Abstract
Perfusion culture of mesenchymal stem cells (MSCs) seeded in biomaterial scaffolds provides nutrients for cell survival, enhances extracellular matrix deposition, and increases osteogenic cell differentiation. However, there is no consensus on the appropriate perfusion duration of cellular constructs in vitro to boost their bone forming capacity in vivo. We investigated this phenomenon by culturing human MSCs in macroporous composite scaffolds in a direct perfusion bioreactor and compared their response to scaffolds in continuous dynamic culture conditions on an XYZ shaker. Cell seeding in continuous perfusion bioreactors resulted in more uniform MSC distribution than static seeding. We observed similar calcium deposition in all composite scaffolds over 21 days of bioreactor culture, regardless of pore size. Compared to scaffolds in dynamic culture, perfused scaffolds exhibited increased DNA content and expression of osteogenic markers up to 14 days in culture that plateaued thereafter. We then evaluated the effect of perfusion culture duration on bone formation when MSC-seeded scaffolds were implanted in a murine ectopic site. Human MSCs persisted in all scaffolds at 2 weeks in vivo, and we observed increased neovascularization in constructs cultured under perfusion for 7 days relative to those cultured for 1 day within each gender. At 8 weeks post-implantation, we observed greater bone volume fraction, bone mineral density, tissue ingrowth, collagen density, and osteoblastic markers in bioreactor constructs cultured for 14 days compared to those cultured for 1 or 7 days, and acellular constructs. Taken together, these data demonstrate that culturing MSCs under perfusion culture for at least 14 days in vitro improves the quantity and quality of bone formation in vivo. This study highlights the need for optimizing in vitro bioreactor culture duration of engineered constructs to achieve the desired level of bone formation.
Keywords: perfusion, bioreactor, composite scaffold, mesenchymal stem cells, osteogenic differentiation, bone formation
INTRODUCTION
Large bone deficits due to trauma, surgery, or resulting from slow and non-healing defects represent a significant clinical problem. More than 1 million bone repair surgeries are performed annually, bringing the financial burden to over $5 billion in the Unites States alone [1, 2]. Autograft bone is the current gold standard for repairing non-healing bone defects, yet its use has numerous drawbacks such as donor site morbidity, prolonged pain, limited availability, and increased risk of infection [3, 4]. Tissue engineered bone constructs represent a promising alternative to autografts. To generate implantable osteogenic grafts, biomaterial scaffolds are commonly seeded with bone-forming cells and cultured in vitro under variable conditions and durations. Compared to static or continuous dynamic culture, scaffolds under continuous perfusion culture exhibit improved cell growth and survival, extracellular matrix (ECM) deposition, and enhanced osteogenic differentiation of mesenchymal stem cells (MSCs) due to increased nutrient availability and application of mechanical stimulation [5–8]. However, the effect of bioreactor culture duration in vitro on the bone forming capacity of MSCs upon transplantation in vivo is largely unknown.
An ideal bone scaffold for bioreactor culture of MSCs must be osteoconductive, providing a structure and network that supports cell attachment, growth, and osteogenic differentiation [9, 10]. Composite scaffolds formed of hydroxyapatite (HA) and poly(lactide-co-glycolide) (PLG) offer tunable biodegradability, osteoconductivity, porosity, and mechanical properties [11–14]. Moreover, HA-PLG scaffolds can regulate osteogenesis and trophic factor secretion by MSCs in vitro and in vivo [11]. We and others reported that the pore size of three-dimensional macroporous scaffolds can regulate cell differentiation under static or dynamic conditions [15–19]. Pore size also directly influences the shear stress experienced by cells when scaffolds are maintained under continuous perfusion, thus regulating osteogenic differentiation of MSCs [10, 17]. Therefore, selecting the appropriate pore size for constructs cultured in perfusion bioreactors is vital for bone tissue engineering applications.
While the exposure of osteoblasts and osteoprogenitor cells to fluid shear stress enhances their bone forming potential in vivo [6, 20], the contribution of perfusion culture duration to bone formation has not been thoroughly investigated. Furthermore, the effect of culture duration on the bone-forming potential of human MSCs, a more clinically relevant cell source compared to osteoblasts, has not been reported in the literature. Bioreactor culture durations ranging from 5 days [21] to 5 weeks [22–24] have been used for creating implantable tissue engineered bone. Shorter culture durations may be sufficient to prime MSCs towards osteogenic differentiation and boost their proangiogenic potential [25]. Extended culture durations such as 2–5 weeks may result in a more mature construct with increased cellularity, differentiated cells and a dense ECM, which can act as a scaffold for host cell infiltration and differentiation [10, 26, 27]. Thus, there is a critical gap in our knowledge for the appropriate in vitro perfusion culture duration to maximize bone formation in vivo with osteogenic grafts.
We hypothesized that HA-PLG scaffolds would serve as effective osteoconductive biomaterials to promote MSC osteogenic differentiation and that longer culture durations would result in mature constructs suitable for implantation. We investigated the role of perfusion on bone formation by culturing human MSCs in porous HA-PLG scaffolds of varying pore sizes and durations in a continuous perfusion bioreactor. Thus, the goals of this study were to 1) determine the effect of pore size of HA-PLG scaffolds on MSC osteogenic differentiation in vitro; 2) characterize the osteogenic response of MSCs in HA-PLG scaffolds when cultured in perfusion bioreactors for up to 21 days; and 3) investigate the effect of in vitro perfusion culture duration on in vivo bone forming capacity of tissue engineered constructs in an ectopic site.
MATERIALS AND METHODS
Scaffold preparation
Scaffolds were fabricated using a gas foaming/particulate leaching method as described [11, 16]. Briefly, microspheres composed of PLG (85:15, DLG 7E; Lakeshore Biomaterials, Birmingham, AL) were prepared using a double-emulsion process and lyophilized to form a free-flowing powder. 9.2 mg of lyophilized microspheres were combined with 23.1 mg of HA crystals (particle size 100 nm, Berkeley Advanced Biomaterials, Berkeley, CA) and 175.6 mg of NaCl to yield a 2.5:1:19 mass ratio of ceramic:polymer:salt. Porogen diameter ranges were achieved by passing salt crystals through sieves of distinct size ranges (small: 125–300 μm, medium: 300–500 μm, and large: 500–850 μm). Control PLG scaffolds were fabricated without HA using 250–425 μm salt crystals, as this is the range currently employed by our group and others to support cellular ingrowth [11, 28–31]. The powdered mixture was then compressed for 1 min into solid disks (final dimensions: 8 mm in diameter and 2 mm in height) using a Carver Press (Carver, Inc., Wabash, IN). Compressed disks were exposed to high pressure CO2 gas (5.5 MPa) for at least 16 hrs followed by rapid pressure release to cause polymer fusion. NaCl particles from the scaffolds were then leached in distilled H2O for 24 hrs. Scaffolds were sterilized by 70% ethanol under gentle vacuum followed by two rinses in sterile PBS and dried in a sterile biosafety cabinet. Prior to cell seeding, scaffolds were soaked in cell culture medium for at least 30 min to facilitate cell adhesion.
Scanning electron microscopy
To visualize gross morphology and pore architecture, scaffolds were gold coated using a sputter coater (Desk II; Denton Vacuum, Moorestown, NJ) and imaged using scanning electron microscopy (Hitachi 3500-N, Hitachi Science Systems Ltd, Tokyo, Japan) at 5 kV. Pore diameter was measured in ImageJ (National Institutes of Health, Bethesda MD). Briefly, pores were measured edge to edge along the maximum and minimum axis and averaged to generate a representative diameter. Eight pores from four separate images per group were measured to determine pore diameter.
Measurement of fluid permeation velocity through scaffolds
Permeation velocity of fluid flow through the scaffolds was measured as an indicator of pore connectivity and indirect measure of fluid shear stress (Fig. 1D, insert). Prior to analysis, scaffold height was measured using digital calipers. Pristine, acellular scaffolds were held in place between two bioreactor adaptors housed between two rubber syringe stoppers in a syringe barrel to create a seal preventing fluid flow around the scaffold. The syringe was filled with media, and the volume that passed through the scaffold was recorded. Permeation velocity was calculated using a derivative of Darcy’s Law [16].
Figure 1. Bioreactor setup and permeation velocity of media through scaffolds.
(A) Scaffolds are held in place by two adaptors that prevent flow of media around scaffolds. Cell suspension and media are injected in the top and bottom port of bioreactors, respectively. (B) Scaffold pore morphology as observed by scanning electron microscopy. (C) Average pore diameter measured from SEM images. (D) Permeation velocity of media through scaffolds of varying pore size and image of experimental setup. Significance is denoted by alphabetical letterings; groups with significance do not share the same letters (n=4 for pore diameter, n=3 for permeation velocity).
Cell culture
Human bone marrow-derived MSCs (Lonza, Walkersville, MD) were expanded without further characterization in growth medium (GM) consisting of minimum essential alpha medium (α-MEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA) and 1% penicillin/streptomycin (Gemini Bio-Products, Sacramento, CA). Cells were cultured under standard conditions (37°C, 21% O2, 5% CO2) and utilized at passages 4–5. For all experiments, cells were initially seeded in GM and then exposed to osteogenic medium (OM: GM supplemented with 10 mM β-glycerophosphate, 50 μg/mL ascorbate-2-phosphate, and 100 nM dexamethasone). Media was changed every 2–3 or 3–4 days for dynamic and bioreactor cultures, respectively.
Cell seeding and maintenance
For characterizing the effect of pore size on cell seeding efficiency, cells were seeded statically or in perfusion bioreactors (Fig. 2A). Scaffolds were soaked in medium for 30 min before seeding. For static seeding, 1.2×106 MSCs were suspended in 75 μL GM and applied dropwise to scaffolds. MSCs were allowed to attach for 3 hrs before transferring scaffolds into well plates with GM. For bioreactor seeding, scaffolds were installed in U-CUP flow perfusion bioreactors (Cellec Biotek, Basel, Switzerland) and 10 mL of GM was injected through the bottom port into the bioreactor (Fig. 1A). 1.2×106 MSCs suspended in 2 mL GM were injected via the top port into the bioreactor, bringing the final media volume in each bioreactor to a total of 12 mL. Up to 10 individual bioreactors were then connected to a syringe pump (Harvard Apparatus, Holliston, MA) to maintain media at a superficial velocity of 3 mL/min for 15–18 hrs.
Figure 2. Cell distribution in HA-PLG scaffolds of varying pore sizes seeded under static or flow conditions.
(A) MSCs were seeded on scaffolds under flow perfusion or statically by pipetting cells directly on top of scaffolds. (B) MTT staining to visualize MSC distribution throughout scaffolds. Diffuse staining and bright purple staining are indicative of well-distributed and locally concentrated cells, respectively. Scale bar represents 5 mm.
For all in vitro studies, scaffolds were seeded using bioreactors to ensure homogenous cell distribution for all experimental groups. After the seeding phase (15–18 hrs), GM was replaced with OM, and constructs were maintained under continuous perfusion culture for up to 21 days. For culture under dynamic conditions, scaffolds were removed from bioreactors after the seeding phase, placed in 24 well plates with 500 μL OM, and maintained on an XYZ shaker (The Belly Button Shaker, IBI Scientific, Peosta, IA) at 30 rpm with the platform having a maximum tilt angle of 4.5 degrees. The shaker was kept in an incubator under standard conditions and was continuously in motion to facilitate media transport throughout the scaffolds.
MTT staining of seeded scaffolds
Scaffolds were stained with 0.5 mg/mL thiazolyl blue tetrazolium bromide (MTT) solution in PBS at 37°C. After 2 hrs, MTT solution was aspirated, scaf folds were washed twice with PBS, and photographs were taken using a digital camera.
In vitro osteogenic differentiation
Scaffolds were retrieved from bioreactors or well plates and washed twice with PBS. A 5 mm biopsy punch (Integra Miltex, York, PA) was used to harvest a disk for qPCR analysis as described [32]. Samples were collected in TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Total RNA was isolated using an RNEasy micro kit (Qiagen, Valencia, CA). RNA was reverse transcribed with the QuantiTect Reverse Transcription kit (Qiagen). RNA purity was examined with a spectrophotometer (NanoDrop 1000, Thermo Fisher Scientific, Waltham, MA), and only samples with high quality (A260/280 >1.6 per manufacturer’s instructions) were used for cDNA synthesis. 250 ng of RNA was used in reverse transcription reactions. qPCR was performed using a QuantiFast Probe PCR kit (Qiagen) on a CFX96 Real-Time System (manufacturer). Primers and probes for RPL13 (HS00204173_m1), SP7 (HS01866874_S1), COL1A1 (HS00164004_M1), and IBSP (HS00173720_M1) were purchased from Applied Biosystems (Foster City, CA). Amplification conditions were 95°C for 3 min, followed by 40 cycles at 95°C for 3 sec and 60°C for 30 min. Quantitative PCR results were normalized to RPL13 transcript levels to yield ΔCt, and fold change in expression relative to the housekeeping gene was calculated using 2−ΔCt. The remainder of the scaffold was collected in 250 μL of passive lysis buffer (Promega, Madison, WI), frozen at −20°C, thawed, sonicated, centrifuged at 5000 rpm for 10 min to pellet the cell debris, and the supernatant was collected for DNA analysis. The supernatant was analyzed for DNA content using the Quant-iT PicoGreen dDNA Assay Kit (Invitrogen) following manufacturer’s instructions. The remaining pellet was resuspended in 0.9 N H2SO4 and incubated overnight at 37°C. Calcium depositio n was quantified by reacting with o-cresolphthalein complexone as previously described [33, 34]. The calcium content of an unused composite scaffold was subtracted from that of cultured composite scaffolds to determine the amount of calcium deposited by MSCs.
To visualize cell distribution and markers of osteogenic differentiation within constructs, samples were washed twice in PBS and fixed in 10% buffered formalin acetate (Fisher Scientific, Fair Lawn, NJ) for 24 hrs at 4°C. Samples were then washed twice in PBS to remove residual formalin acetate and preserved in 70% ethanol at 4°C until further processing. Prior to cryosectioning, samples were soaked in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) overnight to allow OCT to perfuse through the entire scaffold. Constructs were then placed in fresh OCT and frozen on dry ice. 5 μm sections were cut on a Leica CM1850 Cryostat (Leica Microsystems, Bannockburn, IL) and mounted onto microscope slides for analysis. Sections were stained using hematoxylin and eosin (H&E) following standard protocols and immunostained using an anti-osteocalcin (OCN) antibody (1:200, AB13420, Abcam, Cambridge, MA). The percentage area positive for OCN was quantified by creating a mask for brown stain in Photoshop and then calculating percentage of total area (defined by a box drawn around the extremes of the scaffold) in ImageJ.
Murine subcutaneous implantation model
Treatment of experimental animals was in accordance with UC Davis animal care guidelines and all National Institutes of Health animal handling procedures. Male and female eight-week-old nonobese diabetic/severe combined immunodeficient gamma (NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice (Jackson Laboratories, West Sacramento, CA) were anesthetized and maintained under a 2% isoflurane/O2 mixture delivered through a nose cone. Mice exhibited no diabetogenic effects, as streptozotocin was not administered. Each animal received four subcutaneous implants: constructs cultured under perfusion for 1 (upper left), 7 (upper right), or 14 days (lower left), and acellular (lower right) constructs. Unlike constructs cultured under perfusion, which were maintained in OM after the seeding phase, the acellular group consisted of pristine scaffolds that were soaked in GM for 30 min prior to implantation. Animals were euthanized at 2 or 8 weeks, and scaffolds were collected and fixed in 10% buffered formalin acetate for 2 days at room temperature. Samples were then washed twice in PBS to remove residual formalin acetate and preserved in 70% ethanol at 4°C until further processing.
Qualitative and quantitative three-dimensional analysis of explants at 8 weeks was conducted using microCT [35]. Explants were imaged (55 kVp, 145 μA, 300 ms integration time, average of 3 images) using a high-resolution μCT specimen scanner (μCT 35, Scanco Medical; Brüttisellen, Switzerland). Contiguous slices of 2,048 × 2,048 pixels were imaged with 12 μm resolution and slice thickness (voxels). Serial tomograms were reconstructed from raw data of 1000 projections per 180 degrees using a cone beam filtered back projection algorithm [36]. The tomograms were calibrated to 0.0, 99.6, 200.0, 401.0 and 800.3 mg HA/cc concentrations of HA so that grey-values of the images were converted to units of density in mg HA/cc. The entire scaffold was analyzed by contouring to its edges based on a threshold of 256 mg HA/cc. Material in the reconstructed images was partitioned by a threshold of 256–3000 mg HA/cc to discriminate between mineralized and unmineralized tissue. After thresholding, the image noise was reduced using a low pass Gaussian filter (σ=0.8, support=1). Bone volume fraction (BV/TV) was determined by dividing the number of pixels representing bone tissue (BV: bone volume) by the number of pixels in the cylindrical segment (TV: total volume). After microCT analysis, explants were demineralized in Calci-clear (National Diagnostics, Atlanta, GA) for up to 48 hrs, washed twice in PBS, bisected, paraffin-embedded and sectioned at 5 μm thickness. Blood vessel density at 2 weeks was quantified using H&E stained cross-sections and consisted of counting circular structures with well-defined lumens containing erythrocytes. 2-week tissue sections were also immunostained using a human-specific anti-Lamin A/C antibody (1:250, Abcam, Cambridge, MA) to detect the presence of human MSCs. At 8 weeks, H&E and Masson’s trichrome stains of tissue sections were used to visualize tissue ingrowth and collagen distribution within scaffolds, respectively. Sections were also immunostained for bone sialoprotein (BSP) using a human-specific anti-bone sialoprotein antibody (1:200, Abcam).
Statistical analysis
All data are presented as means ± standard deviation of the mean. Statistical analysis was performed using Student’s t-tests, one-way ANOVA and two-way ANOVA with Tukey’s multiple comparison post-hoc test, where applicable. All statistical analysis was performed in Prism 6 software (GraphPad, San Diego, CA), and p values less than 0.05 were considered statistically significant. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
RESULTS
Porogen size increases pore diameter and permeation velocity
SEM images reveal appreciable differences in pore morphology of scaffolds. PLG scaffolds as well as HA-PLG scaffolds with medium and large pores exhibited a uniform honeycomb pattern (Fig. 1B). Scaffolds with small pores appeared collapsed throughout the scaffold. The average pore diameter was in agreement with the range of porogen particles used for each group (Fig. 1C). We also assessed the permeation velocity of media through scaffolds of different pore sizes as an indirect measure of the shear stress experienced by MSCs cultured under flow perfusion (Fig. 1D). We measured increasing permeation velocity with increasing pore size. However, this effect tapered between scaffolds with medium and large pores. Scaffolds with large pores exhibited significantly higher permeation velocity relative to those with small pores or PLG controls.
Pore size differentially affects cell seeding efficiency under static and flow conditions
Scaffolds seeded under perfusion exhibited more homogeneous cell distribution throughout the scaffold than statically seeded scaffolds (Fig. 2B). Although scaffolds with small pores had fewer cells on the underside than scaffolds with medium and large pores, there was no appreciable qualitative difference among scaffolds seeded in bioreactors. For all pore sizes and the PLG control, scaffolds seeded statically exhibited strong staining on only one side of the scaffold, indicating that cells primarily adhered to the top of the scaffold. With increasing pore size, more cells were detected near the center of the scaffold but the underside of the scaffold remained largely devoid of cells.
Pore size of composite scaffolds does not alter MSC response under perfusion
We did not detect significant differences in DNA content, an indicator of cell number, among composite scaffolds of different pore sizes over 21 days in continuous perfusion culture (Fig. 3A), yet scaffolds with small pores had greater DNA content compared to PLG controls. H&E staining revealed relatively uniform cell distribution within scaffolds of all pore sizes. (Fig. 3B). Quantitative assessment of calcium deposition revealed that composite scaffolds of all sizes had significantly higher amounts of calcium compared to PLG controls (Fig. 3C). No significant differences in calcium deposition were observed among scaffolds of different pore sizes. Qualitatively, we observed similar osteocalcin (OCN) expression within pores of scaffolds with medium and large pores (Fig. 3D). Scaffolds with small pores were also brittle and difficult to handle after culture, rendering them unsuitable for implantation. The thin pore walls of scaffolds with small pores corroborated this gross observation (Fig. 1B). Subsequent studies were performed with scaffolds of medium pore size due to similar levels of OCN expression and calcium content to large pore size scaffolds, ease of handling, and the pore size range being closest to that used by our group and others.
Figure 3. Pore diameter does not influence DNA content or osteogenic differentiation of MSCs on HA-PLG scaffolds under perfusion culture.
(A) DNA content. (B) Representative H&E images of scaffolds with increasing pore diameter. (C) Quantification of calcium deposition in MSC-seeded constructs after 21 days. (D) Representative immunohistochemistry for osteocalcin. Black is indicative of scaffold (S); brown is indicative of osteocalcin (OCN) and staining is highlighted by blue arrows. Significance is denoted by alphabetical letterings; groups with significance do not share the same letters (n=5). Scale bar represents 500 μm.
Osteogenic response of MSC-seeded constructs under perfusion culture increases with time
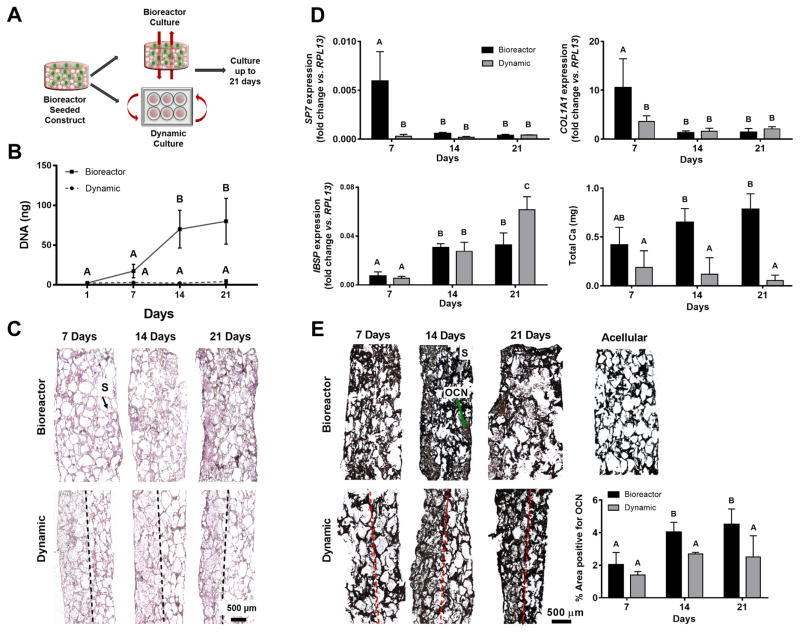
We evaluated the role of continuous dynamic or perfusion culture on the osteogenic response of MSCs seeded on medium pore-sized HA-PLG scaffolds (Fig. 4A). We observed a steady increase in DNA content from 1 to 14 days for scaffolds cultured in perfusion bioreactors (Fig. 4B), yet there was no substantial increase in DNA content beyond 14 days. We did not detect increases in DNA content within scaffolds under dynamic culture over time. H&E–stained sections corroborate DNA quantification and demonstrate increases in cell number with time for bioreactor samples (Fig. 4C). Scaffolds in dynamic culture exhibited spatial restriction of cells to the top surface, which did not increase appreciably over time. In contrast, scaffolds in perfusion culture became more cellularized over time, which was visible throughout the entire scaffold.
Figure 4. DNA content and osteogenic differentiation of MSCs on composite scaffolds plateau after 14 days of bioreactor culture.
(A) MSCs were seeded in HA-PLG scaffolds under perfusion and cultured under dynamic or perfusion conditions for up to 21 days. (B) DNA content. (C) Representative H&E images. Scaffold (S) is denoted by black arrow. Dashed line demarcates the boundary of spatial restriction of cells within a scaffold. (D) Osteogenic gene expression and calcium deposition. (E) Representative immunohistochemistry images for osteocalcin and percentage area positive for osteocalcin. Black is indicative of scaffold (S) and denoted by gray arrow; brown is indicative of osteocalcin (OCN) and denoted by a green arrow. Dashed line demarcates the boundary of spatial restriction of cells within a scaffold. Significance is denoted by alphabetical letterings; groups with significance do not share the same letters (n=5 for DNA and n=4 for osteogenic assays). Scale bar represents 500 μm.
We assessed the osteogenic potential of MSCs by measuring gene expression of osteogenic markers and functional outputs such as calcium deposition (Fig. 4D) and osteocalcin expression. SP7 expression, an early to mid-transcription factor involved in MSC differentiation, was significantly higher in bioreactor samples compared to dynamic samples at 7 days, which was reduced in both experimental groups at 14 and 21 days in culture. COL1A1 expression was also significantly higher in bioreactor samples relative to dynamic samples at 7 days and followed the same trend as SP7. Bone sialoprotein (IBSP), a late marker of osteogenic differentiation, exhibited increased expression at 14 and 21 days in both groups relative to 7 days. Dynamic samples exhibited a significant increase in IBSP expression over bioreactor samples at 21 days. MSCs in bioreactor culture deposited more calcium than those in dynamic culture at 14 and 21 days. We observed a trend for increasing calcium deposition for bioreactor samples over time, although this trend was not significant. Qualitative histological assessment and quantification of these images confirmed trends observed in DNA content and osteogenic gene expression. OCN expression, a late marker of osteogenic differentiation, was more uniform in bioreactor samples compared to dynamic samples (Fig. 4E). OCN expression increased in bioreactor samples from 7 to 14 days, but we observed minimal increase in OCN expression beyond 14 days. Similar to cell distribution, OCN was spatially restricted to the top surface of the scaffold in dynamic samples. Given that 21 days of in vitro culture did not confer significant advantages to the engineered constructs over a 14 day culture period, we selected 14 days as our longest culture duration for in vivo studies.
14 days of perfusion culture in vitro enhances MSC persistence and bone formation in vivo
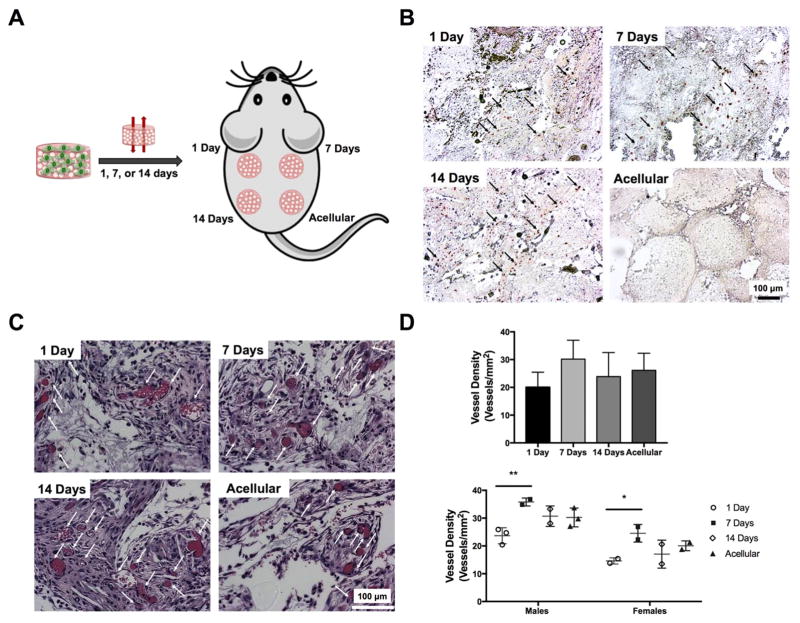
The bone forming potential of perfusion-cultured scaffolds for increasing durations was evaluated in a murine ectopic site (Fig. 5A). Human MSCs persisted in all three cellular groups upon explantation at 2 weeks (Fig. 5B). Explanted scaffolds from all four groups appeared well-vascularized 2 weeks after explantation (Fig. 5C). Quantification of blood vessel density revealed a trend for increased neovascularization in constructs cultured for 7 days vs. 1 day constructs (Fig. 5D, top). Although significant differences were not detected within the full cohort of animals, vascular density was significantly different for constructs cultured for 1 and 7 days in animals of the same gender (Fig. 5D, bottom). Furthermore, the magnitude of vascularization was greater in all scaffolds in male mice compared to female mice.
Figure 5. MSC persistence and scaffold neovascularization is influenced by duration of perfusion culture.
(A) Subcutaneous implantation of constructs maintained in perfusion culture for 1, 7, and 14 days. (B) Human cells are observed in representative immunohistochemistry images for Lamin A/C in constructs 2 weeks post-implantation. Black arrows denote positive staining. (C) H&E staining of explants reveals scaffold neovascularization. White arrows denote blood vessels. (D) Quantification of blood vessel density in scaffolds retrieved from all animals (top) and as a function of gender (bottom) (n=5–6 or n=2–3 for males and females, respectively). *p<0.05 and **p<0.01. Scale bar represents 100 μm.
At 8 weeks post-implantation, we observed human MSCs in all cellular constructs, with the most cells present in constructs cultured under perfusion for 14 days (Supplementary Fig. 1). Qualitatively, fewer human cells were present in explants 8 weeks post-implantation compared to 2 week explants (Fig. 5B, Supplementary Fig. 1). Acellular scaffolds grossly exhibited distinct scaffold boundaries and appeared the least remodeled compared to all cellular scaffolds, and these differences were observable using microCT (Fig. 6A). Constructs cultured for 14 days appeared smaller in diameter than constructs cultured for 1 and 7 days, and acellular scaffolds were the largest. Compared to acellular scaffolds, we detected significant increases in bone volume fraction (BVF) and bone mineral density (BMD) in all cellular scaffolds, regardless of bioreactor culture duration (Fig. 6B–C). Constructs perfused for 14 days exhibited significantly greater BVF and BMD relative to 1 day constructs. In agreement with microCT data, H&E staining revealed that dense connective tissue was visibly greater in scaffolds cultured in vitro for 14 days relative to other groups (Fig. 6D, top; Supplementary Fig. 2). Acellular groups were largely devoid of connective tissue on one side and contained fewer nuclei on the other side relative to cellular groups. Masson’s trichrome staining confirmed the presence of denser collagen with increasing bioreactor culture duration, with 14 day constructs exhibiting the most intense collagen staining compared to other cellular groups (Fig. 6D, center; Supplementary Fig. 2). Acellular explants contained infrequent, small areas of collagen. Immunohistochemistry for human bone sialoprotein (BSP) revealed positive staining in all cellular scaffolds (Fig. 6D, bottom; Supplementary Fig. 2). Constructs cultured for 14 days exhibited larger areas of positive BSP staining compared to 1 and 7 day constructs.
Figure 6. MSC-seeded HA-PLG constructs maintained under flow perfusion for 14 days in vitro result in the greatest bone formation in vivo.
(A) Representative microCT images of explants at 8 weeks post-implantation. Scale bar represents 1 mm. (B) Bone volume fraction and (C) bone mineral density are greatest in 14-day cultured constructs compared to 1 day constructs or acellular controls. (D) Representative H&E images (top) reveal increased tissue ingrowth and density. Masson’s trichrome images (center) exhibit greater collagen content and density with increases in culture duration. Dashed line demarcates areas with distinct levels of connective tissue and cellularization. Representative immunohistochemistry images for bone sialoprotein (BSP) (bottom). Blue arrows indicate pore walls while green arrows mark positive staining for BSP. Scale bar represents 250 μm. Significance is denoted by alphabetical letterings; groups with significance do not share the same letters (n=6).
DISCUSSION
Perfusion bioreactors provide a three-dimensional environment with mechanical stimulation via fluid flow for engineering osteogenic grafts for bone repair. In this study, we sought to determine the appropriate pore size of HA-PLG scaffolds for promoting MSC osteogenic differentiation and the role of continuous perfusion culture duration to generate osteogenic grafts. Larger pore sizes resulted in more uniform cell seeding and distribution, but we did not detect differences in calcium accumulation under perfusion as a function of pore diameter. Compared to PLG controls, HA-PLG scaffolds enhanced the osteogenic differentiation of MSCs. Constructs cultured under continuous perfusion exhibited increased DNA content, cell distribution, and osteogenic markers compared to their dynamic counterparts, yet this favorable cell response to perfusion plateaued after 14 days. Upon implantation into an ectopic site, constructs cultured in perfusion for up to 14 days resulted in significantly greater bone formation. These data demonstrate that optimizing the bioreactor culture duration in vitro can enhance the capacity of MSC-seeded HA-PLG constructs to contribute to bone formation in vivo.
Perfusion culture promotes homogenous cell distribution throughout the construct [37] and efficient transport of critical nutrients by forcing media through the pores of the scaffold. Scaffold pore size was directly associated with seeding efficiency of statically seeded samples, where cells infiltrated farther into scaffolds with larger pore sizes. We observed a similar trend in scaffolds seeded under perfusion, though this effect was less pronounced. Direct perfusion of media through scaffolds also exerts fluid shear stress on cells attached to the pores of scaffolds. Fluid shear stress magnitudes between 0.1 and 0.3 dynes/cm2 are considered stimulatory for osteoblastic cells in flow perfusion bioreactors [38–40]. The flow rate used in this study (3 mL/min) was optimized to reflect a range of fluid shear stress at the time of cell seeding (small pores: 0.35 dynes/cm2, medium pores: 0.2 dynes/cm2, large pores: 0.15 dynes/cm2). Shear stress was calculated from the flow rate, pore diameter, and viscosity of media using previously established methods and assuming uniform pore interconnectivity [22]. These values reflect only a first approximation of shear stress due to limited pore interconnectivity [41] and cell secretion of ECM during prolonged culture [38], thereby reducing conduit diameters for fluid flow and potentially increasing shear stress.
Common synthetic biomaterials under investigation for bioreactor culture include titanium fiber mesh [20], polyurethane [42], β-tricalcium phosphate [43], PLG scaffolds [44], and HA scaffolds [21, 45]. We previously reported that HA-PLG composite scaffolds (pore size 250–425 μm) enhance osteogenesis and trophic factor secretion of human MSCs in static culture compared to PLG scaffolds [11]. In the present study, we demonstrate MSC differentiation is enhanced in HA-PLG scaffolds relative to PLG scaffolds cultured in perfusion bioreactors. While composite scaffolds using HA and polycaprolactone have been employed in perfusion bioreactors [46], this is the first demonstration of the synergistic effect of osteoconductive HA-PLG scaffolds and direct perfusion stimulation of MSCs. Contrary to our hypothesis, we did not detect increases in DNA content or MSC differentiation with increases in pore diameter when cultured under continuous perfusion. Previous findings from our group and others reported that larger pore sizes improve MSC proliferation and differentiation towards the osteogenic and chondrogenic lineages under static or dynamic culture [15, 16, 47, 48]. However, mass transport limitations are mitigated under flow conditions, enabling comparable cell attachment and proliferation in scaffolds of varying sizes. Numerous studies demonstrate that MSC osteogenic differentiation is enhanced in scaffolds with smaller pores, likely due to increased shear stress [10, 17, 38, 49]. However, Holtorf et al. reported that larger pore size promoted early differentiation, while smaller pore size was associated with later differentiation and ECM deposition, confirming the temporal effect of perfusion culture on osteogenic differentiation [17]. Scaffolds formed by the gas foaming method possess macropores with limited interconnectivity, which are supported by numerous micropores that facilitate nutrient transport and may alter permeation velocity of media through scaffolds. Furthermore, scaffolds contain a distribution of pore sizes, which may mask subtle differences in shear stress. Since flow rate was constant among all experimental groups, one might postulate that mass transport had a stronger effect on cell function than shear stress in this study. This phenomenon merits further investigation using composite scaffolds with consistent pore size and geometry. Others who modulate pore size, distribution, and interconnectivity using additive manufacturing to regulate geometry reported the favorable effect of a porosity gradient within scaffolds on MSC osteogenic differentiation [50–53].
Upon establishing an appropriate pore size, we cultured MSCs in bioreactors or dynamic conditions for up to 21 days. While some groups consider short bioreactor culture durations (5 days) sufficient prior to implantation [21], others use moderate durations (10–21 days) [5, 26, 45, 54–57] that are pulsatile or intermittent [58, 59], or up to 5 weeks to generate more mature constructs [22–24]. To minimize delays between harvesting autologous cells and generating an osteogenic graft, we characterized MSC response under perfusion in vitro to select the shortest culture duration necessary for construct maturity. In agreement with other reports [6], DNA content and osteogenic differentiation markers increased up to 14 days and plateaued, motivating our exclusion of constructs cultured for longer periods in vitro. When comparing constructs cultured under dynamic and perfusion conditions, we observed higher IBSP mRNA expression in dynamic samples. This could be attributed to accelerated differentiation in bioreactor samples. Dynamic constructs may be lagging behind in the osteogenic differentiation timeline, and peak IBSP expression in bioreactor samples could have been missed by only examining days 7, 14, and 21. Although two-week culture durations in bioreactors are common for generating osteogenic grafts [6, 45, 55, 57, 60], these data, specifically changes in IBSP gene expression and calcium secretion, highlight the role of temporal changes in MSC differentiation under perfusion culture.
Next, we evaluated the in vivo bone-forming potential of perfusion-cultured osteogenic grafts. To our knowledge, this is the first evaluation of human MSCs osteoinduced on macroporous scaffolds for varying culture durations in a continuous perfusion bioreactor. Sikavitsas et al. demonstrated that the stage of osteoblastic differentiation of rat bone marrow stromal osteoblasts in perfusion culture influences the performance of engineered constructs in vivo [20]. Yeatts et al. reported that human MSCs cultured in tubular perfusion bioreactors for 10 days demonstrated significantly increased bone formation compared to statically cultured or acellular scaffolds [56]. Although tubular perfusion bioreactors enable culture of numerous scaffolds simultaneously, scaffolds were not cultured under direct perfusion to force media through the full scaffold volume, and no other time points were examined. In this study, we observed changes in vascular density in vivo as a function of perfusion culture duration in vitro. Constructs cultured for 1 day contain primarily undifferentiated MSCs. While constructs in perfusion culture for longer durations contain more MSCs, cells are further differentiated toward the osteoblastic lineage. We previously demonstrated in monolayer culture that MSCs secrete more proangiogenic factors when less committed to the osteoblastic phenotype [25, 61]. Thus, it is not surprising that constructs cultured for 7 days exhibited a trend for greater vessel density than 14 day constructs. These data are in accordance with our previous finding of lower vessel density in scaffolds containing osteogenically induced MSCs versus undifferentiated cells [62]. Acellular scaffolds were soaked in growth media for a brief period, much less than those in perfusion culture exposed to osteogenic media. Thus, their osteogenic potential may be due in part to different treatment regimens. The findings in this study suggest a combined effect of more cells due to prolonged culture duration possessing increased osteogenic potential but with blunted proangiogenic potential. We also observed significant increases in vascular density for constructs cultured for 7 days versus 1 day within each gender, emphasizing the need to consider gender-related effects on the proangiogenic nature of MSCs. At 8 weeks post-implantation, we detected a small number of MSCs in all cellular scaffolds, suggesting that human MSCs persist in this model for extended durations. In agreement with our in vitro findings, constructs cultured for 14 days, but not 7 days, exhibited significantly greater bone formation in an ectopic site. Thus, constructs with higher DNA content and human MSCs at a later stage of differentiation at the time of implantation contribute to more robust bone formation in vivo. Mygind et al. also reported that perfusion culture of macrotissues for 14 days led to more homogeneously distributed bone matrix than 7 days [63]. However, our data are contradictory to the findings of Sikavitsas et al. in which bone formation in an orthotopic defect was enhanced in constructs containing stromal osteoblasts at an early stage of differentiation [20]. These differences may relate to the in vivo models used, the biomaterials employed, or species differences in the cell source used to generate osteogenic grafts. The interplay between cell number, differentiation state, trophic factor secretion, and the collective effect on the in vivo bone forming potential of MSCs merits further study.
CONCLUSION
The results of this study demonstrate that HA-PLG scaffolds can augment osteogenic differentiation of MSCs under perfusion. Moreover, this study provides detailed insight into construct cellularity and osteogenic potential of MSCs as a function of bioreactor culture duration. While perfusion culture for only 7 days leads to improved neovascularization, 14 days of bioreactor culture is necessary for robust bone formation. As such, the duration of mechanical stimulation of MSCs seeded in composite scaffolds is an important consideration for future studies aimed at generating osteogenic grafts.
Supplementary Material
Representative immunohistochemistry images for Lamin A/C. Black arrows denote cells that stained positively for human-specific Lamin A/C. Scale bar represents 100 μm.
Full cross-section (scale bar represents 1000 μm) and inset for each image (10X magnification for H&E; 4X magnification for Masson’s Trichrome; 10X magnification for BSP; scale bar represents 250 μm). Blue arrows indicate pore walls while green arrows mark positive staining for BSP.
Acknowledgments
This work was supported by the California Institute of Regenerative Medicine (RT3-07981) and the National Institutes of Health (NIDCR DE025475) to JKL. DM is grateful for financial support through an industry/campus supported fellowship under the Training Program in Biomolecular Technology (T32-GM008799) at the University of California, Davis. JW was supported by a National Science Foundation Graduate Research Fellowship (1650042). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, CIRM or any other agency of the State of California. The funders had no role in the decision to publish, or preparation of the manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors indicate no potential conflicts of interest.
AUTHOR’S CONTRIBUTIONS
D. Mitra: Conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing. J. Whitehead: Collection and/or assembly of data. O.W. Yasui: Collection and/or assembly of data. J.K. Leach: Conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–65. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 2.Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13(5):927–38. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 3.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;(329):300–9. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Seiler JG, 3rd, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc. 2000;9(2):91–7. [PubMed] [Google Scholar]
- 5.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99(20):12600–5. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Uemera T, Dong J, Kojima H, Tanaka J, Tateishi T. Application of perfusion culture system improves in vitro and in vivo osteogenesis of bone marrow-derived osteoblastic cells in porous ceramic materials. Tissue Eng Part A. 2003;9(6):1205–1213. doi: 10.1089/10763270360728116. [DOI] [PubMed] [Google Scholar]
- 7.Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48(2):171–81. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 8.Yeatts AB, Geibel EM, Fears FF, Fisher JP. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol Bioeng. 2012;109(9):2381–91. doi: 10.1002/bit.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrivats AR, McDermott MC, Hollinger JO. Bone tissue engineering: state of the union. Drug Discov Today. 2014;19(6):781–6. doi: 10.1016/j.drudis.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103(8):2488–93. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Genetos DC, Leach JK. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng Part A. 2010;16(1):127–37. doi: 10.1089/ten.tea.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales-Hernandez DG, Genetos DC, Working DM, Murphy KC, Leach JK. Ceramic identity contributes to mechanical properties and osteoblast behavior on macroporous composite scaffolds. J Funct Biomater. 2012;3(2):382–97. doi: 10.3390/jfb3020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(8):1399–409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Tajbakhsh S, Hajiali F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):897–912. doi: 10.1016/j.msec.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Matsiko A, Gleeson JP, O’Brien FJ. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng Part A. 2015;21(3–4):486–97. doi: 10.1089/ten.TEA.2013.0545. [DOI] [PubMed] [Google Scholar]
- 16.Vissers CAB, Harvestine JN, Leach JK. Pore size regulates mesenchymal stem cell response to Bioglass-loaded composite scaffolds. Journal of Materials Chemistry B. 2015;3(44):8650–8658. doi: 10.1039/c5tb00947b. [DOI] [PubMed] [Google Scholar]
- 17.Holtorf HL, Datta N, Jansen JA, Mikos AG. Scaffold mesh size affects the osteoblastic differentiation of seeded marrow stromal cells cultured in a flow perfusion bioreactor. J Biomed Mater Res A. 2005;74(2):171–80. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Park IK, Kim JH, Cho CS, Kim MS. Gas foaming fabrication of porous biphasic calcium phosphate for bone regeneration. Tissue Engineering and Regenerative Medicine. 2012;9(2):63–68. [Google Scholar]
- 19.Gerhardt LC, Boccaccini AR. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials. 2010;3(7):3867–3910. doi: 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sikavitsas VI, van den Dolder J, Bancroft GN, Jansen JA, Mikos AG. Influence of the in vitro culture period on the in vivo performance of cell/titanium bone tissue-engineered constructs using a rat cranial critical size defect model. J Biomed Mater Res A. 2003;67(3):944–51. doi: 10.1002/jbm.a.10126. [DOI] [PubMed] [Google Scholar]
- 21.Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25(7):1823–9. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 22.Grayson WL, Bhumiratana S, Cannizzaro C, Chao PH, Lennon DP, Caplan AI, Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;14(11):1809–20. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grayson WL, Marolt D, Bhumiratana S, Frohlich M, Guo XE, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108(5):1159–70. doi: 10.1002/bit.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peppo GMd, Marcos-Campos In, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110(1):8680–8685. doi: 10.1073/pnas.1301190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7(4):e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhumiratana S, Bernhard JC, Alfi DM, Yeager K, Eton RE, Bova J, Shah F, Gimble JM, Lopez MJ, Eisig SB, Vunjak-Novakovic G. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med. 2016;8(343):343ra83. doi: 10.1126/scitranslmed.aad5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beşkardeş IG, Hayden RS, Glettig DL, Kaplan DL, G̈m̈şderelioğlu M. Bone tissue engineering with scaffold-supported perfusion co-cultures of human stem cell-derived osteoblasts and cell line-derived osteoclasts. Process Biochem. 2016 [Google Scholar]
- 28.Thorrez L, Shansky J, Wang L, Fast L, VandenDriessche T, Chuah M, Mooney D, Vandenburgh H. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials. 2008;29(1):75–84. doi: 10.1016/j.biomaterials.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathi SP, Lin DD, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32(22):5112–22. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J, Decaris ML, Leach JK. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A. 2012;18(13–14):1520–8. doi: 10.1089/ten.tea.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehler RM, Shin S, Fast AG, Gower RM, Shea LD. A PLG/HAp composite scaffold for lentivirus delivery. Biomaterials. 2013;34(21):5431–8. doi: 10.1016/j.biomaterials.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra D, Fatakdawala H, Nguyen-Truong M, Creecy A, Nyman J, Marcu L, Leach JK. Detection of pentosidine cross-links in cell-secreted decellularized matrices using Time Resolved Fluorescence Spectroscopy. ACS Biomater Sci Eng. 2016 doi: 10.1021/acsbiomaterials.6b00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvestine JN, Vollmer N, Ho S, Zikry C, Lee M, Leach K. Extracellular matrix-coated composite scaffolds promote mesenchymal stem cell persistence and bone formation. Biomacromolecules. 2016;22:3524–3531. doi: 10.1021/acs.biomac.6b01005. [DOI] [PubMed] [Google Scholar]
- 34.Murphy KC, Stilhano RS, Mitra D, Zhou D, Batarni S, Silva EA, Leach JK. Hydrogel biophysical properties instruct coculture-mediated osteogenic potential. FASEB J. 2016;30(1):477–86. doi: 10.1096/fj.15-279984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoch AI, Mittal V, Mitra D, Vollmer N, Zikry CA, Leach JK. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials. 2016;74:178–87. doi: 10.1016/j.biomaterials.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A Opt Image Sci Vis. 1984;1(6):612–619. [Google Scholar]
- 37.Keogh MB, Partap S, Daly JS, O’Brien FJ. Three hours of perfusion culture prior to 28 days of static culture, enhances osteogenesis by human cells in a collagen GAG scaffold. Biotechnol Bioeng. 2011;108(5):1203–1210. doi: 10.1002/bit.23032. [DOI] [PubMed] [Google Scholar]
- 38.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100(25):14683–8. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MA, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33(1):63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- 40.Egger D, Spitz S, Fischer M, Handschuh S, Glosmann M, Friemert B, Egerbacher M, Kasper C. Application of a parallelizable perfusion bioreactor for physiologic 3D cell culture. Cells Tissues Organs. 2017;203(5):316–326. doi: 10.1159/000457792. [DOI] [PubMed] [Google Scholar]
- 41.Murphy WL, Dennis RG, Kileny JL, Mooney DJ. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002;8(1):43–52. doi: 10.1089/107632702753503045. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Abedian R, Meister R, Haasper C, Hurschler C, Krettek C, von Lewinski G, Jagodzinski M. Influence of perfusion and compression on the proliferation and differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. Biomaterials. 2012;33(4):1052–64. doi: 10.1016/j.biomaterials.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Jiang H, Wang S, Li H, Zhang H, Zhao L, Peng T, Cao Z, Sun S. Construction of tissue-engineered bone using a bioreactor and platelet-rich plasma. Exp Ther Med. 2014;8(2):413–418. doi: 10.3892/etm.2014.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein AS, Zhu G, Morris GE, Meszlenyi RK, Mikos AG. Effect of osteoblastic culture conditions on the structure of poly(DL-lactic-co-glycolic acid) foam scaffolds. Tissue Eng. 1999;5(5):421–34. doi: 10.1089/ten.1999.5.421. [DOI] [PubMed] [Google Scholar]
- 45.Braccini A, Wendt D, Jaquiery C, Jakob M, Heberer M, Kenins L, Wodnar-Filipowicz A, Quarto R, Martin I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells. 2005;23(8):1066–72. doi: 10.1634/stemcells.2005-0002. [DOI] [PubMed] [Google Scholar]
- 46.Papadimitropoulos A, Riboldi SA, Tonnarelli B, Piccinini E, Woodruff MA, Hutmacher DW, Martin I. A collagen network phase improves cell seeding of open-pore structure scaffolds under perfusion. J Tissue Eng Regen Med. 2013;7(3):183–91. doi: 10.1002/term.506. [DOI] [PubMed] [Google Scholar]
- 47.Schumacher M, Uhl F, Detsch R, Deisinger U, Ziegler G. Static and dynamic cultivation of bone marrow stromal cells on biphasic calcium phosphate scaffolds derived from an indirect rapid prototyping technique. J Mater Sci Mater Med. 2010;21(11):3039–48. doi: 10.1007/s10856-010-4153-y. [DOI] [PubMed] [Google Scholar]
- 48.Yeatts AB, Choquette DT, Fisher JP. Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta. 2013;1830(2):2470–80. doi: 10.1016/j.bbagen.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mygind T, Stiehler M, Baatrup A, Li H, Zou X, Flyvbjerg A, Kassem M, Bunger C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28(6):1036–47. doi: 10.1016/j.biomaterials.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Di Luca A, Longoni A, Criscenti G, Mota C, van Blitterswijk C, Moroni L. Toward mimicking the bone structure: design of novel hierarchical scaffolds with a tailored radial porosity gradient. Biofabrication. 2016;8(4):045007. doi: 10.1088/1758-5090/8/4/045007. [DOI] [PubMed] [Google Scholar]
- 51.Di Luca A, Ostrowska B, Lorenzo-Moldero I, Lepedda A, Swieszkowski W, Van Blitterswijk C, Moroni L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci Rep. 2016;6:22898. doi: 10.1038/srep22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leferink AM, Chng YC, van Blitterswijk CA, Moroni L. Distribution and viability of fetal and adult human bone marrow stromal cells in a biaxial rotating vessel bioreactor after seeding on polymeric 3D additive manufactured scaffolds. Front Bioeng Biotechnol. 2015;3:169. doi: 10.3389/fbioe.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu HS, Won JE, Jin GZ, Kim HW. Construction of mesenchymal stem cell-containing collagen gel with a macrochanneled polycaprolactone scaffold and the flow perfusion culturing for bone tissue engineering. Biores Open Access. 2012;1(3):124–36. doi: 10.1089/biores.2012.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72(3):326–34. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 55.Seitz S, Ern K, Lamper G, Docheva D, Drosse I, Milz S, Mutschler W, Schieker M. Influence of in vitro cultivation on the integration of cell-matrix constructs after subcutaneous implantation. Tissue Eng. 2007;13(5):1059–67. doi: 10.1089/ten.2006.0334. [DOI] [PubMed] [Google Scholar]
- 56.Yeatts AB, Both SK, Yang W, Alghamdi HS, Yang F, Fisher JP, Jansen JA. In vivo bone regeneration using tubular perfusion system bioreactor cultured nanofibrous scaffolds. Tissue Eng Part A. 2014;20(1–2):139–46. doi: 10.1089/ten.tea.2013.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J, Ma T. Bioreactor strategy in bone tissue engineering: pre-culture and osteogenic differentiation under two flow configurations. Tissue Eng Part A. 2012;18(21–22):2354–64. doi: 10.1089/ten.tea.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kavlock KD, Goldstein AS. Effect of pulse frequency on the osteogenic differentiation of mesenchymal stem cells in a pulsatile perfusion bioreactor. J Biomech Eng. 2011;133(9):091005. doi: 10.1115/1.4004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Yu B, Chen J, Tang Z, Zong C, Shen D, Zheng Q, Tong X, Gao C, Wang J. Different effects of intermittent and continuous fluid shear stresses on osteogenic differentiation of human mesenchymal stem cells. Biomech Model Mechanobiol. 2012;11(3–4):391–401. doi: 10.1007/s10237-011-0319-x. [DOI] [PubMed] [Google Scholar]
- 60.Araujo JV, Cunha-Reis C, Rada T, Alves da Silva M, Gomes ME, Yang Y, Ashammakhi N, Reis RL, El-Haj AJ, Neves NM. Dynamic Culture of Osteogenic Cells in Biomimetically Coated Poly(Caprolactone) Nanofibre Mesh Constructs. Tissue Eng Part A. 2010;16(2):557–63. doi: 10.1089/ten.TEA.2009.0223. [DOI] [PubMed] [Google Scholar]
- 61.Binder BY, Genetos DC, Leach JK. Lysophosphatidic acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Eng Part A. 2014;20(7–8):1156–64. doi: 10.1089/ten.tea.2013.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Decaris ML, Binder BY, Soicher MA, Bhat A, Leach JK. Cell-derived matrix coatings for polymeric scaffolds. Tissue Eng Part A. 2012;18(19–20):2148–57. doi: 10.1089/ten.TEA.2011.0677. [DOI] [PubMed] [Google Scholar]
- 63.Chen M, Zhou M, Ye Z, Zhou Y, Tan WS. Ectopic osteogenesis of macroscopic tissue constructs assembled from human mesenchymal stem cell-laden microcarriers through in vitro perfusion culture. PLoS One. 2014;9(10):e109214. doi: 10.1371/journal.pone.0109214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative immunohistochemistry images for Lamin A/C. Black arrows denote cells that stained positively for human-specific Lamin A/C. Scale bar represents 100 μm.
Full cross-section (scale bar represents 1000 μm) and inset for each image (10X magnification for H&E; 4X magnification for Masson’s Trichrome; 10X magnification for BSP; scale bar represents 250 μm). Blue arrows indicate pore walls while green arrows mark positive staining for BSP.