Abstract
Background:
There is a felt need for trying newer therapeutic modalities in patients with chronic spontaneous urticaria, especially in the subset of patients classified as non-responders to antihistamines. Autologous serum therapy is an upcoming modality of treatment, and we decided to study its efficacy by subcutaneous route.
Aims:
To evaluate the effectiveness of subcutaneous autologous serum therapy (AST) in CSU.
Methods:
This was a single blind, placebo-controlled parallel group, randomized, controlled study. Twenty-four patients with CSU (11M: 13 F) were given subcutaneous AST and seventeen patients (7 M: 10F) patients were given subcutaneous injection normal saline (placebo), along with levocetirizine in an on-demand basis in both groups.
Results:
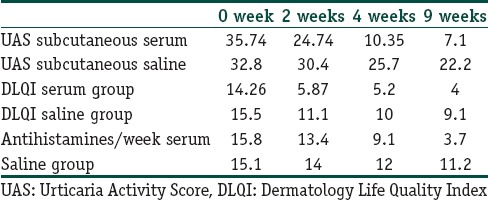
Urticaria activity score (UAS) came down from 35.74 to 7 at the end of 9 weeks and the patients’ requirement of antihistamines also reduced remarkably from 5.8 to 1.7 per week in the serum group. Sub-cutaneous saline group did not show statistically significant fall in UAS. Saline group showed UAS 32.8 at zero week to 22.1 at the end of 9 weeks. DLQI showed significant fall in serum group, from 14.26 to 4 at the end of 9 weeks.
Conclusion:
Subcutaneous autoserum therapy is effective in treatment of CSU.
KEY WORDS: Autologous serum therapy, chronic urticaria, subcutaneous
What was known?
Only half (or lesser) of the patients suffering from CSU respond with complete control of symptoms to licensed doses of H1-antihistamines
Ravaut first described autohemotherapy in 1913 and reported its use in various dermatologic disorders
There has been a resurgence of interest in the use of autologous serum therapy in chronic spontaneous urticaria, with most studies reporting its use by intramuscular route
Introduction
Urticaria is one of the most challenging therapeutic problems faced by dermatologists. A variety of therapeutic modalities have been used for the management of this chronic and often relapsing condition,[1] with various management guidelines having been published in literature.[2,3] Chronic spontaneous urticaria (CSU) has a significant effect on patient's quality of life; hence, there is always a need for trying out newer therapeutic modalities in this disease.
Published studies indicate that only half (or even less) of the patients suffering from CSU respond with complete control of symptoms to licensed doses of H1-antihistamines.[4] Recent research has seen the reemergence of autologous serum therapy (AST) as a treatment modality in urticaria.
Autohemotherapy involves injecting autologous whole blood (AWB) or autologous serum, traditionally into muscle.[5] The procedure was widely used in the treatment of furunculosis and buboes[6] and a variety of diseases including chronic inflammation, allergies, vascular diseases, osteoarthritis, atopic dermatitis,[7] and various other skin disorders in the past.[8]
In 1913, Ravaut[9] and Spiethoff[10] described their use of autohemotherapy for various dermatologic conditions. Ravaut[11] created and obtained the first results with this process. He used to recommend this type of therapy in pruriginous conditions, blistering diseases, urticaria, and certain types of acne associated with pruritus.[11] However, autohemotherapy gradually fell into disuse due to the lack of evidence.
Interest in this modality has resurged in the last 15 years, starting with an initial study published by Staubach et al. in 2006.[12]
It has been demonstrated that around one-third of patients with CSU show a positive response in the autologous serum skin test (ASST), reacting to their own serum.[4] These patients are classified as autoreactive and can be subsumed to suffer from autoreactive CSU.[4] Staubach et al.[12] found that ASST-positive patients required significantly more antihistamines for symptom control when compared to ASST-negative patients.
AST is considered better than autohemotherapy in the treatment of chronic urticaria (CU) as the circulating autoreactive factors which are not present in blood are present in the serum and it is less painful to inject.[13] Furthermore, one has to inject AWB immediately to avoid clotting.[13]
Against this background, we decided to study the efficacy of AST administered as subcutaneous injections in individuals with CSU. The rationale behind this study design was the plausible advantage offered over the intramuscular route – the relative ease of administration, lesser pain, complete absorption of injected serum, and fewer injection site reactions.
Materials and Methods
This study evaluates the effectiveness of subcutaneous AST in CSU and also determines its usefulness. Inclusion criteria were age between 18 and 75 years, cumulative Urticaria Activity Score (UAS) over past 1 week >14 at baseline, and informed written consent. Exclusion criteria were pregnancy or breastfeeding, uncontrolled diabetes mellitus, physical urticaria, and therapy with anticoagulants, for example, warfarin and aspirin.
This was single-blind, placebo-controlled parallel group, randomized, controlled study. Twenty-four patients with CSU (11 M: 13 F) were given subcutaneous AST, and seventeen patients (7 M: 10 F) were given subcutaneous injection normal saline (placebo), along with levocetirizine in an on-demand basis in both groups. Age group was 19–54 years with mean age of 29.7 years. Duration of urticaria was varying from 6 months to 80 months (average duration 18 months).
We started this therapy in the study patients by giving weekly, subcutaneous injections of patients’ own serum or saline in the skin over the abdomen in doses of 0.05 ml/kg body weight. Sterile injection technique with meticulous labeling was used throughout the study.
Basic investigations revealed eosinophilia (five patients), hypothyroidism (four patients), and microcytic hypochromic anemia (two patients). ASST was found to be positive in 11/24 in the serum group and 7/17 in the saline group.
Instruments used in the procedure were centrifuge machine, syringe and needle, vacutainers, and ASST tests requirements (saline and histamine for control). A volume of 5cc of blood was collected in plain (red top) vacutainer. It was centrifuged at 3000 rpm for 10 min. Using 5 cc syringe, 2.5 ml of the separated serum was injected subcutaneously in the abdomen with a 26-gauge needle.
This procedure was repeated at 9 weekly intervals.
Baseline and follow-up UAS were recorded for every patient.
If there was no response after 9 injections, the treatment was discontinued, as per the study protocol.
Observations and Results
As shown in Table 1, UAS came down within few weeks from 35.74 to 7 at the end of 9 weeks, and the patients’ requirement of antihistamines also reduced remarkably from 5.8 to 1.7/week in the serum group. Subcutaneous saline group did not show statistically significant fall in UAS. Saline group showed UAS 32.8 at 0 week to 22.1 at the end of 9 weeks. Dermatology Life Quality Index (DLQI) showed significant fall in serum group.
Table 1.
Observations and Results
Discussion
As per our knowledge, this study is the first report of autoserum therapy given by subcutaneous route. All previously published studies relate to the use of intramuscular autoserum therapy. Advantages of subcutaneous injections are as follows: complete absorption is possible in the absence of comorbidities affecting patient's peripheral circulation, injection is relatively painless, and there are no chances of damage to nerves and vessels.
In the study published by Staubach et al.[12] in 2006, 8 weekly intramuscular injections of AWB were given to ASST-positive and ASST-negative CU patients. Significantly reduced CU activity, decreased use of antihistamines, and improved QOL after AWB treatment were reported.[12]
At least 59.7% of ASST-positive and 46% ASST-negative patients showed significant improvements in signs and symptoms after nine weekly autologous serum skin injections, in the study by Bajaj et al.[13] This improvement was sustained for at least 3–4 months after the last injection.
In the double-blind, randomized controlled study by Debbarman et al.,[14] 54 patients received AST and 57 patients received normal saline injections weekly for 9 weeks, in an intramuscular manner. DLQI showed significant improvement in AST group as compared to placebo group, while total severity score and UAS showed similar improvement.[14]
In contrast, a randomized, three-arm study from Istanbul comparing autologous serum and AWB injections to placebo injections in 88 patients of CU could not establish a statistically significant difference in efficacy between the three methods.[15]
Recently, a systematic review of autohemotherapy as a treatment for urticaria was published.[5] It stated that (1) autohemotherapy does not have major side effects and that minor adverse effects are short lived and similar in frequency to those from placebo injections, (2) overall, autohemotherapy tends to be somewhat more effective in reducing symptoms than control therapy across studies although the advantage is not statistically reliable, (3) AWB and autologous serum injections appear to have similar effectiveness, and (4) urticaria patients who test positive on the ASST respond more favorably to autohemotherapy on average than those who test negative.
Summarizing subcutaneous AST may be a suitable alternative to intramuscular route.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Subcutaneous autoserum therapy is safe and effective in treatment of CSU
May be preferred over intramuscular route due to ease of administration, lesser pain, better absorption
Subcutaenous autoserum therapy is a cheap treatment alternative in refractory cases of CSU
References
- 1.Mehta A, Godse K, Patil S, Nadkarni N, Gautam M. Treatment of refractory chronic urticaria. Indian J Dermatol. 2015;60:230–7. doi: 10.4103/0019-5154.156325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2)LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision and update. Allergy. 2014;69:868–87. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 3.Powell RJ, Leech SC, Till S, Huber PA, Nasser SM, Clark AT. British Society for Allergy and Clinical Immunology. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45:547–65. doi: 10.1111/cea.12494. [DOI] [PubMed] [Google Scholar]
- 4.Maurer M, Weller K, Bindslev-Jensen C, Giménez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA²LEN task force report. Allergy. 2011;66:317–30. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 5.Brewer DD. A Systematic Review of Autohemotherapy as a Treatment for Urticaria and Eczema. Cureus. 2014:6. [Google Scholar]
- 6.Al Aboud K, Al Hawsawi K, Al Alaboud A, Ramesh V, Jain N. Blood transfusion and dermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:20–4. [PubMed] [Google Scholar]
- 7.Pittler MH, Armstrong NC, Cox A, Collier PM, Hart A, Ernst E. Randomized, double-blind, placebo-controlled trial of autologous blood therapy for atopic dermatitis. Br J Dermatol. 2003;148:307–13. doi: 10.1046/j.1365-2133.2003.04921.x. [DOI] [PubMed] [Google Scholar]
- 8.Behl PN. Practice of Dermatology. 7th ed. New Delhi: Oxford Blackwell Scientific Publications; 1990. Autohaemotherapy; p. 76. [Google Scholar]
- 9.Ravaut P. Essay on Autohemotherapy in some dermatoses.(article in French) Ann Dermatol Syphiligr. 1913;4:292–6. [Google Scholar]
- 10.Spiethoff B. Therapeutic use of self-serum (article in German) Münch Med Wochenschr. 1913;60:521. [Google Scholar]
- 11.Ravaut P. Auto-hemotherapie (Therapeutique et Technique) Presse Medicale. 1920:80. [Google Scholar]
- 12.Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: A placebo-controlled trial. Dermatology. 2006;212:150–9. doi: 10.1159/000090656. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: Old wine in a new bottle. Indian J Dermatol Venereol Leprol. 2008;74:109–13. doi: 10.4103/0378-6323.39691. [DOI] [PubMed] [Google Scholar]
- 14.Debbarman P, Sil A, Datta PK, Bandyopadhyay D, Das NK. Autologous serum therapy in chronic urticaria: A promising complement to antihistamines. Indian J Dermatol. 2014;59:375–82. doi: 10.4103/0019-5154.135490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocatürk E, Aktas S, Türkoglu Z, Kavala M, Zindanci I, Koc M, et al. Autologous whole blood and autologous serum injections are equally effective as placebo injections in reducing disease activity in patients with chronic spontaneous urticaria: A placebo controlled, randomized, single-blind study. J Dermatolog Treat. 2012;23:465–71. doi: 10.3109/09546634.2011.593485. [DOI] [PubMed] [Google Scholar]


