Abstract
Antibody-drug conjugates (ADCs), designed to selectively deliver cytotoxic agents to antigen-bearing cells, are poised to become an important class of cancer therapeutics. Human epithelial growth factor receptor (HER2) is considered an effective target for cancer treatment, and a HER2-targeting ADC has shown promising results. Most ADCs undergoing clinical evaluation contain linkers that have a lysosomal protease-cleavable dipeptide, of which the most common is valine-citrulline (VC). However, valine-alanine (VA), another dipeptide comprising two human essential amino acids, has been used in next generation ADCs loading new toxins, but the druggable properties of ADCs loaded the most popular monomethyl auristatin E (MMAE) remain to be further explored. In this study, we generated VA-based ADCs that connected MMAE to an anti-HER2 antibody. We studied the differences in the preparation process, in vitro stability, cathepsin B activity and in vitro cytotoxicity of VA-based ADC compared to the ADC of VC. VA had comparable performance to VC, which preliminarily displays its practicability. Additional efficacy and safety studies in a xenograft model indicate this novel ADC exerted potent anti-tumor activity and negligible toxicity. The results of this study show the application potential of VA-based ADC with MMAE as the payload.
Keywords: antibody-drug conjugate, epithelial growth factor receptor, valine-alanine, monomethyl auristatin E
1. Introduction
Human epithelial growth factor receptor 2 (HER2/ErbB2) is a ligand-less tyrosine kinase receptor that acts as a pro-oncogene in different human cancers [1]. HER2 plays an important role in the development and prognosis of many cancer types such as metastatic breast, gastric, lung, colon, esophageal, and ovarian cancers [2,3]. For instance, it is overexpressed in approximately 20–25% of breast cancers, which were characterized by aggressive proliferation and poor prognosis before the introduction of targeted treatments [4,5]. HER2 is considered an effective target for antibody-based therapy in cancer treatment, especially breast cancer [6,7]. Trastuzumab (Herceptin®) and pertuzumab (Perjeta®) are two approved monoclonal antibody drugs that target the HER2 pathway, and their clinical application has greatly prolonged the survival of patients with metastatic breast cancer [8,9]. Despite these noteworthy advances in HER2-targeted therapy, patients eventually relapse, underscoring the need for alternative therapies [10].
Antibody-drug conjugates (ADCs), which combine the specificity, pharmacokinetics, and biodistribution of a monoclonal antibody (MAB) with the cytotoxic potency of a drug payload, is a promising new therapy for cancer [11]. Ado-trastuzumab emtansine (Kadcyla®), an anti-HER2 ADC that was designed with this new strategy, further prolonged the overall survival of trastuzumab- resistant patients by more than six months [10,12]. The three components of ADCs are in the form of a targeted drug delivery system [13]. In addition to the development of antibodies and cytotoxic drugs, the design of the linker is of essential importance, as it impacts the efficacy and tolerability of the final product.
A suitable linker not only needs to provide sufficient stability during systemic circulation, but must also allow the rapid and efficient release of cytotoxic drug inside the tumor cells [14]. First-generation ADCs often contained unstable linkers, such as unhindered disulfides and hydrazones, which led to their failure [11,15]. On the other hand, next-generation ADCs contain linkers such as peptide linkers, glucuronides, and noncleavable linkers, which increase their stability [16,17]. Cleavage of the commonly used dipeptide linker depends on tumor-associated lysosomal cathepsin B and undergoes rapid hydrolysis, leading to release of the parent drug to kill the tumor [18,19]. The majority of auristatin-based ADCs use a dipeptide (valine-citrulline (VC)) linker conjugated to the auristatin analog monomethyl auristatin E (MMAE) [20,21]. In recent years, valine-alanine (VA), another dipeptide comprising two human essential amino acids, has been used in next generation ADCs loading new toxins such as pyrrolobenzodiazepine (PBD) [22,23]. In addition, the VA-based linker also been mentioned in patents for a novel internalizing ADC that connected MMAE to antibody via a dibromo-containing linker, with any relevant druggable properties were provided [24,25]. The effect of different cytotoxin on the ADC is very significant, in addition to PBD and MMAE being two completely different molecules with different drug release process (Figure 1) [14,26]. The VA peptide has an advantage in the preparation process and cost of materials, and may have the potential to improve ADC stability and decrease product aggregation [27,28]. This has led to interest in exploring the physical and chemical properties, stability, enzymolysis kinetics, and efficacy of VA-based ADCs with MMAE as the potent payload.
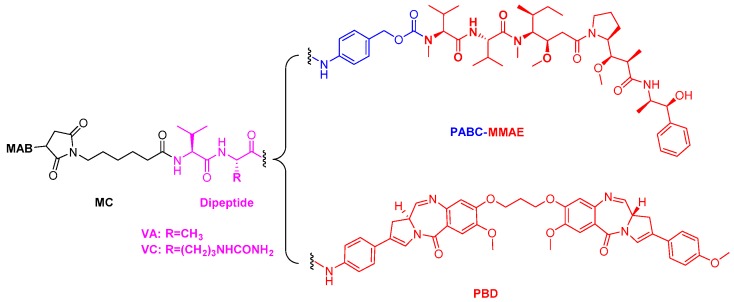
Figure 1.
Structure models of the antibody-drug conjugates (ADCs): MAB-MC-VC-PABC-MMAE and MAB-MC-VA-PBD. MAB: monoclonal antibody; MC: maleimidocaproyl; VC: valine-citrulline; VA: valine-alanine; PABC: p-aminobenzylcarbamate; PBD: pyrrolobenzodiazepine; MMAE: monomethyl auristatin E.
In this study, we generated VA-based ADCs that connected MMAE to an anti-HER2 antibody. Compared to the VC-based ADC which payload been from a clinically approved ADC drug, we studied the preliminarily practicability of the VA-based ADCs in the preparation process, in vitro stability, cathepsin B activity and in vitro cytotoxicity. In addition, we further studied the anti-tumor activity and toxicity of VA-based ADC in a xenograft model. The results demonstrate that the VA-based ADC with MMAE as the payload also has application potential as an antitumor agent.
2. Results and Discussion
2.1. Linker Design
The design of the VA-based linker system was based on acetazolamide-drug conjugates [27]. Compared to counterparts with valine-lysine or valine-arginine linkers, small molecule-drug conjugates (SMDC) with VC and VA linkers exhibited greater stability and superior therapeutic activity. The SMDC containing the VA dipeptide linker not only showed the highest stability in vitro but also the highest efficacy in vivo and in vitro. Applying the linker of this small molecule to ADCs may improve the performance of the final product. In addition, compared to the VC dipeptide, VA may have the potential to reduce ADC aggregation [28].
Both linkers with VA and VC were substrates for cathepsin B, and VA-based ADC could be easily cleaved by enzymes in an antibody-doxorubicin conjugate [28]. The VA dipeptide has also been used in clinical trials of ADCs containing PBD as the cytotoxic payload such as SGN-CD33A [22]. However, the effect of cytotoxin on the ADC is very significant, and the practicability of developing VA for MMAE-based ADC need to be further determined.
We generated VA-based internalizing ADCs with MMAE as the payload, as well as the corresponding VC-based ADC as a comparison. Theoretically, this type of ADC can also release the parent drug in a similar manner (Figure 2A). We replaced the VC dipeptide with VA, with the goal of proving the feasibility of this design strategy. This study also explored the effects of increasing the polarity of the VA linker by shortening the carbon chain (Figure 2B). These data can be used as evidence of the effects of carbon chains on drug release performance.
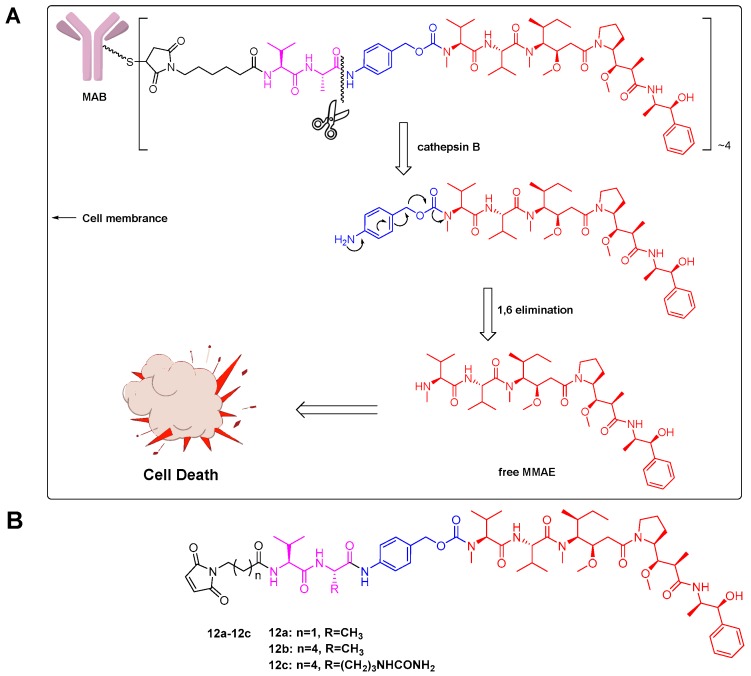
Figure 2.
Linker design: (A) VA-based ADC with MMAE as the payload and its lysosomal processing. Upon internalization of ADC into the lysosomes of target cells, the amide bond between the alanine residue and the p-aminobenzylcarbamate (PABC) portion of the linker was cleaved by cathepsin B. Self-immolation of the PABC portion of the payload-linker via a 1,6-elimination process released free MMAE into the tumor cell, resulting in cell death; (B) Structure of the designed drug payload of MMAE-based ADCs: 12a–12c.
2.2. Evaluation of Antibody-Drug Conjugates (ADC) Preparation
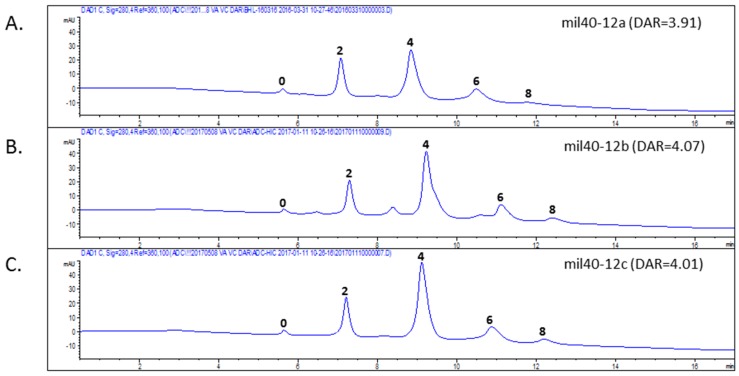
The drug payloads used to make ADCs are typically conjugated to the antibody through cysteine or lysine residues. The number of drug payloads per antibody, commonly referred to as the drug-to-antibody ratio (DAR), can vary between 0 and 8 drugs per antibody. Reduction of inter-molecule chain disulfide bonds to produce free sulfhydryl groups allowed conjunction at specific residues using maleimide-containing linkers, and generated conjugate mixtures at a limited number of defined positions. Hydrophobic interaction chromatography (HIC) analysis of the three ADCs (mil40-12a, mil40-12b, and mil40-12c; mil40 is a humanized monoclonal antibody that binds to HER2) allowed resolution of the conjugates into several major peaks corresponding to 0, 2, 4, 6, and 8 drug molecules per antibody (Figure 3), with an average DAR of about 4.
Figure 3.
HIC analysis of ADCs. HIC allowed resolution of the conjugates into several major peaks corresponding to 0, 2, 4, 6, and 8 drug molecules per antibody. (A) Drug distribution of ADC mil40-12a, DAR = 3.91; (B) Drug distribution of ADC mil40-12b, DAR = 4.07; (C) Drug distribution of ADC mil40-12c, DAR = 4.01. DAR: drug to antibody ratio.
Similar to other biologics, it is important to monitor high molecular weight species in ADC. Size exclusion chromatography (SEC) is a long-established method to measure size variants of proteins, especially high molecular weight species. To confirm the effects of dipeptide type on the degree of aggregation, we synthesized two types of ADCs using VA and VC as linkers. Then, we analyzed aggregation of the two prepared ADCs with an average DAR of about 4. The dimer of the aggregated antibody peak of VA-based ADC (mil40-12b) was 0.27% in the high-performance liquid chromatography (HPLC) spectrum (Agilent 1260; Wilmington, DE, USA), whereas that of VC-based ADC (mil40-12c) was 0.83%. To enlarge this effect, we synthesized the two ADCs with an average DAR of about 7. In these samples, VA-based ADC had no obvious increase in dimeric peak; however, the aggregation of VC-based ADC elevated to 1.80% with an increase in DAR from 4 to 7. In addition, the reaction system of VA-based conjugates had higher limpidity in the conjugate process, leading the recovery rate of the antibody to increase by 8.4%. Thus, VA was chosen over VC to reduce aggregation in ADCs with MMAE as the potent payload.
2.3. Stability Assays In Vitro
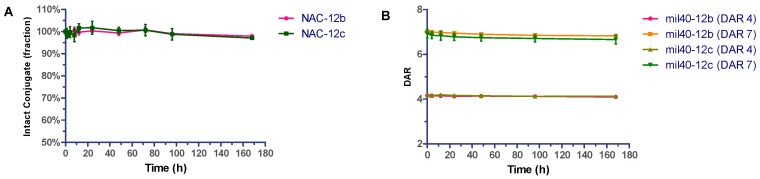
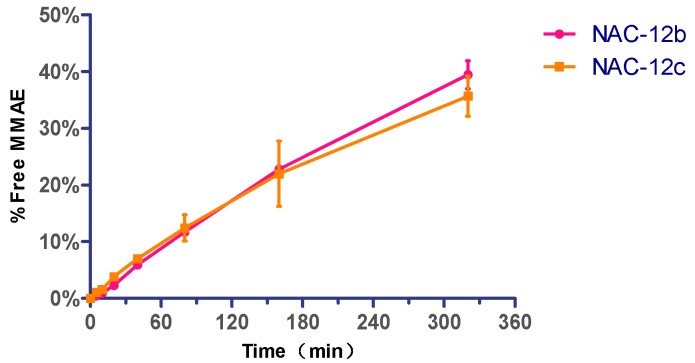
ADCs and their payloads were tested for stability in phosphate-buffer saline (PBS). To compare the linker stability of the two dipeptides, the reactive maleimide double bond of 12b and 12c were reduced with excess N-acetyl-l-cysteine (NAC) to afford NAC-12b and NAC-12c respectively [29]. These compounds were added to PBS buffer and incubated at 37 °C for seven days. Aliquots were taken at various time points and stored at −20 °C before HPLC analysis. Results were based on the area under the curve (AUC) of NAC-12b and NAC-12c at each time point as a percentage of the AUC at t = 0. After seven days, the AUCs for NAC-12b and NAC-12c were 97.92% and 97.05%, respectively, of the two samples taken immediately at t = 0 (Figure 4A). In addition, it could be seen from the HPLC spectrum that the lost fraction was converted to the ring-opened succinimide hydrolysis adduct.
Figure 4.
Stability assays of VA- and VC-based conjugates in PBS. Compound in phosphate buffered saline (PBS) (pH 7.4) were incubated at 37 °C for seven days. Error bars represent standard deviation from three independent experiments (triplicates). Results are shown as the mean ± SD. (A) Stability of NAC-12b and NAC-12c; (B) Stability of VA- and VC-based ADCs loaded with four and seven drugs per antibody.
Next, we analyzed the in vitro stability of these two ADCs with an average DAR of about 4 and 7. Analogously, the two ADCs having 4 and 7 drugs per antibody, respectively, were diluted with PBS (1 mg/mL, pH 7.4) and incubated at 37 °C in a shaking incubator. All of the samples were analyzed by HIC HPLC. As shown in Figure 4B, none of the four ADCs had significant drug off-targets in this test, and VA- and VC-based ADCs that having four payloads decreased stability by 1.58% and 1.34%, respectively, after seven days, whereas those that having seven payloads decreased stability by 2.52% and 3.88%, respectively, under the same test conditions. In short, ADCs with VA- and VC-based linker systems have substantially comparable in vitro stability in PBS.
2.4. Cathepsin B Reactivity
According to previous reports, drug linkers containing VA or VC dipeptides are substrates for cathepsin B [28]. Here, we confirmed the cleavable feasibility of VA-based conjugate under the reaction of cathepsin B. The susceptibility of the dipeptide linker conjugates (NAC-12b and NAC-12c) to enzymatic cleavage was determined by treatment of the acetyl-L-cysteine adduct with cathepsin B from human liver (EC 3.4.22.1, SIGMA-ALDRICH®, St. Louis, MO, USA); NAC-12c was tested in parallel for comparison. The cleavage of dipeptide from both substrates resulted in 1,6-elimination of MMAE, which was identified by liquid chromatography-mass spectrometry (LC-MS; Agilent 1100, Palo Alto, CA, USA). The amount of released MMAE over time is shown in Figure 5. These results demonstrated that the performance of VA-based conjugate is basically the same as VC, and is likely to be a suitable substrate for cathepsin B.
Figure 5.
Cathepsin B reactivity for MMAE release. Typical time course of MMAE cleaved from N-acetyl-l-cysteine (NAC) conjugates based on VA and VC dipeptides. The enzyme-to-substrate ratio was 1:1000. Error bars represent standard deviation from three independent experiments (triplicates). Data are shown as mean ± SD.
2.5. In Vitro Evaluation of Cytotoxic Agents and ADCs
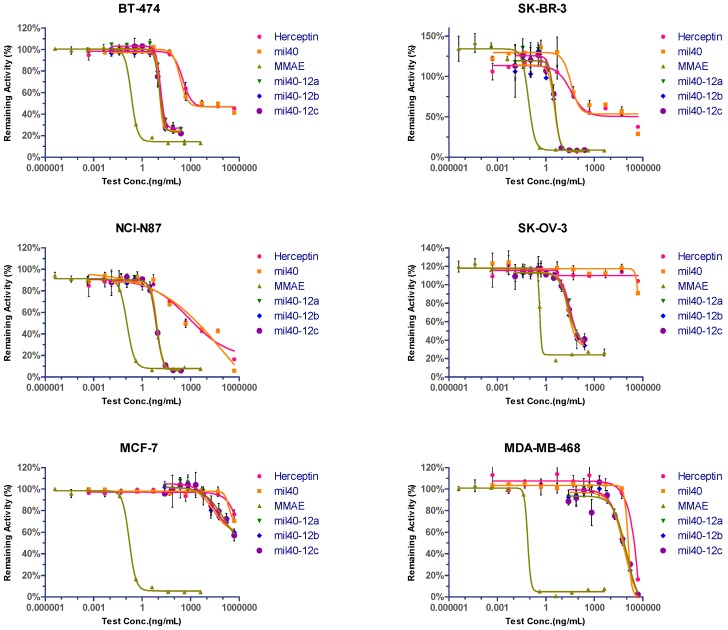
To compare in vitro potency, the HER2-positive cell lines (BT-474, SK-BR-3, SK-OV-3, and NCI-N87), the weakly positive cell line MCF-7 (also known as a cell line expressing normal level of HER2) [30], and the HER2-negative cell line MDA-MB-468 were treated with various ADCs with an average DAR of 4. Both the naked antibodies (mil40 and trastuzumab) had relatively lower inhibitory effects on all HER2-positive cells, and both of them have almost the same activity in all of the tested cell lines. Meanwhile, the three ADCs (mil40-12a, mil40-12b, and mil40-12c) exhibited more potent activity in the HER2-positive cell line, both IC50 and inhibition, while very weak or no inhibitory effects in the HER2-negative cell line, just as antibody drugs, indicating its significant antigen selectivity. As a comparison, MMAE had potent anti-tumor activity in both HER2-positive and HER2-negative cell lines (Figure 6).
Figure 6.
Cytotoxicity and selectivity of ADCs in tumor cells in vitro. Cytotoxicity assay performed in HER2-positive, weakly positive and negative cell lines. Breast cancer cell lines BT-474 and SK-BR-3, gastric cancer cell line NCI-N87, and ovarian cancer cell line SK-OV-3 were HER2-positive. Breast cancer cell lines MCF-7 and MDA-MB-468 were HER2-weakly positive and -negative, respectively. Each group was established three holes and results are shown as mean ± SD. IC50 is presented on Table 1.
The above-mentioned three ADCs had almost identical cytotoxicity in vitro, indicating that the type of dipeptide and length of the carbon chain did not have significant effects on cytotoxicity and drug release in cells. Overall, VA-based ADCs with MMAE exhibited similar efficacy and selectivity to the VC-based ADC, and the selectivity between HER2-positive and HER2-negative cells increased by about 2400–26,000 fold. In addition, both antibodies exhibited very weak cell inhibitory activity in the HER2-positive ovarian cancer cell line SK-OV-3; however, the associated ADCs exhibited similar cytotoxicity to the other cell lines, with an increase in anti-tumor activity of tens of thousands (Table 1).
Table 1.
Cytotoxicity of ADCs, antibodies and MMAE in cancer cell lines in vitro.
| Cell Line | HER2 Status | IC50 (nM) | |||||
|---|---|---|---|---|---|---|---|
| mil40-12a | mil40-12b | mil40-12c | mil40 | Herceptin | MMAE | ||
| BT-474 | HER2+ | 0.09 | 0.08 | 0.08 | 1.54 | 1.75 | 0.30 |
| SK-BR-3 | HER2+ | 0.02 | 0.02 | 0.02 | 0.27 | 0.19 | 0.12 |
| NCI-N87 | HER2+ | 0.05 | 0.06 | 0.05 | 0.36 | 0.43 | 0.16 |
| SK-OV-3 | HER2+ | 0.22 | 0.15 | 0.16 | 2762.61 | >2703 | 0.64 |
| MCF-7 | HER2− | 232.47 | 166.71 | 721.79 | >3438 | >2703 | 0.22 |
| MDA-MB-468 | HER2− | 523.12 | 468.85 | 606.28 | 552.55 | 1558.71 | 0.07 |
2.6. In Vivo Evaluation
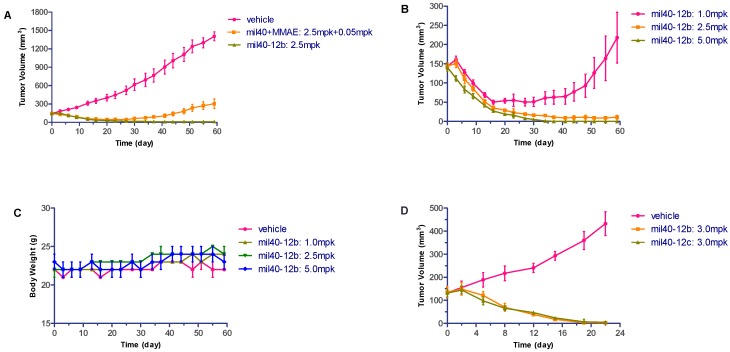
Carrying more payloads usually speeds up the elimination of ADC in vivo [2,31]. Here, the anti-HER2 antibody of mil40 conjugated with an average of four VA-MMAE drug payloads was selected for further in vivo characterization and evaluation. The ADC (mil40-12b) was evaluated in the BT-474 (HTB-20™; ATCC, Maryland, USA) breast tumor model in female non-obese diabetes / severe combined immunodeficiency (NOD/SCID) mice, with naked antibody and unconjugated MMAE as the control group. Compared with vehicle, the control group only caused a partial delay in tumor growth, whereas the ADC treatment group with the same therapeutic dose exhibited very obvious tumor inhibition (Figure 7A). In the ADC medium dose treatment group (2.5 mg/kg), tumors in half of the tumor-bearing animals disappeared (3/6 cures), while all the tumors can still be observed after treatment in the vehicle or mixture treatment group. Furthermore, ADC treatment groups showed significant dose-efficacy dependence, and tumor in all tested animals at 5 mg/kg dose were all disappeared after treatment and no recurrence occurred after one month (Figure 7B). In addition, there was no weight loss observed in all groups treated with ADCs, indicating that the treatments were preliminarily well tolerated (Figure 7C). These results indicate that this VA-based ADC has potent anti-tumor activity and basic safety. Furthermore, the tumor suppression data of ADCs with VA and VC dipeptides showed that VA-based ADC had substantially comparable activity to the ADC of VC (Figure 7D), also without obviously weight loss in all treatment groups (data not shown).
Figure 7.
Xenograft studies of VA-based ADC (mil40-12b). NOD/SCID mice were implanted subcutaneously with BT-474 cells. When the tumors reached ~150 mm3, the animals were given vehicle, the mixture of mil40 and MMAE, and ADCs on: Days 0, 7, 14, and 21 (A–C); or Days 0, 7, and 14 (D). Results are shown as mean ± SD, n = 6/group. (A) Comparison of the efficacy of ADC in the middle dose group with the control group; (B) dose-efficacy dependence of the ADC treatment groups; (C) changes in body weight of the mice during the observation period; (D) comparison of the efficacy of ADCs based on VA and VC dipeptides.
2.7. Hematological Analysis
To further verify the safety of the ADC (mil40-12b), changes in hematology between vehicle and treatment groups were compared at the end of administration and the observation period. The main test indicators included levels of white blood cells (WBCs), red blood cells (RBCs), platelets (PLTs), neutrophils (Neuts), and lymphocytes (Lymph). In contrast to the vehicle group, all of the above hematological parameters from the three ADC treatment groups were similar on Days 28 and 58 (Figure 8).
Figure 8.
Hematological analysis of VA-based ADC (mil40-12b). Numbers “1” and “2” represent the first and second sampling on Days 28 and Days 58, respectively. Red blood cells (RBCs), hemoglobin (HGB), white blood cells (WBCs), platelets (PLTs), neutrophils (Neuts) and lymphocytes (Lymph) were analyzed. Results are shown as mean ± SD, n = 6/group.
Analysis of variance (ANOVA) analysis was conducted on the hematology parameters of the treatment groups on Days 28 and 58, and at the end of drug administration. Unpaired two-tailed t-test for multiple comparisons was used. The level of confidence intervals was set at 95%, and the significance was set at p < 0.05. There was no significant difference between vehicle and ADC treatment groups (1, 2.5, and 5 mg/kg) regarding all parameters from both batches of blood samples (Table 2). Thus, this ADC had low blood toxicity and off-target bone marrow toxicity, which may be the reason it was well tolerated.
Table 2.
The p value in the unpaired t-test between the vehicle and ADC treatment groups.
| Groups | RBC-1 | RBC-2 | HGB-1 | HGB-2 | WBC-1 | WBC-2 | PLT-1 | PLT-2 | Neut-1 | Neut-2 | Lymph-1 | Lymph-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vehicle vs. 1.0 mpk | 0.6519 | 0.3101 | 0.8766 | 0.5107 | 0.6839 | 0.3233 | 0.5315 | 0.8652 | 0.8376 | 0.2773 | 0.8002 | 0.6116 |
| vehicle vs. 2.5 mpk | 0.3300 | 0.4041 | 0.1449 | 0.6756 | 0.2319 | 0.7879 | 0.2542 | 0.8389 | 0.6110 | 0.5141 | 0.2302 | 0.5083 |
| vehicle vs. 5.0 mpk | 0.9090 | 0.4871 | 1.0000 | 0.6930 | 0.5247 | 0.1460 | 0.2221 | 0.4394 | 0.2217 | 0.5862 | 0.3430 | 0.4378 |
2.8. Histopathological Studies
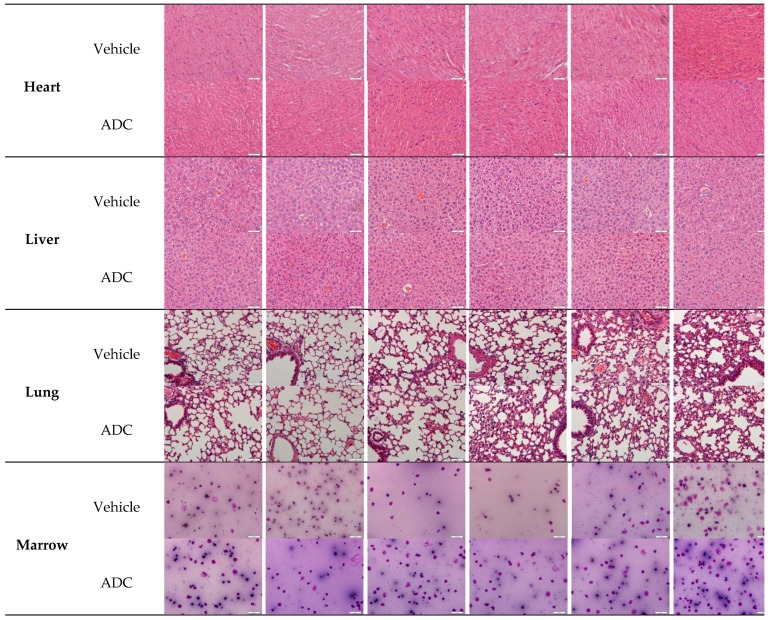
At the end of the xenograft model test, animals in specific groups were used for histopathological studies. Hematoxylin and Eosin (H&E) staining (heart, liver, and lung) and bone marrow smears showed no significant differences in histopathology evaluation, revealing no significant tissue damage produced in animals that received ADC relative to vehicle (Figure 9). This further confirms that this type of VA-based ADC has low systemic toxicity.
Figure 9.
Histopathological studies of VA-based ADC (mil40-12b). At the end of the xenograft test, animals (n = 6/group) in the vehicle and ADC treatment group (2.5 mg/kg) were sacrificed, and the tissue and bone marrow were used for histopathological studies. Scale bar: 46:1; the actual size of each picture is 425 × 320 μm.
3. Materials and Methods
3.1. Chemistry
Unless otherwise indicated, all anhydrous solvents were commercially obtained and stored in Sure-seal bottles under nitrogen. All of the other reagents and solvents were purchased as the highest grade available and used without further purification.
Thin layer chromatography (TLC) was performed using pre-coated silica gel plates (Yantai dexin Bio-Technology Co., Ltd., Yantai, China). Column chromatography was performed using silica gel (200–300 mesh; Yantai Chemical Industry Research Institute, Yantai, China). NMR spectra were recorded on a JNM-ECA-400 400 MHz spectrometer (JEOL Ltd., Tokyo, Japan) using CDCl3 and DMSO-d6 as solvent. Chemical shifts are expressed in (ppm), with tetramethylsilane (TMS) functioning as the internal reference. MS was performed on an API 3000 triple-quadrupole mass spectrometer (AB Sciex, Concord, ON, Canada) equipped with a Turbo Ion Spray electrospray ionization source (AB Sciex, Concord, ON, Canada) that was used for mass analysis and detection. Analyst 1.4 software (AB Sciex, Concord, ON, Canada) was used for data acquisition.
3.2. Synthesis of the Linkers and ADC Payloads
Synthetic routes and analytical data are provided in the experimental protocols.
3.3. Bioconjugation and Purification
Humanized anti-HER2 IgG1 antibodies mil40 (10 mg/mL) in l-Histidine buffer (20 mM), pH 7.5, were treated with tris(2-carboxyethyl)phosphine hydrochloride (TCEP; 2.3 or 4.0 equivalents) at 25 °C for 90 min. To the reduced mAb was added the maleimide drug derivatives (1.2 equivalents /SH group) in ice-cold dimethylacetamide (DMAC) (5% v/v). After 40 min, the reactions were quenched with excess NAC (8 equivalents). The mixture was placed on ice for 30 min before buffer exchange by elution through Sephadex G25, and concentrated by centrifugal ultrafiltration. The conjugates was sterile filtered through a 0.2 μm filter under sterile conditions and stored at −80 °C before use for analysis and testing.
3.4. HPLC Analysis
HIC HPLC was used to determine levels of the molar ratio of drug substitution: 1260 HPLC (Agilent; Wilmington, DE, USA); Butyl-NPR column (2.5 μm, 4.6 × 35 mm, #14947, TOSOH Bioscience; Tokyo, Japan). Here, HIC buffer A was 50 mM potassium phosphate, pH 7.0, and 1.5 M ammonium sulfate; and HIC buffer B was 50 mM potassium phosphate, pH 7.0, 20% isopropanol. The gradient was 100% to 100% B over 15 min; flow rate was 1 mL/min; and UV detection wavelength was 280 nm. The DAR was determined by peak area integration according to the reported method [32].
SEC HPLC was used to determine levels of aggregation within each ADC: 1260 HPLC (Agilent; Wilmington, DE, USA); G3000SWXL analytical column (7.8 mm × 30 cm, #08541, TOSOH Bioscience; Tokyo, Japan). The SEC buffer contained 40 mM sodium phosphate and 150 mM sodium chloride (pH 7.0, 1 mL/min flow rate); and the UV detection wavelength was 280 nm. We performed a needle wash after each injection and include blank runs between each analyte. The aggregation was determined by peak area integration according to the reported method [33].
3.5. Linker Stability Assays In Vitro
The acetyl-l-cysteine (NAC) solution (0.41 mg/mL, PBS, pH 7.4) was added to 12b and 12c (500 mg/mL, DMSO). The reaction mixture was incubated at room temperature for 10 min, after which HPLC analysis revealed complete conversion to NAC-12b and NAC-12c as previously reported [34]. mixtures were incubated at 37 °C in a shaking incubator. Aliquots (200 μL) were collected at subsequent time points (0, 1, 2, 4, 8, 12, 24, 48, 72 h, and 7 days) and frozen at −20 °C. After sampling, all samples were melted at room temperature and analyzed by HPLC. Results were based on the AUC of remaining NAC-12b and NAC-12c at each time point as a percentage of the AUC at t = 0.
Analogously, ADCs that contained different dipeptide linkers were incubated in PBS (pH 7.4; 1 mg/mL) at 37 °C in a shaking incubator. Aliquots (200 μL) were collected at different time points (0, 4, 8, 12, 24, 48, 96, and 168 h) and then stored at −20 °C. All samples were thawed at room temperature before HIC analysis. The DAR of the samples at different time points were calculated as previously described [32].
3.6. Cathepsin B Reactivity
NAC-12b and NAC-12c (500 μg/mL) were prepared according to the above method and the enzymatic reaction temperature was set at 37 °C to mimic physiological conditions. The buffer mixture contained 25 mM sodium acetate, and 1 mM EDTA, at pH 5.5. To 880 μL of the buffer was added the NAC-conjugates solution respectively (100 μL) followed by a solution of Cathepsin B (Sigma: EC 3.4.22.1; #C8571; 20 μL of buffered aqueous solution), and the reaction mixture was incubated at 37 °C [29,35]. Aliquots (100 μL) were taken at subsequent time points (t = 0, 5, 10, 20, 40, 80, 160, and 320 min), and analyzed by LC-MS. Results of the released MMAE was quantified with the standard curve method.
3.7. In Vitro Cytotoxicity
The HER2-positive breast cancer cell line BT-474, SK-BR-3, gastric cancer cell line NCI-N87, ovarian cancer cell line SK-OV-3, weakly positive breast cancer cell line MCF-7 and HER2-negative breast cancer cell line MDA-MB-468 were obtained from ATCC (Manassas, VA, USA) and maintained in RPMI-1640 medium (Cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and Glutamax (Invitrogen). Cells (3.3 × 104 cells/mL) were added to each well of a 384-well plate after which 10 μL compound was also added to the assay plate. The plate was incubated for 4 days at 37 °C, 5% CO2, 95% humidity. Then the plates were incubated at room temperature for about 10 min, 40 μL CTG reagent was added to each well, and the plates were incubated for 30 min at room temperature. Luminescence was detected using the EnSpire Plate Reader, and Prism5 for Windows (Graphpad software, Inc., La Jolla, CA, USA) was used for data analysis, including IC50 calculations.
3.8. Xenograft Studies
Female NOD/SCID mice 6–8 weeks of age, with an average weight of approximately 22 g, were approved by Anikeeper Inc. Female NOD/SCID mice were inoculated subcutaneously with 1 × 107 BT-474 breast cancer cells in 0.2 mL DMEM-Matrigel mixture (1:1 ratio) for tumor development. The treatment started when the mean tumor size reached approximately 150 mm3. The animals were given 1, 2.5, and 5 mg/kg ADC (mil40-12b), antibody + free MMAE, and vehicle alone on Days 0, 7, 14, and 21 or given 3 mg/kg ADCs (mil40-12b and mil40-12c) and vehicle on Days 0, 7, and 14. The animals were monitored twice weekly for body weight and tumor size. Tumor volume was calculated using the formula: TV = a × b2/2, where “a” and “b” are long and short diameters of a tumor, respectively. All procedures related to animal handling, care, and the treatment in this study were performed according to guidelines approved by the Institutional Animal Care and Use Committee of Pharmaron following the guidance of the Association for Assessment and Accreditation of Laboratory Animal Care under the licence number ON-CELL-XEM-06012016 on 1 June 2016.
3.9. Hematology
The animals in the xenograft model were subjected to whole-blood hematological analysis at the end of drug administration (28 days) and after 1 month of recovery (58 days). Then, 100 μL whole blood from each animal was drawn. Some samples were diluted 3 times for a final CBC count because of the volume limitation. ADC treatment-related changes in hematology parameters included RBC, HGB, WBC, PLT, Neut, and Lymph compared to the control, and results are expressed as mean ± SD. The data were analyzed base on the number with corrected dilution factor.
For comparison of hematological indicators, unpaired two-tailed t-test or two-way ANOVA for multiple comparisons was used. The level of significance was set at p < 0.05. Statistical analyses were performed using Prism5 for Windows (Graphpad software, Inc.).
3.10. Histopathology
At the end of the xenograft model test, animals in specific groups were used for histopathological studies. The organs mainly included the heart, liver, and lung. Tissue samples were fixed in 10% buffered formalin for 24 h and transferred into 70% ethanol. Dehydration through 70% ethanol, 85% ethanol, 90% ethanol, 95% ethanol × 2, 100% ethanol × 2 followed by 3 changes of xylene and then paraffin. Finally, the tissues were paraffin block embedded and sectioned. Then, H&E staining, mounting with permount, observation, and photographing under a microscope were performed in addition to bone marrow smears.
4. Experimental Section
4.1. Synthetic Routes
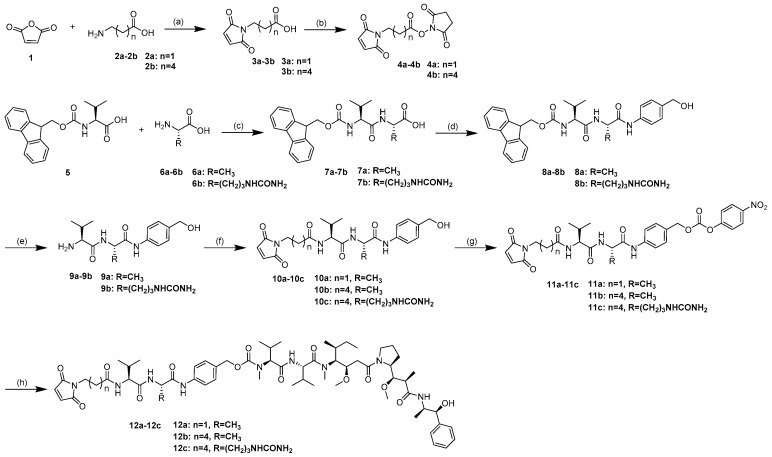
The synthetic routes for the linker-MMAE conjugates 12a–12c are shown in Scheme 1.
Scheme 1.
General synthetic route for synthesis of compounds 12a–12c. Reagents and conditions: (a) AcOH, 120 °C, 6 h, 74–86%; (b) N-Hydroxysuccinimide (SuOH), TFAA, 2,4,6-trimethylpyridine, THF, 0 °C–rt, 1 h, 66–69%; (c) SuOH, DCC, THF, DME, rt, 2 steps, 53–71%; (d) p-Aminobenzyl alcohol, EEDQ, DCM/MeOH, rt, 36 h, 77–92%; (e) Piperidine, DMF, rt, 2 h, 84–90%. (f) 4, DMF, rt, overnight, 79–87%. (g) Bis(4-Nitrophenyl) Carbonate, DIPEA, DMF, rt, 5 h, 86–93%; and (h) MMAE, HOBt, DIPEA, DMF, rt, overnight, 64–80%. rt: room temperature.
4.2. Compound Synthesis and Characterization
3-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)propanoic acid (3a). Maleic anhydride 1 (16.51 g, 168.37 mmol) was added to a solution of the amino acids 2a (10.0 g, 112.25 mmol) in AcOH. The mixture was stirred at 120 °C for 6 h. The reaction mixture was poured into water after cooling to room temperature and extracted with ethyl acetate (3 × 20 mL). The organic layers were combined, washed with brine, dried over anhydrous Na2SO4, and evaporated under reduced pressure to give the crude product. Purification was performed by silica column chromatography in 1:6 ethyl acetate/petroleum ether (v/v). A white solid was obtained (16.37 g, 86%). 1H-NMR (400 MHz, DMSO-d6): δ 2.49 (t, 2H), 3.61 (t, 2H), 7.03 (s, 2H), 12.37 (br, 1H). ESI m/z (M − H)− calculated for C7H7NO4 168.0; found 168.3.
6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanoic acid (3b). Compound 3b was synthesized with the experimental protocols described for 3a. Yield was 74%, as a white solid; 1H-NMR (400 MHz, DMSO-d6): δ 1.24–1.17 (m, 2H), 1.51–1.44 (m, 2H), 2.17 (t, 2H), 3.39 (t, 2H), 7.01 (s, 2H), 11.98 (br, 1H). ESI m/z (M − H)− calculated for C7H7NO4 210.1; found 210.0.
2,5-Dioxopyrrolidin-1-yl 3-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)propanoate (4a). A solution of 3a (4.66 g, 22.0 mmol), 2,4,6-trimethylpyridine (11.6 mL, 88.0 mmol,) and N-Hydroxysuccinimide (SuOH) (5.08 g, 44.0 mmol) in THF (100 mL) was cooled to 0 °C; the trifluoroacetic anhydride (6.12 mL, 44.0 mmol) was added dropwise over 30 min. The reaction mixture was stirred for 1 h at room temperature for 1 h, and then evaporated under reduced pressure and re-dissolved in ethyl acetate (200 mL). The organic layers was washed with 1N HCl and brine, dried over anhydrous Na2SO4, and evaporated under reduced pressure to give the crude product. Purification was performed by silica column chromatography in 1:2 ethyl acetate/petroleum ether (v/v) to give 4.71 g (70%) of 4a as a colorless oil, low temperature placed and converted to a white solid. 1H-NMR (400 MHz, DMSO-d6): δ 2.79 (s, 4H), 3.04 (t, 2H), 3.74 (t, 2H), 7.05 (s, 2H). ESI m/z (M + H)+ calculated for C11H11N2O6 267.1; found 267.1. ESI m/z (M + Na)+ calculated for C11H10N2NaO6 289.0; found 289.1.
2,5-Dioxopyrrolidin-1-yl 6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanoate (4b). Compound 4b was synthesized with the experimental protocols described for 4a. Yield was 69%, as a white solid; 1H-NMR (400 MHz, CDCl3-d1): δ 1.45–1.37 (m, 2H), 1.63 (m, 2H), 1.78 (m, 2H), 2.61 (t, 2H), 2.84 (s, 2H), 3.53 (t, 2H), 6.69 (s, 2H). ESI m/z (M + H)+ calculated for C14H17N2O6 309.1; found 309.4. ESI m/z (M + Na)+ calculated for C14H16N2NaO6 331.1; found 331.2.
(((9H-Fluoren-9-yl)methoxy)carbonyl)-l-valyl-l-alanine (7a). A solution of 5 (25.0 g, 74.0 mmol), SuOH (8.52 g, 74.0 mmol) and DCC (15.27 g, 74.0 mmol) in THF (250 mL) was stirred at room temperature for 16 h. The reaction mixture was cooled to 0 °C for 2 h, and then filtered to remove the insoluble dicyclohexylurea (DCU). The Filter cake was washed with THF; the filtrate was evaporated under reduced pressure to give the crude product as a glassy solid, which was used for the next step directly without further purification. The glassy solid was redissolved in dimethoxyethane (DME) (200 mL) and THF (100 mL), then added the aqueous sodium hydrogencarbonate solution of l-alanine (6.95 g, 78 mmol). The reaction mixture was stirred at room temperature for 16 h, poured into aqueous citric acid solution (400 mL, 15%), and filtered after the filter cake was dried under reduced pressure to give the crude product. The crude product was scattered in ether and purification was performed by ultrasound and filtration 3 times to give 16.2 g (53%) of 7a as a white solid. 1H-NMR (400 MHz, DMSO-d6): δ 0.88 (dd, 6H), 1.27 (d, 3H), 1.96 (m, 1H), 3.89 (q, 1H), 4.22 (m, 4H), 7.33 (t, 2H), 7.43 (m, 3H), 7.74 (t 2H), 7.89 (d, 2H), 8.25 (d, 1H), 12.48 (s, 1H). ESI m/z (M + H)+ calculated for C23H27N2O5 411.2; found 411.3. ESI m/z (M + Na)+ calculated for C23H26N2NaO5 433.2; found 433.4.
(((9H-Fluoren-9-yl)methoxy)carbonyl)-l-valyl-l-Citrulline (7b). Compound 7b was synthesized with the experimental protocols described for 7a. Yield was 70%, as a white solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.89 (dd, 6H), 1.42 (m, 2H), 1.58 (m, 1H), 1.72 (m, 1H), 1.98 (m, 1H), 2.96 (q, 2H), 3.94 (t, 1H), 4.16 (q, 1H), 4.25 (m,3H), 5.43 (s, 2H), 5.98 (t, 1H), 7.33 (t, 2H), 7.43 (q, 3H), 7.76 (t, 2H), 7.89 (d, 2H), 8.21 (d, 1H), 12.61 (s, 1H). ESI m/z (M + H)+ calculated for C26H33N4O6 497.2; found 497.6. ESI m/z (M + Na)+ calculated for C26H32N4NaO6 519.2; found 519.6.
(9H-Fluoren-9-yl)methyl ((S)-1-(((S)-1-((4-(hydroxymethyl)phenyl)amino)-1-oxopropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (8a). p-Aminobenzyl alcohol (3.30 g, 26.8 mmol) and EEDQ (6.63 g, 26.8 mmol) were added to a solution of the 7a (5.50 g, 13.4 mmol) in DCM (150 mL) and methanol (75 mL). The mixture was stirred at room temperature for 36 h, and the reaction mixture was evaporated under reduced pressure to give the crude product. The crude product was scattered in ether and the purification was performed by ultrasound and filtration 3 times to give 5.34 g (77%) of 8a as a white solid. 1H-NMR (400 MHz, DMSO-d6): δ 0.88 (dd, 6H), 1.30 (d, 3H), 2.00 (m, 1H), 3.91 (t, 1H), 4.22 (q, 2H), 4.30 (t, 1H), 4.40 (br, 1H), 4.42 (d, 2H), 5.13 (t, 1H), 7.24 (t, 2H), 7.34 (t, 2H), 7.41 (t, 2H), 7.52 (q, 3H), 7.75 (t, 2H), 7.89 (d, 2H), 8.22 (d, 1H), 9.96 (s, 1H). ESI m/z (M + H)+ calculated for C30H34N3O5 516.2; found 516.4. ESI m/z (M + Na)+ calculated for C30H33N3NaO5 538.2; found 538.3.
(9H-Fluoren-9-yl)methyl ((S)-1-(((S)-1-((4-(hydroxymethyl)phenyl)amino)-1-oxo-5-ureidopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (8b). Compound 8b was synthesized with the experimental protocols described for 8a. Yield was 92%, as a light yellow solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.86 (dd, 6H), 1.41 (m, 2H), 1.57 (m, 1H), 1.69 (m, 1H), 1.98 (m, 1H), 3.00 (m, 2H), 3.92 (q, 1H), 4.23 (q, 2H), 4.30 (q, 1H), 4.41 (q, 3H), 5.12 (t, 1H), 5.42 (s, 2H), 5.98 (t, 1H), 7.23 (d, 2H), 7.32 (m, 2H), 7.41 (m, 2H), 7.46 (d, 1H), 7.54 (d, 2H), 7.74 (t, 2H), 7.89 (d, 2H), 8.12 (d, 1H), 9.99 (s, 1H). ESI m/z (M + Na)+ calculated for C33H39N5NaO6 624.3; found 624.5.
(S)-2-Amino-N-((S)-1-((4-(hydroxymethyl)phenyl)amino)-1-oxopropan-2-yl)-3-methylbutanamide (9a). To a solution of the 8a (4.0 g, 7.76 mmol) in DMF (40 mL) was added piperidine (2 mL). The mixture was stirred at room temperature for 2 h, and evaporated under reduced pressure to give the crude product. Purification was performed by silica column chromatography in 20:1 DCM/methanol (v/v) to give 2.05 g (90%) of 9a as a white solid. 1H-NMR (400 MHz, DMSO-d6): δ 0.85 (dd, 6H), 1.29 (d, 3H), 1.92 (m, 1H), 2.80 (d, 1H), 3.00 (d, 1H), 4.43 (s, 1H), 4.48 (t, 1H), 5.13 (s, 1H), 7.24 (d, 2H), 7.53 (d, 2H), 8.18 (s, 1H), 10.0 (s, 1H). ESI m/z (M + H)+ calculated for C15H24N3O3 294.2; found 294.2. ESI m/z (M + Na)+ calculated for C15H23N3NaO3 316.2; found 316.2.
(S)-2-((S)-2-Amino-3-methylbutanamido)-N-(4-(hydroxymethyl)phenyl)-5-ureidopentanamide (9b). Compound 9b was synthesized with the experimental protocols described for 9a. Yield was 84%, as a light yellow solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.86 (dd, 6H), 1.38 (m, 2H), 1.64 (m, 2H), 1.94 (m, 1H), 2.95 (m, 2H), 3.05 (d, 1H), 4.43 (s, 2H), 4.47 (s, 1H), 5.13 (br, 1H), 5.44 (d, 2H), 6.01 (br, 1H), 7.23 (d, 2H), 7.54 (d, 2H), 8.17 (br, 1H), 10.07 (s, 1H). ESI m/z (M+H)+ calculated for C18H30N5O4 380.2; found 380.3. ESI m/z (M+Na)+ calculated for C18H29N5NaO4 420.2; found 402.3.
(S)-2-(3-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)propanamido)-N-((S)-1-((4-(hydroxymethyl)phenyl)amino)-1-oxopropan-2-yl)-3-methylbutanamide (10a). Compound 4a (1.36 g, 5.11 mmol) was added to a solution of the 9a (1.50 g, 5.11 mmol) in DMF (40 mL). The mixture was stirred at room temperature overnight, and evaporated under reduced pressure to give the crude product. The crude product was scattered in Ether and the purification was performed by ultrasound and filtration 3 times to give 1.84 g (81%) of 10a as a light yellow solid. 1H-NMR (400 MHz, DMSO-d6): δ 0.83 (dd, 6H), 1.30 (d, 3H), 1.93 (m, 1H), 2.44 (t, 2H), 3.36 (m, 2H), 4.12 (q, 1H), 4.37 (m, 1H), 4.42 (d, 2H), 5.12 (t, 1H), 7.01 (s, 2H), 7.23 (d, 2H), 7.54 (d, 2H), 8.05 (d, 1H), 8.19 (d, 1H), 9.83 (s, 1H). ESI m/z (M + H)+ calculated for C22H29N4O6 445.2; found 445.7. ESI m/z (M + Na)+ calculated for C22H28N4NaO6 467.2; found 467.4.
6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)-N-((S)-1-(((S)-1-((4-(hydroxymethyl)phenyl)amino)-1-oxopropan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)hexanamide (10b). Compound 10b was synthesized with the experimental protocols described for 10a. Yield was 87%, as a light yellow solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.84 (dd, 6H), 1.17 (m, 2H), 1.30 (d, 3H), 1.47 (m, 4H), 1.96 (m, 1H), 2.14 (m, 2H), 3.38 ( t, 2H), 4.16 (q, 1H), 4.37 (m, 1H), 4.42 (d, 2H), 5.11 (t, 1H), 7.01 (s, 2H), 7.22 (d, 2H), 7.53 (d, 2H), 7.83 (d, 1H), 8.16 (d, 1H), 9.87 (s, 1H). ESI m/z (M + H)+ calculated for C25H35N4O6 487.3; found 487.5. ESI m/z (M + Na)+ calculated for C25H34N4NaO6 509.2; found 509.4.
6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)-N-((S)-1-(((S)-1-((4-(hydroxymethyl)phenyl)amino)-1-oxo-5-ureidopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)hexanamide (10c). Compound 10c was synthesized with the experimental protocols described for 10a. Yield was 79%, as a light yellow solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.83 (dd, 6H), 1.20 (m, 4H), 1.47 (m, 4H), 1.58 (m, 1H), 1.68 (m, 1H), 1.95 (m, 1H), 2.18 (m, 2H), 2.98 (m, 2H), 3.32 (t, 2H), 4.19 (t, 1H), 4.36 (m, 1H), 4.42 (d, 2H), 5.12 (t, 1H), 5.43 (s, 2H), 5.99 (t, 1H), 7.01 (s, 2H), 7.22 (d, 2H), 7.54 (d, 2H), 7.83 (d, 1H), 8.09 (d, 1H), 9.93 (s, 1H). ESI m/z (M + H)+ calculated for C28H41N6O7 573.3; found 573.6. ESI m/z (M + Na)+ calculated for C25H34N4NaO6 595.3; found 595.7.
4-((S)-2-((S)-2-(3-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)propanamido)-3-methylbutanamido)propanamido)benzyl (4-nitrophenyl) carbonate (11a). Bis(4-Nitrophenyl) Carbonate (0.57 g, 1.89 mmol) and DIPEA (250 μL, 1.42 mmol) were added to a solution of the 10a (0.42 g, 0.95 mmol) in DMF (15 mL). The mixture was stirred at rt for 5 h, and evaporated under reduced pressure to give the crude product. The crude product was scattered in ether and the purification was performed by ultrasound and filtration 3 times to give 0.5 g (86%) of 11a as a light yellow solid. 1H-NMR (400 MHz, DMSO-d6): δ 0.84 (dd, 6H), 1.31 (d, 3H), 1.94 (m, 1H), 2.45 (t, 2H), 3.60 (m, 2H), 4.13 (q, 1H), 4.38 (m, 1H), 5.24 (s, 2H), 7.00 (s, 2H), 7.41 (d, 2H), 7.57 (dt, 2H), 7.64 (d, 2H), 8.02 (d, 1H), 8.19 (d, 1H), 8.31 (dt, 2H), 9.96 (s, 1H). ESI m/z (M + H)+ calculated for C29H32N5O10 610.2; found 610.4. ESI m/z (M + Na)+ calculated for C29H31N5NaO10 632.2; found 632.6.
4-((S)-2-((S)-2-(6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanamido)-3-methylbutanamido)propanamido)benzyl (4-nitrophenyl) carbonate (11b). Compound 11b was synthesized with the experimental protocols described for 11a. Yield was 93%, as a light yellow solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.85 (dd, 6H), 1.18 (m, 2H), 1.31 (d, 3H), 1.47 (m, 4H), 1.96 (m, 1H), 2.15 (m, 2H), 3.36 ( t, 2H), 4.17 (q, 1H), 4.39 (m, 1H), 5.24 (s, 2H), 7.00 (s, 2H), 7.41 (d, 2H), 7.56 (dt, 2H), 7.64 (d, 2H), 7.80 (d, 1H), 8.17 (d, 1H), 8.31 (dt, 2H), 10.00 (s, 1H). ESI m/z (M + H)+ calculated for C32H38N5O10 652.3; found 652.6. ESI m/z (M + Na)+ calculated for C32H37N5NaO10 674.2; found 674.6.
4-((S)-2-((S)-2-(6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanamido)-3-methylbutanamido)-5-ureidopentanamido)benzyl (4-nitrophenyl) carbonate (11c). Compound 11c was synthesized with the experimental protocols described for 11a. Yield was 92%, as a light yellow solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.84 (dd, 6H), 1.22 (m, 4H), 1.47 (m, 4H), 1.58 (m, 1H), 1.68 (m, 1H), 1.95 (m, 1H), 2.17 (m, 2H), 3.00 (m, 2H), 3.28 (t, 2H), 4.19 (t, 1H), 4.38 (m, 1H), 5.24 (s, 2H), 5.44 (s, 2H), 6.01 (t, 1H), 7.01 (s, 2H), 7.41 (d, 2H), 7.56 (dt, 2H), 7.66 (d, 2H), 7.83 (d, 1H), 8.15 (d, 1H), 8.32 (dt, 2H), 10.10 (s, 1H). ESI m/z (M + H)+ calculated for C35H44N7O11 738.3; found 738.6. ESI m/z (M + Na)+ calculated for C35H43N7NaO11 760.3; found 760.6.
4-((S)-2-((S)-2-(3-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)propanamido)-3-methylbutanamido)propanamido)benzyl((S)-1-(((S)-1-(((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(((1S,2R)-1-hydroxy-1-phenylpropan-2-yl)amino)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)(methyl)carbamate (12a). To a solution of the 11a (50.94 mg, 0.0836 mmol), MMAE (40 mg, 0.0557 mmol), HOBt (7.53 mg, 0.0557 mmol) in DMF (3 mL) was added DIPEA (20 μL, 0.1114 mmol). The mixture was stirred at rt overnight, and then poured into water (20 mL). The mixture was extracted with ethyl acetate (3 × 20 mL), the organic layers were combined, washed with brine, dried over anhydrous Na2SO4, and evaporated under reduced pressure to give the crude product. Purification was performed by silica column chromatography in 50:1-10:1 DCM/methanol (v/v) to give 47.6 mg (72%) of 12a as a white solid. 1H-NMR (400 MHz, DMSO-d6): δ 0.88–0.75 (m, 23H), 1.05–0.97 (m, 6H), 1.30–1.23 (m, 6H), 1.54–1.48 (m, 2H), 1.83–1.70 (m, 3H), 1.95–1.91 (m, 2H), 2.14–2.08 (m, 2H), 2.28–2.22 (m, 1H), 2.48–2.38 (m, 4H), 2.89–2.83 (dd, 3H), 2.97 (s, 1H), 3.24–3.12 (m, 8H), 3.33–3.30 (m, 2H), 3.47 (br, 1H), 3.64–3.55 (m, 3H), 4.05–3.92 (m, 2H), 4.13 (t, 1H), 4.26 (t, 1H), 4.49–4.35 (m, 3H), 4.76–4.62 (m, 1H), 5.09–4.96 (m, 2H), 5.44–5.36 (d, 1H), 7.01 (s, 2H), 7.16 (m, 1H), 7.35–7.26 (m, 6H), 7.57 (d, 2H), 7.66 (d, 0.5H), 7.91 (d, 0.5H), 8.05 (d, 1H), 8.12 (d, 0.5H), 8.22 (d, 1H), 8.34 (br, 0.5H), 9.92 (d, 1H). ESI m/z (M + H)+ calculated for C62H94N9O14 1188.6920; found 1188.6913. ESI m/z (M + Na)+ calculated for C62H93N9NaO14 1210.6740; found 1210.6734.
4-((S)-2-((S)-2-(6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanamido)-3-methylbutanamido)propanamido)benzyl((S)-1-(((S)-1-(((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(((1S,2R)-1-hydroxy-1-phenylpropan-2-yl)amino)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)(methyl)carbamate (12b). Compound 12b was synthesized with the experimental protocols described for 12a. Yield was 80%, as a white solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.88–0.73 (m, 25H), 1.05–0.93 (m, 6H), 1.30–1.21 (m, 6H), 1.55–1.43 (m, 6H), 1.81–1.71 (m, 3H), 2.00–1.91 (m, 2H), 2.19–2.06 (m, 4H), 2.28–2.21 (m, 1H), 2.43–2.39 (d, 1H), 2.88–2.83 (dd, 3H), 2.97 (s, 1H), 3.24–3.12 (m, 8H), 3.33–3.30 (m, 2H), 3.49–3.38 (m, 3H), 3.59–3.53 (m, 1H), 4.01 (m, 2H), 4.17 (t, 1H), 4.26 (t, 1H), 4.49–4.36 (m, 3H), 4.75–4.61 (m, 1H), 5.11–4.96 (m, 2H), 5.43–5.36 (dd, 1H), 7.01 (s, 2H), 7.16 (m, 1H), 7.34–7.24 (m, 6H), 7.57 (d, 2H), 7.66 (d, 0.5H), 7.83 (d, 1H), 7.91 (d, 0.5H), 8.10 (d, 0.5H), 8.19 (d, 1H), 8.35 (d, 0.5H), 9.95 (d, 1H). ESI m/z (M + H)+ calculated for C65H100N9O14 1230.7390; found 1230.7380. ESI m/z (M + Na)+ calculated for C65H99N9NaO14 1252.7209; found 1252.7202.
4-((S)-2-((S)-2-(6-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanamido)-3-methylbutanamido)-5-ureidopentanamido)benzyl((S)-1-(((S)-1-(((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(((1S,2R)-1-hydroxy-1-phenylpropan-2-yl)amino)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)(methyl)carbamate (12c). Compound 12c was synthesized with the experimental protocols described for 12a. Yield was 64%, as a white solid; 1H-NMR (400 MHz, DMSO-d6): δ 0.88–0.73 (m, 25H), 1.07–0.97 (m, 6H), 1.59–1.15 (m, 14H), 1.80–1.66 (m, 3H), 1.99–1.93 (m, 2H), 2.20–2.07 (m, 3H), 2.27 (d, 1H), 2.41 (d, 1H), 2.86 (dd, 3H), 3.03–2.94 (m, 3H), 3.32–3.12 (m, 11H), 3.36 (t, 2H), 3.56 (m, 1H), 4.01 (m, 2H), 4.18 (t, 1H), 4.26 (t, 1H), 4.49–4.34 (m, 3H), 4.75–4.62 (m, 1H), 5.11–4.95 (m, 2H), 5.60–5.20 (m, 3H), 6.01 (br, 1H), 7.01 (s, 2H), 7.17 (br, 1H), 7.34–7.24 (m, 6H), 7.41 (br, 0.5H), 7.58 (d, 2H), 7.66 (d, 0.5H), 7.82 (d, 1H), 7.92 (d, 0.5H), 8.12 (d, 1H), 8.35 (br, 0.5H), 10.00 (d, 1H). ESI m/z (M + H)+ calculated for C68H106N11O15 1316.7870; found 1316.7864. ESI m/z (M + Na)+ calculated for C68H105N11NaO15 1338.7689; found 1338.7678.
Mass spectrometry and NMR details of the above synthesized compounds are provided in the Supplementary Materials Figure S1–S38.
5. Conclusions
Currently, ADC is a promising and rapidly growing area of targeted therapeutics for oncology; however, one of the biggest challenges in their development has been the generation of suitable linkers for the conjugation of antibody and cytotoxins. ADCs that load the popular MMAE usually contain VC-based linkers. In this study, a novel ADC that conjugated MMAE to a humanized anti-HER2 antibody via a VA-based cleavable linker was generated to explore its characteristics when used as an anti-tumor drug.
First, VA-containing drug payload exhibits similar performance as VC in terms of in vitro stability and enzymatic activity, and VA-based ADC may be preferred to VC-based ADC on the conjugate process and aggregation. Further, we observed that the VA-based ADC displays favorable potency in vitro and in vivo while not producing significant systemic toxicity during the treatment and recovery period in xenograft studies. In summary, VA-based ADC with MMAE as the payload is shown to be efficacious in the model of HER2-overexpressing cancer, and may have advantages in the generation process and material source of the product compared to current linker technology.
Acknowledgments
We acknowledge the technical support from the Mabworks Biotech Co. Ltd. in conjugating the antibody.
Abbreviations
| ADC | Antibody-drug conjugate |
| DAR | Drug to antibody ratio |
| HER2 | Human epithelial growth factor receptor 2 |
| HIC | Hydrophobic interaction chromatography |
| HPLC | High performance liquid chromatographic |
| MMAE | Auristatin E |
| MS | Mass spectrometric |
| NAC | N-Acetyl-l-cysteine |
| NMR | Nuclear magnetic resonance |
| SEC | Size exclusion chromatography |
| TCEP | tris(2-Carboxyethyl)phosphine hydrochloride |
| VA | Valine-alanine |
| VC | Valine-citrulline |
| RBC | Red blood cell |
| HGB | Hemoglobin |
| WBC | White blood cell |
| PLT | Platelet |
| Neut | Neutrophil |
| Lymph | Lymphocyte |
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/9/1860/s1.
Author Contributions
Song Li, Xinbo Zhou and Wu Zhong conceived the project; Yanming Wang designed the experiments and executed the chemical synthesis; Shiyong Fan performed the pharmacodynamic experiments; Yanming Wang and Shiyong Fan analyzed the data; and Yanming Wang wrote the paper. All of the authors discussed the results and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yan M., Parker B.A., Schwab R., Kurzrock R. HER2 aberrations in cancer: Implications for therapy. Cancer Treat. Rev. 2014;40:770–780. doi: 10.1016/j.ctrv.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Jiang J., Dong L., Wang L., Wang L., Zhang J., Chen F., Zhang X., Huang M., Li S., Ma W., et al. HER2-targeted antibody drug conjugates for ovarian cancer therapy. Eur. J. Pharm. Sci. 2016;93:274–286. doi: 10.1016/j.ejps.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Olayioye M.A. Update on HER2 as a target for cancer therapy—Intracellular signaling pathways of ERBB2/HER2 and family members. Breast Cancer Res. 2001;3:385–389. doi: 10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon D., Eiermann W., Robert N., Pienkowski T., Martin M., Press M., Mackey J., Glaspy J., Chan A., Pawlicki M., et al. Adjuvant trastuzumab in HER2+ breast cancer. N. Engl. J. Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurvitz S.A., Andre F., Jiang Z., Shao Z., Mano M.S., Neciosup S.P., Tseng L.-M., Zhang Q., Shen K., Liu D., et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2+ advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal N., Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S., Sami A., Xiang J. HER2-directed therapy: Current treatment options for her2-positive breast cancer. Breast Cancer. 2015;22:101–116. doi: 10.1007/s12282-015-0587-x. [DOI] [PubMed] [Google Scholar]
- 8.Saini K.S., Azim H.A., Metzger-Filho O., Loi S., Sotiriou C., de Azambuja E., Piccart M. Beyond trastuzumab: New treatment options for HER2+ breast cancer. Breast. 2011;20:S20–S27. doi: 10.1016/S0960-9776(11)70289-2. [DOI] [PubMed] [Google Scholar]
- 9.Molina M.A., Codony-Servat J., Albanell J., Rojo F., Arribas J., Baselga J. Trastuzumab (herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 10.Lambert J.M., Chari R.V. Ado-trastuzumab emtansine (t-DM1): An antibody-drug conjugate (ADC) for HER2+ breast cancer. J. Med. Chem. 2014;57:6949–6964. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 11.Nolting B. Linker technologies for antibody-drug conjugates. In: Ducry L., editor. Antibody-Drug Conjugates. Volume 1045. Humana Press; New York, NY, USA: 2013. pp. 71–100. [DOI] [PubMed] [Google Scholar]
- 12.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., Pegram M., Oh D.Y., Dieras V., Guardino E., et al. Trastuzumab emtansine for HER2+ advanced breast cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zolot R.S., Basu S., Million R.P. Antibody-drug conjugates. Nat. Rev. Drug Discov. 2013;12:259–260. doi: 10.1038/nrd3980. [DOI] [PubMed] [Google Scholar]
- 14.Jain N., Smith S.W., Ghone S., Tomczuk B. Current adc linker chemistry. Pharm. Res. 2015;32:3526–3540. doi: 10.1007/s11095-015-1657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapra P., Hooper A.T., O’Donnell C.J., Gerber H.P. Investigational antibody drug conjugates for solid tumors. Expert. Opin. Investig. Drugs. 2011;20:1131–1149. doi: 10.1517/13543784.2011.582866. [DOI] [PubMed] [Google Scholar]
- 16.De Goeij B.E., Lambert J.M. New developments for antibody-drug conjugate-based therapeutic approaches. Curr. Opin. Immunol. 2016;40:14–23. doi: 10.1016/j.coi.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Drachman J.G., Senter P.D. Antibody-drug conjugates: The chemistry behind empowering antibodies to fight cancer. Hematol. Am. Soc. Hematol. Educ. Program. 2013;2013:306–310. doi: 10.1182/asheducation-2013.1.306. [DOI] [PubMed] [Google Scholar]
- 18.Koblinski J.E., Ahram M., Sloane B.F. Unraveling the role of proteases in cancer. Clin. Chim. Acta. 2000;291:113–135. doi: 10.1016/S0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 19.Dubowchik G.M., Firestone R.A., Padilla L., Willner D., Hofstead S.J., Mosure K., Knipe J.O., Lasch S.J., Trail P.A. Cathepsin b-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: Model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug. Chem. 2002;13:855–869. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 20.Senter P.D., Sievers E.L. The discovery and development of brentuximab vedotin for use in relapsed hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012;30:631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 21.Beck A., Goetsch L., Dumontet C., Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug. Discov. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 22.Kung Sutherland M.S., Walter R.B., Jeffrey S.C., Burke P.J., Yu C., Kostner H., Stone I., Ryan M.C., Sussman D., Lyon R.P., et al. Sgn-cd33a: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant aml. Blood. 2013;122:1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- 23.Tiberghien A.C., Levy J.N., Masterson L.A., Patel N.V., Adams L.R., Corbett S., Williams D.G., Hartley J.A., Howard P.W. Design and synthesis of tesirine, a clinical antibody-drug conjugate pyrrolobenzodiazepine dimer payload. ACS Med. Chem. Lett. 2016;7:983–987. doi: 10.1021/acsmedchemlett.6b00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson D.Y., Ha E., Sauer P., Bowers S., Bruhns M.F., Monteon J., Behrens C., Halcomb R.L. Novel Antibody-Drug Conjugates and Related Compounds, Compositions, and Methods of Use. Patent WO2016064749A2. 2016 Apr 28;
- 25.Theunissen J.-W., Kim S.Y., Presta L.G., Jackson D.Y., Ha E. Preparation of Humanized Anti-Human Protein C16ORF54 Antibodies and Immunoconjugates for Cancer Diagnosis and Therapy. Patent WO2015161247A1. 2015 Octorber;
- 26.Mang Y., Zhao Z., Zeng Z., Wu X., Li Z., Zhang L. Efficient elimination of CD103-expressing cells by anti-CD103 antibody drug conjugates in immunocompetent mice. Int. Immunopharmacol. 2015;24:119–127. doi: 10.1016/j.intimp.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Corso A.D., Neri D. Linker stability influences the anti-tumor activity of acetazolamide-drug conjugates for the therapy of renal cell carcinoma. J. Control. Release. 2016;246:39–45. doi: 10.1016/j.jconrel.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffrey S.C., Nguyen M.T., Andreyka J.B., Meyer D.L., Doronina S.O., Senter P.D. Dipeptide-based highly potent doxorubicin antibody conjugates. Bioorg. Med. Chem. Lett. 2006;16:358–362. doi: 10.1016/j.bmcl.2005.09.081. [DOI] [PubMed] [Google Scholar]
- 29.Gikanga B., Adeniji N.S., Patapoff T.W., Chih H.W., Yi L. Cathepsin b cleavage of vcmmae-based antibody-drug conjugate is not drug location or monoclonal antibody carrier specific. Bioconjug. Chem. 2016;27:1040–1049. doi: 10.1021/acs.bioconjchem.6b00055. [DOI] [PubMed] [Google Scholar]
- 30.Lewis Phillips G.D., Li G., Dugger D.L., Crocker L.M., Parsons K.L., Mai E., Blattler W.A., Lambert J.M., Chari R.V., Lutz R.J., et al. Targeting HER2+ breast cancer with trastuzumab-dm1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 31.Hamblett K.J., Senter P.D., Chace D.F., Sun M.M., Lenox J., Cerveny C.G., Kissler K.M., Bernhardt S.X., Kopcha A.K., Zabinski R.F., et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang J. Drug-to-antibody ratio (DAR) and drug load distribution by hydrophobic interaction chromatography and reversed phase high-performance liquid chromatography. In: Ducry L., editor. Antibody-Drug Conjugates. Volume 1045. Humana Press; New York, NY, USA: 2013. pp. 275–283. [DOI] [PubMed] [Google Scholar]
- 33.Stefano J.E., Busch M., Hou L., Park A., Gianolio D.A. Micro- and mid-scale maleimide-based conjugation of cytotoxic drugs to antibody hinge region thiols for tumor targeting. In: Ducry L., editor. Antibody-Drug Conjugates. Volume 1045. Humana Press; New York, NY, USA: 2013. pp. 145–171. [DOI] [PubMed] [Google Scholar]
- 34.Jeffrey S.C., Andreyka J.B., Bernhardt S.X., Kissler K.M., Kline T., Lenox J.S., Moser R.F., Nguyen M.T., Okeley N.M., Stone I.J., et al. Development and properties of beta-glucuronide linkers for monoclonal antibody-drug conjugates. Bioconjug. Chem. 2006;17:831–840. doi: 10.1021/bc0600214. [DOI] [PubMed] [Google Scholar]
- 35.Hochdorffer K., Abu Ajaj K., Schafer-Obodozie C., Kratz F. Development of novel bisphosphonate prodrugs of doxorubicin for targeting bone metastases that are cleaved ph dependently or by cathepsin b: Synthesis, cleavage properties, and binding properties to hydroxyapatite as well as bone matrix. J. Med. Chem. 2012;55:7502–7515. doi: 10.1021/jm300493m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.