Abstract
Cytomegalovirus (CMV) is a β-herpesvirus that infects most people in the world and is almost always asymptomatic in the healthy host. However, CMV persists for life, requiring continuous immune surveillance to prevent disease and thus, CMV is a frequent complication in immune compromised patients. Many groups have been exploring the potential for adoptive T-cell therapies to control CMV reactivation as well as the progression of solid tumors harboring CMV. In addition, CMV itself is being explored as a vaccine vector for eliciting potent T-cell responses. This review will discuss key features of the basic biology of CMV-specific T cells as well as highlighting unanswered questions and ongoing work in the development of T-cell-based immunotherapies to target CMV.
Keywords: : adoptive immunotherapy, cytomegalovirus, immunotherapy, memory T cells, vaccination
Cytomegalovirus (CMV) is a member of the β-subfamily of herpesviruses, a family of viruses that are thought to have been co-evolving with their hosts for ˜180 million years [1]. CMV establishes an asymptomatic, latent/persistent infection in most people in the world and persists for the life of the host [2]. Importantly, reactivation from latency is thought to occur regularly throughout the body, requiring constant immune surveillance to keep the host disease free. Indeed, patients who are immunosuppressed are at risk for CMV reactivation, which can lead to increased morbidity and mortality (comprehensively reviewed in [3]).
Even in immune-competent individuals, constant CMV reactivations serve as potent stimulators for CMV-specific T cells. Intriguingly, recombinant CMV viruses are now being explored as potential vaccine vectors to generate large numbers of T cells against both infectious diseases and cancer. Importantly, recent work from the Picker lab has demonstrated that CMV-based vaccines were profoundly protective in a nonhuman primate model of HIV infection [4–7]. Thus, a deeper understanding of how CMV-specific immunity is stimulated and maintained will help to improve CMV immune restoration following immune suppression and help to develop new vaccines using attenuated CMV viral vectors.
Our current understanding of the immunology and virology of CMV infections is derived from both human studies and animal models. Unfortunately, in vivo studies using human CMV (HCMV) is difficult because HCMV is often asymptomatic during the acute phase of infection and produces undetectable levels of transcripts during the latent phase. Additionally, CMV species selectivity is so strict that HCMV will not replicate in nonhuman cells [8]. Thus, studies using HCMV are usually performed in vitro and animal studies require species-specific CMV orthologs.
Remarkably, the overall host–pathogen balance has been highly conserved despite divergence between these species-specific CMV viruses. In particular, the natural mouse pathogen murine CMV (MCMV) has been a well-described tool for investigating CMV-specific pathogenesis and immunity [9,10]. It is important to note that HCMV and MCMV differ in multiple ways, including the expression of many unique genes and aspects of viral pathogenesis that are dependent on the host species (e.g., in utero transmission [11]). However, the overall viral life cycles are overlapping and there are several examples of unique viral genes in each virus that have overlapping functions. Importantly for studies of immune control, both HCMV and MCMV use similar mechanisms to evade or limit immune control, both establish latency in the same cell types and both viruses require constant immune surveillance to prevent viral replication and disease [9,12–16]. Although additional studies are needed to further understand and appreciate the similarities and differences between MCMV and HCMV, the MCMV model has provided directly translatable insight into HCMV, particularly in the arena of immune control.
Investigations over the last 20 years with HCMV and animal models of CMV infection have revealed that immune control of CMV is a layered process. Type-I IFN, NK cells, γδ-T cells, B cells, CD4+ T cells and CD8+ T cells all play a recognized (if not yet fully defined) role in suppressing viral activity [3]. In terms of CMV-specific T cells, it is clear that CMV-specific CD8+ T cells can, in isolation, restrict CMV replication as first shown in mice by Reddehase et al. [17] and subsequently in humans by Riddell et al. [18]. Moreover, long-term control of CMV in humans requires both CD4+ and CD8+ T cells [19,20]. Precisely how CMV-specific T cells remain functional and control this infection for life remains a mystery. Importantly, the biology of CMV-specific CD8+ T cells is parallel between mouse and man. In both species, CMV-specific CD8+ T cells persist in large numbers and slowly accumulate over time, a process that has been called ‘memory inflation’ [21,22]. Moreover, the phenotype, genetic signature and half-life of MCMV- and HCMV-specific T cells overlap, indicating the relevance of the MCMV model to study HCMV-specific CD8+ T cells [23–27]. Therefore, the MCMV model is an outstanding tool to explore HCMV-specific T-cell populations.
CMV-specific T cells are at the core of multiple therapeutic approaches designed to control CMV, other pathogens and even cancer. The purpose of this review is to discuss the current state of knowledge about CMV-specific T cells, as well as their use in the settings of adoptive immunotherapy and vaccination.
Part I: CMV reactivation & control
The conditioning regimens necessary for successful hematopoietic stem cell transplantation (HSCT) cause severe immunosuppression in the recipient and, typically, a loss of CMV-specific T cells. Rapid recovery of antiviral CD4+ and CD8+ T cells is critical for viral control and reducing morbidity and mortality [15,19,28]. Studies have shown that it is beneficial for CMV-seropositive patients to receive stem cells from a CMV-seropositive donor, likely because of the presence of donor CMV-specific immune cells that are transplanted with the graft [29,30]. Additional risk factors for CMV disease include: the use of an MHC mis-matched graft, T-cell depletion from the graft and poor T-cell reconstitution after transplant, treatment with high-dose corticosteroids or other immune suppressive drugs (comprehensively reviewed in [31]). Unfortunately, current clinical therapies for CMV replication leave much to be desired [31] and thus, restoring CMV-specific immunity remains the primary goal. A better understanding of CMV-specific immunity, particularly CMV-specific T-cell populations, could enable the development of therapies that promote the recovery of CMV-specific T-cell populations in immune suppressed patients, or improve the development of adoptive T-cell therapies designed to control CMV in these people.
CMV latency & reactivation
CMV is primarily spread via mucosal contact with infected secretions and acutely infects various cell types [2]. After this acute infection and systemic spread, CMV establishes latency throughout the body. Studies using MCMV have shown that the viral genome copy number in various organs is vast compared with the number of detectable transcripts [32], suggesting that the majority of MCMV genomes are transcriptionally silent. Likewise, cells harboring viral DNA can be recovered from humans without evidence of productive viral replication [33].
In order to reactivate from the latent state and produce infectious particles, like all herpesviruses, CMV gene transcription progresses through an ordered cascade beginning with expression of the intermediate-early (IE) genes, which serve as transactivators for further viral gene expression [34]. Following the expression of the IE genes, reactivation progresses to the transcription of the early (E) and late (L) genes that are important for host manipulation, DNA replication and viral packaging (reviewed in [2]). Studies of CMV latency and reactivation in the MCMV model have revealed that IE gene expression occurs continuously throughout latency in a focal and stochastic fashion [35–37]. Thus, it appears there is always a basal level of CMV reactivation. Expression of IE genes is controlled by the major immediate early promoter, which contains consensus binding sites for pro-inflammatory transcription factors (e.g., NF-κB and AP-1) [38–40]. Not surprisingly, inflammatory signals can promote reactivation of HCMV and MCMV [38,41–43]. In this way, CMV is sensitive to its environment and viral activity is directly modulated by the local inflammatory milieu. For this reason, inflammation from a transplant routinely promotes viral reactivation.
T-cell control of CMV latency & reactivation
A substantial amount of data indicates that T cells play a critical role in the control of latent and reactivating CMV. Elegant studies from the Reddehase lab have shown that MCMV-specific CD8+ T cells actively suppress viral reactivation in the lungs of latently infected mice by limiting the cascade of viral gene expression [44]. An additional large body of work from this group has shown that adoptively transferred CD8+ T cells specific for various MCMV proteins could control viral replication (reviewed in [45]). Likewise, infusions of HCMV-specific CD8+ T cells can restore CMV-specific immunity in people, in both prophylactic and therapeutic settings [20,46].
The Reddehase lab has further shown that adoptive transfer of CD4+ T cells was ineffective at limiting viral replication in immune compromised mice [17,47]. Interestingly however, mice lacking CD8+ T cells still controlled viral replication in most organs [48]. Work in humans has suggested that antiviral CD4+ T cells may be important for sustaining HCMV-specific CD8+ T-cell populations in some settings [20] or for directly controlling viral replication through the production of IFN-γ and/or expression of cytolytic machinery [49–51]. Importantly, mice lacking CD4+ T cells have persistent viral replication selectively in the salivary gland [52] where CD4+ T-cell-derived IFN-γ is required to control viral replication [53,54]. The salivary gland is a major site of viral shedding for both MCMV and HCMV. Data from the Oxenius lab suggest that CD8+ T cells cannot recognize virus-infected epithelial cells in the salivary gland as a result of viral immune evasion of class I MHC [53]. Interestingly, these same immune evasion genes have a much more subtle impact in other organs of the body [55], enabling better control of CMV by CD8+ T cells. These data show that CD4+ and CD8+ T cells collaborate to control CMV systemically.
The ongoing immune surveillance of CMV sustains the largest antiviral T-cell populations in the circulation of healthy adults [56]. However, viral transcripts are nearly undetectable. The relationship between the undetectable virus and the overwhelming T-cell response to viral persistence remains one of the major mysteries about CMV immune surveillance. The data suggest that both peak viral titers achieved during acute infection, as well as reactivation and/or reinfection contribute to the overall size of the CMV-specific T-cell pool [57–59]. Moreover, viral spread from the initially infected cells is not required to sustain these large T-cell pools [60]. Together, these data imply that the number of latently infected cells directly influences the size of the CMV-specific T-cell compartment. It is not at all clear, however, that the number of T cells sustained by CMV persistence is necessary to prevent CMV disease. Nevertheless, a minimum threshold must be reached after HSCT to prevent disease (61). It might be argued that the systemic nature of the infection and the sheer number of potentially infected cells demand enormous numbers of T cells to guarantee that some are near to every reactivation event.
Part II: adoptive immunotherapy to control CMV
In humans, there is a clear relationship between generating CD8+ T-cell responses to CMV and limiting CMV infection after HSCT (reviewed in [31]). In a seminal study, Reusser et al. reported that patients that generated a CMV-specific CD8+ cytotoxic T-cell response within the first 3 months after HSCT did not develop CMV pneumonia [15]. Numerous additional studies have shown that adoptive therapy can restore CMV immunity, reduce the risk for CMV infection, reduce the need for antiviral therapy and treat infections that are resistant to antivirals (recently reviewed in [62]). Thus, transferring CMV-specific T cells to HSCT recipients has become an attractive therapeutic option to quickly restore CMV immunity and prevent CMV-related mortality.
In the development of this therapy, it is important to consider the fact that the repeated interactions between CMV-specific T cells and their antigen profoundly effect the T-cell compartment. Indeed, the process of ‘memory inflation’, in which a subset of viral antigens accumulate and persist in large numbers, is thought to be the result of repeated antigen encounter by CMV-specific T cells [21,22,58,63–65]. Importantly, CMV-specific T cells are markedly altered by these repeated antigen encounters. Most CMV-specific CD8+ T cells that undergo memory inflation downregulate an array of molecules associated with memory T cells, and express a phenotype consistent with terminally differentiated effectors, or senescent T cells (e.g., CD62Llow, CD127low, CD27low, CD28low, CD57high, KLRG-1high, NKG2Ahigh, previously reviewed in [66]). Importantly for adoptive therapies, these terminally differentiated CD8+ T cells have a short half-life of ˜45–60 days in mice [24] and humans [25], and have limited proliferative capacity [27,64]. Such cells might be expected to provide only transient protection in an adoptive recipient. Moreover, adoptive immunotherapy protocols have typically involved the expansion of CMV-specific T cells in vitro, followed by their infusion into immune-compromised patients without regards to differentiation status. Presumably, this antigen-driven T-cell expansion in culture further promotes their effector differentiation prior to adoptive transfer, thus potentially limiting their efficacy.
Memory T cells retain superior expansive capacity
Data generated by our group and the Oxenius lab has suggested that a rare population of memory T cells, with substantial proliferative and self-renewal capacity, was responsible for repeatedly producing short-lived effectors in response to viral reactivation in immune competent hosts [27,64] (Figure 1). Theoretically, such memory-like T cells could provide robust, sustained immunity after adoptive therapy. Indeed, multiple studies in humans and mice have found that small subsets of ‘inflationary’ CMV-specific T cells retain a memory-like (CD27hi, CD127hi, KLRG-1low) phenotype. Cells with the same specificities, and even individual T-cell clones, can be distributed across these terminally differentiated and memory-like phenotypes [23,27,64,67,68]. Our recent work showed that the memory-like subset of ‘inflationary’ T cells could support sustained effector populations in response to acute infection or reactivating virus, even after adoptive transfer [27]. Specifically, these memory-like cells could: i) repeatedly produce new effector-like progeny after stimulation, ii) repeatedly produce new memory-like T cells that retained their proliferative capacity even after multiple stimulations (i.e., not undergo terminal differentiation after stimulation) and iii) sustain themselves in the complete absence of antigen and then respond to an infectious challenge or viral reactivation at a later time. All three of these properties would seem to make CMV-specific memory-like T cells an ideal subset for adoptive immunotherapy.
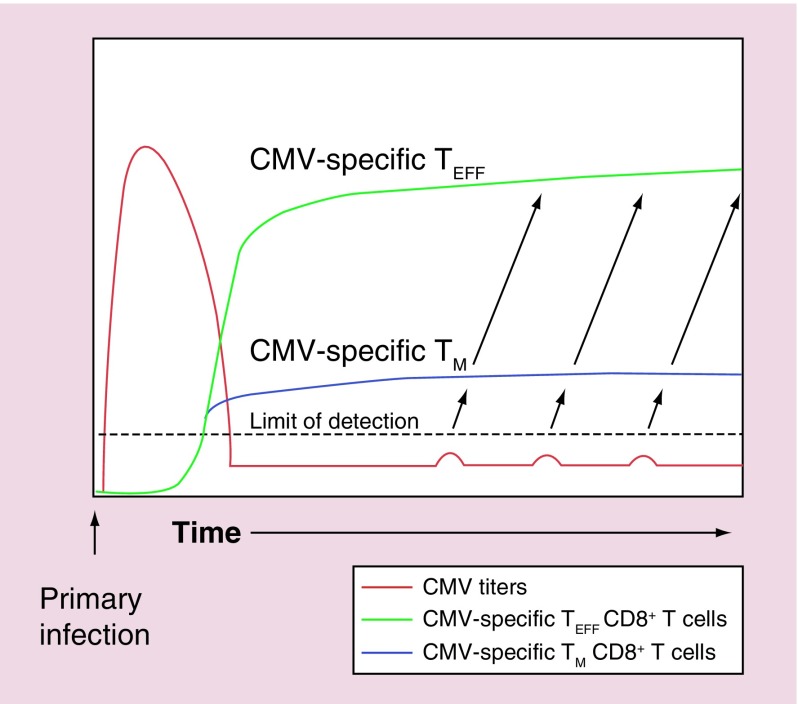
Figure 1. . Model for the maintenance of overwhelming numbers of cytomegalovirus-specific T cells during persistent/latent infection in a healthy host.
After acute infection, CMV titers (red line) fall below the limit of detection. However, stochastic reactivation events stimulate proliferative memory-phenotype T cells (blue line) to divide and differentiate, producing effector T-cell progeny (green line) that are poorly proliferative and have a relatively short half-life.
CMV: Cytomegalovirus; TEFF: Effector T cell; TM: Memory T cell.
There is additional emerging evidence from humans and nonhuman primates that memory-phenotype T cells may be the ideal population for CMV-specific adoptive immunotherapy. First, the CMV-specific repertoire in bone marrow transplant recipients was dominated by transferred CMV-specific T cells that had been memory phenotype in the donor, and not those that were largely effector phenotype before the transplant [68]. These data suggested that the memory phenotype T cells were substantially more expansive than their effector counterparts. Second, CMV-specific T-cell lines derived from central memory cells displayed superior engraftment in a nonhuman primate model of adoptive immunotherapy, relative to T-cell lines derived from effector memory or effector T cells [69]. Together, these data indicate that memory-phenotype T cells maybe far more relevant for establishing long-term immunity after bone marrow transplantation (Figure 2). By extension, we might predict that a preponderance of effector or effector-memory phenotype CMV-specific T cells might identify patients at risk of CMV reactivation and disease. Careful analyses of the CMV-specific T-cell populations in the donor and recipient may help to stratify these individuals into those requiring more or less aggressive therapies. Moreover, approaches to isolate and expand CMV-specific T cells without promoting their terminal differentiation, may be extremely valuable for future adoptive immunotherapies.
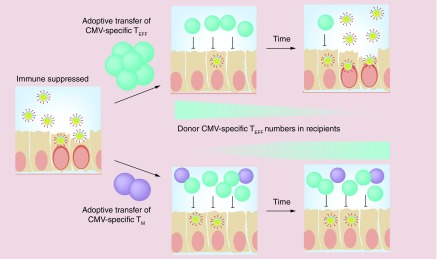
Figure 2. . Adoptive T-cell therapies utilizing memory-phenotype T cells may more robustly sustain cytomegalovirus-specific immunity.
Our data have shown that memory-phenotype murine cytomegalovirus-specific T cells can persist in the absence of antigen, but expand robustly in response to viral reactivation, producing both effector and memory progeny. By contrast, TEFF have a limited half-life and are poorly proliferative. In an adoptive immunotherapy setting, transferring TEFF would be expected to provide transient protection that is lost over time as donor T cells decline (upper panels). By contrast, transferring memory-phenotype T cells would enable long-lasting protection that sustains T-cell numbers (lower panels).
CMV: Cytomegalovirus; TEFF: Effector T cell; TM: Memory T cell.
Part III: CMV & cancer: a new avenue for adoptive immunotherapy
Work over the last several years has shown that HCMV proteins and nucleic acids can be found in samples of human glioblastoma (GBM), colon, breast and prostate cancers and rhabdomyosarcomas [70–74]. Significantly, MCMV infection was shown to induce rhabdomyosarcoma formation in mice heterozygous for the tumor suppressor p53 [74]. Although significant efforts have gone into elucidating this phenomenon, the correlation between CMV and cancer remains controversial, and results have varied by study (reviewed recently Dey et al. [75]). As yet, it is unclear whether the virus causes cellular transformation leading to tumors, exacerbates or accelerates tumor formation or is merely a passenger making use of these rapidly dividing cells. Regardless of cause and effect, the fact that CMV can be found in these tumors has led to a recent push for targeting HCMV to treat GBM [76,77]. Clinical trials have demonstrated increased overall survival in GBM patients treated with Valganciclovir, a common antiviral used to treat CMV infection, in addition to standard therapy [78–80]. Along with this, there are several ongoing clinical trials studying the efficacy of adoptive T-cell therapy using CMV-specific T cells, or dendritic cells pulsed with CMV pp65 RNA to vaccinate GBM patients [76,77,81,82]. Promisingly, in these studies, CMV-specific T cells were able to infiltrate GBM tumors and DCs pulsed with a CMV-derived peptide from the viral pp65 were able to stimulate GBM killing via pp65-specific T cells. Most importantly, vaccination resulted in improved survival of the patients.
It is surprising that boosting CMV-specific T cells, or infusing in vitro expanded T cells could be effective in these individuals given that they were already CMV-positive and thus, likely had large populations of CMV-specific T cells before therapy. Indeed, Crough et al. demonstrated that GBM patients had normal frequencies of CMV-specific T cells when compared with healthy donors [83]. Surprisingly however, unlike healthy donors, large proportions of CMV-specific T cells in GBM patients were dysfunctional. Importantly, upon stimulation in vitro in the presence of cytokines, T-cell function could be restored. Therefore, vaccination and ex vivo T-cell expansion may work by improving the quality of CMV-specific T cells, enabling these cells to kill infected tumors. How and why this might work are fascinating questions to be addressed, and it will be exciting to learn how this therapy progresses in the coming years.
Part IV: CMV as a vaccine platform to promote continuous T-cell immunity
CMV as a vaccine vector
Even though CMV can cause significant morbidity in immune-compromised individuals, and can be found in a variety of human cancers, it has recently drawn interest as a potential vaccine vector because of its ability to induce memory inflation. ‘Inflationary’ CD8+ T cells driven by CMV do not show signs of exhaustion in immune-competent people [26], as commonly seen in several other chronic infections [84]. CMV-driven T cells are also able to migrate into virtually all tissues of the body at steady state [63,85]. Importantly, recombinant CMVs can be used to induce memory inflation of T cells specific for the recombinant antigens in both mice and nonhuman primates [86] and we are beginning to understand how the position of an antigen within the viral genome impacts the T-cell response [87]. Additionally, unlike many viruses, CMV is able to re-infect previously infected individuals [88], allowing for vaccination and boosting with CMV vectors even in CMV-seropositive people. Due to these traits, CMV-based vaccines are currently being developed for clinical use.
To date, CMV has been used as a vaccine vector in a few settings. MCMV was first tested for its ability to induce immunologic contraception using a recombinant virus expressing zona pellucida 3, an ovary antigen, in an attempt to control mouse populations in Australia [89]. Interestingly, this vaccine did not work by inducing zona pellucida 3-specific T cells, but rather antibodies that could sterilize the infected mice [90]. In contrast, infection with recombinant MCMVs expressing the MHC class I restricted viral epitopes from influenza nucleoprotein or LCMV-glycoprotein induced protection against Vaccinia virus expressing nucleoprotein or glycoprotein respectively that correlated with T-cell numbers in the vaccinated mice [86]. More recently, extensive studies have been performed with three recombinant Rhesus Macaque CMVs (RhCMVs) expressing the simian immunodeficiency virus (SIV) epitopes Gag, Retanef or Env [4–7]. In this work, prophylactic vaccination and boosting with these vectors induced protection and clearance of SIV in approximately half of rhesus macaques subsequently infected with SIVmac239. It is still unclear why half of the monkeys were fully protected and half were apparently unprotected. Interestingly, the RhCMV vaccine vectors stimulated SIV-specific CD8+ T cells that were promiscuous and recognized uncommon SIV viral epitopes restricted by MHC II [6]. This effect seems to be related to the altered tropism of the viral vector compared with wild-type RhCMV. Whether these promiscuous T cell populations are essential for protection against SIV awaits further work. As these studies move toward clinical trials, it will be exciting to learn whether similarly constructed HCMV vectors will induce similarly targeted CD8+ T cells.
These successes with CMV as a viral vaccine vector have led several groups to explore CMV-based vaccines for cancer. Although this work is at a much earlier stage, prophylactic and therapeutic vaccination with recombinant MCMV vectors have shown some efficacy in models of prostate cancer [91] and melanoma [92,93]. However, there are many remaining questions. One melanoma-targeting vaccine-induced antibodies with therapeutic efficacy [92] while another induced tumor-specific CD8+ T cells [93]. Whether one is better than the other is unknown. Interestingly, the vaccine promoting melanoma-specific CD8+ T cells required an altered version of the gp100 tumor epitope to be expressed by the vaccine. Native gp100 antigen failed to induce a T-cell response [93]. Addressing whether this is a general problem with MCMV-based vectors inducing self-reactive T cells or whether this is limited to certain antigens will also require further study. Nevertheless, these recent successes using MCMV as an anticancer vaccine vector are encouraging.
Interestingly, there are data that CMV-driven NK cells and γδ-T cells may have anti-tumor effects [94,95]. Indeed, CMV reactivation has been associated with reduced risk for leukemia relapse in HSCT patients [96,97]. Thus, CMV vaccination or using CMV-stimulated NK cells and γδ-T cells in adoptive therapies may have general anticancer effects that could be exploited.
The risks associated with CMV-based vectors & the use of crippled viruses
Most people in the world are already infected with CMV. Indeed, many people are already infected with multiple strains of CMV. Therefore, introducing a CMV-based vaccine into people would not be expected to pose additional risks that are not already inherent in the population. Nevertheless, as discussed in detail above, CMV is dangerous in immune-compromised patients, making therapeutic vaccination for late stage cancer patients possibly dangerous, especially if they are CMV-negative at the time of treatment. In addition, while a vaccine promoting immunity to a foreign pathogen like HIV would seem to pose minimal risk, the same cannot be said of immunity directed against a cancer (self) antigen. Increased safety could be achieved by using crippled viruses as the vaccine vector. We used a spread defective variant of MCMV that lacked the glycoprotein L (ΔgL-MCMV) [60]. Glycoprotein L (gL) forms a heterodimer with the glycoprotein H (gH) within the viral envelope, and this heterodimer is essential for viral entry into cells. We produced the ΔgL-MCMV on complementing cell lines that provided the gL protein in trans, thus producing virions that could go through one round of infection in vivo, but produce only noninfectious viral particles thereafter. Surprisingly, a single inoculation of ΔgL-MCMV led to memory inflation of CD8+ T cells and ‘inflationary’ populations that persisted for over a year. Likewise, a spread-defective variant of MCMV lacking the essential virion protein M94 was shown to persist in the lungs of immune competent mice for at least 1 year after infection [98]. Importantly, the spread-defective ΔgL-MCMV was safe even for completely immune-deficient SCID mice [60], indicating that similarly spread-defective strains of HCMV may be safe, persistent vaccines.
Conclusion & future perspective
CMV-specific T cells are at the core of multiple therapeutic approaches designed to control CMV, other pathogens and even cancer. The use of adoptive immunotherapy to target CMV and other opportunistic viruses is clearly a promising approach and a substantial body of research now stands as proof of principle. The work performed to date demonstrates that cultured antiviral T cells can be effective at limiting CMV reactivation and replication with limited side effects in humans. Ongoing and future work will be needed to optimize the recovery, expansion and infusion of antiviral T cells to generate the most robust and sustained responses. Emerging clinical data and research in animal models, suggest that targeting the most proliferative memory-like T cells may improve the long-term benefits of these adoptive therapies. Thus, the selection and use of defined cell subsets may become more common in the near future. Recent work also suggests that targeting CMV by adoptive therapies or vaccination may be an effective way to target CMV-infected tumor cells. Clinical trials are ongoing with promising initial results and we would expect substantial progress to be made in the coming few years. The use of CMV as a vaccine vector is in its infancy and the first clinical trials are expected to be launched in the next several years. Much work remains to define the advantages and disadvantages of these vectors, as well as mechanistic underpinnings of success or failure in different disease settings. Although these therapies are at various stages of development and testing, they demonstrate the potential for CMV-specific T cells in a diverse group of settings.
Executive summary.
Part I: cytomegalovirus reactivation & control
Cytomegalovirus (CMV) reactivation is a constant event requiring continuous immune surveillance.
CMV-specific T cells play a primary and fundamental role in blocking viral reactivation.
Both CD4+ and CD8+ T cells contribute to the control of CMV.
Part II: adoptive immunotherapy to control CMV
Long-term control of CMV in transplant patients depends on the recovery of CMV-specific CD4+ and CD8+ T-cell populations.
CMV-specific T cells from healthy donors can be expanded or selected and infused into patients at risk of disease.
CMV-specific T cells are largely effector-phenotype, poorly proliferative and short-lived as a result of their ongoing viral immune surveillance. It is unclear whether these effector-like cells contribute to the expansion of CMV-specific T cells or the sustained control of CMV in adoptive recipients.
Small numbers of CMV-specific T cells with memory phenotype and function persist in infected hosts and these cells are superior at responding to CMV reactivation in an animal model.
Part III: CMV & cancer: a new avenue for adoptive immunotherapy
CMV DNA and protein have been found in several human cancers.
Although it is still unclear whether CMV contributed to the development of these tumors, CMV-specific immunotherapy may limit tumor growth by directing immunity to the infected tumor cells. The initial clinical trials have shown promise.
Part IV: CMV as a vaccine platform to promote continuous T-cell immunity
CMV as a vaccine vector
Due to the enormous number of T cells elicited and sustained by CMV infection, CMV-based vectors are being developed as vaccines for infectious disease and cancer.
The most developed CMV-based vaccines are being used to target HIV and these have shown profound efficacy in a nonhuman primate model of HIV infection.
Recent work has highlighted the potential for CMV-based vectors in cancer vaccination.
Crippled (single-cycle) versions of CMV may offer superior safety without sacrificing the sustained T-cell immunity elicited by wild-type vectors.
Conclusion
Adoptive T-cell therapy offers clear benefits for patients at risk of CMV disease and methods will improve as various labs optimize the selection, recovery and expansion of antiviral T cells.
Adoptive T-cell therapy or vaccination may also enable targeting of human cancers infected by CMV.
CMV-based vaccines hold tremendous promise, but much work remains to determine whether this promise will be fulfilled.
Footnotes
Financial & competing interests disclosure
CM Snyder has a financial stake in UbiVac CMV, a company developing spread-defective CMV-based vectors for cancer vaccination. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.McGeoch DJ, Cook S, Dolan A, Jamieson FE, Telford EA. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J. Mol. Biol. 1995;247(3):443–458. doi: 10.1006/jmbi.1995.0152. [DOI] [PubMed] [Google Scholar]
- 2.Mocarski ES, Shenk T, Pass RF. Fields Virology. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. [Google Scholar]
- 3.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 2009;22(1):76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen SG, Ford JC, Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen SG, Sacha JB, Hughes CM, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340(6135):1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen SG, Piatak MJ, Ventura AB, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502(7469):100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Work from the Picker lab exploring cytomegalovirus (CMV)-based vaccines in a nonhuman primate model of HIV infection has really driven the field in recent years. This study demonstrated that protected animals could clear SIV after infectious challenge.
- 8.Smith MG. Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc. Soc. Exp. Biol. Med. 1956;92(2):424–430. doi: 10.3181/00379727-92-22498. [DOI] [PubMed] [Google Scholar]
- 9.Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5(13):1263–1277. doi: 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Curr. Top. Microbiol. Immunol. 2008;325:315–331. doi: 10.1007/978-3-540-77349-8_18. [DOI] [PubMed] [Google Scholar]
- 11.Schleiss MR. Developing a vaccine against congenital cytomegalovirus (CMV) infection: what have we learned from animal models? Where should we go next. Future Virol. 2013;8(12):1161–1182. doi: 10.2217/fvl.13.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis MA, Nelson JA. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr. Opin. Microbiol. 2002;5(4):403–407. doi: 10.1016/s1369-5274(02)00334-x. [DOI] [PubMed] [Google Scholar]
- 13.Koffron AJ, Hummel M, Patterson BK, et al. Cellular localization of latent murine cytomegalovirus. J. Virol. 1998;72(1):95–103. doi: 10.1128/jvi.72.1.95-103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollock JL, Presti RM, Paetzold S, Virgin HWT. Latent murine cytomegalovirus infection in macrophages. Virology. 1997;227(1):168–179. doi: 10.1006/viro.1996.8303. [DOI] [PubMed] [Google Scholar]
- 15.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78(5):1373–1380. [PubMed] [Google Scholar]
- 16.Polic B, Hengel H, Krmpotic A, et al. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 1998;188(6):1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddehase MJ, Weiland F, Munch K, et al. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 1985;55(2):264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]; •• This study was the first description of adoptive immunotherapy for CMV in hematopoietic stem cell transplantation patients.
- 19.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83(7):1971–1979. [PubMed] [Google Scholar]
- 20.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 21.Karrer U, Sierro S, Wagner M, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 2003;170(4):2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu H, Sierro S, V Cuero A, Klenerman P. Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clin Exp Immunol. 2003;134(1):9–12. doi: 10.1046/j.1365-2249.2003.02266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 24.Snyder CM, Cho KS, Bonnett EL, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29(4):650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DL, Masters JE, De Lara CM, et al. Human cytomegalovirus-specific CD8(+) T-cell expansions contain long-lived cells that retain functional capacity in both young and elderly subjects. Immunology. 2011;132(1):27–38. doi: 10.1111/j.1365-2567.2010.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertoghs KM, Moerland PD, van Stijn A, et al. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J. Clin. Invest. 2010;120(11):4077–4090. doi: 10.1172/JCI42758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn M, Turula H, Tandon M, Deslouches B, Moghbeli T, Snyder CM. Memory T cells specific for murine cytomegalovirus re-emerge after multiple challenges and recapitulate immunity in various adoptive transfer scenarios. J. Immunol. 2015;194(4):1726–1736. doi: 10.4049/jimmunol.1402757. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrated that CMV-specific memory-phenotype T cells could survive without antigen, respond repeatedly to infectious challenge and produce both memory and effector progeny in response to viral reactivation.
- 28.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101(2):407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 29.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 30.Ringdén O, Schaffer M, Le Blanc K, et al. Which donor should be chosen for hematopoietic stem cell transplantation among unrelated HLA-A, -B, and -DRB1 genomically identical volunteers. Biol. Blood Marrow Transplant. 2004;10(2):128–134. doi: 10.1016/j.bbmt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol. Oncol. Clin. North Am. 2011;25(1):151–169. doi: 10.1016/j.hoc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurz SK, Rapp M, Steffens HP, et al. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J. Virol. 1999;73(1):482–494. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo K, Kaneshima H, Mocarski ES. Human cytomegalovirus latent infection of granulocyte–macrophage progenitors. Proc. Natl Acad. Sci. USA. 1994;91(25):11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermiston TW, Malone CL, Witte PR, Stinski MF. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J. Virol. 1987;61(10):3214–3221. doi: 10.1128/jvi.61.10.3214-3221.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurz SK, Reddehase MJ. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J. Virol. 1999;73(10):8612–8622. doi: 10.1128/jvi.73.10.8612-8622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study described the continuous and stochastic nature of CMV reactivation using the murine cytomegalovirus model.
- 36.Grzimek NK, Dreis D, Schmalz S, Reddehase MJ. Random, asynchronous, and asymmetric transcriptional activity of enhancer-flanking major immediate-early genes ie1/3 and ie2 during murine cytomegalovirus latency in the lungs. J. Virol. 2001;75(6):2692–2705. doi: 10.1128/JVI.75.6.2692-2705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry SC, Hamilton JD. Detection of murine cytomegalovirus immediate early 1 transcripts in the spleens of latently infected mice. J. Infect. Dis. 1993;167(4):950–954. doi: 10.1093/infdis/167.4.950. [DOI] [PubMed] [Google Scholar]
- 38.Hummel M, Zhang Z, Yan S, et al. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J. Virol. 2001;75(10):4814–4822. doi: 10.1128/JVI.75.10.4814-4822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunninghake GW, Monick MM, Liu B, Stinski MF. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J. Virol. 1989;63(7):3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu B, Stinski MF. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 1992;66(7):4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J. Virol. 2006;80(18):9151–9158. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91(1):119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 43.Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl Acad. Sci. USA. 1998;95(7):3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon CO, Holtappels R, Tervo HM, et al. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J. Virol. 2006;80(21):10436–10456. doi: 10.1128/JVI.01248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holtappels R, Bohm V, Podlech J, Reddehase MJ. CD8 T-cell-based immunotherapy of cytomegalovirus infection: “proof of concept” provided by the murine model. Med. Microbiol. Immunol. 2008;197(2):125–134. doi: 10.1007/s00430-008-0093-2. [DOI] [PubMed] [Google Scholar]
- 46.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 47.Reddehase MJ, Jonjic S, Weiland F, Mutter W, Koszinowski UH. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J. Virol. 1988;62(3):1061–1065. doi: 10.1128/jvi.62.3.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski UH. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 1990;64(11):5457–5464. doi: 10.1128/jvi.64.11.5457-5464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp. Med. 2006;203(13):2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Leeuwen EM, Remmerswaal EB, Vossen MT, et al. Emergence of a CD4+CD28- granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J. Immunol. 2004;173(3):1834–1841. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 51.Gamadia LE, Remmerswaal EB, Weel JF, et al. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101(7):2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 52.Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 1989;169(4):1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A. Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog. 2011;7(8):e1002214. doi: 10.1371/journal.ppat.1002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski UH. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 1992;66(4):1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohm V, Simon CO, Podlech J, et al. The immune evasion paradox: immunoevasins of murine cytomegalovirus enhance priming of CD8 T cells by preventing negative feedback regulation. J. Virol. 2008;82(23):11637–11650. doi: 10.1128/JVI.01510-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redeker A, Welten SP, Arens R. Viral inoculum dose impacts memory T-cell inflation. Eur. J. Immunol. 2014;44(4):1046–1057. doi: 10.1002/eji.201343946. [DOI] [PubMed] [Google Scholar]
- 58.Gamadia LE, van Leeuwen EM, Remmerswaal EB, et al. The size and phenotype of virus-specific T cell populations is determined by repetitive antigenic stimulation and environmental cytokines. J. Immunol. 2004;172(10):6107–6114. doi: 10.4049/jimmunol.172.10.6107. [DOI] [PubMed] [Google Scholar]
- 59.van Leeuwen EM, de Bree GJ, Remmerswaal EB, et al. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106(6):2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 60.Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog. 2011;7(10):e1002295. doi: 10.1371/journal.ppat.1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655–1662. doi: 10.1182/blood-2010-03-273508. [DOI] [PubMed] [Google Scholar]
- 62.Nicholson E, Peggs KS. Cytomegalovirus-specific T-cell therapies: current status and future prospects. Immunotherapy. 2015;7(2):135–146. doi: 10.2217/imt.14.99. [DOI] [PubMed] [Google Scholar]
- 63.Smith CJ, Turula H, Snyder CM. Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS Pathog. 2014;10(7):e1004233. doi: 10.1371/journal.ppat.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torti N, Walton SM, Brocker T, Rulicke T, Oxenius A. Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS Pathog. 2011;7(10):e1002313. doi: 10.1371/journal.ppat.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seckert CK, Schader SI, Ebert S, et al. Antigen-presenting cells of haematopoietic origin prime cytomegalovirus-specific CD8 T-cells but are not sufficient for driving memory inflation during viral latency. J. Gen. Virol. 2011;92(Pt 9):1994–2005. doi: 10.1099/vir.0.031815-0. [DOI] [PubMed] [Google Scholar]
- 66.O'Hara GA, Welten SP, Klenerman P, Arens R. Memory T cell inflation: understanding cause and effect. Trends Immunol. 2012;33(2):84–90. doi: 10.1016/j.it.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Iancu EM, Corthesy P, Baumgaertner P, et al. Clonotype selection and composition of human CD8 T cells specific for persistent herpes viruses varies with differentiation but is stable over time. J. Immunol. 2009;183(1):319–331. doi: 10.4049/jimmunol.0803647. [DOI] [PubMed] [Google Scholar]
- 68.Scheinberg P, Melenhorst JJ, Brenchley JM, et al. The transfer of adaptive immunity to cytomegalovirus (CMV) during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood. 2009;114(24):5071–5080. doi: 10.1182/blood-2009-04-214684. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study showed that, in humans, memory-phenotype CMV-specific T cells within a bone marrow graft, but not effector-phenotype T cells, efficiently reconstituted the recipients.
- 69.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cobbs CS, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62(12):3347–3350. [PubMed] [Google Scholar]; • This study by Cobbs and Harkins demonstrated that human cytomegalovirus could be detected in human cancers.
- 71.Harkins L, Volk AL, Samanta M, et al. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet. 2002;360(9345):1557–1563. doi: 10.1016/S0140-6736(02)11524-8. [DOI] [PubMed] [Google Scholar]; • This study by Cobbs and Harkins demonstrated that human cytomegalovirus could be detected in human cancers.
- 72.Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J. Urol. 2003;170(3):998–1002. doi: 10.1097/01.ju.0000080263.46164.97. [DOI] [PubMed] [Google Scholar]
- 73.Harkins LE, Matlaf LA, Soroceanu L, et al. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae. 2010;1(1):8. doi: 10.1186/2042-4280-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Price RL, Bingmer K, Harkins L, et al. Cytomegalovirus infection leads to pleomorphic rhabdomyosarcomas in Trp53+/- mice. Cancer Res. 2012;72(22):5669–5674. doi: 10.1158/0008-5472.CAN-12-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dey M, Ahmed AU, Lesniak MS. Cytomegalovirus and glioma: putting the cart before the horse. J. Neurol. Neurosurg. Psychiatry. 2015;86(2):191–199. doi: 10.1136/jnnp-2014-307727. [DOI] [PubMed] [Google Scholar]
- 76.Schuessler A, Smith C, Beagley L, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74(13):3466–3476. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 77.Schuessler A, Walker DG, Khanna R. Cytomegalovirus as a novel target for immunotherapy of glioblastoma multiforme. Front. Oncol. 2014;4:275. doi: 10.3389/fonc.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stragliotto G, Rahbar A, Solberg NW, et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: a randomized, double-blind, hypothesis-generating study. Int. J. Cancer. 2013;133(5):1204–1213. doi: 10.1002/ijc.28111. [DOI] [PubMed] [Google Scholar]
- 79.Söderberg-Nauclér C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N. Engl. J. Med. 2013;369(10):985–986. doi: 10.1056/NEJMc1302145. [DOI] [PubMed] [Google Scholar]
- 80.Söderberg-Nauclér C, Peredo I, Stragliotto G. Valganciclovir in patients with glioblastoma. N. Engl. J. Med. 2013;369(21):2066–2067. doi: 10.1056/NEJMc1312413. [DOI] [PubMed] [Google Scholar]
- 81.Nair SK, Sampson JH, Mitchell DA. Immunological targeting of cytomegalovirus for glioblastoma therapy. Oncoimmunology. 2014;3:e29289. doi: 10.4161/onci.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nair SK, De Leon G, Boczkowski D, et al. Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus pp65-specific cytotoxic T cells. Clin. Cancer Res. 2014;20(10):2684–2694. doi: 10.1158/1078-0432.CCR-13-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crough T, Beagley L, Smith C, Jones L, Walker DG, Khanna R. Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme. Immunol. Cell. Biol. 2012;90(9):872–880. doi: 10.1038/icb.2012.19. [DOI] [PubMed] [Google Scholar]
- 84.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 85.Smith CJ, Caldeira-Dantas S, Turula H, Snyder CM. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep. 2015;13(6):1137–1148. doi: 10.1016/j.celrep.2015.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karrer U, Wagner M, Sierro S, et al. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J. Virol. 2004;78(5):2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dekhtiarenko I, Jarvis MA, Ruzsics Z, Cicin-Sain L. The context of gene expression defines the immunodominance hierarchy of cytomegalovirus antigens. J. Immunol. 2013;190(7):3399–3409. doi: 10.4049/jimmunol.1203173. [DOI] [PubMed] [Google Scholar]
- 88.Hansen SG, Powers CJ, Richards R, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328(5974):102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lloyd ML, Shellam GR, Papadimitriou JM, Lawson MA. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol. Reprod. 2003;68(6):2024–2032. doi: 10.1095/biolreprod.102.012880. [DOI] [PubMed] [Google Scholar]
- 90.Lloyd ML, Papadimitriou JM, O'Leary S, Robertson SA, Shellam GR. Immunoglobulin to zona pellucida 3 mediates ovarian damage and infertility after contraceptive vaccination in mice. J. Autoimmun. 2010;35(1):77–85. doi: 10.1016/j.jaut.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 91.Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, et al. A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J. Immunother. 2012;35(5):390–399. doi: 10.1097/CJI.0b013e3182585d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu G, Smith T, Grey F, Hill AB. Cytomegalovirus-based cancer vaccines expressing TRP2 induce rejection of melanoma in mice. Biochem. Biophys. Res. Commun. 2013;437(2):287–291. doi: 10.1016/j.bbrc.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu Z, Huang H, Grenier JM, et al. Cytomegalovirus based vaccine expressing a modified tumor antigen induces potent tumor-specific CD8+ T cell response and protects mice from melanoma. Cancer Immunol. Res. 2015;3(5):536–546. doi: 10.1158/2326-6066.CIR-14-0044. [DOI] [PubMed] [Google Scholar]
- 94.Erlach KC, Bohm V, Knabe M, Deegen P, Reddehase MJ, Podlech J. Activation of hepatic natural killer cells and control of liver-adapted lymphoma in the murine model of cytomegalovirus infection. Med. Microbiol. Immunol. 2008;197(2):167–178. doi: 10.1007/s00430-008-0084-3. [DOI] [PubMed] [Google Scholar]
- 95.Couzi L, Pitard V, Moreau JF, Merville P, Déchanet-Merville J. Direct and indirect effects of cytomegalovirus-induced γδ T cells after kidney transplantation. Front. Immunol. 2015;6:3. doi: 10.3389/fimmu.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 98.Mohr CA, Arapovic J, Muhlbach H, et al. A spread deficient cytomegalovirus for assessment of first target cells in vaccination. J. Virol. 2010;84(15):7730–7742. doi: 10.1128/JVI.02696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]