Abstract
A large number of people in highly industrialized society are employed in night-shift work. Night-shift work interrupts the 24-hour daily cycle known as the circadian rhythm, as well as melatonin synthesis. These disruptions can make the body susceptible to oxidative stress and neural damage. In this regard, it is recommended that employees avoid long-term exposure to night-shift work.
Keywords: Night-shift work, Circadian rhythm, Melatonin, Neurodegenerative disorders
INTRODUCTION
In industrialized countries, a large number of people are engaging in night-shift work. This kind of work is reported to cause various health problems, including sleep disturbances [1], gastrointestinal or cardiovascular dysfunction [2], metabolic syndrome [3], breast cancer [4], colorectal cancer [5], oxidative stress [6], and even stroke [7]. However, night-shift work-induced neurological disorders have not been investigated thoroughly. In this article, our aim is to investigate the neurological susceptibility of night-shift workers and to elucidate the associated risks.
CIRCADIAN RHYTHM REGULATION
The internal environments of living creatures fluctuate on a 24-hour daily cycle known as the circadian rhythm [8]. Among these internal fluctuations, the sleep-wake cycle is the most prominent [9]. In mammals, the circadian rhythm (including the sleep-wake cycle) is regulated by the circadian master clock, the suprachiasmatic nucleus (SCN), which is located in the anterior hypothalamus [9,10]. The SCN consists of a vast population of clock cells that synchronize the 24-hour biological clock by receiving light information through the retinohypothalamic tract [11], followed by secondary orchestration from peripheral oscillators located in other brain regions and peripheral organs [10,12]. In addition to this central master clock, feeding restriction and forced exercise synchronize the peripheral clock [13]. The molecular circadian cycle is maintained by the key transcriptional activators circadian locomotor output cycles kaput (CLOCK) and brain muscle ARNT-like 1 (BMAL). The intracellular CLOCK level remains consistent throughout the day, but the level of BMAL increases in the morning, leading to binding between BMAL and CLOCK [14]. This heterodimer activates the transcription of other clock genes containing E-box cis regulatory sequences, including period circadian protein homologue (per) and cryptochrome (cry) in the daytime [15,16,17]. These accumulated PER and CRY proteins in the cytosol heterodimerize and translocate back to the nucleus at night [16]. Then, the PER-CRY heterodimers terminate their own transcription by inhibiting the CLOCK-BMAL complex, so that CLOCK-BMAL-mediated transcription of PER-CRY no longer occurs [15,16].
CIRCADIAN RHYTHM INTERACTION WITH MELATONIN
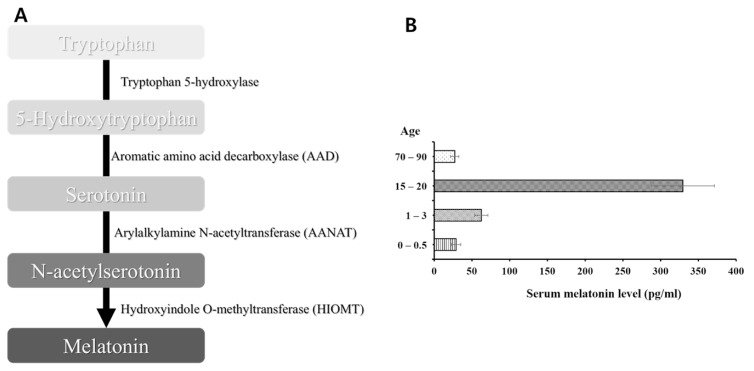
Melatonin is known to modulate the sleep-wake cycle and can alleviate sleep disorders [18]. Tryptophan, the precursor of melatonin, is converted to 5-hydroxytryptophan by tryptophan hydroxylase [19]. In sequence, aromatic amino acid decarboxylase (AAD) converts 5-hydroxytryptophan into serotonin [19]. Next, serotonin is acetylated through the action of arylalkylamine N-acetyltransferase (AANAT) [19]. Then, the acetylated form of serotonin, N-acetylserotonin, is converted to melatonin by the action of hydroxyindole O-methyltransferase (HIOMT) [19] (Fig. 1A).
Fig. 1.
The pineal hormone melatonin. Since melatonin is synthesized circadian-dependently, this hormone is known to be produced robustly at night. (A) Melatonin synthesizing procedures. Aromatic amino acid decarboxylase (AAD) converts 5-hydroxytryptophan into serotonin. Next, serotonin is acetylated through the action of arylalkylamine N-acetyltransferase (AANAT). Then, the acetylated form of serotonin, N-acetylserotonin, is converted to melatonin by the action of hydroxyindole O-methyltransferase (HIOMT) [27]. (B) Nocturnal melatonin synthesis seems to decrease with age. In elderlies, increased vulnerability to neurodegenerative diseases might be due to decreased nocturnal melatonin synthesis [43].
It has been reported that the mammalian SCN regulates the rhythmicity of melatonin synthesis by transmitting the oscillatory information that fluctuates depending on the release of norepinephrine [20]. The release of this neurotransmitter increases at night and decreases during the day [20,21]. The interaction between norepinephrine and β–adrenergic receptors on pinealocytes leads to the synthesis of adenosine 3′,5′-monophosphate (cyclic AMP; cAMP), followed by the induction of arylalkylamine N-acetyltransferase (AANAT) [20].
The mammalian retina is responsible for photic synchronization of the circadian rhythm. The retinal photoreceptors are reported to depolarize at night and hyperpolarize in the daytime [22]. Depolarization of photoreceptors causes Ca2+ influx through voltage-gated channels and increases cAMP level [22]. Consequently, the increase in cAMP level leads to the transcription of the AANAT gene [22], the product of which is known to be a rate-limiting enzyme in melatonin synthesis [23,24]. Thus, the circadian clock affects melatonin synthesis by only enabling cAMP to activate AANAT transcription at a specific time [22,23]. Another study also demonstrated that the BMAL1/CLOCK complex can activate AANAT transcription, suggesting that the circadian rhythm participates in melatonin synthesis [22,25].
NIGHT-SHIFT WORK AND THE CIRCADIAN RHYTHM
Night-shift workers suffer from reduced alertness due to disruption of the circadian rhythm. As described above, this biological rhythm is regulated by the circadian master oscillator, SCN [11]. When the body encounters a new circadian phase, the central and peripheral oscillators begin adjusting to the new schedule, but this adaptation does not occur immediately [10,26]. The circadian adaptation to a new phase is thought to occur at the speed of 1 hour per day [26]. However, this adjustment is impaired in night-shift workers, because it is counteracted by the light cycle [26]. Also, the adjustment speed of the central oscillator is faster than that of the peripheral oscillators [26]. Therefore, this internal desynchronization can cause functional disturbances; one might feel drowsiness in the daytime and be unable to sleep in the nighttime. Also, night-shift workers are likely to suffer from sleep deprivation, which is known to alter not only clock gene expression, but also the DNA binding patterns of BMAL1-CLOCK heterodimers [27,28].
CIRCADIAN DISTURBANCES DUE TO NIGHT-SHIFT WORK AND THEIR IMPLECATIONS FOR NEUROLOGICAL DISORDERS
As melatonin synthesis is affected by the circadian pattern, the release of this hormone might reflect the circadian state. Thus, neurological disorders seem to be associated with circadian disturbances [29]. Therefore, night-shift workers with disrupted circadian patterns and melatonin level are thought to be vulnerable to neurological disorders. Interestingly, nocturnal melatonin synthesis seems to decrease with age [30] (Fig. 1B). This might explain why the increased risk for neurodegenerative disorders has been observed not only in night-shift workers, but also in elderlies. Therefore, it is essential to identify the role of melatonin to better understand the relationship between the circadian rhythm and neurological disorders.
1. Stroke
As mentioned above, melatonin is synthesized circadian-dependently and is known to be synthesized robustly at night [31]. Thus, nighttime workers tend to have low levels of melatonin. In turn, lowered melatonin concentration might increase the risk of neurological disorders, especially stroke [7]. Brown et al. suggested three reasons that reduced melatonin level can increase the risk of stroke [7]. Lack of melatonin can i) increase the risk of atherosclerosis, since atherosclerosis is mainly due to ROS, and melatonin is an antioxidant [7,32], ii) contribute to hypercoagulability [7,33], and iii) reduce blood pressure [7,34]. These protective roles of melatonin cannot be fully exerted if workers are constantly exposed to nighttime work, as melatonin synthesis will be reduced. Other risk factors such as hypertension, diabetes, heart disease, smoking, and obesity might be considered as indirect risk factors for stroke [7].
2. Epilepsy
Patients with epilepsy suffer from seizures and tend to experience seizures at particular times of day [9]. This has been attributed to the circadian fluctuation of stress hormones. The stress hormone cortisol is released in accordance with circadian fluctuations [35,36]. Stress can be a risk factor for epileptic seizures [35,37], and stress hormones can affect the seizure threshold [35]. In this regard, circadian-mediated changes in stress hormone release in night-shift workers can cause diurnal epileptic seizures [35].
3. Alzheimer′s disease (AD)
Circadian rhythm disturbances can easily be seen in AD patients. This disease is characterized by amyloid-β accumulation. The relationship between amyloid-β and the circadian rhythm has been investigated by numerous researchers. It has been suggested that sleep deprivation increases amyloid-β plaque formation and amyloid precursor protein levels, and thus can increase AD risk in night workers [38].
In addition to being a key regulator of the diurnal cycle, the circadian clock might also suppress oxidative stress and synaptic damage [27]. For example, a reduced level of BMAL1, one of the circadian regulating factors, is thought to cause neurodegeneration, as evidenced by the astrogliosis, oxidative damage, and synaptic damage in Bmal1 knockout mice [39]. In this regard, it is expected that the circadian clock regulates amyloid-β level, and that disrupted circadian rhythms make night-shift workers susceptible to amyloid-β accumulation.
4. Parkinson′s disease (PD)
Circadian disruptions and insufficient melatonin synthesis in night-shift workers might make them susceptible to PD, because this disease is also thought to be associated with the pineal hormone melatonin. Previous studies have demonstrated that melatonin level is lower in PD patients than in normal individuals [29,40,41]. Also, clock gene expression seems to be altered in these patients, implying circadian disruption [41]. Melatonin can protect against PD by reducing the neurotoxicity of 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [29,42], thus suppressing α-synuclein assembly [29,43]. Therefore, night shift-work-induced circadian disruptions can increase the risk of PD, while melatonin can counteract this risk.
CONCLUSION
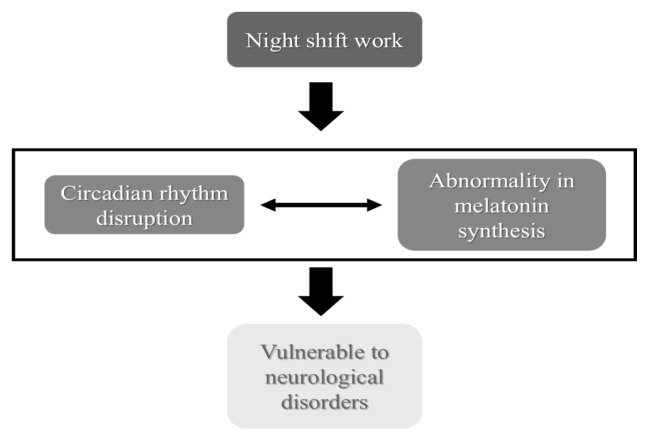
These days, numerous people are employed in night-shift work; however, this kind of work makes people vulnerable to various diseases. Night-shift work can disrupt circadian rhythms, which can alter the synthesis and concentration of melatonin, a hormone that is synthesized circadian-dependently and has neuroprotective/antioxidative roles, thus increasing the risk of neurodegenerative disorders (Fig. 2). Therefore, night-shift workers should be advised to avoid long-term exposure to night-shift work.
Fig. 2.
Night-shift work may increase the risk of neurological disorders. Night-shift work can disrupt circadian rhythms, which can alter the synthesis and concentration of melatonin, a hormone that is synthesized circadian-dependently, and has neuroprotective/antioxidative roles, thus increasing the risk of neurodegenerative disorders.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Research Foundation (NRF-2013R1A2A2A01067169 to Y.H., NRF-2017R1A2A2A01067169 to Y.H.), Republic of Korea. This work was also supported by the 2016 Creative Research Program of Inje University.
REFERENCES
- 1.Garbarino S, Beelke M, Costa G, Violani C, Lucidi F, Ferrillo F, Sannita WG. Brain function and effects of shift work: implications for clinical neuropharmacology. Neuropsychobiology. 2002;45:50–6. doi: 10.1159/000048674. [DOI] [PubMed] [Google Scholar]
- 2.Costa G. The impact of shift and night work on health. Appl Ergon. 1996;27:9–16. doi: 10.1016/0003-6870(95)00047-X. [DOI] [PubMed] [Google Scholar]
- 3.Pietroiusti A, Neri A, Somma G, Coppeta L, Iavicoli I, Bergamaschi A, Magrini A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67:54–7. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 4.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 5.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–8. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 6.Sharifian A, Farahani S, Pasalar P, Gharavi M, Aminian O. Shift work as an oxidative stressor. J Circadian Rhythms. 2005;3:15. doi: 10.1186/1740-3391-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DL, Feskanich D, Sanchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169:1370–7. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turek FW. Circadian rhythms. Horm Res. 1998;49:109–13. doi: 10.1159/000023155. [DOI] [PubMed] [Google Scholar]
- 9.Turek FW, Dugovic C, Zee PC. Current understanding of the circadian clock and the clinical implications for neurological disorders. Arch Neurol. 2001;58:1781–7. doi: 10.1001/archneur.58.11.1781. [DOI] [PubMed] [Google Scholar]
- 10.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17:489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 11.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 12.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Hattori Y, Ikeda Y, Kamagata M, Iwami S, Yasuda S, Tahara Y, Shibata S. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci Rep. 2016;6:27607. doi: 10.1038/srep27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10:683–93. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec 2):R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 16.Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. bHLH-PAS proteins in cancer. Nat Rev Cancer. 2013;13:827–41. doi: 10.1038/nrc3621. [DOI] [PubMed] [Google Scholar]
- 17.Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26:687–94. doi: 10.1002/gps.2582. [DOI] [PubMed] [Google Scholar]
- 19.Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. Sci World J. 2014;2014:586270. doi: 10.1155/2014/586270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi JS, Zatz M. Regulation of circadian rhythmicity. Science. 1982;217:1104–11. doi: 10.1126/science.6287576. [DOI] [PubMed] [Google Scholar]
- 21.Brownstein M, Axelrod J. Pineal gland: 24-hour rhythm in norepinephrine turnover. Science. 1974;184:163–5. doi: 10.1126/science.184.4133.163. [DOI] [PubMed] [Google Scholar]
- 22.Tosini G, Fukuhara C. Photic and circadian regulation of retinal melatonin in mammals. J Neuroendocrinol. 2003;15:364–9. doi: 10.1046/j.1365-2826.2003.00973.x. [DOI] [PubMed] [Google Scholar]
- 23.Niki T, Hamada T, Ohtomi M, Sakamoto K, Suzuki S, Kako K, Hosoya Y, Horikawa K, Ishida N. The localization of the site of arylalkylamine N-acetyltransferase circadian expression in the photoreceptor cells of mammalian retina. Biochem Biophys Res Commun. 1998;248:115–20. doi: 10.1006/bbrc.1998.8916. [DOI] [PubMed] [Google Scholar]
- 24.Klein DC, Roseboom PH, Coon SL. New light is shining on the melatonin rhythm enzyme: the first postcloning view. Trends Endocrinol Metab. 1996;7:106–12. doi: 10.1016/1043-2760(96)00033-1. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Baler R. The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Brain Res Mol Brain Res. 2000;81:43–50. doi: 10.1016/S0169-328X(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 26.Akerstedt T. Work hours, sleepiness and the underlying mechanisms. J Sleep Res. 1995;4:15–22. doi: 10.1111/j.1365-2869.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 27.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mongrain V, La Spada F, Curie T, Franken P. Sleep loss reduces the DNA-binding of BMAL1, CLOCK, and NPAS2 to specific clock genes in the mouse cerebral cortex. PLoS One. 2011;6:e26622. doi: 10.1371/journal.pone.0026622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbott SM, Videnovic A. Chronic sleep disturbance and neural injury: links to neurodegenerative disease. Nat Sci Sleep. 2016;8:55–61. doi: 10.2147/NSS.S78947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldhauser F, Weiszenbacher G, Tatzer E, Gisinger B, Waldhauser M, Schemper M, Frisch H. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J Clin Endocrinol Metab. 1988;66:648–52. doi: 10.1210/jcem-66-3-648. [DOI] [PubMed] [Google Scholar]
- 31.Wurtman RJ, Axelrod J, Phillips LS. Melatonin synthesis in the pineal gland: Control by light. Science. 1963;142:1071–3. doi: 10.1126/science.142.3595.1071. [DOI] [PubMed] [Google Scholar]
- 32.Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 33.Wirtz PH, Spillmann M, Bartschi C, Ehlert U, von Kanel R. Oral melatonin reduces blood coagulation activity: a placebo-controlled study in healthy young men. J Pineal Res. 2008;44:127–33. doi: 10.1111/j.1600-079X.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 34.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–7. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 35.van Campen JS, Valentijn FA, Jansen FE, Joels M, Braun KP. Seizure occurrence and the circadian rhythm of cortisol: a systematic review. Epilepsy Behav. 2015;47:132–7. doi: 10.1016/j.yebeh.2015.04.071. [DOI] [PubMed] [Google Scholar]
- 36.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 37.van Campen JS, Jansen FE, de Graan PN, Braun KP, Joels M. Early life stress in epilepsy: a seizure precipitant and risk factor for epileptogenesis. Epilepsy Behav. 2014;38:160–71. doi: 10.1016/j.yebeh.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 38.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, Rademaker AW, Simuni T, Zadikoff C, Zee PC. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71:463–9. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–95. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acuna-Castroviejo D, Coto-Montes A, Gaia Monti M, Ortiz GG, Reiter RJ. Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sci. 1997;60:PL23–9. doi: 10.1016/s0024-3205(96)00606-6. [DOI] [PubMed] [Google Scholar]
- 43.Ono K, Mochizuki H, Ikeda T, Nihira T, Takasaki J, Teplow DB, Yamada M. Effect of melatonin on alpha-synuclein self-assembly and cytotoxicity. Neurobiol Aging. 2012;33:2172–85. doi: 10.1016/j.neurobiolaging.2011.10.015. [DOI] [PubMed] [Google Scholar]