Abstract
Hepatocellular carcinoma (HCC) is a fatal disease with rising incidence in the world. For advanced HCC, sorafenib, a multikinase inhibitor, is the only systemic therapy with proven survival benefits. Sorafenib is a pan-VEGF receptor inhibitor, and thus many studies have focused its antivascular effects. But VEGF also acts as an immunosuppressive molecule. VEGF can inhibit maturation of dendritic cells, promote immune suppressive cell infiltration and enhance immune checkpoint molecules expression. On the other hand, potent VEGF inhibition may increase tumor hypoxia, which could hinder antitumor immunity or immunotherapy. Thus, achieving synergy when combining anti-VEGF therapy with immunotherapy may require proper polarization of the tumor microenvironment by dose titration or combination with other immunomodulating agents.
Keywords: : CTLA-4, hepatocellular carcinoma, immune checkpoint, LAG-3, PD-1, PD-L1, TIM-3, VEGF, VEGFR2
According to the GLOBOCAN 2012, 782,000 people were diagnosed with liver cancer and 746,000 patients died of the disease in 2012 [1]. Hepatocellular carcinoma (HCC) accounts for 90% of liver malignancies and is currently the second cause of the cancer-related death worldwide. The prognosis of this disease is poor with an overall ratio of mortality to incidence of 0.95. HCC has two unique pathological features, namely ‘chronic inflammation’ and ‘hyper-vascularity'. Carcinogenesis of HCC is strongly related with chronic inflammation of the liver. Hepatitis B virus (HBV) and -C virus (HCV) infection is the most common cause of the disease [2]. However, nonalcoholic steatohepatitis/nonalcoholic fatty liver disease (NASH/NAFLD)-related HCC is increasing in western countries [3]. Other causes include alcohol-induced steatohepatitis, autoimmune hepatitis and exposure to carcinogens such as nitroso compounds or aflatoxin. Persistent inflammation results in exhaustion of immune cells and impaired function, which induce tolerogenicity. The underlying liver disease limits the administration of treatments and compromises the efficacy. In addition, reactivation of hepatitis after anticancer treatment remains a major concern in oncology. HCCs are hypervascularized tumors with predominant arterial blood supply [4]. The fenestrated liver sinusoidal endothelium transforms into a continuous unfenestrated capillary bed (referred to as sinusoidal capillarization, a hallmark of liver disease). Transarterial chemoembolization (TACE) is a common treatment using the tumor-feeding vessels to target HCC. Another common treatment is sorafenib, an antiangiogenic drug with pan anti-VEGF receptor (VEGFR) activity. VEGF/VEGFR2 signaling is one of the most important molecular pathways controlling tumor angiogenesis. However, VEGF/VEGFR axis has multiple functions in tumor microenvironment, including immunosuppression. Thus, VEGFR blockade may have potential immune-modulatory functions.
This is becoming more significant as immunotherapy is successfully being developed against solid malignancies with hundreds of clinical trials currently ongoing. This huge effort became possible with the advent of immune checkpoint blockers, which opened the new era in oncology. However, immunosuppression is present in most tumors as they progress or recur after standard therapies, and is mediated by multiple intricate mechanisms. Overcoming immunosuppression will require an increased understanding of the complex mechanisms underlying it, and rational development of combinations of standard therapies with immunotherapy.
In this review, we will first provide an overview of our current knowledge of immunity in HCC and will discuss the potential of immune checkpoint blockade for HCC treatment. We will also overview the potential immune-modulatory functions of VEGF. Finally, we will discuss the potential benefit of combination therapy of dual blockade of VEGF and immune checkpoint molecules in HCC.
Rationale for immunotherapy for HCC
Accumulating evidence is showing that immunotherapy could be a promising approach for the treatment of HCC. The rationale to target immune checkpoints is based on the fact that HCCs may evade immune system by expression of immune checkpoint molecules, such as PD-1/PD-L1, CTLA-4, TIM-3, LAG-3, among many others. Moreover, immunotherapy in general is attractive for HCC, which is considered as an ‘inflamed,’ potentially immunogenic tumor.
HCCs are potentially immunogenic
Spontaneous antitumor response could be seen in HCC patients. Although rare, there have been reports of cases with spontaneous regressions of HCC, which suggested the development of antitumor immune responses. Indeed, T-cell responses have been documented. Moreover, CD8+ T-cell infiltration into tumor has been reported to correlate with low recurrence rate after resection [5]. Activation of immune responses and T-cell infiltration has also been reported in patients with HCC after percutaneous ethanol injection or radiofrequency ablation [6]. In addition, TAA-specific CD8+ T-cell immune response has been described in HCC patients. GPC-3, NY-ESO-1, SSX-2, MAGE-A and hTERT have all been investigated as tumor antigens in HCC. Recently, Flecken et al. reported that TAA-specific CD8+ T cells infiltrating in HCC could be found in more than 50% of patients and that the ratio of infiltrating cells was correlated with progression-free survival [7]. However, they also found that these TAA-specific CD8+ T cells had impaired ability to produce IFN-γ in vitro, suggesting that the cytokine production rather than proliferation may be defective in HCC-specific CD8+ T cells.
Evasive mechanisms of antitumor immunity in HCC
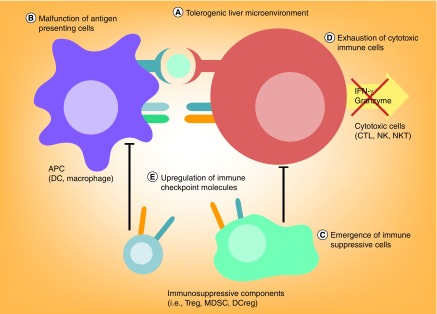
Despite the occurrence of these immune responses in some HCC patients, their impact on tumor growth is often not apparent. The reason may be that HCC evades immunosurveillance through activation of immunosuppressive pathways in their microenvironment (Figure 1). The currently proposed immunosuppressive mechanisms include: first, the tolerogenic liver microenvironment; second, the malfunction of antigen-presenting cells (APCs); third, the emergence of immunosuppressive cell populations; fourth, the exhaustion of effector immune cells (i.e., T cells, natural killer [NK] cells and natural killer T [NKT] cells); and fifth, the upregulation of immune checkpoint molecules.
Figure 1. . Potential mechanisms of immune evasion in hepatocellular carcinoma.
Multiple pathways could mediate immune evasion in HCC.
APC: Antigen-presenting cell; CTL: Cytotoxic lymphocyte; DC: Dendritic cell; DCreg: Regulatory dendritic cell; HCC: Hepatocellular carcinoma; MDSC: Myeloid-derived suppressor cell; NK: Natural killer; NKT: Natural killer T cell; Treg: Regulatory T cell.
Liver itself is anatomically and physiologically in the frontline to the orally taken toxins and pathogens, which account for the innate ‘tolerogenic’ nature of immune responses in the liver. To prevent aberrant immunity in response to these potential antigens absorbed from gut, the liver immune system has evolved to be tolerant. Antigen presentation in liver is maintained by multiple subsets of cells: liver sinusoidal endothelial cells (LSECs), phagocytic cells – in other words, macrophages, dendritic cells (DCs) and monocytes – hepatic stellate cells and hepatocytes. The defective antigen presentation by these cells contributes to the immunosuppressive environment in liver. LSECs are lining the hepatic vasculature, transport exogenous antigens into liver parenchyma and present both major histocompatibility complex class I and II (MHC-I and -II) molecules. Cross-presentation of antigen by LSECs induces antigen-specific CD8+ T-cell tolerance through immune inhibitory molecular interactions of PD-L1 and its receptor PD-1. However, the direct contribution of LSECs to tolerogenicity in HCC is not completely characterized. Among the phagocytic cells, macrophages are most potent to uptake the antigens. Kupffer cells (KCs) are the tissue resident macrophages of the liver. KCs eliminate high-affinity antigen-specific CD8+ T cells, entered into liver, to maintain tolerogenicity. KCs are reported to also have immunomodulatory effect in HCC. KCs can suppress cytotoxic T-cell function through PD-L1/PD-1 interaction. In HBV-related HCC, KCs can express galectin-9, which interacts with TIM-3 on T cells and inhibits immune response in HCC [8,9]. DCs are more specialized cells in terms of antigen presentation than macrophages. DCs are responsible for the T-cell response in liver through IL-10 production [10]. DCs express IL-10, which primes CD4+ T cells and makes the CD4+ T cells to be polarized to Th2. In addition, DCs expand Tregs and induces poor antigen recall responses [10]. DCs have been reported to be functionally impaired in HCC patients due to impaired IL-12 production. Collectively, this ‘tolerogenicity’ of the liver and impaired APC function may help HCC to evade from immunity.
The emergence of immune-suppressive leukocytes – such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) and regulatory DCs – represents another mechanism of immunosuppression. In HCC, Tregs are increased in the circulating blood and tumor-infiltrating leukocytes. Treg accumulation in the tumor correlates with disease progression and poor prognosis [11]. Tregs not only inhibit CD8+ T-cell proliferation and activation but also NK cell activation. MDSCs are a phenotypically heterogeneous population of immature myeloid cells with immunosuppressive activity. MDSCs are identified as CD45+CD11b+CD33+CD14– cell populations. MDSC accumulation is found not only within tumors but also in spleen, blood, bone marrow and liver [12]. MDSCs inhibit the function of effector T cells, decrease NK cell cytotoxicity and cytokine production of NK cells [13]. MDSCs may also promote the expansion of Tregs. It has been suggested that MDSC can interact with KCs and induce PD-L1 expression, which inhibits antigen presentation [12]. Tumor cells can also directly ‘educate’ DCs to promote immunosuppression. Tumor-educated CD11clowCD11bhighMHC-IIlow DCs have been isolated from murine cancers and referred to as regulatory DCs. These regulatory DCs express high amounts of IL-10, nitric oxide (NO), VEGF and arginase I, and thus contribute to immune evasion by tumors [14]. In humans, regulatory DCs have been described as CD14+CD11bhighCTLA-4+PD-1+ and represent less than 13% of peripheral bone marrow-derived cells in HCC patients [15]. Human regulatory DCs have been reported to suppress T-cell activation not only through IL-10 but also indoleamine 2,3-dioxygenase (IDO) production [15].
Cytotoxic immune cell function is also impaired in the HCC microenvironment. While the innate immune cells including NK cells, NKT cells and γδT cells account for 50% of leukocytes in the healthy liver, the proportion is dramatically decreased by the chronic inflammation due to HBV and/or HCV infection. Chronic inflammation also affects NK cell function. Cytotoxicity and cytokine production by NK cells are impaired in chronic HBV or HCV patients by ‘exhaustion’ [16]. Antiviral therapy could restore this functional impairment [17]. Chronic inflammation due to HBV infection has been shown to induce inhibitory costimulatory molecule expression on NK cells, including PD-1 and TIM-3 [18,19]. Similar decrease or suppression of function on NK cells has been observed in HCC. NK cell infiltration is limited in tumor tissue compared with background liver tissue. The number of NK cell infiltration is inversely correlated with the stage of disease progression [20]. Among the infiltrated NK cells, the number of CD56dimCD16+ NK cells, which is related with cytotoxic activity, is dramatically decreased in HCC [21]. While the absolute number of CD56highCD16+ NK cell infiltration is not affected by disease progression, TNF-α and IFN-γ production is severely impaired [21]. Similar to NK cells, the number and function of cytotoxic CD8+ T lymphocytes (CTLs) is also impaired due to chronic inflammation of the liver. In cases with chronic HBV or HCV infection, CTL exhaustion could be induced by continuously high viral loads, exposure to immunosuppressive cytokines such as IL-10 or TGF-β, emergence of Tregs or malfunction of DCs. Expression of multiple immune checkpoint molecules, such as PD-1, CTLA-4, LAG-3, CD244 and TIM-3, is a hallmark of T-cell exhaustion [22,23]. Tbet is an important regulator of immune checkpoint molecule expression in chronic viral inflammation [24]. Exhausted T cells show decreased production of cytokines and cell proliferation, while being more likely to undergo apoptosis [26]. Exhaustion of T cells has been observed in HCC patients [27]. Fas and IFN-γ expression in CD8+ T cells is abnormal in HCC, suggesting defective cytotoxic function. Exhaustion of AFP-specific CD4+ T-cell response has also been reported in advanced HCC patients. CD4+ T cells are critical in priming and expanding the CD8+ memory T cells. A low frequency of AFP-specific CD4+ T cells has been detected in the blood circulation and in tumor tissues from early-stage HCC patients [28]. However, in advanced HCC, blood AFP levels often increase and CD4+ T cells become exhausted and loose their immune supportive function [29].
All the pathways described above cooperate in blocking antitumor immune responses in HCC.
Immune checkpoint molecules as a therapeutic target in HCC
While the immune response to specific antigen is recognized by MHC receptors, the intensity of the response is regulated by costimulatory and coinhibitory molecules. Immune checkpoints are coinhibitory molecules that are physiologically expressed for the maintenance of self-tolerance. Among them PD-1, CTLA-4, TIM-3 and LAG-3 are studied well in chronic hepatitis and HCC.
PD-1
PD-1 is one of CD28 superfamily member coinhibitory receptor of T-cell receptor, which binds to PD-L1 (CD274, also known as B7-H1) or PD-L2 (CD273 and B7-DC). PD-1 is mainly expressed in CD8+ T cells, Tregs, NK cells, MDSC and DCs [30]. PD-1 expression is observed in activated T cells and acts like as ‘brake’ to stop excess immune response. PD-1 is critical for the differentiation and proliferation of Tregs. PD-1 regulates peripheral tolerance and autoimmunity. Chronic exposure to antigen leads to the overexpression of PD-1 in T cells which induce anergy or exhaustion. When PD-1 binds to PD-L1 or PD-L2, T-cell proliferation and cytokine release is inhibited through SHP2, which inactivates ZAP70, a major TCR signaling integrator. T-cell function is differentially affected by the strength of PD-1 signaling [31]. Using the cytokines from tumor-infiltrating lymphocytes expressed into microenvironment, cancer cells express PD-L1 (and sometimes PD-L2) to evade immune surveillance [32]. PD-1/PD-L1 expression has been documented in surgical specimens from resected HCCs. Willimsky et al. reported PD-L1 expression in HCC cells and PD-1 expression in CD8+ T cells [33]. Similarly, Sawada et al. showed that PD-1 is highly expressed on the CTLs of patients vaccinated with GPC3 [34]. PD-1/PD-L1 expression in tumor was significantly correlated with HCC stage, local recurrence rate and poor prognosis [35]. PD-1/PD-L1 expression in circulating cells was reported to correlate with the poor prognosis in HBV-positive HCC patients who underwent cryoablation [36].
PD-L1/PD-1 blockade is already being tested clinically in several trials in HCC patients, some of which are still ongoing. The anti-PD-1 antibody (nivolumab) was tested on 41 HCC patients in a Phase I/II trial [37]. The treatment was well tolerated in this cohort. Interestingly, two cases showed complete response and seven patients showed partial response after nivolumab treatment. Eighteen patients were still on treatment when the study was reported, but the preliminary efficacy and safety data appear promising. Indeed, a Phase III trial comparing nivolumab to sorafenib as first line of treatment in advanced HCC patients has recently been announced [38].
CTLA-4
CTLA-4 (CD152) also acts as a brake for immune response. CTLA-4 is expressed on activated T cells and Tregs and may also be expressed at low levels by naive T cells. CTLA-4 can bind to CD80 and CD86 with much higher affinity than CD28. CTLA-4 outcompetes CD28 for binding to CD80 and CD86 to prevent T-cell activation. CTLA-4 is also known to inhibit the binding of antigen presentation by APCs. Reverse signaling through CD80 or CD86 on APC activates IDO, which degrades tryptophan and suppresses T-cell-mediated antitumor immune responses. CTLA-4 signaling is also reported to stimulate the immune regulatory cytokines such as TGF-β. Inhibition of CD28/CD80 or CD86 binding in APC results in reduced T-cell activation. CTLA-4 knockout in mice is lethal due to autoimmune response with excessive proliferation of CD4+ T cells, suggesting that CTLA-4 function is primarily important in CD4+ T cells. Tregs constitutively express CTLA-4. Treg-specific knockout or blockade of CTLA-4 inhibits their ability to regulate both autoimmunity and anticancer immunity [39]. There are only limited available preclinical data on CTLA-4 blockade in HCC models. Chen et al. combined microwave ablation, local GM-CSF administration and CTLA-4 blockade in subcutaneously injected HCC model [40]. The reimplantation of cancer cells resulted in tumor rejection in 90% of the cases and 50% of the distant lesions were also cured. Antitumor responses were mediated by CD4+ and CD8+ T cells and by NK cells, suggesting successful immunization when using this strategy.
A Phase II trial of the monoclonal antibody against CTLA-4 (tremelimumab) has been previously conducted and reported [41]. The study enrolled 21 patients and showed that treatment was well tolerated. The response rate was 17.6% and the disease control rate was reported as 76.4%. Currently, there is another clinical trial ongoing using tremelimumab with chemoembolization or radiofrequency ablation in advanced HCC patients [42].
TIM-3
TIM-3 is a member of the T-cell immunoglobulin and mucin-domain-containing family of type I membrane glycoproteins. Like PD-1, TIM-3 is expressed on IFN-γ-secreting CD4+ T-helper 1 cells (Th1), CTLs and NK cells [23,43]. Coexpression of TIM-3 and PD-1 on CTLs is a hallmark of T-cell exhaustion. HBV or HCV infection can induce TIM-3 expression on T cells [8,44]. TIM-3 binds to its ligand galectin-9, which is expressed on KCs and other myeloid cells, and negatively regulates T-cell response [9]. HCV-infected hepatocytes also express galectin-9 and induce CD4+CD25+FOXP3+ Treg infiltration in the diseased liver [45]. Li et al. showed that TIM-3 expression is increased in the T lymphocytes infiltrating in HCC [9]. TIM-3-expressing T lymphocytes are senescent and display impaired IFN-γ production. Blockade of TIM-3 in T cells cultured in vitro restored proliferation and IL-2 and IFN-γ production [9]. Recently, Yan et al. showed that TIM-3 is highly expressed by monocytes and macrophage in HCC patients [46]. TIM-3 expression in macrophage was enhanced by TGF-β stimulation. They also showed that TIM-3 knockdown in macrophages resulted suppression of tumor growth both in vitro and in vivo, suggesting that TIM-3 may be a candidate for immunotherapy in HCC, which should be confirmed in future clinical trials.
LAG-3
LAG-3 is a member of immunoglobulin superfamily protein and expressed on NK cells, naive T cells, B cells and Tregs. LAG-3 binds MHC-II and is often coexpressed with PD-1 after chronic stimulation, thus suppressing T-cell activity [47]. The immunosuppressive role of LAG-3 has been studied in viral hepatitis and HCC. In both pathologies, LAG-3-positive CTLs expressed exhaustion markers and their cytokine production was impaired [48]. Currently, dual blockade of LAG-3 with anti-PD-1 treatment is being tested in a Phase I trial (NCT01968109) [49].
Autoimmune response as a ‘double-edged sword’
While immune checkpoint blockade seems promising as an anti-HCC treatment, the potential risk of autoimmune disease remains a concern. Autoimmune hepatitis has been reported in cases without previous liver diseases [50]. As most of the Phase III trials of immune checkpoint blockade for cancer excluded the acute or chronic hepatitis patients, the real risk of reactivation of hepatitis among HBV- or HCV-positive cases is unknown. Theoretically, as the immune checkpoint molecules exist on the immunosuppressive cells (such as CTLA-4 on Tregs), the risk of reactivating autoimmune hepatitis does exist. In fact, among the HCC patients with HCV infection, more than 45% of the cases experienced transient elevation of serum transaminase (>grade 3) in the Phase I/II trial of tremelimumab [41]. A few cases of ipilimumab-induced autoimmune hepatitis with preceding HBV or HCV hepatitis have also been reported [51]. All these patients responded well to high-dose methylpredonisolone treatment. Another case series of patients with HBV or HCV infection treated with ipilimumab reported that the risk of autoimmune hepatitis was not different from nonhepatitis cohorts [52].
On the other hand, immune checkpoint blockade might also promote viral elimination. Viral elimination depends primarily on CTL function. In chronic HBV or HCV hepatitis patients, T cells are exhausted and dysfunctional. Blocking immune checkpoint molecules may also restore antiviral response. Anti-PD-1 antibody treatment has been tested as an antiviral treatment in a Phase I/II trial [53]. Among the 45 cases included, five patients showed a clinical response. Only one case experienced elevation of serum transaminase (grade 4). In summary, the current knowledge on whether immune checkpoint blockade is beneficial or harmful among the chronic hepatitis patients is limited. Further careful examination of this critical issue is warranted.
VEGF overexpression in cancer may lead to immunosuppression
The multitargeted kinase inhibitor sorafenib remains the only available systemic treatment for advanced HCC since its approval in 2008 [54]. The precise mechanism of how sorafenib treatment benefits HCC patients remains largely unclear. Sorafenib's targets include VEGFRs, PDGFRs and RAF kinases. Sorafenib and other VEGF inhibitors clearly affect angiogenesis and vascular permeability in HCC [55]. The antivascular effect of sorafenib has been proposed as a mechanism of action. Unfortunately, all the other antiangiogenic agents tested in randomized Phase III trials have failed to match the efficacy of sorafenib in HCC. Thus, the significance of VEGF pathway inhibition in advanced HCC is currently unknown. In addition to promoting angiogenesis and vascular permeability, VEGF can also exert immunosuppressive effects in tumors (Figure 2). For example, VEGF inhibits maturation of DCs and induces accumulation of immunosuppressive inflammatory cells [56,57]. The complex mechanisms that may mediate anti-VEGF treatment resistance in HCC, and their implications for immunotherapy are discussed below.
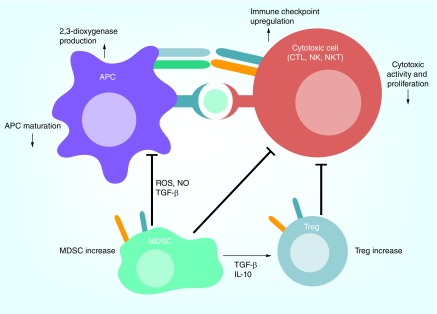
Figure 2. . VEGF is an immunosuppressive factor.
VEGF may act as an immunosuppressive molecule via multiple mechanisms: VEGF can inhibit maturation of dendritic cells. Dendritic cells secrete 2,3-dioxygenase, which inhibits immune response; VEGF can promote the infiltration of regulatory T cell (Treg) and myeloid-derived suppressor cell (MDSC). MDSCs inhibit both antigen presentation and CD8+ cytotoxic T cell (CTL) activity; VEGF can also enhance expression of immune checkpoint molecules expression on CTLs, which suppress the activity of CTLs.
VEGF regulates DC maturation & its function
Appropriate antigen presentation is a prerequisite for the development of antitumor immune responses. Immature DCs lack efficient antigen presentation, which results in immune tolerance.
VEGF is known to inhibit DC maturation in vitro and in vivo through the activation of NF-κB. Alfaro et al. showed that VEGF pathway inhibition using bevacizumab or sorafenib in vitro abrogated the differentiation of monocytes to DCs [58]. Another in vitro study used embryonic stem cells to show that the DC maturation mainly depends on VEGFR1 activation [59]. However, mature DCs function to abrogate T cells by VEGF may depend on VEGFR2 activation [60]. Recently, Marti et al. showed that VEGF can not only regulate DC maturation but also induce immunosuppressive phenotype through the production of IDO [61]. Although plasmacytoid DC (pDC) function in cancer remains unclear, Agudo et al. recently reported the role of VEGF signaling in pDC maturation in lymphoid tissues, which is mediated through an miRNA, miR-126 [62]. They showed that miR-126 is controlling the number of pDCs in secondary lymphoid organs and is regulating the immune function of pDCs, including type I interferon production. miR-126 also promotes VEGFR2 expression in pDCs. Interestingly, VEGFR2 is only expressed by pDCs resident in tissues but not in peripheral blood or bone marrow pDCs, suggesting that VEGFR2 may be a maturation marker for these cells. However, despite the accumulating evidence that VEGF could inhibiting the maturation and activity of DC populations, blockade of VEGF signaling alone may not be sufficient to promote DC maturation [63], likely due to other immunosuppressive cues.
VEGF overexpression promotes intratumoral accumulation of immunosuppressive cells
Tregs are negative regulators of antitumor T-cell immune responses. Multiple studies have shown that Tregs are increased in the peripheral blood of cancer patients and accumulated in tumors grown in mice. Terme et al. showed that VEGF pathway inhibition using either sunitinib or a VEGF-specific antibody resulted in decrease in Tregs in a mouse model of colorectal cancer, indicating that Treg proliferation may be mediated by VEGF/VEGFR2 signaling [64]. Interestingly, the inhibition of Treg proliferation could be observed only in tumor-bearing mice but not in tumor naive mice.
Another immunosuppressive cell population is formed by MDSCs, which can suppress both innate and acquired immunity. In mouse models of liver cancer, MDSCs were shown to inhibit NK cell activation through TGF-β and NKp30 [13] and to regulate T-cell activation by reducing extracellular levels of l-arginine, which is essential for T-cell proliferation [65]. In addition, MDSCs could produce immunosuppressive chemicals like reactive oxygen species, NO and IDO. Accumulation of these chemicals into tumor microenvironment also inhibits T-cell proliferation. Increased intratumoral MDSC infiltration was shown to correlate with blood-circulating VEGF levels in several types of cancers, including HCC [66]. More direct evidence was provided by studies showing that continuous injection of recombinant VEGF increased MDSC population in vivo [67], and that liver-specific overexpression of VEGF led to accumulation of proangiogenic MDSCs in a SDF1α/CXCR4 pathway-dependent manner [68].
VEGF modulates T-cell differentiation & cytotoxic T-cell function
Antitumor immune responses are mediated by cytotoxic immune cells, including CTLs. VEGF may also regulate T-cell differentiation and its cytotoxic function. For example, exogenous VEGF injection into tumor-bearing mice – to achieve concentrations similar to those seen in advanced cancer patients – led to thymic atrophy [69]. This atrophy was due to the lack of thymocyte maturation mediated through VEGFR2 pathway [70]. In cancer, hypoxia and low pH – characteristics of tumor microenvironments – directly promote VEGF production. Activated T cells express VEGFR2 [71], and VEGF overexpression in tumor microenvironment could directly inhibit CTL function thorough inactivation of STAT3 [72]. Indeed, VEGF neutralization could promote CTL activity.
In addition, these characteristics of tumor microenvironment could also inhibit T-cell infiltration and function in an indirect manner. Tumor vessels are a major port of entry for T-cell infiltration into tumors, but they are usually highly abnormal in their structure and function [73]. Hamzah et al. showed that maintaining a normal tumor vessel structure can be achieved by knocking down G-protein signaling 5 (Rgs5) in pericytes, a master regulator of vascular maturation. Loss of Rgs5 resulted in pericyte maturation, marked reductions in tumor hypoxia and vessel leakiness. As a consequence, vascular normalization led to an improvement in antitumor T-lymphocyte trafficking and function, and mouse survival [74]. Finally, Motz et al. recently showed that VEGF could induce Fas ligand (FasL) expression in tumor endothelial cells, which resulted in inhibition of CTL infiltration into tumors and reduced their cytotoxic activity [75].
Anti-VEGF treatment could promote antitumor immune responses
As discussed above, the production of excessive VEGF within the tumor microenvironment may help cancer cells evade the immune system in multiple direct and indirect ways. Importantly, as shown both in preclinical and clinical studies, anti-VEGF treatment may facilitate, in certain circumstances, the antitumor immune responses.
One mechanism is promotion of DC maturation by anti-VEGF treatment. Anti-VEGF treatment improved the number and function of lymph node and spleen DCs in tumor-bearing mice. This finding was in line with data from studies of peripheral blood of patients who underwent bevacizumab treatment [76].
Blockade VEGF signal with sunitinib may also decrease the number of MDSC in bone marrow, spleen and tumor [77,78]. Other preclinical studies showed in liver cancer models that sorafenib can decrease MDSC levels in spleen, bone marrow and tumor [79]. Decrease of MDSC population by sunitinib was also reported clinically in cases with metastatic renal cell carcinoma. The correlation between plasma VEGF levels and the absolute number of MDSC in peripheral blood was seen in patients with head and neck cancer, breast cancer, non-small cell lung cancer and GI cancers. Kusmartsev et al. reported a decrease of CD11b+VEGFR1+ cells in peripheral blood after treatment with bevacizumab in the case with renal cell carcinoma [80]. They showed that CD11b+VEGFR1+ cells could inhibit T-cell activation through induction of oxidative stress.
Anti-VEGF therapy may also affect intratumoral Tregs. For example, sorafenib treatment decreased intratumoral Treg density and inhibited their function in mouse models of liver cancer [81]. Terme et al. showed that anti-VEGF therapy with either bevacizumab or sunitinib inhibits proliferation of Tregs in mouse models of colorectal cancer [64]. This decrease in Tregs depended on VEGFR2 signaling. Clinical data support the decrease in Treg by anti-VEGF therapy among renal cell carcinoma and colorectal carcinoma [64].
CTL infiltration and activity may also be promoted by anti-VEGF therapy. In the mouse model of breast cancer, VEGFR2 blockade by neutralizing antibody inhibited tumor growth through the increase of CTL infiltration and activation [82]. Sunitinib also enhanced intratumoral infiltration of T lymphocytes in mouse models [77]. Recently, Voron et al. showed that VEGF induces exhaustion in intratumoral CTLs by promoting expression of several immune checkpoint molecules, including PD-1 [83]. This inhibitory signaling was mediated via the VEGFR2-PLCγ-calcineurin-NFAT pathway.
Unfortunately, this complex activation of antitumor immunity rarely translates in meaningful benefits after anti-VEGF therapy across tumor types and in HCC. The reasons are likely also very complex and may be related to profound immunosuppression in most cancer lesions, which could also be enhanced by anti-VEGF therapy.
Immunosuppression after anti-VEGF therapy by increased tumor hypoxia
While anti-VEGF therapy may facilitate restoration of immune responses through the mechanisms described above, excessive pruning of tumor vasculature over time could aggravate hypoxia in the tumor microenvironment, and consequently increase immunosuppression. Hypoxia induces immunosuppression by decreasing the activity of cytotoxic cells, by increasing the infiltration of immunosuppressive cell populations and promoting the expression of immunosuppressive cytokines.
In a hypoxic tumor microenvironment, dead cells release adenosine. Adenosine strongly impairs development of effector T cells, especially those that produce IFN-γ and are cytotoxic to tumor cells [84]. Sun et al. reported that hypoxia can induce T-cell apoptosis through adenosine/adenosine (2) receptor signaling in vitro [85]. Cytotoxic activity by NK cells is also restrained by hypoxia. Hypoxia induces the polarization toward an immunosuppressive microenvironment through the induction of immunosuppressive cells and expression of cytokines such as CCL22 and CCL28. Treg expansion and function is activated by adenosine (2) receptor pathway [86]. Both CCL22 and CCL28 promote intratumoral infiltration of Tregs [87]. TGF-β is also upregulated in the microenvironment and promotes Treg accumulation [88]. Tumor cell-derived oncostatin M and eotaxin induce immunosuppressive via macrophages [89]. Finally, hypoxia may upregulate the expression of the immune checkpoint molecules. PD-L1 has been shown to be downstream hypoxia inducible factor 1 alpha (HIF1α) activation in MDSCs, DCs and cancer cells [90].
Overcoming immunosuppression induced by VEGF as well as by its blockade may be potentially achieved by using different strategies. One approach could be careful titration of VEGF inhibition to inhibit VEGF pathway and angiogenesis while avoiding excessive pruning and hypoxia. Another approach could be rational combinations with immunotherapeutics using optimal treatment schedules. For example, one could take advantage of the immunostimulation after anti-VEGF therapy by defining a certain ‘time-window’ when VEGF blockade and immunotherapy could synergize. Finally, one could stimulate antitumor immunity by using additional immune modulators, in the face of increasing hypoxia. These strategies are supported by preclinical data.
For example, Huang et al. showed that dose-titrated anti-VEGFR2 antibody therapy can alleviate hypoxia (via vascular normalization) and potentiate the effects of vaccination in a mouse model of breast cancer [91]. By using a low-dose anti-VEGFR2 antibody, effector T-cell infiltration increased compared with high-dose anti-VEGFR2 treatment. In addition, tumor-infiltrating macrophages showed a more immune stimulatory (M1) phenotype. Similar results have reported using TNF-α blockade in the mouse models. While TNF-α blockade itself can alleviate immune suppressive phenotype on immune cells, vascular destruction by TNF-α blockade resulted in increased tumor hypoxia and paradoxically caused immune suppression [92]. Finally, Shrimali et al. showed that normalization of tumor vasculature through blockade of the VEGF/VEGFR2 increased extravasation of adoptively transferred T cells into the tumor and improved the efficacy of adoptive cell transfer-based immunotherapy [93]. The ‘normalization’ of the vascular and immune environment in tumors after various therapies will clearly be context-dependent and will need to be defined for HCCs.
Combination treatment of anti-VEGF therapy with immune checkpoint blockade
As VEGF and the immune checkpoints are controlling different steps of the immune responses, it is conceivable that their combined blockade could have synergistic antitumor effects (Figure 3). Indeed, simultaneous anti-VEGF therapy and immunotherapy has shown promising synergy in some animal models and in clinical studies. Yasuda et al. tested a combination therapy of anti-VEGFR2 and anti-PD-1 antibodies in a mouse model with subcutaneous implanted tumors [94]. They showed that combination treatment can enhance IFN-γ, TNF-α and granzyme B production – suggesting the enhancement of immune responses. We have proposed a strategy to combine sorafenib therapy with immune checkpoint blockade in HCC. Sorafenib treatment increased tumor hypoxia, which induced the expression of SDF1α and the accumulation of MDSCs in HCC in mice [95]. Pharmacologic inhibition of the CXCR4 receptor using the small-molecule drug AMD3100 in combination with sorafenib treatment resulted in inhibition of tumor growth [95]. In this model, we also showed that immune checkpoint molecule, PD-L1 expression in the tumor was increased after sorafenib treatment and potentially mediated immune evasion. Indeed, triple combination therapy with sorafenib, AMD3100 and anti-PD-1 antibody enhanced the infiltration of activated CTL inside the tumor and significantly delayed tumor growth and metastasis [96]. Thus, sorafenib (and potentially other antiangiogenic agents) may be effective in combination with immune checkpoint inhibition in HCC in the face of increased hypoxia with appropriate inhibition of immunosuppression. Recently, a clinical study of a combination therapy using ipilimumab (an anti-CTLA4 antibody) with bevacizumab (an anti-VEGF antibody) reported promising initial efficacy in melanoma patients [97]. There are currently at least three Phase I or II studies ongoing in different types of tumors (Table 1).
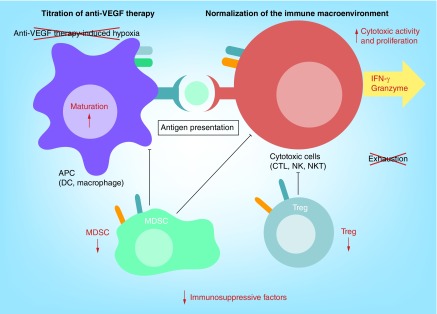
Figure 3. . Potential strategies to achieve synergy between anti-VEGF therapy and immune checkpoint blockade.
Appropriate dosing of anti-VEGF therapy can ‘normalize’ tumor microenvironment, which stabilizes the vasculature and does not increase hypoxia. VEGF blockade could enhance antigen-presenting cell (APC) maturation and cytotoxic T cell (CTL) activation, and reduce immunosuppressive cell function. Addition of immune checkpoint blockade could improve antigen presentation from APC to CTL and reduce exhaustion of CTLs, which could directly promote tumor elimination.
Table 1. . Clinical trials of anti-VEGF therapy in combination with immune checkpoint blockade.
| Anti-VEGF drug | Immune checkpoint blocker | Tumor type | ClinicalTrials.gov ID | Phase | Status | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Anti-VEGF antibody (bevacizumab) | Anti-CTLA-4 antibody (ipilimumab) | Melanoma | NA | I/II | Completed and reported in 2014 | Disease control rate: 67.4% | [81] |
| |
|
|
|
|
|
Median survival: 25.1 months |
|
| Anti-VEGF antibody (bevacizumab) |
Anti-CTLA-4 antibody (ipilimumab) |
Melanoma |
NCT01950390 |
II |
Recruiting |
NA |
|
| Anti-VEGF antibody (bevacizumab)† |
Anti-PD-1 antibody (nivolumab) |
Metastatic renal cell carcinoma |
NCT02210117 |
II |
Recruiting |
NA |
|
| Anti-VEGFR2 antibody (ramucirumab) | Anti-PD-1 antibody (pembrolizumab) | Gastric cancer, non-small cell lung cancer or transitional cell carcinoma of the urothelium | NCT02443324 | I | Recruiting | NA |
†This study compares nivolumab + bevacizumab vs nivolumab + ipilimumab.
NA: Not available.
Conclusion & future perspective
In the context of the immune microenvironment, VEGF blockade is a double-edged sword because it may both increase and reduce immunosuppression. Thus, several elements will be critical for increasing the efficacy of antiangiogenic and immune checkpoint inhibitors when used in combination for HCC and other malignancies. A key element will be establishing the safety of this approach. Another aspect will be gaining further understanding of the molecular and pathophysiological mechanisms of action of these treatment modalities. Finally, it will be critically important to determine the effects of these therapies and their timing, to establishing and validating biomarkers of response and to exploit the normalization of the vascular and immune microenvironment to achieve durable responses and increase the survival of HCC patients.
Executive summary.
Rationale for immunotherapy for hepatocellular carcinoma
Hepatocellular carcinoma (HCC) can be immunogenic. Occasional spontaneous regression of the tumor and cytotoxic CD8+ T lymphocyte (CTL) responses against tumor-associated antigens support this hypothesis.
However, immunosuppressive factors block the antitumor immune responses. These include tumor infiltration predominantly by immunosuppressive cells, insufficient antigen presentation and suppression of cytotoxic T-cell activation, impaired CD4+ T-cell function and upregulation of immune checkpoint expression in cancer and stromal cells.
Immune checkpoints in HCC
Expression of immune checkpoint molecules, including PD-1/PD-L1, CTLA-4, TIM-3, LAG-3, etc., has been documented in HCC. However, the therapeutic relevance of blocking these factors remains unclear.
Immune checkpoint molecule expression is highly associated with chronic liver inflammation, including viral hepatitis, which is a precursor of HCC.
PD-1 or CTLA-4 blockade is currently being tested in Phase II trials in HCC patients with intriguing initial efficacy and safety signals.
VEGF as a immune suppressive molecule
VEGF not only regulates tumor angiogenesis but also has important immune-modulatory functions.
VEGF can inhibit maturation of dendritic cells, promote immune suppressive cell infiltration and enhance immune checkpoint molecules expression.
Potential synergy in anti-VEGF therapy with immune checkpoint blockade
As VEGF and immune checkpoint blockade govern different parts of immune response, dual blockade of these factors may have synergistic effects.
However, anti-VEGF therapy may also increase immunosuppression due to treatment-induced hypoxia.
Combination of anti-VEGF therapy with CTLA-4 blockade is being tested in melanoma patients with promising initial results.
Conclusion & future perspective
The safety prolife of immune checkpoint blockade in HCC should be confirmed in larger clinical trials.
The synergy of anti-VEGF therapy with immune checkpoint blockade should be explored in larger clinical trials.
Biomarkers of response will be crucial for the development of these combinatorial strategies.
Footnotes
Financial & competing interests disclosure
This study is supported by the National Institutes of Health (grant nos. P01-CA080124, R01-CA159258 and R21-CA139168), a National Cancer Institute/Proton Beam Federal Share Program award and the American Cancer Society (grant no. 120733-RSG-11–073–01-TBG [to DG Duda]). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.GLOBOCAN. 2012. http://globocan.iarc.fr/old/FactSheets/cancers/liver-new.asp
- 2.De Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190–1200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 4.Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat. Rec. (Hoboken) 2008;291(6):721–734. doi: 10.1002/ar.20668. [DOI] [PubMed] [Google Scholar]
- 5.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27(2):407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 6.Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–1457. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 7.Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59(4):1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nebbia G, Peppa D, Schurich A, et al. Upregulation of the TIM-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS ONE. 2012;7(10):e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Wu K, Tao K, et al. TIM-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56(4):1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 10.Bamboat ZM, Stableford JA, Plitas G, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J. Immunol. 2009;182(4):1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE. 2011;6(9):e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69(13):5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50(3):799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J. Immunol. 2009;182(10):6207–6216. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 15.Han Y, Chen Z, Yang Y, et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59(2):567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 16.Ahlenstiel G, Titerence RH, Koh C, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138(1):325–335. doi: 10.1053/j.gastro.2009.08.066. e321–e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peppa D, Micco L, Javaid A, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6(12):e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju Y, Hou N, Meng J, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3) mediates natural killer cell suppression in chronic hepatitis B. J. Hepatol. 2010;52(3):322–329. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Cao D, Xu H, Guo G, et al. Intrahepatic expression of programmed death-1 and its ligands in patients with HBV-related acute-on-chronic liver failure. Inflammation. 2013;36(1):110–120. doi: 10.1007/s10753-012-9525-7. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Kuang DM, Pan WD, et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57(3):1107–1116. doi: 10.1002/hep.26192. [DOI] [PubMed] [Google Scholar]
- 21.Cai L, Zhang Z, Zhou L, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin. Immunol. 2008;129(3):428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kroy DC, Ciuffreda D, Cooperrider JH, et al. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology. 2014;146(2):550–561. doi: 10.1053/j.gastro.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owusu Sekyere S, Suneetha PV, Kraft AR, et al. A heterogeneous hierarchy of co-regulatory receptors regulates exhaustion of HCV-specific CD8 T cells in patients with chronic hepatitis C. J. Hepatol. 2015;62(1):31–40. doi: 10.1016/j.jhep.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Kao C, Oestreich KJ, Paley MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 2011;12(7):663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penna A, Pilli M, Zerbini A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45(3):588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 26.Barathan M, Gopal K, Mohamed R, et al. Chronic hepatitis C virus infection triggers spontaneous differential expression of biosignatures associated with T cell exhaustion and apoptosis signaling in peripheral blood mononucleocytes. Apoptosis. 2015;20(4):466–480. doi: 10.1007/s10495-014-1084-y. [DOI] [PubMed] [Google Scholar]
- 27.Gehring AJ, Ho ZZ, Tan AT, et al. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2009;137(2):682–690. doi: 10.1053/j.gastro.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 28.Behboudi S, Pereira SP. Alpha-fetoprotein specific CD4 and CD8 T cell responses in patients with hepatocellular carcinoma. World J. Hepatol. 2010;2(7):256–260. doi: 10.4254/wjh.v2.i7.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witkowski M, Spangenberg HC, Neumann-Haefelin C, et al. Lack of ex vivo peripheral and intrahepatic alpha-fetoprotein-specific CD4+ responses in hepatocellular carcinoma. Int. J. Cancer. 2011;129(9):2171–2182. doi: 10.1002/ijc.25866. [DOI] [PubMed] [Google Scholar]
- 30.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei F, Zhong S, Ma Z, et al. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc. Natl Acad. Sci. USA. 2013;110(27):E2480–E2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 33.Willimsky G, Schmidt K, Loddenkemper C, Gellermann J, Blankenstein T. Virus-induced hepatocellular carcinomas cause antigen-specific local tolerance. J. Clin. Invest. 2013;123(3):1032–1043. doi: 10.1172/JCI64742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawada Y, Yoshikawa T, Shimomura M, Iwama T, Endo I, Nakatsura T. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 2015;46(1):28–36. doi: 10.3892/ijo.2014.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer. 2011;128(4):887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Z, Shi F, Zhou L, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS ONE. 2011;6(9):e23621. doi: 10.1371/journal.pone.0023621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Khoueiry AB, Melero I, Crocenzi TS, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209–040. J. Clin. Oncol. 2015;33(Suppl.) Abstract LBA101. [Google Scholar]
- 38.A study of nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. https://clinicaltrials.gov/ct2/show/NCT02576509?term=hepatocellular+carcinoma+nivolumab&rank=3
- 39.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Shen S, Peng B, Tao J. Intratumoural GM-CSF microspheres and CTLA-4 blockade enhance the antitumour immunity induced by thermal ablation in a subcutaneous murine hepatoma model. Int. J. Hyperthermia. 2009;25(5):374–382. doi: 10.1080/02656730902976807. [DOI] [PubMed] [Google Scholar]
- 41.Sangro B, Gomez-Martin C, De La Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013;59(1):81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 42.A pilot study of tremelimumab – a monoclonal antibody against CTLA-4 in combination with Trans-Arterial Catheter Chemoembolization (TACE), Radiofrequency Ablation (RFA), Stereotactic Body Radiation Therapy (SBRT) or Cryoablation in Subjects with Hepatocellular Carcinoma (HCC) or Biliary Tract Carcinomas (BTC) http://clinicalstudies.info.nih.gov/cgi/detail.cgi?A_2013-C-0120.html
- 43.Rong YH, Wan ZH, Song H, et al. TIM-3 expression on peripheral monocytes and CD3+CD16/CD56+natural killer-like T cells in patients with chronic hepatitis B. Tissue Antigens. 2014;83(2):76–81. doi: 10.1111/tan.12278. [DOI] [PubMed] [Google Scholar]
- 44.Dolina JS, Braciale TJ, Hahn YS. Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice. Hepatology. 2014;59(4):1351–1365. doi: 10.1002/hep.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moorman JP, Wang JM, Zhang Y, et al. TIM-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J. Immunol. 2012;189(2):755–766. doi: 10.4049/jimmunol.1200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan W, Liu X, Ma H, et al. TIM-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut. 2015;64(10):1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 47.Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen N, Liu Y, Guo Y, Chen Y, Liu X, Liu M. LAG-3 negatively regulates the function of intrahepatic HCV-specific CD8 T cells. J. Gastroenterol. Hepatol. 2015;30(12):1788–1795. doi: 10.1111/jgh.13017. [DOI] [PubMed] [Google Scholar]
- 49.Safety study of anti-LAG-3 with and without anti-PD-1 in the treatment of solid tumors. https://clinicaltrials.gov/ct2/show/NCT01968109
- 50.Johncilla M, Misdraji J, Pratt DS, et al. Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am. J. Surg. Pathol. 2015;39(8):1075–1084. doi: 10.1097/PAS.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 51.Cheng R, Cooper A, Kench J, et al. Ipilimumab-induced toxicities and the gastroenterologist. J. Gastroenterol. Hepatol. 2015;30(4):657–666. doi: 10.1111/jgh.12888. [DOI] [PubMed] [Google Scholar]
- 52.Ravi S, Spencer K, Ruisi M, et al. Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: a multicenter, retrospective case series. J. Immunother. Cancer. 2014;2(1):33. doi: 10.1186/s40425-014-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardiner D, Lalezari J, Lawitz E, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE. 2013;8(5):e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 55.Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat. Rev. Clin. Oncol. 2011;8(5):292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terme M, Colussi O, Marcheteau E, Tanchot C, Tartour E, Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin. Dev. Immunol. 2012;2012:492920. doi: 10.1155/2012/492920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 2015;11(4):577–591. doi: 10.1016/j.celrep.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alfaro C, Suarez N, Gonzalez A, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br. J. Cancer. 2009;100(7):1111–1119. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dikov MM, Ohm JE, Ray N, et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J. Immunol. 2005;174(1):215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 60.Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol. Immunother. 2007;56(6):761–770. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marti LC, Pavon L, Severino P, Sibov T, Guilhen D, Moreira-Filho CA. Vascular endothelial growth factor-A enhances indoleamine 2,3-dioxygenase expression by dendritic cells and subsequently impacts lymphocyte proliferation. Mem. Inst. Oswaldo Cruz. 2014;109(1):70–79. doi: 10.1590/0074-0276130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agudo J, Ruzo A, Tung N, et al. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014;15(1):54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer. Res. 2007;13(16):4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 64.Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arihara F, Mizukoshi E, Kitahara M, et al. Increase in CD14+HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 2013;62(8):1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo . Blood. 1998;92(11):4150–4166. [PubMed] [Google Scholar]; •• This report provided evidence that VEGF may act as an immunosuppressive factor.
- 68.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 69.Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 70.Huang Y, Chen X, Dikov MM, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110(2):624–631. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J. Immunol. 2004;172(7):4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 72.Noman MZ, Buart S, Van Pelt J, et al. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J. Immunol. 2009;182(6):3510–3521. doi: 10.4049/jimmunol.0800854. [DOI] [PubMed] [Google Scholar]
- 73.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453(7193):410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]; •• This report provided genetic evidence that the lack of maturation of tumor vasculature is a key factor in immune evasion in cancer.
- 75.Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20(6):607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osada T, Chong G, Tansik R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol. Immunother. 2008;57(8):1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69(6):2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao M, Xu Y, Youn JI, et al. Kinase inhibitor sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab. Invest. 2011;91(4):598–608. doi: 10.1038/labinvest.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kusmartsev S, Eruslanov E, Kubler H, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 2008;181(1):346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 81.Chen ML, Yan BS, Lu WC, et al. Sorafenib relieves cell-intrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int. J. Cancer. 2014;134(2):319–331. doi: 10.1002/ijc.28362. [DOI] [PubMed] [Google Scholar]
- 82.Manning EA, Ullman JG, Leatherman JM, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin. Cancer. Res. 2007;13(13):3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 83.Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohta A, Ohta A, Madasu M, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J. Immunol. 2009;183(9):5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 85.Sun J, Zhang Y, Yang M, et al. Hypoxia induces T-cell apoptosis by inhibiting chemokine C receptor 7 expression: the role of adenosine receptor A(2) Cell. Mol. Immunol. 2010;7(1):77–82. doi: 10.1038/cmi.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]; • This report provided evidence that tumor hypoxia mediates both angiogenesis and immunosuppression in cancer via secretion of CCL28 and Treg infiltration in tumors.
- 88.Chen W, Konkel JE. TGF-beta and ‘adaptive’ Foxp3(+) regulatory T cells. J. Mol. Cell. Biol. 2010;2(1):30–36. doi: 10.1093/jmcb/mjp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tripathi C, Tewari BN, Kanchan RK, et al. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines oncostatin M and eotaxin. Oncotarget. 2014;5(14):5350–5368. doi: 10.18632/oncotarget.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211(5):781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This report showed that PD-L1 may be directly activated by hypoxia-inducible factor 1-alpha, and its blockade led to CTL activation.
- 91.Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This report showed the dose-dependent effect of VEGFR2 blockade on the immune microenvironment of tumors in mice, and its impact on vaccine immunotherapy.
- 92.Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc. Natl Acad. Sci. USA. 2012;109(20):7841–7846. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15):6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo . Clin. Exp. Immunol. 2013;172(3):500–506. doi: 10.1111/cei.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y, Huang Y, Reiberger T, et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology. 2014;59(4):1435–1447. doi: 10.1002/hep.26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y, Ramjiawan RR, Reiberger T, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61(5):1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This report showed that CXCR4 inhibition could reprogram the immune microenvironment and facilitate checkpoint blockade in the face of hypoxia after sorafenib treatment in HCC models in mice with liver fibrosis.
- 97.Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol. Res. 2014;2(7):632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This report showed the feasibility of anti-VEGF therapy and checkpoint blockade in cancer patients.