Abstract
Being an Al-accumulating crop, buckwheat detoxifies and tolerates Al not only in roots but also in leaves. While much progress has recently been made toward Al toxicity and resistance mechanisms in roots, little is known about the molecular basis responsible for detoxification and tolerance processes in leaves. Here, we carried out transcriptome analysis of buckwheat leaves in response to Al stress (20 µM, 24 h). We obtained 33,931 unigenes with 26,300 unigenes annotated in the NCBI database, and identified 1063 upregulated and 944 downregulated genes under Al stress. Functional category analysis revealed that genes related to protein translation, processing, degradation and metabolism comprised the biological processes most affected by Al, suggesting that buckwheat leaves maintain flexibility under Al stress by rapidly reprogramming their physiology and metabolism. Analysis of genes related to transcription regulation revealed that a large proportion of chromatin-regulation genes are specifically downregulated by Al stress, whereas transcription factor genes are overwhelmingly upregulated. Furthermore, we identified 78 upregulated and 22 downregulated genes that encode transporters. Intriguingly, only a few genes were overlapped with root Al-regulated transporter genes, which include homologs of AtMATE, ALS1, STAR1, ALS3 and a divalent ion symporter. In addition, we identified a subset of genes involved in development, in which genes associated with flowering regulation were important. Based on these data, it is proposed that buckwheat leaves develop conserved and distinct mechanisms to cope with Al toxicity.
Keywords: aluminum toxicity, buckwheat, cell wall, chromatin modification, transcription regulation, transporter
1. Introduction
Acidic soils comprise approximately 50% of potentially arable lands worldwide, and only 4.5% of the total acidic soil area is exploited for agriculture [1]. Among the various factors limiting growth in acidic soils, the high Al concentrations are considered the major constraint to plant productivity [2]. Nonetheless, there is a great variation among different plant species, or cultivars within the same species, in resistance to Al toxicity [3,4].
Al resistance refers to the ability of the plant to maintain productivity at elevated Al concentrations, relying on either Al avoidance or Al tolerance [5]. In general, most crop plants resist Al toxicity by means of Al avoidance, whereby Al is excluded from the growing root tip through exudation of Al-chelating ligands such as organic acid anions. Thus, the Al concentration in aboveground parts is very low (<100 µg·(g·dry·wt)−1) [6,7,8]. Species that accumulate Al, by contrast, can accumulate more than 1000 mg·kg−1 A1 in the leaves [7]. For example, Matsumoto et al. reported that tea (Camellia sinensis) plants grown in the field can accumulate 30,000 mg·kg−1 A1 in old leaves, although only 600 mg·kg−1 A1 in young leaves [9]. After 6 weeks cultured in a nutrient solution containing 0.5 mM Al, Melastoma malabathricum accumulated 10,000 mg·kg−1 Al and 7000 mg·kg−1 Al in the old and young leaves, respectively [10]. Hydrangea (Hydrangea macrophylla) plants can accumulate 5000 mg·kg−1 Al in the leaves [11]. However, the molecular mechanisms by which these plant species accumulate and tolerate high concentrations of Al in leaves remain poorly understood.
Buckwheat (Fagopyrum esculentum Moench) grows well in acidic soils by means of different strategies to deal with Al toxicity. Buckwheat secretes oxalate rapidly from the root tip, in response to Al toxicity, to chelate rhizosphere Al and prevent Al entry into the root tip cells [12,13,14]. Once taken up, Al is bound as an Al-oxalate complex that is either compartmentalized into vacuoles or is transported radially into the xylem. Thus, buckwheat has evolved both avoidance and tolerance mechanisms to cope with Al toxicity in roots. In addition, buckwheat is characterized as an Al accumulator [15]. Buckwheat leaves accumulate more than 400 mg·kg−1 Al after 5 days cultured in nutrient solution containing Al [15], and as much as 15,000 mg·kg−1 Al when grown in an acidic soil [16]. Thus, in addition to Al resistance in the root, buckwheat is also able to accumulate Al within leaves, thereby resisting Al in the leaves. These features rank buckwheat as an excellent model for unravelling Al resistance mechanisms in plants, which will be important for the genetic improvement of Al resistance in other crops through biotechnological approaches.
Recently, Yokosho et al. carried out transcriptome analysis of buckwheat leaves in response to short-term moderate Al stress, and singled out possible transporter genes involved in Al resistance in buckwheat leaves [17]. Among 25 candidates, only FeSTAR1 (sensitive to Al rhizotoxicity 1) might be involved in Al resistance by interacting with ALS3 (aluminum sensitive 3) to protect the cell wall from damaging Al interactions [18]. Therefore, it remains largely unknown how buckwheat leaves accumulate and tolerate Al.
In the present study, we carried out genome-wide transcriptome analysis of Al-regulated genes in buckwheat leaves. We identified a total of 33,931 unigenes from buckwheat leaves. Functional classification of Al-responsive genes revealed critical events involved in the adaptation to Al stress in buckwheat leaves. By comparing genes encoding transcription factors (TFs) and transporters between roots and leaves, we not only reveal some conserved mechanisms of Al resistance, but also suggest candidate genes which play distinct roles in these mechanisms.
2. Results
2.1. Buckwheat Leaves Rapidly Accumulate Al
In order to carry out RNA sequencing (RNA-seq) of buckwheat leaves in response to Al stress, we first examined the ability of buckwheat leaves to accumulate Al. To reach this purpose, we compared Al concentrations in roots and leaves between rice bean and buckwheat. Rice bean is an Al excluder that does not accumulate Al in aboveground parts [19], but buckwheat is an Al accumulator [15]. There was a little amount of Al in the roots and shoots in the control treatment, but no differences were observed between rice bean and buckwheat (Figure S1). After treatment with 20 µM Al for 24 h, both rice bean and buckwheat accumulated a substantial amount of Al in their roots (Figure S1A). It seems that buckwheat roots accumulated slightly less (but not significantly less) Al than did rice bean roots (Figure S1). However, the Al concentration in buckwheat leaves was about six times higher than that of rice bean (Figure S1B), confirming that buckwheat can efficiently translocate Al from roots to leaves. We have previously demonstrated that an Al concentration of 20 µM results in the moderate inhibition of root elongation [20]. Thus, we employed an Al concentration of 20 µM Al and a treatment time of 24 h for the construction of RNA-seq libraries.
2.2. De Novo Assembly of the Transcripts and Annotation
After quality control, we obtained approximately 89.5 and 59.1 million clean reads with a mean length of 80 bp from the leaves of the control (−Al) library and Al-treated (+Al) library, respectively (Table 1). The complementary DNA sequence was de novo assembled based on Trinity software, because the buckwheat genome remains unsequenced [21]. We assembled a total of 50,782 transcripts with a mean length of 716 bp, and 33,931 unigenes with a mean length of 677 bp (Table 1). BLASTx was adopted to annotate unigenes in the NCBI non-redundant protein (Nr) database, with a cut-off E-value of 10−5. The result was a total of 26,300 unigenes (77.96%) with high sequence identity to known genes (Table S1).
Table 1.
Summary for the buckwheat leaf transcriptome in control (−Al) and Al-treated (+Al) libraries.
| Assembly Summary | −Al | +Al |
|---|---|---|
| Total number of reads | 89,531,252 | 59,073,878 |
| Total base pairs (bp) | 7,162,500,160 | 4,725,910,240 |
| Average read length (bp) | 80 | 80 |
| Total number of transcripts | 50,782 | |
| Mean length of transcripts | 716 | |
| Total number of unigenes | 33,931 | |
| Mean length of unigenes | 677 | |
| Sequence with E-value | 26,300 | |
2.3. Global Effect of Al Stress on Gene Expression
The changes in the expression of the unigenes was calculated by the reads per kilobase of exon per million mapped reads (RPKM) method [22]. To understand the effects of Al stress on global gene expression in buckwheat leaves, those genes with log2 FC (fold change) ≥ 1 and with absolute values of RPKM ≥ 10 were considered as differentially expressed genes (DEGs). On this basis, a total of 2007 unigenes were considered as DEGs under Al stress, with 1063 and 944 unigenes being up- and downregulated, respectively (Table S2).
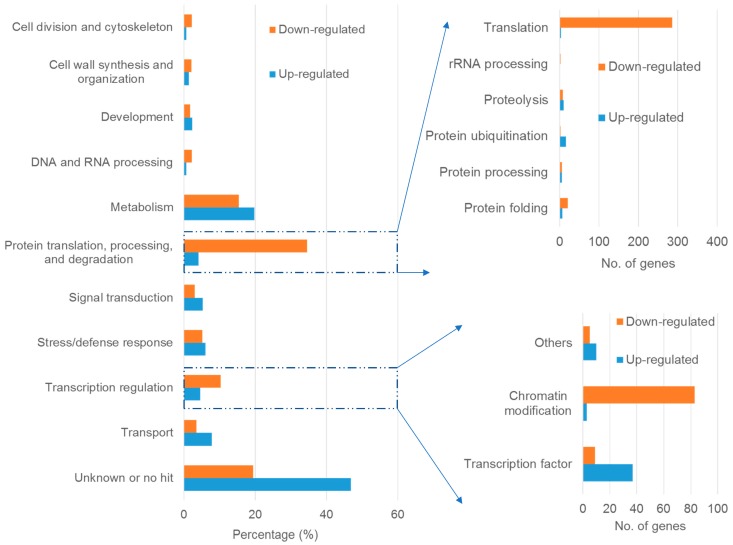
To obtain an overview of these DEGs, BLAST analysis was performed against The Arabidopsis Information Resource (TAIR) database (Available online: http://www.arabidopsis.org/Blast/index.jsp), such that each gene homolog was classified into a specific functional category based on the TAIR gene ontology (GO) biological process (Figure 1). Of the upregulated DEGs, 46.8% were annotated as unknown function or had no hit, and of the downregulated DEGs, 19.4% were annotated as unknown function or had no hit. In the upregulated DEGs, ‘metabolic process’ was the most represented category, which is consistent with our RNA-seq analysis in the buckwheat root tip [20]. The following most abundant categories were ‘transport’, ‘stress/defense response’, and ‘signal transduction’. By contrast, in the downregulated DEGs, genes related to ‘protein translation, processing and degradation’ constituted the most affected category, which is in accordance with our previous study in rice bean [23]. Following were genes involved in ‘metabolism’, ‘transcription regulation’ and ‘stress/defense response’. Except for the downregulated genes involved in ‘protein translation, processing and degradation’, genes related to ‘metabolic process’ were the most affected by Al stress both in up- and downregulated genes, suggesting that buckwheat leaves rapidly reprogram their metabolism to cope with Al toxicity.
Figure 1.
Functional categories of up- and downregulated DEGs. The categorization was performed according to the gene ontology (GO) biological process. The percentage or number of genes in each category is shown.
2.4. Genes Involved in Protein Translation, Processing and Degradation
The proportion of genes associated with ‘protein translation, processing and degradation’ dramatically differentiates the upregulated and downregulated genes (Figure 1). Particularly prominent are genes encoding ribosomal proteins, which are almost exclusively downregulated by Al stress. For example, the expression of 284 genes that encode ribosomal proteins was reduced, whereas only four related genes displayed the opposite pattern (Table S2). On the other hand, genes associated with protein degradation, including protein ubiquitination and proteolysis, were relatively more abundant in upregulated genes than downregulated genes. In accordance with the present results, our previous transcriptional analysis of the rice bean root apex also demonstrated that genes related to ‘protein translation, processing and degradation’ were substantially downregulated by moderate Al stress, in which root elongation was not significantly affected [23]. It appears that the reprogramming of protein metabolism represents a flexibility of plants in response to Al stress.
2.5. Genes Involved in Transcription Regulation
Transcriptional responses of plants to environmental stresses have been extensively investigated, and TFs regulate the expression of genes at the transcriptional level [24]. We identified a total of 49 TF genes (40 upregulated and 9 downregulated) from buckwheat leaf DEGs affected by Al stress (Table 2). The Al-upregulated TFs were categorized into 16 TF families according to the TF family classification proposed by Mitsuda and Ohme-Takagi [24]. By contrast, the downregulated TF genes belong to five TF families. Among these TF genes, a gene (comp30095_c0_seq1) encoding ATAF1 (a NAM, ATAF, and CUC (NAC) TF) was consistently upregulated in both root tips and leaves. However, a gene (comp7947_c0_seq1) encoding a C2H2-like zinc finger TF was downregulated by Al stress in buckwheat leaves. The difference in the transcription regulation of TF genes suggests that transcriptional regulation mechanisms differ between the root apex and leaves under Al stress.
Table 2.
Al-regulated genes encoding transcription factors.
| Gene ID | Fold Change | TAIR ID | Description | Category 1 | Biological Processes 2 |
|---|---|---|---|---|---|
| Upregulated | |||||
| comp24276_c0_seq2 | 1.391 | AT1G46768 | Related to AP2 1 | AP2-EREBP | Ethylene-activated signaling pathway, response to cold, response to water deprivation |
| comp28160_c0_seq1 | 1.029 | AT4G36900 | Related to AP2 10 | AP2-EREBP | Ethylene-activated signaling pathway |
| comp27009_c0_seq1 | 1.045 | ||||
| comp4672_c0_seq1 | 1.329 | AT1G76110 | HMG (high mobility group) box protein with ARID/BRIGHT DNA-binding domain | ARID | Karyogamy, polar nucleus fusion |
| comp13872_c0_seq1 | 1.257 | ||||
| comp28434_c0_seq1 | 1.029 | AT5G49700 | AT-HOOK motif nuclear localized protein 17 | AT-hook | Unknown |
| comp20160_c0_seq1 | 1.651 | AT5G43700 | IAA4 | AUX/IAA | Response to auxin |
| comp32831_c0_seq1 | 1.326 | ||||
| comp27076_c0_seq2 | 1.084 | AT1G09530 | Phytochrome interacting factor 3 | bHLH | De-etiolation, gibberellic acid-mediated signaling pathway, positive regulation of anthocyanin metabolic process, red or far-red light signaling pathway |
| comp17528_c0_seq2 | 1.750 | AT3G19860 | BHLH121 | bHLH | Unknown |
| comp2972_c0_seq1 | 1.898 | ||||
| comp28503_c0_seq1 | 1.511 | AT5G61270 | Basic helix-loop-helix (bHLH) phytochrome interacting factor | bHLH | De-etiolation, red, far-red light phototransduction |
| comp16875_c0_seq1 | 1.294 | AT5G67060 | Encodes a bHLH transcription factor | bHLH | Carpel formation, gynoecium development, regulation of auxin polar transport, transmitting tissue development |
| comp24549_c0_seq1 | 1.005 | AT3G17609 | HY5-homolog | bZIP | Red, far-red light phototransduction, response to UV-B, response to karrikin |
| comp21671_c0_seq1 | 1.015 | ||||
| comp31175_c0_seq1 | 1.074 | AT5G28770 | Basic leucine zipper 63 | bZIP | Response to abscisic acid, response to glucose |
| comp30394_c0_seq1 | 1.113 | ||||
| comp18077_c0_seq1 | 1.106 | AT1G68520 | B-Box domain protein 14 | C2C2-CO-LIKE | Unknown |
| comp31981_c0_seq1 | 1.432 | AT5G24930 | Constans-like 4 | C2C2-CO-LIKE | Regulation of flower development |
| comp31991_c0_seq2 | 1.037 | AT5G57660 | Constans-like 5 | C2C2-CO-LIKE | Regulation of flower development |
| comp31991_c0_seq1 | 1.291 | ||||
| comp5736_c0_seq1 | 1.243 | AT3G21270 | DOF zinc finger protein 2 | C2C2-Dof | Unknown |
| comp13725_c0_seq1 | 1.216 | ||||
| comp60617_c0_seq1 | 1.144 | AT3G47500 | Cycling DOF factor 3 | C2C2-Dof | Flowering |
| comp27353_c0_seq1 | 1.010 | AT2G05160 | CCCH-type zinc finger family protein with RNA-binding domain | C3H | Unknown |
| comp30562_c0_seq1 | 1.351 | AT2G20570 | Golden 2-like 1 | G2-like | Chloroplast organization, negative regulation of flower development, negative regulation of leaf senescence, regulation of chlorophyll biosynthetic process |
| comp32323_c0_seq1 | 1.064 | AT3G04450 | Homeodomain-like superfamily protein | G2-like | Unknown |
| comp25041_c0_seq1 | 1.177 | AT2G46680 | Homeobox 7 | HB | Response to abscisic acid, response to water deprivation |
| comp28163_c0_seq1 | 1.160 | ||||
| comp32393_c0_seq2 | 1.239 | AT5G04760 | Duplicated homeodomain-like superfamily protein | MYB | Unknown |
| comp110794_c0_seq1 | 1.074 | AT2G46830 | Circadian clock-associated 1 | MYB-related (flower) | Flowering |
| comp80633_c0_seq1 | 1.173 | ||||
| comp30095_c0_seq1 | 1.245 | AT1G01720 | ATAF1 | NAC | Negative regulation of abscisic acid-activated signaling pathway |
| comp47425_c0_seq1 | 2.096 | AT3G15500 | ANAC055 | NAC | Jasmonic acid-mediated signaling pathway, response to water deprivation |
| comp26124_c0_seq1 | 2.605 | AT3G15510 | ANAC056 | NAC | Jasmonic acid-mediated signaling pathway, response to water deprivation |
| comp27368_c0_seq1 | 1.295 | AT1G32700 | PLATZ transcription factor family protein | PLATZ | Unknown |
| comp28592_c0_seq2 | 1.021 | AT4G17900 | PLATZ transcription factor family protein | PLATZ | Unknown |
| comp83456_c0_seq1 | 1.048 | AT4G02020 | SET domain-containing protein 10 | SET | Endosperm development |
| comp94475_c0_seq1 | 1.981 | AT5G13080 | WRKY75 | WRKY | Atrichoblast differentiation, lateral root development, induced by Pi starvation |
| comp87094_c0_seq1 | 2.190 | ||||
| Downregulated | |||||
| comp23084_c0_seq1 | −1.004 | AT4G34590 | Basic leucine-zipper 11 | bZIP | Response to sucrose |
| comp7947_c0_seq1 | −1.088 | AT1G75710 | C2H2-like zinc finger protein | C2H2 | Unknown |
| comp18294_c0_seq1 | −1.002 | AT5G28640 | GRF1-interacting factor 1 | GIF | Adaxial/abaxial pattern specification, leaf development |
| comp30187_c0_seq2 | −1.260 | AT4G24540 | Agamous-like 24 | MADS | Flowering |
| comp30099_c0_seq1 | −1.224 | ||||
| comp30187_c0_seq1 | −1.219 | ||||
| comp17187_c0_seq1 | −1.042 | AT1G73230 | Nascent polypeptide-associated complex NAC | TF_GTF | Response to salt stress |
| comp27260_c0_seq1 | −1.051 | ||||
| comp27190_c0_seq1 | −1.004 | ||||
1 Transcription factor category was assigned based on Mitsuda and Ohme-Takagi [24]; 2 biological processes were assumed according to TAIR gene ontology (GO) analysis.
Accumulating evidence suggests that chromatin modification is involved in transcriptional responses [25]. Among 97 downregulated genes involved in transcriptional regulation, 83 were involved in chromatin remodeling and modification. By contrast, only three genes were identified to be involved in chromatin modification out of 50 upregulated transcription regulation genes (Table S2). In our previous RNA-seq analysis of the buckwheat root tip under Al stress, no chromatin remodeling and modification genes were identified [20], suggesting that the chromatin context of transcriptional regulation is specifically involved in the response of buckwheat leaves to Al stress.
2.6. Genes Encoding Transporters
We identified 100 genes from buckwheat leaves that encode transporters, of which 78 were upregulated by Al stress and 22 were downregulated (Table 3). These transporter proteins have diverse roles according to their potential substrate specificity. Noticeably, several genes were found to be conservatively upregulated in both the root tips and leaves (Table 3; Figure 2). These genes include one encoding a divalent ion symporter, three encoding ALS1, four encoding homologs to AtMATE, two encoding STAR1 and two genes encoding ALS3 (Figure 2). Interestingly, five aquaporin genes belonging to the plasma membrane intrinsic proteins (PIPs), the tonoplast intrinsic proteins (TIPs) and the small basic intrinsic proteins (SIPs) subfamilies of the aquaporin family were found to be downregulated, whereas one gene encoding PIP1;5 was transcriptionally upregulated by Al stress (Table 3).
Table 3.
Al-regulated genes that encode putative transporters.
| Gene ID | Fold Change | TAIR ID | Description | Transport Substrate 1 |
|---|---|---|---|---|
| Upregulated | ||||
| comp31346_c0_seq4 | 1.044 | AT1G02260 | Divalent ion symporter | Al |
| comp29520_c0_seq1 | 1.250 | AT5G39040 | Aluminum-sensitive 1 | Al |
| comp3719_c0_seq1 | 1.065 | |||
| comp32696_c0_seq2 | 1.060 | |||
| comp18915_c0_seq1 | 1.333 | AT2G41190 | Transmembrane amino acid transporter family protein | Amino acid |
| comp14037_c0_seq1 | 1.054 | |||
| comp18529_c0_seq1 | 1.266 | AT4G01100 | Adenine nucleotide transporter 1 | AMP; ADP; ATP |
| comp3758_c0_seq1 | 1.016 | AT5G17400 | Endoplasmic reticulum-adenine nucleotide transporter 1 | AMP; ADP; ATP |
| comp29175_c0_seq1 | 1.263 | AT5G15410 | Cyclic nucleotide gated channel 2 | Ca2+ |
| comp29835_c0_seq1 | 1.124 | |||
| comp15571_c0_seq1 | 1.857 | AT2G23790 | Protein of unknown function (DUF607) | Ca2+ |
| comp9425_c0_seq1 | 1.860 | |||
| comp68193_c0_seq1 | 1.851 | |||
| comp31329_c0_seq1 | 1.403 | AT5G61520 | Major facilitator superfamily protein | Carbohydrate |
| comp27968_c0_seq1 | 1.499 | |||
| comp29178_c0_seq1 | 1.403 | AT5G18840 | Major facilitator superfamily protein | Carbohydrate |
| comp7291_c0_seq1 | 1.167 | |||
| comp23114_c0_seq1 | 1.510 | |||
| comp98352_c0_seq1 | 1.678 | AT1G51340 | MATE efflux family protein | Citrate |
| comp11267_c0_seq1 | 2.039 | |||
| comp96590_c0_seq1 | 1.764 | |||
| comp88250_c0_seq1 | 1.705 | |||
| comp81877_c0_seq1 | 1.967 | AT3G21090 | ABCG15 | Cutin |
| comp56152_c0_seq1 | 2.033 | |||
| comp96281_c0_seq1 | 2.336 | |||
| comp22596_c0_seq2 | 1.350 | AT5G16150 | Putative plastidic glucose transporter | Glucose |
| comp22596_c0_seq1 | 1.314 | |||
| comp25276_c0_seq1 | 1.121 | |||
| comp49196_c0_seq1 | 2.598 | AT3G47960 | NRT1/ PTR family 2.10 | Glucosinolate |
| comp26699_c0_seq1 | 2.651 | |||
| comp28002_c0_seq1 | 2.654 | |||
| comp91186_c0_seq1 | 3.385 | |||
| comp24147_c0_seq1 | 1.022 | AT4G24120 | Yellow stripe-like 1 | Iron-nicotianamine; oligopeptide |
| comp106142_c0_seq1 | 1.425 | AT1G31120 | K+ uptake permease 10 | K+ |
| comp30691_c0_seq1 | 1.012 | AT2G35060 | K+ uptake permease 11 | K+ |
| comp12992_c0_seq1 | 2.012 | AT3G02850 | Stellar K+ outward rectifier | K+ |
| comp16659_c0_seq1 | 1.954 | AT5G37500 | Gated outwardly-rectifying K+ channel | K+ |
| comp84025_c0_seq1 | 2.025 | K+ | ||
| comp3908_c0_seq1 | 1.328 | AT5G46240 | Potassium channel protein (KAT1) | K+ |
| comp69280_c0_seq1 | 1.332 | AT3G18830 | Polyol/monosaccharide transporter 5 | Linear polyols; myo-inositol; monosaccharides |
| comp7769_c0_seq1 | 1.787 | AT4G21120 | Cationic amino acid transporter 1 | Lys, Arg and Glu |
| comp10794_c0_seq1 | 1.790 | |||
| comp13115_c0_seq1 | 1.429 | |||
| comp8282_c0_seq1 | 1.398 | |||
| comp25372_c0_seq1 | 1.911 | |||
| comp32005_c0_seq2 | 1.502 | AT1G25480 | Aluminum-activated malate transporter family protein | Malate |
| comp33002_c0_seq1 | 1.168 | AT1G77210 | Sugar transport protein 14 | Monosaccharide |
| comp5166_c0_seq1 | 1.145 | AT3G54140 | Peptide transporter 1 | Peptide |
| comp15597_c0_seq1 | 1.121 | AT2G38940 | Phosphate transporter 1;4 | Pi |
| comp14809_c0_seq1 | 1.128 | |||
| comp32347_c0_seq1 | 1.111 | AT3G26570 | Phosphate transporter 2;1 | Pi |
| comp22508_c0_seq1 | 1.293 | AT5G43350 | Phosphate transporter 1 | Pi |
| comp26614_c0_seq1 | 1.078 | AT5G54800 | Glucose 6-phosphate/phosphate translocator 1 | Pi; PEP, triose phosphate; glucose 6-phosphate |
| comp30882_c0_seq12 | 1.154 | AT4G18210 | Purine permease 10 | Purine; purine derivatives |
| comp30860_c0_seq2 | 1.241 | |||
| comp48026_c0_seq1 | 1.237 | AT1G71880 | Sucrose-proton symporter 1 | Sucrose |
| comp18524_c0_seq1 | 1.234 | AT5G19600 | Sulfate transporter 3;5 | Sulfate |
| comp25104_c0_seq5 | 1.224 | AT3G23560 | Aberrant lateral root formation 5 | Toxins |
| comp25104_c0_seq4 | 1.197 | |||
| comp25244_c0_seq5 | 1.140 | |||
| comp25244_c0_seq2 | 1.123 | |||
| comp26932_c0_seq1 | 2.705 | AT4G30420 | Usually multiple acids move in and out transporter 34 | Unknown |
| comp69634_c0_seq1 | 1.345 | AT4G36670 | Polyol/monosaccharide transporter 6 | Glucose; hexose |
| comp29397_c0_seq1 | 1.032 | AT5G64410 | Oligopeptide transporter 4 | Oligopeptide |
| comp25749_c0_seq1 | 3.018 | AT1G67940 | AtSTAR1 | UDP-glucose |
| comp26709_c0_seq1 | 2.987 | |||
| comp30641_c0_seq1 | 2.916 | AT2G37330 | Aluminum sensitive 3 | UDP-glucose |
| comp30389_c0_seq1 | 2.852 | |||
| comp8633_c0_seq1 | 1.022 | AT5G15640 | Mitochondrial substrate carrier family protein | Unknown |
| comp81941_c0_seq1 | 1.012 | |||
| comp21728_c0_seq1 | 1.064 | AT1G74780 | Nodulin-like/major facilitator superfamily protein | Unknown |
| comp17931_c0_seq1 | 1.231 | AT2G39210 | Major facilitator superfamily protein | Unknown |
| comp5953_c0_seq1 | 1.419 | |||
| comp12616_c0_seq1 | 1.249 | |||
| comp29405_c0_seq1 | 1.204 | AT4G23400 | PIP1;5 | Water |
| comp50926_c0_seq1 | 1.895 | AT1G51500 | ABCG12 | Wax |
| comp6165_c0_seq1 | 1.950 | |||
| comp92864_c0_seq1 | 1.903 | |||
| Downregulated | ||||
| comp29871_c0_seq3 | −1.038 | AT4G28390 | ADP/ATP carrier 3 | ADP; ATP |
| comp30078_c0_seq2 | −1.144 | |||
| comp30078_c0_seq1 | −1.137 | |||
| comp29871_c0_seq1 | −1.003 | |||
| comp31976_c0_seq1 | −1.058 | AT2G36910 | ABCB1/P-glycoprotein 1 | Auxin |
| comp22423_c0_seq1 | −1.066 | AT1G75500 | Usually multiple acids move in and out transporter 5 | Auxin |
| comp28292_c0_seq1 | −1.120 | AT1G53210 | Na+/Ca2+ exchanger | Ca2+ |
| comp33125_c0_seq2 | −2.085 | AT3G51860 | Cation exchanger 3 | Ca2+ and H+ |
| comp23194_c0_seq1 | −1.036 | AT4G32390 | Nucleotide–sugar transporter family protein | Carbohydrate |
| comp25558_c0_seq1 | −1.269 | AT5G19760 | Encodes a novel mitochondrial carrier | Dicarboxylates; tricarboxylates |
| comp27413_c0_seq1 | −1.285 | AT4G27720 | Major facilitator superfamily protein | Mo6+ |
| comp23196_c0_seq1 | −1.057 | AT1G64650 | Major facilitator superfamily protein | Mo6+ |
| comp27697_c0_seq1 | −1.264 | AT2G26690 | NRT1/PTR family 6.2 | Oligopeptide |
| comp32440_c0_seq1 | −1.426 | AT5G33320 | Phosphate/phosphoenolpyruvate translocator | PEP |
| comp20485_c0_seq1 | −1.269 | AT5G14040 | Mitochondrial phosphate transporter 3 | Pi |
| comp25757_c0_seq1 | −1.264 | AT3G01550 | Phosphoenolpyruvate/phosphate translocator 2 | Pi and PEP |
| comp17525_c0_seq1 | −1.012 | AT5G62890 | Xanthine/uracil permease family protein | Unknown |
| comp23073_c0_seq1 | −2.726 | AT4G35100 | PIP2;7 | Water |
| comp27116_c0_seq1 | −1.065 | AT3G16240 | TIP2;1 | Water |
| comp24264_c0_seq1 | −1.383 | AT2G36830 | TIP1;1 | Water |
| comp28684_c0_seq1 | −1.186 | AT3G04090 | SIP 1A | Water |
| comp29502_c0_seq1 | −1.146 | |||
1 Substrate specificity was assumed according to TAIR GO functional analysis.
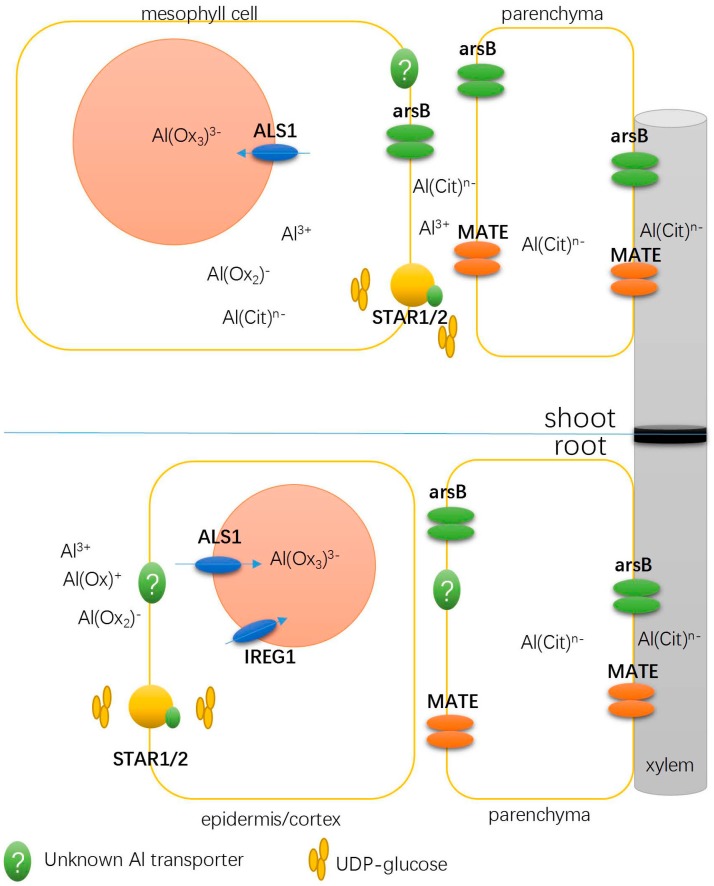
Figure 2.
Illustration of Al-upregulated transporter genes that are conserved between roots and leaves. arsB may function as a bidirectional Al transporter. ALS, aluminum sensitive; arsB, a secondary carrier protein of bacterial arsenic resistance (ars) operons; MATE, multidrug and toxic compound extrusion; STAR, sensitive to Al rhizotoxicity.
2.7. Genes Associated with Development
Different from the root, we specifically identified a group of genes associated with ‘development’ (Figure 1; Table S2). Among 25 upregulated and 17 downregulated development-associated genes, a large proportion was supposed to be involved in flowering.
2.8. Coregulated Genes between Roots and Leaves under Al Stress
Some conserved mechanisms must coexist between roots and shoots, because buckwheat detoxifies Al within both roots and leaves. Thus, we searched for genes in the roots and leaves with the same sequence homologies with Arabidopsis genes. A total of 56 genes were identified, of which 48 were upregulated by Al stress and 8 were downregulated (Table S3). Of great interest were five genes showing similarity with the transporter genes ALS1, AtSTAR1, ALS3, AtMATE and divalent ion transporter. While AtSTAR1/ALS3 could protect the cell wall from Al binding [18], ALS1 is important for the compartmentalization of Al within vacuoles [26,27]. The Arabidopsis AtMATE protein is involved in citrate secretion, to the root apoplast and rhizosphere, to chelate Al [28]. Because buckwheat roots secrete oxalate instead of citrate, the upregulation of the AtMATE homolog gene is likely to facilitate Al translocation in the xylem [29]. Also intriguing is the identification of genes encoding acyl-activating enzyme 3 proteins that have recently been reported to be involved in Al tolerance by regulating cytosolic oxalate homeostasis [30,31].
2.9. Validation of Gene Expression by qRT-PCR Analysis
We validated the RNA-seq data by using quantitative reverse transcription (qRT)-PCR analysis to measure the expression of 20 DEGs upregulated by Al, and 15 DEGs downregulated by Al. The high correlation coefficient (R2 = 0.9324) between RNA-seq data and qRT-PCR results (Figure S2) suggests that the RNA-seq data were reliable.
3. Discussion
Buckwheat displays high Al resistance by employing three different strategies: root tip Al exclusion, Al tolerance based on Al sequestration, and Al transport from the root to the shoot for subsequent sequestration in leaf cell vacuoles. While recent RNA-seq studies on buckwheat roots have greatly improved our understanding of root Al exclusion and tolerance mechanisms, little is known about leaf Al sequestration [17,20,32]. In the present study, we identified a total of 33,931 unigenes from buckwheat leaves, thus providing a platform facilitating future gene function characterization (Table 1). In addition, we identified 1063 upregulated and 944 downregulated genes after exposure of buckwheat to 20 µM Al for 24 h. These DEGs were functionally categorized into a variety of physiological and molecular events (Figure 1), which revealed conserved mechanisms between roots and leaves on the one hand, and distinct adaptive mechanisms in leaves on the other hand.
3.1. Detoxifying Apoplastic Al with a Bacterial-Type ABC Transporter
We identified two genes homologous to OsSTAR1/AtSTAR1 and two genes homologous to OsSTAR2/ALS3 (Table 3). Consistent with our present study, a previous RNA-seq analysis of buckwheat leaves identified a gene encoding FeSTAR1 that was upregulated by Al stress [17]. In rice, OsSTAR1 and OsSTAR2 form a bacterial-type ATP-binding cassette (ABC) transporter to transport uridine diphosphate (UDP)-glucose for the modification of cell walls, which detoxifies Al in the apoplast [18]. Recently, experimental evidence has indicated that Arabidopsis AtSTAR1 and ALS3 could also interact with each other to form a bacterial-type ABC transporter [33]. Given FeSTAR1 and FeALS3 need to work together with respect to Al resistance, it is not surprising in this study that not only FeSTAR1, but also FeALS3, were identified to be coregulated by Al stress. In addition, it is interesting that the expression of FeSTAR1 and FeSTAR2 was induced in both the roots and leaves, which is different from the expression pattern found in rice and Arabidopsis [18,34]. Unlike rice and Arabidopsis, buckwheat accumulates Al within leaves [15] (Figure S1). Thus, it is very likely that the FeSTAR1/FeALS3 protein complex is also involved in protecting the leaf cell apoplast from Al injury.
3.2. Transporters Possibly Involved in Xylem Al Unloading and Sequestration
Sequestration of Al within buckwheat leaf vacuoles involves xylem Al unloading and sequestration into vacuoles across the tonoplast, both of which require the participation of transporters. Unlike in roots, where Al was mostly bound to the cell wall [35], Al in leaves resides mainly in the symplasm [36,37]. Such a difference in Al distribution between the roots and leaves is not surprising, because Al is present in the form of an Al-citrate complex after xylem loading [38]. Thus, it appears that Al is actively transported into leaf cells in the form of an Al-citrate complex. Here, we identified an Al-upregulated gene (comp31346_c0_seq4) encoding a putative divalent ion symporter that is predicted to be a member of the anion permease ArsB/NhD family. Recently, Negishi et al. identified an Al transporter gene, HmPALT2, from the hydrangea sepal, which displays high sequence similarity with the Arabidopsis divalent ion symporter AT1G02260. Functional characterization of HmPALT2 revealed that it can transport Al in the form of Al3+ and Al-citrate complexes [39]. High sequence similarity between comp31346_c0_seq4 and HmPALT2 suggests that this gene is a strong candidate for xylem Al unloading. More intriguingly, in a previous RNA-seq analysis of the buckwheat root apex, we identified three upregulated genes encoding the same divalent ion symporters (Table S3; Figure 2), suggesting conserved mechanisms of Al transport between roots and leaves through transcriptional regulation of this gene. The functional characterization of this gene is currently underway.
Once entering leaf cells, Al is sequestrated into vacuoles [37]. Because almost all the Al in the protoplast was found to be present in vacuoles [36], there must be a tonoplast-localized transporter that actively transports Al into vacuoles. We identified three upregulated genes showing high sequence similarity with ALS1 that could fulfil this function (Table S2). Both rice OsALS1 and Arabidopsis ALS1 are tonoplast-localized transporters that are involved in the sequestration of Al into the vacuole [26,27]. In the buckwheat root tip, we identified a gene encoding FeALS1 and a gene encoding FeIREG1, which is consistent with a previous report [17,20]. Very recently, based on the proteomic analysis of a tonoplast-rich fraction, Lei et al. identified two FeALS1 proteins—FeALS1.1 and FeALS1.2—from buckwheat leaves, and demonstrated their function as tonoplast-localized transporters for Al sequestration into vacuoles [40]. While the expression of FeALS1.1 was induced by Al both in the roots and shoots, FeALS1.2 was constitutively expressed. However, considering the frequent occurrence of gene duplication events in this cross-pollinating species, it is not surprising that we have identified three ALS1 homologs whose expression was induced by Al [20] (Table S2). At present, it remains unknown why FeIREG1 is only involved in root Al sequestration [41], while FeALS1 is conserved between roots and leaves. One plausible explanation may be that FeALS1 is not sufficient for Al sequestration into root vacuoles, and thus an additional transporter, FeIREG1, is required. However, FeALS1 is sufficient for Al sequestration in leaves. Our finding, that there are three homologs of ALS1 involved in buckwheat leaf defense against Al stress, supports our current supposition.
Recently, Negishi et al. identified tonoplast- and plasma membrane-localized Al transporters, HmVALT1 and HmPALT1, from the sepals of the hydrangea, both of which belong to the aquaporin protein family [42]. HmVALT and HmPALT1 belong to the tonoplast-intrinsic proteins (TIPs) and nodulin 26-like intrinsic proteins (NIPs) of aquaporin, respectively. Most recently, NIP1;2 was reported to be a plasma membrane-localized transporter that facilitates Al–malate transport from the root cell wall into the root symplasm [43]. Here, only one gene (comp29405_c0_seq1) displaying sequence similarity with Arabidopsis PIP1;5 was found to be upregulated by Al (Table 3). Clearly, FePIP1;5 belongs to a divergent subfamily of the aquaporin family, and is unlikely to be involved in Al transport. In accordance with this work, our previous study showed that no aquaporin genes were upregulated in the buckwheat root tip by Al stress [20].
3.3. Flowering Regulation in Response to Al Stress
We found that a subset of genes potentially related to flowering are differentially regulated by Al stress. In Arabidopsis, different pathways converge on a few floral integrator genes such as flowering locus T (FT) that promote flowering. During long days, the expression of FT is upregulated by the TF constans (CO), and the FT protein is moved from mature leaves to the meristem to initiate flowering. In the present study, a total of eight upregulated and three downregulated TF genes were found to be associated with flowering (Table 2). For example, one constans-like 4 and two constans-like 5 genes were upregulated by Al stress. Furthermore, a bHLH TF gene encoding HCEATE that is involved in floral organ development was also upregulated by Al stress [44]. However, a gene encoding cycling DOF factor 3 (CDF3), that functions as a transcriptional repressor of the floral integrator genes CO and FT, was also upregulated [45]. In addition to delaying flowering, it was recently reported that Arabidopsis CDF3 is also involved in the mechanisms of tolerance to drought and low temperature [46]. Similarly, the expression of a golden2-like 1 gene that negatively regulates flower development was also inhibited by Al stress. It appears that Al stress differentially affects the expression of flowering-related TFs in buckwheat leaves.
In the upregulated genes, we identified 3 UDP-glucosyl transferase 87A2 (UDPG87A2) genes, five similar to flowering promoting factor1 genes, two ELF4-like genes and two flowering promoting factor1 (FPF1) genes. By contrast, two jasmonate-zim-domain protein1 genes, one calmodulin-like 24 gene, three heat shock protein 90.2 genes, two ELF4 genes, four nucleostemin-like 1 genes and four frigida-like protein genes were identified in the downregulated genes. Among them, UGT87A2, FPF1, similar to flowering promoting factor 1 and nucleostemin-like 1 function as positive regulators that promote flowering [43,47], while the ELF4-like 4 and frigida-like protein genes serve as repressors of flowering, and others may indirectly affect flowering [48,49]. These results suggest that flowering time, or the transition from vegetative to reproduction growth, is affected by Al stress. Clearly, more work is required to understand how buckwheat integrates the Al stress response and flowering time. It is becoming increasingly evident that altering flowering time is an evolutionary strategy by which plants maximize the chances of reproduction under stress conditions. For example, nitrate deficiency promotes flowering in Arabidopsis [50,51]. Under drought stress, plants often accelerate the flowering process to speed life history, thereby avoiding drought [52,53]. Therefore, in the future, it will be interesting to know whether or not buckwheat adapts to Al toxicity by regulating flowering time.
3.4. Chromatin Regulation in Response to Al Stress
We conclude that chromatin regulation may play an important role in the response of buckwheat leaves to Al stress. In addition to the TFs that play a pivotal role controlling gene expression, a large proportion of genes were involved in chromatin regulation, especially among the downregulated DEGs (Table S2). For example, there are 18 genes encoding histone 3.1 and 18 genes encoding histone superfamily protein, respectively; 11 genes encoding histone H2A 12; and 4 genes encoding histone H2A 10 and H2B, respectively. Each nucleosome is an octamer composed of two copies of histones, H2A, H2B, H3 and H4, that package eukaryotic DNA into repeating nucleosomal units. Accumulating evidence suggests that changes in chromatin structure are involved in the regulation of gene expression in response to abiotic stress [25]. Thus, the conspicuous downregulation of a large group of genes related to chromatin remodeling must have a specific role in the response of buckwheat leaves to Al stress, although this requires further investigation.
We also identified genes associated with the post-translational modification of histone N-tails. Al stress depressed the expression of three histone deacetylase 2C genes and two histone deacetylase 3 genes (Table S2). Histone acetylation reduces charge interaction between histones and DNA, thereby facilitating transcription activation. Kim et al. found that some drought-responsive genes such as RD29A, RD29B, RD20 and RAP2.4 were differentially acetylated at K9, K14, K23 and K27 of histone 3 in Arabidopsis under short-term drought stress, and the histone acetylation level was positively correlated with gene expression [53]. The level of H3K9Ac in RD20 and RD29A was quickly reduced after rehydration, which was correlated with gene expression reduction [54]. Apparently, the downregulation of histone deacetylase genes may play an important role in regulating gene expression under Al stress. However, the specific target genes of histone acetylation, and the corresponding phenotypic consequences, have yet to be investigated.
In contrast, a gene encoding histone H3K4-specific methyltransferase was found to be upregulated by Al stress (Table S2). A potential link between histone methylation and gene expression under abiotic stress is emerging. For example, Zong et al. reported that the H3K4me3 modification level was significantly and positively correlated with the transcript level in rice under drought stress, although only 13% of stress-responsive genes were differentially affected by H3K4me3 methylation [55]. A genome-wide study of H3K4 methylation in Arabidopsis plants exposed to drought stress also revealed that H3K4me3 methylation is positively correlated with transcript level [56,57]. Consistent with the downregulation of histone deacetylase genes, the upregulation of histone H3K4-specific methyltransferase is associated with the upregulation of stress-responsive genes, which may be critical for the adaptation of buckwheat leaves to Al stress.
In summary, we provided the transcriptome information for buckwheat leaves in response to Al stress. Functional classification of both up- and downregulated genes, according to the biological processes of homologous genes in Arabidopsis, revealed distinct mechanisms by which buckwheat leaves cope with Al stress. Moreover, comparative analysis of transporter genes between roots and shoots not only revealed the conserved molecular basis of Al resistance between roots and leaves, but also provided us with a basis for the characterization of novel Al resistance genes.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Seeds of an Al-tolerant buckwheat (Fagopyrum esculentum Moench cv. Jiangxi, Jiangxi province, China) were germinated and cultured according to our previous work [20]. On day 3, uniform seedlings were transferred to 1/5 strength Hoagland nutrient solution (pH 5.5) consisting of KNO3 (1.0 mM), Ca(NO3)2 (1.0 mM), MgSO4 (0.4 mM) and (NH4)H2PO4 (0.2 mM), and the micronutrients NaFeEDTA (20 µM), H3BO3 (3.0 µM), MnCl2 (0.5 µM), CuSO4 (0.2 µM), ZnSO4 (0.4 µM) and (NH4)6Mo7O24 (1 µM). The solution was renewed every 3 d. When the true leaf was fully expanded, the seedlings were exposed to the same nutrient solution with (NH4)H2PO4 concentration decreased to 10 µM either in the absence (−Al) or presence (+Al) of 20 µM Al for 24 h. After treatment, the expanded true leaf was collected and immediately frozen in liquid nitrogen for future use. Plants were cultured in an environmentally controlled growth room with a 14 h/26 °C day (light intensity of 300 µmol photons m−2·s−1) and a 10 h/22 °C night regime.
4.2. Determination of Al Accumulation
In order to determine Al accumulation in buckwheat, an Al-resistant rice bean, which is a known Al excluder, was used as a reference [19]. Seeds of rice bean and buckwheat were germinated and cultured following the same procedure. Two-week-old seedlings were exposed to the 1/5 strength nutrient solution with 10 µM (NH4)H2PO4 either in the absence (−Al) or presence (+Al) of 20 µM Al for 24 h. After treatment, roots and shoots were collected and dried at 70 °C in an oven for 2 days. The Al concentration was measured by inductively coupled plasma mass spectrometry after digestion of samples with HNO3.
4.3. RNA Isolation and Solexa Sequencing
The total RNA isolation and sequencing procedures followed our previous work [20].
4.4. Sequence Assembly and Annotation
Sequence assembly and annotation were the same as our previous work [20]. The sequences of all unigenes were deposited in the NCBI database (accession number: SRA589522).
4.5. Differential Gene Expression Analysis and Gene Ontology Biological Processes Analysis
The reads per kb million reads (PRKM) method was used to calculate gene expression levels [22]. To identify Al-induced differentially expressed genes, log2 (fold change) ≥ 1 and RPKM values ≥10 were set as the cut-off. For each group of DEGs, gene ontology (GO) biological processes analysis was performed according to our previous study [20].
4.6. Quantitative RT-PCR Analysis
The total RNA extraction qRT-PCR procedures followed our previous work [20]. Primers for qRT-PCR analysis are listed in Table S4.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (31572193 and 31222049 to Jian Li Yang), the Hangzhou City Government Innovative Program for Science Excellence (20131028), the 973 Project (2014CB441002), the Chang Jiang Scholars Program (Young Scholar), and China Scholarship Council (to Wei Wei Chen). We appreciate Peter Ryan (CSIRO Plant Industry, Canberra, Australia) for polishing our English.
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/9/1859/s1.
Author Contributions
Jian Li Yang conceived the study. Wei Wei Chen and Jian Li Yang designed the experiment. Wei Wei Chen, Jia Meng Xu and Jian Feng Jin performed the experiments. Wei Fan, Jian Li Yang, and He Qiang Lou analyzed the data. Jian Li Yang wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Von Uexküll H.R., Mutert E. Global extent, development and economic impact of acid soils. In: Date R.A., Grundon N.J., Rayment G.E., Probert M.E., editors. Plant-Soil Interactions at Low pH: Principles and Management. Volume 64. Springer; Dordrecht, The Netherlands: 1995. pp. 5–19. [Google Scholar]
- 2.Kochian L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:237–260. doi: 10.1146/annurev.pp.46.060195.001321. [DOI] [Google Scholar]
- 3.Ma J.F., Furukawa J. Recent progress in the research of external Al detoxification in higher plants: A minireview. J. Inorg. Biochem. 2003;97:46–51. doi: 10.1016/S0162-0134(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 4.Kochian L.V., Piñeros M.A., Hoekenga O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil. 2005;274:175–195. doi: 10.1007/s11104-004-1158-7. [DOI] [Google Scholar]
- 5.Liu J., Piñeros M.A., Kochian L.V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 2014;56:221–230. doi: 10.1111/jipb.12162. [DOI] [PubMed] [Google Scholar]
- 6.Ryan P.R., Delhaize E., Jones D.L. Function and mechanism of organic anion exudation from plant roots. Ann. Rev. Plant Mol. Biol. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T., Osaki M. Mechanisms of adaptation to high aluminum condition in native plant species growing in acid soils: A review. Commun. Soil Sci. Plan. 2002;33:1247–1260. doi: 10.1081/CSS-120003885. [DOI] [Google Scholar]
- 8.Kochian L.V., Hoekenga O.A., Piñeros M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto H., Hirasawa E., Morimura S., Takahashi E. Localization of aluminium in tea leaves. Plant Cell Physiol. 1976;17:627–631. [Google Scholar]
- 10.Watanabe T., Osaki M., Tadano T. Aluminum-induced growth stimulation in relation to calcium, magnesium, and silicate nutrition in Melastoma malabathricum L. Soil Sci. Plant Nutr. 1997;43:827–837. doi: 10.1080/00380768.1997.10414649. [DOI] [Google Scholar]
- 11.Ma J.F., Hiradate S., Nomoto K., Iwashita T., Matsumoto H. Internal detoxification mechanism of Al in hydrangea (Identification of Al form in the leaves) Plant Physiol. 1997;113:1033–1039. doi: 10.1104/pp.113.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng S.J., Ma J.F., Matsumoto H. High aluminum resistance in buckwheat. I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998;117:745–751. doi: 10.1104/pp.117.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng S.J., Yang J.L., He Y.F., Yu X.H., Zhang L., You J.F., Shen R.F., Matsumoto H. Immobilization of aluminum with phosphorus in roots is associated with high aluminum resistance in buckwheat. Plant Physiol. 2005;138:297–303. doi: 10.1104/pp.105.059667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J.L., Zheng S.J., He Y.F., You J.F., Zhang L., Yu X.H. Comparative studies on the effect of a protein-synthesis inhibitor on aluminium-induced secretion of organic acids from Fagopyrum esculentum Moench and Cassia tora L. roots. Plant Cell Environ. 2006;29:240–246. doi: 10.1111/j.1365-3040.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- 15.Ma J.F., Zheng S.J., Matsumoto H., Hiradate S. Detoxifying aluminum with buckwheat. Nature. 1997;390:569–570. doi: 10.1038/37518. [DOI] [Google Scholar]
- 16.Shen R., Iwashita T., Ma J.F. Form of Al changes with Al concentration in leaves of buckwheat. J. Exp. Bot. 2004;55:131–136. doi: 10.1093/jxb/erh016. [DOI] [PubMed] [Google Scholar]
- 17.Yokosho K., Yamaji N., Ma J.F. Global transcriptome analysis of Al-induced genes in an Al-accumulating species, common buckwheat (Fagopyrum esculentum Moench) Plant Cell Physiol. 2014;55:2077–2091. doi: 10.1093/pcp/pcu135. [DOI] [PubMed] [Google Scholar]
- 18.Huang C.F., Yamaji N., Mitani N., Yano M., Nagamura Y., Ma J.F. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell. 2009;21:655–667. doi: 10.1105/tpc.108.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J.L., Zhang L., Li Y.Y., You J.F., Wu P., Zheng S.J. Citrate transporters play a critical role in aluminium-stimulated citrate efflux in rice bean (Vigna umbellata) roots. Ann. Bot. 2006;97:579–584. doi: 10.1093/aob/mcl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J.M., Fan W., Jin J.F., Lou H.Q., Chen W.W., Yang J.L., Zheng S.J. Transcriptome analysis of Al-induced genes in buckwheat (Fagopyrum esculentum Moench) root apex: New insight into Al toxicity and resistance mechanisms in an Al accumulating species. Front. Plant Sci. 2017;8:1141. doi: 10.3389/fpls.2017.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 23.Fan W., Lou H.Q., Gong Y.L., Liu M.Y., Wang Z.Q., Yang J.L., Zheng S.J. Identification of early Al-responsive genes in rice bean (Vigna umbellata) roots provides new clues to molecular mechanisms of Al toxicity and tolerance. Plant Cell Environ. 2014;37:1586–1597. doi: 10.1111/pce.12258. [DOI] [PubMed] [Google Scholar]
- 24.Mitsuda N., Ohme-Takagi M. Functional analysis of transcription factors in Arabidopsis. Plant Cell Physiol. 2009;50:1232–1248. doi: 10.1093/pcp/pcp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asensi-Fabado M.A., Amtmann A., Perrella G. Plant responses to abiotic stress: The chromatin context of transcriptional regulation. Biochemi. Biophys. Acta. 2017;1860:106–122. doi: 10.1016/j.bbagrm.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Larsen P.B., Cancel J., Rounds M., Ochoa V. Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta. 2007;225:1447. doi: 10.1007/s00425-006-0452-4. [DOI] [PubMed] [Google Scholar]
- 27.Huang C.F., Yamaji N., Chen Z.C., Ma J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of Al in rice. Plant J. 2011;69:857–867. doi: 10.1111/j.1365-313X.2011.04837.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Magalhaes J.V., Shaff J., Kochian L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–399. doi: 10.1111/j.1365-313X.2008.03696.x. [DOI] [PubMed] [Google Scholar]
- 29.Ma J.F., Ryan P.R., Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001;6:273–278. doi: 10.1016/S1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- 30.Lou H.Q., Fan W., Xu J.M., Gong Y.L., Jin J.F., Chen W.W., Liu L.Y., Hai M.R., Yang J.L., Zheng S.J. An oxalyl-CoA synthetase is involved in oxalate degradation and aluminum tolerance. Plant Physiol. 2016;172:1679–1690. doi: 10.1104/pp.16.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W.W., Fan W., Lou H.Q., Yang J.L., Zheng S.J. Regulating cytoplasmic oxalate homeostasis by acyl activating enzyme 3 is critical for plant Al tolerance. Plant Signal. Behav. 2017;12:e1276688. doi: 10.1080/15592324.2016.1276688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu H., Wang H., Zhu Y., Zou J., Zhao F.J., Huang C.F. Genome-wide transcriptomic and phylogenetic analyses reveal distinct aluminum-tolerance mechanisms in the aluminum-accumulating species buckwheat (Fagopyrum tatarucum) BMC Plant Biol. 2015;15:16. doi: 10.1186/s12870-014-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong J., Piñeros M.A., Li X., Yang H., Liu Y., Muphy A.S., Kochian L.V., Liu D. An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol. Plant. 2017;10:244–259. doi: 10.1016/j.molp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Huang C.F., Yamaji N., Ma J.F. Knockout of a bacterial-type ATP-binding cassette transporter gene, AtSTAR1, results in increased aluminum sensitivity in Arabidopsis. Plant Physiol. 2010;153:1669–1677. doi: 10.1104/pp.110.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klug B., Horst W.J. Oxalate exudation into the root-tip water free space confers protection from aluminum toxicity and allows aluminum accumulation in the symplast in buckwheat (Fagopyrum esculentum) New Phytol. 2010;187:380–391. doi: 10.1111/j.1469-8137.2010.03288.x. [DOI] [PubMed] [Google Scholar]
- 36.Ma J.F., Hiradate S., Matsumoto H. High aluminum resistance in buckwheat II. Oxalic acid detoxifies aluminum internally. Plant Physiol. 1998;117:753–759. doi: 10.1104/pp.117.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen R., Ma J.F., Kyo M., Iwashita T. Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta. 2002;215:394–398. doi: 10.1007/s00425-002-0763-z. [DOI] [PubMed] [Google Scholar]
- 38.Ma J.F., Hiradate S. Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench) Planta. 2000;211:355–360. doi: 10.1007/s004250000292. [DOI] [PubMed] [Google Scholar]
- 39.Negishi T., Oshima K., Hattori M., Yoshida K. Plasma membrane-localized Al-transporter from blue hydrangea sepals is a member of the anion permease family. Genes Cells. 2013;18:341–352. doi: 10.1111/gtc.12041. [DOI] [PubMed] [Google Scholar]
- 40.Lei G.J., Yokosho K., Yamaji N., Fujii-Kashino M., Ma J.F. Functional characterization of two half-size ABC transporter genes in aluminum-accumulating buckwheat. New Phytol. 2017;215:1080–1089. doi: 10.1111/nph.14648. [DOI] [PubMed] [Google Scholar]
- 41.Yokosho K., Yamaji N., Mitani-Ueno N., Shen R.F., Ma J.F. An aluminum-inducible IREG gene is required for internal detoxification of aluminum in buckwheat. Plant Cell Physiol. 2016;57:976–985. doi: 10.1093/pcp/pcw026. [DOI] [PubMed] [Google Scholar]
- 42.Negishi T., Oshima K., Hattori M., Kana M., Mano S., Nishimura M., Yoshida K. Tonoplast- and plasma membrane-localized aquaporin-family transporters in blue hydrangea sepals of aluminum hyperaccumulating plant. PLoS ONE. 2012;7:e43189. doi: 10.1371/journal.pone.0043189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X., Gingrich D.K., Deng Y., Hong Z. A nucleostemin-like GTPase required for normal apical and floral meristem development in Arabidopsis. Mol. Biol. Cell. 2012;23:1446–1456. doi: 10.1091/mbc.E11-09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuster C., Gaillochet C., Lohmann J. U. Arabidopsis HECATE genes function in phytohormone control during gynoecium development. Development. 2015;142:3343–3350. doi: 10.1242/dev.120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawa M., Nusinow D.A., Kay S.A., Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–264. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corrales A.R., Carrillo L., Lasierra P., Nebauer S.G., Dominguez-Figueroa J., Renau-Morata B., Pollmann S., Granell A., Molina R.V., Vicente-Carbajosa J., et al. Multifaceted role of cycling DOF factors 3 (CDF3) in the regulation of flowering time and abiotic stress responses in Arabidopsis. Plant Cell Environ. 2017;40:748–764. doi: 10.1111/pce.12894. [DOI] [PubMed] [Google Scholar]
- 47.Kania T., Russenberger D., Peng S., Apel K., Melzer S. FPF1 promotes flowering in Arabidopsis. Plant Cell. 1997;9:1327–1338. doi: 10.1105/tpc.9.8.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doyle M.R., Davis S.J., Bastow R.M., McWatters H.G., Kozma-Bognár L., Nagy F., Millar A.J., Amasino R.M. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature. 2002;419:74–77. doi: 10.1038/nature00954. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Bermejo E., Balasubramanian S. Natural variation involving deletion alleles of FRIGIDA modulate temperature-sensitive flowering responses in Arabidopsis thaliana. Plant Cell Environ. 2016;39:1353–1365. doi: 10.1111/pce.12690. [DOI] [PubMed] [Google Scholar]
- 50.Castro Marín I., Loef I., Bartetzko L., Searle I., Coupland G., Stitt M., Osuna D. Nitrate regulates floral induction in Arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta. 2010;233:539–552. doi: 10.1007/s00425-010-1316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu T., Li Y., Ren J., Qian Y., Yang X., Duan W., Hou X. Nitrate or NaCl regulates floral induction in Arabidopsis thaliana. Biologia. 2013;68:215–222. doi: 10.2478/s11756-013-0004-x. [DOI] [Google Scholar]
- 52.Franks S.J., Sim S., Weis A.E. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl. Acad. Sci. USA. 2007;104:1278–1282. doi: 10.1073/pnas.0608379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franks S.J. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 2011;190:249–257. doi: 10.1111/j.1469-8137.2010.03603.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim J.M., To T.K., Ishida J., Morosawa T., Kawashima M., Matsui A., Toyoda T., Kimura H., Shinozaki K., Seki M. Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:1580–1588. doi: 10.1093/pcp/pcn133. [DOI] [PubMed] [Google Scholar]
- 55.Kim J. M., To T.K., Ishida J., Matsui A., Kimura H., Seki M. Transition of chromatin status during the process of recovery from drought stress in Arabidopsis thaliana. Plant Cell Physiol. 2012;53:847–856. doi: 10.1093/pcp/pcs053. [DOI] [PubMed] [Google Scholar]
- 56.Zong W., Zhong X., You J., Xiong L. Genome-wide profiling of histone H3K4-tri-methylation and gene expression in rice under drought stress. Plant Mol. Biol. 2013;81:175–188. doi: 10.1007/s11103-012-9990-2. [DOI] [PubMed] [Google Scholar]
- 57.Van Dijk K., Ding Y., Malkaram S., Riethoven J.J., Liu R., Yang J., Laczko P., Chen H., Xia Y., Ladunga I., et al. Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biol. 2010;10:238. doi: 10.1186/1471-2229-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.