Abstract
Cardiovascular disease, a leading cause of death worldwide, is frequently initiated or exacerbated by stress. In fact, chronic stress exposure and heightened reactions to acute psychological stress are both associated with increased cardiovascular morbidity. This brief review focuses on the mechanisms by which corticolimbic nuclei, critical for stress appraisal and emotional reactivity, regulate heart rate and blood pressure responses to psychological stress. Both human and rodent data are examined with a major emphasis on basic studies investigating prefrontal cortex, amygdala, and hippocampus. A detailed literature review reveals substantial limitations in our understanding of this circuitry, as well as significant opportunities for future investigation that may ultimately reduce the burden of cardiovascular illness.
Keywords: Prefrontal cortex, amygdala, hippocampus, blood pressure, heart rate
1. Introduction
1.1 Stress and cardiovascular health
Cardiovascular disease is the most common cause of death worldwide [1] and cardiovascular function is profoundly influenced by stress [2]. In particular, both clinical studies and animal models indicate that chronic stress increases sympathetic drive, promoting tachycardia and elevated arterial pressure [3–6]. Consequently, chronic stress exposure represents a major risk factor for pathological conditions including arrhythmias and cardiac hypertrophy [7]. Furthermore, individuals experiencing depressive illness exhibit autonomic imbalance marked by both sympathetic activation and vagal withdrawal, leading to reduced heart rate variability (HRV) and altered baroreflex sensitivity [8–10]. These depression-related autonomic changes generate a predisposition to co-morbid cardiovascular disease including myocardial ischemia, atherosclerosis, and heart failure [11–13]. Importantly, a case-control study examining potential risk factors for heart disease, the INTERHEART study, found that risk of myocardial infarction more than doubled as a result of psychosocial stress, even after taking into account conventional risk factors such as diabetes, obesity, and lack of exercise [14]. These findings indicate that chronic stress exposure significantly impairs long-term cardiovascular health independent of lifestyle factors that may be associated with stress. Although the biological mechanisms mediating this relationship are not completely understood, stress-associated adverse cardiovascular outcomes likely result from prolonged exposure to neural and endocrine stress responses. In addition to the direct autonomic neural inputs to the heart and vasculature, stress initiates sympathetic-adrenomedullary catecholamine release and corticosteroid secretion by the hypothalamic-pituitary-adrenocortical axis [15]. Collectively, neural and endocrine stress mediators influence many aspects of cellular physiology, including cytokine expression [16–18] and endothelial dysfunction [7,19], both of which predict the incidence of hypertension and heart failure [20].
In addition to chronic stress, exaggerated reactivity to acute stress is also associated with poor cardiovascular outcomes. For instance, increased rates of acute cardiac events occur in association with stressful life events such as bereavement and natural disasters [21,22]. In clinical studies, enhanced heart rate (HR) reactivity to an acute mental stressor predicts future cardiovascular susceptibility, including hypertension, ventricular hypertrophy, and atherosclerosis [23]. Furthermore, short-term corticosteroid concentrations predict long-term cardiovascular mortality relative to all other causes of death and regardless of pre-existing cardiovascular disease [24]. Thus, enhanced acute autonomic and endocrine stress reactivity predict future cardiovascular outcomes. Cardiovascular stress reactivity is proposed to arise at the level of cognitive appraisal, followed by translation to autonomic and endocrine responses by the hypothalamus and brainstem [25,26]; however, the neurobiological mechanisms linking cognitive and stress appraisal circuits with autonomic physiology are incompletely understood. The appraisal of emotion largely occurs in the limbic system, a loosely-defined network of interconnected structures spanning the forebrain. Within the telencephalon, the medial prefrontal cortex (mPFC), amygdala, and hippocampus are the primary limbic cortical structures mediating emotion, memory, and cognition. Consequently, untangling the descending stress-regulatory circuits of these regions represents an important opportunity for delineating the complex relationships between stress and cardiovascular health.
1.2 Central networks mediating cardiovascular risk
Human brain imaging, as well as tract-tracing and electrical stimulation studies in rodents, has identified a collection of interconnected brain structures forming a central autonomic network [27,28]. This network includes subregions of the cerebral cortex, amygdala, hypothalamus, and brainstem [29]. While the major pre-autonomic systems in the hypothalamus and brainstem have been subject to substantial investigation, the corticolimbic regions have received considerably less attention. Recently, a comprehensive meta-analysis was undertaken to determine the human brain regions activated by processing autonomic functions [30]. Across multiple studies and tasks, the mPFC, insular cortex, and amygdala were implicated in responding to autonomic activity. Furthermore, studies by Shoemaker and colleagues (for reviews see [31,32]) have delineated a network of human forebrain structures specifically involved in reflexive cardiovascular control. These studies outline a similar system of cortical structures (e.g. mPFC, amygdala, and insular cortex), as well as the hippocampus, for cardiovascular responsiveness to reflexive challenges such as exercise. The current review focuses specifically on the mPFC, amygdala, and hippocampus due to their essential roles in stress appraisal and reactivity [33–39]. While functional imaging studies indicate that activity in these corticolimbic regions is associated with autonomic cardiovascular regulation, it remains unclear what specific neuronal processes mediate cardiovascular reactivity to acute psychological stress, as well as functional changes associated with chronic stress exposure.
Interestingly, a story is emerging based on presentation of data at a recent scientific meeting [40]. This study engaged a large cohort of individuals in prospective neuroimaging and found that amygdala activity predicted the likelihood of adverse cardiac events. Not only was amygdala activity linked to overall risk for heart attack and stroke, it also predicted the timing of these major cardiac events. Specifically, the magnitude of amygdala activation was positively correlated with immediacy of heart attack and stroke. Although these results remain in the early stages of dissemination, they offer a potential neural substrate for limbic control of cardiovascular risk. Another study examining patients with cortical lesions found that damage specifically within the medial prefrontal region correlated with exaggerated HR responses to mental stress [41]. Thus, there is evidence for an important biological relationship between the activity of corticolimbic brain structures and cardiovascular health.
The initial studies of fronto-temporal control of cardiovascular physiology occurred over 60 years ago [42,43]; however, this is an area of neurobiology that has been somewhat orphaned. While the neurochemical mediators of cardiovascular function in the brainstem and hypothalamus have been studied extensively in animals [44–49], much less is known about the role of telencephalic regions in cardiovascular autonomic control. Much of this work has focused on electric stimulation in anesthetized animals or structural mapping and connectivity, without measuring functional cardiovascular outcomes. However, a number of studies have directly manipulated corticolimbic regions and examined cardiovascular reactivity to acute stress. The areas targeted in these studies, the mPFC, amygdala, and hippocampus, are discussed in the current review with a focus on existing knowledge and questions for future investigation. Importantly, these structures are extremely sensitive to stress and significantly contribute to behavioral stress responsiveness [15,50]. Specifically, chronic stress leads to hypofunction of the mPFC, a structure critical for context appraisal and executive function [51,52]. Prolonged stress has opposite effects in the amygdala, including cellular hypertrophy and dendritic arborization [53,54], representing a potential mechanism for stress-associated anxiety and mood disturbances. Meanwhile, neurons of the hippocampus, crucial for memory and mood, are endangered by chronic stress through exposure to excess corticosteroids [55,56]. The role of these regions in neuroendocrine and behavioral control have been extensively reviewed elsewhere [57,58]; consequently, this brief review focuses on the emerging literature of site-specific studies examining cardiovascular stress responses and questions that remain to be addressed. It is worth noting that, in terms of endocrine, neural, and behavioral responses, systemic and psychogenic stressors engage divergent circuitry. While some cell groups preferentially respond to physiological challenge (e.g. hemorrhage, inflammation, etc.), others are engaged by psychological stressors (e.g. restraint, learned fear, etc.) [59–63]. Given the emphasis of the current review on psychosocial factors influencing cardiovascular physiology, the literature reviewed will be limited to psychogenic stressors.
2. Descending autonomic regulation
The mPFC, amygdala, and hippocampus generate reciprocal, integrated projections that form an interconnected network of emotional and cognitive regulation [35,36,64]. Output of these structures also converges on crucial subcortical sites, primarily within rhomboid and anterolateral subregions of the bed nucleus of the stria terminalis (BST), distinct regions of the hypothalamus (e.g. lateral hypothalamic area [LHA], medial preoptic area [mPOA], and posterior hypothalamus [PH]), and the brainstem (e.g. ventrolateral medulla [VLM], raphe nuclei, and nucleus of the solitary tract [NTS]) [65]. This organization permits the downstream processing of limbic information by nuclei that provide direct synaptic input to sympathetic and parasympathetic preganglionic neurons. Corticolimbic structures provide little direct innervation of stress-effector neurons and this hierarchal organization of subcortical relays provides the intervening synapses necessary to translate emotional processes into physiological reactivity [66]. The vast majority of studies examining this connectivity were conducted in rodents but recent non-human primate research suggests that there is a high level of homology in the descending pathways of autonomic control [67].
The dorsal motor nucleus of the vagus (DMV) is the primary site of preganglionic parasympathetic neurons that target the upper viscera, with the nucleus ambiguus (NA) containing the majority of the cells regulating the heart [15]. In contrast, preganglionic sympathetic neurons arise from the intermediolateral cell column (IML) in the thoracolumbar region of the spinal cord [68,69]. The major stress-regulatory brain regions providing direct inputs to the DMV include the BST, hypothalamus, and hindbrain. Specifically, the DMV in innervated by the anterolateral and rhomboid divisions of the BST [70,71], while hypothalamic inputs arise from the LHA [72], mPOA [73], and paraventricular hypothalamus (PVH) [74]. Although the PVH does not receive direct input from the telencephalon, it is notable as a primary regulator of organismal stress responding with distinct populations of cells that target the pituitary for endocrine responses and others that provide direct input to preganglionic neurons of both the parasympathetic and sympathetic nervous systems [74,75]. Brainstem inputs to the DMV arise from the NTS [76], locus coeruleus (LC) [77], and A1 cell group of the VLM [78]; however, the brainstem exhibits substantial interconnectedness with numerous interactions between the reticular nuclei and vagal complex. There is overlap between inputs to the NA and DMV, with the NA receiving afferents from the rhomboid BST [71], NTS [79], as well as the parabrachial nuclei (PB) [80]. Inputs to the IML appear to be limited largely to the brainstem and hypothalamus [81,82]. Within the brainstem, the VLM, LC, and ventral raphe nuclei project directly to the IML [83,84]; consequently, the influence of many sympatho-modulatory brain structures converge on these nuclei (e.g. the dorsomedial hypothalamus [DMH] and periaqueductal gray [PAG]) [85,86]. Interestingly, a multi-species comparative analysis of hypothalamic afferents to the thoracic IML revealed direct inputs arising specifically from the PVH, LHA, and PH [87]. Accordingly, these pre-autonomic nuclei act as crucial subcortical targets of corticolimbic structures and are well-positioned to integrate affective and cognitive processes with cardiovascular reactivity.
The BST, hypothalamus, and brainstem interact substantially [88–90] and their general effects on markers of sympathetic and parasympathetic activity are well-characterized. There is also a considerable amount of data on the specific roles of these structures for stress-induced cardiovascular responses, which have been reviewed elsewhere [91–95]. Briefly, tonic GABAergic signaling within the PH and neighboring dorsomedial hypothalamus constrains HR and arterial responses to stress and disinhibition of these nuclei increases reactivity [46,96,97]. The LHA inhibits tachycardic responses to stress via the parasympathetic nervous system [98], while the mPOA mediates stress effects of angiotensin on mean arterial pressure (MAP) and vascular resistance [99]. The PVH has multiple mechanisms for bi-directional control of HR and blood pressure responses [100,101], although net synaptic activity in the PVH facilitates cardiovascular stress responses [102]. The NTS is an integral component of autonomic integration and possesses great neurochemical diversity [91,103,104]. Importantly, noradrenergic neurons of the NTS increase HR responses to acute stress and mediate the increase in net sympathetic drive that occurs during chronic stress exposure [105,106]. The NTS is also a site of corticosteroid feedback [107,108] and local glucocorticoid signaling increases HR and MAP responses to both acute and repeated stress [109,110]. Subregion-specific effects have yet to be determined for the extremely heterogeneous BST but evidence suggests that the region generally inhibits HR responses to stress through the parasympathetic nervous system [111]. However, corticotropin-releasing hormone signaling in the BST increases tachycardic and pressor responses to acute stress [112]. Collectively, corticolimbic inputs to these specific regions of the hypothalamus, hindbrain, and BST likely form the neural substrate for corticolimbic-cardiovascular interactions.
3. Medial prefrontal cortex
Multiple cortical areas have been linked to the central autonomic network, namely prefrontal, cingulate, and insular cortices [30,113,114]. Importantly, the mPFC is required for appropriate appraisal and processing of stressful information [115,116], as well as coordinating visceral and behavioral functions [27]. Human studies have correlated decreased mPFC activity with baroreceptor unloading [117] and increased mPFC activation with enhanced HRV [118]. Further, experiments in anesthetized rats have shown that electrical stimulation of the mPFC initiates depressor responses accompanied by inhibition in the VLM [119]. In rodents, the mPFC is generally divided into a dorsal component (prelimbic) and a ventral component (infralimbic), which are homologous to subregions of human prefrontal and cingulate cortices [115]. For detailed comparative analyses of prefrontal/anterior cingulate homologies between rodents and primates see [120,121]. The anatomical distinction within the rodent mPFC is based largely on efferent projections [122] (Fig. 1). Injections of the transneuronal retrograde tracer pseudorabies virus into the stellate ganglion (sympathetic ganglion targeting the heart) or the adrenal gland reveal multisynaptic telencephalic inputs to sympathetic neurons not only from amygdalar and hippocampal regions but also from the infralimbic mPFC (IL) [123]. The IL is well-positioned to influence autonomic stress responses, as the area provides glutamatergic innervation of the NTS, as well as stress-activated inputs to intranuclear GABAergic cells of the PH [88,122,124,125]. Alternatively, the prelimbic mPFC (PL) innervates a population of GABAergic neurons in the anterior BST that inhibit endocrine stress responses [126], as well as the rostral portions of the raphe [122].
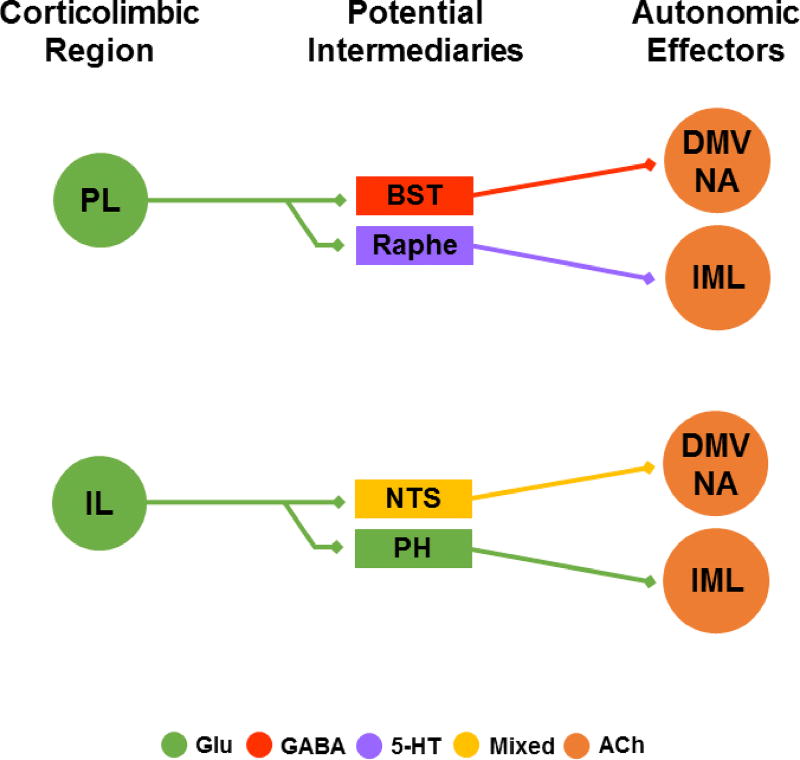
Fig. 1.
Hypothesized anatomical pathways translating mPFC output into autonomic processes. Simplified direct connections are illustrated without indirect multisynaptic circuits. Glutamate outflow from the PL targets GABAergic neurons in the BST and both 5-HT and GABAergic cells in the raphe. These structures then provide direct input to preganglionic neurons of the parasympathetic and sympathetic nervous systems. The IL targets local GABAergic interneurons within the glutamatergic PH to gate stress responding. Additional glutamatergic IL efferents target the NTS, although the neurochemistry of these targets has yet to be determined. See text for references. PL: prelimbic cortex, BST: bed nucleus of the stria terminalis, DMV: dorsal motor nucleus of the vagus, NA: nucleus ambiguus, IML intermediolateral cell column, IL: infralimbic cortex, NTS: nucleus of the solitary tract, PH: posterior hypothalamus, Glu: glutamate, 5-HT: serotonin, ACh: acetylcholine.
Studies of mPFC subregional regulation of cardiovascular stress responses reveal a potential dichotomy between IL and PL. All efferent and afferent neurotransmission can be inhibited within an area via local injections of the non-specific synaptic blocker cobalt chloride. This approach lacks neurochemical and input/output specificity but can be used to determine the functional role of net activity in a given nucleus. Cobalt chloride injections in the PL during acute restraint stress, a common psychogenic stressor, indicate that neurotransmission in the PL inhibits the tachycardiac response to stress without affecting MAP [127]. In contrast, IL injections implicate synaptic signaling in this area in stimulating stress-induced tachycardia. Notably, injections into the neighboring anterior cingulate cortex have no effect [127]. Other studies inhibiting the IL with the GABA agonist muscimol found no effect on HR or MAP responses to any of three acute stressors (cage change, restraint, or air jet) [128]. However, the same study did find that NMDA-mediated activation of the IL decreased HR and MAP responses to air jet stress. Furthermore, cobalt chloride injections globally in the mPFC (PL and IL inclusive) decrease both HR and MAP responses stimulated by exposure to a context previously paired with foot shock [129], indicating the mPFC may be important for generating stress responses based on learned outcomes. This is supported by studies of auditory-cued fear conditioning that found different HR responses to a conditioned stimulus based on lesion placement in the mPFC [130]. Specifically, aspiration lesions of the entire mPFC decreased HR responses to tone. However, aspirations restricted to the dorsal mPFC (including PL) increased tachycardic responses, suggesting the region is symaptho-inhibitory. Excitotoxic chemical lesions of the ventral mPFC (including IL) reduced sympathetic-mediated tachycardia, again suggesting subregions of the mPFC may differentially mediate the cardiovascular consequences of learned fear. Collectively, this literature based on nonspecific synaptic blockade and lesions suggests that the PL likely inhibits acute cardiovascular stress responses, whereas the IL could drive sympathetic activation. However, these effects are equivocal and appear to be context and experience dependent. Ultimately, appropriate regulation of subregional mPFC activity may be vital for reducing stress-related cardiovascular risk, yet more selective studies aimed at specifically targeting the principal neurons of the mPFC, without damaging the local inhibitory circuits or afferents, will provide more definitive conclusions.
4. Amygdala
The amygdala is a collection of many distinct nuclei with developmental, molecular, and organizational heterogeneity [131]. Although there are a variety of circuits and neurochemical mediators within the amygdala, it is generally thought of as a stress-excitatory region that promotes fear and anxiety [132–135]. In fact, activation of some amygdalar regions is sufficient for cardiovascular excitation even in the absence of stress [136]. However, the complex microcircuitry within the amygdala and diverse interactions with other corticolimbic and subcortical nuclei suggest that bidirectional control of stress responses is more likely [137]. Generally, human brain imaging technology cannot identify structure and function of distinct amygdalar nuclei; however, groundbreaking work by Gianaros and colleagues has shown that amygdala activity significantly predicts blood pressure reactivity to stress, as well as preclinical atherosclerosis [113,138,139]. Specifically, individuals with greater MAP stress reactivity show greater amygdala activation and functional connectivity with the perigenual anterior cingulate cortex (homologous to PL) and pontine nuclei [138]. Individual pontine nuclei cannot be resolved but these could potentially include the LC, PB, raphe nuclei, and/or caudal PAG, all of which have been described as critical sites for stress integration [104]. In another study, preclinical atherosclerosis covaried with greater amygdala reactivity and functional connectivity between the amygdala and perigenual anterior cingulate cortex [139]; suggesting that circuit-level interactions between the amygdala and mPFC could be key determinates of susceptibility to cardiovascular pathology. Circuit-mapping in rodents indicates that the lateral, basolateral (BLA), basomedial, and cortical regions of the amygdala provide glutamatergic output to the BST [88] (Fig. 2). Meanwhile, the medial amygdala (MeA) predominately, but not exclusively, releases GABA from projections that target the mPOA, PH, and BST [88,124]. Notably, the central amygdala (CeA) receives input from most other regions of the amygdala and provides abundant stress-related outputs [134,140] (Fig. 3). Not only does the CeA send GABAergic projections to the BST, mPOA, and DMH, it also robustly innervates the brainstem, notably targeting the PB, LC, NTS, raphe, and rostral VLM [88,141–143]. Given this organization, the amygdala has been proposed to disinhibit stress responding through GABA-GABA synaptic connections [33,88,142,144].
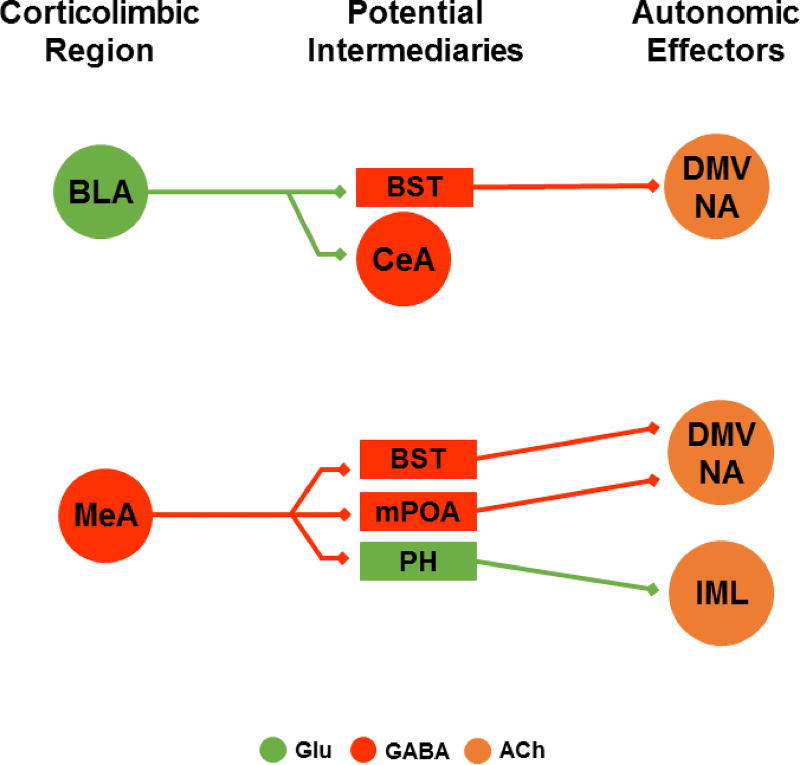
Fig. 2.
Hypothesized anatomical pathways mediating BLA and MeA effects on autonomic outflow. Simplified direct connections are illustrated without indirect multisynaptic circuits. The BLA has limited interaction with cell groups providing direct input to preganglionic neurons; however, the region sends excitatory projections to GABAergic cells in the BST and CeA. The MeA sends predominantly GABAergic projections to the BST, mPOA, and PH, although the neurochemistry of post-synaptic targets has not been directly investigated. See text for references. BLA: basolateral amygdala, BST: bed nucleus of the stria terminalis, CeA: central amygdala, DMV: dorsal motor nucleus of the vagus, NA: nucleus ambiguus, IML intermediolateral cell column, MeA: medial amygdala, mPOA: medial preoptic area, PH: posterior hypothalamus, Glu: glutamate, ACh: acetylcholine.
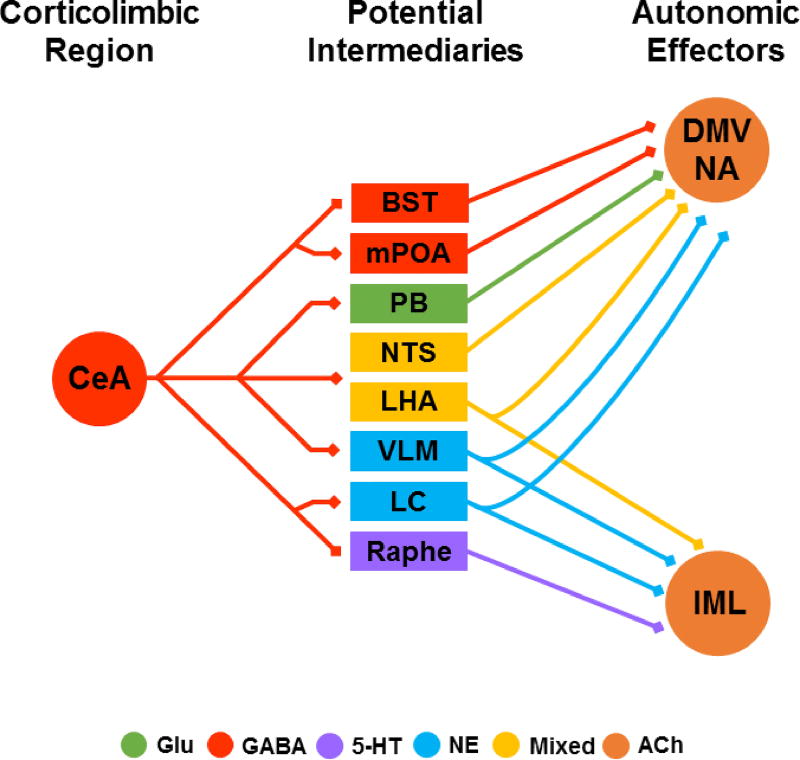
Fig. 3.
Hypothesized anatomical pathways translating CeA output into cardiovascular stress responses. Simplified direct connections are illustrated without indirect multisynaptic circuits. The CeA provides abundant innervation of stress-regulatory centers, particularly in the brainstem, representing a major source of descending limbic outflow. These projections are predominantly GABAergic but also co-release peptides to target a diverse array of postsynaptic cell groups. Generally, the CeA targets the GABAergic BST and mPOA, the neurochemically diverse LHA, and key brainstem centers (PB, NTS, VLM, LC, Raphe). The precise functional outcomes of CeA inputs to these distinct regions remains to be determined. See text for references. CeA: central amygdala, BST: bed nucleus of the stria terminalis, mPOA: medial preoptic area, PB: parabrachial nuclei, NTS: nucleus of the solitary tract, LHA: lateral hypothalamic area, VLM: ventrolateral medulla, LC: locus coeruleus, DMV: dorsal motor nucleus of the vagus, NA: nucleus ambiguus, IML intermediolateral cell column, Glu: glutamate, 5-HT: serotonin, NE: norepinephrine, ACh: acetylcholine.
Based on electrical/chemical stimulation or electrolytic lesion studies, the CeA appears to play an important role in the regulation of blood pressure responses to stress [141]. For instance, borderline hypertensive rats with electrolytic lesions of the CeA have attenuated pressor responses to intermittent foot shock [145]. Furthermore, LeDoux et al. have suggested that the CeA is essential for mediating the autonomic correlates of conditioned fear [146–149]. According to this hypothesis, conditioned cardiovascular responses could be disrupted by destroying areas to which the CeA projects. The authors found that electrolytic or chemical lesions of the LHA interfered with conditioned MAP responses [149]. Although no direct manipulation of amygdala was carried out, the data suggest that a CeA-LHA pathway could be a component of the circuitry mediating fear-related blood pressure reactivity. In contrast, local injections of cobalt chloride into the MeA increase HR responses to acute restraint without altering MAP [150]. A subsequent analysis of noradrenergic signaling in the MeA found that α1-adrenoceptors and α2-adrenoceptors mediate facilitation and inhibition of HR responses to restraint, respectively [151], while β1-adrenoceptors facilitate restraint-evoked tachycardia and β2-adrenoceptors have an inhibitory effect [152]. These studies point to the MeA noradrenergic system as a multifaceted regulator of HR, but not MAP, responses to stress. However, histaminergic receptor antagonism in the MeA impairs the pressor response to acute restraint without altering HR [153]. The BLA has also been implicated in cardiovascular regulation as GABA receptor antagonism in the region increases HR and MAP in the absence of stress, an effect dependent on local NMDA and AMPA signaling [154,155]. Furthermore, the Mas receptor, which binds angiotensin-II metabolite angiotensin-(1–7), acts in the BLA to reduce HR and MAP responses to air jet stress [156]. Although neurochemically diverse mechanisms throughout the amygdala permit bidirectional control of HR and MAP; collectively, the many subnuclei and distinct signaling networks of the amygdala appear to be a critical hub for linking prefrontal, basal forebrain, and brainstem circuits to drive enhanced cardiovascular reactivity. There have been great strides related to predicting cardiovascular responses and outcomes as a function of amygdala activity in humans. Moving forward, basic studies are needed to clarify the complex diversity of intra-amygdalar mechanisms mediating cardiovascular stress reactivity. Furthermore, the net effects of these local circuits on the activity of specific efferent projections to other corticolimbic and subcortical regions may mediate essential aspects of cardiovascular reactivity.
5. Hippocampus
Despite the fact that the hippocampus is implicated in human cardiovascular reflexes [31,32] and that ventral aspects of the structure provide multi-synaptic inputs to sympathetic neurons [123], the hippocampus is not widely considered a component of the central autonomic network. However, the hippocampus is one of the primary regulators of endocrine and behavioral responses to stress [157,158]. Thus, it stands to reason that the structure may play a role in regulating cardiovascular stress responsiveness. In humans, the tachycardic response to exercise is associated with hippocampal and mPFC deactivation [159]. Furthermore, electrical or chemical stimulation of the hippocampus decreases HR and MAP in rodents [160], suggesting that hippocampal activity and HR are inversely related. Importantly, mPFC lesions prevent the cardiovascular consequences of stimulation in the ventral, but not dorsal, hippocampus [160]. Taken together, these findings imply that a ventral hippocampal-mPFC circuit regulates inhibition of HR and MAP, whereas the inhibitory effects of dorsal hippocampal stimulation are likely mediated through a different pathway. The primary stress-regulatory outputs of the hippocampus arise from the ventral subiculum and a portion of these efferents converges on neurons in the anterior BST that are also targeted by the PL [161] (Fig. 4). The ventral hippocampus also innervates the LHA and mPOA, with some projections reaching as far as the medulla [88,162]. The activity of the hippocampus is extensively coupled with emotional and mnemonic processes [163], yet the role of the hippocampus in cardiovascular responses to stress has received little attention. Although, a recent study found that NMDA receptor activation in the dorsal hippocampus facilitated restraint stress-evoked cardiovascular responses [164], contrasting the inhibitory effects of dorsal hippocampal stimulation in the absence of stress [160]. More extensive study of the hippocampal contribution to cardiovascular stress reactivity could elucidate these diverging effects on cardiovascular parameters under basal and stress conditions. Further, examining the effects of specific ventral subicular outputs on HR and MAP responses to stress may provide substantial illumination of the corticolimbic mechanisms of cardiovascular reactivity.
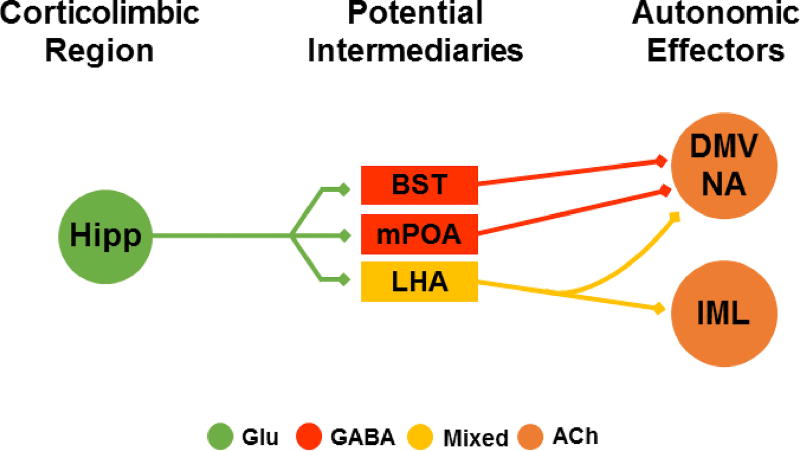
Fig. 4.
Hypothesized anatomical circuitry by which the ventral hippocampus (subiculum and CA1) mediates autonomic responses. Simplified direct connections are illustrated without indirect multisynaptic circuits. The glutamatergic outflow of the ventral hippocampus targets GABAergic cells in the BST and mPOA and diffusely innervates the neurochemically mixed LHA. To date, the functional effects of hippocampal outputs on cardiovascular stress responses have not been investigated. See text for references. Hipp: hippocampus, BST: bed nucleus of the stria terminalis, mPOA: medial preoptic area, LHA: lateral hypothalamic area, DMV: dorsal motor nucleus of the vagus, NA: nucleus ambiguus, IML intermediolateral cell column, Glu: glutamate, ACh: acetylcholine.
6. Summary and future directions
The relationship between psychosocial factors, autonomic imbalance, and cardiovascular disease is supported by numerous human and rodent studies that correlate chronic stress and/or psychiatric illness with reduced HRV, an indicator of sympathovagal imbalance and prominent risk factor for cardiovascular disease [165–168]. Furthermore, the multiple subregions of the mPFC, amygdala, and hippocampus interact to guide cognition and emotion, providing the basis for bidirectional interactions between brain and behavior. However, the consequences for cardiovascular physiology remain an understudied area. Numerous circuit mapping studies indicate that corticolimbic structures target specific basal forebrain and brainstem nuclei that provide direct input to preganglionic sympathetic and parasympathetic neurons. Although a true network-level understanding of this circuitry remains to be determined, even less is known about the functional effects of these pathways on cardiovascular parameters. Our understanding of how the mPFC, amygdala, and hippocampus regulate HR and MAP reactivity to acute stress is based largely on studies employing electrical stimulation, physical/chemical lesions, or nonspecific synaptic blockade. More recent pharmacological investigations of specific neurochemical messengers have revealed a greater complexity to the role of these nuclei in cardiovascular reactivity than anticipated. In fact, there is evidence for opposing effects driven by neighboring cell groups within distinct nuclei, as well as substantial divergence among receptor subtypes within a given region [145,150–153,156]. Based on these factors, few firm conclusions can be drawn about this regulatory network. Some regions of the mPFC seem to supply an inhibitory influence over cardiovascular reactivity, except in the case of aversive associations. Moreover, the CeA promotes blood pressure responses to learned fear, but likely summates inputs from other amygdalar nuclei. Functional connectivity between the amygdala and mPFC has only recently been identified as a predictor of cardiovascular reactivity in human imaging studies [138]. Thus, there are significant unanswered questions regarding our understanding of top-down cardiovascular regulation, as well as the dynamic nature of cardiovascular stress adaptation and pathology.
One question relates to the nature of specific cellular activity and its connection to larger-scale networks. More specifically, what is the role of the microcircuitry generated by interneurons and local collaterals that regulates regional neuronal activity? Further, what is the influence of corticolimbic output neurons and do those effects differ based on postsynaptic targets? These efforts could be greatly advanced by the capabilities of opto- and chemogenetics to manipulate neuronal activity and clarify specific circuits, including interactions between brain areas. There are also tremendous opportunities afforded by conditional genetic manipulations, including Cre-Lox and CRISPR/Cas9, to study specific molecular, cellular, and circuit effects on cardiovascular regulation. This highly-specific control of defined corticolimbic pathways in stress-exposed animals would yield a wealth of data on the mechanisms of cardiovascular stress reactivity. Another area of interest would be the extension of the limbic network for stress appraisal and cardiovascular regulation. Based on human imaging and rodent tract-tracing studies, the insular cortex appears to be an important component of the central autonomic network. Insular cortex has been hypothesized to link with other frontal cortices to form a concerted network receiving viscerosensory information and informing cardiovascular control areas of both physiological and emotional states [169]. This circuitry may then simultaneously encode the magnitude of cardiovascular stress responses and modulate ongoing physiological activity [170]. Accordingly, the area warrants a comprehensive investigation in terms of the neurobiology of stress integration. Stimulation studies suggest that insular cortex has an anterior-posterior gradient that differentially regulates HR and MAP [171], yet the consequences of this gradient for stress reactivity are unknown. Moreover, the functional interactions between the insular cortex, corticolimbic regions, and stress-effector nuclei are not entirely clear.
The studies of corticolimbic stress reactivity outlined in the current review exclusively employ a single acute stressor, as there has been essentially no examination of corticolimbic control of cardiovascular reactivity in the context of chronic stress. In other realms of stress neurobiology the circuitry of acute stress responsiveness frequently differs from the circuitry mediating chronic stress sensitization and/or habituation [125,172–177]. Given the consequences of chronic stress for the cardiovascular system, it is surprising that we have very little knowledge about how the limbic brain encodes the adverse experiences that foster disproportional sympathetic activation and vagal withdrawal. The neurobiological mechanisms mediating interactions between prolonged or repeated stressors and subsequent cardiovascular stress reactivity are critical, yet largely unexplored. Furthermore, there is little evidence to suggest that acute stress hyper-reactivity mediates the cardiovascular burden of chronic stress. In fact, a meta-analysis found that general life stress did not affect HR/BP reactivity and some psychological factors were even associated with reduced cardiovascular reactivity [178]. Specifically, only hostility, aggression, and Type-A behavior associated with increased cardiovascular reactivity, while anxiety, neuroticism, and negative affect were associated with decreased cardiovascular reactivity [178]. Importantly, general life stress, anxiety, neuroticism, and negative affect were all associated with impaired cardiovascular recovery [178]. The measurement of cardiovascular recovery is a largely underreported metric that has significant implications for cumulative exposure to stress mediators. Prolonged recovery following stressful events may relate to cognitive processes such as worry and rumination that negatively impact cardiovascular health [179]. In fact, cardiovascular stress recovery was recently proposed as potential biomarker for resilience to the health effects of psychological stress [180]. Further, studies investigating recovery have the potential to identify mechanisms that differ from those mediating reactivity [23,178,181]. Effect size is an additional consideration for understanding the relative contributions of acute stress responsiveness and long-term stress burden to cardiovascular risk. Effect sizes are frequently reported via different statistical measures across various studies (e.g. odds ratio, correlation, hazard ratio, etc.) making direct comparisons of the risk generated by acute responses vs. chronic life stress difficult. Future studies directly comparing these risk factors would be valuable for guiding the paradigms used in basic research.
Another common feature of the literature outlined in the current review is the exclusive study of males in pre-clinical investigations. Given that the comorbidity of depression and cardiovascular disease is twice the rate in women as men [182,183], sex differences in affective-cardiovascular co-morbidities are of particular importance. The disproportionate female burden of co-occurring depression and cardiovascular disease has been proposed to result from alterations in fetal programming, potentially related to prenatal stress and excess corticosteroid exposure [184,185]. However, ovarian hormones likely play a role in this association as well. In fact, premenopausal women are at lower risk for hypertension, yet the incidence in women surpasses that of men after menopause [186]. It is not entirely clear how fetal programming and/or ovarian factors may alter corticolimbic nuclei but the mPFC, hippocampus, and amygdala express receptors for corticosteroids and estrogens [187–189]. Interestingly, estrogen signaling through estrogen receptor-β in the PVH and rostral VLM protects against salt-induced hypertension in female rats [190], suggesting that further investigations of ovarian hormone signaling in the brain could be important for understanding cardiovascular susceptibility. In addition, the impact of declining levels of ovarian hormones on limbic regulation of stress reactivity could be a critical component of co-occurring depressive and cardiovascular pathologies.
7. Conclusion
Investigating the neurobiological mechanisms linking stress appraisal and reactivity with vulnerability to cardiovascular disease would support ongoing efforts to develop a comprehensive understanding of brain structure-function relationships. Additionally, these studies would likely implicate novel targets to improve health outcomes. There is an ongoing explosion in the technological capabilities of neuroscience that provides new and exciting opportunities to understand the connectivity, chemistry, and functionality of specific neuronal processes. This unprecedented potential could be brought to bear on the critical questions of how chronic stress affects corticolimbic regulation of cardiovascular susceptibility.
Highlights.
Stress and stress-related psychiatric illness greatly increase susceptibility to cardiovascular disease
Corticolimbic nuclei are critical for stress appraisal and reactivity
The organization and integration of corticolimbic circuits for generating cardiovascular stress responses are largely unknown
Acknowledgments
The author is supported by National Institutes of Health Grant K99 HL122454.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012;9:360–70. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 3.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duarte JO, Cruz FC, Leão RM, Planeta CS, Crestani CC. Stress vulnerability during adolescence: comparison of chronic stressors in adolescent and adult rats. Psychosom. Med. 77:186–99. doi: 10.1097/PSY.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 5.Bobrovskaya L, Beard D, Bondarenko E, Beig MI, Jobling P, Walker FR, Day TA, Nalivaiko E. Does exposure to chronic stress influence blood pressure in rats? Auton. Neurosci. 2013;177:217–23. doi: 10.1016/j.autneu.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neurosci. Biobehav. Rev. 1992;16:207–17. doi: 10.1016/s0149-7634(05)80181-2. http://www.ncbi.nlm.nih.gov/pubmed/1630731. [DOI] [PubMed] [Google Scholar]
- 7.Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H1476–98. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 8.Sgoifo A, Carnevali L, de los M, Alfonso AP, Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015;18:343–52. doi: 10.3109/10253890.2015.1045868. [DOI] [PubMed] [Google Scholar]
- 9.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J. Hypertens. 2007;25:2117–24. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 10.Hausberg M, Hillebrand U, Kisters K. Addressing sympathetic overactivity in major depressive disorder. J. Hypertens. 2007;25:2004–5. doi: 10.1097/HJH.0b013e3282ef9819. [DOI] [PubMed] [Google Scholar]
- 11.Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–8. doi: 10.1161/hc4201.097834. http://www.ncbi.nlm.nih.gov/pubmed/11673340. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–79. doi: 10.1161/01.cir.78.4.969. http://www.ncbi.nlm.nih.gov/pubmed/3168199. [DOI] [PubMed] [Google Scholar]
- 13.Tapanainen JM, Thomsen PEB, Køber L, Torp-Pedersen C, Mäkikallio TH, Still A-M, Lindgren KS, Huikuri HV. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am. J. Cardiol. 2002;90:347–52. doi: 10.1016/s0002-9149(02)02488-8. http://www.ncbi.nlm.nih.gov/pubmed/12161220. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 15.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19469025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol. Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 18.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain. Behav. Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Dhar AK, Barton DA. Depression and the Link with Cardiovascular Disease. Front. Psychiatry. 2016;7:33. doi: 10.3389/fpsyt.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brydon L, Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. J. Hypertens. 2005;23:1001–7. doi: 10.1097/01.hjh.0000166841.57474.d0. http://www.ncbi.nlm.nih.gov/pubmed/15834286. [DOI] [PubMed] [Google Scholar]
- 21.Buckley T, McKinley S, Tofler G, Bartrop R. Cardiovascular risk in early bereavement: a literature review and proposed mechanisms. Int. J. Nurs. Stud. 2010;47:229–38. doi: 10.1016/j.ijnurstu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N. Engl. J. Med. 1996;334:413–9. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 23.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 24.Vogelzangs N, Beekman ATF, Milaneschi Y, Bandinelli S, Ferrucci L, Penninx BWJH. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J. Clin. Endocrinol. Metab. 2010;95:4959–64. doi: 10.1210/jc.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosom. Med. 65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. http://www.ncbi.nlm.nih.gov/pubmed/12554814. [DOI] [PubMed] [Google Scholar]
- 26.Lovallo WR. Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. Int. J. Psychophysiol. 58:119–32. doi: 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog. Neurobiol. 1998;54:149–68. doi: 10.1016/s0301-0082(97)00056-7. http://www.ncbi.nlm.nih.gov/pubmed/9481796. [DOI] [PubMed] [Google Scholar]
- 28.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 29.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. http://www.ncbi.nlm.nih.gov/pubmed/8412366. [DOI] [PubMed] [Google Scholar]
- 30.Beissner F, Meissner K, Bär K-J, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 2013;33:10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoemaker JK, Goswami R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Front. Physiol. 2015;6:240. doi: 10.3389/fphys.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoemaker JK, Norton KN, Baker J, Luchyshyn T. Forebrain organization for autonomic cardiovascular control. Auton. Neurosci. 2015;188:5–9. doi: 10.1016/j.autneu.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22302180. [DOI] [PMC free article] [PubMed]
- 34.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front. Neuroendocrinol. 2014;35:180–96. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10415674. [DOI] [PubMed] [Google Scholar]
- 36.Drevets WC. Neuroimaging studies of mood disorders. Biol. Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. http://www.ncbi.nlm.nih.gov/pubmed/11063977. [DOI] [PubMed] [Google Scholar]
- 37.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Desmedt A, Marighetto A, Richter-Levin G, Calandreau L. Adaptive emotional memory: the key hippocampal-amygdalar interaction. Stress. 2015;18:297–308. doi: 10.3109/10253890.2015.1067676. http://www.ncbi.nlm.nih.gov/pubmed/26260664. [DOI] [PubMed] [Google Scholar]
- 39.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15891777. [DOI] [PubMed] [Google Scholar]
- 40.Ishai A, Takx R, Nahrendorf M, Pitman R, Lisa SM, Tawakol A. Greater activity of the brain’s emotional stress center associates with arterial inflammation and predicts subsequent cvd events. J. Am. Coll. Cardiol. 2016;67:2103. doi: 10.1016/S0735-1097(16)32104-0. [DOI] [Google Scholar]
- 41.Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, Thayer JF, Kirschbaum C, Tranel D. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maclean PD. Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain) Electroencephalogr. Clin. Neurophysiol. 1952;4:407–18. doi: 10.1016/0013-4694(52)90073-4. http://www.ncbi.nlm.nih.gov/pubmed/12998590. [DOI] [PubMed] [Google Scholar]
- 43.Anand BK, Dua S. Circulatory and respiratory changes induced by electrical stimulation of limbic system (visceral brain) J. Neurophysiol. 1956;19:393–400. doi: 10.1152/jn.1956.19.5.393. http://www.ncbi.nlm.nih.gov/pubmed/13367870. [DOI] [PubMed] [Google Scholar]
- 44.Ally A. Ventrolateral medullary control of cardiovascular activity during muscle contraction. Neurosci. Biobehav. Rev. 1998;23:65–86. doi: 10.1016/s0149-7634(97)00069-9. http://www.ncbi.nlm.nih.gov/pubmed/9861613. [DOI] [PubMed] [Google Scholar]
- 45.Scislo TJ, O’Leary DS. Vasopressin V1 receptors contribute to hemodynamic and sympathoinhibitory responses evoked by stimulation of adenosine A2a receptors in NTS. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1889–98. doi: 10.1152/ajpheart.01030.2005. [DOI] [PubMed] [Google Scholar]
- 46.Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J. Physiol. 2002;538:941–6. doi: 10.1113/jphysiol.2001.013302. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2290111&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lisa M, Marmo E, Wible JH, Jr, DiMicco JA. Injection of muscimol into posterior hypothalamus blocks stress-induced tachycardia. Am J Physiol. 1989;257:R246–51. doi: 10.1152/ajpregu.1989.257.1.R246. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2750964. [DOI] [PubMed] [Google Scholar]
- 48.Bechtold AG, Scheuer DA. Glucocorticoids act in the dorsal hindbrain to modulate baroreflex control of heart rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1003–11. doi: 10.1152/ajpregu.00345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J. Neurosci. 2013;33:4825–33. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamec RE. Stress effects on limbic function and behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1173–5. doi: 10.1016/j.pnpbp.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 51.McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, Ghosal S, Mahbod P, Packard BA, Myers B, Baccei ML, Herman JP. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. doi:20026655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res. 1985;359:300–5. doi: 10.1016/0006-8993(85)91440-4. http://www.ncbi.nlm.nih.gov/pubmed/4075151. [DOI] [PubMed] [Google Scholar]
- 56.Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J. Neurosci. 1985;5:1228–32. doi: 10.1523/JNEUROSCI.05-05-01228.1985. http://www.ncbi.nlm.nih.gov/pubmed/3998819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziegler DR, Herman JP. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr. Comp. Biol. 2002;42:541–51. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]
- 58.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11683906. [DOI] [PubMed] [Google Scholar]
- 60.Dayas CV, Day TA. Opposing roles for medial and central amygdala in the initiation of noradrenergic cell responses to a psychological stressor. Eur. J. Neurosci. 2002;15:1712–8. doi: 10.1046/j.1460-9568.2001.02011.x. http://www.ncbi.nlm.nih.gov/pubmed/12059979. [DOI] [PubMed] [Google Scholar]
- 61.Spencer SJ, Ebner K, Day TA. Differential involvement of rat medial prefrontal cortex dopamine receptors in modulation of hypothalamic-pituitary-adrenal axis responses to different stressors. Eur. J. Neurosci. 2004;20:1008–16. doi: 10.1111/j.1460-9568.2004.03569.x. [DOI] [PubMed] [Google Scholar]
- 62.Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104:266–271. doi: 10.1016/j.physbeh.2011.03.021. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21443894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9881860. [DOI] [PubMed] [Google Scholar]
- 64.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA. Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron. 2016;89:857–66. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocr. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14596810. [DOI] [PubMed] [Google Scholar]
- 66.Dampney RAL. Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R429–43. doi: 10.1152/ajpregu.00051.2015. [DOI] [PubMed] [Google Scholar]
- 67.Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9922–7. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. Fourth. McGraw-Hill Companies, Incorporated; 2000. https://books.google.com/books?id=yMtpAAAAMAAJ&pgis=1. [Google Scholar]
- 69.Bear MF, Connors BW, Paradiso MA. Neuroscience. Lippincott Williams & Wilkins; 2007. https://books.google.com/books?id=75NgwLzueikC&pgis=1. [Google Scholar]
- 70.Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14648685. [DOI] [PubMed] [Google Scholar]
- 71.Dong H-W, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J. Comp. Neurol. 2003;463:434–72. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- 72.Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res. Rev. 2010;64:14–103. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiba T, Murata Y. Afferent and efferent connections of the medial preoptic area in the rat: a WGA-HRP study. Brain Res. Bull. 1985;14:261–72. doi: 10.1016/0361-9230(85)90091-7. http://www.ncbi.nlm.nih.gov/pubmed/3995367. [DOI] [PubMed] [Google Scholar]
- 74.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980;194:555–70. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 75.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong H-W. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017:208–17. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ter Horst GJ, Toes GJ, Van Willigen JD. Locus coeruleus projections to the dorsal motor vagus nucleus in the rat. Neuroscience. 1991;45:153–60. doi: 10.1016/0306-4522(91)90111-z. http://www.ncbi.nlm.nih.gov/pubmed/1684412. [DOI] [PubMed] [Google Scholar]
- 78.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–7. doi: 10.1126/science.7292008. http://www.ncbi.nlm.nih.gov/pubmed/7292008. [DOI] [PubMed] [Google Scholar]
- 79.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. http://www.ncbi.nlm.nih.gov/pubmed/6756545. [DOI] [PubMed] [Google Scholar]
- 80.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990;293:540–80. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 81.Loewy AD. Descending pathways to sympathetic and parasympathetic preganglionic neurons. J. Auton. Nerv. Syst. 1981;3:265–75. doi: 10.1016/0165-1838(81)90068-0. http://www.ncbi.nlm.nih.gov/pubmed/7276435. [DOI] [PubMed] [Google Scholar]
- 82.Schwanzel-Fukuda M, Morrell JI, Pfaff DW. Localization of forebrain neurons which project directly to the medulla and spinal cord of the rat by retrograde tracing with wheat germ agglutinin. J. Comp. Neurol. 1984;226:1–20. doi: 10.1002/cne.902260102. [DOI] [PubMed] [Google Scholar]
- 83.Amendt K, Czachurski J, Dembowsky K, Seller H. Bulbospinal projections to the intermediolateral cell column: a neuroanatomical study. J. Auton. Nerv. Syst. 1979;1:103–7. doi: 10.1016/0165-1838(79)90009-2. http://www.ncbi.nlm.nih.gov/pubmed/575994. [DOI] [PubMed] [Google Scholar]
- 84.Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- 85.Guyenet PG. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 86.Fontes MA, Xavier CH, de Menezes RC, Dimicco JA. The dorsomedial hypothalamus and the central pathways involved in the cardiovascular response to emotional stress. Neuroscience. 2011;184:64–74. doi: 10.1016/j.neuroscience.2011.03.018. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21435377. [DOI] [PubMed] [Google Scholar]
- 87.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–12. doi: 10.1016/0006-8993(76)90738-1. http://www.ncbi.nlm.nih.gov/pubmed/62600. [DOI] [PubMed] [Google Scholar]
- 88.Myers B, Dolgas CM, Kasckow J, Cullinan WE, Herman JP, Dolgas CM, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct. 2014;219:1287–303. doi: 10.1007/s00429-013-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ziegler DR, Edwards MR, Ulrich-Lai YM, Herman JP, Cullinan WE. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 2012;520:2369–2394. doi: 10.1002/cne.23043. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22247025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front. Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R222–35. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dampney RAL, Horiuchi J, Killinger S, Sheriff MJ, Tan PSP, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin. Exp. Pharmacol. Physiol. 32:419–25. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 94.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002;71:469–80. doi: 10.1016/s0091-3057(01)00689-x. http://www.ncbi.nlm.nih.gov/pubmed/11830181. [DOI] [PubMed] [Google Scholar]
- 95.Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr. Neuropharmacol. 2013;11:141–59. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abshire VM, Hankins KD, Roehr KE, DiMicco JA. Injection of L-allylglycine into the posterior hypothalamus in rats causes decreases in local GABA which correlate with increases in heart rate. Neuropharmacology. 1988;27:1171–1177. doi: 10.1016/0028-3908(88)90013-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3205383. [DOI] [PubMed] [Google Scholar]
- 97.Lisa M, Filippelli A, Marmo E, Wible JH, Jr, DiMicco JA. Microinjection of muscimol into posterior hypothalamus blocks cardiovascular response to experimental stress in rats. Pharmacol Res. 1989;21(Suppl 1):9–10. doi: 10.1016/s1043-6618(89)80027-1. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2633198. [DOI] [PubMed] [Google Scholar]
- 98.Deolindo MV, Reis DG, Crestani CC, Tavares RF, Resstel LBM, Corrêa FMA. NMDA receptors in the lateral hypothalamus have an inhibitory influence on the tachycardiac response to acute restraint stress in rats. Eur. J. Neurosci. 2013;38:2374–81. doi: 10.1111/ejn.12246. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz JA, Reilly NS, Knuepfer MM. Angiotensin and NMDA receptors in the median preoptic nucleus mediate hemodynamic response patterns to stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R155–65. doi: 10.1152/ajpregu.00606.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr. Hypertens. Rep. 2015;17:39. doi: 10.1007/s11906-015-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pyner S. The paraventricular nucleus and heart failure. Exp. Physiol. 2014;99:332–9. doi: 10.1113/expphysiol.2013.072678. [DOI] [PubMed] [Google Scholar]
- 102.Busnardo C, Tavares RF, Resstel LBM, Elias LLK, Correa FMA. Paraventricular nucleus modulates autonomic and neuroendocrine responses to acute restraint stress in rats. Auton. Neurosci. 2010;158:51–7. doi: 10.1016/j.autneu.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 103.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–7. doi: 10.1016/j.physbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daubert DL, McCowan M, Erdos B, Scheuer DA. Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats. J. Physiol. 2012;590:4881–95. doi: 10.1113/jphysiol.2012.232314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bundzikova-Osacka J, Ghosal S, Packard BA, Ulrich-Lai YM, Herman JP. Role of nucleus of the solitary tract noradrenergic neurons in post-stress cardiovascular and hormonal control in male rats. Stress. 2015;18:221–32. doi: 10.3109/10253890.2015.1013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghosal S, Bundzikova-Osacka J, Dolgas CM, Myers B, Herman JP. Glucocorticoid receptors in the nucleus of the solitary tract (NTS) decrease endocrine and behavioral stress responses. Psychoneuroendocrinology. 2014;45:142–53. doi: 10.1016/j.psyneuen.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J. Neurosci. 2010;30:14907–14. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bechtold AG, Patel G, Hochhaus G, Scheuer DA. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1445–54. doi: 10.1152/ajpregu.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H458–67. doi: 10.1152/ajpheart.00824.2003. [DOI] [PubMed] [Google Scholar]
- 111.Crestani CC, Alves FHF, Tavares RF, Corrêa FMA. Role of the bed nucleus of the stria terminalis in the cardiovascular responses to acute restraint stress in rats. Stress. 2009;12:268–78. doi: 10.1080/10253890802331477. [DOI] [PubMed] [Google Scholar]
- 112.Oliveira LA, Almeida J, Benini R, Crestani CC. CRF1 and CRF2 receptors in the bed nucleus of the stria terminalis modulate the cardiovascular responses to acute restraint stress in rats. Pharmacol. Res. 95–96:53–62. doi: 10.1016/j.phrs.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 113.Gianaros PJ, Sheu LK, Remo AM, Christie IC, Crtichley HD, Wang J. Heightened resting neural activity predicts exaggerated stressor-evoked blood pressure reactivity. Hypertension. 2009;53:819–25. doi: 10.1161/HYPERTENSIONAHA.108.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage. 2009;47:922–36. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J. Neuroendocrinol. 2015;27:446–56. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 1):2189–202. doi: 10.1093/brain/123.11.2189. http://www.ncbi.nlm.nih.gov/pubmed/11050020. [DOI] [PubMed] [Google Scholar]
- 117.Kimmerly DS, O’Leary DD, Menon RS, Gati JS, Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J. Physiol. 2005;569:331–45. doi: 10.1113/jphysiol.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ziegler G, Dahnke R, Yeragani VK, Bär K-J. The relation of ventromedial prefrontal cortex activity and heart rate fluctuations at rest. Eur. J. Neurosci. 2009;30:2205–10. doi: 10.1111/j.1460-9568.2009.07008.x. [DOI] [PubMed] [Google Scholar]
- 119.Verberne AJ. Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. Am. J. Physiol. 1996;270:R713–9. doi: 10.1152/ajpregu.1996.270.4.R713. http://www.ncbi.nlm.nih.gov/pubmed/8967398. [DOI] [PubMed] [Google Scholar]
- 120.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.88. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21788943. [DOI] [PMC free article] [PubMed]
- 121.Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav. Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. http://www.ncbi.nlm.nih.gov/pubmed/14643455. [DOI] [PubMed] [Google Scholar]
- 122.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14579424. [DOI] [PubMed] [Google Scholar]
- 123.Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001;903:117–27. doi: 10.1016/s0006-8993(01)02453-2. http://www.ncbi.nlm.nih.gov/pubmed/11382395. [DOI] [PubMed] [Google Scholar]
- 124.Myers B, Carvalho-Netto E, Wick-Carlson D, Wu C, Naser S, Solomon MB, Ulrich-Lai YM, Herman JP. GABAergic Signaling within a Limbic-Hypothalamic Circuit Integrates Social and Anxiety-Like Behavior with Stress Reactivity. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016;6:603–21. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19494154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tavares RF, Corrêa FMA, Resstel LBM. Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J. Neurosci. Res. 2009;87:2601–7. doi: 10.1002/jnr.22070. [DOI] [PubMed] [Google Scholar]
- 128.Müller-Ribeiro FC de F, Zaretsky DV, Zaretskaia MV, Santos RAS, DiMicco JA, Fontes MAP. Contribution of infralimbic cortex in the cardiovascular response to acute stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R639–50. doi: 10.1152/ajpregu.00573.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Resstel LBM, Joca SRL, Guimarães FG, Corrêa FMA. Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience. 2006;143:377–85. doi: 10.1016/j.neuroscience.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–93. doi: 10.1016/0006-8993(94)90024-8. http://www.ncbi.nlm.nih.gov/pubmed/8032913. [DOI] [PubMed] [Google Scholar]
- 131.Sah P, Faber ESL, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 132.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10760490. [DOI] [PubMed] [Google Scholar]
- 133.Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res. 2010;214:465–469. doi: 10.1016/j.bbr.2010.05.049. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20573588. [DOI] [PubMed] [Google Scholar]
- 134.Davis M. Neurobiology of fear responses: the role of the amygdala. J. Neuropsychiatry Clin. Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. http://www.ncbi.nlm.nih.gov/pubmed/9276841. [DOI] [PubMed] [Google Scholar]
- 135.Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 136.Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann. N. Y. Acad. Sci. 2003;985:308–25. doi: 10.1111/j.1749-6632.2003.tb07090.x. http://www.ncbi.nlm.nih.gov/pubmed/12724167. [DOI] [PubMed] [Google Scholar]
- 137.Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci. 2008;28:990–9. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol. Psychiatry. 2009;65:943–50. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin. Exp. Pharmacol. Physiol. 32:450–6. doi: 10.1111/j.1440-1681.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- 142.Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15:173–185. doi: 10.1016/s0891-0618(98)00045-3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9797074. [DOI] [PubMed] [Google Scholar]
- 143.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J. Chem. Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. http://www.ncbi.nlm.nih.gov/pubmed/9271192. [DOI] [PubMed] [Google Scholar]
- 144.Herman JP, Myers B. Neuroendocrinology of Stress. John Wiley & Sons, Ltd; Chichester, UK: 2015. Methods and Approaches to Understand Stress Processing Circuitry. [DOI] [Google Scholar]
- 145.Sanders BJ, Wirtz-Nole C, DeFord SM, Erling BF. Central amygdaloid lesions attenuate cardiovascular responses to acute stress in rats with borderline hypertension. Physiol. Behav. 1994;56:709–13. doi: 10.1016/0031-9384(94)90231-3. http://www.ncbi.nlm.nih.gov/pubmed/7800737. [DOI] [PubMed] [Google Scholar]
- 146.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 147.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 2006;26:12387–96. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn. Mem. 2001;8:156–63. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2854842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fortaleza EAT, Tavares RF, Corrêa FMA. The medial amygdaloid nucleus modulates cardiovascular responses to acute restraint in rats. Neuroscience. 2009;159:717–26. doi: 10.1016/j.neuroscience.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Fortaleza EAT, Scopinho AA, Corrêa FM de Aguiar. α1 and α2-adrenoceptors in the medial amygdaloid nucleus modulate differently the cardiovascular responses to restraint stress in rats. Pharmacol. Res. 2012;66:154–62. doi: 10.1016/j.phrs.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 152.Fortaleza EAT, Scopinho AA, Corrêa FMA. B-adrenoceptors in the medial amygdaloid nucleus modulate the tachycardiac response to restraint stress in rats. Neuroscience. 2012;227:170–9. doi: 10.1016/j.neuroscience.2012.09.048. [DOI] [PubMed] [Google Scholar]
- 153.de Almeida DO, Ferreira HS, Pereira LB, Fregoneze JB. Hypertensive response to stress: the role of histaminergic H1 and H2 receptors in the medial amygdala. Physiol. Behav. 2015;144:95–102. doi: 10.1016/j.physbeh.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 154.Soltis RP, Cook JC, Gregg AE, Sanders BJ. Interaction of GABA and excitatory amino acids in the basolateral amygdala: role in cardiovascular regulation. J. Neurosci. 1997;17:9367–74. doi: 10.1523/JNEUROSCI.17-23-09367.1997. http://www.ncbi.nlm.nih.gov/pubmed/9364082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sajdyk TJ, Shekhar A. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by gamma-aminobutyric acidA receptor blockade in the basolateral amygdala of rats. J. Pharmacol. Exp. Ther. 1997;283:969–77. http://www.ncbi.nlm.nih.gov/pubmed/9411030. [PubMed] [Google Scholar]
- 156.Oscar CG, Müller-Ribeiro FC de F, Martins Lima A, Campagnole-Santos MJ, Santos RAS, Xavier CH, Fontes MAP. Angiotensin-(1–7) in the basolateral amygdala attenuates the cardiovascular response evoked by acute emotional stress. Brain Res. 2015;1594:183–9. doi: 10.1016/j.brainres.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 157.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2070776. [DOI] [PubMed] [Google Scholar]
- 158.Fitzsimons CP, van Hooijdonk LWA, Schouten M, Zalachoras I, Brinks V, Zheng T, Schouten TG, Saaltink DJ, Dijkmans T, Steindler DA, Verhaagen J, Verbeek FJ, Lucassen PJ, de Kloet ER, Meijer OC, Karst H, Joels M, Oitzl MS, Vreugdenhil E. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol. Psychiatry. 2013;18:993–1005. doi: 10.1038/mp.2012.123. [DOI] [PubMed] [Google Scholar]
- 159.Norton KN, Luchyshyn TA, Kevin Shoemaker J. Evidence for a medial prefrontal cortex-hippocampal axis associated with heart rate control in conscious humans. Brain Res. 2013;1538:104–15. doi: 10.1016/j.brainres.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 160.Ruit KG, Neafsey EJ. Cardiovascular and respiratory responses to electrical and chemical stimulation of the hippocampus in anesthetized and awake rats. Brain Res. 1988;457:310–21. doi: 10.1016/0006-8993(88)90701-9. http://www.ncbi.nlm.nih.gov/pubmed/2905918. [DOI] [PubMed] [Google Scholar]
- 161.Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21715634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Köhler C. Subicular projections to the hypothalamus and brainstem: some novel aspects revealed in the rat by the anterograde Phaseolus vulgaris leukoagglutinin (PHA-L) tracing method. Prog. Brain Res. 1990;83:59–69. doi: 10.1016/s0079-6123(08)61241-8. http://www.ncbi.nlm.nih.gov/pubmed/2392571. [DOI] [PubMed] [Google Scholar]
- 163.Ai H, Opmeer EM, Veltman DJ, van der Wee NJA, van Buchem MA, Aleman A, van Tol M-J. Brain Activation During Emotional Memory Processing Associated with Subsequent Course of Depression. Neuropsychopharmacology. 2015;40:2454–63. doi: 10.1038/npp.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moraes-Neto TB, Scopinho AA, Biojone C, Corrêa FMA, Resstel LBM. Involvement of dorsal hippocampus glutamatergic and nitrergic neurotransmission in autonomic responses evoked by acute restraint stress in rats. Neuroscience. 2014;258:364–73. doi: 10.1016/j.neuroscience.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 165.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol. Behav. 2003;78:703–10. doi: 10.1016/s0031-9384(03)00050-7. http://www.ncbi.nlm.nih.gov/pubmed/12782226. [DOI] [PubMed] [Google Scholar]
- 166.Jandackova VK, Britton A, Malik M, Steptoe A. Heart rate variability and depressive symptoms: a cross-lagged analysis over a 10-year period in the Whitehall II study. Psychol. Med. 2016;46:1–11. doi: 10.1017/S003329171600060X. [DOI] [PubMed] [Google Scholar]
- 167.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009;33:81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 168.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36:747–56. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 169.Oppenheimer S, Cechetto D. The Insular Cortex and the Regulation of Cardiac Function. Compr. Physiol. 2016;6:1081–133. doi: 10.1002/cphy.c140076. [DOI] [PubMed] [Google Scholar]
- 170.Gianaros PJ, Wager TD. Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr. Dir. Psychol. Sci. 2015;24:313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J. Comp. Neurol. 1991;303:355–74. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- 172.Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur. J. Neurosci. 2012;36:2547–55. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, Herman JP. Role of Prefrontal Cortex Glucocorticoid Receptors in Stress and Emotion. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.03.024. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23683655. [DOI] [PMC free article] [PubMed]
- 174.Martí O, Armario A. Influence of Regularity of Exposure to Chronic Stress on the Pattern of Habituation of Pituitary-Adrenal Hormones, Prolactin and Glucose. Stress. 1997;1:179–189. doi: 10.3109/10253899709001107. http://www.ncbi.nlm.nih.gov/pubmed/9787243. [DOI] [PubMed] [Google Scholar]
- 175.Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23964212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–39. doi: 10.1016/s0306-4522(97)00577-0. http://www.ncbi.nlm.nih.gov/pubmed/9578393. [DOI] [PubMed] [Google Scholar]
- 177.Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J. Neurosci. 2000;20:5564–73. doi: 10.1523/JNEUROSCI.20-14-05564.2000. http://www.ncbi.nlm.nih.gov/pubmed/10884340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol. Bull. 2008;134:829–85. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- 179.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J. Psychosom. Res. 2006;60:113–24. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 180.Walker FR, Pfingst K, Carnevali L, Sgoifo A, Nalivaiko E. In the search for integrative biomarker of resilience to psychological stress. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 181.Linden W, Earle TL, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: conceptual siblings separated at birth? J. Psychosom. Res. 1997;42:117–35. doi: 10.1016/s0022-3999(96)00240-1. http://www.ncbi.nlm.nih.gov/pubmed/9076640. [DOI] [PubMed] [Google Scholar]
- 182.Goldstein JM, Cherkerzian S, Buka SL, Fitzmaurice G, Hornig M, Gillman M, O’Toole S, Sloan RP. Sex-specific impact of maternal-fetal risk factors on depression and cardiovascular risk 40 years later. J. Dev. Orig. Health Dis. 2011;2:353–64. doi: 10.1017/S2040174411000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Möller-Leimkühler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin. Neurosci. 2007;9:71–83. doi: 10.31887/DCNS.2007.9.1/ammoeller. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3181845&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goldstein JM, Handa RJ, Tobet SA. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front. Neuroendocrinol. 2014;35:140–58. doi: 10.1016/j.yfrne.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tobet SA, Handa RJ, Goldstein JM. Sex-dependent pathophysiology as predictors of comorbidity of major depressive disorder and cardiovascular disease. Pflügers Arch. Eur. J. Physiol. 2013;465:585–94. doi: 10.1007/s00424-013-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hay M. Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin. Sci. (Lond) 2016;130:9–18. doi: 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- 187.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. http://www.ncbi.nlm.nih.gov/pubmed/9388012. [DOI] [PubMed] [Google Scholar]
- 188.Reul JM, de Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986;24:269–272. doi: 10.1016/0022-4731(86)90063-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3702410. [DOI] [PubMed] [Google Scholar]
- 189.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2998738. [DOI] [PubMed] [Google Scholar]
- 190.Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Estrogen receptor-β in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension. 2013;61:1255–62. doi: 10.1161/HYPERTENSIONAHA.111.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]