Abstract
Recent advances in mitochondrial biogenesis have provided the emerging recognition that mitochondria do much more than ‘simply providing energy for cellular function’. Currently, a constantly improving understanding of the mitochondrial structure and function has been providing valuable insights into the contribution of defects in mitochondrial metabolism to various human diseases, including chronic obstructive pulmonary disease and lung cancer. The growing interest in mitochondria research led to development of new biomedical fields in the two main smoking-related lung diseases. However, there is considerable paucity in our understanding of mechanisms by which mitochondrial dynamics regulate lung diseases. In this review, we will discuss our current knowledge on the role of mitochondrial dysfunction in the pathogenesis of chronic obstructive pulmonary disease and non-small-cell lung cancer.
Keywords: : chronic obstructive pulmonary disease, mitochondria, mitochondrial dysfunction, non-small-cell lung cancer
Background
Mitochondria are the only subcellular organelle with their own DNA (mtDNA) that are hallmarks of eukaryotes [1]. The major tasks of mitochondria include the production of ATP and the metabolites necessary to fulfill the bioenergetics and biosynthetic demands of the cell. Based on these crucial roles, mitochondria are regarded as the ‘powerhouse of the cell’ [2]. In addition to later two major roles, mitochondria are involved in other essential tasks, such as cellular signaling, differentiation and cell death, as well as maintaining control of the cell cycle and cell growth [2–4]. Mitochondria are also widely recognized as a major endogenous source of reactive oxygen species (ROS), which have important roles in cell signaling and homeostasis, during their normal oxidative metabolism. Although optimal level of ROS production is necessary for normal oxidative metabolism, excessive ROS has adverse effect on cell integrity and can damage other important molecules such as lipids, proteins and DNA [5–7]. Thus, defects in the complex and dynamic mitochondrial function, including metabolic alterations, have been implicated in several human diseases such as diabetes, atherosclerosis, neurodegenerative diseases including Parkinson's and Alzheimer's diseases, and several inherited conditions [7–11].
The two main smoking-related lung diseases, chronic obstructive pulmonary disease (COPD) and lung cancer, are major health concerns worldwide. Despite the advances in understanding of the molecular processes underlying these diseases, the prognosis remains alarming over the last few decades. Unfortunately, by using current approaches it is difficult to diagnose COPD until it is clinically apparent and moderately advanced [12]. The WHO has predicted a rise of COPD mortality, from the fourth leading cause of death in 2004 to the third in 2030 [13]. Despite current measures for management of COPD patients, such as smoking cessation, combination of inhaled corticosteroid with long-acting β2 agonist or long-acting muscarinic antagonist, and several novel anti-inflammatory drugs, many COPD patients are still suffering from persistent and progressive pulmonary inflammation [14]. Similarly, lung cancer is one of the leading causes of cancer-related mortality. The prognosis of patients with lung cancer has improved only minimally during the past two decades, with overall 5-year survival rates between 14 and 16% [15]. Moreover, most novel therapeutic agents for non-small-cell lung cancer (NSCLC) target a single molecular aberration, while the majority of patients will either not possess that particular mutations or harbor tumors with multiple abnormalities [16]. Eventually, these reasons often lead to drug resistance after initial benefit through a variety of mechanisms. This poses an unmet need to develop new strategies for finding more robust biomarkers of early diagnosis and more effective novel drugs for these two major lung diseases.
COPD and lung cancer are both associated with chronic inflammation and oxidative stress [17–21]. Generally, cigarette smoke (CS) is an environmental pollutant that contains more than 5000 chemical compounds [22], which are well-known sources of ROS such as hydrogen peroxide, superoxide anion and hydroxyl radicals [23]. Majority of these compounds have not only possessed mutagenic properties but also possess carcinogenic activity [22]. In addition to the exogenous source of ROS that implies CS by itself, endogenous production of ROS is also increased among smokers [24]. As mitochondria are the major endogenous source of ROS, it is reasonable to assume that mitochondrial dysfunction may contribute to the pathogenesis of COPD and lung cancer. Over the last decade, studies of mitochondria have contributed to better understanding of its function and led to development of new biomedical fields in several human diseases. Recently, several excellent reviews on mitochondrial biology in relevance to lung diseases, including lung cancer, have been published [25–28]. However, there is considerable paucity in our understanding of mechanisms by which mitochondrial dynamics regulate lung diseases. Therefore, in this review we will discuss our current knowledge on the role of mitochondrial dysfunction in the pathogenesis of COPD and NSCLC.
Mitochondria
Structure of the mitochondria
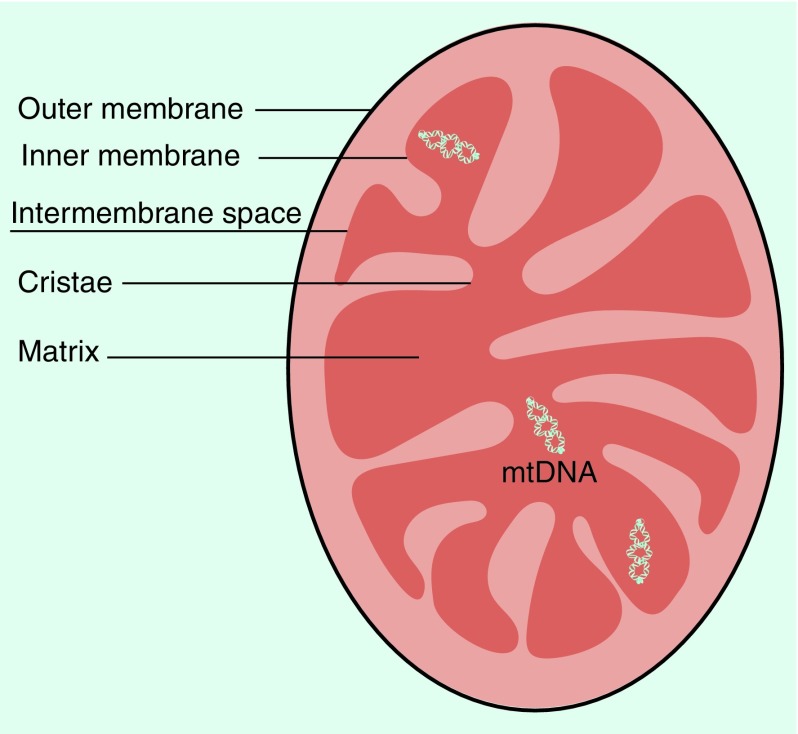
Mitochondria contain two membranes that separate four distinct compartments; the outer membrane (OMM), the inner membrane (IMM), the intermembrane space and the matrix (Figure 1). The IMM is highly folded into cristae, which houses the mega dalton complexes of the electron transport chain and ATP synthase that control the basic rates of cellular metabolism [2]. The production of ATP is generated by means of the electron-transport chain and the oxidative-phosphorylation system (also known as ‘respiratory chain’) [11]. The respiratory chain is located along the IMM and consists of five multimeric protein complexes, termed complex I (nicotinamide adenine dinucleotide-Coenzyme Q oxidoreductase, approximately 46 subunits), complex II (succinate-Coenzyme Q oxidoreductase, 4 subunits), complex III (Q-cytochrome c oxidoreductase 11 subunits), complex IV (cytochrome c oxidase, 13 subunits) and complex V (ATP synthase, approximately 16 subunits) [11,29,30].
Figure 1. . The basic structure of the mitochondrion.
Mitochondria are enveloped by two highly specialized lipid membranes, with four distinct parts; the outer membrane, the inner membrane, the intermembrane space and the matrix. The highly folded and convoluted inner membrane, called cristae, extended into the inner mitochondria matrix.
mtDNA
Mitochondria are the only subcellular organelles with their own mtDNA, and they are under the dual genetic control of both nDNA and mtDNA [31]. The human mtDNA is a double-stranded circular molecule of 16,569 nucleotides, which contains 37 genes. Of these, 24 are encoded for mitochondrial translation (two ribosomal RNAs [rRNAs] and 22 transfer RNAs [tRNAs]) and 13 are encoded subunits for the respiratory complexes I, III and IV; only complex II is solely composed of proteins encoded by nuclear genes [32,33]. mtDNA is exclusively maternally inherited and can replicate independently of nDNA. While nDNA is protected by histones which shield it from a variety of potentially dangerous ROS, mtDNA lack such protection, and it is therefore more ROS susceptible [31,34].
The advantages of mtDNA mutation detection as a potential biomarker for disease come from the differences between mtDNA and nDNA. The mtDNA is characterized by its small size (16.5 kb) harboring 37 densely packed genes and high copy number (several hundreds to thousands copies per cell). It is a distinct advantage over nDNA for the detection in precious clinical samples. In addition, because mitochondria have lacking protective histones and less efficient repair mechanism than nuclear genomes, mutations may accumulate in the coding regions and are more likely to have biological consequences [35]. Therefore, analyses of mtDNA mutation can be used as a potential biomarker for early detection, monitoring of disease progression and response to therapy, or predictive stratification of patients for treatment.
Overview of mitochondrial roles in eukaryotic cells
Mitochondria are found in all eukaryotic cells and play many important roles in human pathophysiology. The most important role of mitochondria is to produce cellular ATP by oxidative phosphorylation, a process that requires the orchestrated actions of five respiratory enzyme complexes [11,36]. The central set of reactions involved in ATP production are collectively known as the citric acid cycle (also known as the tricarboxylic acid cycle or the Krebs cycle). Mitochondria are also involved in orchestrating other metabolic processes, such as signaling through ROS [37], regulation of apoptosis [4], calcium signaling [38], heme and steroid synthesis [39,40], cellular differentiation and growth, and cell cycle control. However, some of those mitochondrial functions are selective for specific cell types [2,8,41].
Mitochondrial dynamics & mitophagy
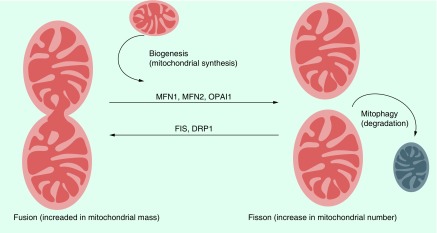
Mitochondria are dynamic intracellular organelle. The structure and number of mitochondria can dramatically change in response to their physiological state through constitutive cycles of fusion and fission (collectively termed mitochondrial dynamics). Mitochondria are also actively transported in cells and can have defined subcellular distributions [42,43]. The field of mitochondrial dynamics was established by Lewis et al., who described it as: “Any one type of mitochondria such as a granule, rod or thread may at times change into any other type or may fuse with another mitochondrion, or it may divide into one or several mitochondria” [8,44]. Biesele et al. first observed the elongation and disjoining of mitochondria caused by coenzyme A, suggesting changes in the metabolic activity of the cell [45]. Mitochondrial fusion, which is a fundamental process in life of eukaryotic cells, plays a central role in maintaining intact mtDNA copies and oxidized proteins, mitochondrial membrane components and matrix metabolites, and results in a more interconnected mitochondrial network. In contrast, mitochondrial fission, creates smaller, more discrete mitochondria and takes part in segregation of dysfunctional mitochondria from the mitochondrial pool [8,42,43,46]. A simplified representation of the mitochondrial fusion–fission cycle is depicted in Figure 2. Mitochondrial fusion and fission are antagonistic activities and mediated by a small number of highly conserved, guanosine triphosphatases (GTPases) [8,47–49]. Thus, the rates of fusion and fission must be tightly controlled to adjust the homeostasis of cell mitochondria depending on changing physiological conditions [8,42,43]. Although this tightly regulated system is not completely understood, some of the key proteins involved have been identified (Table 1). Fusion is mediated by MFN1 and MFN2 isoforms in the OMM and by OPA1 protein in the IMM [8,50]. Fission is mediated by DRP1, a cytosolic protein that translocates to the OMM upon activation. DRP1 is actively targeted to the OMM by non-GTPase receptor proteins such as FIS1, MFF and mitochondrial elongation factor [8,51].
Figure 2. . A simplified fusion–fission cycle of mitochondria.
Mitochondria are dynamically regulated to maintain cellular homeostasis. Mitochondrial biogenesis increases mitochondrial numbers and replaces damaged mitochondria. MFN1 and MFN2 and OPA1-mediated fusion dilutes damage that accumulates in the mitochondria, including mitochondrial DNA mutations and reactive oxygen species. In contrast, the excessive mitochondrial damages can promote fission mediated through DRP1 and FIS1. Repaired polarized mitochondria re-enter the mitochondrial pool by fusion, and remaining depolarized mitochondria are transported to lysosomes for elimination by mitophagy (autophagy).
Table 1. . The key proteins involved in mediation of mitochondrial fusion and fission.
| Protein | Function | Localization | Ref. |
|---|---|---|---|
| Fusion proteins: | [8,50,51] | ||
| –Mitofusin-1 and -2 | GTPase protein, tethers adjacent mitochondria | OMM | |
| –Optic atrophy 1 | GTPase protein, mediates fusion of inner mitochondrial | IMM | |
| –Mitochondrial elongation |
DRP1-targeting protein, recruits DRP1 to mitochondria but inhibits its function, promoting fusion |
OMM |
|
| Fission proteins: | [8,42] | ||
| –Dynamic-related protein 1 | GTPase protein, translocates to the OMM when activated | Mostly cytosol | |
| –Mitochondrial fission 1 | DRP1-targeting protein, recruits DRP1 from the cytosol to the mitochondria | OMM | |
| –Mitochondrial fission factor | DRP1-targeting protein, recruits DRP1 during mitochondrial fission | OMM |
GTPase: Guanosinetriphosphatase; IMM: The inner membrane; OMM: The outer membrane.
Autophagy is a process of self-degradation of cellular components, such as the cytosol, organelles and protein aggregates, through their encapsulation by a double-membrane autophagosome. The specific autophagic elimination of mitochondria is termed as mitochondrial autopghagy (also referred to as mitophagy). Mitophagy is thought to serve as an intrinsic mitochondrial quality control mechanism. Mitochondrial fusion is important for the dissipation of metabolic energy and for the complementation of mtDNA gene products in heteroplasmic cells, whereas damaged mitochondria are separated from the mitochondrial network by fission and subsequently degraded by mitophagy [42,52]. Emerging evidence suggests that mitophagy can be either protective or deleterious in the lung of COPD.
Mitochondrial ROS
ROS are oxygen (O2) derived molecules that can readily oxidize other molecules. In general, free radicals and other ROS are generated in a wide range of normal physiological conditions. Historically, accumulation of ROS and oxidative stress have been linked to multiple human diseases and mitochondrial ROS were exclusively thought to cause cellular damage and were considered to lack a physiological function [6]. However, substantial evidences have demonstrated that production of mitochondrial ROS has evolved as a method of communication between mitochondrial function and other cellular processes to maintain homeostasis and promote adaptation to stress [6,37,53]. The major source of endogenous ROS is mitochondrial electron transport chain during normal metabolism. The content of mitochondrial ROS is determined by the rates of both mitochondrial ROS production and disposal, and it is tightly regulated by a number of physiological cell signaling, such as mitochondrial membrane potential, metabolic state of mitochondria and O2 levels [6,37]. Optimal mitochondrial ROS production has important role in regulating the range of cell homeostasis processes, including adaptation to hypoxia, autophagy, immunity, differentiation and aging [6]. However, the production of mitochondrial ROS has been reported to be increased in a variety of pathologic conditions, such as hypoxia, ischemia, reperfusion, aging and chemical inhibition of mitochondrial respiration [5,54–56]. Recently, accumulating data have demonstrated that the excessive production of mitochondrial ROS may critically mediate several core autophagic pathways in cancer initiation and progression [57].
Mitochondria & apoptosis
Apoptosis is the process of programmed cell death that serves as a major mechanism for the precise regulation of cell numbers, and as a defense mechanism to remove unwanted and potentially dangerous cells. However, the inappropriate activation of apoptotic process may cause or contribute to a variety of pathologic conditions [58–60]. Mitochondria play key roles in regulating apoptosis in mammalian cells [4]. Mitochondria can trigger cell death in a number of ways; disrupting electron transport and energy metabolism, by releasing or activating apoptosis-related proteins such as cytochrome c and apoptosis-inducing factor, and by altering cellular redox potential [4,61,62]. These processes are subjected to a complex regulation involving multiple proteins that, among the other roles, maintain the integrity of mitochondrial compartments. Due to its fundamental importance, the multifaceted role of mitochondria in orchestrating apoptotic machinery has been discussed in great details in a numerous focused review [63–67]. Although significant advances have been made over the last 20 years in our understanding of the mitochondrial apoptotic pathway, a series of recent studies were undertaken to re-evaluate its role in cancer cell growth and progression. While discussing all that is known on the relationship between mitochondrial apoptosis and cancer is beyond the scope of this manuscript, it is crucial to ‘stress out’ that molecular networks underlining this mechanisms may have potential translational applications [68,69]. Development of the new approaches that BH3 mimetic was a milestone moment and is being tested in clinical trials. Thus, studying the role of mitochondria in apoptotic processes may allow further insight into how mitochondrial defects contribute to the pathogenesis of human diseases, including cancer.
Mitochondrial & human diseases
Nearly a century ago, mitochondrial dysfunction has been observed in cancer cells. This observation, known as the ‘Warburg effect’ or ‘aerobic glycolysis’, is that cancer cells take up glucose and produce lactic acid in the presence of oxygen [70–72]. The first human mitochondria disease and pathogenic mtDNA mutations were identified in 1962 and 1988, respectively [73–75]. These observations lead to growing interest for the basic scientist and translational researcher to further study the role of mitochondrial genome in different human diseases and disseminate that knowledge into clinical practice. Currently, more than 250 pathogenic mtDNA mutations have been identified in a wide variety of human diseases including cancer, with a heterogeneity of phenotypes and a variable age of onset [10,31,76]. Primary respiratory chain diseases could be initiated and progress as a result of mtDNA mutations or mutation of nuclear genes encoding respiratory chain subunits. The most common respiratory chain disorders are also known as mitochondrial encephalopathy, and involve brain, nervous system and skeletal muscle [9,11]. In addition, an emerging concept suggests that disordered mitochondrial dynamics are directly or indirectly involved in pathogenesis of complex diseases including cancer, cardiovascular and neurodegenerative conditions, that are not classically considered to involve mitochondria [8,9,25–28,32,77,78].
Mitochondrial dysfunction in COPD
Overview of COPD
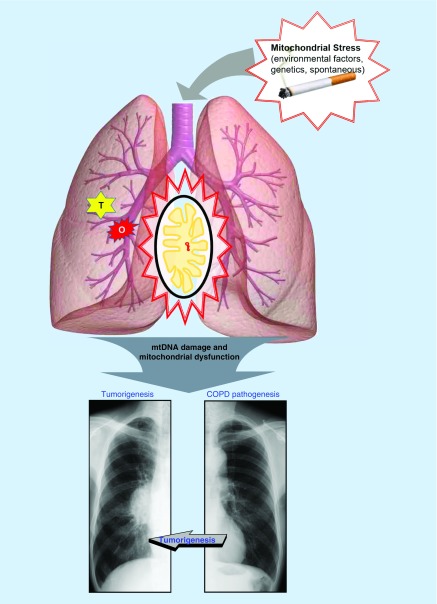
COPD is characterized by progressive, irreversible airflow limitation resulting from an abnormal and chronic inflammatory response of the lungs to noxious particles and gases [12]. COPD is a multidimensional disease, with pathologic changes in the large and small airways (chronic bronchitis and bronchiolitis) and lung parenchyma (emphysema). The pathologic changes vary greatly in their expression among patients. Several pathogenetic mechanisms contribute to the development of COPD. First, influx of noxious particles (such as CS) in the small airway and alveoli may lead to abnormal inflammatory response, provoking activation of various inflammatory mediators (such as macrophages, neutrophils and CD8+ T lymphocytes) that participate in the inflammatory response in the airways of COPD patients. Second, there is a disruption of the balance between proteolytic and antiproteolytic molecules in the lung. An increased proteolytic activity promotes emphysema. This response may be a consequence of inflammation or may arise from genetic factors (e.g., α-1 antitrypsin deficiency). A third mechanism is oxidative stress, which arises as a result of endogenous antioxidant defenses being genetically impaired and/or overwhelmed by the presence of ROS. Oxidants are generated in the airways by CS or are released from inflammatory and epithelial cells. Oxidative stress can lead to cell dysfunction or cell death and can induce damage to the lung extracellular matrix. Recent advances in understanding the COPD pathogenesis have suggested a fourth mechanism; disruption of the balance between apoptosis and clearance of apoptotic cells in the lung. Several studies in COPD patients with emphysema describe an increase in apoptosis, support an important role for apoptosis in the pathogenesis of COPD. These findings suggest that COPD pathology deteriorates even after smoking cessation may be related to increased apoptosis in the airways of COPD patients even after smoking cessation. However, more studies are needed to identify the most important apoptotic pathways in the development of COPD, including also the chronic bronchitis component of COPD [79,80]. These four processes involved in the pathogenesis of COPD are not independent mechanisms and several interactions between these processes occur during disease development [18,80–84]. Thus, by virtue of COPD pathogenesis (e.g., involvement of ROS production and apoptosis), mitochondria may play a distinctive role in the development and progression of COPD (Figure 3).
Figure 3. . The schema of the development of chronic obstructive pulmonary disease and lung cancer.
Mitochondrial stress [48], such as environmental (radiation, toxic chemicals), genetics (mutations in genes for metabolic processes or repair pathways) and spontaneous (ROS generated as byproduct of electron transport), can cause mtDNA damage or mutation, and subsequently lead to mitochondrial dysfunction. The mitochondrial dysfunction in airways and lung parenchyma can enhance the major chronic obstructive pulmonary disease (COPD) pathogenesis, including imbalance of proteolysis, disruption of apoptosis, oxidative stress and chronic inflammation response. These processes eventually lead to COPD, which is characterized by mucous hypersecretion, tissue destruction and/or airway inflammation and fibrosis (x-ray shows hyperinflated lungs, flattened diaphragms, diminished vascular markings suggest emphysema). Mitochondrial dysfunction can be induced by some oncogenes (O) and tumor suppressor genes (T), similar to the major COPD pathogenesis, can induce tumorigenesis in the lung and eventually can lead to lung cancer (x-ray shows right hilar mass which on biopsy came out to be squamous cell carcinoma).
Mitochondrial dysfunction & COPD
Since the first evidence that large aggregates of mitochondria were found in diaphragm muscle of COPD patient [85], many studies of muscle dysfunction in COPD have shown that significant skeletal muscle mitochondrial dysfunction occurred among COPD patients [86]. A study using muscle biopsies of mild-to-moderate COPD reported that skeletal and respiratory muscles of COPD patients demonstrate increased cytochrome oxidase activity and excessive ROS production. These results suggest that mitochondrial dysfunctions occur in relation to systemic factors and already present at early stage of disease [87]. More recent study suggests that there are three major mitochondrial dysfunctions related to muscle dysfunction in COPD [86], including reduction in oxidative capacity [88], enhanced mitochondrial ROS production [89] and increased autophagy and apoptosis [90,91]. The peroxisome proliferator-activated receptors (PPARs) and PPAR-γ coactivator (PGC)-1α are key regulators of mitochondrial biogenesis and hence of skeletal muscle oxidative capacity [92]. In several studies, expression of PPARs and PGC-1α, as well as TFAM, which maintains mtDNA copy number by regulating mtDNA replication and transcription in proportion to muscle oxidative capacity, was shown to be reduced in peripheral skeletal muscle of patients with moderate-to-severe COPD and muscle weakness [86,92,93]. These results proposed that PGC-1α and the PPAR signaling pathways may represent a novel class of potential targets for pharmacological agents that could be helpful in the management of COPD [86,93].
Prohibitin (PHB) complexes, essential components of the mitochondrial fusion machinery, are predominately localized in the IMM, and maintaining normal mitochondrial function and morphology. They have a diverse role in pathogenesis of several diseases such as cancer, inflammatory bowel disease, diabetes and obesity [94,95]. The first study of PHB level in COPD lung tissue showed significant downregulation of PHB1 in COPD and non-COPD smokers in comparison to nonsmoker, especially in the mitochondria of the COPD patients. Moreover, PHB1 levels were associated with the degree of airway obstruction [96]. The iron-regulatory proteins (IRPs) regulate cellular iron homeostasis and serve as the major regulatory proteins in mammalian cells. IRP2 is identified as a leading candidate COPD-susceptibility gene in humans, as it is often increased in the COPD lungs. Furthermore, IRP2 is located on human chromosome 15q25, which has been associated with lung cancer [97]. The subsequent study showed that IRP2 promotes mitochondrial dysfunction in experimental COPD, where mice treated with a mitochondrial iron chelator were protected from CS-induced COPD, suggesting a critical functional role and potential therapeutic intervention for the mitochondrial-iron axis in COPD [98].
The changes of mitochondrial structure in COPD were first studied in bronchial epithelial cells. Mitochondrial changes including depletion of cristae, increased branching and elongation and swelling of the mitochondria were observed GOLD IV COPD epithelium. Furthermore, long-term CS exposure significantly increased the expression of specific fission/fusion markers, oxidative phosphorylation (OXPHOS) proteins and oxidative stress markers. These changes were accompanied by increased levels of the pro-inflammatory mediators [99]. Another study showed that airway smooth muscle cells from COPD patients have decreased mitochondrial membrane potential, ATP content and lower complex protein expression, whereas mitochondrial ROS levels were increased. In addition, in a mouse model of oxidative stress induced by chronic exposure to ozone, prophylactic treatment with mitochondrial-targeted antioxidant MitoQ restored mitochondrial function in lung tissue, prevented airway hyper-responsiveness and precluded the presence of inflammatory cells and mediators [100]. These results further support the notion that mitochondrial dysfunction may play a key role in COPD pathogenesis and potentially provide a novel therapeutic target for COPD maintenance and prevention.
There is now considerable interest in autophagy processes in lung disease, particularly relevant to the effects of inflammation. Recently, in human COPD, lung epithelial cells displayed increased expression of the mitophagy protein PINK1, the necroptosis regulator RIP3 and the fission regulator DRP1. These finding implicate mitophagy-dependent necroptosis in lung emphysematous changes in response to CS exposure [101,102]. Another study showed that CS exposure in vitro augmented cellular senescence in lung epithelial cells and fibroblasts, mouse lung as well as human smokers and patients with COPD. Moreover, mitochondria-targeted antioxidant (Mito-Tempo) restored impaired mitophagy, decreased mitochondrial mass accumulation and delayed cellular senescence in Parkin-overexpressing cells. This finding is the first report that the mechanisms of defective mitophagy lead to accumulation of dysfunctional and damaged mitochondria [103]. These results provide new insight into the role of mitophagy in pathogenesis of COPD and promising therapeutic strategies in COPD.
Mitochondrial dysfunction in NSCLC
Overview of NSCLC
Lung cancer, which arises from the cells of the respiratory epithelium, can be divided into two major histologic categories. Small cell lung cancer is a highly malignant tumor derived from cells exhibiting neuroendocrine characteristics and accounts for 15% of lung cancer cases. NSCLC, which accounts for the remaining 85% of cases, is further divided into three major histologic subtypes: adenocarcinoma (AD), squamous cell carcinoma (SCC) and large cell carcinoma [104,105]. The development of molecular profiling of NSCLC, especially in AD [106], has led to the emergence of ‘personalized therapy’, which provides the potential to tailor medical care both at tumor and patient levels [105]. However, the majority of patients develop resistance to these targeted therapies over time, resulting in disease progression. These facts have given rise to the term ‘precision medicine’, an emerging approach for disease treatment and prevention which takes into account more refined and effective therapies based on individual diagnostic tests and individual genetic analyses [107].
Mitochondrial dysfunction & NSCLC
The causal link between inflammation and cancer have been suggested by Virchow in the 19th century [108]. Currently, genetic instability resulting from chronic inflammation represents the seventh hallmark of tumorigenesis [109]. Chronic inflammation may result in superfluous ROS. ROS-induced mtDNA damage is implicated in a wide range of pathologic process, including carcinogenesis, aging and degenerative diseases. Traditional oncogenes and tumor suppressor genes, such as RAS, MYC and TP53, can induce altered mitochondrial function including increased electron transport, oxygen uptake and ROS production [20,110]. TP53 point mutations are widely evident in the respiratory epithelium of smokers and asbestos-exposed individuals [111].
Several mtDNA mutations have been reported in various types of human cancers including lung [10]. The mtDNA D-loop is important for regulation of mitochondrial genome replication and expression. Some studies reported that D-loop alterations are frequent in NSCLC and SNPs in mtDNA D-loop are found to be independent prognostic markers for NSCLC outcome [112,113]. The prevalence of mtDNA mutations in lung cancers of never-smokers patients, as compared with the current-smokers, was significantly higher, and were associated with mutation at exons 19 and 21 of the EGFR gene [114]. Furthermore, increased mtDNA copy number was found to be associated with subsequent risk of lung cancer among heavy smokers [115]. These results suggest that further studies of signature mtDNA alterations may provide new strategies for detecting and managing lung cancer that occur among nonsmokers and heavy smokers.
Recent study of altered mitochondrial dynamics in lung cancer has reported that human AD tissues and cell lines have reduced MFN2 and increased DRP1 expression, mitochondrial fusion and fission mediators, respectively. Furthermore, lung cancer cells treated with inhibitors of DRP1 or enhancers of MFN2, showed a marked reduction in cancer cell proliferation and increase in spontaneous apoptosis. Whereas in xenotransplantation model, it was found to induce the regression of lung tumor [116]. Another study has reported that increased DRP1 expression in lung AD correlates with poor prognosis [117]. OPA1 is involved in the IMM fusion and anticancer drug-mediated cytotoxicity. High level of OPA1 expression is frequently found in lung AD and is associated with poor prognosis. Notably, OPA1 expression correlates with expression levels of DRP1 and MFN1/2. Silencing of OPA1 led to reduced cisplatin resistance and activation of caspase-dependent apoptotic pathway in cell lines [118]. The focal adhesion protein paxillin (PXN) was reported to be mutated, amplified and overexpressed in NSCLC, with higher levels seen in more advanced stages of the disease [119]. PXN mutants were associated with anti-apoptotic protein BCL-2, which is known to localize to the mitochondria, and with DRP1 overexpression. Although overexpression of either wild-type or mutated paxillin increased cell's resistance to cisplatin, clinically, NSCLC patients with PXN mutations have poorer survival rates relative to patients with wild-type PXN [120]. More recent study showed that inhibition of the mitochondrial chaperone HSP90-like protein is leading to decreased NSCLC cell migration and invasion in vitro [121]. These data suggest that mitochondrial dynamics play a complex role in cancer cell proliferation, migration and survival.
Mitochondrial dysfunction in NSCLC with COPD
The relationship between COPD & NSCLC
It is well established that CS is a major contributor to COPD and NSCLC. Patients with these conditions share a common environmental risk factor (such as CS) as well as genetic predisposition represented by the incidence of these diseases in only a fraction of smokers (Figure 3) [122]. Clinically, both can remain silent until late in the diseases process. Generally, cancer is characterized by anti-apoptotic processes, unlimited cell proliferation and sustained angiogenesis, whereas COPD is characterized by increased apoptosis, extracellular matrix degradation and limited angiogenesis [122]. In this regard, these diseases have been considered as two different manifestations of the same condition, two sides of the same coin [122–124]. Thus, understanding of molecular alterations that are either shared or difference between COPD and NSCLC may be extremely informative for managing both conditions.
Interestingly, COPD itself is an independent risk factor for lung cancer. The COPD increases the risk of lung cancer up to 4.5-fold [125–127]. Furthermore, the presence of emphysema is associated with poor prognosis in lung cancer patients [128]. Particularly, smokers with COPD have a higher risk of developing an SCC histological subtype of NSCLC [129]. Recently, large international case–control consortium reported an investigation into the association between multiple previous respiratory diseases (PRDs) and lung cancer. Findings of this study have shown that co-occurrence of chronic bronchitis and emphysema and/or pneumonia had a stronger positive association with lung cancer than chronic bronchitis ‘only’, and emphysema was found to have stronger association with lung cancer, compared with chronic bronchitis as well as other PRDs. These findings are consistent with previous pooled analysis [130]. Lung cancer is also a leading cause of morbidity and mortality in COPD patients [131]. Thus, a better understanding of the potential pathogenetic mechanisms, including field carcinogenesis, epithelial–mesenchymal transition, chronic inflammation, genetic or epigenetic abnormalities including DNA methylation and telomere shortening, and pulmonary stem cells [19,122,123,132], leading to lung cancer in COPD is of great clinical interest.
Although little is currently known about epigenetic mechanism linking lung cancer and COPD susceptibility, a few data indicate that CS induce cancer associated epigenomic alterations in respiratory epithelium. These findings include activation of Wnt signaling, a diminution of H4K16Ac and H4K20Me3 marks, increasing levels of H3K27Me3, and hypomethylation including that of D4Z4, NBL2 and LINE-1, repetitive DNA sequences along with gene-specific hypermethylation of tumor suppressor genes (TSGs) such as RASSF1A and RAR-β. Furthermore, aberrant methylation of the p16 tumor suppressor gene and/or O6-methylguanine-DNA methyltransferase promoters, and the DNA hypermethylation status of the p16, CDH13, RASSF1A and APC genes have been proposed as a biomarker for early detection of NSCLC [133].
Mitochondrial dysfunction & NSCLC with COPD
A study of gene expression signature in lung SCC patients with and without COPD has demonstrated a significant overexpression of genes related to mitochondrial localization in SCC patients with COPD [134]. Another study reported that expression of mtTFA, which promotes transcription of mtDNA and regulates mtDNA replication, is lower in the SCC with COPD group when compared with SCC patients without COPD history. Notably, expression of the mtTFA protein positively correlated with pulmonary function (FEV1), and negatively correlated with apoptic and smoke index [135].
Conclusion & future perspective
It has been over a century since mitochondria first captured the attention of scientists [70,72]. Although an interest in mitochondria was waning over the years, accumulating evidence of novel, previously unknown roles of mitochondria, such as regulation of cellular apoptosis, has led to a turning point and provoked growing interest in mitochondria research [4,58,62]. Recent advances in genetics and biology of mitochondria have provided the emerging recognition that mitochondria do much more than ‘simply providing energy for cellular function’. Currently, mitochondria are being increasingly viewed as the major organelle for maintaining cellular homeostasis. In addition, a constantly improving understanding of the mitochondrial structure and function has been providing valuable insights into the contribution of defects in mitochondrial metabolism to various human diseases, including COPD and NSCLC.
As the COPD and NSCLC are major inflammation-related lung diseases, the importance of mitochondria is obvious. Over the past years, defects of the complex and dynamic mitochondrial function, including metabolic alterations, have been identified as the cause of pathogenesis in COPD and NSCLC. The proteins related to mitochondrial dysfunction in COPD and NSCLC discussed in the present overview are summarized in Table 2. However, to date there are no clinically approved mitochondria-targeted therapies available for treatment and maintenance of these diseases. Nevertheless, a number of prospective clinical trials of metformin, oxidative phosphorylation inhibitors, are currently underway, including the use of metformin as a chemoprevention agent and as a therapeutic in combination with other standard lung cancer treatment regimens [25]. Based on the constantly increasing number of studies on mitochondrial metabolic profiling in COPD and NSCLC, it is quite plausible that in the future, mitochondria-based early diagnostic biomarkers and therapeutic regimens for these conditions will emerge.
Table 2. . Summary of proteins related to mitochondrial dysfunction in chronic obstructive pulmonary disease and non-small-cell lung cancer noted in this article.
| Protein | Function | Results and a potential role as biomarker | Ref. |
|---|---|---|---|
| PPARs |
Key regulators of mitochondrial biogenesis |
COPD muscle (↓), potential therapeutic target |
[86,92,93] |
| PGC-1α |
Key regulators of mitochondrial biogenesis |
|
– |
| TFAM |
Maintains mtDNA copy number |
|
– |
| Prohibitin |
Maintaining normal mitochondrial function |
COPD lung (↓), poor prognostic factor |
[96] |
| IRP |
Regulate cellular iron homeostasis |
COPD lung (↑), potential therapeutic target |
[98] |
| MFN2 |
Fusion regulatory protein |
AD tissues and cell lines (↓) |
[116] |
| DRP1 |
Fission regulatory protein |
AD tissues and cell lines (↑), poor prognostic factor |
[116,117] |
| OPA1 |
Fusion regulatory protein |
AD tissues (↑), poor prognostic factor |
[118] |
| PXN |
Associated with anti-apoptotic protein B cell lymphoma 2 and Drp-1 protein overexpression |
NSCLC tissues (↑), poor prognostic factor |
[119,120] |
| HSP90-like protein |
Mitochondrial chaperone |
NSCLC cell migration and invasion (↓) |
[121] |
| mtTFA | Promotes transcription of mtDNA and regulates mtDNA replication | SCC tissue with COPD (↓), good prognostic factor | [135] |
(↑): Overexpression or increased; (↓): Downregulation or decreased.
AD: Adenocarcinoma; COPD: Chronic obstructive pulmonary disease; IRP: Iron-regulatory protein; mtDNA: Mitochondrial DNA; mtTFA: Mitochondrial transcription factor A; NSCLC: Non-small-cell lung cancer; PPAR: Peroxisome proliferator-activated receptor; PXN: Protein paxillin; SCC: Squamous cell carcinoma; TFAM: Transcription factor A mitochondrial.
Executive summary.
Mitochondria
Mitochondria are double-membrane subcellular organelles with their own DNA (mtDNA) that are essential for cell survival in eukaryotes. Their fusion–fission cycle (collectively termed mitochondrial dynamics) is important for the maintenance of mitochondrial functions.
Mitochondria are the major organelle for ATP production and for maintaining cellular homeostasis, such as signaling through ROS, regulation of apoptosis, calcium signaling, heme and steroid synthesis, cellular differentiation and growth, and cell cycle control.
Mitochondrial dysfunction in chronic obstructive pulmonary disease or non-small-cell lung cancer
The two main smoking-related lung disease, chronic obstructive pulmonary disease (COPD) and non-small-cell lung cancer (NSCLC), are associated with chronic inflammation and oxidative stress.
Defects of the complex and dynamic mitochondrial function have been identified as the cause of pathogenesis in COPD and NSCLC.
The proteins related to mitochondrial dysfunction in COPD are as follows: PPARs, PGC-1α, TFAM, prohibitin and IRP.
The proteins related to mitochondrial dysfunction in NSCLC are as follows: MFN2, DRP1, OPA1, PXN and HSP90-like protein.
The proteins related to mitochondrial dysfunction in NSCLC with COPD are as follows: genes related to mitochondrial localization and mtTFA.
Conclusion
The importance of mitochondria in the cause of pathogenesis in COPD and NSCLC is as clear as daylight.
The growing interest in mitochondrial metabolic profiling in COPD and NSCLC will emerge in mitochondria-based early diagnostic biomarkers and therapeutic regimens in the near future.
Footnotes
Financial & competing interests disclosure
MO Hoque was supported by the Clinical Innovative Award #103015_CIA from FAMRI (Miami, FL, USA) and by the National Cancer Institute grant R01CA208709 (grant 1R01CA208709-01). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Henze K, Martin W. Evolutionary biology: essence of mitochondria. Nature. 2003;426(6963):127–128. doi: 10.1038/426127a. [DOI] [PubMed] [Google Scholar]
- 2.Mcbride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr. Biol. 2006;16(14):R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11(1):9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A detailed paper on targeting mitochondrial metabolism for the treatment of cancer.
- 4.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]; •• Provides accumulating evidence of novel roles that mitochondria play in the regulation of apoptosis.
- 5.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross CE, Halliwell B, Borish ET, et al. Oxygen radicals and human disease. Ann. Intern. Med. 1987;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 8.Archer SL. Mitochondrial dynamics – mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013;369(23):2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]; •• This paper is a review that details mitochondrial dynamics defects in several human diseases and its potential as a therapeutic target.
- 9.Schapira AH. Mitochondrial diseases. Lancet. 2012;379(9828):1825–1834. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee A, Mambo E, Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25(34):4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]; • Provides a detailed summary of mtDNA in human cancer.
- 11.Dimauro S, Schon EA. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003;348(26):2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 13.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J. Glob. Health. 2015;5(2):020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loukides S, Bartziokas K, Vestbo J, Singh D. Novel anti-inflammatory agents in COPD: targeting lung and systemic inflammation. Curr. Drug Targets. 2013;14(2):235–245. doi: 10.2174/1389450111314020008. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA. Cancer J. Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 16.Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer – is it becoming a reality? Nat. Rev. Clin. Oncol. 2010;7(7):401–414. doi: 10.1038/nrclinonc.2010.64. [DOI] [PubMed] [Google Scholar]
- 17.Bozinovski S, Vlahos R, Anthony D, et al. COPD and squamous cell lung cancer: aberrant inflammation and immunity is the common link. Br. J. Pharmacol. 2016;173(4):635–648. doi: 10.1111/bph.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 19.Houghton AM. Mechanistic links between COPD and lung cancer. Nat. Rev. Cancer. 2013;13(4):233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 20.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology. 2011;25(5):400–410. 413. [PubMed] [Google Scholar]
- 21.Lawless MW, O'Byrne KJ, Gray SG. Oxidative stress induced lung cancer and COPD: opportunities for epigenetic therapy. J. Cell Mol. Med. 2009;13(9A):2800–2821. doi: 10.1111/j.1582-4934.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talhout R, Schulz T, Florek E, Van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health. 2011;8(2):613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons MJ, Gibson JF, Ingram DJ. Free-radicals produced in cigarette smoke. Nature. 1958;181(4614):1003–1004. doi: 10.1038/1811003a0. [DOI] [PubMed] [Google Scholar]
- 24.Miro O, Alonso JR, Jarreta D, Casademont J, Urbano-Marquez A, Cardellach F. Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis. 1999;20(7):1331–1336. doi: 10.1093/carcin/20.7.1331. [DOI] [PubMed] [Google Scholar]
- 25.Lennon FE, Salgia R. Mitochondrial dynamics: biology and therapy in lung cancer. Expert Opin. Investig. Drugs. 2014;23(5):675–692. doi: 10.1517/13543784.2014.899350. [DOI] [PubMed] [Google Scholar]
- 26.Sureshbabu A, Bhandari V. Targeting mitochondrial dysfunction in lung diseases: emphasis on mitophagy. Front. Physiol. 2013;4:384. doi: 10.3389/fphys.2013.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts ER, Thomas KJ. The role of mitochondria in the development and progression of lung cancer. Comput. Struct. Biotechnol. J. 2013;6:e201303019. doi: 10.5936/csbj.201303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aravamudan B, Thompson MA, Pabelick CM, Prakash YS. Mitochondria in lung diseases. Expert Rev. Respir. Med. 2013;7(6):631–646. doi: 10.1586/17476348.2013.834252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeitink J, Van Den Heuvel L, Dimauro S. The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2001;2(5):342–352. doi: 10.1038/35072063. [DOI] [PubMed] [Google Scholar]
- 30.Chance B, Williams GR. A method for the localization of sites for oxidative phosphorylation. Nature. 1955;176(4475):250–254. doi: 10.1038/176250a0. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005;6(5):389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Anderson S, Bankier AT, Barrell BG, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 34.Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009;276(20):5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakupciak JP, Wang W, Markowitz ME, et al. Mitochondrial DNA as a cancer biomarker. J. Mol. Diagn. 2005;7(2):258–267. doi: 10.1016/S1525-1578(10)60553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemarie A, Grimm S. Mitochondrial respiratory chain complexes: apoptosis sensors mutated in cancer? Oncogene. 2011;30(38):3985–4003. doi: 10.1038/onc.2011.167. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. Am. J. Hematol. Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajnoczky G, Csordas G, Das S, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40(5–6):553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossier MF. T channels and steroid biosynthesis: in search of a link with mitochondria. Cell Calcium. 2006;40(2):155–164. doi: 10.1016/j.ceca.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim. Biophys. Acta. 2006;1763(7):723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 42.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010;11(12):872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 43.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8(11):870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 44.Lewis MR, Lewis WH. Mitochondria in tissue culture. Science. 1914;39(1000):330–333. doi: 10.1126/science.39.1000.330. [DOI] [PubMed] [Google Scholar]
- 45.Biesele JJ, Tobioka M. Mitochondria in living cells: an analysis of movements. J. Biophys. Biochem. Cytol. 1956;2(4 Suppl.):319–324. doi: 10.1083/jcb.2.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper is first report that demonstrates the elongation and disjoining of mitochondria caused by coenzyme A.
- 46.Berman SB, Pineda FJ, Hardwick JM. Mitochondrial fission and fusion dynamics: the long and short of it. Cell Death Differ. 2008;15(7):1147–1152. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bleazard W, Mccaffery JM, King EJ, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat. Cell Biol. 1999;1(5):298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Youle RJ, Van Der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 1999;147(4):699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zorzano A, Liesa M, Sebastian D, Segales J, Palacin M. Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin. Cell Dev. Biol. 2010;21(6):566–574. doi: 10.1016/j.semcdb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhao J, Liu T, Jin S, et al. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J. 2011;30(14):2762–2778. doi: 10.1038/emboj.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins Y, Chouchani ET, James AM, Menger KE, Cocheme HM, Murphy MP. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012;125(Pt 4):801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 54.Ambrosio G, Zweier JL, Duilio C, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 1993;268(25):18532–18541. [PubMed] [Google Scholar]
- 55.Moghaddas S, Hoppel CL, Lesnefsky EJ. Aging defect at the QO site of complex III augments oxyradical production in rat heart interfibrillar mitochondria. Arch. Biochem. Biophys. 2003;414(1):59–66. doi: 10.1016/s0003-9861(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278(38):36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 57.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 2011;36(1):30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur. J. Biochem. 1998;252(1):1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 59.Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging. 2012;4(5):330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 61.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922–2933. [PubMed] [Google Scholar]
- 62.Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94(6):695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 63.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper is a detailed review of how the mitochondrial fission and fusion machinery intersects apoptosis pathways.
- 64.Pradelli LA, Beneteau M, Ricci JE. Mitochondrial control of caspase-dependent and -independent cell death. Cell Mol. Life Sci. 2010;67(10):1589–1597. doi: 10.1007/s00018-010-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria – specificity in membrane targeting for death. Biochim. Biophys. Acta. 2011;1813(4):532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]; • This review details the various interactions that may drive the targeting of Bcl-2 proteins to mitochondria and their subsequent activity in apoptosis.
- 66.Chalah A, Khosravi-Far R. The mitochondrial death pathway. Adv. Exp. Med. Biol. 2008;615:25–45. doi: 10.1007/978-1-4020-6554-5_3. [DOI] [PubMed] [Google Scholar]
- 67.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009;21(6):871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Scatena R. Mitochondria and cancer: a growing role in apoptosis, cancer cell metabolism and dedifferentiation. Adv. Exp. Med. Biol. 2012;942:287–308. doi: 10.1007/978-94-007-2869-1_13. [DOI] [PubMed] [Google Scholar]
- 69.Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br. J. Cancer. 2015;112(6):957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper is a review for better understanding of the ‘Warburg effect’ and the mechanistic links between cellular metabolism and growth control.
- 72.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 73.Luft R, Ikkos D, Palmieri G, Ernster L, Afzelius B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J. Clin. Invest. 1962;41:1776–1804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper is the first mitochondrial disease case report in human.
- 74.Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 75.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 76.Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 2010;1797(2):113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007;83(1):84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plataki M, Tzortzaki E, Rytila P, Demosthenes M, Koutsopoulos A, Siafakas NM. Apoptotic mechanisms in the pathogenesis of COPD. Int. J. Chron. Obstruct Pulmon. Dis. 2006;1(2):161–171. doi: 10.2147/copd.2006.1.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006;7:53. doi: 10.1186/1465-9921-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukaro VR, Hodge S. Airway clearance of apoptotic cells in COPD. Curr. Drug Targets. 2011;12(4):460–468. doi: 10.2174/138945011794751609. [DOI] [PubMed] [Google Scholar]
- 82.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 83.Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am. J. Respir. Cell Mol. Biol. 2005;32(5):367–372. doi: 10.1165/rcmb.F296. [DOI] [PubMed] [Google Scholar]
- 84.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur. Respir. J. 2003;22(4):672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 85.Lloreta J, Orozco M, Gea J, Corominas JM, Serrano S. Selective diaphragmatic mitochondrial abnormalities in a patient with marked air flow obstruction. Ultrastruct. Pathol. 1996;20(1):67–71. doi: 10.3109/01913129609023240. [DOI] [PubMed] [Google Scholar]
- 86.Taivassalo T, Hussain SN. Contribution of the mitochondria to locomotor muscle dysfunction in COPD patients. Chest. 2015;149(5):1302–1312. doi: 10.1016/j.chest.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Puente-Maestu L, Perez-Parra J, Godoy R, et al. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur. Respir. J. 2009;33(5):1045–1052. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]
- 88.Gosker HR, Hesselink MK, Duimel H, Ward KA, Schols AM. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur. Respir. J. 2007;30(1):73–79. doi: 10.1183/09031936.00146906. [DOI] [PubMed] [Google Scholar]
- 89.Picard M, Godin R, Sinnreich M, et al. The mitochondrial phenotype of peripheral muscle in chronic obstructive pulmonary disease: disuse or dysfunction? Am. J. Respir. Crit. Care Med. 2008;178(10):1040–1047. doi: 10.1164/rccm.200807-1005OC. [DOI] [PubMed] [Google Scholar]
- 90.Agusti AG, Sauleda J, Miralles C, et al. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166(4):485–489. doi: 10.1164/rccm.2108013. [DOI] [PubMed] [Google Scholar]
- 91.Guo Y, Gosker HR, Schols AM, et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188(11):1313–1320. doi: 10.1164/rccm.201304-0732OC. [DOI] [PubMed] [Google Scholar]
- 92.Remels AH, Schrauwen P, Broekhuizen R, et al. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur. Respir. J. 2007;30(2):245–252. doi: 10.1183/09031936.00144106. [DOI] [PubMed] [Google Scholar]
- 93.Remels AH, Gosker HR, Schrauwen P, Langen RC, Schols AM. Peroxisome proliferator-activated receptors: a therapeutic target in COPD? Eur. Respir. J. 2008;31(3):502–508. doi: 10.1183/09031936.00068207. [DOI] [PubMed] [Google Scholar]
- 94.Theiss AL, Sitaraman SV. The role and therapeutic potential of prohibitin in disease. Biochim. Biophys. Acta. 2011;1813(6):1137–1143. doi: 10.1016/j.bbamcr.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kasashima K, Sumitani M, Satoh M, Endo H. Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp. Cell. Res. 2008;314(5):988–996. doi: 10.1016/j.yexcr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Soulitzis N, Neofytou E, Psarrou M, et al. Downregulation of lung mitochondrial prohibitin in COPD. Respir. Med. 2012;106(7):954–961. doi: 10.1016/j.rmed.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 97.Demeo DL, Mariani T, Bhattacharya S, et al. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. Am. J. Hum. Genet. 2009;85(4):493–502. doi: 10.1016/j.ajhg.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cloonan SM, Glass K, Laucho-Contreras ME, et al. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat. Med. 2016;22(2):163–174. doi: 10.1038/nm.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper strongly supports the use of mitochondrial–iron chelators as novel therapeutic approaches for chronic obstructive pulmonary disease.
- 99.Hoffmann RF, Zarrintan S, Brandenburg SM, et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 2013;14:97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wiegman CH, Michaeloudes C, Haji G, et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015;136(3):769–780. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Indicates that treatments that target mitochondria-derived oxidative stress represent a promising new therapeutic strategy in chronic obstructive pulmonary disease.
- 101.Ryter SW, Choi AM. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol. 2015;4:215–225. doi: 10.1016/j.redox.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mizumura K, Cloonan SM, Nakahira K, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Invest. 2014;124(9):3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmad T, Sundar IK, Lerner CA, et al. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29(7):2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin. Chest Med. 2011;32(4):605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N. Engl. J. Med. 2008;359(13):1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. N. Engl. J. Med. 2012;366(6):489–491. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 108.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 109.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 110.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation – why mitochondria are targets for cancer therapy. Mol. Aspects Med. 2010;31(2):145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 111.Husgafvel-Pursiainen K, Kannio A, Oksa P, et al. Mutations, tissue accumulations, and serum levels of p53 in patients with occupational cancers from asbestos and silica exposure. Environ. Mol. Mutagen. 1997;30(2):224–230. [PubMed] [Google Scholar]
- 112.Suzuki M, Toyooka S, Miyajima K, et al. Alterations in the mitochondrial displacement loop in lung cancers. Clin. Cancer Res. 2003;9(15):5636–5641. [PubMed] [Google Scholar]
- 113.Ding C, Li R, Wang P, Fan H, Guo Z. Sequence polymorphisms of the mitochondrial displacement loop and outcome of non-small cell lung cancer. Exp. Ther. Med. 2012;3(5):861–864. doi: 10.3892/etm.2012.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dasgupta S, Soudry E, Mukhopadhyay N, et al. Mitochondrial DNA mutations in respiratory complex-I in never-smoker lung cancer patients contribute to lung cancer progression and associated with EGFR gene mutation. J. Cell. Physiol. 2012;227(6):2451–2460. doi: 10.1002/jcp.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosgood HD, 3rd, Liu CS, Rothman N, et al. Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis. 2010;31(5):847–849. doi: 10.1093/carcin/bgq045. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides evidence that mtDNA copy number is associated with future development of lung cancer among heavy smokers.
- 116.Rehman J, Zhang HJ, Toth PT, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26(5):2175–2186. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiang YY, Chen SL, Hsiao YT, et al. Nuclear expression of dynamin-related protein 1 in lung adenocarcinomas. Mod. Pathol. 2009;22(9):1139–1150. doi: 10.1038/modpathol.2009.83. [DOI] [PubMed] [Google Scholar]
- 118.Fang HY, Chen CY, Chiou SH, et al. Overexpression of optic atrophy 1 protein increases cisplatin resistance via inactivation of caspase-dependent apoptosis in lung adenocarcinoma cells. Hum. Pathol. 2012;43(1):105–114. doi: 10.1016/j.humpath.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 119.Jagadeeswaran R, Surawska H, Krishnaswamy S, et al. Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res. 2008;68(1):132–142. doi: 10.1158/0008-5472.CAN-07-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawada I, Hasina R, Lennon FE, et al. Paxillin mutations affect focal adhesions and lead to altered mitochondrial dynamics: relevance to lung cancer. Cancer Biol. Ther. 2013;14(7):679–691. doi: 10.4161/cbt.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caino MC, Chae YC, Vaira V, et al. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J. Clin. Invest. 2013;123(7):2907–2920. doi: 10.1172/JCI67841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J. Natl Cancer Inst. 2009;101(8):554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer. 2015;90(2):121–127. doi: 10.1016/j.lungcan.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Petty TL. Are COPD and lung cancer two manifestations of the same disease? Chest. 2005;128(4):1895–1897. doi: 10.1378/chest.128.4.1895. [DOI] [PubMed] [Google Scholar]
- 125.Purdue MP, Gold L, Jarvholm B, Alavanja MC, Ward MH, Vermeulen R. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax. 2007;62(1):51–56. doi: 10.1136/thx.2006.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch. Intern Med. 2003;163(12):1475–1480. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- 127.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann. Intern. Med. 1986;105(4):503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 128.Ueda K, Jinbo M, Li TS, Yagi T, Suga K, Hamano K. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin. Cancer Res. 2006;12(22):6730–6736. doi: 10.1158/1078-0432.CCR-06-1196. [DOI] [PubMed] [Google Scholar]
- 129.Papi A, Casoni G, Caramori G, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax. 2004;59(8):679–681. doi: 10.1136/thx.2003.018291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Denholm R, Schuz J, Straif K, et al. Is previous respiratory disease a risk factor for lung cancer? Am. J. Respir. Crit. Care Med. 2014;190(5):549–559. doi: 10.1164/rccm.201402-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann. Intern. Med. 2005;142(4):233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 132.Caramori G, Casolari P, Cavallesco GN, Giuffre S, Adcock I, Papi A. Mechanisms involved in lung cancer development in COPD. Int. J. Biochem. Cell Biol. 2011;43(7):1030–1044. doi: 10.1016/j.biocel.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 133.Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration. 2011;81(4):265–284. doi: 10.1159/000324601. [DOI] [PubMed] [Google Scholar]
- 134.Boelens MC, Gustafson AM, Postma DS, et al. A chronic obstructive pulmonary disease related signature in squamous cell lung cancer. Lung Cancer. 2011;72(2):177–183. doi: 10.1016/j.lungcan.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 135.Peng H, Yang M, Chen ZY, et al. Expression and methylation of mitochondrial transcription factor a in chronic obstructive pulmonary disease patients with lung cancer. PLoS ONE. 2013;8(12):e82739. doi: 10.1371/journal.pone.0082739. [DOI] [PMC free article] [PubMed] [Google Scholar]