Abstract
Tumor-specific monoclonal antibodies (mAbs) have demonstrated efficacy in the clinic, becoming an important approach for cancer immunotherapy. Due to its limited expression on normal tissue, the GD2 disialogangloside expressed on neuroblastoma cells is an excellent candidate for mAb therapy. In 2015, dinutuximab (an anti-GD2 mAb) was approved by the US FDA and is currently used in a combination immunotherapeutic regimen for the treatment of children with high-risk neuroblastoma. Here, we review the extensive preclinical and clinical development of anti-GD2 mAbs and the different mechanisms by which they mediate tumor cell killing. In addition, we discuss different mAb-based strategies that capitalize on the targeting ability of anti-GD2 mAbs to potentially deliver, as monotherapy, or in combination with other treatments, improved antitumor efficacy.
Keywords: : cancer immunotherapy, monoclonal antibodies, neuroblastoma
Aside from the debilitating and sometimes life-threatening toxicities associated with surgery, chemotherapy and radiotherapy, the thoughtful implementation of these therapeutic modalities has greatly improved the prognosis for many cancers [1]. However, achieving long-term cancer remission using these therapies alone remains a challenge, in part due to the nonspecific nature of conventional therapy. One approach, with demonstrated efficacy in a growing number of clinical settings, is tumor-reactive monoclonal antibody (mAb) therapy. High-risk pediatric neuroblastoma (NBL) is a childhood cancer that is often refractory to conventional therapy and is characterized by nearly uniform expression of cell surface disialoganglioside GD2 [2]. Careful preclinical and clinical investigations have enabled the development of therapy regimens involving tumor-reactive anti-GD2 mAbs with a beneficial impact on the clinical management of NBL. Dinutuximab, an anti-GD2 mAb, recently received USA and European approval for the treatment of high-risk NBL making it the first approved tumor reactive mAb directed at a glycolipid or intended for use specifically in treatment of a childhood malignancy [3].
Continuing investigations into the mechanisms underlying the efficacy of anti-GD2 mAb therapy have led to the exploration of a number of ‘next-generation’ anti-GD2 mAbs (and mAb-based agents) designed to increase treatment efficacy and decrease toxicity. While these agents specifically target GD2 for use in GD2 expressing malignancies, there is reason to believe that these new modalities, or the concepts they represent, can be adapted and applied to a wide variety of adult and pediatric malignancies.
Tumor-reactive mAb therapy
Tumor-reactive mAb cancer therapy involves a lab-generated mAb designed to recognize and help destroy tumor. Ideally, the tumor antigen targeted by mAb therapy is found preferentially on neoplastic cells as compared with healthy tissues. Most tumor-reactive mAbs target antigens conserved by a cancer-type and shared by a substantial subpopulation of patients with the targeted type of cancer. As a result, mAbs that recognize a cell-surface molecule that is overexpressed, or selectively expressed on a fraction of cancers from patients with a given histological type of cancer can be more readily incorporated into specific cancer treatment regimens without the need to tailor the therapy to the individual. To date, tumor-reactive mAb therapy has been successfully implemented into the treatment regimen of a number of distinct adult and pediatric cancers (Table 1); additional mAb targets, combinations and off-label trials are currently in the pipeline.
Table 1. . US FDA-approved unconjugated monospecific tumor-reactive monoclonal antibodies.
| mAb | Target | Oncologic indication(s) |
|---|---|---|
| Alemtuzumab |
CD52 |
Chronic lymphocytic leukemia |
| Cetuximab |
EGFR |
Head and neck cancer, colorectal carcinoma |
| Daratumumab |
CD38 |
Multiple myeloma |
| Dinutuximab |
GD2 |
High-risk neuroblastoma |
| Elotuzumab |
CD319 |
Multiple myeloma |
| Necitumumab |
EGFR |
Non-small-cell lung cancer |
| Obinutuzumab |
CD20 |
Chronic lymphocytic leukemia |
| Ofatumumab |
CD20 |
Chronic lymphocytic leukemia |
| Panitumumab |
EGFR |
Colorectal carcinoma |
| Pertuzumab |
HER2 |
Breast carcinoma |
| Rituximab |
CD20 |
Chronic lymphocytic leukemia, non-Hodgkin lymphoma |
| Tositumomab |
CD20 |
Non-Hodgkin lymphoma |
| Trastuzumab | HER2 | Breast carcinoma, gastric or gastroesophageal junction cancers |
Major mechanisms of action of unconjugated tumor-reactive mAbs
Antibody-dependent cell-mediated cytotoxicity (ADCC)
The process of ADCC in mAb therapy involves: recognition of a tumor antigen by the mAb; engagement of NK cells, neutrophils and macrophages via their cell surface Fcγ-receptors; tumor cell phagocytosis (macrophages) or destruction (NK cells and neutrophils). ADCC is likely the major antitumor mechanism of most tumor-reactive mAbs. Several investigations have demonstrated correlations between high affinity Fcγ-receptor and favorable clinical response to therapy with a variety of tumor-reactive mAbs, including rituximab [4,5], trastuzumab [6], cetuximab [7] and anti-GD2 mAb [8]. However, other studies have not found such associations [9–11]. The role of NK cells in this clinical response to mAbs is supported by correlations between NK-cell inhibitory receptor repertoire and clinical response [12,13].
Complement-dependent cytotoxicity
In mAb-mediated complement dependent cytotoxicity (CDC), presynthesized, inactive serum proteins are rapidly activated upon contact with tumor-bound mAb resulting in opsonization and lysis of complement-coated tumor cells. Despite data demonstrating a significant role for mAb-mediated CDC in vitro, some cancers (including lung, ovarian and colon) disrupt CDC in vivo, such that the contribution of CDC to the in vivo antitumor effect of most clinically studied mAb seems modest compared with the ADCC [14,15].
Direct cytotoxicity
In the case of tumor antigens that serve a required cell-survival function, blockade of these receptors via mAb targeting can result in cytotoxicity in addition to ADCC. This can include blockade of growth factor receptors overexpressed on malignancies [16]. Intriguingly, some mAbs targeting tumor markers, not classically associated with the induction of cell death (such as GD2), can induce direct tumor cell death [17–19]. Certain data suggest that this may involve cross-linking of cell surface structures by the mAb, as certain structural forms of mAbs and certain membrane antigens, are more susceptible to this type of mAb-induced cell death [20].
Anti-GD2 mAb immunotherapy of high-risk neuroblastoma
NBL, a malignancy of neuro-ectodermal embryonic origin, is the commonest extracranial solid tumor of childhood. Nearly half of newly diagnosed NBL cases are determined to be ‘high-risk', based on clinical features of age, tumor stage, histology, biology and genetic features [21–23]. For children diagnosed with high-risk disease, an aggressive multi-modal therapy regimen comprising multi-agent chemotherapy, surgery, autologous hematopoietic stem cell transplant (HSCT), local radiation therapy and treatment with isotretinoin still results in refractory or relapsed disease for approximately two-thirds of patients [24]. While these nonimmunotherapeutic-based approaches are usually effective in significantly reducing tumor burden, they are unable to completely eradicate malignant cells from most patients. These relatively few surviving cells are capable of producing relapse with a tumor that is now more resistant to previous therapies. Several investigators reasoned that these children would benefit from a targeted therapeutic approach aimed at the detection and elimination of residual NBL cells.
GD2 is a cell surface glycolipid conserved among tumors of neuroectodermal origin including NBL, melanoma (MEL; adult and pediatric) and some osteosarcomas, ewings sarcomas and certain other cancers. It is also expressed at low levels on normal melanocytes and peripheral nerve fibers [2,25]. In malignant cells the biological function of GD2 is incompletely understood, but may modulate cellular adhesion/invasion and/or proliferation [26,27]. The preferential expression of GD2 on tumor cells makes it an attractive target for mAb therapy. The sections that follow summarize preclinical and clinical investigations involved in the development and refinement of anti-GD2 mAb in NBL therapy. Table 2 summarizes the selected published clinical trials with the different anti-GD2 strategies reviewed here. Additionally, other anti-GD2 immunotherapeutic approaches currently under development are discussed, see Figure 1 for a visual representation of these approaches.
Table 2. . Selected summary of published clinical trials targeting GD2 molecules.
| Intervention | Phase | Disease (number of pts) | Clinical response | Ref. |
|---|---|---|---|---|
|
Murine Abs | ||||
| 3F8 |
I |
Metastatic NBL or malignant MEL (17) |
Antitumor responses occurred in 7/17 pts, ranging from CR to MR |
[34] |
| 3F8 |
II |
Stage 4 NBL (16) |
3 pts were long-term survivors between 79 and 130 months after treatment |
[35] |
| 3F8 |
II |
Stage 4 NBL (34) |
14 pts are alive and 13 pts are PF 40–130 months. 38% long-term PFS |
[36] |
| 3F8 + GM-CSF |
II |
NBL (136) |
Median follow-up for survivors was 34 and 36 months for OS and PFS. Outcome at 2 yrs, 97% patients with prior relapse progressed, 62% with no prior relapse progressed |
[8] |
| 3F8 + GM-CSF |
II |
NBL (45) |
12/15 pts treated for NBL-resistant induction therapy had CR, 5/10 patients treated for recurrent NB resistant to retrieval therapy had CR |
[40] |
| 3F8 + GM-CSF + CRA |
II |
Stage 4 NBL (169) |
At 5 yrs from the start, PFS improved from 44% for patients in regimen A to 56% and 62% to patients in regimen B and C. OS was 49, 61 and 81%, respectively |
[41] |
| 3F8 + GM-CSF |
II |
NBL in metastatic osteomedullary sites (79) |
CR 87% by histology and 38% by MIBG. Five-year PFS was 24% |
[42] |
| 14.G2a |
I |
Stage 4 NBL (9) |
CR in 2 pts and PR in 2 pts |
[47] |
| 14.G2a |
I |
Metastatic MEL (12) |
PR in 1 pt, MR in 1 pt and SD in 1 pt |
[48] |
| 14.G2a |
I |
Refractory MEL, NBL or Osteosarcoma (18) |
MR in 3 pts and PR in 2 pts |
[49] |
| 14.G2a + IL2 |
I/IB |
GD2+ malignancies (33) |
PR in 1 pt and CR in 1 pt |
[50] |
|
Chimeric Abs | ||||
| ch14.18 |
I |
Refractory NBL (10) and osteosarcoma (1) |
Although MTD was not reached, clinical responses were observed: 1 PR, 4 MR and 1 SD |
[56] |
| ch14.18 |
I |
Stage 4 NBL (9) |
CR in 2 pts, PR in 2 pts, MR in 1 pt, SD in 1 pt |
[159] |
| ch14.18 |
I |
Stage 4 NBL, under 1 yr (59) |
3-yr EF: 80.5, 87.5 and 75.0% and 3-yr OS: 90.1, 93.8 and 91.7% for patients treated with antibody ch14.18, MT and no further therapy, respectively. |
[59] |
| ch14.18 |
II |
Stage 4 NBL, over 1 yr (334) |
3-yr OS, Ch14.18 (68.5%) was superior to maintenance chemotherapy (56.6%) and no additional therapy (46.85) |
[58] |
| ch14.18 |
II |
Stage 4 NBL, over 1 yr (334) |
The 9-yr EFS rates were 41 ± 4%, 31 ± 5% and 32 ± 6% for MAB ch14.18, NB90 MT and no consolidation, respectively |
[60] |
| ch14.18 + GM-CSF |
I |
NBL |
No objective responses |
[61] |
| ch14.18 + IL2 |
IB |
MEL (24) |
1 CR, 1 PR, 8 SD, 1 pt with >50% decrease in metastases |
[63] |
| ch14.18 + R24 + IL2 |
I |
MEL or Sarcoma (27) |
PR in 2 pts, SD in 4 pts and 1 pt without evidence of disease |
[64] |
| ch14.18 + GM-CSF + IL2 |
I |
NBL (25) |
The estimated 3-yr survival probability was 78% |
[65] |
| ch14.18 + GM-CSF + IL2 + CRA |
II |
High-risk NBL (126) |
Immunotherapy was superior to standard therapy with EFS (66 ± 5% vs 46 ± 5% at 2 yrs) and OS (86 ± 4% vs 75 ± 5% at 2 yrs) |
[66] |
|
Humanized mAbs | ||||
| hu14.18K322A |
I |
Refractory/recurrent NBL (38) |
CR in 4 pts, PR in 2 pts |
[79] |
|
Immunocytokines | ||||
| hu14.18-IL2 |
I |
Refractory/recurrent NBL (27) and MEL (1) |
No measurable responses; 3 pts showed evidence of antitumor activity |
[96] |
| hu14.18-IL2 |
I |
Metastatic MEL (33) |
No measurable responses; 8 pts had SD |
[97] |
| hu14.18-IL2 |
I/II |
Stage 4 MEL (9) |
No measurable responses; 2 pts had SD |
[98] |
| hu14.18-IL2 |
II |
Metastatic MEL (14) |
PR in 1 pt, SD in 4 pts |
[99] |
| Hu14.18-IL2 |
II |
Relapsed/refractory NBL (38) |
CR in 5 pts |
[100] |
|
Radiolabeled mAbs | ||||
| 131I-3F8 |
I |
GD2+ tumors metastasized to CNS and leptomeninges (15) |
3 pts with objective radiographic and/or cytologic responses. 2 pts in remission for more than 3.5 yrs |
[160] |
| 131I-3F8 or 131I-8H9 |
I |
Recurrent NBL metastatic to the CNS (21) |
17 pts are alive 7–74 months (median 33 months) since CNS relapse, with all 17 remaining free of CNS NBL |
[117] |
|
CAR T cells | ||||
| GD2-CAR T cells |
I |
High-risk NBL (19) |
CR in 3 pts |
[136] |
| GD2-CAR T cells |
I |
Recurrent/refractory advanced-stage NBL (11) |
CR in 1 pt and 4 pts had evidence of tumor necrosis or regressions |
[161] |
|
Anti-idiotype vaccine | ||||
| TriGem | I | Advanced MEL (47) | The Kaplan–Meier derived overall median survival was not reached | [162] |
Abs: Antibodies; CAR: Chimeric antigen receptor; CR: Complete remission; CRA: 13-cis-retinoic acid; CNS: Central nervous system; EFS: Event-free survival; MEL: Melanoma; MIBG: Metaiodobenzylguanidine; MR: Mixed responses; MRD: Minimal residual disease; MT: Maintenance chemotherapy; MTD: Maximum tolerated dose; NBL: Neuroblastoma; No: Number; OS: Overall survival; PF: Progression free; PFS: Progression-free survival; PR: Partial remission; pts: Patients; SD: Stable disease; Yr: Year.
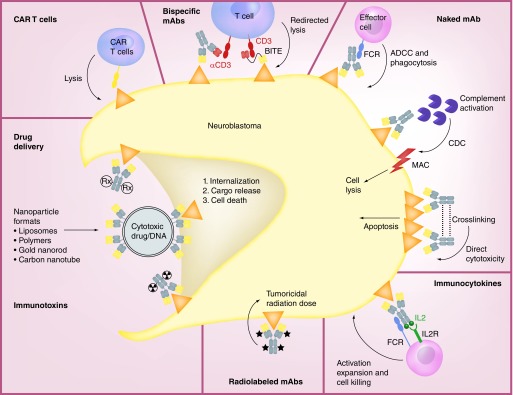
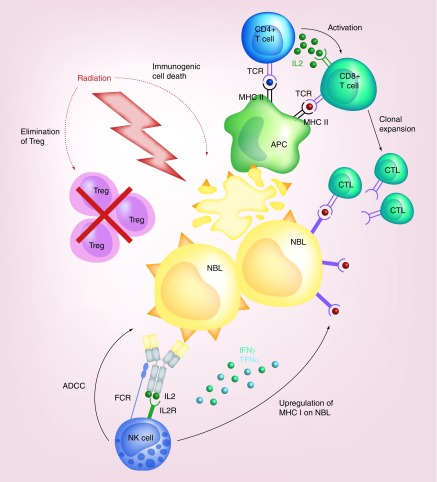
Figure 1. . Effector mechanisms of anti-GD2 mAbs and mAb-based approaches.
Binding of naked anti-GD2 mAbs to the surface of NBL cells leads to three possible effector mechanisms: the recruitment of FCR expressing effector cells such as NK cells and granulocytes to mediate ADCC and monocytes and macrophages to mediate phagocytosis; induction of CDC by binding of C1q to the mAb, subsequent activation of the complement cascade and delivery of MAC to the tumor cell membrane; and direct cytotoxicity by crosslinking of the mAbs and the induction of apoptosis. IL2-based immunocytokines concentrate IL2 at the tumor site and lead to the activation of effector cells for tumor cell killing via engagement of both their IL2R and FCRs. Radiolebled mAbs serve as markers for radioimmunodetection and can deliver tumoricidal doses of radiation to the tumor cells for cell death. Immunotoxins and drug conjugates deliver different agents to the cell membrane where they are internalized allowing the intracellular release of the toxic cargo leading to subsequent cell death. CAR T cells have been ex vivo engineered to recognize the tumor antigen and lead to tumor cell lysis. Bispecific mAbs exist in various formats, such as hybrid bifunctional mAbs with two different antigen-specific regions or as BITE with the main goal of simultaneously engaging tumor antigen and costimulatory molecules, such as CD3 to redirect T cells for tumor lysis.
ADCC: Antibody-dependent cell cytotoxicity; BITE: Bispecific T-cell engagers; CAR: Chimeric antigen receptor; CDC: Complement-dependent cytotoxicity; FCR: Fc-receptor; IL2R: IL2 receptor; mAb: Monoclonal antibody; MAC: Membrane attack complex.
Development of anti-GD2 mAbs
Murine anti-GD2 mAbs
IgG3 - 3F8 & 14.18
The murine IgG3 antibodies, 14.18 and 3F8, were among the first anti-GD2 mAbs described in the 1980s. These became promising candidates for clinical applicability due to their high GD2-binding affinity, prolonged stability once bound to antigen and the ability to kill tumor cells [2]. Preclinical testing demonstrated that 3F8 leads to dose-dependent killing of tumor cells through immune effector mechanisms such as: CDC [28]; ADCC [13,29,30]; and phagocytosis of mAb-opsonized tumor cells [31]. Concurrently, 14.18 was shown to have in vitro activity by effectively lysing tumor cells by ADCC and CDC [32,33]. Importantly, 14.18 demonstrated antitumor effects in vivo by suppressing the establishment and growth of human NBL tumors in mice [2]. Together, these data provided rationale for the implementation of anti-GD2 mAb therapy in clinical trials.
3F8 became the first anti-GD2 mAb to be tested in patients with NBL and MEL [34]. Clinical Phase I and II studies demonstrated the antitumor potential of 3F8 as a single agent in the setting of minimal residual disease (MRD), by raising event-free survival and achieving long-term remissions in some patients [34–36]. These clinical studies revealed two major considerations for the further development of anti-GD2 immunotherapy. First, 3F8 infusion was accompanied by substantial neuropathic pain; that although reversible and tolerable with analgesics, limited the dose administered to patients. Second, 3F8 proved to be immunogenic, with the majority of patients developing human anti-mouse antibodies (HAMA) after 3F8 infusion. Patients with high HAMA titers did not exhibit side effects or antitumor responses following 3F8 treatment, suggesting that high levels of HAMA in the serum interfered with 3F8 activity [34].
In an effort to amplify the antitumor effect of 3F8, cytokines (such as IL2 and GM-CSF) were added to the immunotherapeutic regimen to expand the effector cell populations involved in tumor cell killing and activate them for greater cytotoxicity. Preclinical data showed an increase in activation markers and enhancement of ADCC when combining IL2 [37,38] and GM-CSF [39] with 3F8 in vitro. Phase II clinical trials of combination therapy with 3F8 and GM-CSF demonstrated that treatment was well tolerated showing promise for the treatment of minimal residual NBL [8,40]. In a retrospective analysis of consecutive Phase II trials over 20 years, the combination of 3F8 with GM-CSF and cis-retinoic acid (CRA) was effective against chemotherapy-resistant marrow MRD, improving overall survival over time (see Figure 2) [41]. A recent, large Phase II trial revealed that 3F8 treatment with prolonged exposure to GM-CSF via subcutaneous (sc.) injection of GM-CSF appeared superior to a historical control group receiving 3F8 together with intravenous administration of GM-CSF, raising 5-year progression-free-survival from 11% in the intravenous group to 24% in the subcutaneous group [42]. Phase II studies of 3F8 + GM-CSF combination therapy are still ongoing [43].
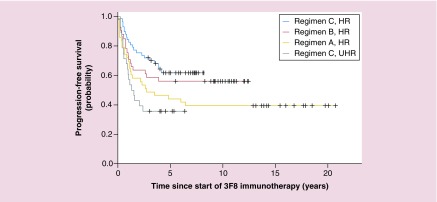
Figure 2. . Progression-free survival over two decades for 169 patients with stage 4 neuroblastoma in first remission after consecutive immunotherapy regimens.
3F8 alone (regimen A – HR; n = 43), 3F8 + intravenous GM-CSF + CRA (regimen B – HR; n = 41) and 3F8 + subcutaneous GM-CSF + CRA (regimen C – HR; n; = 57 and regimen C – UHR; n = 28); p = 0.018 (derived from log-rank test to compare progression-free survival among these four groups).
CRA: 13-cis-retinoic acid; HR: High risk; UHR: Ultra high risk.
Adapted with permission from [41] © American Society of Clinical Oncology (2012). All rights reserved.
IgG2a – 14.G2a & ME36.1
To potentially increase the antitumor efficacy of anti-GD2 mAbs, isotype switch variant hybridomas were used to produce murine IgG2a subclasses, which have been associated with enhanced ADCC. ME36.1, an IgG3 mouse mAb that was class switched to IgG1 and IgG2a, recognizes both GD2 and GD3 [44]. Preclinical work demonstrated that ME36.1 inhibited growth of primary and metastatic MEL in nude mice [45]. 14.G2a, a class switch IgG2a variant of 14.18, was developed for clinical testing after demonstrating better ADCC in vitro when compared with 14.18 and effectively suppressing the growth of human NBL xenografts in immunodeficient mice [46]. Comparable to 3F8, Phase I studies of 14.G2a in patients with NBL and MEL showed excellent tumor localization of radiolabeled 14.G2a, modest antitumor activity accompanied by dose-limiting toxicities and the development of HAMA in virtually all patients [47–49]. Combination treatment with IL2 and 14.G2a was tolerable in NBL patients resulting in modest antitumor activity [50].
Chimeric & humanized mAbs
ch14.18
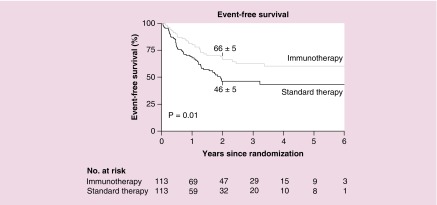
In order to circumvent the immunogenicity associated with mouse mAbs, a human/murine chimeric antibody was created by joining the mouse variable genes of the 14.18 mAb with the human constant IgG1 and κ genes [51]. The ch14.18 mAb has comparable binding affinity to GD2, equal ability to mediate CDC and superior ability to mediate ADCC [52,53]. Initial studies demonstrated that ch14.18 has a longer half-life and is less immunogenic when compared with murine mAbs [54,55]. Phase I and II clinical trials using ch14.18 as a single agent resulted in a similar toxicity and efficacy profile to 14.G2a with severe pain continuing to be a common adverse effect [56–60]. The first large evaluation of ch14.18 monotherapy for patients following completion of conventional therapy, evaluated in a nonrandomized trial, showed no significant advantage of the ch14.18 therapy [58]. Subsequent retrospective analysis of 11 years of follow-up did demonstrate significant benefit of ch14.18 on overall survival over chemotherapy and no maintenance therapy [60]. Ch14.18 was combined with GM-CSF [61,62] or with IL2 [63,64], where Phase I studies demonstrated that simultaneous administration of the mAb with either of these cytokines was safe. To capitalize on the activation potential of both of these cytokines, a treatment regimen with ch14.18 + GM-CSF + IL2 + CRA was developed by The Children’s Oncology Group (COG) [65]. The regimen had substantial mAb and cytokine related toxicity, but was tolerated acceptably by the majority of patients. Thus COG chose to initiate a large randomized trial of this regimen for patients within 100 days of completing the HSCT component of their front-line NBL treatment. This randomized trial indicated that the immunotherapy arm was superior to standard therapy with respect to event-free survival (66 ± 5% vs 46 ± 5%) and overall survival (86 ± 4% vs 75 ± 5%) at 2 years (see Figure 3) [65,66]. Based largely on these data, the ch14.18 mAb, as part of this regimen with IL2 and GM-CSF and isotretinoin has been approved by the US FDA and by the EMA, and is now known, generically as dinutuximab [66]. While improvements in OS and EFS are still very much needed, at least in the USA, where COG represents approximately 90% of institutions treating children with cancer, this regimen is the current ‘standard’ for children with newly diagnosed high-risk NBL following HSCT.
Figure 3. . Event-free survival for 226 patients randomized into the treatment groups.
13-cis-retinoic acid (CRA) alone (standard therapy) versus immunotherapy (ch14.18 monoclonal antibody + GM-CSF + IL-2 + CRA) for high-risk neuroblastoma. Data are shown for event-free survival for all 226 patients. The estimated survival (±SE) at 2 years is indicated in the plot.
Adapted with permission from [66] © Massachusetts Medical Society (2010). Reprinted with permission from Massachusetts Medical Society.
Continued research is evaluating the components of this regimen clinically. As substantial toxicity is associated with the IL2 courses, the European Neuroblastoma Consortium, SIOPN, has evaluated a separate regimen of IL2 by subcutaneous injection [67] and more recently combined this with ch14.18 mAb. Initial analyses, based on intent to treat assignments, suggest no added benefit for including the IL2, from this study. However the toxicity associated with the IL2 in this study prompted more of the IL2 patients to receive less of the assigned mAb. For those patients that completed all assigned therapy, there was a trend toward benefit from the IL2 [68]. In addition, Lode et al. have found that the neuropathic pain associated with four daily infusions of ch14.18 is substantially lessened when the same total mAb dose per course is given as a 10-day constant infusion [69]. SIOPN is now evaluating this 10-day mAb regimen, with and without a modified IL2 subcutaneous regimen. Ongoing studies by COG are evaluating the potential for using the ch14.18 mAb in the same courses as chemotherapy, rather than after completion of chemotherapy [70] (clinicaltrials.gov NCT01767194).
Hu14.18K322A mAb
Chimeric mAbs are less immunogenic than murine mAbs but the remaining mouse component in the antigen-binding region of ch14.18 can still stimulate human anti-chimeric antibodies (HACA) [54,63,65,71,72]. To reduce the HACA response, humanized mAbs have been developed by introducing the hypervariable regions of the murine 14.18 mAb into the human IgG1 mAb framework. By limiting the rodent characteristics to only the hypervariable regions the percentage of original mouse mAb capable of inducing allergic reactions is decreased. The hu14.18K322A mAb was created with three major differences aimed at improving clinical applicability: humanized mAb to be less immunogenic [73], single amino acid substitution in the Fc region virtually abrogating complement activation to induce less neuropathic toxicity [74] and production in YB2/O cell lines to decrease fucosylation in the Fc region to augment interaction with FcRs and increase ADCC [75]. Preclinical work demonstrated that in combination with CD40/CpG, hu14.18K322A induced a better antitumor effect in mice through interactions with myeloid and NK cells [76]. Clinical studies confirmed that the murine hypervariable regions within hu14.18K322A retained antigen specificity, with hu14.18K322A being able to reach tumor targets without nonspecific uptake as seen for murine and chimeric mAbs [77]. Studies evaluating the toxicity of hu14.18K322A demonstrated a dramatic decrease in CDC while retaining ADCC activity in vitro and a significant decrease in pain in patients. This reduction in pain made therapy more tolerable allowing an increase in the maximum tolerated dose [73,78,79]. Pilot clinical studies are underway to test combination treatments predicted to augment antitumor effects, such as the addition of standard chemotherapy, IL2, GM-CSF and infusion of NK cells (clinicaltrials.gov NCT02130869, NCT01576692, NCT01857934).
Hu3F8 mAb
The original murine 3F8 (mu3F8) mAb has also been humanized to circumvent HAMA responses. In vitro analysis demonstrated that hu3F8 was 200-fold more efficient in mediating ADCC than m3F8, but had substantially lower ability to activate complement. Since CDC has been associated with the downregulation of ADCC and implicated in the pain side effects observed in anti-GD2 therapy, this selective increase in ADCC could potentially translate into a more effective and tolerable agent in the clinic [80]. Early results from Phase I clinical trials demonstrate low immunogenicity, favorable pharmacokinetics and less dose limiting pain side effects for hu3F8 than for the mu3F8. [43]
Anti-O-Acetyl GD2
O-acetyl-GD2 (OAc-GD2) is a naturally occurring GD2 derivative that is highly expressed on the surface of GD2 positive tumor cells, somewhat comparable to GD2 on those tumor cells [81,82]. Appealingly, unlike GD2, OAc-GD2 appears to not be expressed on peripheral nerves. Therefore, targeting this antigen might reduce the neuropathic pain associated with anti-GD2 mAb therapy while retaining antitumor efficacy. 8B6 is an anti-OAc-GD2 mAb with no cross reaction to GD2 [82,83]. When compared with 14.G2a, 8B6 demonstrates comparable epitope binding affinity and in vitro suppression of tumor growth [82]. In animal models, 8B6 is as efficient but better tolerated than 14.G2a, highlighting the potential use of this mAb to avoid anti-GD2 associated neuropathic side effects [82].
The less immunogenic chimeric c.8B6 with a human IgG1 isotype, retains the binding affinity and has been shown to have similar functional properties to ch14.18 both in vitro and in vivo, sharing the same anti-NBL effects but with much less allodynic activity [84]. The lack of OAc-GD2 expression on nerves and the lack of pain associated with c.8B6 provide a rationale for the future clinical application of c.8B6 in high-risk NBL patients.
Anti-GD2 mAb-based fusion strategies to enhance therapeutic potential
Immunocytokines
Numerous preclinical and clinical trials administering anti-GD2 mAbs together with cytokines, such as IL2, IL15 and GM-CSF, have demonstrated clinical benefit over mAb monotherapy [41,42,50,61–66]. In order to capitalize on the antitumor effects observed with these combination therapies immunocytokines (ICs) were created. By combining a tumor-specific mAb and an immunostimulatory cytokine into one fusion protein, ICs concentrate cytokines within the tumor microenvironment leading to localized immune cell activation and the potential reduction of systemic cytokine-induced toxicities [85].
IL2-based ICs
The ch14.18-IL2 fusion protein was the first anti-GD2 IC created by combining one human IL2 molecule to each of the heavy chains of an intact ch14.18 mAb [86]. ch14.18-IL2 is able to bind GD2 expressed on the surface of cells indicating that it retains its antigen binding ability and can effectively target IL2 to the tumor site leading to tumor lysis [87]. In vitro analyses showed that ch14.18-IL2 activated human effector cells to the same extent as soluble human IL2 and was able to mediate ADCC effectively; demonstrating that both the IL2 and Fc components of the fusion protein remained intact and able to interact with their respective receptors [86,88]. When tested in mice, pharmacokinetics studies demonstrated that fusion protein prolonged the half-life of IL2 when compared with soluble IL2, highlighting the potential for ch14.18-IL2 to lead to more efficient IL2-induced immune cell activation in vivo [89]. Moreover, ch.14.18-IL2 demonstrated superior antitumor effects when compared with equivalent amounts of the ch14.18 or hu14.18 mAb and IL2 administered simultaneously as separate agents [87,90,91]. The promising preclinical data with ch14.18-IL2 led to the development of a hu14.18-IL2, a humanized IC, to recapitulate in patients the antitumor effects observed with ch14.18-IL2 while minimizing the immunogenicity associated with the chimeric mAb [92]. Mice studies in various tumor models indicated that hu14.18-IL2 elicited a prolonged antitumor response via NK- and T-cell involvement, eradicated metastases and exhibited protective immunity to tumor re-challenge [93–95]. Accordingly, Phase I trials in children with refractory or recurrent NBL and adults with advanced MEL demonstrated that intravenous hu14.18-IL2 could be administered safely with reversible toxicities at doses that induced immune activation [96,97]. Nevertheless, no complete or partial responses were seen, suggesting that greater antitumor activity might be seen in patients with less bulky tumors [96]. Furthermore, Phase II trials in patients with MEL demonstrated immune activation with reversible toxicities [98,99] and some patients with refractory NBL that only exhibited disease evaluable by metaiodobenzylguanidine or bone marrow histology demonstrated clear evidence for antitumor activity in the setting of nonbulky disease [100].
In an effort to further decrease IL2 related toxicities and enhance efficacy, a new generation of ICs has been recently created by fusing the IL2 molecules to the C-terminus of the light chains on the hu14.18 mAb rather than on the heavy chains [101]. This modification creates a sequestering effect that results in hindered binding of the IL2 to intermediate affinity IL2Rs by limiting access to a critical contact residue of IL2 by the IL2R β-chain, but maintaining binding and activation of the high affinity IL2Rs [101]. Changing the location of the IL2 molecules allows for preferential targeting and activation of high affinity IL2Rs (associated with antitumor effects) and reduced stimulation of intermediate affinity IL2Rs (associated with IL2-induced toxicity). This new platform for constructing ICs with varying degrees of affinity for IL2Rs represents an innovative strategy to potentially reduce dose-limiting side effects while retaining the desired efficacy.
IL15-based ICs
IL15 has similar biologic activity to IL2, including the generation and persistence of cytotoxic T cells and NK cells associated with antitumor benefit [102]. Unlike IL2, IL15 does not activate T regulatory cells capable of attenuating the desired immune responses [103]. Due to the nature of IL15 trans-presentation to the IL15 receptor (IL15R), the soluble IL15/IL15Rα complex exerts higher immune stimulatory effects than IL15 alone [103]. The c.60C3-RLI IC was created by fusing one RLI (IL15Rα/IL15 fusion protein) molecule to each of the heavy chains of the anti-GD2 mAb c.60C3 [104]. Preclinical studies demonstrated that the c.60C3-RLI retained the cytokine activity of RLI as well as the mAb effector functions (ADCC and CDC), displaying strong antitumor effects in mouse models. Like the IL2-based ICs, its therapeutic potency was higher than those of RLI and anti-GD2 alone or in combination [104,105]. The potential decrease in Treg activation and the retained efficacy expected from IL15-based ICs indicates these may be attractive candidates for clinical application.
Immunotoxins
Immunotoxins were designed to capitalize on the highly specific targeting potential of anti-GD2 mAbs by delivering toxins of plant or bacterial origin directly into malignant cells. These molecules are predicted to kill tumor cells by binding to the surface of tumor cells through antigen recognition and entering the cells via endocytosis; leading to release of the toxin and destruction of the target cell by intracellular toxin-induced death pathways [106].
Initial experiments using 14.G2a-RA, created by conjugating Ricin A (a plant-derived ribosome inactivating lectin) to 14.G2a, demonstrated that GD2 expression and internalization rate by tumor cells were key factors in determining cytotoxic potency in vitro [107]. Further work with various chemically linked ricin A anti-GD2 ICs demonstrated significant in vivo antitumor activity and increased survival in the human NBL model in SCID mice [108,109]. Alternatively, 14.G2a has also been coupled to gelonin, a separate ribosome-inactivating plant toxin reported to localize better within tumors and exert more potent effects than ricin A [110]. Other strategies utilizing the fusion of single chain anti-GD2 antibody Fv fragments (scFvs) with bacterial toxins aim to mediate targeted cell death independent of the host’s immune function. Fusions comprising anti-GD2 scFvs linked to toxins include: DT5F11 (containing the catalytic domain of diphtheria toxin) and 14.18-ETA (containing a deletion mutant of pseudomonas exotoxin A). These were each shown to directly kill GD2+ tumor cells through inhibition of protein synthesis [111,112].
Radiolabeled mAbs
Radioimmunotherapy refers to the use of mAbs as carriers to deliver radioactivity to the tumor site, either as tracers or as targeted therapeutics [113]. This targeted approach is appealing for radiation sensitive tumors such as NBL and decreases the toxicity associated with external beam radiation.
Anti-GD2 radiolabeled mAbs have been tested in the clinic for radioimmunodetection of primary and metastatic disease. 131I-3F8 was sensitive and specific at detecting sites with metastatic disease as shown by CT/MRI, and identifying tumors that were confirmed histologically as NBLs [114]. Likewise, 99Tc-14.18 demonstrated superior sensitivity in the detection of tumor recurrence and metastases when compared with metaiodobenzylguanidine, demonstrating the value of these reagents for the assessment of therapy response, disease progression and early detection of metastases [115].
Radioimmunotherapy seeks to kill malignant cells directly by delivering tumoricidal doses of radioactivity to the tumor site, particularly against MRD. Early preclinical studies demonstrated that 3F8 could target 131I to established NBL xenograft in nude mice leading to 95% tumor shrinkage and tolerable toxicities [116]. In a Phase I study of patients with recurrent metastatic CNS NBL, 131I-3F8 was well tolerated and significantly improved survival when compared with historical results [117].
Recently, 5F11-scFv-SA, an anti-GD2 single chain variable fragment coupled to Streptavidin was created with the purpose of pretargeting biotin-conjugated radioisotopes. This multi-step approach involves the administration of 5F11-scFv-SA, followed by a chemical clearing agent to remove unbound mAb and then 111In-DOTA-biotin (DOTA is a molecule that chelates radionuclides) [118]. By separating the administration of the mAb from the cytotoxic agent, unwanted systemic radiation damage due to slow plasma clearance of radiolabeled mAbs can be decreased. Initial studies demonstrated selective tumor uptake of 5F11-scFv-SA and favorable tumor-to-nontumor ratios when compared with single step radiolabeled-mAb treatment [118].
Drug delivery via anti-GD2 mAbs
Anti-GD2 mAbs have also been used in the development of drug delivery systems designed to facilitate tumor-specific delivery of active agents while reducing systemic toxicities associated with conventional ‘free’ drugs. One approach known as antibody–drug conjugates combines cytotoxic drugs through a linker region with the tumor specific mAb. The anti-GD2 antibody–drug conjugate created by linking 14.G2a to a chemically stable calicheamicin analog (DNA-damaging antibiotic) was found to suppress tumor growth and dissemination of liver metastasis in a syngeneic model of murine NBL [119].
Furthermore, anti-GD2 mAbs can be used to impart selectivity to nanoparticle-based carriers which have the potential to load and transport higher amounts of drugs, increase penetration to the tumor and metastatic sites and overcome the limiting pharmacokinetic properties that result in insufficient drug delivery associated with other agents [120]. Different nanoparticle formats that have been bioconjugated with anti-GD2 full size mAbs or Fab fragments include: liposomes, which have small diameter and are able to differentially accumulate and penetrate into solid tumors (with highly permeable capillaries) relative to normal tissue (with less permeable tight junctions) [121–124]; porous silica nanoparticles, which are inert, biodegradable, nontoxic and more stable due to uniform particle size [125]; gold nanorods and carbon nanotubes capable of absorbing near-infrared laser light leading to in vitro photothermal destruction of tumor cells [126,127].; and iron-based nanoparticles that enable direct delivery of drugs carried by the nanoparticle [128].
Nanoparticles can be loaded with a variety of cytotoxic or other anti-cancer agents. There have been many studied, including: doxorubicin (chemotherapeutic drug) [122]; antisense oligonucleotide (downregulate c-myb and c-myc proto-oncogenes) [121,123].; fenretinide (HPR), a synthetic retinoid acid derivative that promotes apoptosis of NBL cells in vitro [124]; miR-34a (multi-gene targeting microRNA capable activating caspase-mediated apopototic pathways) [125]. Some of these targeted nanoparticles showed promising antitumor effects in vivo.
Redirecting T cells for tumor cell killing via anti-GD2 mAbs
Bi-specific mAbs
Bi-specific mAbs (biAbs) have two different binding specificities able to crosslink different target antigens expressed on the same cell or two different cells. This dual specificity allows biAbs to engage tumors through antigen binding while simultaneously activating immune effector cells by targeting costimulatory molecules or receptors [129]. Due to their lack of Fc receptors, the potent tumoricidal capacity of T cells is underutilized in traditional mAb therapy. Therefore, most biAbs under development target the CD3 complex expressed on T cells which initiates the signaling cascades that lead to T-cell activation. This strategy bridges uncommitted T cells with tumor targets, forming effective immune synapses and redirecting the cytotoxic potential of T cells in order to kill cancer cells in a non-MHC-restricted manner [130]. Preclinical data with biAbs produced by fusing anti-CD3 scFvs to anti-GD2 IgG mAbs [131] or heteroconjugating full anti-CD3 and anti-GD2 mAbs [109,132] have demonstrated their ability to cross-link GD2+ tumors to CD3+ CTLs in the absence of MHC expression by the tumor cell, leading to specific cytolysis of tumor cells via perforin and granzyme B injection.
Second generation biAbs under development include a tri-functional biAb containing a chimeric mouse IgG2a/rat IgG2b Fc region which has been genetically engineered to preferentially bind activating FcRs in order to effectively engage antigen presenting cells, such as macrophages and dendritic cells [133]. Recently, Cheng et al. optimized an anti-GD2/anti-CD3 biAb using the monovalent scFv platform which joins two scFvs as a single polypeptide chain in tandem. Also referred to as bispecific T-cell engager (BITE) technology, these BITE biAbs seek to improve therapeutic efficacy by monovalently binding T cells with lower affinity thus avoiding nonspecific T-cell activation until being presented in a multivalent manner by a target cell. Preclinical work using this anti-GD2 BITE biAb was successfully validated in vitro against a panel of GD2+ cell lines and in vivo in xenograft tumor models [134].
Anti-GD2 chimeric antigen receptor (CAR) T cells
In the anti-GD2 CAR T-cell immunotherapeutic strategy, peripheral T cells are collected from a patient, genetically altered to express a monoclonal scFv specific to GD2 that is linked to intracellular costimulatory molecule(s), grown in cell culture to high number and re-infused into the same patient [135]. This approach allows T cells to recognize and destroy GD2+ tumor cells irrespective of HLA restriction. Studies show that anti-GD2 CAR-T cells induce long-term antitumor efficacy without the neuropathic pain associated with anti-GD2 mAb infusion. [136]. In 11 high-risk NBL patients with active disease at the time of treatment with anti-GD2 CAR T cells, three patients achieved complete remission; two of whom remained without evidence of disease at 5 years post treatment [136]. In these patients, the CAR-T’s were potent EBV-reactive T cells designed (by gene transfer) to also recognize GD2 expressing cells, in an effort to promote in vivo CAR-T-cell expansion. This proved effective for some patients and favorable long-term clinical responses/remission was correlated with CAR-T persistence in circulation [137]. Current clinical trials involving anti-GD2 targeting CAR-T include: NCT01953900 (varicella zoster virus and GD2 specific CAR-T), NCT01822652 (anti-GD2 CAR-T + anti-PD1 checkpoint blockade mAb), NCT02107963 (anti-GD2 CAR-T) and NCT02439788 (anti-GD2 NKT CAR). While undoubtedly more complicated and less ‘widely available’ than mAb-based therapies, as the CAR-T approach requires individualization of therapy, the CAR-T approach to GD2 positive malignancies has produced some very exciting results warranting cautious optimism.
Anti-idiotype vaccines
As previously discussed, clinical study of the host response to mAb therapy has demonstrated that a subset of patients mount an antibody response against the therapeutic mAb. When such an endogenous antibody is directed against the mAb’s antigen binding region (idiotype or ID) it is termed anti-ID. In tumor-reactive mAb therapy, high levels of anti-ID mAbs are a concerning development as they can decrease the detectable level of mAb in serum and interfere with desired mechanisms of antitumor action of the mAb [138,139].
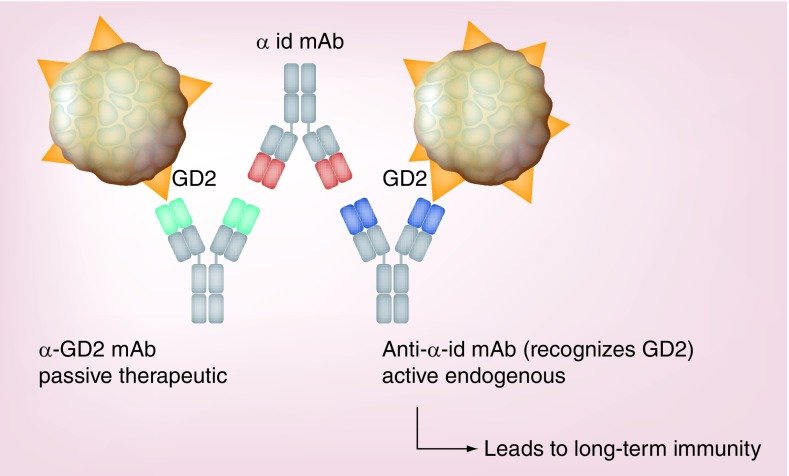
Though at first counterintuitive, some studies have shown the development anti-ID antibody after tumor-reactive mAb therapy to be correlated with clinically evident antitumor activity [140]. One explanation for this paradoxical phenomenon relies on the idiotype network theory [141]. Briefly, this theory suggests that after the development of an anti-ID Ab by the treated patient, the immune response continues and subsequently develops an antibody response capable of recognizing the specific idiotype component of the anti-ID Abs (see Figure 4). In some cases, this patient-derived antibody is not only an ‘anti-anti-ID’, but can also recognize the original tumor antigen that was recognized by the therapeutic mAb. As this is now an ‘active’ immune response, generated by the patient’s immune system (rather than a passive antibody reaction relying only on the presence of the therapeutic mAb), it has the potential for resulting in long-term antitumor immunity. In fact, in a trial of NBL patients treated with anti-GD2 mAb, long-term progression-free survival and overall survival were significantly correlated with the induction of an anti-anti-ID antibody network [142].
Figure 4. . Development of an anti-anti-idiotypic monoclonal antibodies network.
The α-GD2 therapeutic mAb (green) acts as an antigen, eliciting the production of an α-id mAb (red) that recognizes the antigen-binding site of the α-GD2 mAb. This α-id mAb mimics the structure of GD2 leading to the generation of an anti-α-id mAb (purple) with parental antigenic specificity capable of recognizing the antigen-binding site of the α-id mAb and the original antigen, GD2.
The anti-anti-ID approach to cancer immunotherapy has been supported in a number of preclinical settings. In preclinical models of NBL, vaccination with 1A7 (a murine mAb directed against the 14.18-idiotype anti-GD2 mAbs) resulted in sera with GD2 reactivity capable of mediating ADCC in vitro [143]. When tested in 47 patients with advanced MEL, 1A7 was well tolerated with virtually no toxicity, detectable levels of GD2-specific antisera in 40/47 patients and some signs of a possible antitumor effect [144]. In a separate approach, racotumomab, an anti-ID mAb mimicking a different but similar ganglioside to GD2, was evaluated in a Phase I trial demonstrating its relative safety, though this study was not designed to evaluate efficacy [145].
Using anti-GD2 mAb injected intratumorally to function as an ‘in situ’ vaccine & induce an adaptive T-cell response
While one purpose of tumor-reactive mAb therapy is to enable systemic delivery to target microscopic undetected metastasis and induce CDC or ADCC against them, certain additional immune activation can be obtained by direct intratumoral delivery of mAbs. When an anti-GD2 based IC (hu14.18-IL2) is injected intratumorally, a superior antitumor effect is seen than when the same amount of IC is injected intravenous [146]. In mice bearing relatively small GD2+ tumors, intratumorally was superior to intravenous treatment resulting in the resolution of both primary and distant tumors, increasing numbers of tumor infiltrating lymphocytes, achieving better IC retention, enhancing the antitumor effect and improving mice survival [146,147]. Importantly, tumor burden appeared as a strong predictor of IC-induced lymphocyte infiltration and treatment outcome, with smaller tumors responding better [148]. This antitumor effect involves both NK cells and T cells [147,148].
More recent data indicate that pretreatment with ‘palliative dose’ radiation therapy (12 Gy) can allow intratumoral injection of the hu14.18-IL2 to eradicate macroscopic (200 mm3) GD2+ murine tumors in syngeneic mice, leaving most mice tumor free [149,150]. These tumor-free mice subsequently demonstrate a tumor specific, T-cell mediated, memory response, enabling them to reject rechallenge with GD2+ or GD2-variants of the GD2+ MEL that they initially destroyed. In this setting, the anti-GD2 IC, when combined with radiotherapy, is enabling the existing tumor to function as an ‘in situ vaccine’, which that results in protective, functional adaptive immunity (see Figure 5). Further preclinical development is ongoing in order to move this concept into clinical testing.
Figure 5. . Combination of radiation therapy and intratumoral injection of anti-GD2 monoclonal antibodies as an ‘in situ’ vaccine to induce an adaptive immune response.
Pretreatment with palliative doses of radiation therapy leads to the elimination of T regulatory cells and immunogenic tumor cell death. Followed by mAb/IL2 therapy, which facilitates tumor cytotoxicity and creates a favorable environment for antigen presentation and the induction of adaptive immunity with the creation of long-term tumor-specific memory.
ADCC: Antibody-dependent cell cytotoxicity; APC: Antigen-presenting cell; CTL: Cytotoxic T lymphocyte; FCR: Fc-receptor; IL2R: IL2-receptor; MHC: Major histocompatibility complex; NBL: Neuroblastoma; NK cell: Natural killer cell; TCR: T cell receptor; Treg: T regulatory cell.
Future perspective
In some patients with clinically evident cancer, histologic analyses show evidence for an adaptive, albeit ineffective, endogenous antitumor adaptive immune response. It appears that these patients are the most likely to benefit from the implementation of ‘checkpoint blockade’ via targeting of the CTLA-4 and/or PD-1 axis [151]. This strategy has shown promising results in a subset of patients for almost every type of solid tumor studied [152–154]. The expression of these surface molecules on the tumor cells is required for the success of this therapeutic strategy. Therefore, the fact that there is evidence demonstrating that PD-L1 is upregulated in NB cell lines [155] as well as in tumor cells isolated from NB patients [156], supports the use of this strategy in the clinic. In contrast, patients whose tumors show little evidence for an ongoing adaptive immune response are less likely to benefit from checkpoint blockade [157]. These patients may benefit from approaches designed to engage the innate immune defenses toward tumor eradication, via tumor recognition facilitated through tumor-directed mAbs and their derivatives. After recognition of the tumor by the mAb-derived treatment, these patients may become candidates for additional therapies to subsequently augment their adaptive immune response. One possibility is the combination of treatments that target additional NB-associated surface molecules in order to increase the possibility of binding tumor cells that might selectively downregulate surface molecules and evade one of the targeting mAbs. One example of this strategy is the recent work by Kramer et al., combining radio-iodinated anti-GD2 and anti-B7-H3 mAbs [117].
The clinical benefit observed with dinutuximab implementation into the therapeutic regimen for high-risk NBL occurred only after careful analysis of preclinical and clinical data, which revealed: the patient population (patients with MRD) most likely to benefit; the predominantly NK and neutrophil–macrophage-associated ADCC responsible for antitumor effect; the NK cell and neutrophil-macrophage activating effects of IL2 and GM-CSF, respectively; and the timing of these interventions. As the various forms of anti-GD2 therapies move into extended clinical development, questions of patient selection, therapeutic combinations and timing of immunotherapy will require more focus to further improve antitumor efficacy. Which patients are most likely to benefit from a particular form of anti-GD2 treatment? Might genotyping for polymorphisms of immune function or more detailed tumor characterization identify separate groups of patients that respond differently to different forms of treatment? How should these distinct anti-GD2 therapies be combined with other immunotherapies, with conventional chemotherapy, radiation therapy and surgery, or with newer small molecule targeted therapies? In addition, when in a patient’s treatment course is the optimal time to be incorporating these distinct forms of anti-GD2-based immunotherapy?
Conclusion
In 2013, immunotherapy was recognized as the scientific breakthrough of the year [158]. The excitement and scientific energy invested into immunotherapy is typified by the immunotherapeutic approaches targeting GD2. After scientific advances allowed for the generation of monoclonal antibodies, early work revealed the potential for an antitumor effect achievable by targeting GD2. Even so, translation into clinical benefit in the setting of an aggressive malignancy proved to be a substantial challenge. The unrelenting study, diligent data examination and worldwide collaboration by numerous effective teams and collaborative groups has led to the safe and clinically beneficial implementation of anti-GD2 mAb therapy for NBL. While the current clinical results show benefit, the majority of children with high risk NBL are still dying of relapsed or refractory disease. More improvements in treatment outcome are urgently needed. As the increasingly effective application of cancer immunotherapy continues forward, further advances in our understanding of the immune system, cancer biology and bio-manufacturing capabilities are essential. All involved are hopeful that substantial improvements in treatment outcome, now visible on the horizon, will soon become reality.
Executive summary.
Tumor-reactive monoclonal antibody therapy
Tumor-specific mAbs recognize antigens expressed on tumor cells, leading to the recruitment and activation of immune cells that destroy the targeted tumor cells.
Targeting conserved cancer antigens shared by populations of patients, allows for a more broadly applicable treatment regimen that is not limited to individual tailoring.
Major mechanisms of action of unconjugated tumor-reactive mAbs
-
Antibody-dependent cell-mediated cytotoxicity (ADCC)
– Likely the major antitumor mechanism of tumor reactive mAbs.
– Mediated by immune effector cells that express Fc receptors, such as natural killer (NK) cells, neutrophils and macrophages. Engagement of Fc receptors on these cells leads to tumor cell destruction or phagocytosis, respectively.
-
Complement-dependent cytotoxicity
– Mediated by inactive serum proteins that become activated by tumor bound mAb, leading to lysis of the tumor cell.
Anti-GD2 mAb immunotherapy of high-risk neuroblastoma
Neuroblastoma (NBL) is the most common extracranial pediatric solid tumor, with half of newly diagnosed patients determined to be ‘high-risk’.
GD2 is highly expressed on NBL tumors with low expression on normal tissue, thus it can be targeted by anti-GD2 monoclonal mAbs.
Development of anti-GD2 mAbs
-
IgG3 - 3F8 & 14.18
– Demonstrated GD2 binding affinity, stability and the ability to kill tumor cells via ADCC, complement dependent cytotoxicity and phagocytosis.
– In vivo experiments showed antitumor effects with the suppression of human NBL in mice providing rationale for clinical trials.
– 3F8 was the first anti-GD2 mAb to be tested in the clinic, demonstrating antitumor potential as monotherapy and better efficacy when combined with immune activating cytokines such as IL2 and GM-CSF.
-
IgG2a – 14.G2a & ME36.1
– IgG3 mAbs were class switched to IgG2a subclasses in order to increase antitumor efficacy since IgG2a is associated with enhanced ADCC.
– 14.G2a demonstrated better ADCC in vitro and effectively suppressed NBL tumors in immunodeficient mice.
– In clinical trials, 14.G2a showed comparable antitumor activity to 3F8 both as a monotherapy and when combined with IL2.
-
Chimeric and humanized mAbs
– Ch14.18, a human/chimeric anti-GD2 mAb created to reduce immunogenicity in patients, was US FDA approved in 2015 for the treatment of high-risk NBL.
– Hu14.18K322A, a humanized anti-GD2 mAb, appears to be less immunogenic and less painful in patients.
Anti-O-acetyl-GD2 targets a GD2 derivative that is highly expressed on tumor cells but not on peripheral nerves, therefore targeting this antigen might reduce neuropathic pain.
Anti-GD2 mAb based fusion strategies to enhance therapeutic potential
Immunocytokines (ICs) combine a tumor-specific mAb and a cytokine into one fusion protein able to concentrate cytokines within the tumor microenvironment and locally activate immune cells while reducing systemic toxicities.
Immunotoxins deliver toxins directly to the tumors by binding malignant cells, undergoing endocytosis and intracellularly releasing the toxin to induce cell death.
Radiolabeled mAbs are used for radioimmunodetection of disease and can also serve as carriers of tumoricidal doses of radioactivity directly into radiosensitive tumors.
Drug delivery via anti-GD2 mAbs
Antibody–drug conjugates combine cytotoxic drugs through a linker region with an anti-GD2 mAb in order to facilitate tumor specific delivery of the active agent while reducing the toxicity of the ‘free drug’.
Nanoparticles-based carriers that load and transport higher amounts of drugs are bioconjugated with anti-GD2 mAbs to impart tumor specificity and have shown promising preclinical antitumor effects in vivo.
Redirecting T cells for tumor cell killing via anti-GD2 mAbs
Bi-specific antibodies combining anti-CD3 with anti-GD2 mAbs are able to cross-link GD2+tumors to CD3+ CTLs in the absence of MHC leading to efficient inhibition of tumor growth in various mouse tumor models.
Anti-GD2 CAR-T cells can destroy GD2+ tumors irrespective of HLA restrictions, potentially leading to long-term antitumor efficacy without neuropathic pain.
Anti-anti-ID mAbs that recognize the anti-ID component of anti-ID mAbs (generated by the patients in response to the anti-GD2 mAb) and also GD2 have the potential for long-term antitumor immunity.
Using anti-GD2 injected intratumorally to function as an ‘in situ’ vaccine & induce and adaptive T-cell response
Additional immune activation is obtained when ICs are injected intratumorally, resulting in a superior antitumor effect involving NK and T cells.
When combined with radiation, intratumorally IC is capable of eradicating macroscopic tumors producing long-term immunity that protects mice against tumor rechallenge.
Acknowledgements
The authors thank Robert Gordon for his computer graphic contributions, in creating Figures 1, 4 and 5.
Footnotes
Disclaimer
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Financial & competing interests disclosure
This research was supported by Hyundai Hope on Wheels Grant; Midwest Athletes Against Childhood; Stand Up 2 Cancer; The St. Baldrick’s Foundation; American Association of Cancer Research; University of Wisconsin-Madison Carbone Cancer Center; and supported in part by Public Health Service Grants CA21115, CA23318, CA66636, CA180820, CA180794, CA21076, CA180799, CA14958, CA180816, CA166105 and CA197078; from the National Cancer Institute. The Advanced Opportunity Fellowship through SciMed Graduate Research Scholars and the Molecular Bioscience Training Grant (MBTG) T32 GM07215 at University of Wisconsin – Madison, provided funding for ZP Horta and The Howard Hughes Medical Scholars Fellowship Program provided funding for JL Goldberg. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Mujoo K, Cheresh DA, Yang HM, Reisfeld RA. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res. 1987;47(4):1098–1104. [PubMed] [Google Scholar]
- 3.US FDA. FDA approves first therapy for high-risk neuroblastoma [press release] 10 March (2015). www.fda.gov/newsevents/newsroom/pressannouncements/ucm437460.htm
- 4.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 5.Weng W-K, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu–positive metastatic breast cancer. J. Clin. Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor-expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J. Clin. Oncol. 2007;25(24):3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 8.Cheung N-KV, Sowers R, Vickers AJ, Cheung IY, Kushner BH, Gorlick R. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J. Clin. Oncol. 2006;24(18):2885–2890. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 9.Farag SS, Flinn IW, Modali R, Lehman TA, Young D, Byrd JC. Fc gamma RIIIa and Fc gamma RIIa polymorphisms do not predict response to rituximab in B-cell chronic lymphocytic leukemia. Blood. 2004;103(4):1472–1474. doi: 10.1182/blood-2003-07-2548. [DOI] [PubMed] [Google Scholar]
- 10.Hurvitz SA, Betting DJ, Stern HM, et al. Analysis of Fcγ receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin. Cancer Res. 2012;18(12):3478–3486. doi: 10.1158/1078-0432.CCR-11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paez D, Pare L, Espinosa I, et al. Immunoglobulin G fragment C receptor polymorphisms and KRAS mutations: are they useful biomarkers of clinical outcome in advanced colorectal cancer treated with anti-EGFR-based therapy? Cancer Sci. 2010;101(9):2048–2053. doi: 10.1111/j.1349-7006.2010.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado DC, Hank JA, Kolesar J, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14. 18-IL2 immunotherapy. Cancer Res. 2010;70(23):9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarek N, Le Luduec J-B, Gallagher MM, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J. Clin. Invest. 2012;122(9):3260. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor RP, Lindorfer MA. The role of complement in mAb-based therapies of cancer. Methods. 2014;65(1):18–27. doi: 10.1016/j.ymeth.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Meyer S, Leusen JH, Boross P. Regulation of complement and modulation of its activity in monoclonal antibody therapy of cancer. MAbs. 2014;6(5):1133–1144. doi: 10.4161/mabs.29670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19(56):6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 17.Honeychurch J, Alduaij W, Azizyan M, et al. Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood. 2012;119(15):3523–3533. doi: 10.1182/blood-2011-12-395541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doronin Ii, Vishnyakova PA, Kholodenko IV, et al. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer. 2014;14:295. doi: 10.1186/1471-2407-14-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signalling through lipid rafts. Immunology. 2002;107(2):176–182. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodeur GM, Maris JM, Yamashiro DJ, Hogarty MD, White PS. Biology and genetics of human neuroblastomas. J. Pediat. Hematol. Onc. 1997;19(2):93–101. doi: 10.1097/00043426-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. New. Engl. J. Med. 1985;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 23.Shimada H, Stram DO, Chatten J, et al. Identification of subsets of neuroblastomas by combined histopathologic and N-myc analysis. J. Natl. Cancer I. 1995;87(19):1470–1476. doi: 10.1093/jnci/87.19.1470. [DOI] [PubMed] [Google Scholar]
- 24.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N. Engl. J. Med. 1999;341(16):1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 25.Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44(12 Part 1):5914–5920. [PubMed] [Google Scholar]
- 26.Yoshida S, Fukumoto S, Kawaguchi H, Sato S, Ueda R, Furukawa K. Ganglioside GD2 in small cell lung cancer cell lines enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001;61(10):4244–4252. [PubMed] [Google Scholar]
- 27.Shibuya H, Hamamura K, Hotta H, et al. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci. 2012;103(9):1656–1664. doi: 10.1111/j.1349-7006.2012.02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saarinen UM, Coccia PF, Gerson SL, Pelley R, Cheung N-KV. Eradication of neuroblastoma cells in vitro by monoclonal antibody and human complement: method for purging autologous bone marrow. Cancer Res. 1985;45(11 Part 2):5969–5975. [PubMed] [Google Scholar]
- 29.Kushner BH, Cheung N-KV. Clinically effective monoclonal antibody 3F8 mediates nonoxidative lysis of human neuroectodermal tumor cells by polymorphonuclear leukocytes. Cancer Res. 1991;51(18):4865–4870. [PubMed] [Google Scholar]
- 30.Munn D, Garnick M, Cheung N. Effects of parenteral recombinant human macrophage colony-stimulating factor on monocyte number, phenotype, and antitumor cytotoxicity in nonhuman primates. Blood. 1990;75(10):2042–2048. [PubMed] [Google Scholar]
- 31.Munn DH, Cheung N. Phagocytosis of tumor cells by human monocytes cultured in recombinant macrophage colony-stimulating factor. J. Exp. Med. 1990;172(1):231–237. doi: 10.1084/jem.172.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honsik CJ, Jung G, Reisfeld RA. Lymphokine-activated killer cells targeted by monoclonal antibodies to the disialogangliosides GD2 and GD3 specifically lyse human tumor cells of neuroectodermal origin. Proc. Natl Acad. Sci. USA. 1986;83(20):7893–7897. doi: 10.1073/pnas.83.20.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harel W, Shau H, Hadley CG, et al. Increased lysis of melanoma by in vivo-elicited human lymphokine-activated killer cells after addition of antiganglioside antibodies in vitro . Cancer Res. 1990;50(19):6311–6315. [PubMed] [Google Scholar]
- 34.Cheung N-KV, Lazarus H, Miraldi FD, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a Phase I study in patients with neuroblastoma and malignant melanoma. J. Clin. Oncol. 1987;5(9):1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 35.Cheung N, Kushner BH, Yeh S, Larson SM. 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: a Phase II study. Int. J. Oncol. 1998;12(6):1299–1605. doi: 10.3892/ijo.12.6.1299. [DOI] [PubMed] [Google Scholar]
- 36.Cheung N, Kushner BH, Cheung IY, et al. Anti-G (D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J. Clin. Oncol. 1998;16(9):3053–3060. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 37.Hank JA, Robinson RR, Surfus J, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50(17):5234–5239. [PubMed] [Google Scholar]
- 38.Munn DH, Cheung N-KV. Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res. 1987;47(24 Part 1):6600–6605. [PubMed] [Google Scholar]
- 39.Kushner BH, Cheung N. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73(7):1936–1941. [PubMed] [Google Scholar]
- 40.Kushner BH, Kramer K, Cheung N-KV. Phase II trial of the anti-GD2 monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J. Clin. Oncol. 2001;19(22):4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 41.Cheung N-KV, Cheung IY, Kushner BH, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung NKV, Cheung IY, Kramer K, et al. Key role for myeloid cells: Phase II results of anti-GD2 antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int. J. Cancer. 2014;135(9):2199–2205. doi: 10.1002/ijc.28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobrenkov K, Cheung N-KV. GD2-targeted immunotherapy and radioimmunotherapy. Semin. Oncol. 2014;41(5):589–612. doi: 10.1053/j.seminoncol.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurin J, Thurin M, Kimoto Y, et al. Monoclonal antibody-defined correlations in melanoma between levels of GD2 and GD3 antigens and antibody-mediated cytotoxicity. Cancer Res. 1987;47(5):1229–1233. [PubMed] [Google Scholar]
- 45.Iliopoulos D, Ernst C, Steplewski Z, et al. Inhibition of metastases of a human melanoma xenograft by monoclonal antibody to the GD2/GD3 gangliosides. J. Natl Cancer Inst. 1989;81(6):440–444. doi: 10.1093/jnci/81.6.440. [DOI] [PubMed] [Google Scholar]
- 46.Mujoo K, Kipps TJ, Yang HM, et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49(11):2857–2861. [PubMed] [Google Scholar]
- 47.Handgretinger R, Baader P, Dopfer R, et al. A Phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14. G2a. Cancer Immunol. Immun. 1992;35(3):199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleh MN, Khazaeli M, Wheeler RH, et al. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992;52(16):4342–4347. [PubMed] [Google Scholar]
- 49.Murray JL, Cunningham JE, Brewer H, et al. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. J. Clin. Oncol. 1994;12(1):184–193. doi: 10.1200/JCO.1994.12.1.184. [DOI] [PubMed] [Google Scholar]
- 50.Frost JD, Hank JA, Reaman GH, et al. A Phase I/IB trial of murine monoclonal anti-GD2 antibody 14. G2a plus interleukin-2 in children with refractory neuroblastoma. Cancer. 1997;80(2):317–333. doi: 10.1002/(sici)1097-0142(19970715)80:2<317::aid-cncr21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 51.Gillies SD, Lo K-M, Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J. Immunol. Methods. 1989;125(1):191–202. doi: 10.1016/0022-1759(89)90093-8. [DOI] [PubMed] [Google Scholar]
- 52.Mueller BM, Romerdahl CA, Gillies SD, Reisfeld RA. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J. Immunol. 1990;144(4):1382–1386. [PubMed] [Google Scholar]
- 53.Barker E, Mueller BM, Handgretinger R, Herter M, Alice LY, Reisfeld RA. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51(1):144–149. [PubMed] [Google Scholar]
- 54.Albertini MR, Gan J, Jaeger P, et al. Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J. Immunother. 1996;19(4):278–295. doi: 10.1097/00002371-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Uttenreuther-Fischer M, Huang C, Yu A. Pharmacokinetics of human-mouse chimeric anti-GD2 mAb ch14. 18 in a Phase I trial in neuroblastoma patients. Cancer Immunol. Immun. 1995;41(6):331–338. doi: 10.1007/BF01526552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu A, Uttenreuther-Fischer MM, Huang C-S, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14. 18 in patients with refractory neuroblastoma and osteosarcoma. J. Clin. Oncol. 1998;16(6):2169–2180. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 57.Handgretinger R, Anderson K, Lang P, et al. A Phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14. 18 in patients with neuroblastoma. Eur. J. Cancer. 1995;31(2):261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 58.Simon T, Hero B, Faldum A, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14. 18 in children older than 1 year with metastatic neuroblastoma. J. Clin. Oncol. 2004;22(17):3549–3557. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 59.Simon T, Hero B, Faldum A, et al. Infants with stage 4 neuroblastoma: the impact of the chimeric anti-GD2-antibody ch14. 18 consolidation therapy. Klin. Padiatr. 2004;217(3):147–152. doi: 10.1055/s-2005-836518. [DOI] [PubMed] [Google Scholar]
- 60.Simon T, Hero B, Faldum A, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14. 18 or oral metronomic chemotherapy. BMC Cancer. 2011;11(1):21. doi: 10.1186/1471-2407-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti–ganglioside GD2 monoclonal antibody (ch14. 18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a children’s cancer group study. J. Clin. Oncol. 2000;18(24):4077–4085. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 62.Desai AV, Fox E, Smith LM, Lim AP, Maris JM, Balis FM. Pharmacokinetics of the chimeric anti-GD2 antibody, ch14. 18, in children with high-risk neuroblastoma. Cancer Chemoth. Pharm. 2014;74(5):1047–1055. doi: 10.1007/s00280-014-2575-9. [DOI] [PubMed] [Google Scholar]
- 63.Albertini MR, Hank JA, Schiller JH, et al. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin. Cancer Res. 1997;3(8):1277–1288. [PubMed] [Google Scholar]
- 64.Choi BS, Sondel PM, Hank JA, et al. Phase I trial of combined treatment with ch14. 18 and R24 monoclonal antibodies and interleukin-2 for patients with melanoma or sarcoma. Cancer Immunol. Immun. 2006;55(7):761–774. doi: 10.1007/s00262-005-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14. 18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children’s Oncology Group. J. Clin. Oncol. 2009;27(1):85–91. doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ladenstein R, Pötschger U, Siabalis D, et al. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after megatherapy and autologous stem-cell reinfusion. J. Clin. Oncol. 2010;29(4):441–448. doi: 10.1200/JCO.2009.23.5465. [DOI] [PubMed] [Google Scholar]
- 68.Ladenstein RL, Poetschger U, Luksch R, et al. Immunotherapy (IT) with ch14. 18/CHO for high-risk neuroblastoma: first results from the randomised HR-NBL1/SIOPEN trial. J. Clin. Oncol. 2014;32(Suppl. 5s) Abstract 10026. [Google Scholar]
- 69.Lode HN, Valteau-Couanet D, Garaventa A, et al. Long-term infusion of anti-GD2 antibody ch14. 18/CHO in combination with interleukin-2 (IL2) activity and efficacy in high-risk relapsed/refractory neuroblastoma patients. J. Clin. Oncol. 2015;33(Suppl.) Abstract TPS10080. [Google Scholar]
- 70.Park JR, Bagatell R, London WB, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr. Blood Cancer. 2013;60(6):985–993. doi: 10.1002/pbc.24433. [DOI] [PubMed] [Google Scholar]
- 71.Saleh MN, Khazaeli M, Wheeler RH, et al. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14. 18 in patients with malignant melanoma. Hum. ABS. 1992;3(1):19–24. [PubMed] [Google Scholar]
- 72.Brüggemann M, Winter G, Waldmann H, Neuberger M. The immunogenicity of chimeric antibodies. J. Exp. Med. 1989;170(6):2153–2157. doi: 10.1084/jem.170.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorkin LS, Otto M, Baldwin WM, et al. Anti-GD 2 with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149(1):135–142. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thommesen JE, Michaelsen TE, Løset GÅ, Sandlie I, Brekke OH. Lysine 322 in the human IgG3 C H 2 domain is crucial for antibody dependent complement activation. Mol. Immunol. 2000;37(16):995–1004. doi: 10.1016/s0161-5890(01)00010-4. [DOI] [PubMed] [Google Scholar]
- 75.Ito A, Ishida T, Yano H, et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rγnull mouse model. Cancer Immunol. Immun. 2009;58(8):1195–1206. doi: 10.1007/s00262-008-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alderson KL, Luangrath M, Elsenheimer MM, et al. Enhancement of the anti-melanoma response of Hu14. 18K322A by αCD40+ CpG. Cancer Immunol. Immun. 2013;62(4):665–675. doi: 10.1007/s00262-012-1372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vāvere AL, Butch ER, Dearling JL, et al. 64Cu-p-NH2-Bn-DOTA-hu14. 18K322A, a PET radiotracer targeting neuroblastoma and melanoma. J. Nucl. Med. 2012;53(11):1772–1778. doi: 10.2967/jnumed.112.104208. [DOI] [PubMed] [Google Scholar]
- 78.Anghelescu DL, Goldberg JL, Faughnan LG, et al. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr. Blood Cancer. 2015;62(2):224–228. doi: 10.1002/pbc.25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Navid F, Sondel PM, Barfield R, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14. 18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol. 2014;32(14):1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheung N-KV, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo . Oncoimmunology. 2012;1(4):477–486. doi: 10.4161/onci.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye JN, Cheung NKV. A novel O-acetylated ganglioside detected by anti-GD2 monoclonal antibodies. Int. J. Cancer. 1992;50(2):197–201. doi: 10.1002/ijc.2910500207. [DOI] [PubMed] [Google Scholar]
- 82.Alvarez-Rueda N, Desselle A, Cochonneau D, et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent antitumor activity without peripheral nervous system cross-reactivity. PLoS One. 2011;6(9):e25220. doi: 10.1371/journal.pone.0025220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerato E, Birkle S, Portoukalian J, Mezazigh A, Chatal J-F, Aubry J. Variable region gene segments of nine monoclonal antibodies specific to disialogangliosides (GD2, GD3) and their O-acetylated derivatives. Hybridoma. 1997;16(4):307–316. doi: 10.1089/hyb.1997.16.307. [DOI] [PubMed] [Google Scholar]
- 84.Terme M, Dorvillius M, Cochonneau D, et al. Chimeric antibody c. 8B6 to O-acetyl-GD2 mediates the same efficient anti-neuroblastoma effects as therapeutic ch14. 18 antibody to GD2 without antibody induced allodynia. PLoS One. 2014;9(2):e87210. doi: 10.1371/journal.pone.0087210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sondel PM, Gillies SD. Immunocytokines for cancer immunotherapy. In: Morse MA, Clay TM, Lyerly HK, editors. Handbook Of Cancer Vaccines. Humana Press; NYUSA: 2004. pp. 341–358. [Google Scholar]
- 86.Gillies SD, Reilly EB, Lo K-M, Reisfeld RA. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc. Natl Acad. Sci. USA. 1992;89(4):1428–1432. doi: 10.1073/pnas.89.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sabzevari H, Gillies SD, Mueller BM, Pancook JD, Reisfeld RA. A recombinant antibody–interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc. Natl Acad. Sci. USA. 1994;91(20):9626–9630. doi: 10.1073/pnas.91.20.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hank JA, Surfus JE, Gan J, et al. Activation of human effector cells by a tumor reactive recombinant anti-ganglioside GD2 interleukin-2 fusion protein (ch14. 18-IL2) Clin. Cancer Res. 1996;2(12):1951–1959. [PubMed] [Google Scholar]
- 89.Kendra K, Gan J, Ricci M, et al. Pharmacokinetics and stability of the ch14.18–interleukin-2 fusion protein in mice. Cancer Immunol. Immun. 1999;48(5):219–229. doi: 10.1007/s002620050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91(5):1706–1715. [PubMed] [Google Scholar]
- 91.Lode HN, Xiang R, Dolman CS, Reisfeld RA, Varki NM, Gillies SD. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J. Natl Cancer Inst. 1997;89(21):1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 92.Niculescu-Duvaz I. Technology evaluation: EMD-273063, EMD Lexigen. Curr. Opin. Mol. Ther. 2004;6(5):559–566. [PubMed] [Google Scholar]
- 93.Lode HN, Xiang R, Pertl U, et al. Melanoma immunotherapy by targeted IL-2 depends on CD4+ T-cell help mediated by CD40/CD40L interaction. J. Clin. Invest. 2000;105(11):1623. doi: 10.1172/JCI9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neal ZC, Yang JC, Rakhmilevich AL, et al. Enhanced activity of hu14. 18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin. Cancer Res. 2004;10(14):4839–4847. doi: 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 95.Neal ZC, Imboden M, Rakhmilevich AL, et al. NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy. Cancer Immunol. Immun. 2004;53(1):41–52. doi: 10.1007/s00262-003-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Osenga KL, Hank JA, Albertini MR, et al. A Phase I clinical trial of the hu14. 18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin. Cancer Res. 2006;12(6):1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.King DM, Albertini MR, Schalch H, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J. Clin. Oncol. 2004;22(22):4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribas A, Kirkwood JM, Atkins MB, et al. Phase I/II open-label study of the biologic effects of the interleukin-2 immunocytokine EMD 273063 (hu14. 18-IL2) in patients with metastatic malignant melanoma. J. Transl. Med. 2009;7:68. doi: 10.1186/1479-5876-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Albertini MR, Hank JA, Gadbaw B, et al. Phase II trial of hu14. 18-IL2 for patients with metastatic melanoma. Cancer Immunol. Immun. 2012;61(12):2261–2271. doi: 10.1007/s00262-012-1286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) Phase II study. J. Clin. Oncol. 2010;28(33):4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gillies SD. A new platform for constructing antibody-cytokine fusion proteins (immunocytokines) with improved biological properties and adaptable cytokine activity. Protein Eng. Des. Sel. 2013;26(10):561–569. doi: 10.1093/protein/gzt045. [DOI] [PubMed] [Google Scholar]
- 102.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth F. R. 2002;13(2):169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 103.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trend. Pharmacol. Sci. 2012;33(1):35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vincent M, Bessard A, Cochonneau D, et al. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int. J. Cancer. 2013;133(3):757–765. doi: 10.1002/ijc.28059. [DOI] [PubMed] [Google Scholar]
- 105.Vincent M, Quéméner A, Jacques Y. Antitumor activity of an immunocytokine composed of an anti-GD2 antibody and the IL-15 superagonist RLI. Oncoimmunology. 2013;2(11):e26441. doi: 10.4161/onci.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu. Rev. Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 107.Wargalla UC, Reisfeld RA. Rate of internalization of an immunotoxin correlates with cytotoxic activity against human tumor cells. Proc. Natl Acad. Sci. USA. 1989;86(13):5146–5150. doi: 10.1073/pnas.86.13.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gottstein C, Schön G, Tawadros S, et al. Antidisialoganglioside ricin A-chain immunotoxins show potent antitumor effects in vitro and in a disseminated human neuroblastoma severe combined immunodeficiency mouse model. Cancer Res. 1994;54(23):6186–6193. [PubMed] [Google Scholar]
- 109.Manzke O, Russello O, Leenen C, Diehl V, Bohlen H, Berthold F. Immunotherapeutic strategies in neuroblastoma: antitumoral activity of deglycosylated ricin A conjugated anti-GD2 antibodies and anti-CD3xanti-GD2 bispecific antibodies. Med. Pediatr. Oncol. 2001;36(1):185–189. doi: 10.1002/1096-911X(20010101)36:1<185::AID-MPO1044>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 110.Mujoo K, Reisfeld RA, Cheung L, Rosenblum MG. A potent and specific immunotoxin for tumor cells expressing disialoganglioside GD2. Cancer Immunol. Immun. 1991;34(3):198–204. doi: 10.1007/BF01742313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomas PB, Delatte SJ, Sutphin A, Frankel AE, Tagge EP. Effective targeted cytotoxicity of neuroblastoma cells. J. Pediatr. Surg. 2002;37(3):539–544. doi: 10.1053/jpsu.2002.30856. [DOI] [PubMed] [Google Scholar]
- 112.Tur MK, Sasse S, Stocker M, et al. An anti-GD2 single chain Fv selected by phage display and fused to Pseudomonas exotoxin A develops specific cytotoxic activity against neuroblastoma derived cell lines. Int. J. Mol. Med. 2001;8(5):579–584. doi: 10.3892/ijmm.8.5.579. [DOI] [PubMed] [Google Scholar]
- 113.Larson SM, Carrasquillo JA, Cheung N-KV, Press OW. Radioimmunotherapy of human tumours. Nat. Rev. Cancer. 2015;15(6):347–360. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yeh S, Larson SM, Burch L, et al. Radioimmunodetection of neuroblastoma with iodine-131–3F8: correlation with biopsy, iodine-131-metaiodobenzylguanidine and standard diagnostic modalities. J. Nucl. Med. 1991;32(5):769–776. [PubMed] [Google Scholar]
- 115.Reuland P, Geiger L, Thelen MH, et al. Follow-up in neuroblastoma: comparison of metaiodobenzylguanidine and a chimeric anti-GD2 antibody for detection of tumor relapse and therapy response. J. Pediat. Hematol. Oncol. 2001;23(7):437–442. doi: 10.1097/00043426-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 116.Cheung N-KV, Landmeier B, Neely J, et al. Complete tumor ablation with iodine 131-radiolabeled disialoganglioside GD2-specific monoclonal antibody against human neuroblastoma xenografted in nude mice. J. Natl Cancer Inst. 1986;77(3):739–745. doi: 10.1093/jnci/77.3.739. [DOI] [PubMed] [Google Scholar]
- 117.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J. Neurooncol. 2010;97(3):409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheung N-KV, Modak S, Lin Y, et al. Single-chain Fv-streptavidin substantially improved therapeutic index in multistep targeting directed at disialoganglioside GD2. J. Nucl. Med. 2004;45(5):867–877. [PubMed] [Google Scholar]
- 119.Lode HN, Reisfeld RA, Handgretinger R, Nicolaou KC, Gaedicke G, Wrasidlo W. Targeted therapy with a novel enediyene antibiotic calicheamicin ϑI1 effectively suppresses growth and dissemination of liver metastases in a syngeneic model of murine neuroblastoma. Cancer Res. 1998;58(14):2925–2928. [PubMed] [Google Scholar]
- 120.Pastorino F, Brignole C, Loi M, et al. Nanocarrier-mediated targeting of tumor and tumor vascular cells improves uptake and penetration of drugs into neuroblastoma. Front. Oncol. 2013;3:190. doi: 10.3389/fonc.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pastorino F, Brignole C, Marimpietri D, et al. Targeted liposomal c-myc antisense oligodeoxynucleotides induce apoptosis and inhibit tumor growth and metastases in human melanoma models. Clin. Cancer Res. 2003;9(12):4595–4605. [PubMed] [Google Scholar]
- 122.Pastorino F, Brignole C, Marimpietri D, et al. Doxorubicin-loaded Fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63(1):86–92. [PubMed] [Google Scholar]
- 123.Brignole C, Pastorino F, Marimpietri D, et al. Immune cell–mediated antitumor activities of GD2-targeted liposomal c-myb antisense oligonucleotides containing CpG motifs. J. Natl Cancer Inst. 2004;96(15):1171–1180. doi: 10.1093/jnci/djh221. [DOI] [PubMed] [Google Scholar]
- 124.Raffaghello L, Pagnan G, Pastorino F, et al. In vitro and in vivo antitumor activity of liposomal Fenretinide targeted to human neuroblastoma. Int. J. Cancer. 2003;104(5):559–567. doi: 10.1002/ijc.10991. [DOI] [PubMed] [Google Scholar]
- 125.Tivnan A, Orr WS, Gubala V, et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS One. 2012;7(5):e38129. doi: 10.1371/journal.pone.0038129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng C-A, Wang C-H. Anti-neuroblastoma activity of gold nanorods bound with GD2 monoclonal antibody under near-infrared laser irradiation. Cancers. 2011;3(1):227–240. doi: 10.3390/cancers3010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang C-H, Huang Y-J, Chang C-W, Hsu W-M, Peng C-A. In vitro photothermal destruction of neuroblastoma cells using carbon nanotubes conjugated with GD2 monoclonal antibody. Nanotechnology. 2009;20(31):315101. doi: 10.1088/0957-4484/20/31/315101. [DOI] [PubMed] [Google Scholar]
- 128.Xu Y, Baiu DC, Sherwood JA, et al. Linker-free conjugation and specific cell targeting of antibody functionalized iron-oxide nanoparticles. J. Mater. Chem. B. 2014;2(37):6198–6206. doi: 10.1039/C4TB00840E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thakur A, Lum LG. Cancer therapy with bispecific antibodies: clinical experience. Curr. Opin. Mol. Ther. 2010;12(3):340. [PMC free article] [PubMed] [Google Scholar]
- 130.Suzuki M, Curran KJ, Cheung NKV. Chimeric antigen receptors and bispecific antibodies to retarget T cells in pediatric oncology. Pediatr. Blood Cancer. 2015;62(8):1326–1336. doi: 10.1002/pbc.25513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu H, Cheng M, Guo H, Chen Y, Huse M, Cheung N-KV. Retargeting T cells to GD2 pentasaccharide on human tumors using bispecific humanized antibody. Cancer Immunol. Res. 2014;3(3):266–277. doi: 10.1158/2326-6066.CIR-14-0230-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yankelevich M, Kondadasula SV, Thakur A, Buck S, Cheung NKV, Lum LG. Anti-CD3× anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr. Blood Cancer. 2012;59(7):1198–1205. doi: 10.1002/pbc.24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deppisch N, Ruf P, Eissler N, et al. Efficacy and tolerability of a GD2-directed trifunctional bispecific antibody in a preclinical model: subcutaneous administration is superior to intravenous delivery. Mol. Cancer Ther. 2015;14(8):1877–1883. doi: 10.1158/1535-7163.MCT-15-0156. [DOI] [PubMed] [Google Scholar]
- 134.Cheng M, Ahmed M, Xu H, Cheung NKV. Structural design of disialoganglioside GD2 and CD3-bispecific antibodies to redirect T cells for tumor therapy. Int. J. Cancer. 2015;136(2):476–486. doi: 10.1002/ijc.29007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev. 2015 doi: 10.1016/j.blre.2015.10.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 136.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hank JA, Gan J, Ryu H, et al. Immunogenicity of the hu14. 18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin. Cancer Res. 2009;15(18):5923–5930. doi: 10.1158/1078-0432.CCR-08-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Siebert N, Seidel D, Eger C, Juttner M, Lode HN. Functional bioassays for immune monitoring of high-risk neuroblastoma patients treated with ch14.18/CHO anti-GD2 antibody. PLoS One. 2014;9(9):e107692. doi: 10.1371/journal.pone.0107692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mirick G, Bradt B, Denardo S, Denardo G. A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q. J. Nucl. Med. Mol. Imaging. 2004;48(4):251. [PubMed] [Google Scholar]
- 141.Kieber-Emmons T, Monzavi-Karbassi B, Pashov A, Saha S, Murali R, Kohler H. The promise of the anti-idiotype concept. Front. Oncol. 2012;2:196. doi: 10.3389/fonc.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheung N-KV, Guo H-F, Heller G, Cheung IY. Induction of Ab3 and Ab3′ antibody was associated with long-term survival after anti-GD2 antibody therapy of stage 4 neuroblastoma. Clin. Cancer Res. 2000;6(7):2653–2660. [PubMed] [Google Scholar]
- 143.Sen G, Chakraborty M, Foon KA, Reisfeld RA, Bhattacharya-Chatterjee M. Preclinical evaluation in nonhuman primates of murine monoclonal anti-idiotype antibody that mimics the disialoganglioside GD2. Clin. Cancer Res. 1997;3(11):1969–1976. [PubMed] [Google Scholar]
- 144.Foon KA, Lutzky J, Baral RN, et al. Clinical and immune responses in advanced melanoma patients immunized with an anti-idiotype antibody mimicking disialoganglioside GD2. J. Clin. Oncol. 2000;18(2):376–376. doi: 10.1200/JCO.2000.18.2.376. [DOI] [PubMed] [Google Scholar]
- 145.Cacciavillano W, Sampor C, Venier C, et al. A phase I study of the anti-idiotype vaccine racotumomab in neuroblastoma and other pediatric refractory malignancies. Pediatr. Blood Cancer. 2015;62(12):2120–2124. doi: 10.1002/pbc.25631. [DOI] [PubMed] [Google Scholar]
- 146.Johnson EE, Lum HD, Rakhmilevich AL, et al. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol. Immun. 2008;57(12):1891–1902. doi: 10.1007/s00262-008-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, et al. Intratumoral hu14. 18–IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK Cells as well as enhanced IC retention. J. Immunol. 2012;189(5):2656–2664. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, et al. Intratumoral treatment of smaller mouse neuroblastoma tumors with a recombinant protein consisting of IL-2 linked to the hu14. 18 antibody increases intratumoral CD8+ T and NK cells and improves survival. Cancer Immunol. Immun. 2013;62(8):1303–1313. doi: 10.1007/s00262-013-1430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morris ZS, Guy EI, Francis DM, et al. Immunocytokine augments local and abscopal response and animal survival when added to radiation and CTLA-4 checkpoint inhibition in a murine melanoma model. J. Immunother. Cancer. 2015;3(Suppl. 2):P308. [Google Scholar]
- 150.Morris Z, Francis D, Gressett M, et al. Combining local radiation and tumor-specific antibody or immunocytokine to elicit in situ tumor vaccination. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-2644. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ribas A. Releasing the brakes on cancer immunotherapy. N. Engl. J. Med. 2015;373(16):1490–1492. doi: 10.1056/NEJMp1510079. [DOI] [PubMed] [Google Scholar]
- 152.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J. Immunother. Cancer. 2015;3(1):1–13. doi: 10.1186/s40425-015-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Merchant M, Wright M, Baird K, et al. Phase 1 clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin. Cancer Res. 2015;22(6):1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boes M, Meyer-Wentrup F. TLR3 triggering regulates PD-L1 (CD274) expression in human neuroblastoma cells. Cancer Lett. 2015;361(1):49–56. doi: 10.1016/j.canlet.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 156.Dondero A, Pastorino F, Chiesa MD, et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. OncoImmunology. 2016;5(1):e1064578. doi: 10.1080/2162402X.2015.1064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16(4):399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 158.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 159.Handgretinger R, Anderson K, Lang P, et al. A Phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14. 18 in patients with neuroblastoma. Eur. J. Cancer. 1995;31(2):261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 160.Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J. Clin. Oncol. 2007;25(34):5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]
- 161.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Foon KA, Lutzky J, Baral RN, et al. Clinical and immune responses in advanced melanoma patients immunized with an anti-idiotype antibody mimicking disialoganglioside GD2. J. Clin. Oncol. 2000;18(2):376–384. doi: 10.1200/JCO.2000.18.2.376. [DOI] [PubMed] [Google Scholar]