Abstract
Mutable viruses, such as HIV, pose difficult obstacles to prevention and/or control by vaccination. Mutable viruses rapidly diversify in populations and in individuals, impeding development of effective vaccines. We devised the ‘mutable vaccine’ to appropriate the properties of mutable viruses that undermine conventional strategies. The vaccine consists of a DNA construct encoding viral antigen and regulatory sequences that upon delivery to B cells target the enzymatic apparatus of ‘somatic hypermutation’ causing the construct to mutate one million-times baseline rates and allowing production and presentation of antigen variants. We postulate the mutable vaccine might thus anticipate diversification of mutable viruses, allowing direct control or slowing of evolution. Initial work presented here should encourage consideration of this novel approach.
Keywords: : antigen, B cell, escape variant, HIV, mutable virus, somatic hypermutation, vaccine, viral evolution, virus
Vaccination has been used with great success to prevent some of the most dreaded pathogens and/or to neutralize their products. Effective vaccines must be immunogenic and the immunity must anticipate the organisms and variants that might infect the vaccinated individual in the future. Vaccines that confer the most enduring protection thus target organisms or products that are genetically stable and relatively nonpolymorphic (e.g., paramyxoviruses). Vaccines can effectively target organisms that exist as multiple variants, such as influenza virus if variants that will be prevalent can be anticipated and represented. However, vaccines have never effectively targeted highly mutable viruses, such as HIV or hepatitis C [1–3]. Here, we describe an approach we devised for potentially overcoming the barriers to generating protective immunity against mutable viruses and other genetically capricious entities. Although the efficacy of the mutable vaccine is yet to be tested, we present the concept and our perspective on the potential mechanisms at this juncture because vaccines of conventional design have never proven effective in prophylaxis of mutable viruses [4–6].
Four daunting barriers preclude the development of effective vaccines for mutable viruses such as HIV. First, the rapid rate of viral replication and mutation of the viral genome (∼4.1 ± 1.7 × 10-3/base/cell, corresponding to one viral generation in an infected cell [7]) generates numerous viral variants in the host and in the population [8–11]. Second, relative indifference of the viral life cycle to changes in protein sequence and expression and tropism for cell types that are relatively inured to genomic plasticity (lymphocytes for HIV and hepatocytes for hepatitis C) [12,13] allows diverse clades to be generated; potentially to be transmitted in the population [10,11] and to rapidly evolve [10,14–16]. Hence, a vaccine representing the most prevalent variants at one point in time will not protect against all variants existing when the vaccine is administered and immunity develops. Third, although HIV and other mutable viruses are immunogenic, and although immunity can decrease viral replication and reduce disease severity [8,9,11,17–24], the rate of viral diversification exceeds the rate of development of protective immunity, allowing selection and expansion of variants that can occupy lacunae in immune recognition, in other words, ‘escape variants’ [2,3,8,9,11]. Fourth, even if broadly protective immunity can be generated, HIV and similar viruses are still not eradicated because some viral envelope epitopes are hidden during most of the virus life cycle [4], because infected cells may evade the actions of cytotoxic cells (by decreasing expression of MHC or by ‘accommodation’ as in transplants [25,26]) and because reservoirs of latent virus are not subject to immune-mediated clearance [27–30].
Given these challenges, we reasoned that conventional approaches to vaccination would inevitably fail and designed an entirely new approach that potentially anticipates and slows viral evolution, allowing sterilizing immunity to develop to control and potentially eradicate the virus. We call our vaccine ‘the mutable vaccine’ [31]. The mutable vaccine consists of a DNA construct encoding one or more viral antigens, an adjuvant and immunoglobulin (Ig) enhancer sequences (Figure 1). After introduction into stimulated B cells, the vaccine co-opts the enzymatic apparatus that supports somatic hypermutation of Ig genes and expression of the products [32,33].
Figure 1. . A schematic model of the mutable vaccine.
The vaccine is a DNA construct encoding one or more viral antigens (HIV gp140 is shown as an example) fused with C3d and regulated by immunoglobulin promoter and enhancer sequences.
Mutation of the HIV genome is at least in part generated by cytidine deamination by APOBEC3-G, resulting in G to A mutations in the sense (+) strand [34,35], possibly by other cytidine deaminases acting directly on the integrated viral genome causing C to T transitions in the sense strand [36,37] and by errors introduced by the reverse transcriptase [38]. HIV-1 reverse transcriptase mutations are thought to contribute no more than 2% of viral mutations [7]. Like mutation of HIV genes, somatic hypermutation is initiated by deamination of cytosine to uracil in the variable region of the Ig genes and in other genes under the control of Ig enhancers [32] owing to ‘activation-induced cytidine deaminase’, an enzyme sharing extensive homology with APOBEC3-G. In addition, mismatches created by cytidine deamination are often resolved by error-prone polymerases, and hence the mutations target all four nucleotides (and because activation-induced cytidine deaminase is expressed only transiently in B cells), mutations become ‘fixed’ as B cells differentiate further to plasma cells [32]. The mutable vaccine also appropriates the secretory apparatus of B cells so that variants can be produced and secreted in abundance (up to 15 pg Ig/cell/day). Stimulation of the B cells when the vaccine is introduced and possibly at later times increases mutation and secretion of products. Finally, the variants produced by vaccinated B cells can generate peptides that are taken up and effectively presented to the T cells [39]. We thus reasoned that by generating antigenic variants in B cells, which are capable of secreting and/or presenting antigen with adjuvant, the vaccine might anticipate and generate immunity to viral variants that arise during the first rounds of viral replication (Figure 2).
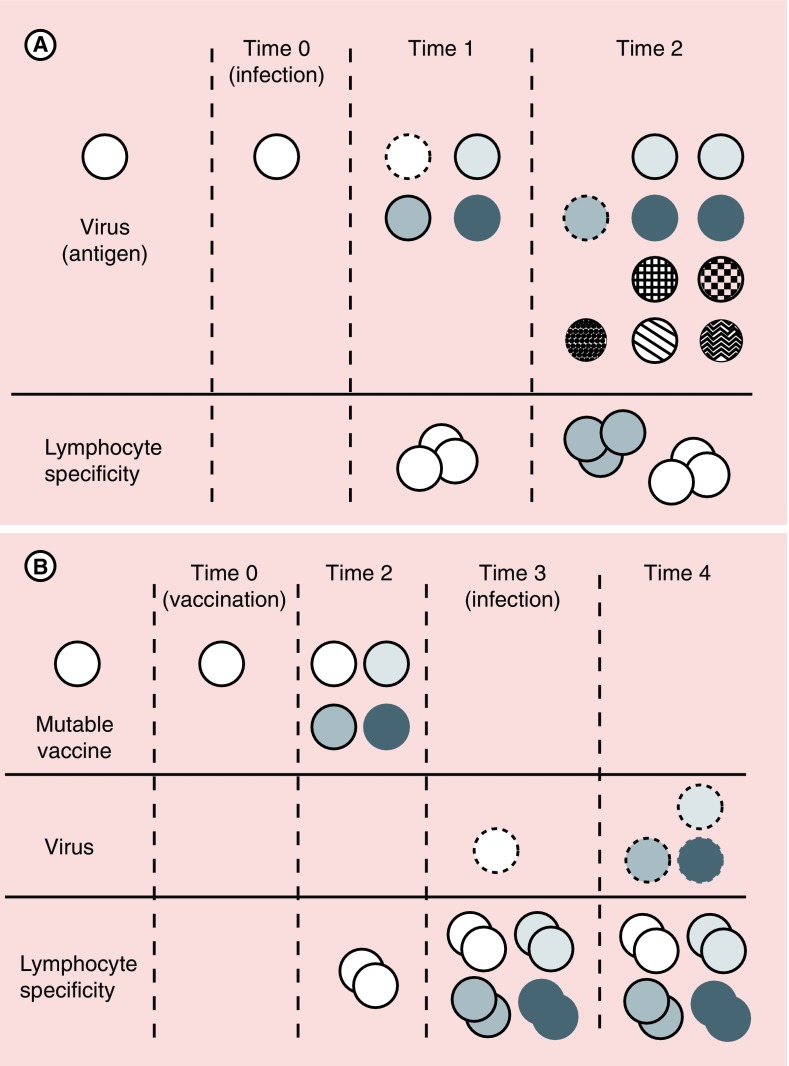
Figure 2. . Model for viral evolution and control by vaccination with the mutable vaccine.
(A) Evolution of a mutable virus and the adaptive immune response. After infection at time 0, the virus diversifies over time (time 1 and 2). Immunity reflecting lymphocyte specificity to the infecting virus is manifested at time 1 (dashed line), but the virus has already diversified, leaving variants that escape control by adaptive immunity. Further diversification (time 2) generates still more variants at time 2, some of which might be controlled by lymphocytes specific for viral variants at time 1 (dashed lines), but many variants escape control, leading to progressive viral diversification, despite T-cell and B-cell responses. (B) Potential impact of the mutable vaccine on viral evolution. The mutable vaccine administered at time 0 evokes immunity against the encoded antigen symbolized an open circle. The vaccine antigen diversifies by time 2, at which time lymphocytes responding to the initial antigen are proliferating. At time 3, infection occurs and lymphocyte effector cells can eradicate infecting virus. If infecting virus is not entirely cleared and begins to diversify by time 4, the expanded effector lymphocytes against initial variants may exert partial control, slowing viral evolution and allowing immunity specific for antigens of the infecting virus other than those encoded in the vaccine to emerge (not illustrated).
The mutable vaccine thus differs from conventional vaccines in that it does not employ a fixed antigen or set of antigens, but rather it provides one or more ‘founder’ antigens that diversify over time. The vaccine diversifies through some of the same stepwise catalytic pathways and by the same kinetics as a mutable virus. If, in this way, the mutable vaccine anticipates diversification of a virus, it might evoke immunity to antigenic variants that would be generated in series after infection, that is, it might provide a novel dynamic type of anticipatory immunity. Ultimately, if the vaccine generates immunity against variants that would develop during early generations of viral replication, it might block the establishment of chronic infection. However, such ‘perfect’ efficacy might fail in some (or many instances) either because the vaccine fails to anticipate all variants or because protective immunity fails to be elicited against some variants. However, we speculate that protection might nonetheless occur if the mutable vaccine anticipates enough viral variants to slow evolution of the virus. Slowing of viral evolution could allow the adaptive immune response of the host to ‘catch up’ with and clear the more slowly evolving virus. This later possibility, although still theoretical, might be promoted if virus-induced inflammation activates B cells harboring the vaccine thereby triggering further rounds of vaccine diversification, vitiating selection of escape variants.
A prototype of the mutable vaccine
We designed and have begun to test a prototype of the mutable vaccine. The prototype consists of: the full sequence of HIV Env cloned under the control of the λ (Ig) light chain promoter to direct B-cell expression [40]; three copies of complement (in frame with env) to decrease the threshold for immunogenicity in T cell-dependent B-cell responses [41] and to enhance cell-mediated immunity independent of B cells [42]; and the Ig heavy chain large intronic enhancer (Eμ) to confer hypermutability. The gene product is assembled and secreted as a trimer, enhancing immunogenicity still further [40].
The rate of mutation of the mutable vaccine
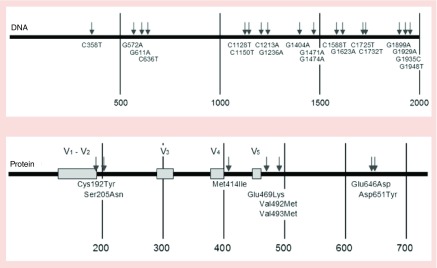
To determine the rate of mutation of the mutable vaccine, a hypermutation competent B-cell line, generated by transformation with the Abelson murine leukemia virus (A-MuLV) [43] was stably transfected with the mutable vaccine. To determine rate of mutation, we employed an approach involving direct sequencing of transduced B-cell clones generated by sequential limiting dilution, as we previously described [44]. Sequencing env in vaccinated B-cell clones revealed mutations throughout the length of the env gene encoded in the construct (Figure 3) [40,44]. Thus, 1 month after the vaccination, the env contained 19 single unique point mutations in the 2000-bp length of the gene. Analysis of serial generations revealed a rate of mutation of 10-5 bp/B-cell generation, equivalent to the rate of somatic hypermutation of Ig genes, as hoped.
Figure 3. . Extent of mutation in the mutable vaccine.
Tumor B cells, hypermutation-competent tumor B cells (of the 18.81 lineage [43]) were transduced with the mutable vaccine and the env sequences and deduced amino acid sequences were determined in single cell clones derived from the original tumor cells following three sequential limiting dilutions [44]. (A) Sites of mutation of env in clonal B cells expressing the mutable vaccine. Arrows denote nucleotide changes from the original vaccine sequence. The type of mutation and the position in the sequence are shown. (B) Amino acid changes relative to the variable (V) domains of the Env. Arrows and numbers indicate the position of changes in the sequence. Numbering was according to the YU-2 protein (UniProtKB: locus ENV_HV1Y2, accession P35961).
Adapted in part from [44].
Anticipation of natural variants of capsular (Env) protein
Out of the mutations shown in Figure 3, eight encoded amino acid changes and of these, six represented natural variants of HIV-1 Env (Table 1). What is especially remarkable, however, is that three of the mutations exactly match natural variants of Env found in a different clade. The Env of HIV-1 O.CM.91.MVP5180, a virus of clade O, has Lys at position 469. The Env of HIV-1 C-BW.00.00BW07621, a virus of clade C; G.GH.03GH175G, clade G virus; and O.CM.96.CMABB637 a clade O virus, all have Asn at position 205. Interestingly, mutation E646D can be found in many (more than 20) HIV-1 Env variants of group M including clades A1, A2, several strains of clade B, C, in one strain of clade D, F2, G, H, J, K, N, O, in several recombinant circulating forms (e.g., AE and AG); and in all but one of the primary clade C isolates published [45] and in primary clade B isolates PVO.4 and TRJO4551.58 [46]. These results indicate that Env variants generated by the mutable vaccine prototype anticipated at least some of the evolution that occurred in human subjects harboring HIV virus. Although it is impossible to predict all of the variants of env (or any gene) that would be generated over time, the mutable vaccine prototype appears capable of generating at least some of these variants. We speculate that immunity against some, yet unknown, fraction of variants would slow that viral evolution, allowing protective immunity to develop against variants not incorporated in the vaccine and hence possibly control.
Table 1. . Representation of natural HIV Env variants among variants deduced from env mutants generated by the mutable vaccine.
| Amino acid changes (numbering is according to YU-2 reference) |
Naturally occurring strains with correspondent amino acid mutation as compared with HXB2 The sequences analyzed were obtained from the HIV sequence compendium 2009 published compiled from http://www.hiv.lanl.gov/content/sequence/alignN/align.html and with published sequences obtained from primary isolates of strains B [46] and C [45] |
| Cys192Tyr |
Cys mutated but not to Tyr in many naturally occurring variants |
| Ser205Asn |
CB.W.00.00.BW07621, G.GH.03.03GH175G, O.CM.96.CMABB637 Ser mutated to other residues in many other strains including primary isolates of clade C viruses [45] |
| Met414Ile |
Met mutated but not to Ile in many naturally occurring variants |
| Glu469Lys |
O.CM.91.MVP5180 |
| Val492Met |
Val mutated but not to Met in many naturally occurring variants |
| Val493Met |
Val mutated but not to Met in many naturally occurring variants |
| Glu646Asp |
Most naturally occurring variants have Asp in the corresponding position. Those include envelopes of different clades of group M including clade A1, A2 in few B strains, C, in one D strain analyzed, F2, G, H, J, K N, O, in several recombinant circulating forms (AE and AG as examples), in all but one of the primary clade C isolates published recently [40] and in primary clade B isolates PVO.4 and TRJO4551.58 [46] |
| Asp651Tyr | Asp mutated in many naturally occurring variants |
Immunogenicity of the mutable vaccine
For what may be obvious reasons, we have yet to determine whether and to which extent the mutable vaccine can protect against a mutating pathogen. Testing efficacy of the mutable vaccine will be far more challenging than testing efficacy of conventional vaccines because the evolution of vaccine-originated variants will vary over time in each immunized subject and in cohorts of immunized individuals and it will be difficult or impossible to know a priori which variants would have evoked manifestly protective immunity. The same challenges will impede optimization of dosage and route (e.g., subcutaneous vs mucosal), which may be needed to ascertain efficacy. We have ascertained the vaccine prototype evokes antigen specific B-cell and T-cell responses, but determining whether those responses, once optimized, can protect against or can slow evolution of a mutable organism likely cannot be predicted by analysis of antibody affinity or virus neutralization and might rather depend on more complex in vivo analysis of viral persistence and pathogenicity.
We have determined that the mutable vaccine elicits detectable anti-Env antibody responses (Figure 4). After delivery of the vaccine to mice, we measured by ELISA YU-2 Env-specific antibodies in serum at various times thereafter. For the ELISA, YU-2 Env was immobilized onto hCD4-coated plates and binding of antibodies in serial dilutions of sera obtained before and after vaccination assayed using goat antimouse IgG–HRP conjugated. We do not know whether these antibody levels or the affinity of these antibodies would confer protection. Or do we know the levels or affinities of interaction between anti-ENV antibodies and ENV variants. However, titers approximately at this magnitude, whether or not protective, have been taken to indicate positive responses in clinical trials of ENV peptide vaccines [47].
Figure 4. . Antibody response to the mutable vaccine.
Mice vaccinated with tumor B cells expressing the mutable vaccine produce anti-ENV antibodies. The levels of anti-ENV antibodies before and after vaccination were measured using a capture ELISA and displayed as absorbance (y-axis) versus dilution (x-axis). Gray indicates post-immune sera and black pre-immune sera. The graph is representative of five independent experiments.
We next asked whether the mutable vaccine can generate ‘protective’ cellular immunity. To address that question, we introduced the B cells expressing the vaccine, ‘vaccinated’ cells or B cells not expressing the vaccine, ‘unvaccinated’ controls into syngeneic mice, asking whether cellular immunity slows tumor growth. We reasoned that since these tumor cells support somatic hypermutation of Ig endogenous genes at rates similar to the rate of mutation of mutable viruses and since the tumor cells at baseline grow and kill syngeneic mice, protection conferred by a vaccine might offer a first, if still remote, glimpse at potential efficacy, independent of antibody responses [26]. As Figure 5 shows, mice given B cells without the vaccine rapidly developed tumors and died whereas mice given B cells with the vaccine exhibited significantly slower formation of tumors (p < 0.0001) (Figure 5A) and 25% of the vaccinated mice survived indefinitely (Figure 5B). The protection afforded by the vaccine depended on adaptive immunity as vaccinated immunodeficient (RAG-2 deficient, γc-/-) mice developed tumors as rapidly as unvaccinated mice (Figure 5B & C). Thus, the mutable vaccine prototype rapidly generates a robust protective immune response. We also found that JH-/- κ-/- mice given vaccinated B cells survived twice as long as JH-/- κ-/- mice given control B cells, supporting the concept that the mutable vaccine generates cell-mediated immunity (Figure 5D).
Figure 5. . The mutable vaccine generates protective immunity.
Immune-competent syngeneic (A) or RAG-/- γc-/- mice (B) were injected with 2 × 107 tumor B cells expressing (+HIV-ENV) or not expressing the mutable vaccine (-HIV-ENV) in the peritoneum and tumor volume (in mm3) was determined on day 10. Figure 5C–E depict survival of immune-competent syngeneic (C), RAG-/- γc-/- mice (E) or B-cell-deficient mice (JH-/- κ -/-) following injection with 2 × 107 tumor B cells expressing (+HIV-ENV) or not expressing the mutable vaccine (-HIV-ENV) in the peritoneum.
We do not as yet know whether an immune response that effectively slows tumor growth also can effectively eradicate or slow the evolution of a virus. Nor do we know to what extent protective immunity would extend to variants of the immunizing antigen. However, we have performed preliminary work indicating that activation of B cells, as might be induced by vaccination or by infection, increases expression of env mRNA and production of protein in B cells (not shown).
Discussion
We have developed and here describe an entirely novel vaccine, the mutable vaccine that might be of value in addressing the challenges posed by mutable organisms, such as HIV. Although mutable organisms like HIV can be highly immunogenic, classical approaches to vaccination, typically optimizing the selection of antigen(s) and manner of co-stimulation and regimen of delivery, to generate anticipatory protective immunity, have always failed [1,16,28,30]. Instead of taking a classical approach, we exploited key aspects of virus–host interaction in fashioning a dynamic, mutable vaccine we hope might prove of value where classical vaccines fail.
Novel aspects of the mutable vaccine include the cellular target, the rapid generation of antigenic variants and the potential for evolving the immunogen in anticipation of or in parallel with a mutating organism. Thus, B cells targeted by the mutable vaccine foster ‘hypermutation’ of encoded antigen(s), thrive in conjunction with mutation and recombination and present and/or secrete antigens. Hypermutation of the vaccine appropriates the enzymatic apparatus of B cells, in part via the same pathways used by mutable viruses. Indeed, as our preliminary work suggests, mutation of the vaccine can potentially generate HIV Env variants that would emerge during chronic infection with HIV. We have also found that C3d encoded in the vaccine can amplify protective cell-mediated immune responses to tumor antigens in target cells independent of responses to antigens encoded in the construct [42]. We do not assume the vaccine will anticipate all or even most antigenic variants that would be generated after infection occurs. Rather, the vaccine would hopefully anticipate enough variants either to eradicate the virus before extensive replication occurs or to slow viral evolution enough to allow immunity to recognize and control those variants that ‘escape’ representation by the vaccine. It is also possible that enhanced cellular immunity conferred by C3d [42] could slow viral replication and hence evolution independent of the particular variants produced.
Given the diversity of HIV in the community and the reliance of the vaccine (much like the virus) on random errors, we think it unlikely that even a fully optimized mutable vaccine could anticipate and prevent infection by all viral variants in the community. However, when infection does occur, we speculate the dynamic function of the vaccine might add significantly to resistance and viral clearance. We recently devised and will soon report a new approach for specific delivery of DNA constructs to B cells and for achieving persistent expression of encoded protein for many months. Effective application of the mutable vaccine might allow vaccine elements to persist to the time of infection when virus-induced inflammation might drive increased diversification and production of antigen. Persistent expression might also facilitate the generation of broadly neutralizing antibodies, which otherwise are not detected until months or years after infection, if at all.
Efficacy of the mutable vaccine might not require expression of and immunization against all viral variants. Because HIV and other mutable viruses are immunogenic, we reason that ongoing targeting of a small fraction of viral variants expressed by the vaccine might slow viral evolution (by directly eliminating some variants and by diverting immunity decreasing selection pressure) increasing the impact of immunity evoked by the virus itself.
We do not presume to know which mutable virus(es) and which viral antigens could be optimally targeted and whether obstacles yet unknown will slow progress. However, we communicate the concept and preliminary experience with the mutable vaccine hoping to encourage the design and pursuit of nonconventional efforts to address the vexing problem mutable viruses present.
Conclusions
The mutable vaccine represents an entirely new approach to the generation protective immunity against mutable viruses. Instead of relying on a fixed antigen, the mutable vaccine delivered to B cells provides an antigen that changes over time, potentially anticipating changes in the virus. This property and responsiveness of the vaccine to inflammation might circumvent or efface viral evolution. The fundamental principles underlying the mutable vaccine have been tested, but whether and to which extent efficacy can be achieved remains to be determined.
Future perspective
Mutable viruses are usually not eradicated by protective immunity, in part because viral evolution outstrips protective immunity. Conventional vaccines, including vaccines with multiple antigens while potentially effective against parental clades, ultimately fail unless a mutable virus is immediately and completely eradicated. We suspect that a successful strategy for prophylaxis against mutable viruses and possibly for treatment will exploit the genetic instability of the viruses, at least in part by attacking viral evolution. The mutable vaccine was designed with such a strategy in mind. The most immediate challenge to adopting this strategy is the designing of systems for testing efficacy, since conventional standards, e.g. protective immunity against the parental virus strain, will fail to ascertain the unique properties of the approach.
Executive summary.
Mutable viruses, such as HIV, pose a daunting hurdle to effective control by vaccination. The hurdle reflects in part the considerable diversity of viruses in the community and in part the rapid diversification that occurs after infection.
Conventional vaccines might generate immunity to some strains in the community but ultimately fail diversification and selection after infection allows the virus to escape immune control.
The mutable vaccine described in this communication takes a novel approach. The vaccine exploits unique biological properties of B cells including the ability to support “hypermutation” of Ig genes, the responsiveness to inflammation, and capacity to produce and secrete proteins and to present protein fragments to T cells.
The mutable vaccine consists of a DNA construct that encodes one or more viral antigens and C3d, which promotes B cell and T cell responses to antigen. After introduction into B cells, the antigen mutates rapidly, potentially recapitulating and hence anticipating viral variants found in infected individuals. A model for subverting viral evolution based on full or partial anticipation of new viral variants is proposed.
The mutable vaccine is immunogenic but whether it will prevent or control a mutable virus remains to be tested.
Footnotes
Financial & competing interests disclosure
The work described in this manuscript was supported in part by grants from the National Institutes of Health (AI061100) and from the Bill and Melinda Gates Foundation (52090). M Cascalho and JL Platt hold a patent for the mutable vaccine, as cited in the manuscript and a provisional patent (62/328,131) for C3d cellular and acellular vaccines for prevention and treatment of cancer. No other conflicts of interest are pertinent to this communication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Stephenson KE, D'couto HT, Barouch DH. New concepts in HIV-1 vaccine development. Curr. Opin. Immunol. 2016;41:39–46. doi: 10.1016/j.coi.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reitz MS, Jr, Wilson C, Naugle C, Gallo RC, Robert-Guroff M. Generation of a neutralization-resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell. 1988;54(1):57–63. doi: 10.1016/0092-8674(88)90179-1. [DOI] [PubMed] [Google Scholar]
- 3.Narayan O, Clements JE, Griffin DE, Wolinsky JS. Neutralizing antibody spectrum determines the antigenic profiles of emerging mutants of visna virus. Infect. Immun. 1981;32(3):1045–1050. doi: 10.1128/iai.32.3.1045-1050.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes BF. New approaches to HIV vaccine development. Curr. Opin. Immunol. 2015;35:39–47. doi: 10.1016/j.coi.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escolano A, Dosenovic P, Nussenzweig MC. Progress toward active or passive HIV-1 vaccination. J. Exp. Med. 2017;214(1):3–16. doi: 10.1084/jem.20161765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada S, Yoshimura K. Driving HIV-1 into a vulnerable corner by taking advantage of viral adaptation and evolution. Front. Microbiol. 2017;8:390. doi: 10.3389/fmicb.2017.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuevas JM, Geller R, Garijo R, Lopez-Aldeguer J, Sanjuan R. Extremely high mutation rate of HIV-1 in vivo . PLoS Biol. 2015;13(9):e1002251. doi: 10.1371/journal.pbio.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker BD, Korber BT. Immune control of HIV: the obstacles of HLA and viral diversity. Nat. Immunol. 2001;2(6):473–475. doi: 10.1038/88656. [DOI] [PubMed] [Google Scholar]
- 9.Kourtis AP, Ibegbu C, Nahmias AJ, et al. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 1996;335(19):1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 10.Williamson S. Adaptation in the env gene of HIV-1 and evolutionary theories of disease progression. Mol. Biol. Evol. 2003;20(8):1318–1325. doi: 10.1093/molbev/msg144. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 1995;332(4):201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 12.Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. Nature Rev. Molec. Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 13.Platt JL, Zhou X, Lefferts AR, Cascalho M. Cell fusion in the war on cancer: a perspective on the inception of malignancy. Int. J. Mol. Sci. 2016;17(7) doi: 10.3390/ijms17071118. pii: E1118. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankarappa R, Margolick JB, Gange SJ, et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 1999;73(12):10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowland-Jones S, Pinheiro S, Kaul R. New insights into host factors in HIV-1 pathogenesis. Cell. 2001;104(4):473–476. doi: 10.1016/s0092-8674(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 16.Bonsignori M, Liao HX, Gao F, et al. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol. Rev. 2017;275(1):145–160. doi: 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs A, Connors M. HIV-1 and immune control: can we change the course of HIV-1? Lancet. 2004;363(9412):833–834. doi: 10.1016/S0140-6736(04)15770-X. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407(6803):523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 19.Ye P, Kirschner DE, Kourtis AP. The thymus during HIV disease: role in pathogenesis and in immune recovery. Curr. HIV Res. 2004;2(2):177–183. doi: 10.2174/1570162043484898. [DOI] [PubMed] [Google Scholar]
- 20.Bernardin F, Kong D, Peddada L, Baxter-Lowe LA, Delwart E. Human immunodeficiency virus mutations during the first month of infection are preferentially found in known cytotoxic T-lymphocyte epitopes. J. Virol. 2005;79(17):11523–11528. doi: 10.1128/JVI.79.17.11523-11528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009;206(6):1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 2009;206(6):1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson NA, Reed J, Napoe GS, et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 2006;80(12):5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mcmichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 2010;10(1):11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J. Immunol. 2004;172(9):5143–5148. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 26.Cascalho MI, Chen BJ, Kain M, Platt JL. The paradoxical functions of B cells and antibodies in transplantation. J. Immunol. 2013;190(3):875–879. doi: 10.4049/jimmunol.1100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimata JT, Rice AP, Wang J. Challenges and strategies for the eradication of the HIV reservoir. Curr. Opin. Immunol. 2016;42:65–70. doi: 10.1016/j.coi.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierson T, Mcarthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Ann. Rev. Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 29.Maldarelli F. The role of HIV integration in viral persistence: no more whistling past the proviral graveyard. J. Clin. Invest. 2016;126(2):438–447. doi: 10.1172/JCI80564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boritz EA, Darko S, Swaszek L, et al. Multiple origins of virus persistence during natural control of HIV infection. Cell. 2016;166(4):1004–1015. doi: 10.1016/j.cell.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cascalho MI, Platt JL. 2010. US7776321 B2.
- 32.Cascalho M. Advantages and disadvantages of cytidine deamination. J. Immunol. 2004;172:6513–6518. doi: 10.4049/jimmunol.172.11.6513. [DOI] [PubMed] [Google Scholar]
- 33.Cascalho M, Wong J, Steinberg C, Wabl M. Mismatch repair co-opted by hypermutation. Science. 1998;279(5354):1207–1210. doi: 10.1126/science.279.5354.1207. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424(6944):94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424(6944):99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 36.Janini M, Rogers M, Birx DR, Mccutchan FE. Human immunodeficiency virus type 1 DNA sequences genetically damaged by hypermutation are often abundant in patient peripheral blood mononuclear cells and may be generated during near-simultaneous infection and activation of CD4(+) T cells. J. Virol. 2001;75(17):7973–7986. doi: 10.1128/JVI.75.17.7973-7986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ball JK, Holmes EC, Whitwell H, Desselberger U. Genomic variation of human immunodeficiency virus type 1 (HIV-1): molecular analyses of HIV-1 in sequential blood samples and various organs obtained at autopsy. J. Gen. Virol. 1994;75(Pt 4):67–79. doi: 10.1099/0022-1317-75-4-867. [DOI] [PubMed] [Google Scholar]
- 38.Mansky LM, Temin HM. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 1995;69(8):5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 40.Balin SJ, Ross TM, Platt JL, Cascalho M. HIV genes diversify in B cells. Current HIV Res. 2008;6:10–18. doi: 10.2174/157016208783571919. [DOI] [PubMed] [Google Scholar]
- 41.Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat. Immunol. 2000;1(2):127–131. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt JL, Silva I, Balin SJ, et al. C3d regulates immune checkpoint blockade and enhances antitumor immunity. JCI Insight. 2017 doi: 10.1172/jci.insight.90201. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wabl M, Burrows PD, Von Gabain A, Steinberg C. Hypermutation at the immunoglobulin heavy chain locus in a pre-B-cell line. Proc. Natl Acad. Sci. USA. 1985;82(2):479–482. doi: 10.1073/pnas.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balin SJ, Cascalho M. The rate of mutation of a single gene. Nucleic Acids Res. 2010;38(5):1575–1582. doi: 10.1093/nar/gkp1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Salazar-Gonzalez JF, Derdeyn CA, et al. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 2006;80(23):11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert PB, Peterson ML, Follmann D, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a Phase III HIV-1 preventive vaccine trial. J. Infect. Dis. 2005;191(5):666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]