Abstract
Aim:
The objective of this study was to assess if avibactam, a new β-lactamase inhibitor, can restore the potency of carbapenems, a sub-class of β-lactams, against Mycobacterium abscessus clinical isolates.
Materials & methods:
28 M. abscessus clinical isolates that are resistant to multiple drugs currently used to treat its infection were included. MIC of carbapenems alone and in combination with avibactam against these strains were determined.
Results:
Tebipenem, an oral carbapenem, and ertapenem and panipenem exhibited the greatest shift in MIC when supplemented with avibactam.
Conclusion:
Avibactam restores MICs of tebipenem, ertapenem and panipenem against M. abscessus to therapeutically achievable concentrations and raises the possibility of usefulness of these carbapenems to treat drug-resistant M. abscessus infections.
KEYWORDS : avibactam, carbapenem, ertapenem, Mycobacterium abscessus, panipenem, tebipenem
Mycobacterium abscessus is a rapidly growing nontuberculous mycobacterium found widely in soil and water and can cause a spectrum of infections [1]. Prevalence of M. abscessus infections in the lungs of people with chronic conditions, such as cystic fibrosis is significant and can often lead to serious morbidity [2]. A survey revealed that M. abscessus is present in the sputum of approximately 13% of cystic fibrosis patients in the USA [3]. Among nontuberculous mycobacterium lung infections, M. abscessus is one of the prevalent species and often leads to a chronic and incurable disease [4–6]. Drug resistance in M. abscessus is steadily rising globally, making it increasingly difficult to manage infections with these strains [7]. Therefore, new drugs and novel regimens are acutely needed to treat infections with M. abscessus. An ideal new drug would inhibit a novel target so that it can be effective against M. abscessus strains that are resistant to currently used drugs.
The peptidoglycan is an Achilles’ heel of bacteria as agents that inhibit its biosynthesis, namely β-lactams and glycopeptides, comprise some of the most widely used class of antibacterials in modern medicine. β-lactams derive their activity by preventing formation of linkage between peptide side chains by inhibiting the transpeptidases that catalyze this reaction [8]. Recently it was demonstrated that majority of the linkages in the peptidoglycan layer of M. abscessus are generated by LD-transpeptidases [9] and that this class of enzyme is selectively more susceptible to the carbapenem class of β-lactams [10–12]. Imipenem, a carbapenem, has superior activity compared with cefoxitin against clinical strains of M. abscessus isolated from cystic fibrosis patients [13]. M. abscessus harbors a chromosomally encoded β-lactamase that is highly active and therefore is of major concern while considering β-lactams for treatment of M. abscessus infections [14,15]. Here, we have studied if avibactam, a recently developed β-lactamase inhibitor, can alter the potency of the carbapenem class of β-lactams against M. abscessus. Recent studies have provided insight into the activities of some older carbapenems with or without avibactam against M. abscessus [16–18]. We have included all commercially available carbapenems, most importantly new and oral carbapenems, and a collection of clinically isolated M. abscessus strains most of which are resistant to multiple drugs currently deployed to treat infection by this pathogen. Activities of the combinations of clavulanate, a β-lactamase inhibitor and carbapenems, were recently reported [19]; therefore clavulanate was excluded from this study.
Materials & methods
• Bacterial strains
Twenty-eight unique clinical isolates of M. abscessus were used in this study. These strains were obtained de-identified from the archive of the Clinical Microbiology Laboratory of the Johns Hopkins University Hospital as per institutional ethical guidelines. They were isolated over a 10-year period, from 2005 to 2015, from patients that were temporally and geographically unrelated. No two isolates are from the same patient. Those displaying a high level of resistance to antibacterials used for M. abscessus infection were selected for this study. All strains obtained prior to 2014 were identified to the M. abscessus complex level using a variety of methods including 16S rDNA sequencing in conjunction with selected biochemical testing, such as sodium citrate. More recent isolates (those isolated after 2014) were identified using MALDI ToF MS in which a Bruker MicroFlex LT (MicroFlex LT, Bruker, Bremen, Germany) mass spectrometer and Bruker Biotyper software and existing database (version 2.0, Bruker) were employed. Subspeciation within the M. abscessus complex, which helps to distinguish between M. abscessus sensu stricto, M. massiliense and M. bolletii, was not done as this is not per the current standard of care at the Johns Hopkins Clinical Mycobacteriology Laboratory (MD, USA). Distinguishing between M. abscessus sensu stricto and M. massiliense is most often performed to guide therapy since M. massiliense is known to have a nonfunctional erm gene and is therefore susceptible to macrolides. However, due to the high number of macrolide-resistant M. abscessus complex isolates recovered at Johns Hopkins, most patient isolates are subjected to drug susceptibility testing, making speciation within the complex of lesser importance. Thus, the proportion of each subspecies within the M. abscessus complex for the Johns Hopkins strain collection is not known. M. abscessus ATCC 19977 was included as a reference drug-sensitive strain.
• Growth conditions & MIC
All strains were initially grown in 7H9 complete medium composed of Middlebrook 7H9 broth (BD Diagnostics, MD, USA) supplemented with 0.5% glycerol, 10% oleic acid-albumin-dextrose-catalase and 0.05% Tween-80 at 37°C with constant shaking. A standard broth microdilution method [20] was used to determine MIC. Briefly, M. abscessus strains were grown as described above and these cultures, at exponential phase (A600nm ∼0.6–0.8), were used to inoculate 105 colony-forming units into each well of microtiter culture plates containing a carbapenem at twofold serial dilutions ranging from 256 to 0.25 µg/ml. An identical setup but with wells containing 4 µg/ml of avibactam was used to assess the effect of this agent on each carbapenem. Carbapenems studied were procured commercially from Sigma-Aldrich (Sigma-Aldrich, MO, USA) and include ertapenem, meropenem, imipenem, doripenem, biapenem, faropenem, tebipenem and panipenem. β-lactamase inhibitors sulbactam, tazobactam and avibactam were also procured from Sigma-Aldrich. In addition, to establish baseline antibacterial susceptibility for each strain, we also determined MICs for drugs currently used to treat M. abscessus infections. They are linezolid, cefoxitin, kanamycin, ciprofloxacin, moxifloxacin, amikacin, tigecycline, imipenem, tobramycin, trimethoprim/sulfamethoxazole and clarithromycin. Mycobacterium abscessus growth was evaluated by visual inspection of pellets after 3 days of incubation (the exception being clarithromycin for which incubation was extended to 14 days) at 30°C without shaking as per Clinical Laboratory Standard Institute guidelines [21]. In addition to assessment in 7H9 complete medium, we also used cation adjusted Mueller–Hinton broth (BD) for MIC studies for the reference strain M. abscessus ATCC 19977. All experiments were repeated and the final data represent the average of two biological replicates.
Results
We studied the utility of β-lactamase inhibitors sulbactam, tazobactam and avibactam in restoring in vitro potencies of carbapenems against M. abscessus using the reference drug-susceptible strain ATCC 19977. We determined MICs of ertapenem, meropenem, imipenem, doripenem, biapenem, faropenem, tebipenem and panipenem alone or in the presence of the aforementioned three β-lactamase inhibitors. In general, addition of sulbactam and tazobactam failed to reduce the MICs of the carbapenems (Table 1). However, avibactam (4 µg/ml) consistently reduced the MICs of carbapenems by 2- to 32-fold. Based on these data, we selected avibactam for further consideration.
Table 1. . Minimum inhibitory concentrations (in µg/ml) of carbapenems with and without β-lactamase inhibitors against Mycobacterium abscessus ATCC 19977.
| Drug | 7H9 broth | 7H9 + sulbactam | 7H9 + tazobactam | 7H9 + avibactam | CAMHB only | CAMHB + avibactam |
|---|---|---|---|---|---|---|
| Ertapenem |
64–128 |
>64 |
32–64 |
4–8 |
128–256 |
8–16 |
| Meropenem |
8–16 |
8–16 |
8–16 |
2–4 |
32–64 |
4–8 |
| Imipenem |
4–8 |
4–8 |
2–4 |
2–4 |
8–16 |
4–8 |
| Doripenem |
8–16 |
8–16 |
8–16 |
2–4 |
16–32 |
4–8 |
| Biapenem |
8–16 |
4–8 |
8–16 |
2–4 |
16–32 |
8–16 |
| Faropenem |
32–64 |
16–32 |
32–64 |
8–16 |
64–128 |
16–32 |
| Tebipenem |
128–256 |
>64 |
>64 |
4–8 |
128–256 |
8–16 |
| Panipenem |
64–128 |
16–32 |
32–64 |
8–16 |
64–128 |
8–16 |
| Sulbactam |
>64 |
ND |
ND |
ND |
ND |
ND |
| Tazobactam |
>64 |
ND |
ND |
ND |
ND |
ND |
| Avibactam | >256 | ND | ND | ND | >256 | ND |
β-lactamase inhibitors sulbactam, tazobactam and avibactam were used at a fixed concentration of 4 µg/ml.
CAMHB: Cation-adjusted Mueller–Hinton broth; ND: Not determined.
The 28 clinical isolates included in this study exhibited a wide range of susceptibilities and resistance to the antibacterials used to treat M. abscessus infections (Table 2). This baseline profile illustrates that these clinical isolates are mostly resistant to the drugs that are part of the current recommendation for treatment of M. abscessus infections, with tigecycline being the only exception. The MIC of carbapenems against the drug-sensitive control strain M. abscessus ATCC 19977 were as expected. They were 128, 16, 8, 16, 16, 64, 256 and 128 µg/ml for ertapenem, meropenem, imipenem, doripenem, biapenem, faropenem, tebipenem and panipenem, respectively. Broth microdilution breakpoints for imipenem and meropenem have been established for rapidly growing mycobacteria, including M. abscessus and are the same for both drugs: susceptible, ≤4 µg/ml, intermediate 8–16 µg/ml and resistant ≥32 µg/ml. However, breakpoints for M. abscessus have not been established for any of the other carbapenems tested in this study. In such cases, comparison of individual MICs to average peak plasma level and half-life may be useful clinically (ertapenem: average peak plasma concentration ∼150 µg/ml with a half-life of ∼4 h [22]; tebipenem: average peak plasma concentration ∼8 µg/ml with a half-life of 1 h [23]; panipenem: average peak plasma concentration ∼22 µg/ml with a half-life of 40–70 min [24]).
Table 2. . Antimicrobial susceptibility profiles of 28 Mycobacterium abscessus clinical strains against carbapenems (with and without avibactam, 4 µg/ml) and antibacterials that are currently used to treat M. abscessus infection and where available currently used Clinical Laboratory Standard Institute breakpoints for interpretation.
| M. abscessus clinical strain |
MIC of carbapenems with and without avibactam |
Susceptibility profile for antibacterials used for treatment of M. abscessus infections |
Avi | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erta | Erta + Avi | Mero | Mero + Avi | Imi | Imi + Avi | Dori | Dori + Avi | Bia | Bia + Avi | Faro | Faro + Avi | Tebi | Tebi + Avi | Pani | Pani + Avi | CLR | SXT | CIP | MOX | FOX | AMI | LZD | IMI | TOB | TGC | KAN | ||
| 1N |
128 |
16 |
16 |
4 |
8 |
8 |
16 |
4 |
16 |
4 |
64 |
32 |
256 |
4 |
128 |
16 |
I |
R |
R |
R |
I |
R |
R |
I |
R |
4 |
8 |
>256 |
| 2N |
256 |
8 |
8 |
4 |
8 |
8 |
16 |
8 |
16 |
4 |
128 |
32 |
256 |
8 |
64 |
8 |
R |
R |
R |
R |
I |
S |
R |
I |
R |
0.5 |
16 |
>256 |
| 3N |
128 |
16 |
16 |
4 |
8 |
8 |
32 |
4 |
16 |
4 |
64 |
32 |
256 |
4 |
64 |
16 |
R |
ND |
ND |
R |
I |
ND |
R |
I |
ND |
ND |
16 |
>256 |
| 4N |
64 |
16 |
16 |
8 |
16 |
16 |
16 |
8 |
16 |
8 |
64 |
16 |
256 |
4 |
32 |
16 |
R |
R |
R |
R |
I |
R |
R |
I |
R |
1 |
8 |
>256 |
| 5N |
64 |
16 |
16 |
4 |
32 |
32 |
16 |
4 |
16 |
8 |
64 |
16 |
256 |
8 |
128 |
32 |
I |
R |
R |
R |
I |
R |
R |
R |
R |
1 |
16 |
>256 |
| 6N |
128 |
16 |
16 |
4 |
8 |
8 |
16 |
4 |
16 |
4 |
128 |
32 |
256 |
4 |
128 |
8 |
R |
ND |
ND |
R |
I |
ND |
R |
I |
ND |
ND |
16 |
>256 |
| 11N |
>256 |
32 |
128 |
8 |
32 |
32 |
128 |
8 |
128 |
8 |
128 |
64 |
>256 |
16 |
128 |
64 |
R |
R |
R |
R |
I |
R |
R |
R |
R |
4 |
>256 |
>256 |
| 12N |
>256 |
16 |
16 |
4 |
16 |
8 |
32 |
4 |
16 |
4 |
128 |
32 |
>256 |
4 |
128 |
16 |
R |
S |
R |
R |
I |
S |
R |
I |
R |
0.25 |
16 |
>256 |
| 13N |
256 |
8 |
32 |
8 |
32 |
16 |
16 |
8 |
16 |
8 |
64 |
32 |
128 |
8 |
64 |
16 |
S |
R |
R |
R |
S |
S |
I |
R |
R |
1 |
8 |
>256 |
| 14N |
>256 |
32 |
128 |
8 |
32 |
8 |
32 |
8 |
64 |
8 |
128 |
64 |
>256 |
16 |
256 |
32 |
I |
R |
R |
R |
I |
S |
R |
R |
R |
4 |
16 |
>256 |
| 19N |
256 |
8 |
16 |
4 |
4 |
4 |
16 |
4 |
16 |
4 |
128 |
32 |
128 |
4 |
64 |
4 |
R |
R |
R |
R |
I |
S |
R |
S |
R |
1 |
8 |
>256 |
| 201 |
256 |
16 |
32 |
4 |
16 |
8 |
32 |
4 |
32 |
8 |
256 |
64 |
>256 |
4 |
128 |
8 |
R |
R |
R |
R |
I |
R |
R |
I |
R |
4 |
16 |
>256 |
| 202 |
>256 |
32 |
128 |
8 |
32 |
8 |
64 |
8 |
128 |
8 |
128 |
64 |
>256 |
8 |
256 |
16 |
R |
R |
R |
R |
I |
R |
R |
R |
R |
1 |
16 |
>256 |
| 203 |
>256 |
32 |
64 |
8 |
16 |
16 |
64 |
8 |
64 |
8 |
128 |
32 |
128 |
8 |
64 |
16 |
R |
R |
R |
R |
I |
R |
R |
I |
R |
2 |
64 |
>256 |
| 204 |
>256 |
32 |
128 |
8 |
32 |
16 |
128 |
8 |
64 |
16 |
256 |
64 |
>256 |
8 |
256 |
16 |
R |
R |
R |
R |
I |
S |
R |
R |
R |
4 |
32 |
>256 |
| 206 |
>256 |
16 |
32 |
4 |
16 |
8 |
32 |
8 |
32 |
8 |
128 |
32 |
256 |
4 |
128 |
8 |
I |
R |
R |
R |
I |
S |
R |
I |
R |
0.5 |
32 |
>256 |
| 208 |
256 |
16 |
16 |
4 |
16 |
8 |
32 |
8 |
16 |
8 |
64 |
32 |
256 |
4 |
64 |
8 |
R |
R |
R |
R |
I |
R |
R |
I |
R |
0.5 |
32 |
>256 |
| 210 |
256 |
8 |
16 |
8 |
8 |
8 |
32 |
4 |
32 |
8 |
128 |
32 |
256 |
8 |
64 |
8 |
R |
R |
R |
R |
I |
R |
R |
S |
R |
1 |
32 |
>256 |
| 211 |
>256 |
16 |
32 |
4 |
16 |
8 |
32 |
4 |
16 |
4 |
128 |
64 |
>256 |
4 |
64 |
16 |
R |
R |
R |
R |
I |
R |
R |
I |
R |
0.5 |
16 |
>256 |
| 212 |
>256 |
32 |
16 |
8 |
16 |
8 |
16 |
8 |
16 |
8 |
64 |
32 |
256 |
8 |
64 |
16 |
R |
R |
R |
R |
I |
R |
R |
I |
R |
0.5 |
16 |
>256 |
| 214 |
64 |
16 |
16 |
8 |
32 |
32 |
8 |
4 |
16 |
8 |
64 |
16 |
128 |
8 |
64 |
32 |
S |
R |
R |
R |
S |
R |
R |
R |
R |
1 |
16 |
>256 |
| 215 |
64 |
8 |
16 |
8 |
32 |
32 |
16 |
8 |
16 |
8 |
64 |
16 |
256 |
16 |
64 |
32 |
R |
R |
R |
R |
I |
R |
R |
R |
R |
2 |
32 |
>256 |
| 216 |
128 |
16 |
16 |
8 |
16 |
16 |
16 |
8 |
32 |
8 |
128 |
64 |
256 |
16 |
64 |
16 |
R |
R |
R |
R |
I |
R |
R |
I |
R |
1 |
8 |
>256 |
| 218 |
128 |
16 |
16 |
4 |
8 |
8 |
8 |
4 |
16 |
8 |
64 |
32 |
256 |
8 |
32 |
16 |
R |
R |
S |
R |
I |
S |
R |
I |
R |
1 |
8 |
>256 |
| JHH2 |
>256 |
32 |
128 |
8 |
16 |
16 |
32 |
8 |
32 |
8 |
256 |
64 |
>256 |
16 |
256 |
16 |
R |
ND |
R |
R |
I |
I |
R |
I |
R |
≤4 |
>256 |
>256 |
| JHH4 |
128 |
16 |
16 |
4 |
16 |
8 |
16 |
4 |
16 |
8 |
64 |
32 |
256 |
8 |
64 |
16 |
R |
ND |
R |
R |
I |
R |
R |
I |
R |
>4 |
16 |
>256 |
| JHH9 |
128 |
8 |
16 |
4 |
16 |
8 |
16 |
4 |
16 |
4 |
64 |
32 |
256 |
4 |
64 |
16 |
R |
ND |
R |
R |
I |
R |
R |
I |
R |
>4 |
16 |
>256 |
| JHHKB | >256 | 64 | 128 | 16 | 32 | 16 | 128 | 8 | 128 | 16 | 64 | 32 | 128 | 16 | 256 | 32 | R | ND | ND | R | I | ND | R | R | ND | ND | >256 | >256 |
AMI: Amikacin (susceptible, 1–16 µg/ml, intermediate: 32 µg/ml, resistant: ≥64 µg/ml); Avi: Avibactam; Bia: Biapenem; CIP: Ciprofloxacin (susceptible: 0.12–1 µg/ml, intermediate: 2 µg/ml, resistant: ≥4 µg/ml); CLR: Clarithromycin (susceptible: 0.06–2 µg/ml, intermediate: 4 µg/ml, resistant: ≥8 µg/ml); Dori: Doripenem; Erta: Ertapenem; Faro: Faropenem; FOX: Cefoxitin (susceptible, 4–16 µg/ml, intermediate: 32–64 µg/ml, resistant: ≥128 µg/ml); I: Intermediate; Imi or IMI: Imipenem (susceptible, 2–4 µg/ml, intermediate: 8–16 µg/ml, resistant: ≥32 µg/ml); KAN: Kanamycin (no interpretation for this drug currently available, however, an MIC >5 µg/ml for M. tuberculosis is considered resistant in broth; LZD: Linezolid (susceptible: 1–8 µg/ml, intermediate: 16 µg/ml, resistant: ≥32 µg/ml); Mero: Meropenem; MXF: Moxifloxacin (susceptible: 0.25–1 µg/ml, intermediate: 2 µg/ml, resistant: ≥4 µg/ml); ND: Not determined; Pani: Panipenem; R: Resistant; S: Susceptible; SXT: Trimethoprim/sulfamethoxazole (susceptible: 0.25/4.75–2/38 µg/ml, resistant: ≥4/76 µg/ml); Tebi: Tebipenem; TGC: Tigecycline (no interpretation for this drug currently available, however an MIC >4 µg/ml for M. tuberculosis is considered resistant in broth); TOB: Tobramycin (susceptible, 1–2 µg/ml, intermediate: 4 µg/ml, resistant: ≥8 µg/ml).
S, I and R are classifications based on CLSI breakpoints.
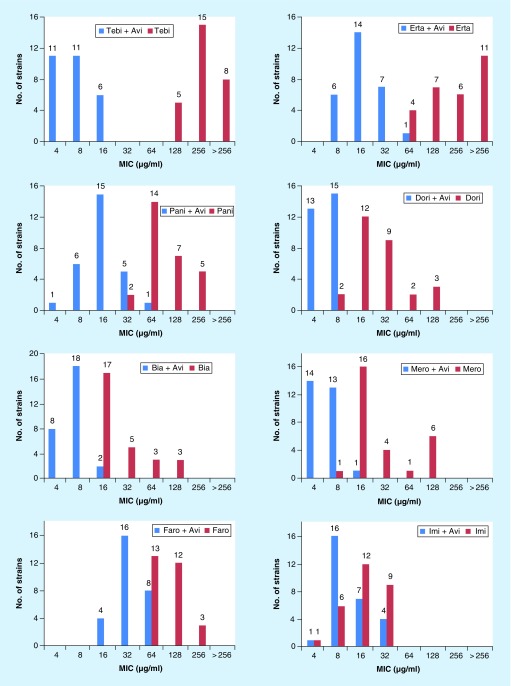
Compared with ATCC 19977, the 28 clinical isolates were equally or more resistant to the carbapenems with a few exceptions (Table 2). The average reduction in MICs of the carbapenems, in combination with avibactam, against the 28 clinical strains is as follows: imipenem (twofold), faropenem (threefold), biapenem (fivefold), doripenem (sixfold), meropenem (sixfold), panipenem (eightfold), ertapenem (>13-fold) and tebipenem (>38-fold) (Figure 1). While avibactam was least effective in reducing the MICs of imipenem and faropenem against M. abscessus, it was able to restore the MICs of panipenem, ertapenem and tebipenem to levels that are therapeutically relevant. These data also show an interesting pattern: irrespective of how high or low is the MIC of a carbapenem alone, avibactam can reduce the final MIC to no lower than 4–8 µg/ml. Therefore, it appears that there is a lower limit for MIC of carbapenem and avibactam combination.
Figure 1. . Distribution of susceptibility of Mycobacterium abscessus strains (n 28) against carbapenems with or without 4 µg/ml avibactam.
The data labels in each plot represent the number of isolates.
Avi: Avibactam; Bia: Biapenem; Dori: Doripenem; Erta: Ertapenem; Faro: Faropenem; Imi: Imipenem; Mero: Meropenem; Pani: Panipenem; Tebi: Tebipenem.
Discussion
M. abscessus is considered to be the most virulent of the rapidly growing mycobacteria. This organism most commonly affects immunocompromised hosts, for example, those with cystic fibrosis and lung transplant recipients, and is largely considered to be a chronic and incurable disease [6]. Effective antimicrobial treatment is sometimes the only thing that stands between these patients and overwhelming infection or even death.
The treatment regimen for M. abscessus pulmonary disease generally involves combination therapy with multiple agents for an extended course of several months [25]. Many of these antibiotics are poorly tolerated and are associated with significant cytotoxic effects [6]. The high prevalence of both intrinsic and acquired antimicrobial resistance further complicates this regimen, making development of novel treatment strategies crucial. M. abscessus genome encodes a β-lactamase, BlaMab, which is not effectively inhibited by clavulanate, sulbactam and tazobactam [14]. We have also failed to observe significant reduction in MIC of carbapenems against M. abscessus when supplemented with clavulanate [19] or sulbactam or tazobactam (Table 1). BlaMab has been shown to hydrolyze β-lactams with high efficiency, especially imipenem. While this study suggested that avibactam could potentially protect imipenem from BlaMab [14], in our study, the MIC of imipenem was least altered by avibactam (Figure 1 & Table 2).Therefore, it appears that M. abscessus likely possess an additional way to degrade imipenem even when it is protected from BlaMab by avibactam. On the other hand, it is plausible that tebipenem, ertapenem and panipenem are efficiently hydrolyzed by BlaMab and by any additional mechanism present in M. abscessus, and avibactam effectively protects these carbapenems.
Our study showed that the addition of avibactam to various carbapenem antibiotics effectively reduced the MICs of carbapenem-resistant M. abscessus isolates to within therapeutically achievable levels in vitro. Additionally, the largest reduction in MIC was achieved with tebipenem, which is available in an oral formulation and may further simplify the multidrug treatment regimen.
Both carbapenems and β-lactamase inhibitors are US FDA approved, widely available and generally well tolerated. The results of our study are highly promising, as they denote a potential new treatment strategy for carbapenem-resistant M. abscessus that could be easily implemented in clinical practice. Recently, a combination of avibactam and ceftazidime, a β-lactam of cephalosporin subclass, was developed to restore potency of ceftazidime against a range of β-lactam resistant bacteria [26]. This combination, available under trade name Avycaz, is FDA-approved for interabdominal and urinary tract infections, in cases where treatment options are limited. Given this precedent, further study of combinations of avibactam and carbapenems, especially tebipenem, ertapenem and panipenem, in a clinical setting would be a step toward the possibility of adding new options for treatment of drug-resistant M. abscessus.
Conclusion
Avibactam is the most active β-lactamase inhibitor in reducing the MICs of tebipenem, ertapenem and panipenem; if it were not for avibactam, the MIC of these carbapenems would be well outside the clinically relevant therapeutic window. For those carbapenems that exhibit high MIC against M. abscessus, addition of avibactam usually reduces the MIC. However, it appears that avibactam reduces the MIC to 4–8 µg/ml, but not below this range, irrespective of how high the MIC of a carbapenem alone is.
EXECUTIVE SUMMARY.
Our study assessed if avibactam can restore potency of carbapenems against Mycobacterium abscessus. Using a full panel of carbapenems against a collection of independent M. abscessus clinical isolates, we observed that for selected carbapenems, avibactam greatly reduces their MIC.
Avibactam reduces the MIC of tebipenem against M. abscessus to 4–8 µg/ml, representing a 32–64 fold decrease. This concentration is achievable in the blood making tebipenem–avibactam combination a potentially new regimen for treatment of M. abscessus infection.
Avibactam also reduces the MIC of ertapenem and panipenem against M. abscessus to levels achievable in the blood.
Further study of combinations of avibactam and tebipenem or ertapenem or panipenem, in a clinical setting, would be a step toward the possibility of adding new options for treatment of drug-resistant M. abscessus.
Acknowledgements
The authors thank EL Nuermberger for input in selection of M. abscessus clinical isolates.
Footnotes
Financial & competing interests disclosure
This study was supported by awards from the Cystic Fibrosis Foundation LAMICH16GO and NIH R21AI121805 to GL. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Jonsson BE, Gilljam M, Lindblad A, Ridell M, Wold AE, Welinder-Olsson C. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 2007;45(5):1497–1504. doi: 10.1128/JCM.02592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martiniano SL, Nick JA, Daley CL. Nontuberculous mycobacterial infections in cystic fibrosis. Clin. Chest Med. 2016;37(1):83–96. doi: 10.1016/j.ccm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Olivier KN, Weber DJ, Wallace RJ, Jr, et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;167(6):828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 4.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 2010;182(7):970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winthrop KL, Mcnelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am. J. Respir. Crit. Care Med. 2010;182(7):977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care. Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 7.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. J. Antimicrobial. Chemother. 2012;67(4):810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 8.Walsh C. Antibiotics: Actions, Origins, Resistance. ASM Press; DC, USA: 2003. p. 3. [Google Scholar]
- 9.Lavollay M, Fourgeaud M, Herrmann JL, et al. The peptidoglycan of Mycobacterium abscessus is predominantly cross-linked by L, D-transpeptidases. J. Bacteriol. 2011;193(3):778–782. doi: 10.1128/JB.00606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubee V, Triboulet S, Mainardi JL, et al. Inactivation of Mycobacterium tuberculosis L, D-transpeptidase LdtMt(1) by carbapenems and cephalosporins. Antimicrob. Agents. Chemother. 2012;56(8):4189–4195. doi: 10.1128/AAC.00665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordillot M, Dubee V, Triboulet S, et al. In vitro cross-linking of peptidoglycan by Mycobacterium tuberculosis L, D-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob. Agents. Chemother. 2013;57(12):5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar P, Kaushik A, Lloyd EP, et al. Non-classical transpeptidases yield insight into new antibacterials. Nat. Chem. Biol. 2017;13(1):54–61. doi: 10.1038/nchembio.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavollay M, Dubee V, Heym B, et al. In vitro activity of cefoxitin and imipenem against Mycobacterium abscessus complex. Clin. Microbiol. Infect. 2014;20(5):O297–O300. doi: 10.1111/1469-0691.12405. [DOI] [PubMed] [Google Scholar]; • Report of activity of carbapenems against Mycobacterium abscessus.
- 14.Soroka D, Dubee V, Soulier-Escrihuela O, et al. Characterization of broad-spectrum Mycobacterium abscessus class A β-lactamase. J. Antimicrobial. Chemother. 2014;69(3):691–696. doi: 10.1093/jac/dkt410. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre AL, Dubee V, Cortes M, Dorchene D, Arthur M, Mainardi JL. Bactericidal and intracellular activity of β-lactams against Mycobacterium abscessus . J. Antimicrobial. Chemother. 2016;71(6):1556–1563. doi: 10.1093/jac/dkw022. [DOI] [PubMed] [Google Scholar]; • Profile of activity of β-lactams against M. abscessus.
- 16.Ito K, Hashimoto K, Ogata H. Activity of cephems and carbapenems against clinically isolated Mycobacterium abscessus . Kekkaku. 2003;78(9):587–590. [PubMed] [Google Scholar]
- 17.Dubee V, Bernut A, Cortes M, et al. β-lactamase inhibition by avibactam in Mycobacterium abscessus . J. Antimicrobial. Chemother. 2015;70(4):1051–1058. doi: 10.1093/jac/dku510. [DOI] [PubMed] [Google Scholar]; •• A report of utility of avibactam as β-lactam companion against M. abscessus.
- 18.Soroka D, Ourghanlian C, Compain F, et al. Inhibition of β-lactamases of mycobacteria by avibactam and clavulanate. J. Antimicrobial. Chemother. 2016 doi: 10.1093/jac/dkw546. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Kaushik A, Makkar N, Pandey P, Parrish N, Singh U, Lamichhane G. Carbapenems and rifampin exhibit synergy against Mycobacterium tuberculosis and Mycobacterium abscessus . Antimicrob. Agents. Chemother. 2015;59(10):6561–6567. doi: 10.1128/AAC.01158-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavan TL, Town MA. A microdilution method for antibiotic susceptibility testing: an evaluation. Am. J. Clin. Pathol. y. 1970;53(6):880–885. doi: 10.1093/ajcp/53.6.880. [DOI] [PubMed] [Google Scholar]
- 21.Desmond E. Susceptibility testing of Mycobacteria, Nocardiae and other aerobic Actinomycetes. Clinical Laboratory Standard Institute. 2011:M24–A2. http://shop.clsi.org/site/Sample_pdf/M24A2_sample.pdf [PubMed] [Google Scholar]
- 22.Nix DE, Majumdar AK, Dinubile MJ. Pharmacokinetics and pharmacodynamics of ertapenem: an overview for clinicians. J. Antimicrobial. Chemother. 2004;53(Suppl. 2):ii23–ii28. doi: 10.1093/jac/dkh205. [DOI] [PubMed] [Google Scholar]
- 23.Kijima K, Sato N, Koresawa T, et al. Pharmacokinetics analysis of tebipenem pivoxil in a Phase II clinical trial in otolaryngological infections. Jpn. J. Antibiot. 2009;62(2):143–154. [PubMed] [Google Scholar]
- 24.Goa KL, Noble S. Panipenem/betamipron. Drugs. 2003;63(9):913–925. doi: 10.2165/00003495-200363090-00005. discussion 926. [DOI] [PubMed] [Google Scholar]
- 25.Brown-Elliott BA, Nash KA, Wallace RJ., Jr Antimicrobial Susceptibility Testing, Drug Resistance Mechanisms, and Therapy of Infections with Nontuberculous Mycobacteria. Clin. Microbiol. Rev. 2012;25(3):545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright GD. Empowering older antibiotics. Cell. 2016;167(2):301. doi: 10.1016/j.cell.2016.09.032. [DOI] [PubMed] [Google Scholar]