Abstract
Over the past 30 years, at least 30 new infectious diseases have emerged to threaten the health of millions of people across the globe. The major challenge to combat these infections is that for many of them, there is no specific treatment or cure or vaccine. There is limited scope of preventing or controlling them. The contributory factors include urbanization and destruction of natural habitats, climate change and changing ecosystems, changes in population of reservoir hosts or intermediate insect vectors and microbial genetic mutation, international trade and commerce, change in human demographics and behavior, lack of public health services and infrastructure, and antibiotic resistance. It is clear by now that the problem of emerging infectious disease (EID) is not restricted to any single country, and a strong and sustainable international collaboration will be needed in their prevention and control. India along with other countries in the South-East Asian region will continue to bear the brunt of the burden of EIDs in years to come.
KEY WORDS: Emerging infectious diseases, IDSP, International Health Regulations
What was known?
Emerging infectious diseases caused by numerous micro-organisms have been affecting a region, country and sometimes the entire globe from time to time sporadically or in the form of small outbreaks to global pandemic like swine flu. Many diseases which were once considered to be no longer a threat to the public health have once again begun to re-emerge.
Introduction
The 21st century has witnessed the emergence of many new infectious diseases of serious public health implications from severe acute respiratory syndrome (SARS) to avian influenza (AI) A humans by a large number. This major public health threat has huge socioeconomic impact and more so in the developing countries like India.[1,2]
“An emerging infectious disease (EID) is one that has appeared and affected a population for the first time, or has existed previously but is rapidly increasing, either in terms of the number of new cases within a population, or its spread to new geographical areas” (e.g. SARS).[3] They also include infectious diseases that have affected a given area in the past, declined with passage of time or were controlled, but again reappeared in increasing numbers.[1]
Sometimes, an old disease reappears in a new clinical form that may often be severe or fatal. These are known as re-emerging diseases, and chikungunya in India is a recent example of such diseases.[1]
Literature reveals that around 60% of all human infectious diseases recognized so far, and approximately 75% of the EIDs that have affected humankind over the past three decades were zoonotic in nature.[4] Scientific research conducted by Jones et al. on 335 emerging diseases between 1940 and 2004 indicated that certain areas of the world are more likely to experience the emergence of new infectious diseases.[5] Among these global “hotspots” for EIDs are countries related to the Indo-Gangetic Plain and the Mekong River Basin. Nipah virus (NiV), Crimean-Congo hemorrhagic fever (CCHF), and AI A (H5N1) are examples of diseases that have recently emerged and have affected the World Health Organization (WHO) South-East Asia Region.[1,5]
Causative Agents
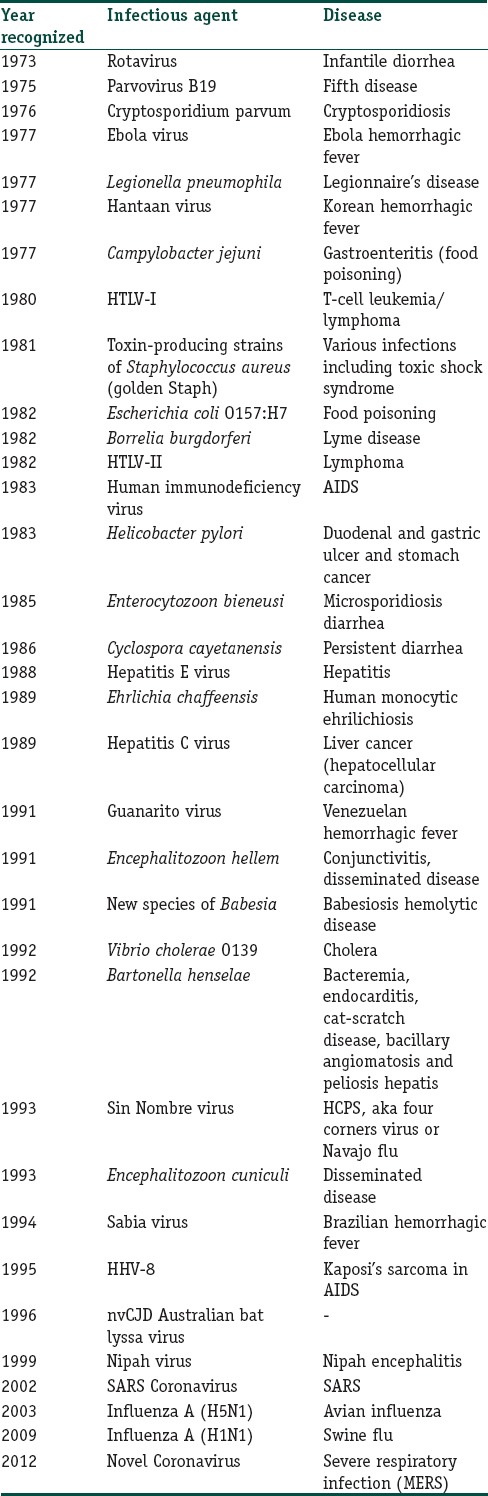
Over the past 30 years, at least 30 new infectious diseases have emerged to threaten the health of millions of people across the globe[3,6,7,8] [Table 1]. The major challenge to combat these infections is that for many of them, there is no specific treatment or cure or vaccine. Moreover, there is limited scope of preventing or controlling them.[6]
Table 1.
New infectious diseases of public health importance since 1973
The WHO recently published a list of top emerging diseases likely to cause major epidemics. The initial list of disease priorities needing urgent research and development (R&D) attention comprised CCHF, Ebola virus disease (EVD) and Marburg, Lassa fever, MERS and SARS coronavirus diseases, Nipah, and Rift Valley fever. It was proposed that the list will be reviewed annually or when new diseases emerge. This priority list forms the backbone of the new WHO Blueprint for R&D preparedness by focusing accelerated R&D on dangerous pathogens which are the most prone to cause epidemics. A further three diseases were also listed as serious, necessitating further action as soon as possible: chikungunya, severe fever with thrombocytopenia syndrome, and Zika.[9]
Contributing Factors Responsible for Emergence and Re-Emergence
There is interplay of a large number of factors behind emergence and reemergence [Table 2].
Table 2.
Factors responsible for emergence and re-emergence

It appears that most of the EIDs are caused by pathogens that are already present in the environment unnoticed. They tend to emerge when provided with favorable conditions and infect a new host. On rare instances, some of them evolve into a new variant and cause a new disease.[10] The contributory factors that enable the infectious agents to evolve into new ecological niches, to reach and adapt to new hosts, and to spread more easily among the new hosts include urbanization and destruction of natural habitats, leading to close contact of humans with animals, climate change and changing ecosystems, changes in population of reservoir hosts or intermediate insect vectors, and microbial genetic mutation.[1] International trade and commerce, change in human demographics and behavior, lack of public health services and infrastructure, and antibiotic resistance have also contributed significantly in the emergence and re-emergence of infectious diseases.[10]
Global Scenario
Infectious diseases are periodically emerging and re-emerging in nearly every corner of earth, many with the potential to cause pandemic. Global examples are just overwhelming [Figure 1].
Figure 1.
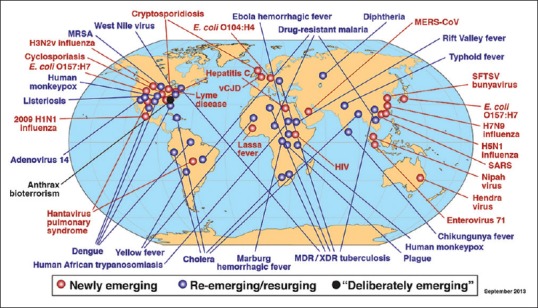
Global examples of emerging, re-emerging and deliberately emerging (linked to bioterrorism) disease (Adapted from Ref 13)
For a large part of these EIDs, Asia remains the epicenter.[12]
“Communicable diseases do not respect the geopolitical boundaries of nation states.”[14] Many recent pandemics provide testimonials to this statement.
A completely new strain of cholera, O139, appeared in southeastern India and since then has spread to other parts of the country and crossed borders to reach China, Thailand, and other areas in South-East Asia.[6] Since 1997, AI (H5N1) having emerged in Hong Kong spread rapidly to Cambodia, Indonesia, Thailand, and Viet Nam.[15]
The new SARS coronavirus after emerging from Guangdong Province of China in November 2002, spread rapidly to 30 countries across Asia, the Americas, and Europe, with a total of 8439 cases and 812 deaths in less than a year.[15] “SARS demonstrates dramatically the global havoc that can be wreaked by a newly EID.”[16] In March 2009, cases of H1N1 influenza were first reported in Mexico, which then spread to the United States and finally to rest of the world including India. By the next 6 months, almost all countries had reported H1N1 virus to the WHO, with more than 18,000 deaths, 67% of that in the USA alone.[17] Appearing for the first time in Sudan and Democratic Republic of Congo in 1976, the EVD, previously called Ebola hemorrhagic fever, then occurred periodically.
“The current outbreak in West Africa, (first cases notified in March 2014), is the largest and most complex Ebola outbreak since the Ebola virus was first discovered in 1976. There have been more cases and deaths in this outbreak than all others combined. It has also spread between countries starting in Guinea then spreading across land borders to Sierra Leone and Liberia, by air (1 traveller) to Nigeria and USA (1 traveller), and by land to Senegal (1 traveller) and Mali (2 travellers).”[18] EVD in West Africa was first reported during early March 2014 in Guinea's 3 southeastern prefectures which share borders with Liberia and Sierra Leone.[19,20,21,22] It then rapidly spread through most parts of Guinea and then to Liberia and Sierra Leone, causing the largest EVD epidemic ever.[20,21,23] Till November 1, 2015, West Africa had reported more than 28,000 EVD cases, of which over 3800 (including greater than 2500 deaths) were reported from Guinea alone.[24]
Zika virus, a mosquito-borne flavivirus, was first identified in Uganda in 1947 in monkeys. It was later identified in humans in 1952 in Uganda and the United Republic of Tanzania. Outbreaks of Zika virus disease were recorded in Africa, the Americas, Asia, and the Pacific. From the 1960s to 1980s, human infections were found across Africa and Asia, typically accompanied by mild illness. The first large outbreak of disease caused by Zika infection was reported from the Island of Yap (Federated States of Micronesia) in 2007. In July 2015, Brazil reported an association between Zika virus infection and Guillain–Barré syndrome. In October 2015, Brazil also reported an association between Zika virus infection and microcephaly.[25]
Indian Scenario
India in the recent past has seen a large number of outbreaks of EIDs, many of which are zoonotic and majority of the infectious agents being virus.[7] However, this does not belittle the role of other infectious agents in causing outbreaks in India.
In 1994, there was a pneumonic plague epidemic in Surat, India, causing 54 deaths and the migration of about 300,000 residents who fled from the affected areas. India again reported an outbreak of pneumonic plague in February 2002 in Himachal Pradesh. Again a localized outbreak of bubonic plague occurred in Dangud village in district Uttarkashi, Uttarakhand, in October 2004.[26]
In 1992, a wide-scale cholera outbreak occurred in India first starting in southern India and spread both inland and along coastal areas of Bay of Bengal.[7] It was found that a new serogroup of Vibrio cholerae O139 was associated with this epidemic cholera.[27] Since then, reports of cholera outbreak due to this new serogroup came from many parts of the country.[28,29,30,31,32,33] Between 1997 to 2006, several states reported outbreaks of cholera [Figure 2].
Figure 2.
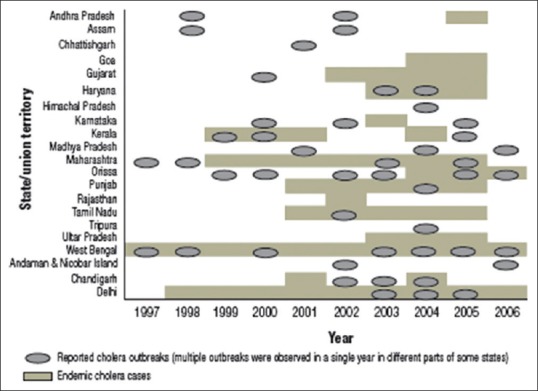
Distribution of cholera cases and outbreaks in India, 1997–2006 (Adapted from Ref 34)
The states of Andhra Pradesh, Assam, Haryana, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Odisha, and West Bengal and the union territories of Andaman and Nicobar Islands, New Delhi, and Chandigarh had cholera outbreaks during >1 year. The states which had the highest number of reported outbreaks were West Bengal, Odisha, Maharashtra, and Kerala, together accounted for 60% of all reported outbreaks. Of all the cholera cases that occurred during these outbreaks, 91% were identified in the states or union territories of Odisha, West Bengal, Andaman and Nicobar Islands, Assam, and Chhattisgarh. During this 10-year period, 823 deaths were reported with an overall case fatality rate of 0.37%.[34]
India accounted for 19%–84% of the global burden of Diphtheria from 1998 to 2008. Several outbreaks of Diphtheria were reported from New Delhi, Andhra Pradesh, Assam, Maharashtra, Chandigarh, and Gujarat.[35,36,37,38,39] These outbreaks revealed an epidemiological age shift with the disease currently affecting older children of 5–19 years age group and adults, and mostly involved children who were unimmunized or partially immunized against diphtheria.[7]
Outbreaks of scrub typhus caused by intracellular Gram-negative bacterium Orientia tsutsugamushi have been reported from the Sub-Himalayan belt, from Jammu to Nagaland.[40] Such outbreaks occurred in southern India during cooler months (October 2001 to February 2002)[41] and from Himachal Pradesh, Sikkim, and Darjeeling district of West Bengal during 2003, 2004, and 2007.[42] An outbreak of scrub typhus in Uttarakhand has been reported in 2005[43] and then again in 2012.[40]
India reported two outbreaks of NiV encephalitis (fruit bats being natural host) in West Bengal, bordering Bangladesh, in 2001 and 2007. Seventy-one cases with 50 deaths (70% of the cases) were reported in the two outbreaks. During January and February 2001, an outbreak of febrile illness with neurological symptoms was observed in Siliguri, West Bengal. A second outbreak was reported in 2007 in Nadia district of West Bengal.[44]
In 1965, Chandipura virus belonging to Rhabdoviridae family and transmitted by female sandfly bite was first isolated in the Chandipura region (Nagpur) from a fever case.[45] However, its potential to become a dangerous EID was established only after the outbreak in Andhra Pradesh in 2003 where it was associated with causing large-scale encephalitis outbreak in children 9–15 years in various districts of Andhra Pradesh with high case fatality rate (55.6).[46] During the same period in the Nagpur region of Maharashtra, 33 encephalitis cases were confirmed as Chandipura with a case fatality rate of 41%.[47,48] Focal outbreaks were also reported from Gujarat in 2004[49] and Maharashtra in 2005[50] and 2007[51] and again in Gujarat in 2014.[52]
AI A (H5N1) was first recognized in humans in 1997 during poultry outbreak in Hong Kong. Since its re-emergence in 2003, outbreaks of AI have been reported from poultry in Asia to Europe and Africa. According to the WHO, 16 countries have reported 846 laboratory confirmed human cases of AI including 449 deaths since 2003 through January 2016. Of these 16 countries, 4 were in the South-East Asia Region, namely, Bangladesh, Myanmar, Indonesia, and Thailand. In India, the first outbreak of AI occurred in Maharashtra and Gujarat in February 2006. Since then, 25 episodes of AI A (H5N1) were reported from 15 states and union territories till January 2015. Fortunately, no human case of AI A is detected in India so far. However, it is kept under rigorous surveillance under Integrated Disease Surveillance Programme (IDSP).[53]
In April 2009, the first case of influenza A H1N1 was reported from Mexico.[54] Subsequently, the infection spread across 74 countries with 30,000 confirmed cases by June 2009.[55] Altogether 214 countries were affected by the pandemic.[56] The first case of H1N1 positive in India was reported on May 2009, from a passenger who traveled from the USA arriving at Hyderabad airport.[57] After that, the virus soon became endemic and spread to almost all major cities in India.[58] “The highest number of cases were reported in 2009 (27,236), followed by 2010 (20,604) and 2012 (5,054 cases). The highest number of swine flu deaths took place in 2011 (1,763), followed by 2009 (981) and 2012 (405).”[59] During 2013, India reported 5,253 cases and 699 deaths with a case fatality rate of 13.3%.[60] The country again reported 937 cases and 218 deaths from swine flu in the year 2014. By mid-February 2015, the reported cases and deaths in 2015 had surpassed the previous numbers (33,000 cases with 2000 deaths).[61]
CCHF is a widespread disease caused by a tick-borne virus (Nairovirus) of the Bunyaviridae family. The CCHF virus causes severe viral hemorrhagic fever outbreaks, with a case fatality rate of 10%–40%. CCHF is endemic in Africa, the Balkans, the Middle East and Asian countries.[62] In India, the first confirmed case of CCHF was reported during a nosocomial (hospital acquired) outbreak in Ahmedabad, Gujarat, in January 2011. Subsequently, outbreaks were reported from different districts of this state every year. During 2012–2015, several outbreaks and cases of CCHF transmitted by ticks via livestock and several nosocomial infections were reported in the states of Gujarat (6 districts) and Rajasthan (3 districts). CCHF outbreaks pose a threat to public health services of its epidemic potential, its high case fatality ratio (10%–40%), its potential for nosocomial outbreaks, and the difficulties in its treatment and prevention.[63]
The risk of Dengue has increased in recent years due to rapid urbanization, and deficient water management including improper water storage practices in urban, peri-urban, and rural areas, leading to proliferation of mosquito breeding sites. The cases peak after monsoon and it is not uniformly distributed throughout the year. However, in the southern states and Gujarat, the transmission is perennial. Currently, it is endemic in 35 states/UTs. After 1996 outbreak (total 16,517 cases and 545 deaths), rising number of cases were recorded in 2003, 2005, 2008, 2010, 2012, and 2013. In 2010, a total of 28,292 cases and 110 deaths have been reported. During 2012, 50,222 cases and 242 deaths and during 2013, 75,808 cases and 193 deaths were reported. Highest number of deaths were reported by Maharashtra (48) followed by Kerala (29) and Punjab (25). During 2014, 33,320 cases and 86 deaths have been reported.[64] In the year 2015, maximum number of cases was reported from New Delhi followed by Punjab, Haryana, West Bengal, Gujarat, Karnataka, Maharashtra, and other states.[65]
Chikungunya re-emerged in the country after a gap of three decades. During 2006, total 1.39 million clinically suspected chikungunya cases were reported in the country. Out of 35 states/UTs, 16 were affected, namely Andhra Pradesh, Karnataka, Maharashtra, Tamil Nadu, Madhya Pradesh, Gujarat, Kerala, Andaman and Nicobar Islands, New Delhi, Rajasthan, Puducherry, Goa, Odisha, West Bengal, Lakshadweep, and Uttar Pradesh. There were no reported deaths directly related to chikungunya. In 2007, altogether 14 states were affected and reported 59,535 suspected chikungunya fever cases with no mortality. Subsequently, in 2008, 2009, 2010, 2011, and 2012, 95,091, 73,288, 48,176, 20,402, and 15,977 suspected chikungunya fever cases with no mortality were reported. During 2013, 18,840 suspected chikungunya cases were reported whereas during 2014 (till November) 12,694 suspected chikungunya cases have been reported.[64] Acute encephalitis syndrome (AES) is found to be a growing problem in India. Outbreaks predominantly occur in rural communities with poor access to healthcare and are quite often not notified as AES cases. In about 50% of the cases, the causative agent remains elusive while around 15.5% are diagnosed as cases of Japanese Encephalitis (JE).[66] JE is endemic in 179 districts of 21 states of which Assam, Bihar, Tamil Nadu, Uttar Pradesh, and West Bengal have been reporting more than 80% of disease burden. During 2011, 8249 cases and 1169 deaths and during 2012, 8344 cases and 1256 deaths due to AES including JE were reported. During 2013, 7825 cases and 1273 deaths due to AES including JE have been reported. While in 2014, reported cases of AES including JE were 9693 including 1490 deaths [Figure 3].[64]
Figure 3.
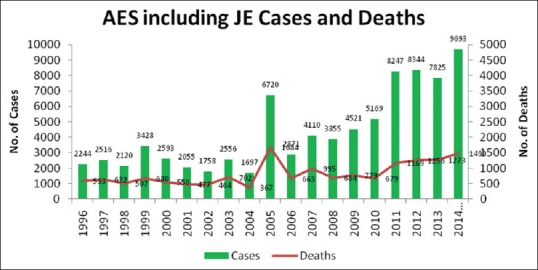
Acute encephalitis syndrome and Japanese Encephalitis cases with deaths in India from 1996 to 2014 (Adapted from Ref 64)
Strategies to Combat Emerging Infectious Diseases
Controlling EIDs poses a daunting challenge for the public health infrastructure of any country and more so for a developing country like India. The dire need is to develop an effective plan of action with a clear-cut strategic vision.
The WHO promulgates five strategic elements that are needed to combat EIDs. These include (1) epidemic preparedness and rapid response, (2) strengthen public health infrastructure, (3) exchange information with public and mass media through risk communication, (4) improve research and its utilization, and (5) continuing advocacy for political commitment and partnership building.[3]
India's Response
To strengthen the disease surveillance in the country by establishing a decentralized state-based surveillance system for epidemic-prone diseases to detect the early warning signals, Integrated Disease Surveillance Programme (IDSP) was launched in India with World Bank assistance in November 2004 to detect and respond to disease outbreaks quickly.[67] The program continues during 12th Plan (2012–2017) under the National Health Mission (NHM). Under IDSP, surveillance units have been established in all states/districts and central surveillance unit is established and integrated into the National Centre for Disease Control (NCDC), New Delhi. Training of state/district surveillance teams and rapid response teams has been completed for all 35 states/UTs. Information technology network connecting 776 sites in states/district headquarters and premier institutes has been established with the help of National Informatics Centre and Indian Space Research Organization for data entry, training, video conferencing, and outbreak discussion. Weekly disease surveillance data on epidemic-prone disease are being collected from reporting units such as subcenters, primary health centers, community health centers, and hospitals including government and private sector hospitals and medical colleges. Media scanning and verification cell established under IDSP in July 2008 detects and shares media alerts with the concerned states/districts for verification and response. District laboratories are being strengthened for diagnosis of epidemic-prone diseases. These laboratories are also being supported by a contractual microbiologist to manage the laboratories. In 9 States, a referral lab network has been established by utilizing the existing 65 functional laboratories in the medical colleges and various other major centers in the states and linking them with adjoining districts for providing diagnostic services for epidemic-prone diseases during outbreaks. Based on the experience gained, the plan will be implemented in the remaining 26 states/UTs. Considering the nonavailability of health professionals in the field of epidemiology, microbiology, and entomology at district and state levels, the Ministry of Health and Family Welfare (MOHFW) approved the recruitment of trained professionals under NHM in order to strengthen the disease surveillance and response system by placing one epidemiologist each at state/district headquarters, one microbiologist and entomologist each at the state headquarters. The post of a Veterinary Consultant at State Surveillance Unit has been approved by the MOHFW recognizing the Mission Statement of One Health Initiative (multidisciplinary collaborative approach to solving global and environmental health challenges).[67]
Compliance to International Health Regulations
The SARS outbreak in 2002 made it clear to the global health authority that international surveillance and disease notification could not be just limited to plague, cholera, and yellow fever and this revelation led to the formation of new International Health Regulations (IHR [2005]) with purpose and scope “to prevent, protect against, control and provide a public health response to the international spread of disease in ways that are commensurate with and restricted to public health risks, and which avoid unnecessary interference with international traffic and trade.”[68]
Because the IHR (2005) are not limited to specific diseases but apply to new and ever-changing public health risks, they are intended to have long-lasting relevance in international response to the emergence and spread of disease. They also provide the legal basis for important health documents applicable to the international travel and transport and sanitary protections for the users of international airports, ports and ground crossings.[68]
“India being one of the signatories has begun to implement various provisions of the IHR (2005) to establish national and thereby global public health security by preventing and responding to acute public health risks having potential to cross borders and pose threat to other countries.”[7]
Research Initiative
In October 2007, the Government of India created a new Department of Health Research within MOHFW, contemplating to bring research to the core activity of the health development and provide technical support for dealing with epidemics and investigation of outbreaks due to new and exotic agents and development of tools for prevention.[69] The Indian Council of Medical Research continues to play a major role in providing research inputs along with detection and management guidelines through its network of institutes devoted to specific diseases and a chain of regional centers.[7,69]
World Health Organization Response
In 2000, the WHO and partners established the Global Outbreak Alert and Response Network (GOARN) to ensure countries have rapid access to the most appropriate resources and experts for the identification, assessment, and response to public health emergencies of international importance.[70] GOARN provides a global operational framework linking a broad range of public health capacities and expertise to keep the international community alert to the threat of outbreaks, and ready to coordinate support to countries and effectively deploy emergency response teams. GOARN is basically a multidisciplinary technical collaboration of over 200 technical institutions and networks that works with over 600 partners worldwide, including national public health institutions and hospitals, ministries of health, academic, research and technical institutions, networks, such as laboratories, surveillance initiatives and research agencies, United Nations, and international and nongovernmental organizations.[70]
Global Disease Detection Program: India
The US Centers for Disease Control and Prevention collaborates with the government of India to address priority public health burdens in the region. In 2009, the India Global Disease Detection (GDD) Regional Center was established. The focus of the GDD center, located at India's NCDC, is to establish the India Epidemic Intelligence Service and to work with local, regional, and global public health entities to better detect, identify, and contain EID threats. From 2009 to 2015, the GDD center in India supported effective response to 74 outbreaks, including hepatitis A, dengue, measles, avian flu, anthrax, cholera, and malaria, training of over 2000 public health officials in short-term public health exercises, including epidemiology and laboratory, rapid response, and risk communication and graduation of 13 future global health leaders as part of the 2-year Field Epidemiology Training Program.[71]
Future Needs
India along with other countries in SEAR will continue to bear the brunt of the burden of EIDs in years to come. Strengthening of surveillance and timely reporting systems, rapid response to the occasion, and a revamped health infrastructure at all levels of health care empowered by qualified, experienced and dedicated workforce are needed as countermeasure to fight this menace. Involvement of mass media will be of paramount importance in spreading awareness and involving community particularly in mitigating panic. Private sector would also play a significant role in reporting and complying with government guidelines and thereby reducing underreporting. Comprehensive training of physicians in public health and epidemiology, basic microbiology, and clinical medicine appears to be the need of the hour. It is clear by now that the problem of EID is not restricted to any single country and a strong and sustainable international collaboration will be needed in their prevention and control.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Incidence of numerous EIDs show a rising trend. Many new viral agents are adding to the pool of infections. Newer surveillance strategies and global diseases detection programme and international cooperation form the cornerstone in prevention and control of this menace.
References
- 1.World Health Organization, Regional Office for South-East Asia. A Brief Guide to Emerging Infectious Diseases and Zoonoses. New Delhi: WHO-SEARO; 2014. [Google Scholar]
- 2.Centre for Disease Control and Prevention. Operationalizing “One Health”: A Policy Perspective – Taking Stock and Shaping an Implementation Roadmap: Meeting Overview. 4-6 May, 2010. Stone Mountain, Georgia, Atlanta: CDS; 2011. [Last accessed on 2017 Feb 22]. Available from: http://www.stacks.cdc.gov/view/cdc/22020 . [Google Scholar]
- 3.World Health Organization, Regional Office for South-East Asia. Combating Emerging Infectious Diseases in the South-East Asia Region. New Delhi: WHO-SEARO; 2005. [Google Scholar]
- 4.World Health Organization, South-East Asia Region, Western Pacific Region. Asia Pacific Strategy for Emerging Diseases: 2010. New Delhi, Manila: WHO-SEARO, WHO-WPRO; 2011. [Last accessed on 2014 Feb 22]. Available from: http://www.wpro.who.int/emerging_diseases/documents/docs/ASPED_2010.pdf . [Google Scholar]
- 5.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–3. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park K. Park's Textbook of Preventive and Social Medicine. 23rd ed. Jabalpur, India: M/s Banarasidas Bhanot Publishers; 2015. Emerging and re-emerging infectious diseases; pp. 355–9. [Google Scholar]
- 7.Dikid T, Jain SK, Sharma A, Kumar A, Narain JP. Emerging and re-emerging infections in India: An overview. Indian J Med Res. 2013;138:19–31. [PMC free article] [PubMed] [Google Scholar]
- 8.Mackey TK, Liang BA, Cuomo R, Hafen R, Brouwer KC, Lee DE, et al. Emerging and reemerging neglected tropical diseases: A review of key characteristics, risk factors, and the policy and innovation environment. Clin Microbiol Rev. 2014;27:949–79. doi: 10.1128/CMR.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Blueprint for R&D Preparedness and Response to Public Health Emergencies due to Highly Infectious Pathogens. Geneva: WHO; 2015. Dec 8-9, [Last accessed on 2017 Mar 02]. Available from: http://www.who.int/entity/csr/research-and-development/meeting-report-prioritization.pdf?ua=1 . [Google Scholar]
- 10.Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morens DM, Fauci AS. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog. 2013;9:e1003467. doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Office for South-East Asia Region and Western-Pacific Region: Asia Pacific Strategy for Emerging Diseases. WHO; 2005. [Google Scholar]
- 13.Fauci SA, David Quammen D. NEIDL Symposium: Talking about Infectious Diseases. [Last accessed on 2017 Mar 05]. Available from: https://www.bu.edu/today/2016/neidl-symposium-infectious-diseases/ Available from: https://www.bu.edu/today/files/2016/09/global-emerging-infectious-diseases-map.jpg .
- 14.Aginam O. International law and communicable diseases. Bull World Health Organ. 2002;80:946–51. [PMC free article] [PubMed] [Google Scholar]
- 15.Summary Table of SARS Cases by Country. Geneva: World Health Organization; 2002. Nov 1, [Last accessed on 2017 Mar 05]. 5 7 August, 2003. Available from: http://www.who.int/csr/sars/country/2003_08_15/en/index.html . [Google Scholar]
- 16.World Health Organization. Weekly Epidemiological Record No. 49/50. 2005 Oct;80:425–32. [Google Scholar]
- 17.World Health Organization. Situation Updates – Pandemic (H1N1) 2009 – Update 112. Geneva: WHO; [Last accessed on 2017 Mar 05]. Available from: http://www.who.int/csr/don/2010_08_06/en/ [Google Scholar]
- 18.World Health Organization (WHO). Ebola Virus Disease, Fact Sheet No 103. Geneva: WHO; 2014. Sep, [Last accessed on 2017 Mar 07]. Available from: https://www.who.int/mediacentre/factsheets/fs103/en/ [Google Scholar]
- 19.World Health Organization. Global Alert and Response: Ebola Virus Disease in Guinea. 2014. [Last accessed on 2017 Mar 07]. Available from: http://www.who.int/csr/don/2014_03_23_ebola/en/
- 20.Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, Sall AA, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–7. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 21.Dixon MG, Schafer IJ. Centers for Disease Control and Prevention (CDC). Ebola viral disease outbreak – West Africa, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:548–51. [PMC free article] [PubMed] [Google Scholar]
- 22.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–25. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 23.Barry M, Traoré FA, Sako FB, Kpamy DO, Bah EI, Poncin M, et al. Ebola outbreak in Conakry, Guinea: Epidemiological, clinical, and outcome features. Med Mal Infect. 2014;44:491–4. doi: 10.1016/j.medmal.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Ebola Situation Report. 2015. Nov 4, [Last accessed on 2017 Mar 07]. Available from: http://www.apps.who.int/ebola/current-situation/ebola-situation-report-4-november-2015 .
- 25.World Health Organization. Zika Virus Fact Sheet Updated. Geneva: WHO; 2016. Sep 6, [Last accessed on 2017 Mar 08]. Available from: http://www.who.int/mediacentre/factsheets/zika/en/ [Google Scholar]
- 26.World Health Organization Regional Office for South-East Asia. Frequently Asked Questions on Plague. New Delhi: WHO-SEARO; 2004. [Google Scholar]
- 27.Ramamurthy T, Yamasaki S, Takeda Y, Nair GB. Vibrio cholerae O139 Bengal: Odyssey of a fortuitous variant. Microbes Infect. 2003;5:329–44. doi: 10.1016/s1286-4579(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 28.Mitra R, Basu A, Dutta D, Nair GB, Takeda Y. Resurgence of Vibrio cholerae O139 Bengal with altered antibiogram in Calcutta, India. Lancet. 1996;348:1181. doi: 10.1016/s0140-6736(05)65326-3. [DOI] [PubMed] [Google Scholar]
- 29.Jalgaonkar SV, Ingole KV, Ambhore NA, Fule RP. Re-emergence of Vibrio cholerae serogroup O139 during June-August, 1997 in Yavatmal (Maharashtra) Indian J Med Res. 1998;108:1–2. [PubMed] [Google Scholar]
- 30.Lahiri KK, Ayyagari A. Vibrio cholerae O139 in Lucknow. Indian J Med Microbiol. 1998;16:133. [Google Scholar]
- 31.Kaur H, Lal M. Reappearance of Vibrio cholerae O139 during March-August, 1998 in Ludhiana (Punjab), India. Indian J Med Res. 1999;109:3–4. [PubMed] [Google Scholar]
- 32.Raut S, Jalgaonkar SV, Tankhiwale NS, Agarwal VA. Re-emergence of Vibrio cholerae O139 serogroup during 1998 in Nagpur (Maharashtra), India. Indian J Med Res. 1999;109:1–2. [PubMed] [Google Scholar]
- 33.Agrawal G, Jalgaonkar SV, Jagtap PM, Kamlakar UP, Deogade NG. Emergence and re-emergence of Vibrio cholerae O139: An epidemiological study during 1993-2002 at Nagpur, Central India. Indian J Med Sci. 2003;57:155–7. [PubMed] [Google Scholar]
- 34.Kanungo S, Sah BK, Lopez AL, Sung JS, Paisley AM, Sur D, et al. Cholera in India: An analysis of reports, 1997-2006. Bull World Health Organ. 2010;88:185–91. doi: 10.2471/BLT.09.073460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murhekar MV, Bitragunta S. Persistence of diphtheria in India. Indian J Community Med. 2011;36:164–5. doi: 10.4103/0970-0218.84141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lodha R, Dash NR, Kapil A, Kabra SK. Diphtheria in urban slums in North India. Lancet. 2000;355:204. doi: 10.1016/S0140-6736(99)04847-3. [DOI] [PubMed] [Google Scholar]
- 37.Khan N, Shastri J, Aigal U, Doctor B. Resurgence of diphtheria in the vaccination era. Indian J Med Microbiol. 2007;25:434. doi: 10.4103/0255-0857.37367. [DOI] [PubMed] [Google Scholar]
- 38.Ray SK, Das Gupta S, Saha I. A report of diphtheria surveillance from a rural medical college hospital. J Indian Med Assoc. 1998;96:236–8. [PubMed] [Google Scholar]
- 39.Nath B, Mahanta TG. Investigation of an outbreak of diphtheria in borborooah block of Dibrugarh district, Assam. Indian J Community Med. 2010;35:436–8. doi: 10.4103/0970-0218.69282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan F, Mittal G, Agarwal RK, Ahmad S, Gupta S, Shadab M. Prevalence of scrub typhus a cause of concern in Uttarakhand Region, India. Int J Curr Microbiol App Sci. 2015;Special Issue-1:101–9. [Google Scholar]
- 41.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, et al. Outbreak of scrub typhus in Southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359–64. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 42.Rajendran A. Scrub typhus in pediatric age group: A report from a tertiary care hospital. J Pediatr Sci. 2011;3:e82. [Google Scholar]
- 43.Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the Himalayan region of India. Jpn J Infect Dis. 2005;58:208–10. [PubMed] [Google Scholar]
- 44.World Health Organization, Regional Office for South-East Asia. Nipah Virus Outbreaks in the WHO South-East Asia Region. New Delhi: WHO-SEARO; (undated); [Last accessed on 2017 Mar 08]. Available from: http://www.searo.who.int/entity/emerging_diseases/links/nipah_virus_outbreaks_sear/en/ [Google Scholar]
- 45.Bhatt PN, Rodrigues FM. Chandipura: A new arbovirus isolated in India from patients with febrile illness. Indian J Med Res. 1967;55:1295–305. [PubMed] [Google Scholar]
- 46.Rao BL, Basu A, Wairagkar NS, Gore MM, Arankalle VA, Thakare JP, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869–74. doi: 10.1016/S0140-6736(04)16982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra AC. Chandipura encephalitis: A newly recognized disease of public health importance. In: Mishra AC, editor. National Institute of Virology Commemorative Compendium. Pune: NIV, Golden Jubilee Publication; 2004. pp. 1–20. [Google Scholar]
- 48.Investigation of Outbreak of Encephalitis in Nanded District and some Districts of Vidarbha Region of Maharashtra. National Institute of Virology, Pune. Annual Report 2003-2004. Pune: NIV; 2004. pp. 3–4. [Google Scholar]
- 49.Chadha MS, Arankalle VA, Jadi RS, Joshi MV, Thakare JP, Mahadev PV, et al. An outbreak of Chandipura virus encephalitis in the Eastern districts of Gujarat State, India. Am J Trop Med Hyg. 2005;73:566–70. [PubMed] [Google Scholar]
- 50.Encephalitis in Bhandara & Nagpur, Maharashtra. National Institute of Virology, Pune. Annual Report 2005-2006. Pune: NIV; 2006. p. 8. [Google Scholar]
- 51.Gurav YK, Tandale BV, Jadi RS, Gunjikar RS, Tikute SS, Jamgaonkar AV, et al. Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian J Med Res. 2010;132:395–9. [PubMed] [Google Scholar]
- 52.Disease Alerts/Outbreaks Reported and Responded to by States/UTs through Integrated Disease Surveillance Programme (IDSP) 2014. Jul, [Last accessed on 2017 Mar 10]. Available from: http//:www.idsp.nic.in/WriteReadData/DOB2014/30th_wk14.pdf .
- 53.National Health Portal. Avian Influenza in Humans. India, Delhi: NHP, MOHFW; 2016. Mar, [Last accessed on 2017 Mar 14]. Available from: https://www.nhp.gov.in/disease/communicable-disease/avian-influenza-in-humans . [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC). Update: novel influenza A (H1N1) virus infection – Mexico, March-May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:585–9. [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization. World now at the start of 2009 Influenza Pandemic. Statement to the Press by WHO Director General – Dr Margaret Chan. [Last accessed on 2017 Mar 14]. Available from: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html .
- 56.Choudhry A, Singh S, Khare S, Rai A, Rawat DS, Aggarwal RK, et al. Emergence of pandemic 2009 influenza A H1N1, India. Indian J Med Res. 2012;135:534–7. [PMC free article] [PubMed] [Google Scholar]
- 57.John TJ, Moorthy M. 2009 pandemic influenza in India. Indian Pediatr. 2010;47:25–31. doi: 10.1007/s13312-010-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee A, Roy T, Agrawal AS, Sarkar M, Lal R, Chakrabarti S. Prevalence and epidemiology of pandemic H1N1 strains in hospitals of Eastern India. J Public Health Epidemiol. 2010;2:171–4. [Google Scholar]
- 59.Kawanpure H, Ugargol AR, Padmanabha BV. A study to assess knowledge, attitude and practice regarding swine flu. Int J Health Sci Res. 2014;4:6–11. [Google Scholar]
- 60.Ghosh A, Philip S. Five H1N1 Deaths in Kerala, Surge in Gujarat and Rajasthan Push Fatality Rate to Over 20%. 2014. Jun, [Last accessed on 2017 Mar 15]. Available from: http://www.indianexpress.com/article/india/indiaothers/five-h1n1-deaths-in-kerala-surge-in-gujarat-andrajasthan-push-fatality-rate-to-over-20/
- 61.Swine flu outbreak: 743 deaths, 12,000 cases set alarm bells ringing. Hindustan Times. 2015. Feb 21, [Last accessed on 2017 Mar 16]. Available from: http://www.hindustantimes.com/india/swine-flu-outbreak-774-deaths-13-000-cases-set-alarm-bells-ringing/story-qKeRWZ9QocPLll3Oif09RL.html .
- 62.World Health Organization. Crimean-Congo Haemorrhagic Fever Fact Sheet. Geneva: WHO; 2013. Jan, [Last accessed on 2017 Mar 17]. Available from: http://www.who.int/mediacentre/factsheets/fs208/en/ [Google Scholar]
- 63.National Health Portal. Crimean-Congo Haemorragic Fever (CCHF) India, Delhi: MOHFW, NHP; 2016. Jan, [Last accessed on 2017 Mar 17]. Available from: https://www.nhp.gov.in/disease/blood-lymphatic/crimean-congo-haemorrhagic-fever-cchf . [Google Scholar]
- 64.National Vector Borne Disease Control Programme. Annual Report 2014-15. Delhi: NVBDCP, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; [Google Scholar]
- 65.National Health Portal. National Health Portal. Dengue Fever. India, Delhi: MOHFW, NHP; 2016. Feb, [Last accessed on 2017 Mar 19]. Available from: https://www.nhp.gov.in/disease/musculo-skeletal-bone-joints-/dengue-feverBnvnbv . [Google Scholar]
- 66.Kelly R. Acute Encephalitis Syndrome Outbreaks in India – An Ongoing Puzzle. School of Public Health and Community Medicine, UNSW Medicine, Australia. 2017. Mar, [Last accessed on 2017 Mar 20]. Available from: https://www.sphcm.med.unsw.edu.au/infectious-diseases-blog/acute-encephalitis-syndrome-outbreaks-india-%E2%80%93-ongoing-puzzle .
- 67.Integrated Disease Surveillance Programme (IDSP). National Centre for Diseases Control, Directorate General of Health Services. Delhi: Ministry of Health and Family Welfare, Government of India; [Last accessed on 2017 Mar 20]. Available from: http://www.idsp.nic.in/ [Google Scholar]
- 68.World Health Organization. International Health Regulations (2005) 2nd ed. Reprinted. Geneva: WHO; 2008. [Google Scholar]
- 69.Kant L. Combating emerging infectious diseases in India: Orchestrating a symphony. J Biosci. 2008;33:425–7. doi: 10.1007/s12038-008-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. International Health Regulations Global Outbreak Alert and Response Network (GOARN) Geneva: WHO; 2015. [Google Scholar]
- 71.Centers for Disease Control and Prevention. Global Disease Detection Program. India CDC, Atlanta, USA: [Last accessed on 2017 Mar 24]. Available from: https://www.cdc.gov/globalhealth/healthprotection/gdd/india.html . [Google Scholar]


