Abstract
Tumor-associated macrophages (TAMs), representing most of the leukocyte population in solid tumors, demonstrate great phenotypic heterogeneity and diverse functional capabilities under the influence of the local tumor microenvironment. These anti-inflammatory and protumorigenic macrophages modulate the local microenvironment to facilitate tumor growth and metastasis. In this review, we examine the origin of TAMs and the complex regulatory networks within the tumor microenvironment that facilitate the polarization of TAMs toward a protumoral phenotype. More extensively, we evaluate the mechanisms by which TAMs mediate angiogenesis, metastasis, chemotherapeutic resistance and immune evasion. Lastly, we will highlight novel interventional strategies targeting TAMs in preclinical studies and in early clinical trials that have significant potential in improving efficacy of current chemotherapeutic and/or immunotherapeutic approaches.
Keywords: : immunotherapy, tumor-associated macrophages (TAM), tumor microenvironment
Nonresolving inflammation caused by cancer cells within the tumor microenvironment (TME) is a hallmark of cancer [1]. It is now widely accepted that in all stages of neoplastic development, tumor cells co-inhabit with various types of immune cells at densities ranging from subtle infiltration to gross inflammation. Though these immune cells were originally thought to be part of the antitumoral responses by the immune system to eradicate tumors, more evidences have pointed to the fact that the tumor-associated inflammatory response has a paradoxical effect of enhancing tumor initiation, progression and ultimately metastasis [2]. More importantly, research has demonstrated the important tumor-promoting effects that innate immune system, largely represented by the abundant tumor-associated macrophages (TAMs) within the stroma, has on neoplastic progression. At the primary tumor site, macrophages enhance tumor cell growth directly by promoting angiogenesis and tumor cell chemotherapy resistance and indirectly by inducing immune dysfunctions through interactions with other immune cells within the TME, resulting in immune evasion of cancer cells [3,4]. Abundant infiltration of macrophages is correlated with poor clinical prognosis in most human cancers [4]. Because of the critical roles macrophages play in the TME in support of tumor progression, they have emerged as a novel target for immunotherapy. This review focuses on the diverse functional roles of macrophages in the TME and the implications for cancer immunotherapy.
Macrophages
Classified as part of the mononuclear phagocytic system (MPS), macrophages are a group of terminally differentiated cells that play critical roles in tissue homeostasis, inflammation and protection against pathogenic infections. In the classical model of macrophage development, hematopoietic stem cells within the bone marrow give rise to myeloid progenitor cells, which ultimately become circulating monocytes through sequential steps of differentiation. Two subcategories of monocytes exist, including the inflammatory monocytes which mediate extravascular inflammatory responses and the patrolling monocytes which are tasked with clearance of damaged cells and debris intravascularly. Upon extravasation into the tissue through the endothelium, monocytes can differentiate into macrophages [5].
As macrophages are one of the most plastic cells in the MPS, they can acquire distinct functional phenotypes depending on environmental cues. Various signals activate a broad array of intracellular transcriptional networks in macrophages resulting in the polarization of functions ranging from proinflammatory responses to inflammation resolution and tissue repair. The M1/M2 nomenclature for macrophages defined macrophages into two polarized phenotypes – the classically activated M1 macrophages versus the alternatively activated M2 macrophages [6]. Though these states mirror the T helper 1 (Th1)-Th2 states of T cells, the M1/M2 phenotypes are not stably differentiated dichotomous subsets in the same way as Th1 and Th2 cells. Rather, macrophages in mouse and human can exhibit phenotypes in a spectrum of possible forms with two extremes defined by M1 and M2 [7]. Activated by IFN-γ, microbial products (e.g., lipopolysaccharides) or GM-CSF, the M1-polarized macrophages express high levels of proinflammatory cytokines: IL-1, IL-6, IL-12 and TNF, produce reactive nitrogen and reactive oxygen species, have high capacity to present antigen and consequently promote Th1 response. Therefore, M1 macrophages are considered strongly microbicidal and antitumoral. In contrast, M2 macrophages, activated by IL-4, IL-13, IL-10 and M-CSF/CSF-1, are generally characterized by low production of proinflammatory cytokines, high production of anti-inflammatory cytokines IL-10 and COX-2 derived prostaglandin E2 (PGE2), high expression of mannose (CD206) and scavenger (CD163) receptors and increased release of ornithine and polyamines through the arginase pathway [6,8]. Furthermore, distinct chemokine profiles are associated with M1 and M2 macrophage activation. M1 macrophages characteristically express high levels of CXCL9, CXCL10 and CCL5 that amplify cytotoxic T-cell activation and resistance to intracellular pathogens and tumors. On the other hand, M2 subset of chemokines, including CCL2, CCL17 and CCL22 promote recruitment of regulatory T cells (Treg), tissue repair and remodeling and tumor progression (Figure 1) [8].
Figure 1. . Tumor microenvironment stimuli control tumor-associated macrophage polarization.
M1-like TAM activation is stimulated by LPS, IFN-γ and/or GM-CSF. These molecules act through transcription factors Stat1, IRF1 and/or IRF5 to induce production of iNOS and a variety of proinflammatory and thereby antitumoral cytokines and chemokines. Tumor-derived IL-4 &IL-13, IL-10, M-CSF and/or lactic acid supports the activation of M2-like TAM. This activates an arginase1-dependent metabolism of arginine via the actions of transcription factors Stat3/6, Klf2/4 and/or IRF3/5. Consequently, M2-TAMs express scavenger and mannose receptors, CD163 and CD206, respectively, and secrete a spectrum of anti-inflammatory and protumoral cytokines, chemokines and signaling molecules.
iNOS: Inducible nitric oxide synthase; LPS: Lipopolysaccharide; RNS: Reactive nitrogen species; ROS: Reactive oxygen species; TAM: Tumor-associated macrophage.
Tumor-associated macrophages
Though often considered synonymous with M2-macrophages, TAMs are a group of protumoral macrophages that have distinct transcriptional and phenotypic characteristics from those of M1 and M2 macrophages [9]. Therefore, the M1-M2 dichotomy is excessively simplistic for TAM classification and does not consider a variety of factors influencing TAM functions, including location of the microenvironment, type of cancer and stage of the tumor. Because of the functional and phenotypic heterogeneity reflected in the tumor-infiltrating macrophages, it is a complex process to identify specific subgroups of TAMs with overlapping features based on one set of specific markers. Furthermore, whether diverse TAM populations originate from distinct precursors or are results of differential microenvironmental stimuli is still largely unknown. In addition to classifying TAMs based on previously described surface markers for M2 macrophages such as MHC class II molecules, CD163 and CD206 or cytokines/chemokines such as IL-10 and CCL-2, recent approaches to categorize TAMs based on their functional role in the TME has also gained momentum [4]. Specific markers expressed or cytokines/chemokines released by a subset of TAMs have been mapped to a protumoral function, such as the expression of VEGFR1 on angiogenic as well as metastasis-promoting TAMs. However, it remains a difficult task to identify specific markers that truly define distinct subpopulations of TAMs in vivo because of the complexity of TME and macrophage plasticity.
In addition to difficulties encountered in identifying unique subsets of TAMs in vivo, the origin of TAMs also remains controversial. Though the historic description of the MPS proposed that tissue-resident macrophages in adulthood is continuously replenished by bone marrow (BM) derived blood monocytes, recent evidences have demonstrated that the majority of macrophages that reside in healthy tissues arise from yolk sac progenitors prenatally and the long-term persistence of these tissue macrophages rely on self-renewal rather than replenishment by circulating BM monocytes [10]. This raises the question regarding the origin of TAMs and their methods of recruitment and differentiation to the tumor stroma. Evidence to date supported that TAMs are replaced by circulating Ly6ChiCCR2+ monocytes in grafted mammary carcinoma, in MMTV-neu mouse model of primary mammary carcinogenesis and in the Lewis lung carcinoma syngeneic transplant model [11–15]. Circulating inflammatory Ly6ChiCD11c−MHCII−CD11bhiCCR2+ monocytes are derived from BM hematopoietic stem cells, recruited to tumor sites and later differentiated into TAMs by the actions of Notch signaling pathway and its downstream transcription factor recombinant signal-binding protein for the IgκJ region (RBPJ). After differentiation, Ly6ChiCCR2+ monocytes undergo downregulation of Ly6C and CD11b while upregulate expressions of MHCII and Vcam1 to become Ly6C−CD11c+MHCII+CD11bloVcam1+ TAMs [14,15]. In addition, tumor-infiltrating macrophages are often short-lived and require constant replenishment from the circulation. Using mouse models that enable the tracking of BM-derived monocytes, Shand et al. showed that TAMs were derived and rapidly replenished from the BM in tumor-bearing mice [16]. Nonetheless, TAM expansion and polarization within the tumor stroma require factors that promote myeloid progenitor recruitment and expansion and factors that are driving the polarization into the immunosuppressive TAM. CSF1, VEGF-A and CCL2, among others have been shown to be potent chemoattractants for macrophages and macrophage progenitors, driving the accumulation of TAMs. Increased levels of CCL2, CSF1 and VEGF-A are correlated with macrophage accumulation at the tumor site in a wide spectrum of human cancers, including those of breast, prostate, ovarian and lung [2,17,18]. Once recruited to the primary tumor site, various signaling pathways, including JAK/Stat and PI3K/Akt orchestrate the polarization under the influence of various tumor-derived or stroma-derived factors [19]. IL-4 and IL-13, synthesized by CD4+ T cells and/or tumor cells act on infiltrating macrophages through intracellular Stat6 and PI3K signaling to promote an immunosuppressive TAM phenotype while IL-10 produced by regulatory T cells (Treg) also participates in the activation of the TAM phenotype via the actions of Stat3. Other cytokines secreted by the tumor cells, including CSF1 and TGF-β also strongly promote M2-polarization of TAMs [19,20]. In addition, a recent report also highlighted that lactic acid, a tumor cell-derived aerobic glycolysis byproduct, induces TAM functional polarization through the action of hypoxia inducible factor 1α (HIF-1α) resulting in subsequent promotion of tumorigenesis (Figure 1) [21]. However, TAM polarization is not a defined process but demonstrates great functional and phenotypic diversity depending on the cancer type and tumor stage. Currently, there is a lack of comprehensive understanding of the origins of macrophages within the tumor stroma in different types and stages of cancer. In addition, the mechanisms of recruitment, retention and differentiation of this population of cells remain unclear. Nonetheless, extensive research has highlighted the dynamic functional roles TAMs play within the TME.
The functions of TAMs
The presence of TAM is associated with poor clinical outcome in patients with glioma, cholangiocarcinoma, ovarian, breast cancer and Hodgkin's lymphoma [22]. Extensive literature also demonstrated that TAMs within the TME play pivotal roles in promoting tumorigenesis and metastasis via both nonimmune and immune mechanisms. The supportive roles of TAM in angiogenesis, metastasis and cancer stem cell (CSC) functions are discussed below.
Angiogenesis
Angiogenesis is a coordinated process orchestrated by cancer and cancer stromal cells as a prerequisite for metastatic disease [1]. It requires the degradation of the basement membrane along with endothelial cell proliferation and migration. TAMs are extensively involved in each step of the angiogenic process: degradation of the basement membrane through the production of matrix metalloproteinases (MMPs) and cathepsins [23]; and secretion of proangiogenic growth factors such as VEGF, PDGF, basic FGF (bFGF) and the chemokines CCL2 and CXCL8 that provide the vascular network necessary not only for maintaining cancer cell growth but also to promote dissemination [24]. In addition, the degradation of tissue matrix surrounding the tumor cells by MMPs releases heparin-bound growth factors, such as VEGF-A to further support angiogenesis. Last, VEGFA and CCL2 also act as strong chemotactic factors for the recruitment of monocytes and their expressions are positively correlated with increased TAM accumulation and high levels of tumor vascularization in human invasive ductal breast carcinoma (Figure 2) [25]. Therefore, TAMs represent an indirect mechanism of amplification of angiogenesis by cancer cells and work to promote tumor neovascularization in concert with tumor-derived angiogenic factors.
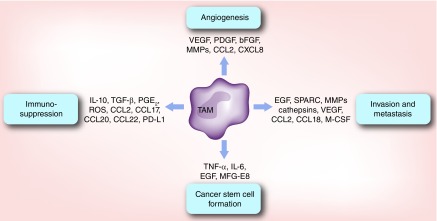
Figure 2. . Protumoral functions of tumor-associated macrophages.
TAMs promote tumorigenesis through several aspects. First, TAMs release numerous angiogenic factors, including VEGF, PDGF and bFGF that stimulate angiogenesis within the tumor. Second, TAMs secrete many signaling molecules, growth factors and MMPs that consequently activate tumor cells epithelial-to-mesenchymal transition, invasion and metastasis. Third, TAMs promote the formation and maintenance of cancer stem cells through the actions of various cytokines and molecules. Last, TAMs negatively regulate cytotoxic effector cells, such as CD8+, NK and NKT cells while simultaneously promote the expansion of immunosuppressive Treg and MDSCs via cytokines, metabolic enzymes and/or surface receptor interactions.
MDSC: Myeloid-derived suppressor cell; MMP: Matrix metalloproteinase; ROS: Reactive oxygen species; TAM: Tumor-associated macrophage.
Interestingly, though we currently do not fully understand how the process termed ‘angiogenic switch’ where tumor cells acquire vasculature to provide oxygenation and waste disposal to sustain growth beyond a certain size is regulated [1], a unique subset of Tie2+ macrophages have been shown to regulate this switch in the PyMT model [26]. CSF-1 upregulates Tie2 expression on TAMs [27]. Expression of angiopoietin 1, ligand for Tie2 on endothelial cells then allows for the alignment of Tie2+ macrophage along the abluminal surface of blood vessels, resulting in macrophage-synthesized WNT7b targeting of vascular endothelial cells, their production of VEGF and ultimately activation of the angiogenic switch [28]. Collectively, these evidences highlighted the critical role of TAMs in activating angiogenic switch and promoting neovasculature formation throughout tumorigenesis.
Metastasis
Metastatic spreading of cancer from primary tumor site requires tumor cells to undergo sequential steps of epithelial-to-mesenchymal transition (EMT), intravasation into and transit through circulation or lymphatics and extravasation at a distant site sui for the survival and colonization [1]. Extensive evidences have demonstrated correlation between TAMs recruitment and increase tumor cell invasiveness and distal spreading [29]. Therefore, it is not surprising to learn that TAMs not only contribute to early EMT of tumor cells but also help prepare premetastatic niche – a distant site primed to support metastatic growth. Using two solid tumor models, researchers showed that macrophage-derived TGF-β induced EMT-mediating pathway in tumor cells, resulting in increased expression of mesenchymal markers and an invasive phenotype [30]. Furthermore, direct interactions with TAMs is also required for malignant cell migration and intravasation in the absence of angiogenesis based on intravital imaging studies using multiphoton microscopy [31]. The paracrine signaling loop of tumor-derived CSF-1 and macrophage-synthesized EGF results in the coordinate movement of cancer cells and perivascular macrophages to stream along collagen fibers to cluster around blood vessels. Once arrived at the blood vessels, TAMs engage in several other mechanisms to facilitate cancer cells’ escape. TAM-derived Secreted Protein Acidic and Rich in Cysteine enhances cancer cell migration to other ECM proteins [32]. TAMs further modify the TME composition to favor tumor escape through the collective actions of proteolytic enzymes cathepsins B or S, MMPs (MMP-2, -7, -9 and -12) and serine proteases. These TAM-derived enzymes degrade the basement membrane and surrounding ECM to aid in the creation of a premetastatic or metastatic niche [33]. TAMs also abundantly produce CCL18 which triggers integrin clustering around human breast cancer cells and allows for their adherence to ECM through interactions with the phosphatidylinositol transfer protein, membrane-associated 3 receptor, resulting in enhanced intravasation, metastasis and reduced patient survival (Figure 2) [34]. Last, the identification of a distinct population of VEGFR1highCX3CR1highCCR2high but Tie2− macrophage at the lung metastatic site in a spontaneous breast cancer model demonstrated that macrophages not only serve to prepare the prematastatic niche to increase metastatic efficiency but also foster cancer cell survival and growth at the distant site [35]. Depletion of Tie2+ macrophages chemically or through genetic manipulations was found to interfere with metastatic seeding [18]. Together, TAMs were demonstrated to be an important player in the complex multistep process resulting in tumor metastasis and, therefore, have become an increasingly attractive therapeutic target in cancer immunotherapy.
Cancer cell stemness & resistance to therapy
The concept of CSC proposed that a population of cancer cells that acquire stem cell-like or tumor-initiating properties can proliferate indefinitely, contributing to tumor progression, enhanced resistance to cytotoxic therapy and metastasis [36]. As normal stem cells are highly influenced by their niche, CSCs interact with and, in turn, are regulated by stromal cells in the TME. Though it is still unclear how different populations of immune cells within the TME promote CSCs growth, several lines of evidence support that inflammatory signals derived from TAMs facilitate CSC expansion and thereby poor response to chemotherapy [37]. Indeed, the release of proinflammatory cytokines TNF-α by TAMs triggers the expansion of a group of stem-like cancer cells through the NF-κB pathway in a colon cancer model while TAM-derived IL-6 promotes CSCs growth via Stat3 signaling in human hepatocellular carcinoma [38,39]. Akt/mTOR signaling in clear cell renal cell carcinoma was also found to increase EMT and stem cell-like populations under the influence of TAMs [40]. Additionally, juxtacrine signaling between CSCs and TAMs through the CD90 and Eph4A receptors activate Src and NF-κB in carcinoma cells to sustain the CSC niche in breast cancer [41]. The significant influence of TAMs on cancer cell chemotherapeutic resistance was also found to be associated with its promotion of CSC properties. Gene signatures representing CSC properties (Sox-2, Oct-4, Nanog, AbcG2 and Sca-1) were found to be upregulated by TAMs via a paracrine EGFR/Stat3/Sox2 signaling pathway, resulting in increased drug efflux capacity and resistance to chemotherapy in murine breast cancer cells [42]. Recent studies also demonstrated that TAM-derived milk fat globule-epidermal growth factor 8 (MFG-E8) favors CSC reservoir survival during chemotherapeutic treatments via activation of Sonic Hedgehog and Stat3 pathways (Figure 2) [43]. Not surprisingly, targeting TAMs and tumor stromal monocytes by inhibiting CSF1R or CCR2 arrests the proliferation of CSCs, resulting in improved chemotherapeutic responses in pancreatic carcinoma [44]. Therefore, these studies suggest an important therapeutic implication in developing immunotherapeutic methods to target TAMs, which may rescue resistance conferred by CSCs, leading to synergistic therapeutic effects.
In addition to chemotherapeutic resistance conferred by TAM-modulated CSCs, TAMs also directly influence the outcomes of cancer cell-targeting chemo- and radio-therapies. In vitro and in vivo evidence suggested that chemotherapy-elicited tissue damage promotes TAM-dependent tumor-protective tissue repair which leads to dampened chemotherapeutic responses. Such misdirected responses are promoted by increased CSF1-dependent recruitment and polarization of TAMs, increased TAM proteolytic activities as well as the induction of drug-metabolism enzymes in the presence of a variety of conventional chemotherapeutic agents such as doxorubicin, gemcitabine and paclitaxel, etc. Additionally, the continuous influx of TAMs with release of immunosuppressive and profibrotic cytokines such as IL-10 and TGF-β after radiation treatments contributes to tumor recurrence and poor therapeutic outcome [45,46]. Together, these evidences highlighted the crucial role of TAMs in limiting cancer therapy efficacy and the importance of TAM-targeting therapeutic options that may provide a solution to overcome such limitations.
Immunosuppressive role of TAMs
The coordinated interplay between the innate immune system, represented by macrophages and dendritic cells and the adaptive immune system, largely consisting of T lymphocytes, is essential in preventing the development and progression of neoplastic cells [47]. While the process of immune surveillance is operating normal in noncancer bearing hosts, a key issue in tumor immunology is to combat the immunosuppressive factors within the TME taming the normal antitumoral responses. Substantial evidence has supported that TAMs, when skewed to a protumoral phenotype, subvert tumor-infiltrating T lymphocytes functions such that protumoral immunoregulatory functions are favored over antitumoral effector functions. TAMs further modify the immune cell composition within the TME to decrease antitumoral immune cells while simultaneously increase the presence of immunosuppressive cell types to accelerate tumorigenesis. The immunosuppressive effects of TAMs consist of direct interactions with cytotoxic T cells in an antigen-specific and antigen-nonspecific manner or indirect suppression of effector T cells by the expansion of Treg will be discussed below.
Activation of CD8+ effector T cells has long been recognized as one of the major mechanisms of antitumor immunity. The destruction of tumor cells recognized by CD8+ T cells is mediated through: activation of the apoptosis cascades within malignant cells via death receptors; cell lysis by the release of cytotoxic/cytolytic granules; and, production of proinflammatory cytokines and chemokines to establish an unfavorable microenvironment for cancer cell growth. In fact, in a variety of human solid tumors, including renal cell carcinoma, melanoma, breast and ovarian cancer, colorectal carcinoma and gastrointestinal stromal tumors, the presence of activated CD8+ T cells within the tumor stroma has been shown to have clinically significant prognostic value [48]. However, in the case of metastatic melanoma, functional analysis of tumor-infiltrating CD8+ T cells ex vivo revealed blunted proliferation and cytokine production that can be restored with cytokine stimulation in vitro, suggesting that dominant inhibitory pathways are operational within the TME, blunting the function of cytotoxic T cells and allowing for tumor expansion [49]. As the clinical importance of effective CD8+ T-cell function in immune surveillance and in eliminating malignant cells when combined with traditional cancer treatments is incalculable, it is imperative to understand the mechanisms of suppression on T cells in the TME to better design immunotherapeutic approaches.
TAMs suppress CD8+ T-cell activation via several major mechanisms:
Depletion of metabolites essential for T-cell proliferation;
Inhibition of T-cell functions by production of anti-inflammatory cytokines;
Activation of T-cell checkpoint blockade through engagement of inhibitory receptors.
Metabolism of L-arginine and L-tryptophan, catalyzed by TAM-derived arginase-1 and indoleamine dioxygenase 1/2 (IDO 1/2), respectively, results in the failure to re-express CD3 ζ-chain in the T-cell receptor complex and inability to respond to tumor antigen, thus suppressing effector T-cell activation [50–52]. Interestingly, the dichotomy of L-arginine catabolism, mediated by arginase-1 and inducible nitric oxide synthase both lead to T-cell suppression through different mechanisms [53]. In addition, production of anti-inflammatory cytokines, including IL-10, TGF-β and prostaglandin-E2 (PGE2) by TAMs under the influence of tumor-derived factors, further inhibits cytotoxic CD8+, Th1 and Th2 CD4+ cells to establish a self-propagating immunosuppressive TME (Figure 2) [54–56]. It is important to note, however, evidence showing direct cytokine-mediated CD8+ T-cell suppression remains elusive. Last, TAMs further promote the dysfunction of tumor-infiltrating lymphocytes by expressing the ligands of the inhibitory receptors programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) [57,58]. Originally discovered for their importance in regulating the intensity of immune responses as well as response to self-antigens, their potential in treating a broad spectrum of advanced human cancers has just now emerged. When activated by ligands, PD-L1, PD-L2 and B7–1 (CD80), B7–2 (CD86), PD-1 and CTLA-4, respectively, inhibit downstream T-cell receptor and B-cell receptor (BCR) signaling, T-cell activation, proliferation and cytotoxic functions. Under the influence of HIF-1α, TAMs and myeloid-derived suppressor cells (MDSCs) upregulate the expression of PD-L1, thus inducing T-cell suppression in hypoxic tumor regions [59]. However, it is difficult to decipher the relative contribution of TAM-expressed PD-L1 on T-cell suppression in the TME as tumor cells and various other cell types also express PD-L1 [60]. Therefore, future studies aimed at dissecting how PD1–PDL1 interactions between effector T cells and TAMs contribute to the overall immunosuppression in the TME in vivo will improve current understanding of TAMs.
Though overall evidence is lacking, some studies suggest that TAMs also induce NK and NK T-cell dysfunctions. TAM-derived IL-10 inhibits local IL-12 production, a cytokine essential for NK-cell cytotoxicity [61]. A recent study also showed that TAMs induce a CD27lowCD11bhigh-exhausted NK-cell phenotype and inhibit NK cytotoxicity in a cell contact-dependent manner requiring TGF-β signaling [62]. Additionally, tumor-induced inflammation and hypoxia increase TAM-dependent production of CCL20 via activation of the NF-κB signaling pathway. CCL20, in turn, promotes the migration of Vα24-invariant NKT cells to the hypoxic tumor regions, where NK T-cell viability and antitumor function were suppressed [63]. Collectively, these evidences suggested that TAMs are a major force in disrupting antitumoral responses by effector cells in the TME and remain a significant obstacle to effective immunotherapy.
Recruitment of other immunosuppressive cells
Chronic inflammation is a characteristic of cancer and can result in the recruitment of immunosuppressive and protissue repair cell types to the TME. TAMs abundantly produce a variety of cytokines and chemokines that attract or induce immunoregulatory/immunosuppressive cells at the tumor site. Treg, a subset of CD4+ T cells characterized by its ability to negatively regulate immune responses, has been shown to suppress effector T-cell functions in the TME. Natural Treg cells are attracted to the tumor stroma via chemokine receptors CCR4, CCR5, CCR6 and CCR10 [64]. TAMs in ovarian and colorectal cancers produce CCL17/CCL22 and CCL20, which act on CCR4+ and CCD6+ Treg, respectively, to induce Treg migration to the TME (Figure 2). In these cases, the accumulation of Treg is associated with reduced patient survival [65,66]. In addition to recruitment of natural Treg to the tumor stroma, TAMs are also involved in the induction of Treg cells in the TME though the process is incompletely understood. As the regulatory functions of Treg is governed by a pivotal transcription factor, Foxp3 in CD4+ T cells, it is not surprising to find that TAMs promote the expression of Foxp3 via IL-10 and TGF-β signaling [4,64,67,68]. Direct evidence also showed the induction of Foxp3 and CTLA4 expression in CD4+ T cells by TAMs in human renal cell carcinoma in vivo, further emphasizing the critical role of TAMs in cancer-related inflammation, immunosuppression and malignant progression [69].
Last, TAMs-dependent recruitment of MDSCs, another group of potent immunosuppressive cells of myeloid origin can further suppress innate and adaptive immune responses to cancer. MDSCs are a heterogeneous group of cells consisting of immature precursors of monocytes and granulocytes. Although similar to TAMs in many aspects, gene expression analysis showed that MDSCs are a separate entity from TAMs [70,71]. However, tumor-infiltrating monocytic MDSCs can differentiate into TAMs by CSF-1 and HIF-1α [72,73]. TAMs, like M2-polarized macrophages, abundantly produce CCL2 which promotes CCR2+ monocytic MDSCs trafficking from bone marrow to tumor [74]. In human glioblastoma, elevated levels of CCL2 expression correlated with reduced overall survival (OS) of patients and CD163+ TAMs were found to be a major source of CCL2 [75]. Together, these studies demonstrated that TAMs not only suppress antitumoral responses but also promote protumorigenic immune dysfunctions to enhance cancer progression, thus presenting a difficult multifaceted challenge to the development of immunotherapeutics.
Implication for immunotherapy
In recent years, TAMs have emerged as an attractive target for cancer immunotherapy. Its heavy presence in the tumor stroma across a panel of human malignancies, combined with its unique ability to modulate a variety of immune cell functions and nonimmune processes in cancer suggest targeting TAMs may provide novel treatment options to cancer types currently unresponsive to conventional chemotherapeutics and exciting synergism to existing immunotherapies. To overcome the immunosuppressive and protumoral functions of TAMs, current therapeutic strategies have focused on three major aspects:
Reduction of TAMs presence by depleting existing TAMs and/or precursors;
Prevention of TAMs accumulation by blocking their trafficking to the tumor site;
Induction of TAM reprogramming to favor antitumoral functions.
Recent studies have shown these interventional approaches are feasible and effective both in preclinical studies using mouse models and in clinical trials. We will focus on some of these recent successes below and summarize ongoing or complete clinical trial findings in Table 1.
Table 1. . Therapeutic agents targeting macrophages for the treatment of cancer.
| Drugs | Mechanism of action | Trial results or ongoing studies |
|---|---|---|
|
CCL2-CCR2 axis inhibitors | ||
| PF-04136309 |
Interferes with CCL2 binding to CCR2 |
PF-04136309 plus FOLFIRINOX reduce TAMs presence in the stroma and have better treatment response in patients with locally advance PDAC [72] Ongoing Phase Ib/II trial in combination with gemcitabine and nab-paclitaxel in first-line metastatic pancreatic cancer patients (NCT02732938) |
| Carlumab |
Anti-CCL2 IgG1κ neutralizing monoclonal antibody |
Carlumab showed preliminary antitumor activity in patients with advanced solid tumors [73,74] |
|
CSF1-CSF1R signaling blockers | ||
| BLZ945 |
Selective CSF1R inhibitor |
Ongoing Phase I/II trial of BLZ945 alone or in combination with PDR001 (anti-PD-1 mAb) in advanced solid tumors (NCT02829723) |
| PLX3397 |
Selective inhibitor of CSF1R and two other tyrosine kinase receptors KIT and FLT3 |
PLX3397 led to partial response in 52% and s disease in 32% of patients with tenosynovial giant cell tumor in a Phase I/II study [81]
Ongoing Phase I trial with paclitaxel in advance solid tumors (NCT01525602) Ongoing Phase Ib/II trial with eribulin in metastatic breast cancer (NCT01596751) |
| Emactuzumab (RG7155) |
Anti-CSF1R neutralizing antibodies |
RG7155 demonstrated therapeutic efficacy in patients with diffuse-type giant cell tumor [82] |
| AMG-820 |
Anti-CSF1R neutralizing antibodies |
Ongoing Phase I trial in advanced solid tumors (NCT01444404) |
| IMC-CS4 |
Anti-CSF1R neutralizing antibodies |
Ongoing Phase I trial in advanced solid tumors (NCT01346358) |
|
TLR agonists | ||
| Imiquimod |
TLR7 agonist |
Completed Phase I trial in combination with multipeptide vaccination for cutaneous metastases of melanoma (NCT01264731) Completed Phase II trial in breast cancer patients with chest wall recurrence or skin metastases (NCT00899574) Ongoing Phase II trial in combination with abraxane in advanced breast cancer (NCT00821964) |
| 852A |
TLR7 agonist |
Phase II trial in breast, ovarian, endometrial and cervical cancers (NCT00319748) |
|
Multifunctional agent | ||
| Trabectedin | Binds the minor groove of DNA to induce cell cycle arrest and death selectively in monocytes and macrophages | Phase III clinical trial confirmed trabectedin is superior to dacarbazine in treating advance soft tissue sarcoma [85] Phase III study showed trabectedin plus PLD improves progression-free survival and overall survival than PLD alone in recurrent ovarian cancer [86] Ongoing Phase II trial in malignant pleural mesothelioma (NCT02194231) Ongoing Phase III trial in locally advanced or metastatic liposarcoma or leiomyosarcoma (NCT01692678) |
PDAC: Pancreatic ductal adenocarcinoma; PLD: Pegylated liposomal doxorubicin; TAM: Tumor-associated macrophage; TLR: Toll-like receptor.
CCL2–CCR2 axis inhibitors
Trafficking of monocytes from bone marrow to the tumor site requires the CCL2–CCR2 signaling axis. In various human neoplasia, increased levels of CCL2 are correlated with increased occurrences of metastasis and decreased OS [18,75,76]. In mouse models, inactivation of CCL2–CCR2 signaling achieved by gene knockout or inhibition of CCR2 by a small molecule inhibitor PF-04136309 reduced tumor growth and metastatic spread [76,77]. PF-04136309 prevents CCR2 activation and downstream signaling by interfering with the binding of CCL2 to its receptor CCR2. In a Phase-Ib clinical trial comparing a standard chemotherapy regimen FOLFIRINOX (oxaliplatin, irinotecan, leucovorin and fluorouracil) plus PF-04136309 to FOLFIRINOX alone in patients with locally advanced pancreatic ductal adenocarcinoma, the addition of PF-04136309 was safe and resulted in a reduction in the infiltration of TAMs and Treg while an increase in CD4+ and CD8+ effector cell's presence in the tumor stroma was observed. Patients treated with PF-04136309 plus FOLFIRINOX also showed better treatment responses than FOLFIRINOX alone, providing strong evidence supporting the clinical advantage of inhibiting CCL2–CCR2-mediated TAM recruitment [78]. In addition, carlumab, a human anti-CCL2 IgG1κ mAb was evaluated in patients with solid tumors and showed preliminary antitumor activity. However, carlumab only transiently suppress free CCL2 in patients, thus limiting its clinical efficacy [79,80].
CSF1–CSF1R signaling blockers
Inhibition of CSF1–CSF1R signaling by neutralizing antibodies or small molecule antagonists has shown exciting promise in developing therapeutics to effectively target TAM recruitment and activation. In several preclinical studies using murine breast cancer and glioblastoma models, researchers have demonstrated CSF1–CSF1R signaling blockade slowed primary tumor growth, reduced metastatic potential and improved long-term survival of tumor-bearing mice [81–83]. Treatments with BLZ945, a potent and selective CSF-1R inhibitor attenuated tumor growth correlated with decreased TAMs presence and enriched CD8+ T cells in tumor stroma in a murine K14-HPV-16 transgenic mouse model of cervical carcinogenesis [83]. This has been translated to a first-in-human Phase I/II study of BLZ945 alone or in combination with PDR001, a monoclonal antibody against PD-1 in advanced solid tumors (NCT02829723). Another agent, PLX3397, selectively inhibits CSF1R and two other tyrosine kinase receptors KIT and FLT3 [84]. Blockage of CSF1R with PLX3397 not only improved the efficacy of adoptive cell therapy through inhibition of immunosuppressive macrophage recruitment and activation in murine melanoma but also potentiated the response of xenograft glioblastoma to ionizing radiation (IR) by preventing differentiation and protumoral activation of IR-recruited monocytes in mice [85,86]. In a human Phase I/II study evaluating the safety and efficacy of PLX3397 alone in tenosynovial giant-cell tumor, treatment with PLX3397 led to partial response in 52% of patients while 32% achieved stable disease [87]. Additionally, a neutralizing antibody emactuzumab (RG7155) against CSF1R not only reduced macrophage infiltration in mouse models but also demonstrated similar therapeutics effects against diffuse-type giant cell tumor in patients [88]. Two other neutralizing antibodies against CSF1R – AMG 820 (NCT01444404) and IMC-CS4 (NCT01346358) are also currently under Phase I clinical trials for their efficacy in treatment of advanced solid tumors. These data confirmed the clinical merit of CSF1-CSF1R signaling blockade alone or as a part of combinatorial therapies to offer synergistic immunotherapeutic effects in treatments of human cancers.
Trabectedin
Approved in Europe as a single-agent chemotherapeutic treatment for soft tissue sarcoma, trabectedin has long been recognized for its ability to bind the minor groove of DNA to induce cell-cycle arrest and death. Its unique ability to selectively kill human monocytes and/or macrophages while inhibiting CCL2 and IL-6 production in vitro provided initial evidence for its clinical relevance in targeting TAMs in vivo [89]. In preclinical studies using four different mouse models, trabectedin showed selective killing of mononuclear phagocytes in blood, spleen and tumors while sparing neutrophils and lymphocytes via a caspase-8-dependent apoptosis pathway. In addition, murine tumors showed downregulation of CCL2 and VEGF after trabectedin treatment, resulting in a decrease in the recruitment of monocytes to the tumor site and reduced angiogenic sprouting, respectively. Patients with soft tissue sarcoma treated with trabectedin also showed a strong decrease in TAM density correlated with a reduction in angiogenesis in comparing pre- versus post-treatment biopsies [90]. Currently, the preclinical mechanistic studies and success in mouse models of trabectedin have been translated to numerous clinical trials evaluating its safety and efficacy in single-agent or combinational treatments against a variety of human cancers. To date, at least two large-scale multicenter Phase III clinical trials have confirmed its therapeutic advantage. One trial concluded that trabectedin is superior to dacarbazine in treating advance soft tissue sarcoma while the others showed trabectedin plus pegylated liposomal doxorubicin improves progression-free survival and OS than pegylated liposomal doxorubicin alone in recurrent ovarian cancer patients as a second-line treatment [91,92].
Toll-like receptor agonists
Functional comparisons of stromal macrophages in early- versus late-stage tumors revealed that prolonged interactions with the TME skew macrophages toward an immunosuppressive phenotype with tumorigenic properties [73]. This suggests mechanisms to educate TAMs to regain their tumoricidal functionalities may have the potential be utilized in immunotherapy. Because activation of Toll-like receptors (TLRs) expressed on macrophages leads to M1 polarization and robust production of proinflammatory cytokines such as IFN-γ, TNF-α and IL-12 through NF-κB signaling, TLR agonists have been evaluated in various mouse models for their ability to reprogram TAM functions to destroy tumor cells [93]. Imiquimod, a US FDA-approved topical TLR7 agonist, not only increased effect T-cell tumor infiltration and inhibited mouse breast cancer growth alone but also synergized with IR to achieve complete regression of tumor [94]. Local delivery of a TLR7/8 agonist 3M-052 boosted systemic antitumor immunity by repolarizing TAMs to a M1 NO-producing phenotype and resulted in eradication of murine metastatic melanoma. In addition, combining 3M-052 with antibodies against CTLA4 and PD-L1 is synergistic in targeting established murine B16.F10 melanoma by rescuing TAM and cytotoxic CD8+ T cell tumoricidal functions [95]. Though clinical evidence demonstrating the efficacy of TLR agonists is currently insufficient, multiple Phase I or II trials are currently underway to evaluate the therapeutic value of TLR-dependent reprogramming of TAM functions [1].
Last, though therapeutic approaches using small molecule inhibitors or neutralizing antibodies to deplete TAMs or suppress its functions have been the focus of TAM-targeting therapies, recent literatures also proposed using TAMs as a novel target to deliver chemotherapy in triple-negative breast cancer (TNBC), a therapeutically resistant subtype of human breast cancer. As TNBC is often infiltrated with high numbers of TAMs, Niu et al. showed that doxorubicin-containing nanoparticles targeting TAMs via mannose-mannose receptor recognition reduced TAM density and inhibited tumor growth in an orthotopic mouse model of TNBC. In addition, the effectiveness of these nanoparticles is found to be dependent on their ability to target TAMs within TNBC stroma [96]. Other novel approaches to target TAMs in vivo include the use of DNA vaccines against TAM-derived protein legumain in several murine cancer models [97] as well as the success of using clodronate/liposome to delete TAMs as an adjuvant therapy to boost antitumor immunity induced by a TLR agonist-conjugated peptide Pam2IDG [98]. Together, these evidences emerge as exciting new proofs that the high presence of TAMs within cancer stroma can be further exploited by targeted therapeutic approaches in the future.
Conclusion & future perspective
The human innate and adaptive immune systems are powerful protective mechanisms against pathogenic infections and are crucial in cancer immunosurveillance. Recent efforts to augment immune responses in treatments of various human cancers have gained considerable success. Therefore, a deeper understanding of the pathologic mechanisms underlying immunosuppression within the TME will benefit design and development of therapeutics for cancer patients. As TAMs are highly prevalent in the stroma of many solid tumors, they represent a crucial promoting force in cancer progression via angiogenesis, metastasis, chemotherapeutic resistance and immune evasion. Disrupting the vicious interactions between TAMs and cancer cells could potentially have significant implications for improving patient survival. In addition, rescuing TAM-mediated immune dysfunctions by ablation or repolarization, in combination of immunotherapies aim at augmenting cytotoxic CD8+ T-cell functions, have shown promising results and possess immense potential.
Though promising, many current approaches targeting TAMs proved to be insufficient to eradicate tumor or too toxic to patients. Targeting a single signaling axis that promotes the immunosuppressive and protumoral functions of macrophages is inadequate as there are multiple signals involved in the communication between tumor cells and TAMs. Identifying and inhibiting key driver pathways, which are critical for both cancer cell survival and TAM activation, may offer therapeutic advantages as it disrupts the vicious positive feedback loop between tumor and TAMs. Consequently, a more comprehensive understanding of the major driving forces underlying macrophage polarization within the TME will provide a mechanistic foundation for developing future therapies. Genomic, epigenomic, proteomic and metabolomics analyses to delineate the extensive regulatory networks governing TAM functions may also reveal novel therapeutic targets. Furthermore, future studies aim at further defining TAM subpopulation in different cancer or tissue types in different stages of tumor development is needed to refine our current approaches targeting TAMs. In conclusion, therapies targeting TAMs, a major force in modulating tumorigenesis and immunity in the tumor stroma may improve efficacy of current immunotherapeutic approaches.
Executive summary.
Macrophages, one of the most plastic cell types of the hematopoietic system, are functionally heterogeneous depending on the local microenvironment in which they reside.
Tumor-associated macrophages (TAMs), representing most of the immune cell population within the tumor stroma, can be polarized to an anti-inflammatory and protumoral phenotype under the influence of the tumor microenvironment.
The presence of TAMs is associated with poor clinical outcome in a variety of human cancers due to the pivotal roles they play in promoting tumorigenesis and metastasis via both nonimmune and immune mechanisms.
Through interactions with neoplastic cells, endothelial cells and the extracellular matrix, TAMs promote angiogenesis, metastasis, cancer cell stemness and chemotherapeutic resistance.
TAMs have direct immunosuppressive functions by interfering with effector T-cell functions at the tumor site. In addition, TAMs promote the recruitment of other immunosuppressive cell types, including Treg and myeloid-derived suppressor cells to further disrupt local antitumor immune responses.
Current therapeutic approaches to target TAMs focus on selective ablation or reprogramming of this population of cells.
Combining inhibitors that target the CCL2-CCR2 and CSF1-CSF1R signaling axis critical for macrophage migration and protumoral activation achieved synergistic effects when combined with standard chemotherapeutic regimen in early phase clinical trials.
Selective killing of monocytes and/or macrophages with trabectedin demonstrated clinical efficacy as a single agent and in a combinatorial regimen in treatments of human soft tissue sarcoma and recurrent ovarian cancer, respectively.
Development of future therapeutic approaches to target TAMs requires a more comprehensive understanding of the major driving forces underlying macrophage polarization within the tumor microenvironment. In addition, better defining TAMs in different cancer or tissue types in different stages of tumor development may reveal novel therapeutic opportunities and refine current strategies in targeting TAMs.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants CA136934, CA186973 and CA193167. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 4.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014;14(6):392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 6.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 9.Biswas SK, Gangi L, Paul S, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell. Mol. Immunol. 2015;12(1):1–4. doi: 10.1038/cmi.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025. doi: 10.1038/npjbcancer.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin RA, Li MO. Ontogeny of tumor-associated macrophages and its implication in cancer regulation. Trends. Cancer. 2016;2(1):20–34. doi: 10.1016/j.trecan.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 15.Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that tumor-associated macrophages (TAMs) are functionally and phenotypically distinguishable from tissue macrophages in a mouse mammary tumor model.
- 16.Shand FHW, Ueha S, Otsuji M, et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. PNAS. 2014;111(21):7771–7776. doi: 10.1073/pnas.1402914111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linde N, Lederle W, Depner S, Van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J. Pathol. 2012;227(1):17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 18.Qian B-Z, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization; enabling diversity with identity. Nat. Rev. Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 21.Colegio OR, Chu N-Q, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates the role of tumor-derived lactic acid in promoting M2-polarization of TAMs via the action of HIF-1α.
- 22.Heusinkveld M, Van Der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer. 2008;8(8):618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 25.Valkovic T, Dobrila F, Melato M, Sasso F, Rizzardi C, Jonjic N. Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch. 2002;440(6):583–588. doi: 10.1007/s004280100458. [DOI] [PubMed] [Google Scholar]
- 26.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67(11):5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 27.Forget MA, Voorhees JL, Cole SL, et al. Macrophage colony-stimulating factor augments tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PLoS ONE. 2014;9(6):e98623. doi: 10.1371/journal.pone.0098623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Yeo EJ, Cassetta L, Qian BZ, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74(11):2962–2973. doi: 10.1158/0008-5472.CAN-13-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyckoff JB, Wang Y, Lin EY, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]; •• Very first multiphoton microscopic study that provided direct visual evidence that TAMs assist tumor-cell intravasation in the absence of local angiogenesis.
- 32.Sangaletti S, Di Carlo E, Gariboldi S, et al. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Res. 2008;68(21):9050–9059. doi: 10.1158/0008-5472.CAN-08-1327. [DOI] [PubMed] [Google Scholar]
- 33.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Yao Y, Gong C, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19(4):541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian B, Deng Y, Im JH, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE. 2009;4(8):e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clin. Cancer Res. 2011;17(19):6125–6129. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwitalla S, Fingerle AA, Cammareri P, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152(1–2):25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z, Xie H, He D, Li L. Infiltrating macrophages increase RCC epithelial mesenchymal transition (EMT) and stem cell-like populations via AKT and mTOR signaling. Oncotarget. 2016;7(28):44478–44491. doi: 10.18632/oncotarget.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu H, Clauser KR, Tam WL, et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014;16(11):1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Liao D, Chen C, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31(2):248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 43.Jinushi M, Chiba S, Yoshiyama H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. PNAS. 2011;108(30):12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 2012;4(4):376–389. [PMC free article] [PubMed] [Google Scholar]
- 47.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 48.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mortarini R, Piris A, Maurichi A, et al. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63(10):2535–2545. [PubMed] [Google Scholar]
- 50.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez PC, Zea AH, Desalvo J, et al. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol. 2003;171(3):1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 53.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 2002;168(2):689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 54.Kalinski P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012;188(1):21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh SA, Li MO. TGF-beta: guardian of T cell function. J. Immunol. 2013;191(8):3973–3979. doi: 10.4049/jimmunol.1301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruffell B, Chang-Strachan D, Chan V, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009;206(6):1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am. J. Pathol. 2009;174(3):1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T-cell activation. J. Exp. Med. 2014;211(5):781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121(5):734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sica A, Saccani A, Bottazzi B, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J. Immunol. 2000;164(2):762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 62.Krneta T, Gillgrass A, Poznanski S, et al. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J. Leukoc. Biol. 2016 doi: 10.1189/jlb.3A1215-552R. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; • Demonstrated the immunosuppressive effects of TAMs on other effector cells within the tumor microenvironment, notably NK cells in a contact-dependent mechanism involving TGF-β signaling.
- 63.Liu D, Song L, Wei J, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J. Clin. Invest. 2012;122(6):2221–2233. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front. Immunol. 2013;4:190. doi: 10.3389/fimmu.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Zhang N, Li Q, et al. Tumor-associated macrophages recruit ccr6+ regulatory t cells and promote the development of colorectal cancer via enhancing ccl20 production in mice. PLoS ONE. 2011;6(4):e19495. doi: 10.1371/journal.pone.0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature Immunol. 2007;8(10):1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 68.Savage ND, De Boer T, Walburg KV, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J. Immunol. 2008;181(3):2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 69.Daurkin I, Eruslanov E, Stoffs T, et al. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71(20):6400–6409. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- 70.Pucci F, Venneri MA, Biziato D, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood ‘resident’ monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114(4):901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 71.Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Invest. 2015;125(9):3365–3376. doi: 10.1172/JCI80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corzo CA, Condamine T, Lu L, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lesokhin AM, Hohl TM, Kitano S, et al. Monocytic CCR2+ myeloid derived suppressor cells promote immune escape by limiting activated CD8 T cell infiltration into the tumor microenvironment. Cancer Res. 2012;72(4):876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang AL, Miska J, Wainwright DA, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5671–5682. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 2013;19(13):3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu X, Kang Y. Chemokine (C-C Motif) ligand 2 engages CCR2(+) stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J. Biol. Chem. 2009;284(42):29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resec and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, Phase 1b trial. Lancet Oncol. 2016;17(5):651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows clinical efficacy of combinatorial treatment of CCR2 inhibition with standard chemotherapeutic regimen in treatment of advanced pancreatic cancer.
- 79.Brana I, Calles A, Lorusso PM, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter Phase 1b study. Targeted Oncol. 2015;10(1):111–123. doi: 10.1007/s11523-014-0320-2. [DOI] [PubMed] [Google Scholar]
- 80.Sandhu SK, Papadopoulos K, Fong PC, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother. Pharmacol. 2013;71(4):1041–1050. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 81.Denardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strachan DC, Ruffell B, Oei Y, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells. Oncoimmunology. 2013;2(12):e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang C, Ibrahim PN, Zhang J, et al. Design and pharmacology of a highly specific dual FMS and KIT kinase inhibitor. PNAS. 2013;110(14):5689–5694. doi: 10.1073/pnas.1219457110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mok S, Koya RC, Tsui C, et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74(1):153–161. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stafford JH, Hirai T, Deng L, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro-oncology. 2016;18(6):797–806. doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of csf1r kinase in tenosynovial giant-cell tumor. N. Engl. J. Med. 2015;373(5):428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 88.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 89.Germano G, Frapolli R, Simone M, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70(6):2235–2244. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 90.Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]; •• Showed strong evidence in support of selective killing of mononuclear phagocytes, including TAMs by trabectedin and treatment advantages this agent offers.
- 91.Demetri GD, Von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a Phase III randomized multicenter clinical trial. J. Clin. Oncol. 2016;34(8):786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monk BJ, Herzog TJ, Kaye SB, et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J. Clin. Oncol. 2010;28(19):3107–3114. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]
- 93.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dewan MZ, Vanpouille-Box C, Kawashima N, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin. Cancer Res. 2012;18(24):6668–6678. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh M, Khong H, Dai Z, et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J. Immunol. 2014;193(9):4722–4731. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niu M, Valdes S, Naguib YW, Hursting SD, Cui Z. Tumor-associated macrophage-mediated targeted therapy of triple-negative breast cancer. Mol. Pharm. 2016;13(6):1833–1842. doi: 10.1021/acs.molpharmaceut.5b00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J. Clin. Invest. 2006;116(8):2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen K-Y, Song Y-C, Chen IH, Chong P, Liu S-J. Depletion of tumor-associated macrophages enhances the anti-tumor immunity induced by a Toll-like receptor agonist-conjugated peptide. Hum. Vaccin. Immunother. 2014;10(11):3241–3250. doi: 10.4161/hv.29275. [DOI] [PMC free article] [PubMed] [Google Scholar]