Abstract
Renal podocytes form the main filtration barrier possessing unique phenotype maintained by proteins including podocalyxin and nephrin, which are modulated in pathological conditions. In diabetic nephropathy (DN), podocytes become structurally and functionally compromised. Nephrin, a structural backbone protein of the slit diaphragm, acts as regulator of podocyte intracellular signalling with renoprotective role. Vitamin D3 through its receptor, VDR, provides renal protection in DN but limited data exist about its effect on podocytes. In this study, we used isolated rat glomeruli to assess podocalyxin and nephrin expression after treatment with the 1,25‐dihydroxyvitamin D3 analogue paricalcitol in the presence of normal and diabetic glucose levels. The role of 1,25‐dihydroxyvitamin D3 (calcitriol) and its analogue, paricalcitol, on podocyte morphology and survival was also investigated in the streptozotocin (STZ)‐diabetic animal model. In our ex vivo model, glomeruli exhibited high glucose‐mediated down‐regulation of podocalyxin, and nephrin, while paricalcitol reversed the high glucose‐induced decrease of nephrin and podocalyxin expression. Paricalcitol treatment enhanced VDR expression and promoted VDR and RXR co‐localization in the nucleus. Our data also indicated that hyperglycaemia impaired survival of cultured glomeruli and suggested that the implemented nephrin down‐regulation was reversed by paricalcitol treatment, initiating Akt signal transduction which may be involved in glomerular survival. Our findings were further verified in vivo, as in the STZ‐diabetic animal model, calcitriol and paricalcitol treatment resulted in significant amelioration of hyperglycaemia and restoration of nephrin signalling, suggesting that calcitriol and paricalcitol may provide molecular bases for protection against loss of the permselective renal barrier in DN.
Keywords: Diabetic nephropathy, glomerulus, nephrin, podocalyxin, Vitamin D3, Vitamin D3 Receptor, high glucose, paricalcitol, STZ
Introduction
Active vitamin D3 is an important hormone implicated in prevention and treatment of hyperparathyroidism in dialysis patients. Increasing amount of data suggests a beneficial role of vitamin D3 in chronic kidney disease (CKD), associated with increased survival of patients 1, 2. One of the most common causes of end‐stage renal disease is DN 3. Vitamin D may provide renoprotection in DN in part via the renin‐angiotensin system 4, 5, 6. DN is characterized by pathological changes, such as thickening of the glomerular basement membrane and mesangial expansion and by the clinical hallmark of proteinuria 7. The field of research on DN has mainly focused on podocytes which are important for maintaining glomerular permselectivity 8.
Interestingly, podocytes lose their negative surface charge or slit diaphragm integrity, leading to disruption of the filtration barrier and subsequently to DN 9, 10. The slit diaphragm, a specialized cell junction between adjacent podocytes, is required for functional glomerular filtration, and its central component is nephrin. The nephrin‐associated protein complex includes several molecules, for example CD2‐associated protein (CD2AP), podocin and zonula occludens‐1 (ZO‐1) 11. It should be noted that the slit diaphragm proteins nephrin, CD2AP and podocin, in addition to their structural functions, are able to initiate PI3K/AKT‐dependent signal transduction in glomerular podocytes 12. It is well known that PI3K/p‐Akt signalling pathway protects glomerular podocytes and ameliorates proteinuria 13, 14, 15. However, there is no direct evidence to date to conclusively demonstrate that activation of the PI3K/AKT pathway by nephrin in the podocyte protects against apoptosis.
The anti‐proteinuric activity of vitamin D3 analogue has been confirmed in a number of clinical trials in diabetic patients with CKD 16. The renoprotective property of vitamin D3 has been confirmed through studies with various animal models of kidney disease 17. Vitamin D receptor (VDR), a member of the nuclear receptor superfamily, mediates the biological activities of vitamin D3 by forming a heterodimer with retinoid X receptor (RXR) and binding to vitamin D response elements (VDREs) in the regulatory region of target genes to regulate gene expression 18.
For example, we reported that vitamin D3 and its analogue, paricalcitol, maintained and induced the expression levels of specific components of human podocytes in vitro, restoring structural and functional integrity in a VDR‐dependent manner 19. Paricalcitol is a selective VDR activator (VDRA) with minimal hypercalcemic effects that reduces proteinuria in DN 16.
In this study, we explore the therapeutic role of paricalcitol in vivo and ex vivo, in isolated rat glomeruli exposed to diabetic conditions and provide insights into the molecular mechanisms activated via paricalcitol and the nephrin‐PI3K/pAkt signalling pathway in podocyte injury.
Materials and methods
Experimental animals
Wistar rats (180–220 g) were used for all the studies performed. Rats were housed in the animal facility of NCSR ‘Demokritos’ (EL25 BIO 019020022) and were maintained with free access to water and rat chow with a 12‐hrs light–dark cycle. Experimental protocols were approved by the Institutional Animal Care, and all animal experimentations were carried out in agreement with the ethical recommendations of the European Communities Council Directive of 22 September 2010 (2010/63/EU), animal welfare assurance number: protocol nbr: 6464. All procedures were performed under phenobarbital anaesthesia.
Antibodies and reagents
Goat polyclonal anti‐nephrin N20, mouse monoclonal anti‐VDR (D‐6) and rabbit polyclonal anti‐RXR (C‐20) antibodies were purchased from Santa Cruz Biotechnology. Rabbit polyclonal anti‐PODXL antibody was purchased from Thermo Fisher Scientific (MA, USA). Rabbit monoclonal anti‐p85α/PI3 kinase antibody was purchased from Millipore (MA, USA). Rabbit polyclonal anti‐Akt, rabbit monoclonal anti‐phospho‐Ser473 Akt, rabbit polyclonal anti‐cleaved caspase‐3 and anti‐PARP antibodies were purchased from Cell Signaling. Mouse monoclonal anti‐tubulin antibody was purchased from Sigma‐Aldrich Ltd., (Gillingham, UK) Fluorescent secondary antibodies, donkey anti‐rabbit Alexa Fluor 594, donkey anti‐goat Alexa Fluor 488, donkey antimouse Alexa Fluor 594 and donkey antimouse Alexa Fluor 488 were purchased from Molecular Probes, Invitrogen Thermo Fisher Scientific, MA, USA. STZ was purchased from Sigma‐Aldrich.
Isolation and culture of rat glomeruli
Glomeruli isolation was carried out as described by Sharma et al. 20. Briefly, a high mid‐line incision on the abdomen exposed the abdominal cavity and total bilateral nephrectomy was performed to anesthetized animals. The kidney capsules were removed, and glomeruli were isolated following consecutive passage of the mashed cortexes through screens of 80‐ and 200‐mesh size. Glomeruli were recovered from atop the 200‐mesh screen into Dulbecco's modified Eagle's medium (DMEM; Biochrom Gmb, Berlin, Germany) supplemented with 10% foetal calf serum (FCS; Biochrom Gmb),15 mM HEPES (Biochrom Gmb), 5 mg/ml insulin‐transferrin‐sodium selenite (ITS; Sigma‐Aldrich), 50 nM dexamethasone (Sigma‐Aldrich), 2 mM glutamine (Biochrom Gmb) and 5 mM or 25 mM D‐glucose (Sigma‐Aldrich) and incubated at 37°C in a 5% (v/v) CO2 humidified chamber for 4 or 8 days. The isolated rat glomeruli were treated with 150 nM or 300 nM paricalcitol (VDRA; Abbott, Hellas (BGP, Attika, Greece)) for 4 days in presence of normal (5 mM) or high (25 mM) glucose levels.
Western blotting and immunoprecipitation
For Western blotting, following appropriate treatment where applicable, samples were lysed in modified RIPA lysis buffer [(150 mmol/l NaCl, 50 mmol/l Tris‐HCl (pH 7.4), 1 mmol/l 129 Na2EDTA, 1% (v/v) Triton X‐100, 0.25% (w/v) sodium deoxycholate and 0.1% (w/v) 130 sodium dodecyl sulphate (SDS), 1 × PhosSTOP (cocktail of phosphatase inhibitors, Roche, Mannheim, Germany) and 1 × Protease inhibitors cocktail (Roche)]. Protein concentration was determined by the Bradford colorimetric assay (Pierce). Immunoblot analysis of cell lysates was performed as previously described 19. To ensure equal amounts of protein loading, the blots were stripped (Re‐Blot Plus Mild Solution; Millipore) and re‐probed with anti‐β‐tubulin monoclonal antibody. Blots were developed using the ECL detection system (Thermo Fisher Scientific Inc.) and the ImageQuant™ LAS 4000 (GE Healthcare, UK). Quantification was performed using ImageJ software (IJ 1.46R).
Immunoprecipitation of nephrin and nephrin‐associated proteins from lysates was performed using 400 μg of total protein from each sample of isolated rat glomeruli. Briefly, 400 μg of total protein from each lysate was incubated overnight at 4°C with anti‐nephrin N20 antibody. Immune complexes were captured with protein G‐agarose (Merck KGaA) for 4 hrs at 4°C, followed by three washes in ice‐cold PBS. Immune complexes were extracted with 60 μl boiling 2×Laemmli sample buffer containing β‐mercaptoethanol. Fifty microlitres of each supernatant fraction was processed for Western blot analysis.
Immunocytochemistry and fluorescence labelling of isolated glomeruli
Glomeruli cultured on glass coverslips were fixed with 4% (w/v) paraformaldehyde in PBS pH 7.4, for 15 min. at room temperature. After 10 min. quenching with 50 mmol/lt NH4Cl, cells were permeabilized with 0.25% (v/v) Triton X‐100 in PBS for 10 min., washed with PBS and blocked with 1% (w/v) BSA in PBS for 120 min. at room temperature. Glomeruli were then incubated with anti‐nephrin N‐20 antibody, antip85α/PI3 kinase antibody, anti‐phospho‐Ser473 Akt, anti‐Akt, anti‐VDR and anti‐RXR antibody overnight at 4°C and finally with the appropriate fluorescent secondary antibodies for 1 hr at room temperature (all antibodies in PBS containing 1% (w/v) BSA). Finally, coverslips were washed with PBS, incubated with DAPI (0.7 μg/ml) in PBS for 5 min. and washed again with PBS before mounting them with Dako Fluorescent Mounting Medium. Fluorescent specimens were examined with a confocal laser‐scanning microscope (TCS SP5 Confocal System, Leica Mannheim, Germany). Images were obtained and processed with Adobe Photoshop CS4 version 11.0, software.
In situ cell death detection—TUNEL assay
Isolated rat grown on glass coverslips was fixed with 4% (w/v) paraformaldehyde in PBS pH 7.4, for 30 min. at room temperature, permeabilized for 30 min. at room temperature with 0.1% Triton X in 0.1% sodium citrate (pH6.0) and finally incubated with 50 μl TUNEL reaction mixture (TdT enzyme and fluorochrome labelling solution) for 1 hr at 37°C in the dark [In Situ Cell Death Detection Kit, TMR red (Roche Diagnostics)]. Finally, the isolated glomeruli were washed with PBS and incubated with DAPI in PBS for 5 min., before mounting with Dako Fluorescent Mounting Medium (Dako). Specimens were examined with a confocal laser‐scanning microscope (TCS SP5 Confocal System; Leica). Images were obtained and processed with Adobe Photoshop CS4 version 11.0, software.
Animal treatment and design
To establish the diabetic model, STZ (Sigma‐Aldrich) dissolved in 0.1 mol/l citrate buffer (pH 4.5) at 65 mg/kg was single‐intraperitoneally injected (n = 15), while control rats received only the citrate buffer solution (n = 5). Blood glucose levels were derived from the caudal vein and measured 72 hrs after the injection using the Contour Link Meter (Bayer Diabetes, Canada). The model standard was confirmed after blood glucose measurements greater than 400 mg/dl. After 1 week of acclimatization, rats were randomly divided into four groups: control (C) (n = 5), STZ diabetic (DN) (n = 5), STZ rats treated with 400 ng/kg paricalcitol daily (DN‐P) (n = 5) and STZ rats treated with 100 ng/kg calcitriol daily (DN‐C) (n = 5). All animals were sacrificed at 5 weeks after STZ injection. At the end of the experiment and prior to sacrifice, bodyweights, blood samples and 24‐hrs urine samples were collected in metabolic cages. The kidneys were used for glomeruli isolation and for histological assessments.
Histopathological analysis
Kidneys were fixed in 10% formalin and embedded in paraffin for histological investigation. Kidney sections were stained with sirius red reagent (Sigma‐Aldrich) for collagen determination and examined by light microscopy in a blinded manner. We analysed randomly selected non‐overlapping stained glomeruli from each group.
Statistics
Experiments were performed at least three times, and results were expressed as means ± S.D. The Student's t‐test method was used to determine the statistical significance, and the P‐values are indicated in the figures, where (*) represents P < 0.05, (**) represents P < 0.01 and (***) represents P < 0.001. Statistical analysis for assessment of VDRA treatment in DN animal model was performed by one‐way anova with Greenhouse‐Geisser correction using Graph Prism version 7 (GraphPad software, CA, USA).
Results
VDRA enhanced the expression levels of podocyte markers and VDR in isolated glomeruli
Accumulating evidence suggests that VDRA, such as paricalcitol, can restore glucose‐mediated down‐regulation of nephrin and podocalyxin (PODXL) expression in T‐SV40‐Immortalized Human Glomerular Epithelial Cells 19. In an effort to determine whether VDRA may also affect functioning glomeruli, we analysed nephrin, PODXL and VDR expression levels in isolated rat glomeruli cultured in normal (5 mM) or diabetic (25 mM) glucose levels in the presence of 150 or 300 nM paricalcitol. Glomeruli cultured in the presence of 5 mM glucose displayed enhanced nephrin and PODXL expression levels when they were exposed to VDRA (Fig. 1A). Diabetic glucose levels induced a statistically significant decrease in nephrin and PODXL protein levels in isolated glomeruli (Fig. 1A). Interestingly, we observed that in glomeruli exposed to 25 mM glucose, VDRA increased the expression levels of nephrin and PODXL which were apparently restored to normal levels (Fig. 1B). In a similar pattern, VDRA enhanced VDR expression levels in either glucose condition (Fig. 1A and B).
Figure 1.
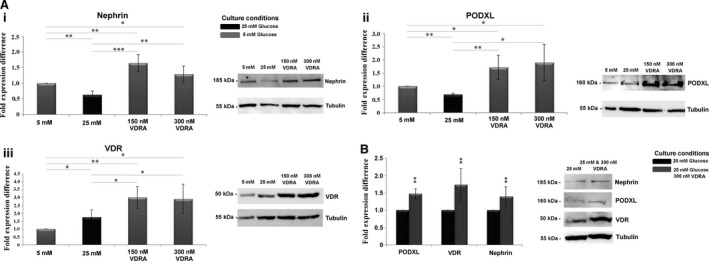
VDRA increased the expression levels of Nephrin, PODXL and VDR in isolated rat glomeruli. (A) Western blot analysis in isolated rat glomeruli cultured in 5 mM or 25 mM glucose in presence of 150 nM or 300 nM VDRA (paricalcitol) for (i) nephrin, (ii) PODXL and (iii) VDR. (B) Western blot analysis in isolated rat glomeruli cultured in 25 mM glucose or in 25 mM glucose in presence of VDRA for nephrin, PODXL and VDR. Western blot quantification was performed using ImageJ software, and the results were normalized to the tubulin positive control and then to isolated rat glomeruli cultured in 5 mM glucose. The values are presented as the mean ± S.D. of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001 Student's t‐test).
VDRA induced nuclear translocation of VDR and co‐localization with RXR
The subcellular localization of VDR and RXR in podocytes was analysed by immunofluorescence analysis in isolated rat glomeruli cultured in normal or hyperglycaemic conditions in presence or absence of 300 nM VDRA. In both normal and high glucose levels, these proteins partly co‐localized mainly in the nucleus (Fig. 2). Treatment with VDRA greatly enhanced VDR and RXR co‐localization in the nucleus (Fig. 2). Additionally, immunofluorescence staining of nephrin was performed, to verify that the VDR‐expressing cells were podocytes.
Figure 2.
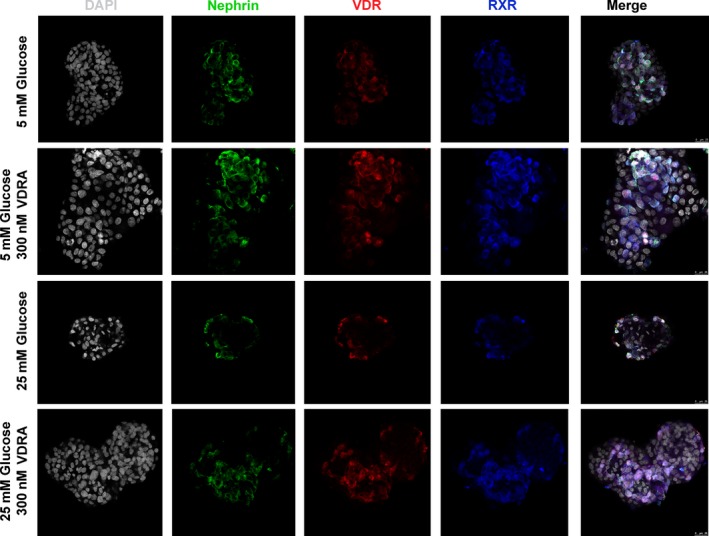
VDRA induced nuclear translocation of VDR and co‐localization with RXR. Representative confocal microscopy images of triple staining for Nephrin (green), VDR (red), RXR (blue) and DAPI (grey) in isolated rat glomeruli cultured in 5 mM glucose, 25 mM glucose and in presence of 300 nM VDRA. Original magnifications, 63×.
High glucose impaired nephrin‐mediated PI3K‐pAkt signalling pathway
Isolated glomeruli exhibited glucose‐dependent down‐regulation of nephrin expression. In view of the fact that nephrin signalling in podocytes is mediated via PI3K activation, we investigated the association of nephrin with the p85 regulatory subunit of PI3K in isolated glomeruli. Immunofluorescence co‐localization studies with nephrin and p85α‐PI3K in normo‐glycemic and hyperglycaemic glomeruli showed that nephrin is partially co‐localized with p85α‐PI3K after treatment with VDRA (Fig. 3A). These findings were further verified by co‐immunoprecipitation experiments with nephrin‐specific antibodies that showed that endogenous nephrin was associated with the p85α regulatory subunit of PI3K (Fig. 3B). We next examined whether this association may trigger PI3K‐dependent Akt signalling. Western blot analysis revealed that phosphorylation of Akt was significantly reduced in the presence of diabetic culture levels compared to glomeruli exposed to normal glucose conditions (Fig. 4A and B). Isolated glomeruli exposed to VDRA, exhibited a twofold increase of Ser473 Akt phosphorylation compared to non‐treated glomeruli (Fig. 4A and B), whereas basal Akt levels remained unaltered (Fig. S1). These results indicated that VDRA mediated enhancement of nephrin expression, may participate in PI3K activation and Akt phosphorylation in rat glomeruli ex vivo, thus inducing activation of the PI3K‐Akt signalling pathway.
Figure 3.
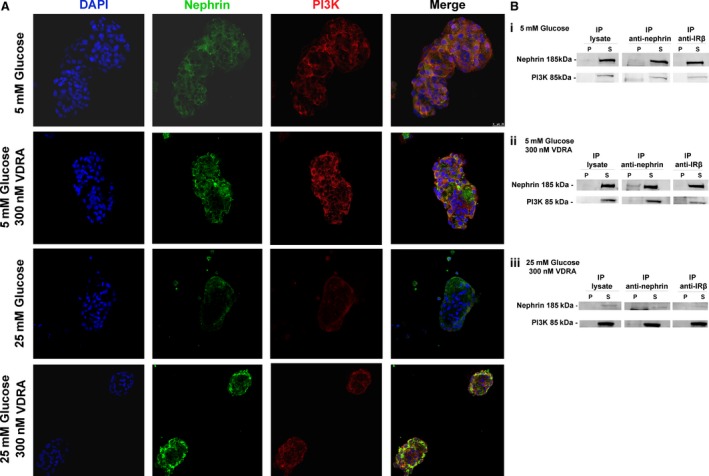
Nephrin and PI3K are co‐immunoprecipitated in isolated rat glomeruli lysate. (A) Representative confocal microscopy images of double staining for Nephrin (green), PI3K (red) and DAPI (blue) in isolated rat glomeruli cultured in 5 mM glucose, 25 mM glucose and in presence of VDRA. Original magnifications, 63×. (B) Immunoprecipitation (IP) with anti‐nephrin in isolated rat glomeruli cultured in (i) 5 mM glucose, (ii) 5 mM glucose in presence of VDRA and (iii) 25 mM glucose in presence of VDRA. No interactions were found with anti‐CBP, serving as negative control. P, precipitate; S, supernatant.
Figure 4.
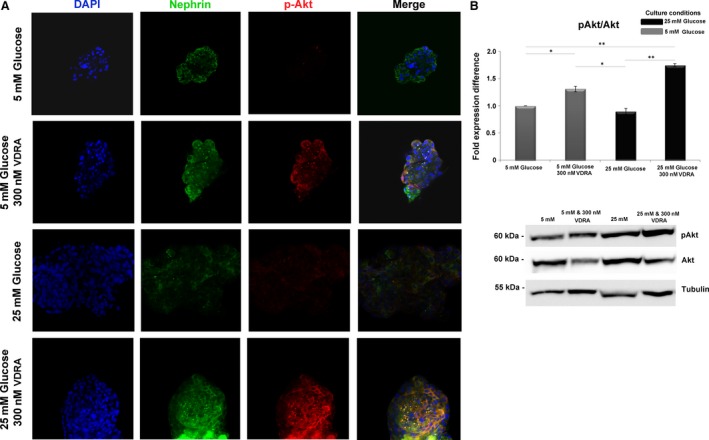
Paricalcitol enhanced Akt phosphorylation. (A)Representative confocal microscopy images of double staining for nephrin (green), pAkt (red) and DAPI (blue) in isolated rat glomeruli cultured in 5 mM glucose, 25 mM glucose and in presence of VDRA. Original magnifications, 63×. (B) Western blot analysis of pAkt/Akt in isolated rat glomeruli cultured in 5 mM glucose, 25 mM glucose and in presence of VDRA. Western blot quantification was performed using ImageJ software, and the results were normalized to the tubulin positive control and then to isolated rat glomeruli cultured in 5 mM glucose. The values are presented as the mean ± S.D. of three independent experiments (*P < 0.05; **P < 0.01 Student's t‐test).
VDRA rescues glomeruli from glucose‐induced apoptosis
In sequence, we investigated the effect of VDRA on cell survival. Caspase‐3 activity was monitored by caspase‐processing and cleavage of its downstream target, poly (ADP‐ribose) polymerase (PARP). Detection of caspase‐mediated cleavage of PARP has been established as a hallmark of apoptosis 21. Western blotting analysis demonstrated that upon exposure to 25 mM glucose levels, isolated glomeruli displayed increased levels of the large fragment (19 kD) of activated caspase‐3 and increased ratio of cleaved/uncleaved PARP, compared to glomeruli grown in the presence of 5 mM glucose levels (Fig. 5B and C). In addition, the degree of apoptosis was evaluated by detection of DNA strand breaks (in situ TUNEL and DAPI staining). Analysis of glomerular cells for DNA fragmentation (TUNEL‐positive cells) revealed that no TUNEL‐positive cells were observed in isolated glomeruli cultured in normal glucose levels with or without VDRA (Fig. 5A). However, increased glucose concentration resulted in significant increase in the number of TUNEL‐positive cells (Fig. 5A). This glucose‐induced susceptibility to apoptosis was inhibited by VDRA treatment (Fig. 5A). Taken together these findings clearly confirm the pivotal role of VDRA on cell survival.
Figure 5.
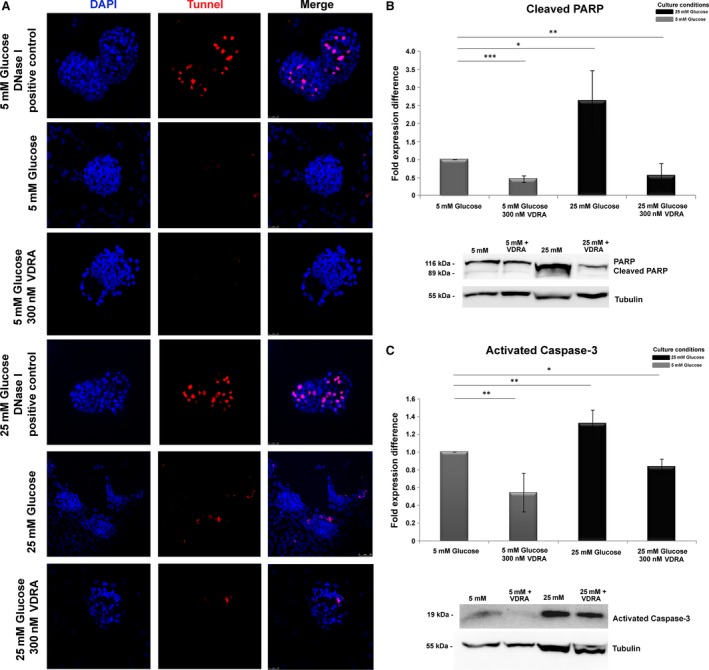
Determination of apoptosis in isolated rat glomeruli by TUNEL assay. (A) Representative fluorescent images of TUNEL‐positive cells in isolated rat glomeruli cultured in 5 mM glucose, 25 mM glucose and in presence of VDRA. Original magnifications, 63×. (B) Western blot analysis of cleaved PARP in isolated rat glomeruli cultured in 5 mM glucose, 25 mM glucose and in presence of VDRA. (C) Western blot analysis of activated caspase‐3 in isolated rat glomeruli cultured in 5 mM glucose, 25 mM glucose and in presence of VDRA. Western blot quantification was performed using ImageJ software, and the results were normalized to the tubulin positive control and then to isolated rat glomeruli cultured in 5 mM glucose. The values are presented as the mean ± S.D. of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0,001 Student's t‐test).
In vivo administration of VDRA in STZ animal model
To investigate the effect of VDRA on glomerular function in vivo, we performed studies in the streptozotocin‐induced (STZ) diabetic animal model. This is a widely used model for diabetes that has been employed for assessing the mechanisms of diabetes as well as screening potential therapies for the treatment of this condition, and evaluation of therapeutic options.
To assess the renal protective effect of VDRA, fasting blood glucose levels and bodyweights were measured starting from 3 days after STZ injection. As shown in Figure 6A, the rats in the DN group demonstrated elevated blood glucose levels, which were significantly higher than those of the non‐diabetic, control rats (P < 0.001). The kidney/bodyweight of the DN rats presented a significant increase compared to control rats (Fig. 6Bi, ii). During the study period, this increase was slightly abated in the DN‐P rats, but still remained elevated compared to non‐diabetic, control rats (Fig. 6Bi, ii). Interestingly, the 24‐hrs urine levels of DN‐P and DN‐C rats were significantly improved, in comparison with the DN group (P < 0.0001; Fig. 6C). Additionally, general parameters, namely urine creatinine and urine nitrogen, were also estimated. Specifically, evidence of VDRA‐induced podocyte protection was obtained from the urine nitrogen and urine creatinine levels that were significantly improved in comparison with the DN group (Fig. 6Di, ii). The above data suggest that VDRA treatment significantly reduced proteinuria and protected podocytes in the rats with experimentally induced diabetes.
Figure 6.
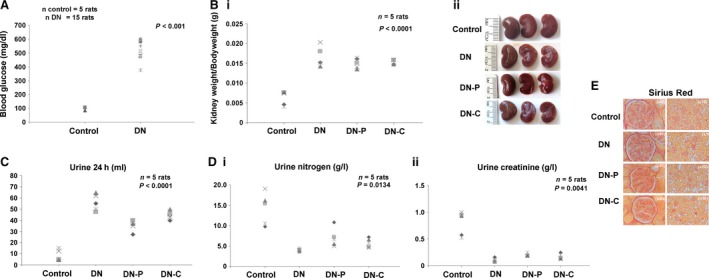
STZ model animals. Rats were randomly divided into four groups: control (C) (n = 5), STZ diabetic (DN) (n = 5), STZ rats treated with 400 ng/kg paricalcitol daily (DN‐P) (n = 5) and STZ rats treated with 100 ng/kg calcitriol daily (DN‐C) (n = 5). (A) Blood glucose levels (mg/ml) of control (C) and STZ‐diabetic animals (DN). (B) (i) Kidney weight/Bodyweight, (ii) Representative images of kidneys of control, STZ and diabetic animals after in vivo treatment with paricalcitol (DN‐P) or calcitriol (DN‐C). (C) Volume of urine 24 hrs (D) (i) urine nitrogen (g/l), (ii) urine creatinine (g/l). (E) Representative images of sirius red staining of C, DN, DN‐P and DN‐C animals. Original magnifications, 40× and 10×.
Effect of VDRA on histological changes
Using light microscopy, we evaluated the efficacy of VDRA in controlling DN in STZ rats. Glomeruli of STZ rats demonstrated increased nearly onefold accumulation of sirius red‐positive extracellular matrix proteins and the glomerular surface area became larger compared with that of normal rats. The above‐mentioned morphological kidney lesions were significantly attenuated after VDRA treatment (Fig. 6E).
VDRA induces changes in the expression levels of nephrin and VDR in the glomeruli of rats with DN
Western blot analysis revealed a reduction in the expression of nephrin and VDR proteins in the glomeruli of rats with DN (Fig. 7). Following administration of VDRA, the protein levels of nephrin (Fig. 7A) and VDR (Fig. 7B) were markedly restored.
Figure 7.
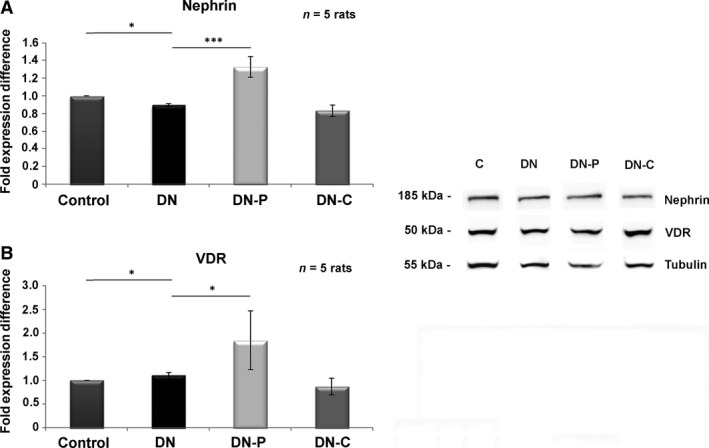
In vivo assessment of nephrin and VDR expression levels. Western blot analysis in isolated rat glomeruli after 5 weeks in vivo treatment with paricalcitol or calcitriol for (A) nephrin and (B) VDR. Western blot quantification was performed using ImageJ software, and the results were normalized to the positive control and then to isolated rat glomeruli from control animals, respectively. The values are presented as the mean ± S.D. of three independent experiments (*P < 0.05; ***P < 0.001 Student's t‐test).
Discussion
Active vitamin D is an important hormone implicated in prevention and treatment of hyperparathyroidism in dialysis patients. Increasing amount of data suggests a beneficial role of vitamin D in CKD, associated with increased survival of patients 1, 2. Vitamin D is important for ameliorating CKD and has been reported to reduce proteinuria in diabetic patients. Vitamin D regulates gene expression through its receptor, VDR, which interacts with VDREs located within regulatory regions of target genes. Mouse podocytes in vitro and in vivo and rat podocytes in vivo express VDR constitutively 22, 23, 24, 25. Our results provided evidence that VDR is also constitutively expressed ex vivo, in isolated rat glomeruli, and demonstrated for the first time that in our ex vivo model VDR expression and transport to the nucleus were affected by hyperglycaemia and were increased after VDRA treatment. In glomeruli cultured in high glucose levels, a modest but consistent increase of nuclear VDR was observed, compared to glomeruli cultured in normal glucose levels. This glucose‐mediated increase of VDR has also been reported in mouse podocytes in vitro 22. Glucose has been reported to interact with VDR resulting in impairment of its DNA binding and function within cells 26. Therefore, glucose‐mediated VDR increase in our ex vivo model could represent a compensatory mechanism against the hyperglycaemia‐induced inhibition of VDR binding on VDRE. This could also explain the fact that VDR expression levels in the STZ‐induced diabetic rats displaying DN were significantly decreased, suggesting that the compensatory mechanism is not applied in the in vivo situation.
It is known that VDR and RXR form heterodimers in the absence of VDRA regulating the basal transcriptional activity of target genes 27. In accordance with other findings we herein demonstrated that addition of VDRA stabilizes VDR‐RXR heterodimers and promote translocation of VDR to the nucleus. In accordance with our results, other reports have demonstrated that VDR is partly located in nuclei even in the absence of calcitriol and becomes accumulated to a greater extent in nuclei following addition of the ligand 19, 28. One important protein for sustaining normal podocyte structure and function is podocalyxin 29. Podocalyxin was permanently suppressed in immortalized human podocytes cultured in high glucose levels and this suppression was impeded following VDRA treatment 19, 30. We herein report for the first time that in isolated glomeruli VDRA managed to relatively restore the hyperglycaemia‐induced loss of podocalyxin, thus exerting a significant beneficial effect by maintaining and additionally restoring the differentiated podocytic phenotype. In our previous work, we have suggested that VDR is not necessarily associated with maintaining podocalyxin expression in normal conditions. However, VDR becomes apparently pivotal for re‐starting the expression of podocalyxin which has been previously suppressed by high glucose; this effect could be directly mediated by VDR or by a transcriptional complex of which VDR is a part 19. It has been also suggested that advanced glycation end products (AGEs) are partly involved in glucose‐dependent podocalyxin down‐regulation, since aminoguanidine (which prevents AGE formation), preserved podocalyxin expression 30. Vitamin D also moderates the deleterious impact of AGEs on endothelial cells by preventing the AGE‐induced elevation of NF‐kB‐p65‐DNA binding activity 31. Hence, an additional protective effect of VDRA in podocytes could be through moderation of AGE‐induced adverse effects or inhibition of AGE formation.
Nephrin is a cell surface receptor participating in cell–cell adhesion and signalling functions in glomerular podocytes 32. The present study focused on the role of VDRA on nephrin signal transduction in isolated rat glomeruli. We demonstrated that nephrin interacted/associated with the p85α regulatory subunit of PI3K ex vivo in isolated glomeruli. Similar interactions were also reported to regulate renal podocyte function in vitro 33. In our model PI3K association with nephrin resulted in activation of PI3K, as shown by activation of Akt phosphorylation. Phosphorylation of the intracellular domain of nephrin mediates interactions with several proteins, including Neph1, podocin, CD2AP, PI3K, that transmit nephrin signalling to the actin cytoskeleton and regulate several podocytic functions including survival 34, 35. The significance of nephrin signalling in vivo was previously well documented in glomerular podocytes. Specifically, CD2AP was reported to interact with both nephrin and PI3K in vivo, stimulating anti‐apoptotic Akt signalling in podocytes 12. Chronic hyperglycaemia disrupts signal transduction pathways involved in glomerular survival 36. Our previous work 37, as well as work by others 38 demonstrated a decrease of glomerular nephrin expression as an early event in hyperglycaemic conditions. We demonstrated herein that short‐term exposure of isolated glomeruli to high glucose affected nephrin expression and impaired phosphorylation and Akt activation.
Our data showed that nephrin expression was up‐regulated following VDRA treatment in glomeruli exposed to normal glucose levels. Similarly, immunofluorescence and Western blot studies revealed that glomeruli cultured in the presence of high glucose exhibited reduced total and cell surface nephrin expression. However, treatment with VDRA restored nephrin expression to levels similar to those observed in glomeruli cultured in normal glucose concentration. These findings were further verified by Western blot studies in glomeruli of STZ‐treated rats revealing and an in vivo reduction of nephrin expression which was rescued by VDRA treatment of the STZ‐treated rats. Additional studies have also indicated that vitamin D increased nephrin in vivo in murine and human podocytes 19, 39, 40, 41 and in rats with puromycin aminonucleoside‐induced nephrosis 42, 43, 44. Furthermore, paricalcitol increased nephrin expression in vivo, in adriamycin‐induced nephropathy 45 as well as in STZ‐treated rats 46. However, we herein demonstrate for the first time that hyperglycaemia‐induced nephrin induction is an early event in STZ‐induced DN, established within 5 weeks and VDRA treatment, can rescue nephrin expression at the initial stages of DN establishment.
An important finding of the present study was that VDRA treatment rescues podocytes from hyperglycaemia‐induced apoptosis by influencing the nephrin‐PI3K‐Akt signalling pathway (Fig. 8). Calcitriol has also been reported to ameliorate podocyte injury in DN rats via the PI3K/p‐Akt signalling pathway 13. In addition, the renoprotective effects of vitamin D3 and its analogues may be inducing alternative pathways leading to the production of monocyte chemoattractant protein 1 (MCP1), as has been suggested for both glomerular epithelial cells 47 and mesangial cells 48.
Figure 8.
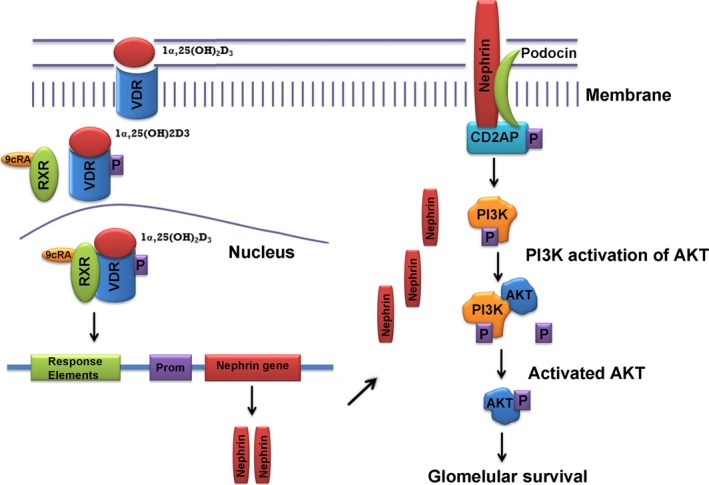
Molecular basis of VDRA beneficiary action through the nephrin signalling pathway. VDRA treatment enhances VDR expression and promotes VDR and RXR co‐localization in the nucleus, leading to increased nephrin expression, which participates in PI3K‐mediated Akt activation thus promoting glomerular survival.
In summary, the work described herein unravelled a pivotal role for VDRA in glomerular survival signalling, pointing to an anti‐apoptotic function that may contribute to an important inhibitory action on DN progression. Gradual reduction of nephrin expression accompanied by decreased Akt activity due to hyperglycaemia may further affect glomerular survival. Our data suggest an important role for additional factors, calcitriol and paricalcitol which may contribute to attenuation of podocytic damage and/or loss. Further understanding of the mechanisms involved at the molecular level will contribute to the use of these drug/therapeutic targets for the treatment of DN.
Author contributions
OT performed acquisition of data, analysed and interpreted the data and drafted the manuscript. EFT performed study conception and design, analysed and interpreted the data, performed funding acquisition and critically revised the article. AC Analysed and interpreted the data and critically revised the article. CI analysed and interpreted the data, critically revised the article and performed provision of VDRA. GD performed study conception and design, and acquisition of data, analysed and interpreted the data, performed funding acquisition, drafted the manuscript and critically revised the article.
Conflict of interest
The authors indicate no potential conflict of interests. This work was partly supported by a grant from Abbvie Pharmaceuticals S.A. (Hellas) that did not have any involvement in designing experiments, collecting, analysing or interpreting data and drafting or submitting the manuscript.
Supporting information
Figure S1. Expression levels of Akt.
Acknowledgements
O.T. was funded by Action: ‘KRHPIS’, Title: ‘Identification of targets for diagnosis and treatment of diseases’ (DIAS OPS 448325). We are grateful to Dr. Eleni Rigana for technical assistance with confocal microscopy and to Mrs Anna Agapaki for her valuable assistance in the histochemistry unit of BRFAA. We also thank Dr. Panagiotis Politis for providing useful reagents.
References
- 1. Marco MP, Craver L, Betriu A, et al Influence of vitamin D receptor gene polymorphisms on mortality risk in hemodialysis patients. Am J Kidney Dis. 2001; 38: 965–74. [DOI] [PubMed] [Google Scholar]
- 2. Tentori F, Hunt WC, Stidley CA, et al Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006; 70: 1858–65. [DOI] [PubMed] [Google Scholar]
- 3. Wang AY. Cardiovascular risk in diabetic end‐stage renal disease patients. J Diabetes. 2011; 3: 119–31. [DOI] [PubMed] [Google Scholar]
- 4. Li YC, Kong J, Wei M, et al 1,25‐Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin‐angiotensin system. J Clin Invest. 2002; 110: 229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Z, Zhang Y, Ning G, et al Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci USA. 2008; 105: 15896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Z, Sun L, Wang Y, et al Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008; 73: 163–71. [DOI] [PubMed] [Google Scholar]
- 7. Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008; 4: 444–52. [DOI] [PubMed] [Google Scholar]
- 8. Li JJ, Kwak SJ, Jung DS, et al Podocyte biology in diabetic nephropathy. Kidney Int Suppl. 2007; S36–42. [DOI] [PubMed] [Google Scholar]
- 9. Faul C, Asanuma K, Yanagida‐Asanuma E, et al Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007; 17: 428–37. [DOI] [PubMed] [Google Scholar]
- 10. Allison SJ. Podocyte biology: a new regulator of podocytes. Nat Rev Nephrol. 2012; 8 (12): 683–683. [DOI] [PubMed] [Google Scholar]
- 11. Patari‐Sampo A, Ihalmo P, Holthofer H. Molecular basis of the glomerular filtration: nephrin and the emerging protein complex at the podocyte slit diaphragm. Ann Med. 2006; 38: 483–92. [DOI] [PubMed] [Google Scholar]
- 12. Huber TB, Hartleben B, Kim J, et al Nephrin and CD2AP associate with phosphoinositide 3‐OH kinase and stimulate AKT‐dependent signaling. Mol Cell Biol. 2003; 23 (14): 4917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song Z, Guo Y, Zhou M, et al The PI3K/p‐Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism. 2014; 63: 1324–33. [DOI] [PubMed] [Google Scholar]
- 14. Zhu J, Sun N, Aoudjit L, et al Nephrin mediates actin reorganization via phosphoinositide 3‐kinase in podocytes. Kidney Int. 2008; 73: 556–66. [DOI] [PubMed] [Google Scholar]
- 15. Xing L, Liu Q, Fu S, et al PTEN Inhibits High Glucose‐Induced Phenotypic Transition in Podocytes. J Cell Biochem. 2015; 116: 1776–84. [DOI] [PubMed] [Google Scholar]
- 16. de Zeeuw D, Agarwal R, Amdahl M, et al Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010; 376: 1543–51. [DOI] [PubMed] [Google Scholar]
- 17. Li YC. Renoprotective effects of vitamin D analogs. Kidney Int. 2009; 78: 134–9. [DOI] [PubMed] [Google Scholar]
- 18. Haussler MR, Whitfield GK, Haussler CA, et al The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998; 13: 325–49. [DOI] [PubMed] [Google Scholar]
- 19. Verouti SN, Tsilibary EC, Fragopoulou E, et al Vitamin D receptor activators upregulate and rescue podocalyxin expression in high glucose‐treated human podocytes. Nephron Exp Nephrol. 2012; 122: 36–50. [DOI] [PubMed] [Google Scholar]
- 20. Sharma M, Zhou Z, Miura H, et al ADMA injures the glomerular filtration barrier: role of nitric oxide and superoxide. Am J Physiol Renal Physiol. 2009; 296: F1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP‐ribose) and PARP‐1. Genes Dev. 2012; 26: 417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Zhou J, Minto AW, et al Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 2006; 70: 882–91. [DOI] [PubMed] [Google Scholar]
- 23. Matsui I, Hamano T, Tomida K, et al Active vitamin D and its analogue, 22‐oxacalcitriol, ameliorate puromycin aminonucleoside‐induced nephrosis in rats. Nephrol Dial Transplant. 2009; 24: 2354–61. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Borchert ML, Deluca HF. Identification of the vitamin D receptor in various cells of the mouse kidney. Kidney Int. 2012; 81: 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li YC. Podocytes as target of vitamin D. Curr Diabetes Rev. 2011; 7: 35–40. [DOI] [PubMed] [Google Scholar]
- 26. Patel SR, Xu Y, Koenig RJ, et al Effect of glucose on the function of the calcitriol receptor and vitamin D metabolism. Kidney Int. 1997; 52: 79–86. [DOI] [PubMed] [Google Scholar]
- 27. Tagami T, Lutz WH, Kumar R, et al The interaction of the vitamin D receptor with nuclear receptor corepressors and coactivators. Biochem Biophys Res Commun. 1998; 253: 358–63. [DOI] [PubMed] [Google Scholar]
- 28. Milde P, Merke J, Ritz E, et al Immunohistochemical detection of 1,25‐dihydroxyvitamin D3 receptors and estrogen receptors by monoclonal antibodies: comparison of four immunoperoxidase methods. J Histochem Cytochem. 1989; 37: 1609–17. [DOI] [PubMed] [Google Scholar]
- 29. Takeda T, McQuistan T, Orlando RA, et al Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001; 108: 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drossopoulou GI, Tsotakos NE, Tsilibary EC. Impaired transcription factor interplay in addition to advanced glycation end products suppress podocalyxin expression in high glucose‐treated human podocytes. Am J Physiol Renal Physiol. 2009; 297: F594–603. [DOI] [PubMed] [Google Scholar]
- 31. Talmor YGE, Benchetrit S, Bernheim J, et al Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am J Physiol Renal Physiol. 2008; 294: F1059–64. [DOI] [PubMed] [Google Scholar]
- 32. Welsh GI, Saleem MA. Nephrin‐signature molecule of the glomerular podocyte? J Pathol. 2010; 220: 328–37. [DOI] [PubMed] [Google Scholar]
- 33. Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol. 2004; 15: 1382–91. [DOI] [PubMed] [Google Scholar]
- 34. Huber TB, Kottgen M, Schilling B, et al Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001; 276: 41543–6. [DOI] [PubMed] [Google Scholar]
- 35. Lehtonen S, Zhao F, Lehtonen E. CD2‐associated protein directly interacts with the actin cytoskeleton. Am J Physiol Renal Physiol. 2002; 283: F734–43. [DOI] [PubMed] [Google Scholar]
- 36. Katsoulieris EN, Drossopoulou GI, Kotsopoulou ES, et al high glucose impairs insulin signaling in the glomerulus: an in vitro and ex vivo approach. PLoS One. 2016; 11: e0158873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsotakos NE, Sagnou M, Kotsopoulou ES, et al Glucose‐induced gradual phenotypic modulation of cultured human glomerular epithelial cells may be independent of Wilms’ tumor 1 (WT1). BMC Cell Biol. 2013; 14: 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doublier S, Salvidio G, Lupia E, et al Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003; 52: 1023–30. [DOI] [PubMed] [Google Scholar]
- 39. Okamura M, Takano Y, Saito Y, et al Induction of nephrin gene expression by selective cooperation of the retinoic acid receptor and the vitamin D receptor. Nephrol Dial Transplant. 2009; 24: 3006–12. [DOI] [PubMed] [Google Scholar]
- 40. Saito Y, Okamura M, Nakajima S, et al Suppression of nephrin expression by TNF‐alpha via interfering with the cAMP‐retinoic acid receptor pathway. Am J Physiol Renal Physiol. 2010; 298: F1436–44. [DOI] [PubMed] [Google Scholar]
- 41. Takano Y, Yamauchi K, Hiramatsu N, et al Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol. 2007; 292: F1573–82. [DOI] [PubMed] [Google Scholar]
- 42. Migliori M, Giovannini L, Panichi V, et al Treatment with 1,25‐dihydroxyvitamin D3 preserves glomerular slit diaphragm‐associated protein expression in experimental glomerulonephritis. Int J Immunopathol Pharmacol. 2005; 18: 779–90. [DOI] [PubMed] [Google Scholar]
- 43. Xiao HQ, Shi W, Liu SX, et al Podocyte injury is suppressed by 1,25‐dihydroxyvitamin D via modulation of transforming growth factor‐beta 1/bone morphogenetic protein‐7 signalling in puromycin aminonucleoside nephropathy rats. Clin Exp Pharmacol Physiol. 2009; 36: 682–9. [DOI] [PubMed] [Google Scholar]
- 44. Xiao H, Shi W, Liu S, et al 1,25‐Dihydroxyvitamin D(3) prevents puromycin aminonucleoside‐induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3‐kinase/Akt‐signaling pathway. Am J Nephrol. 2009; 30: 34–43. [DOI] [PubMed] [Google Scholar]
- 45. He W, Kang YS, Dai C, et al Blockade of Wnt/beta‐catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011; 22: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X, Song Z, Guo Y, et al The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ‐induced diabetic rats. Mol Cell Biochem. 2015; 399: 155–65. [DOI] [PubMed] [Google Scholar]
- 47. Zhang ZYW, Sun L, Szeto FL, et al 1,25‐Dihydroxyvitamin D3 targeting of NF‐kappaB suppresses high glucose‐induced MCP‐1 expression in mesangial cells. Kidney Int. 2007; 72: 193–201. [DOI] [PubMed] [Google Scholar]
- 48. Sanchez‐Niño MDBM, Córdoba‐Lanús E, Valcheva P, et al Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2012; 302: 647–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression levels of Akt.


