., Spencer D.D. Adenosine: A potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 48.Lee K.S., Schubert P., Heinemann U. The anticonvulsive action of adenosine: A postsynaptic, dendritic action by a possible endogenous anticonvulsant. Brain Res. 1984;321:160–164. doi: 10.1016/0006-8993(84)90694-2. [DOI] [PubMed] [Google Scholar]
- 49.Dulla C.G., Frenguelli B.G., Staley K.J., Masino S.A. Intracellular Acidification Causes Adenosine Release During States of Hyperexcitability in the Hippocampus. J. Neurophysiol. 2009;102:1984–1993. doi: 10.1152/jn.90695.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frenguelli B.G., Wall M.J. Combined electrophysiological and biosensor approaches to study purinergic regulation of epileptiform activity in cortical tissue. J. Neurosci. Methods. 2016;260:202–214. doi: 10.1016/j.jneumeth.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Lindquist B.E., Shuttleworth C.W. Adenosine receptor activation is responsible for prolonged depression of synaptic transmission after spreading depolarization in brain slices. Neuroscience. 2012;223:365–376. doi: 10.1016/j.neuroscience.2012.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jarosch M.S., Gebhardt C., Fano S., Huchzermeyer C., Ul Haq R., Behrens C.J. Heinemann U Early adenosine release contributes to hypoxia-induced disruption of stimulus-induced sharp wave-ripple complexes in rat hippocampal area CA3. Eur. J. Neurosci. 2015;42:1808–1817. doi: 10.1111/ejn.12941. [DOI] [PubMed] [Google Scholar]
- 53.Brennan A.M., Connor J.A., Shuttleworth C.W. Modulation of the amplitude of NAD(P)H fluorescence transients after synaptic stimulation. J. Neurosci. Res. 2007;85:3233–3243. doi: 10.1002/jnr.21288. [DOI] [PubMed] [Google Scholar]
- 54.Aronica E., Zurolo E., Iyer A., de Groot M., Anink J., Carbonell C., van Vliet E.A., Baayen J.C., Boison D., Gorter J.A. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia. 2011;52:1645–1655. doi: 10.1111/j.1528-1167.2011.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boison D. Adenosine and Epilepsy: From Therapeutic Rationale to New Therapeutic Strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- 56.Klaft Z.J., Hollnagel J.O., Salar S., Calişkan G., Schulz S.B., Schneider U.C., Horn P., Koch A., Holtkamp M., Gabriel S., et al. Adenosine A1 receptor-mediated suppression of carbamazepine-resistant seizure-like events in human neocortical slices. Epilepsia. 2016;57:746–756. doi: 10.1111/epi.13360. [DOI] [PubMed] [Google Scholar]
- 57.Stafstrom C.E., Roopra A., Sutula T.P. Seizure suppression via glycolysis inhibition with 2-deoxy-d-glucose (2DG) Epilepsia. 2008;49:97–100. doi: 10.1111/j.1528-1167.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 58.Shao L.R., Stafstrom C.E. Glycolytic inhibition by 2-deoxy-d-glucose abolishes both neuronal and network bursts in an in vitro seizure model. J. Neurophysiol. 2017;118:103–113. doi: 10.1152/jn.00100.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samokhina E., Popova I., Malkov A., Ivanov A.I., Papadia D., Osypov A., Molchanov M., Paskevich S., Fisahn A., Zilberter M., et al. Chronic inhibition of brain glycolysis initiates epileptogenesis. J. Neurosci. Res. 2017 doi: 10.1002/jnr.24019. [DOI] [PubMed] [Google Scholar]
- 60.Sada N., Lee S., Katsu T., Otsuki T., Inoue T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–1367. doi: 10.1126/science.aaa1299. [DOI] [PubMed] [Google Scholar]
- 61.Bough K.J., Rho J.M. Anticonvulsant Mechanisms of the Ketogenic Diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 62.Rogawski M.A., Löscher W., Rho J.M. Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb. Perspect. Med. 2016;6:a022780. doi: 10.1101/cshperspect.a022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daniel P.M., Love E.R., Moorhouse S.R., Pratt O.E. The influence of age on the influx of ketone bodies into the brain of the rat. J. Physiol. 1977;268:15P–16P. [PubMed] [Google Scholar]
- 64.Leino R.L., Gerhart D.Z., Duelli R., Enerson B.E., Drewes L.R. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 2001;38:519–527. doi: 10.1016/S0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]