Abstract
Invasion of macrophages and replication within an acidic and degradative phagolysosome-like vacuole are essential for disease pathogenesis by Coxiella burnetii, the bacterial agent of human Q fever. Previous experimental constraints imposed by the obligate intracellular nature of Coxiella limited knowledge of pathogen strategies that promote infection. Fortunately, new genetic tools facilitated by axenic culture now allow allelic exchange and transposon mutagenesis approaches for virulence gene discovery. Phenotypic screens have illuminated the critical importance of Coxiella's type 4B secretion system in host cell subversion and discovered genes encoding translocated effector proteins that manipulate critical infection events. Here, we highlight the cellular microbiology and genetics of Coxiella and how recent technical advances now make Coxiella a model organism to study macrophage parasitism.
KEYWORDS : apoptosis, autophagy, Coxiella burnetii, effector protein, host cell invasion, macrophages, mutagenesis, phagolysosome, type 4B secretion, vacuole remodeling
Coxiella: a wide-ranging zoonotic pathogen
The expansive geographical distribution and host range of Coxiella burnetii, a Gram-negative intracellular bacterium, was noted soon after its discovery as the causative agent of human Q fever. Acute Q fever typically presents as an incapacitating but self-limited flu-like illness with other common clinical presentations being atypical pneumonia and/or hepatitis. Rare chronic infections also occur that normally manifest as endocarditis or vascular disease, with increased incidence associated with valvular heart disease, vascular pathology and/or a compromised immune system [1,2]. Evidence for infection exists in a multitude of vertebrate and invertebrate hosts [3]. Infected domestic livestock, especially goats, sheep and dairy cattle are responsible for the vast majority of human Q fever cases. These zoonotic reservoirs shed Coxiella into the environment that can fuel human outbreaks [4]. Especially relevant to pathogen transmission are the vast numbers of bacteria present in birth products. Desiccation of placental material promotes aerosol transmission of highly infectious, environmentally stable Coxiella that can infect individuals miles away from a single point source [5]. Exacerbating pathogen dissemination are abortion waves in goats and sheep caused by Coxiella infection [6]. Detection of the Coxiella using PCR amplification of chromosomal IS1111 repetitive elements has revealed the extent to which zoonotic reservoirs have dispersed Coxiella into the USA, with 23.8% of over 1600 random environmental samples containing Coxiella DNA [7]. Two caveats of this study are that IS1111-like sequences are widespread in Coxiella-like endosymbionts [8], and that PCR does not distinguish between live and dead bacteria. However, mice were infected with material from several field samples, indicating environmental samples can contain viable C. burnetii [7]. Q fever outbreaks can have substantial financial impact as illustrated by the 2007–2011 Netherlands outbreak (>4000 cases) where human disease burden and infection control measures were estimated to cost 307 million Euro [9].
Although Coxiella is frequently referenced as a close relative of the γ-proteobacterium Legionella pneumophila [10], the two bacteria actually have a distant relationship [11]. A new study shows the evolutionary progenitor of C. burnetii is in fact an ancient monophyletic group of Coxiella-like endosymbionts of ticks [12]. Genetic diversity among C. burnetii strains suggests it recently emerged from this group as a parasite of vertebrate cells, possibly through acquisition of virulence genes from a coinfecting pathogen [12]. A rooted phylogeny of sequenced C. burnetii strains using SNPs implies the ancestor of Coxiella was more likely to cause persistent or chronic infection [11]. Further pathoadaptive evolution within the genus, driven by expansion of insertion sequence elements, accumulation of pseudogenes and extensive genomic rearrangements, led to more acutely virulent strains [10,11,13]. The observation that the naturally attenuated Dugway 5J108-111 strain has the largest genome with the fewest pseudogenes suggests this strain lineage is at a more primitive stage of pathoadaptation [10,11]. It is interesting to speculate that novel gene content of the Dugway strain includes antivirulence genes enabling persistent, subclinical infection of animal hosts [11,14].
Animal models of acute Q fever clearly demonstrate a relationship between genome content and pathotype [15,16]. SNP-based typing has revealed 35 unique genotypes of Coxiella [11,17,18]. Some genotypes have defined associations with animal reservoirs and disease in humans. For example, genotype 8 has a goat reservoir and is associated with human chronic disease [18]. Researchers hypothesize that this genotype is more likely to cause a subclinical infection that goes untreated, thereby persisting and causing chronic disease such as endocarditis [18]. Genotype 17 is associated with severe, acute Q fever pneumonia in French Guiana [19]. In the USA, millions of dairy cows appear exclusively infected with genotype 20 strains. However, despite tremendous shedding of these strains into the environment, they have not been isolated from human Q fever cases and thus may be attenuated in causing human disease [20].
Pressure exerted by serial laboratory passage also drives Coxiella strain variation. Coxiella isolated from natural sources and infections produces full-length lipopolysaccharide (LPS) that is required for virulence. During serial passage in immunoincompetent hosts, such as embryonated hen's eggs and tissue culture cells, or synthetic media [21], variants emerge within the population that produce truncated LPS. Extensive passage (˜90 passages) culminates in avirulent organisms with a severely truncated, deep-rough LPS consisting of lipid A and core sugars, but no O-antigen [22–24]. This change in LPS structure corresponds with a change in reactivity to postvaccination sera referred to as phase variation. Organisms displaying full-length and severely truncated LPS are serologically in Phase I and Phase II, respectively. O-antigen is the primary surface antigen recognized by Phase I antiserum [25]. Some, but not all, Phase II strains contain a chromosomal deletion eliminating roughly 20 genes associated with sugar metabolism [26–28]. Moreover, deep-rough LPS of Phase II strains can be antigenically distinct [29]. Collectively, these observations suggest multiple mutational pathways can lead to phase transition. Nonetheless, a plaque-cloned Phase II variant (NMII) of the Nine Mile Phase I (NMI) strain containing a large chromosomal deletion is widely used by Coxiella researchers, as the strain is considered nonrevertable to full virulence and thus suitable for use at biosafety level-2 [23,30]. All other strains are considered biosafety level-3 organisms. Importantly, the growth kinetics and intracellular trafficking of NMI and NMII in human macrophages appear indistinguishable, making infection by NMII a relevant model for ex vivo pathogen–host interaction studies [31,32]. NMII also has the advantage of being roughly 500-fold more infectious on a per particle basis for nonprofessional phagocytic cells, such as Vero epithelial cells, aiding cell biology studies [33]. Increased surface hydrophobicity and protein exposure due to missing O-antigen are thought to facilitate NMII–host plasma membrane interactions [34,35]. Greater exposure of lipoprotein, a potent TLR-2 ligand, may also explain the unusual growth restriction of NMII, but not NMI, by primary mouse macrophages [36,37]. Ligation of TLR-2 triggers synthesis of the proinflammatory cytokine tumor necrosis factor, which in turn elicits production of the antibacterial effector nitric oxide that inhibits NMII replication [37–39]. Although potentiated innate immune signaling also occurs during NMII interactions ex vivo with primary human macrophages and dendritic cells [32,40], no growth defects relative to NMI are observed. However, the resulting robust immune response elicited by NMII during primary infection of mammals is thought to render the strain avirulent [37,40].
Making a home in host cells
As an intracellular bacterial pathogen, Coxiella must be internalized by host cells to cause disease. The primary target of Coxiella during natural infection is alveolar macrophages [32,41–43]. The organism can subsequently disseminate to colonize and replicate in resident macrophages of different tissues and organs [44]. Trophoblasts of infected females can also become heavily infected with adipoctyes potentially serving as a reservoir of persistent Coxiella [45,46]. Ex vivo, multiple cell lines have been used to investigate Coxiella infections including primary macrophages, macrophage-like cells, epithelial cells and fibroblasts [47]. Macrophages and fibroblasts internalize live or dead Coxiella with equal efficiency, suggesting Coxiella plays a passive role in cellular uptake by both phagocytic and nonphagocytic cells [48,49]. Because protease treatment of Coxiella inhibits uptake, surface proteins likely serve as invasins [48]. Indeed, as described below, OmpA has recently been identified as the first Coxiella invasin inducing cellular uptake, specifically by nonprofessional phagocytes [50]. Two monocyte surface receptors are known for Coxiella: αVβ3 integrin, engaged by NMI and NMII, and complement receptor 3, co-engaged by NMII [51]. Membrane protrusions induced during attachment by NMI are thought to restrict interactions with CR3, suggesting LPS plays a role in Coxiella infection of human phagocytes [51,52]. Consistent with the induction of membrane projections, Coxiella internalization requires F (filamentous)-actin rearrangements [48,51–53]. The signaling cascade associated with Coxiella uptake by phagocytic and nonphagocytic cells involves Rho family GTPases. RhoA appears particularly important considering the activity of the downstream effectors ROCK, a protein kinase and mDia1, an actin nucleating factor, are required for optimal internalization [53]. Src-family kinases [54] and the F-actin binding protein cortactin [55] also play roles in internalization of Coxiella by phagocytic and nonphagocytic cells, respectively.
Following uptake, Coxiella is confined to a nascent vacuole that transits through the default endocytic maturation pathway from early endosome to phagolysosome [31,56,57]. Full maturation of the Coxiella-containing vacuole (CCV) to fusion with lysosomes is indicated by the presence of active cathepsins [31,56–60]. Coxiella is uniquely adapted among intravacuolar bacterial pathogens to replicate in an acidic phagolysosome-like compartment that retains significant hydrolytic activity [31,57]. Consequently, the pathogen does not deploy the sophisticated mechanisms used by other pathogens to avoid death by lysosome [61,62]. In fact, Coxiella requires the acidic conditions of the vacuole for metabolic activation and production of proteins that manipulate infection events, such as vacuole expansion and inhibition of apoptosis [63–65]. Roughly coincident with the onset of Coxiella replication (˜1–2 days postinfection), the CCV becomes highly fusogenic, a property that generally produces a single vacuole that occupies the majority of the host cell cytoplasm [65]. Studies have recently highlighted CCV engagement with several cellular vesicular trafficking pathways predicted to provide membrane and/or nutrients to the Coxiella replicative niche.
Much attention has been devoted to defining the role of autophagy in Coxiella infection as the pathway can exert both pro- and anti-microbial effects [66]. Several lines of evidence indicate the CCV fuses with autophagosomes, and that this interaction benefits Coxiella. The CCV clearly decorates with the autophagy markers LC3 [56,57,59,60,67–69], Beclin 1 [59,70], Rab24 [70] and p62 [67]. Autophagosome interactions with the CCV occur within a few hours of infection and require Coxiella protein synthesis and an active type 4B secretion system (T4BSS) [56,67]. Although levels of lipidated LC3 (LC3-II) increase during Coxiella infection [56,60,67], the overall rate of autophagic flux does not increase as evidenced by the absence of p62 turnover [60,67]. As the CCV expands, endogenous LC3 continues to accumulate in the vacuole lumen, but not the limiting membrane, suggesting constitutive fusion between the CCV and autophagosomes delivering LC3-labeled cargo [60,71]. CCV fusion with autophagosomes is presumed to assist Coxiella by providing membrane for vacuole expansion and nutrients for pathogen growth [67]. However, there are conflicting data on the overall benefit of autophagy to Coxiella infection. Inhibition of autophagy with 3-methyladenine reduces vacuole expansion and pathogen growth whereas the opposite occurs with induction of autophagy by host cell starvation or treatment with the mTOR inhibitor, rapamycin [68,70]. Others have shown siRNA knockdown of essential autophagy proteins results in multiple CCV without negatively impacting Coxiella replication, and that a Coxiella mutant in the effector gene cbu0021 (cvpB, cig2) (discussed in the ‘The hard part: effector function’ section) replicates normally in an LC3-negative CCV [60,71].
Interactions with secretory and endocytic pathways also contribute to CCV development. The vacuole decorates with Rab1b, a small GTPase that regulates transport between the ER and Golgi apparatus [69]. Expression of a dominant-negative version of Rab1 or siRNA knockdown inhibits CCV biogenesis. Similar effects are observed if the secretory pathway is disrupted by brefeldin A treatment or expression of a dominant-negative version of Sar1. Separate studies have shown disruption of Rab5 or Rab7, GTPases that direct endocytic maturation, negatively impacts CCV formation [56,68,70,72]. Clathrin-mediated vesicular trafficking is an important component of CCV endosome recruitment as depletion of clathrin or the clathrin-adaptor protein AP2, inhibits vacuole expansion and Coxiella replication [73]. Through an unknown mechanism, the major lysosmal membrane proteins LAMP-1 and LAMP-2 also participate in CCV formation by increasing the rate of endosome fusion [57].
A genome-wide siRNA screen using a HeLa cell infection model identified several components of the retromer, a complex that retrieves receptors from the endolysosomal system, that contribute to CCV biogenesis and Coxiella replication [72]. The same study identified soluble NSF attachment receptor (SNARE) syntaxin-17 as a key host factor mediating homotypic fusion of CCVs. Multiple nascent CCVs that occur in cells infected with more than one bacterium typically fuse to form a single large and spacious vacuole [65]. The multivacuolar phenotypes observed after depletion of syntaxin-17 or ATG5/12 are similar, suggesting constitutive fusion between the CCV and autophagosomes maintains the vacuole in fusogenic, autophagolysosomal state [60,72]. Indeed, syntaxin-17 has recently been described as an autophagosome-associated SNARE required for proper autophagosome–lysosome fusion [74]. The endocytic SNAREs VAMP3, 7 and 8 also localize to the CCV [75]. Optimal recruitment of VAMP7 requires Coxiella protein synthesis and disruption of VAMP7 activity disrupts CCV hetero- and homo-typic fusion events and Coxiella replication [75].
The CCV is surrounded by F-actin puncta that potentially provide structural integrity and/or tracks for vesicular fusion events [58]. Chemical disruption of F-actin results in aberrantly small CCVs that fully mature through the endocytic pathway but cannot fuse with latex bead-containing phagosomes. The actin-associated small GTPases Cdc42 and RhoA traffic to the CCV, with peak recruitment requiring Coxiella protein synthesis Expression of a dominant-negative RhoA results in a small, multivacuole phenotype reminiscent of that associated with disrupted autophagy or syntaxin 17 activity [58,60].
The Coxiella infection cycle occurs over several days with minimal cytopathic effect and no concerted egress event [76]. Thus, the organism is adept at maintaining host cell viability by disrupting both intrinsic and extrinsic apoptotic cell death signaling [63,77]. Apoptosis inhibition involves pathogen activation of the prosurvival kinases Akt, Erk1/2 and PKA [32,78,79]. Host cell maintenance requires active Coxiella protein synthesis and a functional T4BSS [63,77,80]. Indeed, recent data show PKA is recruited to the CCV membrane in a T4BSS-dependent fashion. PKA inactivates the proapoptotic protein Bad by phosphorylation, a modification that also stimulates localization of Bad to the CCV [79]. PKA activity in general is also required for optimal CCV development and bacterial replication [81]. A similar dual functionality is observed with CCV-localized Beclin 1, which benefits Coxiella by promoting autophagy and by binding the antiapoptotic protein Bcl-2 and modulating its activity [59].
Type 4 home remodeling
As discussed above, successful infection by Coxiella requires pathogen-directed manipulation of several host cell processes including apoptosis, autophagy, secretion and endolysosomal trafficking. It is now recognized that translocation of proteins with effector functions by a Dot/Icm T4BSS is critical for co-opting host cell functions. The Coxiella NMI genome encodes 23 homologs of the 26 L. pneumophila dot/icm genes [82,83]. Predicted protein identities range from 20.8 (IcmD) to 63.4% (DotB) [84]. Four of ten dot/icm genes tested complemented the corresponding mutant in L. pneumophila, suggesting functional similarity between the two systems [85,86]. Consequently, and owing to the lack of genetic tools for Coxiella, L. pneumophila was extensively used as a surrogate host to identify Coxiella T4BSS substrates using both CyaA and BlaM cytosolic translocation reporter assays [87–96]. Indeed, of the 143 currently recognized Coxiella Dot/Icm effectors, 125 were initially identified in L. pneumophila. Gain-of-function studies using L. pneumophila also helped define Coxiella effector function [97,98].
Although L. pneumophila has proven utility as a heterologous system to examine Coxiella Dot/Icm functions, traditional gene mutation/complementation studies in Coxiella are necessary to validate surrogate results and to fulfill molecular Koch's postulates [99]. However, Coxiella's obligate intracellular lifestyle made development of genetic methods technically challenging. Reliance on a eukaryotic host cell for growth dramatically complicates selection, scoring, cloning and expansion of genetic transformants [100]. Moreover, mutants with lowered intracellular growth fitness may not be recovered. Despite these obstacles, a mutagenesis protocol for Coxiella was developed using the mariner-based Himar1 transposon (Tn) and Vero host cell system [101]. Promptly thereafter, Coxiella was liberated from its host cell by growth in the synthetic medium, acidified citrate cysteine medium (ACCM) [102,103]. Under microaerobic conditions, second-generation ACCM-2 now supports robust axenic growth in liquid media and development of small (˜0.5 mm in diameter) colonies on solid media [103]. Axenic growth dramatically accelerated several advances in Coxiella genetic manipulation. Multiple methods for gene inactivation are now available including random mutagenesis using Himar1 [80] and targeted mutagenesis using a ‘loop in/loop out’ strategy coupled with sacB counterselection or a Cre-lox approach that allows deletion of large chromosomal regions [104]. For single and multiple copy complementation, a Tn7-based system and RSF1010 ori-based shuttle vectors, respectively, are available [80,88,91,100]. Additional tools include inducible gene expression using the anhydrotetracycline-inducible promoter tetA [80] and a luciferase-based gene reporter system [105]. A method of nonantibiotic positive selection of Coxiella genetic transformants has recently been reported that uses nutritionally defined ACCM (ACCM-D) and complementation of Coxiella's natural arginine auxotrophy by expression of heterologous arginine biosynthesis genes capable of converting citrulline into arginine [106].
Confirmation that Coxiella translocates T4BSS substrates originally identified using L. pneumophila was first accomplished using shuttle vector technology [87,88,91]. The essential nature of the T4BSS for productive infection was then demonstrated by showing strains harboring Himar1 insertions in icmD or icmL or targeted deletions in dotA or dotB, had severe intracellular growth defects associated with failure to secrete effector proteins in a dot/icm-dependent fashion [80,87,104]. Direct screening of potential Dot/Icm substrates in Coxiella is now feasible [60,73,87,94,107,108]. Moreover, targeted mutagenesis [73,98,107] and large-scale Tn screens [50,60] have revealed the importance of individual effectors in host cell colonization and helped resolve their function.
Mining for effectors
The bulk of Coxiella Dot/Icm substrates have been identified using bioinformatic criteria. Eukaryotic-like features (ELF), a C-terminal Dot/Icm translocation signal and similarity to known Coxiella effectors are strong predictors of effector proteins. Indicators of effector genes are upstream PmrA regulatory motifs and genomic location [73,87–92,94–96,107]. When assembling candidate effector gene lists, the predictive power of these features is amplified when multiple criteria are combined in machine learning [95] and manual approaches [88,94]. Ultimately, Dot/Icm substrates must be empirically validated using translocation assays. In an unbiased screen using translocation as a positive readout, Carey et al. [87] generated a library of random Coxiella genomic fragments cloned downstream from the cyaA reporter gene. Screening of L. pneumophila transformants in pools of 50 identified 7 Dot/Icm substrates. This list was expanded to 18 upon screening predicted paralogs of identified effectors and proximal genes.
Studies of L. pneumophila Dot/Icm substrates revealed a C-terminal translocation signal that varies substantially in primary sequence, but exhibits an overall regular distribution of charged, polar and hydrophobic amino acids [109–111]. Computational modeling of the complex physiochemical parameters of amino acids comprising translocation signals demonstrated enrichment of hydrophobic amino acids within the three carboxyl terminal residues, serine and threonine at positions -4 to -11 and negatively charged amino acids between residues -8 and -18 [111]. A subsequent analysis revealed a motif within some Dot/Icm substrates comprised of 3–6 glutamate residues at position -11 to -18 termed the E-block [112]. This information was applied in a hidden semi-Markov model that predicted 44 Coxiella effector genes, 28 of which encoded bona fide Dot/Icm substrates [89].
Translocated effector proteins of pathogenic bacteria operate at the pathogen–host interface. Effectors have consequently evolved to contain ELFs in the form of domains or motifs that functionally mimic eukaryotic host cell proteins [113]. The higher frequency of ELFs in bacterial effectors versus noneffectors provides a powerful predictive tool for assembling candidate effector lists [73,88,90–92,94–96,107,113]. Indeed, recognition of a cohort of Coxiella genes encoding 33 amino acid ankyrin repeat domains (ARDs) resulted in identification of the first class of Coxiella Dot/Icm effectors [90,92]. ARDs, along with tetratricopeptide and leucine-rich repeats, are structural recognition modules typically found in eukaryotic proteins. Coiled-coil and helix–loop–helix domains, as well as transmembrane helices, are not specifically ELFs, but because they frequently engage proteins, lipids and/or nucleotides, they have been included in Coxiella effector searches. Although the presence of these structural modules is useful in assembling candidate effector gene lists, they provide little information on effector function. ELFs associated with biochemical functions include regulator of chromosome condensation 1-like domains linked with guanine nucleotide exchange, HAD-like domains found in phosphotransferases, filamentation induced by cAMP domains involved in AMPylation, serine/threonine protein kinase domains associated with phosphorylation and HECT-like E3 ligase and F-Box domains involved in ubiquitination. Another type of ELF found in effectors, termed short linear motifs (SLMs), consist of short amino acid sequences (3–10 residues long). The protein binding activities of SLMs can be used to unravel effector function. For example, endocytic sorting SLMs within the effector CvpA were critical for establishing its interaction with clathrin-coated vesicles [73].
At the nucleic acid level, a strong predictor of effector genes is the presence of an upstream PmrA regulatory element [88,95,114]. PmrA is a response regulator that couples with the sensory histidine kinase PmrB to form a two-component system. Work in the Legionella system demonstrated PmrA regulates expression of approximately 15% of known effector genes by binding the tandem repeat sequence cTTAATatT-N2-cTTAATatT [114,115]. Functional expression of Coxiella PmrA in Legionella was also achieved [114]. As detailed below, Coxiella PmrAB has subsequently been shown by multiple groups to be essential for productive infection by Coxiella [50,60,105].
A final effector screen relies on binding to DotF in a bacterial-two hybrid system [88]. DotF, which comprises the core transmembrane complex of the T4BSS [116], was previously shown to bind a subset of Legionella effectors [117]. Screening of a genomic library revealed six Coxiella DotF-binding proteins that were validated as Dot/Icm substrates using the CyaA translocation assay [88]. The small number of effectors identified in both Legionella and Coxiella screens may reflect new data indicating DotF is not a major receptor of cytoplasmically expressed Dot/Icm substrates [116].
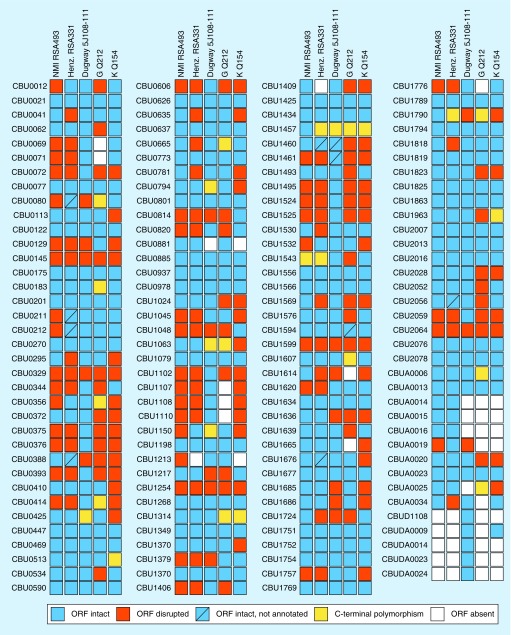
As effector lists were assembled, it was quickly recognized that considerable heterogeneity exists among effector gene repertoires of Coxiella genotypes associated with distinct virulence potential in animal models of Q fever [87,92,94]. The vast majority (>96%) of Dot/Icm substrates have been identified using the genome of the NMI RSA493 strain (multispacer sequencing typing genotype 18, 26), which is highly virulent [16,18]. Cross-genome comparisons with Henzerling RSA331 (high virulence; genotype 18, 25), G Q212 (moderate virulence; genotype 21), K Q154 (low virulence; genotype 8) and Dugway 5J108-111 (low virulence; genotype 111) [16,18,118], showed that many effector genes are pseudogeneized, due to insertion/deletions resulting in frameshifts, or completely missing (Figure 1). Indeed, only 44 out of 143 identified effector genes are intact among all 5 strains. Several annotated effector genes encode proteins with small N-terminal truncations that may still be functional if synthesized and critical domains are not disrupted. Effectors with altered C-terminal sequences are likely nonfunctional due to failed translocation, although a Legionella Dot/Icm effector with an internal translocation signal has recently been described [119]. An interesting subset of Coxiella effectors exhibiting strain variation is plasmid encoded [91]. All Coxiella strains examined to date harbor a moderately sized (˜37–54 kb) plasmid or have approximately 17.5 kb of plasmid DNA integrated into their chromosome [10]. Plasmids share 22 conserved genes and have eight to 13 unique genes each. Thirteen of 15 genes within the integrated plasmid sequences are also conserved [10]. Screening of all plasmid-encoded hypothetical proteins uncovered 12 effector genes. Three effector genes are conserved between all plasmids and the integrated plasmid sequences [93], while three and four are uniquely encoded by QpH1 (carried by Nine Mile and Henzerling strains) and QpDG (carried by the Dugway strain), respectively [93]. The strict maintenance of plasmid sequences suggests they serve critical functions in Coxiella biology, with conserved effector genes predicted to play important roles in host cell manipulation [93]. By analyzing genome sequences from 10 Coxiella strains, Pearson et al. [11] recently defined the Coxiella pan genome as consisting of 2148 genes, with 341 genes not conserved among all strains. The attenuated Dugway strain contains the largest number of unique genes with 98. Some undoubtedly encode novel effector proteins that possibly contribute to infection outcome. Testing of effector mutants in animal models will provide needed insight into the contributions of effector pools to pathotype-specific virulence.
Figure 1. . Polymorphisms of Coxiella effector genes.
ORFs encoding validated Dot/Icm substrates were used to BLAST the genomes of the Nine Mile Phase I (NMI) RSA493, Henzerling RSA331, Dugway 5J108-111, G Q212 and K Q154 strains for homologous sequences. Effector ORFs were classified as intact (blue) if the length of the translated amino acid sequence was at least 90% of the longest representative amino acid sequence. Homologous ORFs with polymorphisms leading to protein sequence lengths less than 90% of the longest representative amino acid sequence were considered disrupted (red). In some cases, an intact effector gene was present but not annotated in GenBank (blue with slash), or completely missing (white). Amino acid sequences encoded by intact ORFs were further inspected for C-terminal polymorphisms, resulting largely from frame shift mutations, predicted to disrupt the translocation signal (yellow). Only 44 effector ORFs are conserved among the Coxiella strains in this analysis. The ORF identifiers CBU and CBUA correspond to chromosomal and plasmid genes, respectively, of the NMI RSA493 strain. The ORF identifiers CBUD and CBUDA correspond to chromosomal and plasmid genes, respectively, of the Dugway 5J108-111 strain.
ORF: Open reading frame.
Finally, it is worth noting that, despite similar Dot/Icm apparatuses, effectors of Coxiella and Legionella are unique to each bacterium with virtually no homologs in other proteobacteria. Their distinct effector cohorts reflect disparate intracellular lifestyles, host range and acquisition of new genetic material by unknown horizontal gene transfer mechanisms that benefit lifestyle changes.
The hard part: effector function
With the greater part of the Coxiella Dot/Icm effector cohort known, focus has now shifted toward functional analysis. Effectors target host systems with defined roles in Coxiella cellular parasitism, and identifying their subcellular destinations can help design laboratory inquiries into function. However, detection of native effectors is exceedingly difficult; thus, ectopic expression of effector–fluorescent protein chimeras has been extensively used for trafficking studies, despite known artifacts of this procedure [120]. Effectors traffic to mitochondria [87,92,94,121], endoplasmic reticulum [91,94,96,122], Golgi apparatus [87], lysosomes [87,92,107,122], autophagosomes [91,93], endocytic vesicles [73,107], nucleus [87,94,121–123], microtubules [92,122] and ubiquitinated proteins [91,93]. An interesting subset of five effectors designated Cvp (Coxiella vacuolar protein) also traffic to the CCV [73,107]. The vast majority of effectors display a nonspecific, diffuse or punctate cytoplasmic appearance. Also relying on ectopic expression is the SEAP assay used to identify effectors that potentially disturb mammalian secretory trafficking. Several effectors inhibit release of SEAP in a HEK293T reporter cell system including CBU0635 [87], CBU1566 (CvpC), CBU1825, CBUA0019 [94] and CBUD1884 (ElpA) [96].
For a handful of effectors, functional insight has benefited from ectopic expression that confers gain-of-function and phenotype changes. Biochemical assays are then conducted to define pathway involvement. Because effectors often modulate the functions of eukaryotic pathways conserved between higher and lower eukaryotes, the yeast Saccharomyces cerevisiae is commonly used as a surrogate system to provide clues concerning effector function. Specific synthetic inhibition phenotypes observed in yeast ectopically expressing bacterial proteins can provide insight into effector identity and/or the cell processes they target [124]. Four studies have used yeast toxicity as a screening tool for Coxiella effectors [87,94,95,122]. Lifshitz et al. [95] specifically examined growth phenotypes associated with medium containing caffeine, a compound that activates the yeast cell wall integrity MAPK pathway. Interestingly, expression of CBU0885 (CetCb4) or CBU1676 (Cem9) potentiates yeast growth inhibition in the presence of caffeine, whereas CBU0388 (CetCb2) suppresses growth inhibition. Enhanced caffeine toxicity induced by Cem9 is rescued by coexpression of CetCb4 [95], suggesting antagonizing roles in modulating the cell wall integrity MAPK pathway. CetCb4 and Cem9 both contain an HAD domain required for activity. Whether these effectors promote Coxiella infection by coordinately regulating MAPKs, such as Erk1/2, through phosphorylation events remains to be determined.
Pathogen-associated molecular patterns (PAMPs) of invading microbes are sensed by pathogen recognition receptors of macrophages. Among the innate immune responses elicited by these interactions are apoptotic and pyroptotic programmed cell death [125]. Coxiella has a lengthy intracellular replication cycle lasting over a week [31], and thus deploys mechanisms to prevent both extrinsic (death receptor-mediated) and intrinsic (mitochondria-mediated) apoptosis [63,77]. The potent antiapoptotic activity of Coxiella requires a functional Dot/Icm apparatus [80]. A phenotypic screen of mammalian cells ectopically expressing Coxiella ARD effectors revealed that CBU0781 (AnkG) protects cells against staurosporine-induced intrinsic apoptosis [90,97]. In gain-of-function experiments, Legionella expressing AnkG replicated in mouse dendritic cells that normally die apoptotically following infection [97]. Subcellular localization of AnkG to the mitochondria coincides with its binding to p32, an interaction that is required for AnkG translocation to the nucleus and antiapoptotic activity [97,121]. A similar screen of known Coxiella effectors identified CBU1524 (CaeA) and CBU1532 (CaeB) as antiapoptotic effectors [87,126]. CaeA localizes to the nucleus and reduces both intrinsic and extrinsic apoptotic cascades between the initiator caspase-9 and the executioner caspase-7 by a mechanism requiring a CaeA glutamate/lysine repeat region [127]. More potent inhibition of intrinsic apoptosis is observed with CaeB that localizes to mitochondria and blocks mitochondrial outer membrane permeabilization in response to staurosporine treatment by a mechanism independent of p32 [126]. Prolonged activation of the prosurvival kinases Erk1/2 and Akt by Coxiella is also associated with antiapoptotic activity [78].
Genetic dissection of the importance of effectors implicated in Coxiella apoptosis inhibition and MAPK signaling has not been conducted. Advances in Coxiella genetic manipulation now make mutant generation and phenotyping an increasingly important requirement for publication of effector studies. Allelic exchange was described in a recent report by Cunha et al. [98] on a Coxiella effector involved in inflammasome inhibition. As opposed to noninflammatory apoptosis, pyroptosis is an inflammatory process mediated by inflammasome activation of caspase-1. Essential for inflammasome activity is PAMP recognition by cytosolic Nod-like receptors. Coxiella infection of primary mouse macrophages fails to induce caspase-1 and capase-11 activation and the subsequent release of IL-1 cytokines [37,98]. Furthermore, Coxiella inhibits induction of caspase-11-mediated, noncanonical activation of the inflammasome by L. pneumophila in macrophage coinfection experiments [98]. By expressing a panel of Coxiella effectors in L. pneumophila, CBU1823 (IcaA) was found to inhibit caspase-11 activation [98]. Importantly, a Coxiella icaA mutant failed to suppress caspase-11-mediated inflammasome activation induced by L. pneumophila in coinfection experiments. Cytosolic delivery of a Coxiella extract enriched in LPS induces caspase-11 dependent pore formation, indicating the bacterium produces molecules that induce pyroptosis. However, infection with the Coxiella icaA mutant alone fails to activate the inflammasome, suggesting that, like antiapoptotic effectors, Coxiella deploys functionally redundant effectors that contribute to protection [98]. A potential caveat is related to the observation that human primary alveolar macrophages infected with NMII, but not virulent strains of Coxiella, release mature IL-1β [32]. Thus, inflammasome activation may be Coxiella strain and/or host cell specific.
Functional insight has been gained for CvpA (CBU0665) [73,107]. A ΔcvpA mutant shows poor intracellular growth in unusually small CCVs. CvpA contains several dileucine ([DERQ]XXXL[L,I]) and tyrosine (YXXϕ, ϕ-bulky hydrophobic residue) SLMs that interact in pull-down assays with clathrin and the heterotetrameric clathrin adaptor complex AP2, suggesting CvpA targets clathrin-mediated vesicular trafficking originating from the plasma membrane [128]. Consistent with this hypothesis, ectopically expressed mCherry-CvpA localizes with clathrin on the cell periphery and endocytic recycling vesicles in the pericentrosomal region in uninfected cells, known trafficking itineraries of endogenous endocytic cargo proteins. mCherry-CvpA expression also inhibits endocytosis of transferrin [73], a cargo molecule internalized at the plasma membrane by clathrin-mediated endocytosis [129,130]. Coxiella replication and CCV biogenesis are dramatically impaired in cells depleted of AP2 or clathrin. Collectively, these data suggest CvpA targets clathrin-mediated vesicular trafficking that contributes to CCV biogenesis.
Three groups have demonstrated that mutation of the effector gene cbu0021 (cvpB, cig2) results in aberrant CCV formation and/or Coxiella growth [50,60,107,131]. Vacuoles harboring cbu0021 mutants do not homotypically fuse, resulting in a multivacuole phenotype [60,107,131]. In two investigations, cbu0021 mutants also display deficient replication [50,107]. Autophagosome interactions with the CCV require an active T4BSS [67] and CCV recruitment of the autophagy protein LC3 requires functional CBU0021 [60]. Depletion of the autophagy regulators syntaxin 7, ATG5 or ATG7 during infection with wild-type bacteria results in a multiple vacuole phenotype similar to that associated the cbu0021 mutant, suggesting a functional link between the Coxiella effector, autophagy and CCV fusogenic properties [60]. Indeed, CBU0021 binds phosphoinositides on host cell membranes and perturbs the activity of the phosphatidylinositol 3-kinase PIKfyve to enrich phosphatidylinositol 3-phosphate (PI[3]P) on CCVs [131]. This favors association of autophagosomal machinery with CCVs and their homotypic fusion [131].
Allelic exchange demonstrated the importance of the remaining cvp genes (cvpC, -D and -E) in host cell parasitism [107]. Seven cir (Coxiella effector for intracellular replication) genes with Tn insertions that result in intracellular growth defects by Coxiella were also recovered from a library following bioinformatics-based effector discovery [94]. With the exception of cbu0021, cbu1751 (described below) and cvpA, -D and -E, effector mutants have not been genetically complemented to eliminate potential contributions of polar effects and/or secondary mutations on phenotypes. Nonetheless, thus far a surprisingly high percentage of Coxiella mutants in effector genes show discernable intracellular growth defects when compared with L. pneumophila effector gene mutants. There is extensive functional redundancy in the effector pool of L. pneumophila, maintenance of which is attributed to selective pressure to survive in a multiple species of amoebae [132]. Supplementary Table 1 summarizes characteristics of known Coxiella Dot/Icm substrates and the strategies used for identification.
Blasting the chromosome with Himar1
The past few years have witnessed remarkable advances in microscopy-based high-content screening applied to the study of infectious diseases [133,134]. Multiphenotypic screens can be developed that simultaneously extrapolate a large number of features from bacteria and host cells [133]. The creation of an exceptionally large, single vacuole filled with bacteria makes the Coxiella infectious process an ideal system for this type of analysis. When combined with robust Himar1-mediated insertional mutagenesis, large and ordered libraries of Coxiella mutants can be generated and screened in a high-throughput manner for mutations that confer infection deficiencies. Mapping Tn insertions on the Coxiella genome identifies the repertoire of coding and noncoding regions that promote infection. Moreover, genes with no insertions potentially represent those universally required for growth.
The first application of this technology was reported by Martinez et al. [50] who evaluated 1082 Coxiella Tn mutants for the capacity to invade, replicate within and/or protect host cells from apoptosis. Mutagenesis was conducted using a Himar1-derived Tn designed to express green fluorescent protein [101]. Automated fluorescence microscopy and image analysis were employed to score nuclei features (apoptosis), Coxiella features (intracellular growth) and the number of CCV per host nuclei (internalization) from an average of 15,000 infected Vero cells per mutant. Intracellular growth was scored by measuring both CCV number and Coxiella colony size. The Tn insertion point for each isolated mutant, regardless of phenotype, was mapped using single primer PCR coupled to DNA sequencing to provide a global view of putative virulence determinants and Coxiella genome architecture. Mutation of 483 of the 1831 NMII coding sequences (26.1%) was reported, with a remarkable 83.2% of mutated genes conferring a mild or strong phenotype in one or more scored feature. As predicted, 40 Tn insertions in 16 dot/icm genes resulted in severe intracellular growth defects. Furthermore, mutation of 12 of 20 genes encoding confirmed Dot/Icm substrates produced severe intracellular replication phenotypes, results that allow prioritization of effectors for further study. Critical transcription factors were identified, including PmrA (CBU1227) and OmpR (CBU2006), which are response regulators of two-component systems. Interestingly, insertions in 32 annotated pseudogenes and 11 intergenic regions predicted to encode sRNA, resulted in strong replication defects, suggesting these genomic regions produce products important for host cell colonization. Alternatively, these Tn insertions could exert polar effects on, or disrupt promoter regions of, adjacent genes involved in intracellular growth.
A seminal finding of Martinez et al. [50] was identification of OmpA (CBU1260) as a Coxiella invasin mediating internalization by nonphagocytic cells. Structure predictions suggest OmpA exposes four unstructured loops at the surface of Coxiella with deletion analysis implicating loop 1 as essential for internalization. Purified OmpA, or antibodies targeting the extracellular domains of OmpA, neutralize Coxiella infectivity, supporting a receptor/ligand model of host cell entry. Interestingly, macrophages efficiently internalize the Coxiella ompA Tn mutant; however, the organisms display intracellular replication defects. OmpA may benefit Coxiella growth in macrophages by modulating innate immune signaling as demonstrated for OmpA-like proteins of other intracellular pathogens [135].
A second Tn screen was conducted by Newton et al. [60] using Himar1 modified to express mCherry fluorescent protein. Over 3237 arrayed insertion mutants were recovered and the Tn insertion points of 460 mutants determined. Using a visual fluorescence microscopy screen incorporating LAMP-1 staining for demarcation of the CCV, mutants were isolated that displayed no intracellular replication (21 genes, 6 intergenic regions), moderate intracellular replication defects (24 genes, 1 intergenic region), a multivacuole phenotype (1 gene) or a filamentous phenotype (seven genes). Again, dot/icm genes were highly represented (16 of 21) among inactivated genes that confer a null growth phenotype, with the two-component system pmrAB also included in this group. Additional regulators, such as the GacS-like sensor kinase CBU1761, were identified as needed for optimal intracellular growth. Mutation of genes encoding the previously described Dot/Icm substrates CBU1461, CBU1751 (Cig57) and CBU1754 [88,89,94] resulted in moderate intracellular replication defects whereas insertions interrupting production of CBU0021 produced an unusual multivacuole phenotype without an overall replication defect. A novel effector protein (CBU1780) was also identified (null replication phenotype). Mutants in 15 additional effector genes showed no growth deficiency, suggesting functional redundancy among this effector cohort.
The Tn mutant screens of Martinez et al. [50] and Newton et al. [60] are notable in identifying the same factors as critical for successful host cell parasitism, such as the Dot/Icm T4BSS and its regulator PmrAB and the effectors CBU0021 and CBU1780. However, disparate results were also obtained, primarily with respect to mutated genes conferring moderate to strong replication defects in the study by Martinez et al. [50] with no discernable phenotype in the study by Newton et al. [60]. These discordant results could be attributable to the use of different microscopic scoring systems, host cell lines (Vero versus HeLa) and/or lengths of infection. Also, it is unclear whether some replication defects might be attributable to a defect in overall metabolic fitness that manifests both extra- and intra-cellularly. Nonetheless, both screens are landmark studies that revealed novel determinants of intracellular growth and/or CCV development.
Table 1 summarizes the characteristics of mutated Coxiella effector genes and their associated phenotypes. Figure 2 illustrates steps in Coxiella infection and CCV formation, and depicts host cell processes potentially modulated by Coxiella effector proteins.
Table 1. . Intracellular growth phenotypes of Coxiella burnetii effector gene mutants.
| ORF | Aliases | Intracellular growth phenotype | Ref. |
|---|---|---|---|
| CBU0012 |
|
Growth defect (50, 94) |
[50,94] |
| CBU0021 |
cvpB/cig2 |
Growth defect (50, 107); CCV fusion defect (107, 60, 131) |
[50,60,107,131] |
| CBU0041 |
cirA |
Growth defect (94) |
[94] |
| CBU0069-CBU0071 |
ankP |
No growth defect (60); growth defect (50) |
[50,60] |
| CBU0072 |
ankA |
No growth defect (60); growth defect (50) |
[50,60] |
| CBU0077 |
|
No growth defect (50) |
[50] |
| CBU0145 |
ankB |
No growth defect (60) |
[60] |
| CBU0295 |
|
No growth defect (60) |
[60] |
| CBU0388 |
|
Growth defect (94) |
[94] |
| CBU0425 |
cirB |
Growth defect (94); no growth defect (50) |
[50,94] |
| CBU0447 |
ankF |
No growth defect (50) |
[50] |
| CBU0626 |
|
Growth defect (50) |
[50] |
| CBU0635 |
|
No growth defect (50) |
[50] |
| CBU0665 |
cvpA |
Growth and CCV fusion defects (73) |
[73] |
| CBU0937 |
cirC |
Growth defect (50, 94) |
[50,94] |
| CBU1079 |
|
No growth defect (60) |
[60] |
| CBU1198 |
|
Growth defect (94) |
[94] |
| CBU1217 |
|
No growth defect (50) |
[50] |
| CBU1251 |
|
No growth defect (60) |
[60] |
| CBU1376 |
coxK2 |
No growth defect (60) |
[60] |
| CBU1457 |
|
Growth defect (94) |
[94] |
| CBU1460 |
|
Growth defect (50) |
[50] |
| CBU1461 |
coxCC8 |
Growth defect (60) |
[60] |
| CBU1525 |
|
Growth defect (50) |
[50] |
| CBU1556 |
cvpC |
Growth defect (107) |
[107] |
| CBU1569 |
coxCC12 |
No growth defect (60) |
[60] |
| CBU1639 |
|
No growth defect (50) |
[50] |
| CBU1665 |
|
Growth defect (50) |
[50,60] |
| CBU1751 |
cig57 |
Growth defect (60); no growth defect (50) |
[50] |
| CBU1754 |
|
Growth defect (60) |
[60] |
| CBU1780 |
|
Growth defect (60) |
[60] |
| CBU1789 |
|
No growth defect (60) |
[60] |
| CBU1818 |
cvpD |
Growth and CCV fusion defects (107) |
[107] |
| CBU1823 |
icaA |
No growth defect reported (98) |
[98] |
| CBU1863 |
cvpE |
Growth and CCV fusion defects (107) |
[107] |
| CBU2013 |
|
Growth defect (94) |
[94] |
| CBU2016 |
|
Growth defect (50) |
[50] |
| CBU2028 |
|
No growth defect (60) |
[60] |
| CBU2052 |
cirD |
Growth defect (94); no growth defect (60) |
[60,94] |
| CBU2056 |
|
No growth defect (60) |
[60] |
| CBU2059 |
cirE |
Growth defect (94) |
[94] |
| CBU2076 |
|
No growth defect (60) |
[60] |
| CBU2078 |
|
No growth defect (50, 60) |
[50,60] |
| CBU2082 |
|
No growth defect (60) |
[60] |
| CBUA0023 | cpeF | Growth defect (50) | [50] |
CCV: Coxiella-containing vacuole; ORF: Open reading frame.
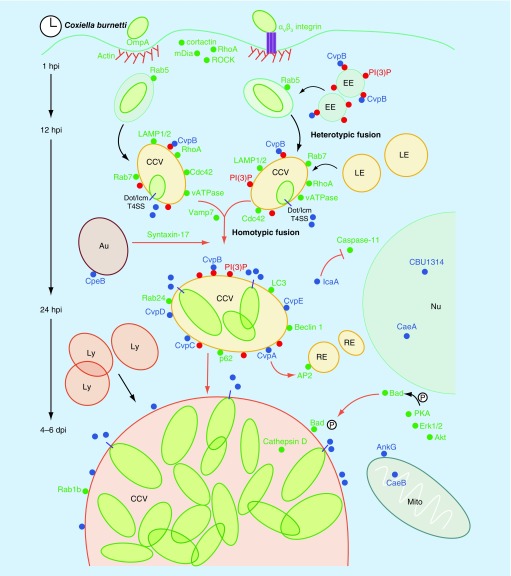
Figure 2. . Steps in Coxiella infection and Coxiella-containing vacuole formation and host cell processes potentially modulated by Coxiella effector proteins.
Coxiella internalization is facilitated by the rearrangement of the host actin cytoskeleton, mediated by the actin-binding proteins cortactin and mDia and requiring the activation of the small GTP-ases RhoA and ROCK. The Coxiella invasin OmpA mediates bacterial internalization by nonprofessional phagocytes, whereas internalization by macrophages requires interaction with cell surface αVβ3 integrins. Inside cells bacteria reside within CCVs that progressively acquire endosomal markers by heterotypic fusion with early and late endosomes and lysosomes (EE, LE and Ly, respectively). CCV acidification activates bacterial metabolism, which likely coincides with translocation of effectors (blue dots) by the Dot/Icm T4BSS (purple rods). This is required for vacuole expansion and for the recruitment of the autophagosomal and endocytic SNARE proteins syntaxin-17 and Vamp7, mediating the homotypic fusion of multiple CCVs into a single vacuole. CCV maturation requires Coxiella effectors to reroute several vesicle trafficking pathways that contribute membrane to the expanding CCV. The Coxiella effector CvpA interacts with AP2 on RE. The effector CvpB localizes at EE and CCVs, where it binds PI(3)P (red dots) and manipulates its metabolism to favor vacuole biogenesis and autophagy (Au)-mediated homotypic fusion of CCVs. Vacuole decoration by the small GTPase Rab1b suggests that other Coxiella effectors manipulate ER-to-Golgi traffic. Additional Coxiella effectors inhibit the intrinsic and extrinsic apoptotic pathways of infected cells. Dot/Icm-mediated translocation of yet unidentified effectors is required to activate the prosurvival kinases Akt, Erk1/2 and PKA. In turn, PKA phosphorylates and inactivates the proapoptotic protein Bad, triggering its recruitment by the CCV. The effectors AnkG and CaeA localize at Mito where they prevent membrane permeabilization induced by staurosporin. CaeA localizes at the Nu of infected cells where it prevents apoptosis by preventing caspase-9 and -7 cleavage whereas the effector IcaA has been recently associated with the inhibition of caspase-11 cleavage in macrophages to prevent pyroptosis. Finally, the Coxiella effector CBU1314 localizes at the nucleus of infected cells where it may act as nucleomodulin. Green dots indicate host proteins implicated in Coxiella infection events. Black arrows indicate events that do not require the translocation of Coxiella effectors; red arrows indicate processes mediated by Coxiella effectors.
CCV: Coxiella-containing vacuole; EE: Early endosome; LE: Late endosome; Mito: Mitochondria; Nu: Nucleus; RE: Recycling endosome; SNARE: Soluble NSF attachment receptor.
Regulating type 4 translocation
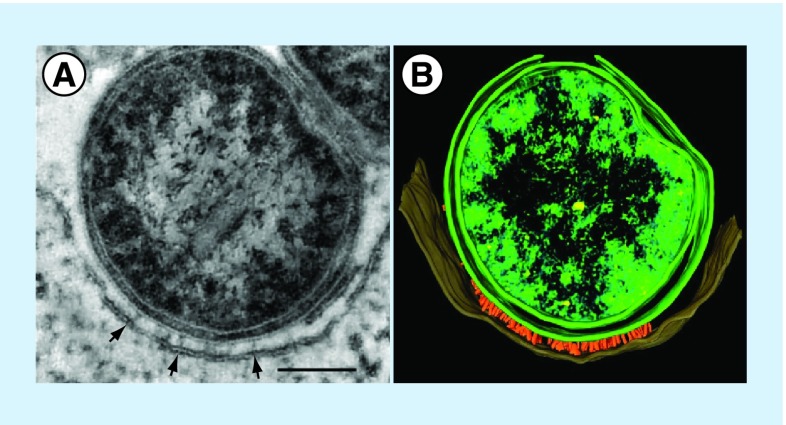
Current data support a model whereby effector translocation requires both host cell membrane contact and pathogen metabolism. Evidence for host membrane contact is based on the observation that verified effector proteins are not detected by mass spectrometry in ACCM-2 following Coxiella culture [136] and electron micrographs showing protrusions emanating from the Coxiella surface that appear to penetrate the CCV lipid bilayer (Figure 3). The presence of Dot/Icm proteins with Walker box ATPase motifs, such as DotB, suggests substrate translocation is an energy-requiring process that requires active pathogen metabolism [84]. Coxiella is metabolically active only at moderately acidic pH [64]. As such, disruption of host factors, such as Rab5 and Rab7, that regulate endolysosomal maturation and acidification, also inhibit Dot/Icm-mediated secretion [108]. Of note, effector translocation is detected earlier in mouse macrophages (4 h postinfection) than HeLa cells (8 h postinfection), suggesting the CCV maturation/acidification process occurs more rapidly in the former cell type [108]. Inhibiting transcription with rifampicin upon infection inhibits effector release, but it is unclear whether this effect is Dot/Icm specific or due to a general decline in metabolic fitness [108]. Induction of the T4BSS as late as one day postinfection initiates Coxiella replication, showing secretion is not required during transit through the endolysosomal cascade for productive infection [80]. This result, and data showing nonreplicating Coxiella remain viable in LAMP-1-positive vacuoles of host cells treated with bacteriostatic antibiotics for 2 days [65], indicate Coxiella is inherently resistant to digestion by lysosomal hydrolases. Intimate membrane contact and energy-dependent translocation of preformed effectors is invoked for the L. pnenomophila T4BSS [137]; however, unlike Coxiella, Legionella effector translocation must occur coincident with pathogen internalization to promote development of an ER-derived vacuole that avoids lysosome fusion and supports growth [137,138]. The environmental cue(s) that activates the Coxiella T4BSS is unknown, but could involve inorganic ions or pH as demonstrated for other specialized secretion systems [139,140].
Figure 3. . Association of Coxiella cell envelope projections with the Coxiella-containing vacuole membrane.
(A) Transmission electron micrograph and (B) 3D pseudocolored tomograph of a THP-1 macrophage infected for 48 h with Coxiella. Cell envelope-associated needle-like structures (˜20 nm in length) at points of Coxiella–coxiella-containing vacuole membrane contact (arrows) are evident that are likely components of the T4BSS. Bar =100 nm.
A critical regulator of the Coxiella T4BSS is the two-component system PmrAB [50,60,105]. Genetic inactivation of the system by three independent groups resulted in strong intracellular growth defects [50,60,105]. Using a luciferase reporter assay and a Coxiella ΔpmrA mutant, Beare et al. [105] demonstrated PmrA is a critical transcriptional regulator of Dot/Icm operons and effector genes containing predicted PmrA regulatory elements. Coincidently, functional PmrA is required for effector translocation [60,105]. RNA sequencing of the ΔpmrA mutant revealed several pmrA-regulated genes, including a few new effector genes, lacking PmrA regulatory elements. Cross-talk between PmrAB and other Coxiella regulatory factors may control transcription of these genes. Of much interest are the host cell signal(s) that activate the sensory kinase PmrB.
In addition to a C-terminal translocation signal, many Dot/Icm substrates require recognition of an internal signal by the chaperone (adaptor) complex IcmSW for optimal transport [92,141]. IcmS dependency was demonstrated for 7 of 11 Coxiella ARD effectors in the surrogate L. pneumophila system [92]. Tn mutagenesis of icmS and icmW results in a moderate [50,60] and strong [60] intracellular growth defects, respectively. A working model of IcmSW engagement suggests chaperone binding maintains effectors in an unfolded state that facilitates recognition of the C-terminal signal sequence by the translocation apparatus [142]. Whether IcmSW targets effector subsets with common functions or temporal constraints remains to be determined.
Conclusion
The phagolysosome-like niche of Coxiella is unique among intracellular bacteria and represents a fascinating example of pathogen–host adaptation. The CCV can expand to occupy the majority of host cell cytoplasm and accommodate hundreds of replicating Coxiella without causing obvious cytoxicity. In fact, persistently infected mouse L929 cells display a normal cell cycle and doubling time [143]. Habitation of a vacuole that fully matures through the endolysosomal pathways ensures sufficient acidity to drive Coxiella metabolism. In contrast, metabolic quiescence associated with the relatively neutral extracellular environmental likely contributes to prolonged maintenance of Coxiella as an infectious form. The mature Coxiella vacuole is clearly a specialized compartment that engages multiple vesicular trafficking pathways for acquisition of membrane for vacuole enlargement and presumably nutrients for pathogen growth. The newfound ability to genetically manipulate Coxiella now establishes Coxiella as a model system of macrophage parasitism. Most predominantly, genetics have unraveled the essential nature of the Dot/Icm T4BSS in macrophage colonization and are beginning to elucidate functions of the associated effector pool. Effector mutations that result in defects in endosome fusion, autophagosome interactions and apoptosis/inflammasome inhibition have been identified, although their biochemical activities remain unresolved. Tn mutagenesis screens have revealed numerous Coxiella genes assisting host invasion and intracellular replication, including OmpA, the first identified Coxiella invasin.
Future perspective
The burgeoning tractability of Coxiella as host–pathogen model system has enticed new researchers into the field for what should be the golden age of Coxiella virulence factor discovery. However, Coxiella researchers would benefit from further refinement of existing tools and development of new technologies. Saturation-level Tn mutagenesis of Coxiella strains that cause clinical disease in animal models of Q fever would allow high-throughput Tn-insertion sequencing strategies for identification of the global gene repertoire required for animal infection [144]. Tn-based promoter traps could also define genome-wide transcriptional responses under diverse environmental conditions. Existing Cre-lox technology should be explored for generation of attenuated, nonrevertable variants of Coxiella pathotypes for use in pathogen–host interaction studies at biosafety level 2. Restricted use of antibiotic resistance genes as selectable markers make development of nonantibiotic based selection schemes and markerless allelic exchange methods desirable. From the host cell side, genome editing using CRISPR technology now simplifies generation of knockout cell lines to identify host cell functions participating in infection and effector activity [145]. Macrophage transcriptional responses to infection by different Coxiella pathotypes [15,16] will shed light on strain-specific host cell interactions.
Defining T4BSS effector function is central to understand Q fever pathogenesis. Unfortunately, the field has only scratched the surface in understanding effector activities, the relevance of effector gene polymorphisms, strain specificity in effector cohorts and potential effector redundancies. Contributing to these gaps in knowledge is an incomplete grasp of the host cell systems co-opted for Coxiella parasitism. Apoptosis and inflammasome inhibition are clearly strategies to sustain the macrophage host for Coxiella's lengthy infectious cycle, with subversion of several vesicular trafficking pathways providing the CCV membrane and nutrients. However, the complex effector pool of Coxiella undoubtedly modulates additional eukaryotic systems for pathogen benefit such as the cytoskeleton [146], ubiquitination [147] and nuclear processes via the activity of an emerging class of effectors known as nucleomodulins [148,149]. Elucidating signal transduction events that trigger effector translocation, the temporal reliance on effector groups during infection and whether effector–effector regulation occurs [150,151], are of much interest to the field.
Despite progress in genetic dissection of effector functions using tissue culture models of infection, LPS still remains the only genetically defined virulence factor evaluated using immunocompetent rodent models (mice and guinea pigs) [33,152]. To date, the attenuated NMII strain has been exclusively used to generate mutants using transformation. Because of biocontainment constraints, many Coxiella laboratories are restricted to using this strain and therefore have employed infection of immune-deficient mice to glean information on Coxiella pathogenesis [153,154]. New invertebrate models of infection have also shown promise in assessing virulence attributes of NMII mutants [155]. Nonetheless, effector mutations may only manifest during infection of mammals with intact innate and adaptive immune responses, requiring mutant generation and subsequent animal testing of clinically relevant strains of Coxiella.
EXECUTIVE SUMMARY.
Coxiella: a wide-ranging zoonotic pathogen
Coxiella burnetii has worldwide distribution with a broad range of animal hosts. Shedding by domestic livestock is primarily responsible for aerosol transmission of Q fever to humans.
Pathoadaptation appears to drive strain variation within the genus Coxiella with some genotypes showing distinct animal reservoirs and disease associations.
Making a home in host cells
Coxiella is unique among intracellular bacteria in replicating within a macrophage vacuole that phenotypically resembles a large phagolysosome. The Coxiella-containing vacuole (CCV) fuses with endolysosomal, autophagic and secretory vesicles.
Coxiella exerts a potent antiapoptotic effect on host cells that benefits its slow intracellular growth rate.
Type 4 home remodeling
Host cell-free growth revolutionized the study of Coxiella, in large part by accelerating the development of advanced genetic tools.
Conventional mutation/complementation strategies are now available to directly examine Coxiella gene function, including Dot/Icm and effector-encoding genes.
Mining for effectors
Expression in L. pneumophila of a random library of Coxiella proteins N-terminally fused to the CyaA cytosolic translocation reporter revealed multiple Coxiella Dot/Icm substrates.
Strong bioinformatics predictors of T4BSS substrates include eukaryotic-like motifs and C-terminal positively charged E-blocks, as well as upstream PmrA regulatory sequences on encoding genes.
Full-length effector genes conserved between Coxiella strains presumably perform functions required for macrophage colonization.
The hard part: effector function
To date, mutation of 24 of 46 Dot/Icm effector genes confer defects in intracellular growth and/or CCV formation.
Ectopic expression in mammalian cells and/or gain of function in L. pneumophila has shed light on antiapoptotic functions of the effectors AnkG, CaeA and CaeB.
Like antiapoptosis effectors, inhibition of the inflammasome by the effector IcaA is another strategy employed by Coxiella to prolong host cell viability.
Synthetic inhibition or potentiation of yeast growth have identified CBU0885 (CetCb4), CBU1676 (Cem9) and CBU0388 (CetCb2) as potential modulators of the mammalian MAP kinase pathway.
Cumulative data suggest the effectors CBU0021 (CvpB, Cig2) and CBU0665 (CvpA) modulate vesicular trafficking events.
Blasting the chromosome with Himar1
Screening of Coxiella Himar1 Tn mutant libraries has provided a genome-wide view of genes required for optimal intracellular growth and/or CCV formation.
High-content screening for internalization defects revealed OmpA as a Coxiella invasin mediating internalization by epithelial cells.
Regulating type 4 translocation
Regulation of the T4BSS by Coxella is multifactorial with transcriptional control involving the two-component sensory system PmrAB and post-translational control involving the chaperone pair IcmSW and a C-terminal translocation signal.
CCV acidification that activates Coxiella metabolism is required for effector translocation by the Dot/Icm system.
Supplementary Material
Acknowledgements
The authors thank A Mora for graphics support and B Hansen for electron microscopy.
Footnotes
Financial & competing interests disclosure
This work was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (RA Heinzen) and by grants from the Agence Nationale de la Recherche (ANR) (ANR-14-CE14-0012-01; Project AttaQ), ERA-NET Infect-ERA (ANR-13-IFEC-0003; Project EUGENPATH) and the ATIP-AVENIR programme (M Bonazzi). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special notes have been highlighted as: • of interest
- 1.Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005;5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 2.Kampschreur LM, Delsing CE, Groenwold RH, et al. Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: results from the Dutch chronic Q fever database. J. Clin. Microbiol. 2014;52:1637–1643. doi: 10.1128/JCM.03221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babudieri C. Q fever: a zoonosis. Adv. Vet. Sci. 1959;5:81–84. [Google Scholar]
- 4.Angelakis E, Raoult D. Q fever. Vet. Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Hackert VH, Van Der Hoek W, Dukers-Muijrers N, et al. Q fever: single-point source outbreak with high attack rates and massive numbers of undetected infections across an entire region. Clin. Infect. Dis. 2012;55:1591–1599. doi: 10.1093/cid/cis734. [DOI] [PubMed] [Google Scholar]
- 6.Palmer NC, Kierstead M, Key DW, Williams JC, Peacock MG, Vellend H. Placentitis and abortion in goats and sheep in Ontario caused by Coxiella burnetii . Can. Vet. J. 1983;24:60–61. [PMC free article] [PubMed] [Google Scholar]
- 7.Kersh GJ, Wolfe TM, Fitzpatrick KA, et al. Presence of Coxiella burnetii DNA in the environment of the United States, 2006 to 2008. Appl. Environ. Microbiol. 2010;76:4469–4475. doi: 10.1128/AEM.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duron O. The IS1111 insertion sequence used for detection of Coxiella burnetii is widespread in Coxiella-like endosymbionts of ticks. FEMS Microbiol. Lett. 2015;362:fnv132. doi: 10.1093/femsle/fnv132. [DOI] [PubMed] [Google Scholar]
- 9.Van Asseldonk MA, Prins J, Bergevoet RH. Economic assessment of Q fever in the Netherlands. Prev. Vet. Med. 2013;112:27–34. doi: 10.1016/j.prevetmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Beare PA, Unsworth N, Andoh M, et al. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella . Infect. Immun. 2009;77:642–656. doi: 10.1128/IAI.01141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson T, Hornstra HM, Sahl JW, et al. When outgroups fail; phylogenomics of rooting the emerging pathogen. Coxiella burnetii. Syst. Biol. 2013;62:752–762. doi: 10.1093/sysbio/syt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duron O, Noel V, Mccoy KD, et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen. Coxiella burnetii. PLoS Pathog. 2015;11:e1004892. doi: 10.1371/journal.ppat.1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallen MJ, Wren BW. Bacterial pathogenomics. Nature. 2007;449:835–842. doi: 10.1038/nature06248. [DOI] [PubMed] [Google Scholar]
- 14.Bliven KA, Maurelli AT. Antivirulence genes: insights into pathogen evolution through gene loss. Infect. Immun. 2012;80:4061–4070. doi: 10.1128/IAI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii . Nat. Rev. Microbiol. 2013;11:561–573. doi: 10.1038/nrmicro3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell-Lodrigue KE, Andoh M, Poels MW, et al. Coxiella burnetii isolates cause genogroup-specific virulence in mouse and guinea pig models of acute Q fever. Infect. Immun. 2009;77:5640–5650. doi: 10.1128/IAI.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glazunova O, Roux V, Freylikman O, et al. Coxiella burnetii genotyping. Emerg. Infect. Dis. 2005;11:1211–1217. doi: 10.3201/eid1108.041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornstra HM, Priestley RA, Georgia SM, et al. Rapid typing of Coxiella burnetii . PLoS ONE. 2011;6:e26201. doi: 10.1371/journal.pone.0026201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahamat A, Edouard S, Demar M, et al. Unique clone of Coxiella burnetii causing severe Q fever, French Guiana. Emerg. Infect. Dis. 2013;19:1102–1104. doi: 10.3201/eid1907.130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson T, Hornstra HM, Hilsabeck R, et al. High prevalence and two dominant host-specific genotypes of Coxiella burnetii in U.S. milk. BMC Microbiol. 2014;14:41. doi: 10.1186/1471-2180-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kersh GJ, Oliver LD, Self JS, Fitzpatrick KA, Massung RF. Virulence of pathogenic Coxiella burnetii strains after growth in the absence of host cells. Vector Borne Zoonotic Dis. 2011;11:1433–1438. doi: 10.1089/vbz.2011.0670. [DOI] [PubMed] [Google Scholar]
- 22.Toman R, Skultety L. Structural study on a lipopolysaccharide from Coxiella burnetii strain Nine Mile in avirulent Phase II. Carbohydr. Res. 1996;283:175–185. doi: 10.1016/0008-6215(96)87610-5. [DOI] [PubMed] [Google Scholar]
- 23.Amano K, Williams JC. Chemical and immunological characterization of lipopolysaccharides from Phase I and Phase II Coxiella burnetii . J. Bacteriol. 1984;160:994–1002. doi: 10.1128/jb.160.3.994-1002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ftacek P, Skultety L, Toman R. Phase variation of Coxiella burnetii strain Priscilla: influence of this phenomenon on biochemical features of its lipopolysaccharide. J. Endotoxin. Res. 2000;6:369–376. [PubMed] [Google Scholar]
- 25.Hackstadt T, Peacock MG, Hitchcock PJ, Cole RL. Lipopolysaccharide variation in Coxiella burnetii: intrastrain heterogeneity in structure and antigenicity. Infect. Immun. 1985;48:359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beare PA, Samuel JE, Howe D, Virtaneva K, Porcella SF, Heinzen RA. Genetic diversity of the Q fever agent, Coxiella burnetii assessed by microarray-based whole-genome comparisons. J. Bacteriol. 2006;188:2309–2324. doi: 10.1128/JB.188.7.2309-2324.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denison AM, Massung RF, Thompson HA. Analysis of the O-antigen biosynthesis regions of Phase II isolates of Coxiella burnetii . FEMS Microbiol. Lett. 2007;267:102–107. doi: 10.1111/j.1574-6968.2006.00544.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuley R, Smith HE, Frangoulidis D, Smits MA, Jan Roest HI, Bossers A. Cell-free propagation of Coxiella burnetii does not affect its relative virulence. PLoS ONE. 2015;10:e0121661. doi: 10.1371/journal.pone.0121661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beare PA, Howe D, Cockrell DC, Heinzen RA. Efficient method of cloning the obligate intracellular bacterium Coxiella burnetii . Appl. Environ. Microbiol. 2007;73:4048–4054. doi: 10.1128/AEM.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hackstadt T. Biosafety concerns and Coxiella burnetii . Trends Microbiol. 1996;4:341–342. doi: 10.1016/0966-842x(96)81555-1. [DOI] [PubMed] [Google Scholar]
- 31.Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. Coxiella burnetii Phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect. Immun. 2010;78:3465–3474. doi: 10.1128/IAI.00406-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham JG, Macdonald LJ, Hussain SK, Sharma UM, Kurten RC, Voth DE. Virulent Coxiella burnetii pathotypes productively infect primary human alveolar macrophages. Cell. Microbiol. 2013;15:1012–1025. doi: 10.1111/cmi.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moos A, Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 1987;55:1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JC, Peacock MG, Mccaul TF. Immunological and biological characterization of Coxiella burnetii Phases I and II, separated from host components. Infect. Immun. 1981;32:840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackstadt T. Steric hindrance of antibody binding to surface proteins of Coxiella burnetti by Phase I lipopolysaccharide. Infect. Immun. 1988;56:802–807. doi: 10.1128/iai.56.4.802-807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamboni DS, Campos MA, Torrecilhas AC, et al. Stimulation of toll-like receptor 2 by Coxiella burnetii is required for macrophage production of pro-inflammatory cytokines and resistance to infection. J. Biol. Chem. 2004;279:54405–54415. doi: 10.1074/jbc.M410340200. [DOI] [PubMed] [Google Scholar]
- 37.Bradley WP, Boyer MA, Nguyen HT, et al. Primary role for TLR-driven TNF rather than cytosolic immune detection in restricting Coxiella burnetii Phase II replication within mouse macrophages. Infect. Immun. 2016;84:998–1015. doi: 10.1128/IAI.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamboni DS, Rabinovitch M. Phagocytosis of apoptotic cells increases the susceptibility of macrophages to infection with Coxiella burnetii Phase II through down-modulation of nitric oxide production. Infect. Immun. 2004;72:2075–2080. doi: 10.1128/IAI.72.4.2075-2080.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamboni DS. Genetic control of natural resistance of mouse macrophages to Coxiella burnetii infection in vitro: macrophages from restrictive strains control parasitophorous vacuole maturation. Infect. Immun. 2004;72:2395–2399. doi: 10.1128/IAI.72.4.2395-2399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon JG, Howe D, Heinzen RA. Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc. Natl Acad. Sci. USA. 2005;102:8722–8727. doi: 10.1073/pnas.0501863102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khavkin T, Tabibzadeh SS. Histologic, immunofluorescence, and electron microscopic study of infectious process in mouse lung after intranasal challenge with Coxiella burnetii . Infect. Immun. 1988;56:1792–1799. doi: 10.1128/iai.56.7.1792-1799.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham JG, Winchell CG, Kurten RC, Voth DE. Development of an ex vivo tissue platform to study the human lung response to Coxiella burnetii . Infect. Immun. 2016;84(5):1438–1445. doi: 10.1128/IAI.00012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calverley M, Erickson S, Read AJ, Harmsen AG. Resident alveolar macrophages are susceptible to and permissive of Coxiella burnetii infection. PLoS ONE. 2012;7:e51941. doi: 10.1371/journal.pone.0051941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein A, Louveau C, Lepidi H, et al. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 2005;73:2469–2477. doi: 10.1128/IAI.73.4.2469-2477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez J, Souriau A, Buendia AJ, et al. Experimental Coxiella burnetii infection in pregnant goats: a histopathological and immunohistochemical study. J. Comp. Pathol. 2006;135:108–115. doi: 10.1016/j.jcpa.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Bechah Y, Verneau J, Ben Amara A, et al. Persistence of Coxiella burnetii the agent of Q fever, in murine adipose tissue. PLoS ONE. 2014;9:e97503. doi: 10.1371/journal.pone.0097503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voth DE, Heinzen RA. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii . Cell. Microbiol. 2007;9:829–840. doi: 10.1111/j.1462-5822.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 48.Baca OG, Klassen DA, Aragon AS. Entry of Coxiella burnetii into host cells. Acta Virol. 1993;37:143–155. [PubMed] [Google Scholar]
- 49.Tujulin E, Macellaro A, Lilliehook B, Norlander L. Effect of endocytosis inhibitors on Coxiella burnetii interaction with host cells. Acta Virol. 1998;42:125–131. [PubMed] [Google Scholar]
- 50.Martinez E, Cantet F, Fava L, Norville I, Bonazzi M. Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi-phenotypic high-content screening. PLoS Pathog. 2014;10:e1004013. doi: 10.1371/journal.ppat.1004013. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes a high-throughput microscopy-based screen of Coxiella Tn mutants that identified genes required for optimal host cell invasion and growth, including the first Coxiella invasin (OmpA).
- 51.Capo C, Lindberg FP, Meconi S, et al. Subversion of monocyte functions by Coxiella burnetii: impairment of the cross-talk between avb3 integrin and CR3. J. Immunol. 1999;163:6078–6085. [PubMed] [Google Scholar]
- 52.Meconi S, Jacomo V, Boquet P, Raoult D, Mege JL, Capo C. Coxiella burnetii induces reorganization of the actin cytoskeleton in human monocytes. Infect. Immun. 1998;66:5527–5533. doi: 10.1128/iai.66.11.5527-5533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salinas RP, Ortiz Flores RM, Distel JS, Aguilera MO, Colombo MI, Beron W. Coxiella burnetii phagocytosis Is regulated by GTPases of the Rho family and the RhoA effectors mDia1 and ROCK. PLoS ONE. 2015;10:e0145211. doi: 10.1371/journal.pone.0145211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meconi S, Capo C, Remacle-Bonnet M, Pommier G, Raoult D, Mege JL. Activation of protein tyrosine kinases by Coxiella burnetii: role in actin cytoskeleton reorganization and bacterial phagocytosis. Infect. Immun. 2001;69:2520–2526. doi: 10.1128/IAI.69.4.2520-2526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosales EM, Aguilera MO, Salinas RP, et al. Cortactin is involved in the entry of Coxiella burnetii into nonphagocytic cells. PLoS ONE. 2012;7:e39348. doi: 10.1371/journal.pone.0039348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. The autophagic pathway is actively modulated by Phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 2007;9:891–909. doi: 10.1111/j.1462-5822.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 57.Schulze-Luehrmann J, Eckart RA, Olke M, Saftig P, Liebler-Tenorio E, Luhrmann A. LAMP proteins account for the maturation delay during the establishment of the Coxiella burnetii-containing vacuole. Cell. Microbiol. 2016;18:181–194. doi: 10.1111/cmi.12494. [DOI] [PubMed] [Google Scholar]
- 58.Aguilera M, Salinas R, Rosales E, Carminati S, Colombo MI, Beron W. Actin dynamics and Rho GTPases regulate the size and formation of parasitophorous vacuoles containing Coxiella burnetii . Infect. Immun. 2009;77:4609–4620. doi: 10.1128/IAI.00301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez CL, Colombo MI. Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell. Death Differ. 2010;17:421–438. doi: 10.1038/cdd.2009.129. [DOI] [PubMed] [Google Scholar]; • Demonstrates that Coxiella-containing vacuole (CCV)-localized Beclin 1 benefits Coxiella by promoting autophagy and inhibiting cell death through interactions with the antiapoptotic protein Bcl-2.
- 60.Newton HJ, Kohler LJ, Mcdonough JA, et al. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog. 2014;10:e1004286. doi: 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes a microscopy-based visual screen of Coxiella Tn mutants that identified genes required for robust CCV generation and intracellular replication by Coxiella, including the effector gene cig2 (cvpB) that promotes CCV homotypic fusion via a process that engages the autophagy pathway.
- 61.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 62.Case ED, Smith JA, Ficht TA, Samuel JE, De Figueiredo P. Space: a final frontier for vacuolar pathogens. Traffic. 2016;17:461–474. doi: 10.1111/tra.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voth DE, Howe D, Heinzen RA. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect. Immun. 2007;75:4263–4271. doi: 10.1128/IAI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hackstadt T, Williams JC. Incorporation of macromolecular precursors by Coxiella burnetii in an axenic medium. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and Rickettsial Diseases. Academic Press, Inc.; NY, USA: 1981. pp. 431–440. [Google Scholar]
- 65.Howe D, Melnicakova J, Barak I, Heinzen RA. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell Microbiol. 2003;5:469–480. doi: 10.1046/j.1462-5822.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 66.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winchell CG, Graham JG, Kurten RC, Voth DE. Coxiella burnetii type IV secretion-dependent recruitment of macrophage autophagosomes. Infect. Immun. 2014;82:2229–2238. doi: 10.1128/IAI.01236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beron W, Gutierrez MG, Rabinovitch M, Colombo MI. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect. Immun. 2002;70:5816–5821. doi: 10.1128/IAI.70.10.5816-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campoy EM, Zoppino FC, Colombo MI. The early secretory pathway contributes to the growth of the Coxiella-replicative niche. Infect. Immun. 2011;79:402–413. doi: 10.1128/IAI.00688-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gutierrez MG, Vazquez CL, Munafo DB, et al. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell. Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 71.Kohler LJ, Roy CR. Biogenesis of the lysosome-derived vacuole containing Coxiella burnetii . Microbes Infect. 2015;17:766–771. doi: 10.1016/j.micinf.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mcdonough JA, Newton HJ, Klum S, Swiss R, Agaisse H, Roy CR. Host pathways important for Coxiella burnetii infection revealed by genome-wide RNA interference screening. MBio. 2013;4:e00606–e00612. doi: 10.1128/mBio.00606-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larson CL, Beare PA, Howe D, Heinzen RA. Coxiella burnetii effector protein subverts clathrin-mediated vesicular trafficking for pathogen vacuole biogenesis. Proc. Natl Acad. Sci. USA. 2013;110:E4770–E4779. doi: 10.1073/pnas.1309195110. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates roles for clathrin and the clathrin adapter protein AP-2 in formation of the CCV and provides evidence that the Coxiella effector CvpA manipulates endocytic recycling by a process involving clathrin binding.
- 74.Itakura E, Mizushima N. Syntaxin 17: the autophagosomal SNARE. Autophagy. 2013;9:917–919. doi: 10.4161/auto.24109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campoy EM, Mansilla ME, Colombo MI. Endocytic SNAREs are involved in optimal Coxiella burnetii vacuole development. Cell. Microbiol. 2013;15:922–941. doi: 10.1111/cmi.12087. [DOI] [PubMed] [Google Scholar]
- 76.Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA. Temporal analysis of Coxiella burnetii morphological differentiation. J. Bacteriol. 2004;186:7344–7352. doi: 10.1128/JB.186.21.7344-7352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luhrmann A, Roy CR. Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect. Immun. 2007;75:5282–5289. doi: 10.1128/IAI.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voth DE, Heinzen RA. Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii antiapoptotic activity. Infect. Immun. 2009;77:205–213. doi: 10.1128/IAI.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macdonald LJ, Graham JG, Kurten RC, Voth DE. Coxiella burnetii exploits host cAMP-dependent protein kinase signalling to promote macrophage survival. Cell Microbiol. 2014;16:146–159. doi: 10.1111/cmi.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beare PA, Gilk SD, Larson CL, et al. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. MBio. 2011;2:e00175–00111. doi: 10.1128/mBio.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates using Coxiella genetics that the T4BSS is required for productive infection and apoptosis inhibition, and that pathogen replication ensues following significant delays in secretion system deployment.
- 81.Macdonald LJ, Kurten RC, Voth DE. Coxiella burnetii alters cyclic AMP-dependent protein kinase signaling during growth in macrophages. Infect. Immun. 2012;80:1980–1986. doi: 10.1128/IAI.00101-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seshadri R, Paulsen IT, Eisen JA, et al. Complete genome sequence of the Q-fever pathogen Coxiella burnetii . Proc. Natl Acad. Sci. USA. 2003;100:5455–5460. doi: 10.1073/pnas.0931379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Segal G, Shuman HA. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii . Mol. Microbiol. 1999;33:669–670. doi: 10.1046/j.1365-2958.1999.01511.x. [DOI] [PubMed] [Google Scholar]
- 84.Segal G, Feldman M, Zusman T. The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii . FEMS Microbiol. Rev. 2005;29:65–81. doi: 10.1016/j.femsre.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Zusman T, Yerushalmi G, Segal G. Functional similarities between the icm/dot pathogenesis systems of Coxiella burnetii and Legionella pneumophila . Infect. Immun. 2003;71:3714–3723. doi: 10.1128/IAI.71.7.3714-3723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zamboni DS, Mcgrath S, Rabinovitch M, Roy CR. Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system. Mol. Microbiol. 2003;49:965–976. doi: 10.1046/j.1365-2958.2003.03626.x. [DOI] [PubMed] [Google Scholar]
- 87.Carey KL, Newton HJ, Luhrmann A, Roy CR. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 2011;7:e1002056. doi: 10.1371/journal.ppat.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First validation using a Coxiella mutant that the T4BSS is essential for host cell colonization and that effectors translocated by L. pneumophila Dot/Icm are also translocated by Coxiella in T4BSS-dependent fashion.
- 88.Chen C, Banga S, Mertens K, et al. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii . Proc. Natl Acad. Sci. USA. 2010;107:21755–21760. doi: 10.1073/pnas.1010485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lifshitz Z, Burstein D, Peeri M, et al. Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc. Natl Acad. Sci. USA. 2013;110:707–715. doi: 10.1073/pnas.1215278110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–1654. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voth DE, Beare PA, Howe D, et al. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J. Bacteriol. 2011;193:1493–1503. doi: 10.1128/JB.01359-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voth DE, Howe D, Beare PA, et al. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 2009;191:4232–4242. doi: 10.1128/JB.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maturana P, Graham JG, Sharma UM, Voth DE. Refining the plasmid-encoded type IV secretion system substrate repertoire of Coxiella burnetii . J. Bacteriol. 2013;195:3269–3276. doi: 10.1128/JB.00180-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weber MM, Chen C, Rowin K, et al. Identification of Coxiella burnetii type IV secretion substrates required for intracellular replication and Coxiella-containing vacuole formation. J. Bacteriol. 2013;195:3914–3924. doi: 10.1128/JB.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lifshitz Z, Burstein D, Schwartz K, Shuman HA, Pupko T, Segal G. Identification of novel Coxiella burnetii Icm/Dot effectors and genetic analysis of their involvement in modulating a mitogen-activated protein kinase pathway. Infect. Immun. 2014;82:3740–3752. doi: 10.1128/IAI.01729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Graham JG, Winchell CG, Sharma UM, Voth DE. Identification of ElpA, a Coxiella burnetii pathotype-specific Dot/Icm type IV secretion system substrate. Infect. Immun. 2015;83:1190–1198. doi: 10.1128/IAI.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luhrmann A, Nogueira CV, Carey KL, Roy CR. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc. Natl Acad. Sci. USA. 2010;107:18997–19001. doi: 10.1073/pnas.1004380107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First demonstration of a Coxiella T4BSS effector protein (AnkG) that inhibits apoptosis, a process involving binding of the mitochondrial protein p32.
- 98.Cunha LD, Ribeiro JM, Fernandes TD, et al. Inhibition of inflammasome activation by Coxiella burnetii type IV secretion system effector IcaA. Nat. Commun. 2015;6:10205. doi: 10.1038/ncomms10205. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes a Coxiella T4BSS effector protein (IcaA) that disrupts caspase-11-mediated noncanonical activation of the inflammasome.
- 99.Falkow S. Molecular Koch's postulates applied to bacterial pathogenicity – a personal recollection 15 years later. Nat. Rev. Microbiol. 2004;2:67–72. doi: 10.1038/nrmicro799. [DOI] [PubMed] [Google Scholar]
- 100.Beare PA, Sandoz KM, Omsland A, Rockey DD, Heinzen RA. Advances in genetic manipulation of obligate intracellular bacterial pathogens. Front. Microbiol. 2011;2:97. doi: 10.3389/fmicb.2011.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beare PA, Howe D, Cockrell DC, Omsland A, Hansen B, Heinzen RA. Characterization of a Coxiella burnetii ftsZ mutant generated by Himar1 transposon mutagenesis. J. Bacteriol. 2009;191:1369–1381. doi: 10.1128/JB.01580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Omsland A, Cockrell DC, Howe D, et al. Host cell-free growth of the Q fever bacterium Coxiella burnetii . Proc. Natl Acad. Sci. USA. 2009;106:4430–4434. doi: 10.1073/pnas.0812074106. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes host cell-free (axenic) growth of Coxiella in a synthetic medium under microaerobic conditions.
- 103.Omsland A, Beare PA, Hill J, et al. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl. Environ. Microbiol. 2011;77:3720–3725. doi: 10.1128/AEM.02826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beare PA, Larson CL, Gilk SD, Heinzen RA. Two systems for targeted gene deletion in Coxiella burnetii . Appl. Environ. Microbiol. 2012;78:4580–4589. doi: 10.1128/AEM.00881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beare PA, Sandoz KM, Larson CL, Howe D, Kronmiller B, Heinzen RA. Essential role for the response regulator PmrA in Coxiella burnetii type 4B secretion and colonization of mammalian host cells. J. Bacteriol. 2014;196:1925–1940. doi: 10.1128/JB.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sandoz KM, Beare PA, Cockrell DC, Heinzen RA. A defined axenic medium allows complementation of arginine auxotrophy for genetic transformation of Coxiella burnetii . Appl. Environ. Microbiol. 2016;82:3042–3051. doi: 10.1128/AEM.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Larson CL, Beare PA, Voth DE, et al. Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infect. Immun. 2015;83:661–670. doi: 10.1128/IAI.02763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Newton HJ, Mcdonough JA, Roy CR. Effector protein translocation by the Coxiella burnetii Dot/Icm type IV secretion system requires endocytic maturation of the pathogen-occupied vacuole. PLoS ONE. 2013;8:e54566. doi: 10.1371/journal.pone.0054566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kubori T, Hyakutake A, Nagai H. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol. Microbiol. 2008;67:1307–1319. doi: 10.1111/j.1365-2958.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- 110.Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl Acad. Sci. USA. 2005;102:826–831. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burstein D, Zusman T, Degtyar E, Viner R, Segal G, Pupko T. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 2009;5:e1000508. doi: 10.1371/journal.ppat.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang L, Boyd D, Amyot WM, et al. The E Block motif is associated with Legionella pneumophila translocated substrates. Cell Microbiol. 2011;13:227–245. doi: 10.1111/j.1462-5822.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Felipe KS, Pampou S, Jovanovic OS, et al. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J. Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zusman T, Aloni G, Halperin E, et al. The response regulator PmrA is a major regulator of the icm/dot type IV secretion system in Legionella pneumophila and Coxiella burnetii . Mol. Microbiol. 2007;63:1508–1523. doi: 10.1111/j.1365-2958.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- 115.Segal G. The Legionella pneumophila two-component regulatory systems that participate in the regulation of Icm/Dot effectors. Curr. Top. Microbiol. Immunol. 2013;376:35–52. doi: 10.1007/82_2013_346. [DOI] [PubMed] [Google Scholar]
- 116.Sutherland MC, Binder KA, Cualing PY, Vogel JP. Reassessing the role of DotF in the Legionella pneumophila type IV secretion system. PLoS ONE. 2013;8:e65529. doi: 10.1371/journal.pone.0065529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl Acad. Sci. USA. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Waag DM, Byrne WR, Estep J, Gibbs P, Pitt ML, Banfield CM. Evaluation of cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) monkeys as experimental models of acute Q fever after aerosol exposure to Phase-I Coxiella burnetii . Lab. Anim. Sci. 1999;49:634–638. [PubMed] [Google Scholar]
- 119.Jeong KC, Sutherland MC, Vogel JP. Novel export control of a Legionella Dot/Icm substrate is mediated by dual, independent signal sequences. Mol. Microbiol. 2015;96:175–188. doi: 10.1111/mmi.12928. [DOI] [PubMed] [Google Scholar]
- 120.Schnell U, Dijk F, Sjollema KA, Giepmans BN. Immunolabeling artifacts and the need for live-cell imaging. Nat. Methods. 2012;9:152–158. doi: 10.1038/nmeth.1855. [DOI] [PubMed] [Google Scholar]
- 121.Eckart RA, Bisle S, Schulze-Luehrmann J, et al. Antiapoptotic activity of Coxiella burnetii effector protein AnkG is controlled by p32-dependent trafficking. Infect. Immun. 2014;82:2763–2771. doi: 10.1128/IAI.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodriguez-Escudero M, Cid VJ, Molina M, Schulze-Luehrmann J, Luhrmann A, Rodriguez-Escudero I. Studying Coxiella burnetii type IV substrates in the yeast Saccharomyces cerevisiae: focus on subcellular localization and protein aggregation. PLoS ONE. 2016;11:e0148032. doi: 10.1371/journal.pone.0148032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weber MM, Faris R, Mclachlan J, et al. Modulation of the host transcriptome by Coxiella burnetii nuclear effector Cbu1314. Microbes Infect. 2016;18(5):336–345. doi: 10.1016/j.micinf.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 124.Siggers KA, Lesser CF. The yeast Saccharomyces cerevisiae: a versatile model system for the identification and characterization of bacterial virulence proteins. Cell Host Microbe. 2008;4:8–15. doi: 10.1016/j.chom.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ashida H, Mimuro H, Ogawa M, et al. Cell death and infection: a double-edged sword for host and pathogen survival. J. Cell Biol. 2011;195:931–942. doi: 10.1083/jcb.201108081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klingenbeck L, Eckart RA, Berens C, Luhrmann A. The Coxiella burnetii type IV secretion system substrate CaeB inhibits intrinsic apoptosis at the mitochondrial level. Cell Microbiol. 2013;15:675–687. doi: 10.1111/cmi.12066. [DOI] [PubMed] [Google Scholar]
- 127.Bisle S, Klingenbeck L, Borges V, et al. The Inhibition of the apoptosis pathway by the Coxiella burnetii effector protein CaeA requires the EK repetition motif, but is independent of survivin. Virulence. 2016;7(4):400–412. doi: 10.1080/21505594.2016.1139280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 129.Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martinez E, Allombert J, Cantet F, et al. The Coxiella burnetii effector CvpB modulates phosphoinositide metabolism for optimal vacuole development. Proc. Natl Acad. Sci. USA. 2016;113:E3260–E3269. doi: 10.1073/pnas.1522811113. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that the effector CvpB promotes interactions with autophagosomes that favor CCV homotypic fusion by manipulating CCV phosphoinositides.
- 132.O'Connor TJ, Adepoju Y, Boyd D, Isberg RR. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc. Natl Acad. Sci. USA. 2011;108:14733–14740. doi: 10.1073/pnas.1111678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brodin P, Christophe T. High-content screening in infectious diseases. Curr. Opin. Chem. Biol. 2011;15:534–539. doi: 10.1016/j.cbpa.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 134.Boutros M, Heigwer F, Laufer C. Microscopy-based high-content screening. Cell. 2015;163:1314–1325. doi: 10.1016/j.cell.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 135.Selvaraj SK, Prasadarao NV. Escherichia coli K1 inhibits proinflammatory cytokine induction in monocytes by preventing NF-kappaB activation. J. Leukoc. Biol. 2005;78:544–554. doi: 10.1189/jlb.0904516. [DOI] [PubMed] [Google Scholar]
- 136.Stead CM, Omsland A, Beare PA, Sandoz KM, Heinzen RA. Sec-mediated secretion by Coxiella burnetii . BMC Microbiol. 2013;13:222. doi: 10.1186/1471-2180-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Charpentier X, Gabay JE, Reyes M, Zhu JW, Weiss A, Shuman HA. Chemical genetics reveals bacterial and host cell functions critical for type IV effector translocation by Legionella pneumophila . PLoS Pathog. 2009;5:e1000501. doi: 10.1371/journal.ppat.1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roy CR, Berger KH, Isberg RR. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 139.Yu XJ, Mcgourty K, Liu M, Unsworth KE, Holden DW. pH sensing by intracellular Salmonella induces effector translocation. Science. 2010;328:1040–1043. doi: 10.1126/science.1189000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deng W, Li Y, Hardwidge PR, et al. Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infect. Immun. 2005;73:2135–2146. doi: 10.1128/IAI.73.4.2135-2146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cambronne ED, Roy CR. The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation. PLoS Pathog. 2007;3:e188. doi: 10.1371/journal.ppat.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sutherland MC, Nguyen TL, Tseng V, Vogel JP. The Legionella IcmSW complex directly interacts with DotL to mediate translocation of adaptor-dependent substrates. PLoS Pathog. 2012;8:e1002910. doi: 10.1371/journal.ppat.1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baca OG, Scott TO, Akporiaye ET, Deblassie R, Crissman HA. Cell cycle distribution patterns and generation times of L929 fibroblast cells persistently infected with Coxiella burnetii . Infect. Immun. 1985;47:366–369. doi: 10.1128/iai.47.2.366-369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barquist L, Boinett CJ, Cain AK. Approaches to querying bacterial genomes with transposon-insertion sequencing. RNA Biol. 2013;10:1161–1169. doi: 10.4161/rna.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wright AV, Nunez JK, Doudna JA. Biology and applications of CRISPR systems: harnessing nature's toolbox for genome engineering. Cell. 2016;164:29–44. doi: 10.1016/j.cell.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 146.Michard C, Sperandio D, Bailo N, et al. The Legionella kinase LegK2 targets the ARP2/3 complex to inhibit actin nucleation on phagosomes and allow bacterial evasion of the late endocytic pathway. MBio. 2015;6:e00354–e00315. doi: 10.1128/mBio.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou Y, Zhu Y. Diversity of bacterial manipulation of the host ubiquitin pathways. Cell. Microbiol. 2015;17:26–34. doi: 10.1111/cmi.12384. [DOI] [PubMed] [Google Scholar]
- 148.Rolando M, Sanulli S, Rusniok C, et al. Legionella pneumophila effector RomA uniquely modifies host chromatin to repress gene expression and promote intracellular bacterial replication. Cell Host Microbe. 2013;13:395–405. doi: 10.1016/j.chom.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 149.Bierne H, Cossart P. When bacteria target the nucleus: the emerging family of nucleomodulins. Cell. Microbiol. 2012;14:622–633. doi: 10.1111/j.1462-5822.2012.01758.x. [DOI] [PubMed] [Google Scholar]
- 150.Ensminger AW. Legionella pneumophila armed to the hilt: justifying the largest arsenal of effectors in the bacterial world. Curr. Opin. Microbiol. 2015;29:74–80. doi: 10.1016/j.mib.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 151.Jeong KC, Sexton JA, Vogel JP. Spatiotemporal regulation of a Legionella pneumophila T4SS substrate by the metaeffector SidJ. PLoS Pathog. 2015;11:e1004695. doi: 10.1371/journal.ppat.1004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect. Immun. 2007;75:3245–3255. doi: 10.1128/IAI.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Islam A, Lockhart M, Stenos J, Graves S. The attenuated Nine Mile Phase II clone 4/RSA439 strain of Coxiella burnetii is highly virulent for severe combined immunodeficient (SCID) mice. Am. J. Trop. Med. Hyg. 2013;89:800–803. doi: 10.4269/ajtmh.12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ochoa-Reparaz J, Sentissi J, Trunkle T, Riccardi C, Pascual DW. Attenuated Coxiella burnetii Phase II causes a febrile response in gamma interferon knockout and Toll-like receptor 2 knockout mice and protects against reinfection. Infect. Immun. 2007;75:5845–5858. doi: 10.1128/IAI.00901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Norville IH, Hartley MG, Martinez E, Cantet F, Bonazzi M, Atkins TP. Galleria mellonella as an alternative model of Coxiella burnetii infection. Microbiology. 2014;160:1175–1181. doi: 10.1099/mic.0.077230-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.