Abstract
Aim:
We previously reported that incidence rates for chronic lymphocytic leukemia (CLL) among US states are significantly correlated with levels of residential radon (RR). Because these correlations could be influenced by confounding and/or misclassification among large geographic units, we reinvestigated them using smaller geographic units that better reflect exposure and disease at the individual level.
Methods:
We examined the relationships between CLL and RR per county in 478 counties with publicly-available data.
Results:
After adjustment for ultraviolet radiation, a possible risk factor for CLL, county rates for CLL and RR were significantly correlated among males and females both together and separately (p < 0.0001).
Conclusion:
CLL is significantly associated with RR at the county level.
KEYWORDS : chronic lymphocytic leukemia, epidemiology, ionizing radiation, latitude, radon
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in western populations [1], with approximately 18,960 new cases and 4660 related deaths annually in the USA [2]. CLL is predominantly a disease of the elderly, with a median age at diagnosis of 71 years. It is more common among Caucasians and its incidence is almost twice as high among men than women [3–5 ]. The etiology of CLL is poorly understood. A family history of hematologic malignancy is an established risk factor [6,7]. Atopic conditions (e.g., allergy, hay fever, asthma and eczema) appear to be protective [8]. Environmental risk factors for CLL have not been consistently identified. Pesticides have been implicated in several [9–11] but not all [12] studies. An increased risk in association with exposure to sunlight/ultraviolet radiation (UVR) has been reported for non-Hodgkin lymphomas as a group [13,14]. Conversely, a protective effect of UVR has been reported for CLL individually [8,15].
An etiologic role for ionizing radiation in CLL is controversial [16]. Increased risks for CLL have not been observed among atomic bomb survivors and among other highly radiation-exposed populations, leading to the long-standing view that CLL is not radiogenic [17,18]. However, that view has been challenged by findings of significant associations between ionizing radiation and CLL among Czech uranium miners and Chernobyl cleanup workers [19–23]. The most important source of ionizing radiation for the general public is exposure to radon at home [24]. Radon is a naturally-occurring gas that is the product of radioactive elements present in rocks and soil. The gas enters homes through cracks in the foundation and can be trapped inside, particularly during winter when homes are sealed. Radon is the most important cause of death from lung cancer after smoking [25]. The WHO report of 2009 [26], as well as other reports [27,28], did not discount possible effects of radon on cancers other than lung cancer. Radon has been examined in several studies of non-lung cancers, including esophageal cancer [29], stomach cancer [27,30] and leukemia [e.g., 28,31–33]. A large case-control study of radon and childhood leukemia in Denmark found a significant association that was not confirmed in population-based studies in Switzerland and Norway [34–36]. However, a study of leukemia in relation to homes built with construction materials containing uranium as well as results of a recent meta-analysis, support an etiologic role for radon in childhood leukemia [31,37].
We recently reported significant positive correlations between levels of residential radon (RR) and CLL incidence rates in the USA at the level of the state [38]. A limitation of that study is that states are relatively large ecologic units and the correlations observed could be influenced by extraneous factors (confounding) and by misclassification. For example, although there is a strong correlation between per capita dietary fat consumption and breast cancer rates among countries, fat intake is not associated with breast cancer risk among individuals [39]. Because the potential effects of confounding and misclassification generally are reduced among smaller ecologic units [40], we re-examined the association between RR and CLL at a finer scale, the county. We report that incidence rates for CLL in the USA are significantly correlated with RR at the county level and remain so after controlling for UVR.
Methods
We investigated RR and CLL incidence rates among US states that had publicly-available data. Age-adjusted CLL incidence rates per county were computed for a 15 year time period, 1999–2013 [41,42]. Rates for counties in North Dakota (ND) were obtained from the ND Statewide Cancer Registry [43]. All rates were age-adjusted to the 2000 US standard population and were calculated per 100,000 persons (males and females together) and separately by gender [44]. Rates for counties with fewer than ten cases were considered unstable and were censored. However, data for counties in Texas (TX) were censored for ≤ 16 cases. The difference in the censoring cut-points reflects the reporting policies of individual state registries.
Residential radon levels per county were obtained from RR surveys conducted by the USA Environmental Protection Agency (EPA) using short-term radon detectors placed in the lowest livable area of the residence for 2–7 days. Radon measurements were recorded as picocuries per liter (1 pCi/l = 37 Bq/m3; for details of radon measurements see [45–48]). Counties with radon measurements performed on fewer than five homes were excluded.
Because exposure to UVR may be a risk factor for CLL, we included data on geographic latitude, a variable that is highly correlated with UVR and often used as a surrogate for it [49]. County-level latitude was derived from a digital map of US counties and equivalent boundaries from the Environmental Systems Research Institute [50].
• Statistical analyses
We used generalized linear models (unadjusted and latitude-adjusted) to evaluate the correlation between county-level radon and CLL incidence rates. The interaction between gender and radon was estimated and if significant, an analysis was performed separately for each gender. Radon levels were categorized in three groups: ‘Low’, ≤ 2 pCi/l; ‘Medium’, > 2 pCi/l and ≤ 4 pCi/l; and ‘High’, > 4 pCi/l (the EPA level recommended for remediation of homes [51]). All analyses were repeated with radon as a categorical variable with ‘Low’ as the reference. Statistical analyses used SAS v9.4 (SAS Institute, NC, USA). The statistical significance level was set at p < 0.05.
Results
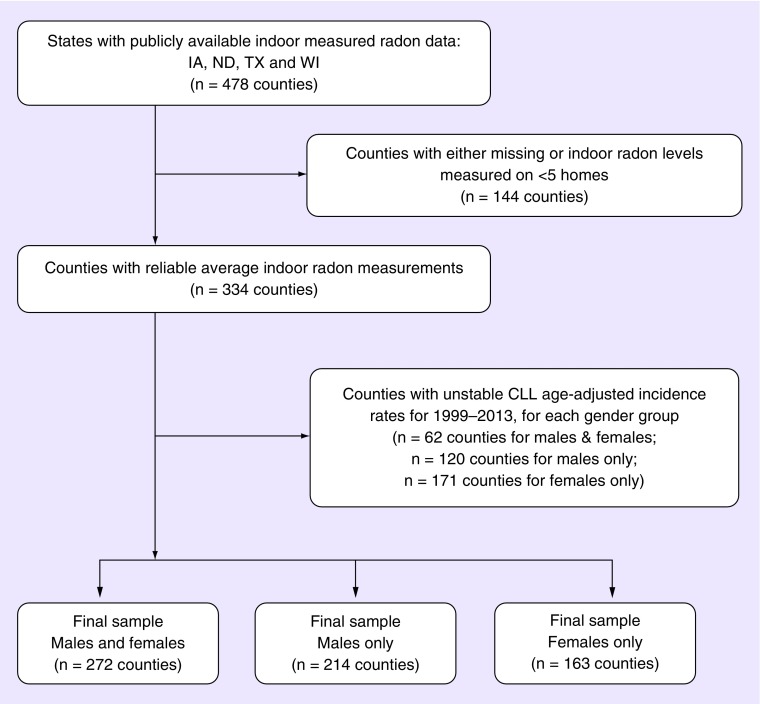
Data on county-level incidence rates and on radon levels were available for four states: Iowa (IA) [45], North Dakota (ND) [46], Texas (TX) [47] and Wisconsin (WI) [48], comprising 478 counties. Seventy (70) % of these counties had radon measurements performed on at least five residences. In total, 92, 86, 25 and 4% of counties had a mean residential level exceeding 4 pCi/l (respectively). Stable CLL age-adjusted incidence rates for 1999–2013 could be computed for 81% of counties for males and females, and for 64 and 49% of males and females only (Figure 1). The total number of CLL cases included was 22,811; 13,002 males and 8387 females (Table 1) (numbers do sum exactly due to the county censoring for each of the groups, as noted).
Figure 1. . Derivation of study population.
Table 1. . Numbers of CLL cases and age-adjusted incidence rates, 1999–2013.
|
Gender |
State |
Number of cases |
CLL age-adjusted incidence per 100,000 |
95% CI |
||
|---|---|---|---|---|---|---|
| Total | Median by county | IQR by county | ||||
| Males and Females |
Iowa |
3425 |
23 |
(17, 37) |
6.5 |
(6.2, 6.7) |
| Males and Females |
North Dakota |
639 |
22 |
(12, 40) |
6.7 |
(6.3, 7.2) |
| Males and Females |
Texas |
13605 |
52 |
(28, 117) |
5.0 |
(4.9, 5.1) |
| Males and Females |
Wisconsin |
5142 |
53 |
(31, 104) |
6.0 |
(5.9, 6.2) |
| Males |
Iowa |
1870 |
17 |
(13, 27) |
9.0 |
(8.6, 9.4) |
| Males |
North Dakota |
337 |
22 |
(11,44) |
9.2 |
(8.4, 10.1) |
| Males |
Texas |
7686 |
44 |
(26,93) |
6.8 |
(6.6, 6.9) |
| Males |
Wisconsin |
3109 |
32 |
(22, 70) |
8.4 |
(8.1, 8.7) |
| Females |
Iowa |
1090 |
15 |
(12, 24) |
4.5 |
(4.3, 4.8) |
| Females |
North Dakota |
194 |
26 |
(12, 31) |
4.7 |
(4.2, 5.3) |
| Females |
Texas |
5168 |
38 |
(24,74) |
3.7 |
(3.6, 3.8) |
| Females | Wisconsin | 1935 | 26 | (17,52) | 4.2 | (4.0, 4.3) |
CI: Confidence Interval for the age-adjusted CLL incidence; IQR: Inter-Quartile Range.
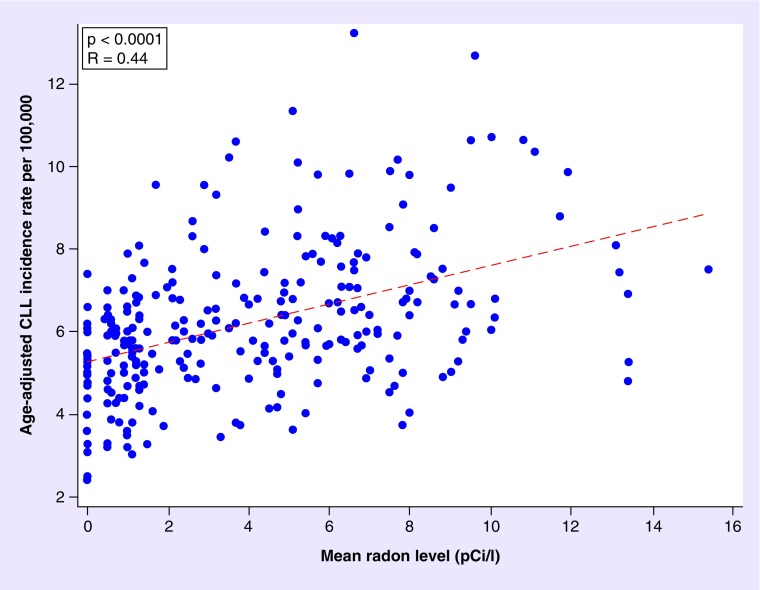
In bivariate analyses, age-adjusted CLL incidence rates were significantly positively correlated with mean RR overall (p < 0.0001) (Figure 2) and for males and females separately (p < 0.0001 and p < 0.0001). In multivariable analyses, the overall age-adjusted CLL incidence rate was significantly correlated with RR and this effect persisted after adjustment for latitude (p < 0.0001). We observed a significant interaction between gender and radon, adjusting for latitude (p = 0.0009). A one-unit increase in radon (in pCi/l) at a constant latitude was associated with an increase of approximately two cases of CLL/106 among females (95% CI: 1–3), and approximately four cases/106 among males (95% CI: 2–5) (Table 2). Conversely, latitude was not significantly correlated with CLL among males or females (p = 0.31 and p = 0.86, respectively).
Figure 2. . Indoor radon exposure levels and age adjusted chronic lymphocytic leukemia incidence rates in Iowa, North Dakota, Texas and Wisconsin during 1999-2013, by county, for both males and females.
Table 2. . The association between indoor radon exposure (using continuous measurement values) and age-adjusted CLL incidence, after adjustment for geographic latitude.
| Population | n | Variable | PE | 95% CI for the PE | p-value | Adjusted R-value |
|---|---|---|---|---|---|---|
| Males and Females | 272 | Radon level | 0.169 | (0.094, 0.244) | < 0.0001 | 0.46 |
| |
|
Latitude |
0.052 |
(0.012, 0.093) |
0.012 |
|
| Males only | 214 | Radon level | 0.364 | (0.237, 0.492) | < 0.0001 | 0.49 |
| |
|
Latitude |
0.036 |
(-0.033, 0.104) |
0.305 |
|
| Females only | 163 | Radon level | 0.198 | (0.111, 0.284) | < 0.0001 | 0.40 |
| Latitude | -0.004 | (-0.047, 0.039) | 0.859 |
CI: Confidence Interval; PE: Parameter estimate.
The results were similar using radon as a categorical variable, adjusting for latitude (Table 3). For males and females together, there were approximately 12 more cases of CLL/106 among counties with an RR value of >4 versus <2 pCi/l (p = 0.002). There was a significant adjusted interaction between both genders together and radon (p = 0.009). Among males, there were approximately 17 more cases of CLL/106 with an RR exposure level of >4 versus <2 pCi/l (p = 0.006), adjusting for latitude. Among females, there were approximately nine more cases of CLL/106 for an RR exposure of >4 versus <2 pCi/l (p = 0.033). There was no significant difference in the mean CLL incidence rates among counties with ‘Low’ versus ‘Medium’ RR exposure levels, either for both genders together or separately. Latitude was not significantly associated with CLL in any of the multivariable models.
Table 3. . The association between indoor radon exposure (using categorical measurement values) and age-adjusted CLL incidence, after adjusting for latitude.
| Population | n | Variable | PE | 95% CI for the PE | p-value | Adjusted R-value |
|---|---|---|---|---|---|---|
| Males and Females | 272 | Radon level | 0.43 | |||
| Low | REF | |||||
| Medium | 0.657 | (-0.150, 1.464) | 0.110 | |||
| High | 1.165 | (0.440, 1.891) | 0.002 | |||
| |
|
Latitude |
0.045 |
(-0.011, 0.101) |
0.113 |
|
| Males only | 214 | Radon level | 0.43 | |||
| Low | REF | |||||
| Medium | 0.248 | (-1.116, 1.611) | 0.721 | |||
| High | 1.699 | (0.481, 2.916) | 0.006 | |||
| |
|
Latitude |
0.077 |
(-0.018, 0.172) |
0.110 |
|
| Females only | 163 | Radon level | 0.31 | |||
| Low | REF | |||||
| Medium | 0.226 | (-0.652, 1.105) | 0.611 | |||
| High | 0.871 | (0.072, 1.671) | 0.033 | |||
| Latitude | 0.016 | (-0.046, 0.078) | 0.606 |
CI: Confidence Interval; PE: Parameter estimate.
Discussion
We studied counties from four states, including two (ND and IA) with the highest RR in the USA [38]. We found positive correlations between RR and CLL incidence rates for both genders, both together and individually. These associations remained significant after adjusting for UVR.
Given that we recently reported on the relationship between RR and CLL incidence rates at the state level, why re-examine it at the county level? The answer is that relationships observed at the state level do not guarantee similar relationships at lower levels. A vivid illustration that associations do not generalize ‘downward’ is the case of an indecisive jury: a jury that is indecisive does not imply that individual jurors are indecisive. Indeed, it is the resoluteness of the individual jurors that results in an indecisive jury. Similarly, it is possible that the significant correlations between radon and CLL rates across states are due to (confounding) factors other than a relationship at the county level. Thus, our findings that, in actuality, CLL levels increase as radon levels increase at the level of the county are an important extension of our previous findings.
These results are consistent with several prior ecological studies in the USA at the state level [38] and with findings from England and Wales at the county level [52]. Smith et al. [33] found a (nonsignificant) positive association between CLL incidence rates and county level radon values within Iowa. A recent cohort study among the Cancer Prevention cohort by Teras et al. [53] reported a significantly increased risk for interpolated radon levels by county and hematologic cancers in general but did not find a significant association for CLL. However, that cohort may have been underpowered to detect associations with high radon levels as it included few residents of high radon states. Conversely, our study included radon measurements from 99% of counties in IA and 93% of counties in ND, the states with the highest radon levels in the USA.
Studies of individuals exposed to short-term and high levels of ionizing radiation, such as Japanese atomic bomb survivors [54] or individuals exposed to therapeutic radiation [18,55,56] do not support an etiologic role for ionizing radiation in CLL. This contrasts with studies of uranium miners [19] and of participants in the cleanup at the Chernobyl nuclear site [21–23,57]. These conflicting results could be resolved if the mechanism for radon's effects on CLL differed from the mechanisms by which of other types of ionizing radiation cause cancer. One way in which radon differs from other forms of ionizing radiation is that the relevant route of exposure, with respect to lung cancer, is via inhalation. That is, radon-induced lung cancer involves the deposition of radon decay products in the bronchial epithelium where α particles damage the DNA of bronchial epithelial cells. B lymphocytes also are prevalent in the tracheobronchial epithelium and these lymphocytes could be affected analogously to bronchial epithelial cells [58]. Although the cell of origin of CLL is unknown, evidence suggests that it is a mature B lymphocyte [59]. Additionally, an increased number of apparently normal B cells (monoclonal B cell lymphocytosis) is a recognized precursor to CLL [60]. Rats exposed to radon show an increase in incorporation of bromodeoxyuridine (indicating increased proliferation) in respiratory tract macrophages. Similar findings have been described in normal human lung fibroblasts exposed to a low dose of α particles [61]. This suggests that another mechanism of radon's carcinogenicity on B lymphocytes may be via its role as a mitogen [62].
Our study has several limitations. First, this is an ecological (group level) study and its results cannot be generalized to individuals [63]. Second, paired data on county RR and CLL incidence rates were available only for a subset of the counties in the USA (478/3144 ≈ 15.2%). We had no information on residential history. Thus, it is possible that some individuals with CLL were exposed to leukemogens in one location but migrated to another where they were diagnosed. However, the effect of migration would be to reduce the size of any association, as it is unlikely that individuals with subclinical CLL migrate selectively to counties with high RR. Radon measurements were obtained via charcoal canisters placed in the residence for 2–7 days. These short term measurements are less accurate than long-term measurements (3 months to 1 year) and may introduce information bias. However, this bias also should be nondifferential and would skew the association between RR and CLL toward the null. We could not address the effects of other potential confounders, such as agricultural exposures. However, unlike CLL rates, which vary three-fold across ND and IA, 90% of the land in these states is farmland [64]. Thus, variation in agricultural exposures in these states is small and seems unlikely to account for the large differences in CLL incidence rates.
Conversely, our study has several strengths. To our knowledge, it is the first to investigate the association between RR exposure and CLL adjusting for the effects of UVR. Our ecologic study complements others [53] as it includes states with the highest levels of RR in the USA. Also, unlike studies in which radon levels were estimated or imputed [53,65], our study used actual radon measurements. Finally, we suggest plausible means by which CLL could be caused by radon.
Conclusion
Our findings at the county level confirm prior findings at the state level [38] and add to a growing body of literature that suggests that radon plays an etiologic role in CLL.
Future perspective
The epidemiologic investigation of cancers of unknown etiology often begins with studies of ‘place’ – locations where disease rates are unusually high, and ends with studies of ‘persons’. This approach has been successful in mesothelioma, where high rates in US counties with ship-building industries led to the identification of exposure to asbestos (used in insulating ships) as the cause [66]. Incidence rates for CLL in the USA are highest in Iowa and North Dakota, rural states where specialty medical care is scarce. Thus, increased healthcare-utilization is an unlikely explanation for these rates. Our analyses confirm that CLL incidence rates at a small scale are highly correlated with RR. The next generation of studies most move from ‘place’ to ‘person’ and determine whether exposure to radon among individuals is associated with increased risk of CLL.
SUMMARY POINTS.
The etiology of chronic lymphocytic leukemia (CLL), the most common adult leukemia in western populations, remains unknown. However, ionizing radiation is an etiologic factor in other leukemias and the traditional view that of CLL is an exception to this rule (i.e., is nonradiogenic) has been questioned.
There is a significant positive linear association between measured indoor radon levels and age-adjusted CLL incidence rates at the county level.
These findings are consistent for both men and women and remain so after adjusting for the possible confounding effect of ultraviolet radiation.
These ecological findings support the view that CLL may be radiogenic.
Acknowledgements
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103442.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Nabhan C, Rosen ST. Chronic lymphocytic leukemia: a clinical review. JAMA. 2014;312(21):2265–2276. doi: 10.1001/jama.2014.14553. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute; SEER Cancer Statistics Factsheets: Chronic lymphocytic leukemia.http://seer.cancer.gov/statfacts/html/clyl.html [Google Scholar]
- 4.Shanafelt TD, Rabe KG, Kay NE, et al. Age at diagnosis and the utility of prognostic testing in patients with chronic lymphocytic leukemia. Cancer. 2010;116(20):4777–4787. doi: 10.1002/cncr.25292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diehl LF, Karnell LH, Menck HR. The American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Data Base report on age, gender, treatment, and outcomes of patients with chronic lymphocytic leukemia. Cancer. 1999;86(12):2684–2692. [PubMed] [Google Scholar]
- 6.Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;109(8):3479–3488. doi: 10.1182/blood-2006-06-031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldin LR, Björkholm M, Kristinsson SY, Turesson I, Landgren O. Elevated risk of chronic lymphocytic leukemia and other indolent non-Hodgkin's lymphomas among relatives of patients with chronic lymphocytic leukemia. Haematologica. 2009;94(5):647–653. doi: 10.3324/haematol.2008.003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slager SL, Benavente Y, Blair A, et al. Medical history, lifestyle, family history, and occupational risk factors for chronic lymphocytic leukemia/small lymphocytic lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI. 2014;(48):41–51. doi: 10.1093/jncimonographs/lgu001. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alavanja MC, Hofmann JN, Lynch CF, et al. Non-Hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PLoS ONE. 2014;9(10):e109332. doi: 10.1371/journal.pone.0109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schinasi LH, De Roos AJ, Ray RM, et al. Insecticide exposure and farm history in relation to risk of lymphomas and leukemias in the Women's Health Initiative observational study cohort. Ann. Epidemiol. 2015;25(11):803–810. doi: 10.1016/j.annepidem.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karakosta M, Delicha EM, Kouraklis G, Manola KN. Association of various risk factors with chronic lymphocytic leukemia and its cytogenetic characteristics. Arch. Environ. Occup. Health. 2016;71(6):317–329. doi: 10.1080/19338244.2015.1116429. [DOI] [PubMed] [Google Scholar]
- 12.Cocco P, Brennan P, Ibba A, et al. Plasma polychlorobiphenyl and organochlorine pesticide level and risk of major lymphoma subtypes. Occup. Environ. Med. 2008;65(2):132–140. doi: 10.1136/oem.2007.033548. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand KA, Chang ET, Abel GA, et al. Sunlight exposure, vitamin D, and risk of non-Hodgkin lymphoma in the Nurses’ Health Study. Cancer Causes Control. 2011;22(12):1731–1741. doi: 10.1007/s10552-011-9849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Holford TR, Leaderer B, et al. Ultraviolet radiation exposure and risk of non-Hodgkin's lymphoma. Am. J. Epidemiol. 2007;165(11):1255–1264. doi: 10.1093/aje/kwm020. [DOI] [PubMed] [Google Scholar]
- 15.Cahoon EK, Pfeiffer RM, Wheeler DC, et al. Relationship between ambient ultraviolet radiation and non-Hodgkin lymphoma subtypes: a U.S. population-based study of racial and ethnic groups. Int. J. Cancer. 2015;136(5):E432–E441. doi: 10.1002/ijc.29237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamblin TJ. Have we been wrong about ionizing radiation and chronic lymphocytic leukemia? Leuk. Res. 2008;32(4):523–525. doi: 10.1016/j.leukres.2007.08.015. [DOI] [PubMed] [Google Scholar]; • The author, an established authority on chronic lymphocytic leukemia (CLL), concedes that the association between ionizing radiation and the incidence of CLL may have been dismissed with ‘less than adequate evidence’.
- 17.Linet MS, Schubauer-Berigan MK, Weisenburger DD, et al. Chronic lymphocytic leukaemia: an overview of aetiology in light of recent developments in classification and pathogenesis. Br. J. Haematol. 2007;139(5):672–686. doi: 10.1111/j.1365-2141.2007.06847.x. [DOI] [PubMed] [Google Scholar]
- 18.United Nations. Sources and Effects of Ionizing Radiation : United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2000 report to the General Assembly, with scientific annexes. United Nations; New York, NY, USA: 2000. Scientific Committee on the Effects of Atomic Radiation. [Google Scholar]
- 19.Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ. Health Perspect. 2006;114(6):818–822. doi: 10.1289/ehp.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson DB, Wing S, Schroeder J, Schmitz-Feuerhake I, Hoffmann W. Ionizing radiation and chronic lymphocytic leukemia. Environ. Health Perspect. 2005;113(1):1–5. doi: 10.1289/ehp.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramenko I, Bilous N, Chumak A, et al. Chronic lymphocytic leukemia patients exposed to ionizing radiation due to the Chernobyl NPP accident--with focus on immunoglobulin heavy chain gene analysis. Leuk. Res. 2008;32(4):535–545. doi: 10.1016/j.leukres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Romanenko AY, Finch SC, Hatch M, et al. The Ukrainian-American study of leukemia and related disorders among Chernobyl cleanup workers from Ukraine: III. Radiation risks. Radiat. Res. 2008;170(6):711–720. doi: 10.1667/RR1404.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zablotska LB, Bazyka D, Lubin JH, et al. Radiation and the risk of chronic lymphocytic and other leukemias among Chernobyl cleanup workers. Environ. Health Perspect. 2013;121(1):59–65. doi: 10.1289/ehp.1204996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Environmental Protection Agency. EPA assessment of risk from radon in homes. 2003. http://www2.epa.gov/sites/production/files/2015–05/documents/402-r-03–003.pdf
- 25.Agency for Toxic Substances and Disease Registry. ToxFAQs for radon. 2012. http://www.atsdr.cdc.gov/toxfaqs/tfacts145.pdf [PubMed]
- 26.WHO Handbook on Indoor Radon: A Public Health Perspective. Geneva: 2009. http://apps.who.int/iris/bitstream/10665/44149/1/9789241547673_eng.pdf [PubMed] [Google Scholar]
- 27.Barbosa-Lorenzo R, Barros-Dios JM, Raices Aldrey M, Cerdeira Carames S, Ruano-Ravina A. Residential radon and cancers other than lung cancer: a cohort study in Galicia, a Spanish radon-prone area. Eur. J. Epidemiol. 2016;31(4):437–441. doi: 10.1007/s10654-016-0134-x. [DOI] [PubMed] [Google Scholar]
- 28.Turner MC, Krewski D, Chen Y, Pope CA, 3rd, Gapstur SM, Thun MJ. Radon and nonrespiratory mortality in the American Cancer Society cohort. Am. J. Epidemiol. 2012;176(9):808–814. doi: 10.1093/aje/kws198. [DOI] [PubMed] [Google Scholar]
- 29.Ruano-Ravina A, Aragones N, Perez-Rios M, Lopez-Abente G, Barros-Dios JM. Residential radon exposure and esophageal cancer. An ecological study from an area with high indoor radon concentration (Galicia, Spain) Int. J. Radiat. Biol. 2014;90(4):299–305. doi: 10.3109/09553002.2014.886792. [DOI] [PubMed] [Google Scholar]
- 30.Messier KP, Serre ML. Lung and stomach cancer associations with groundwater radon in North Carolina, USA. Int. J. Epidemiol. 2017;46(2):676–685. doi: 10.1093/ije/dyw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axelson O, Fredrikson M, Akerblom G, Hardell L. Leukemia in childhood and adolescence and exposure to ionizing radiation in homes built from uranium-containing alum shale concrete. Epidemiology. 2002;13(2):146–150. doi: 10.1097/00001648-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Lubin JH, Linet MS, Boice JD, Jr, et al. Case-control study of childhood acute lymphoblastic leukemia and residential radon exposure. J. Natl Cancer Inst. 1998;90(4):294–300. doi: 10.1093/jnci/90.4.294. [DOI] [PubMed] [Google Scholar]
- 33.Smith BJ, Zhang L, Field RW. Iowa radon leukaemia study: a hierarchical population risk model for spatially correlated exposure measured with error. Stat. Med. 2007;26(25):4619–4642. doi: 10.1002/sim.2884. [DOI] [PubMed] [Google Scholar]
- 34.Raaschou-Nielsen O, Andersen CE, Andersen HP, et al. Domestic radon and childhood cancer in Denmark. Epidemiology. 2008;19(4):536–543. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- 35.Hauri D, Spycher B, Huss A, et al. Domestic radon exposure and risk of childhood cancer: a prospective census-based cohort study. Environ. Health Perspect. 2013;121(10):1239–1244. doi: 10.1289/ehp.1306500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Risco Kollerud R, Blaasaas KG, Claussen B. Risk of leukaemia or cancer in the central nervous system among children living in an area with high indoor radon concentrations: results from a cohort study in Norway. Br. J. Cancer. 2014;111(7):1413–1420. doi: 10.1038/bjc.2014.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong J, Qin L, Cao Y, et al. Environmental radon exposure and childhood leukemia. J. Toxicol. Environ. Health B Crit. Rev. 2012;15(5):332–347. doi: 10.1080/10937404.2012.689555. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz GG, Klug MG. Incidence rates of chronic lymphocytic leukemia in US states are associated with residential radon levels. Future Oncol. 2016;12(2):165–174. doi: 10.2217/fon.15.275. [DOI] [PubMed] [Google Scholar]; •• Reports a significant correlation between US state level CLL incidence rates and weighted state level residential radon.
- 39.Willett WC. Dietary fat intake and cancer risk: a controversial and instructive story. Semin. Cancer Biol. 1998;8(4):245–253. doi: 10.1006/scbi.1998.0076. [DOI] [PubMed] [Google Scholar]
- 40.Elliott P, Savitz DA. Design issues in small-area studies of environment and health. Environ. Health Perspect. 2008;116(8):1098. doi: 10.1289/ehp.10817. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows that the effects of confounding and misclassification inherent in correlations between large ecologic units are reduced among smaller ecologic units.
- 41.Cancer rates info home. http://www.cancer-rates.info/
- 42.National Cancer Institute; surveillance, epidemiology, and end results program (SEER); SEER*Stat databases. 2015. http://seer.cancer.gov/data/seerstat/nov2015/
- 43.North Dakota Statewide Cancer Registry. https://ndcancer.org/index.html
- 44.Day JC. U.S. Bureau of the Census, Current Population Reports; 1996. Population projections of the United States by age, sex, race, and hispanic origin: 1995 to 2050; pp. P25–1130. [Google Scholar]
- 45.Iowa Department of Public Health. Radon data by county. 2010. http://idph.iowa.gov/radon/resources
- 46.North Dakota Department of Health; Radon home survey in North Dakota - 1988. https://www.ndhealth.gov/aq/iaq/radon/Home88.htm
- 47.Texas Department of Health and Southwest Texas State University; Final report of the Texas indoor radon survey. 1994. pp. 15–20.
- 48.EPA's Map of Radon Zones; Wisconsin; 1993. U.S. Environmental Protection Agency; Office of radiation and indoor air; radon division; pp. 81–82. [Google Scholar]
- 49.Hu S, Ma F, Collado-Mesa F, Kirsner RS. Ultraviolet radiation and incidence of non-Hodgkin's lymphoma among hispanics in the United States. Cancer Epidemiol. Biomarkers Prev. 2004;13(1):59–64. doi: 10.1158/1055-9965.epi-03-0187. [DOI] [PubMed] [Google Scholar]
- 50.Maguire DJ. Encyclopedia of geographic information science. 2008.
- 51.U.S. Environmental Protection Agency. A citizen's guide to radon - the guide to protecting yourself and your family from radon. 2012. https://www.epa.gov/sites/production/files/2016–02/documents/2012_a_citizens_guide_to_radon.pdf
- 52.Alexander FE, Mckinney PA, Certwright RA. Radon and leukaemia. Lancet. 1990;335:1336–1337. [Google Scholar]
- 53.Teras LR, Diver WR, Turner MC, et al. Residential radon exposure and risk of incident hematologic malignancies in the cancer prevention study-II nutrition cohort. Environ. Res. 2016;148:46–54. doi: 10.1016/j.envres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Little MP, Weiss HA, Boice JD, Jr, Darby SC, Day NE, Muirhead CR. Risks of leukemia in Japanese atomic bomb survivors, in women treated for cervical cancer, and in patients treated for ankylosing spondylitis. Radiat. Res. 1999;152(3):280–292. [PubMed] [Google Scholar]
- 55.Radivoyevitch T, Sachs RK, Gale RP, Smith MR, Hill BT. Ionizing radiation exposures in treatments of solid neoplasms are not associated with subsequent increased risks of chronic lymphocytic leukemia. Leuk. Res. 2016;43:9–12. doi: 10.1016/j.leukres.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Silver SR, Hiratzka SL, Schubauer-Berigan MK, Daniels RD. Chronic lymphocytic leukemia radiogenicity: a systematic review. Cancer Causes Control. 2007;18(10):1077–1093. doi: 10.1007/s10552-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 57.Kesminiene A, Evrard AS, Ivanov VK, et al. Risk of hematological malignancies among chernobyl liquidators. Radiat. Res. 2008;170(6):721–735. doi: 10.1667/RR1231.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harley NH, Robbins ES. Radon and leukemia in the Danish study: another source of dose. Health Phys. 2009;97(4):343–347. doi: 10.1097/HP.0b013e3181ad8018. [DOI] [PubMed] [Google Scholar]
- 59.Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117(6):1781–1791. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parikh SA, Kay NE, Shanafelt TD. Monoclonal B-cell lymphocytosis: update on diagnosis, clinical outcome, and counseling. Clin. Adv. Hematol. Oncol. 2013;11(11):720–729. [PubMed] [Google Scholar]
- 61.Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60(5):1290–1298. [PubMed] [Google Scholar]
- 62.Taya A, Morgan A, Baker ST, Humphreys JA, Bisson M, Collier CG. Changes in the rat lung after exposure to radon and its progeny: effects on incorporation of bromodeoxyuridine in epithelial cells and on the incidence of nuclear aberrations in alveolar macrophages. Radiat. Res. 1994;139(2):170–177. [PubMed] [Google Scholar]
- 63.Porta M. A Dictionary of Epidemiology. Oxford University Press; Oxford, UK: 2008. [Google Scholar]
- 64.States by percentage of farmland. www.stuffaboutstates.com/agriculture/farm_by_percent.htm
- 65.Collman GW, Loomis DP, Sandler DP. Childhood cancer mortality and radon concentration in drinking water in North Carolina. Br. J. Cancer. 1991;63(4):626–629. doi: 10.1038/bjc.1991.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tagnon I, Blot WJ, Stroube RB, et al. Mesothelioma associated with the shipbuilding industry in coastal Virginia. Cancer Res. 1980;40(11):3875–3879. [PubMed] [Google Scholar]