Abstract
Obesity is associated with an increased risk of a number of serious medical conditions, including cancer. As far as prostate cancer is concerned, obesity is associated with an increased risk of high-grade tumors, which is possibly related to lower androgen levels. Diet may also affect prostate cancer risk since countries with a higher dietary fat intake also present higher prostate cancer mortality rates. Interestingly, prostate cancer is associated with a number of metabolic alterations that may provide valuable diagnostic and therapeutic targets. This review explores the available clinical as well as biological evidence supporting the relationship between obesity, diet, alteration in metabolic pathways and prostate cancer.
KEYWORDS : diet, obesity, urologic/prostate
Overweight and obesity are respectively defined as a BMI of 25–29 kg/m2, and of greater than 30 kg/m2, and are characterized by an abnormal/excessive fat deposits in the body [1]. As a result of their increasing prevalence, which rose by 47% in children and by 28% in adults since 1980–2013, currently an estimated 2.1 billion people are either overweight or obese worldwide and, by the year 2030, 58% of the world’s adult population is expected to be overweight or obese [1]. Not only is obesity associated with an increased risk of a variety of chronic medical conditions including hypertension, coronary artery disease, diabetes, musculoskeletal disorders (especially osteoarthritis), gastroesophageal reflux disease and obstructive sleep apnea syndrome, but it also increases the risk of cancer [2–4]. In particular, obese individuals are more frequently diagnosed with cancer [5], and also have a worse cancer-specific survival with respect to nonobese controls. The complex biological mechanisms underlying the association between cancer and obesity include a number of different factors, such as the increased secretion of steroid hormones, chronic high insulin levels, insulin resistance and a persistent inflammatory state [6,7]. Prostate cancer is a highly prevalent tumor, accounting for approximately 25% of all malignancies diagnosed in men in the USA [8]. Treatment for localized as well as advanced disease is rapidly evolving [9,10]. Early detection of prostate cancer can be achieved in older asymptomatic men by the use of prostate-specific antigen (PSA)-based screening, which is associated with a substantial risk of overdiagnosis and overtreatment. The low diagnostic accuracy of PSA may be improved by the incorporation of clinical risk factors, biological markers and radiological assessment into screening procedures [11–17]. Obesity is an established risk factor of prostate cancer, since it is associated with an increased incidence of high-risk or aggressive prostate cancer [18] and an increased incidence of disease relapse [19]. A higher BMI has also been associated with increased risk of upstaging and upgrading in male candidates for active surveillance [20]. Intake of dietary fats, which is related to obesity [21], may be an independent risk factor [22]. In addition to being a risk factor in prostate cancer patients, obesity may also be a consequence of the androgen deprivation therapy administered to patients with locally advanced or metastatic prostate cancer [23]. Changes in body composition can affect the quality of life in patients with prostate cancer and increase the risk of cardiovascular diseases [24]. The interplay between obesity and prostate cancer has profound consequences from an epidemiologic, social or clinical point of view. We here review the clinical as well as preclinical evidence regarding the association between obesity and prostate cancer, with a focus on the potential role of dietary factors.
Obesity & prostate cancer
• Clinical evidence
A large body of evidence suggests that the BMI does not only influence PSA levels in healthy individuals, but it also affects the risk of being diagnosed with high-grade tumors, which has important prognostic implications. In a large prospective cohort [25] of 15,827 men without prostate cancer assessed for both serum PSA levels and BMI at baseline during the years 2010–2012 and followed up until 2015, 735 men were diagnosed with prostate cancer and 282 patients (38.4%) presented high-grade cancers. An inverse relationship between BMI and serum PSA levels was reported, with PSA levels decreasing by 1.6% (95% CI: -2.1 to -1.1) for each one unit increase in BMI. With respect to the reference (BMI: 18.5 to <25 kg/m2), men having a BMI of 25 to <30, 30 to <35 and ≥35 kg/m2, respectively, showed decreases of 3.7, 11.7 and 32.3% of PSA levels. A decreased risk of low-grade prostate cancer in men with a BMI of 30 to <35 kg/m2 or >35 kg/m2 along with an increased risk of high-grade prostate cancer among men with a BMI of 30 to <35 kg/m2 was also reported [25]. These findings are consistent with the results obtained in a meta-analysis of 12 studies including 19,130 cases of localized prostate cancer in a sample of 1,033,009 men and of 13 studies including 7067 cases of advanced prostate cancer in a sample of 1,080,790 men. A higher BMI was associated with a decreased risk of localized prostate cancer (relative risk [RR]: 0.94 [95% CI: 0.91–0.97] for every 5 kg/m2 increase) and with an increased risk of advanced prostate cancer (RR: 1.09 [95% CI: 1.02–1.16] for every 5 kg/m2 increase) [18]. Several additional cohort studies indicated that prostate cancer was associated with a higher BMI [26–28]. Although other studies failed to do so [29–31], one meta-analysis [23] including 55,521 cases reported among 2,818,767 men enrolled in 31 studies, and 13,232 cases and 16,317 controls enrolled in 25 case–control studies showed an overall RR of prostate cancer of 1.05 (95% CI: 1.01–1.08) per 5 kg/m2 BMI increment, with a significant RR of 1.12 per 5 kg/m2 increment (95% CI: 1.01–1.23) for advanced disease compared with a nonsignificant RR of 0.96 per 5 kg/m2 increment (95% CI: 0.89–1.03) for localized disease. Other studies have highlighted the positive and inverse relationship of BMI with high- and low-grade tumors, respectively [24–26]. Of note, in the Prostate Cancer Prevention trial [25], obese men (BMI: ≥30 kg/m2) presented an 18% lower risk of low-grade prostate cancer, but also a 78% increased risk of high-grade tumors compared with men who had a BMI of <25 kg/m2. In men who have been diagnosed with prostate cancer, a higher BMI has a detrimental prognostic effect, as shown in a retrospective study including 3161 prostate cancer patients followed up for 11 years [32]. In this patient cohort, a BMI of ≥27.5 kg/m2 and also a BMI of <22.5 kg/m2 were associated with an increased risk of dying of prostate cancer (hazard ratio [HR]: 1.44; 95% CI: 1.09–1.90 and HR: 1.33; 95% CI: 1.02–1.74, respectively) compared with the reference group (BMI: 22.5 to <25 kg/m2). Nevertheless, if patients dying within the first 2 years of follow-up were not included in the analyses, the excess of risk of dying of prostate cancer remained statistically significant in men with a BMI of ≥27.5 kg/m2, but not in men with a BMI of <22.5 kg/m2. In this regard, it is noteworthy that lower serum testosterone levels are both associated with a higher BMI [33] and with a detrimental prognostic effect in patients with advanced disease [34]. In a retrospective analysis of patients with advanced prostate cancer enrolled in the COU-AA-301 trial testing abiraterone plus prednisone versus prednisone alone, patients with higher baseline androgen levels showed a more favorable outcome, with median survival significantly increasing with each quartile increase in testosterone level in both treatment arms [34]. Nevertheless, the detrimental effects of obesity may be only partially dependent on its effect on androgen levels, as prostate cancer incidence does not appear to be related to endogenous testosterone levels [35]. In fact, apart from the fact that lower androgen levels in obese men may contribute to select more aggressive androgen-independent clones [32,36], obesity may also make early diagnosis more difficult due to its association with lower PSA levels and larger prostates. The mechanisms underlying the relationship of obesity with prostate cancer are yet to be fully elucidated.
• Biological mechanisms
Systemic inflammation, alterations in the insulin and IGF-1 axis, and variations in sex hormone levels and adipokines are among the underlying mechanisms of the relationship between obesity and prostate cancer. In particular, hyperinsulinemia was reported to accelerate tumor growth in different prostate cancer xenograft models [37,38] and primary human prostate cancer commonly expresses the insulin receptor [39], suggesting that insulin may stimulate human prostate cancer growth. Population studies have also suggested an association between the blood levels of IGF-1 and IGFBPs, and the risk of prostate cancer [40]. As mentioned before, obesity is also associated with decreased androgen levels [41], which may cause the conditions for the selection of neoplastic clones less dependent on androgens, with a shorter duration of the castration sensitivity state and worse prognosis [42]. Furthermore, obesity itself is associated with a chronic inflammatory condition that results in alteration of serum levels of leptin and adipokines. Although leptin exerts a predominantly protumor effect in human androgen-independent PC-3 and DU145 prostate cancer cell lines, with increased proliferation, decreased apoptosis [43–45] and increased migration [46], adiponectin exerts a potent antitumor effect. Although serum leptin levels are elevated in obese individuals, adiponectin levels were found to be reduced significantly in metastatic prostate cancer patients versus those with organ-confined disease [47]. Cytokines, such as IL-6, TNF-α and VEGF, may also play a role, since their serum levels are reported to be increased in prostate cancer versus controls and in patients with advanced versus localized prostate cancer [48–50]. Obesity may also mediate a more aggressive phenotype in certain cancers via a paracrine effect. Adipocytes are recognized to be part of the tumor microenvironment in a variety of cancers. The term ‘cancer-associated adipocytes’ has been used to describe the abnormal adipocytes adjacent to cancer cells. These adipocytes show lower lipid content, decreased levels of markers (e.g., hormone-sensitive lipase, adiponectin and resistin) of adipocyte differentiation and overexpression of several inflammatory cytokines (e.g., IL-6 and IL-1β). Peritumoral adipocytes and tumor cells present intersecting signaling pathways. In one study assessing prostate cancer specimens, the infiltration of adipocytes was associated with cancer grade, and 90 versus 20% of high- versus low-grade specimens showed infiltrative fat [51]. Cancer-associated adipocytes may also act as an energy source for tumor cells [52]. Obesity increases the odds that the tumor invades the periprostatic adipose tissue (PPAT) surrounding the prostate gland, and extraprostatic extension is a widely acknowledged adverse factor in prostate cancer [53]. The prostate gland is surrounded by PPAT, which represents an active endocrine organ and an energy source. The existing correlation between the abundance of PPAT and tumor aggressiveness suggests a paracrine role during tumorigenesis [54]. Mature adipocytes are able to support prostate cancer cell malignant phenotype. Recently, Laurent et al. suggested that the chemokine CCL7 released by adipocyte of PPAT increased migration of prostate cancer cells [55]. Interestingly, Ribeiro et al. found that prostate cancer-released factors can regulate the expression of protumorigenic adipokines (osteopontin, TNF-α, IL-6 and adiponectin), matrix metalloproteinase activity and mitochondrial DNA in PPAT [56]. A role for diet-induced obesity in promoting prostate tumor growth has also been documented in the transgenic adenocarcinoma of the prostate mouse model [57].
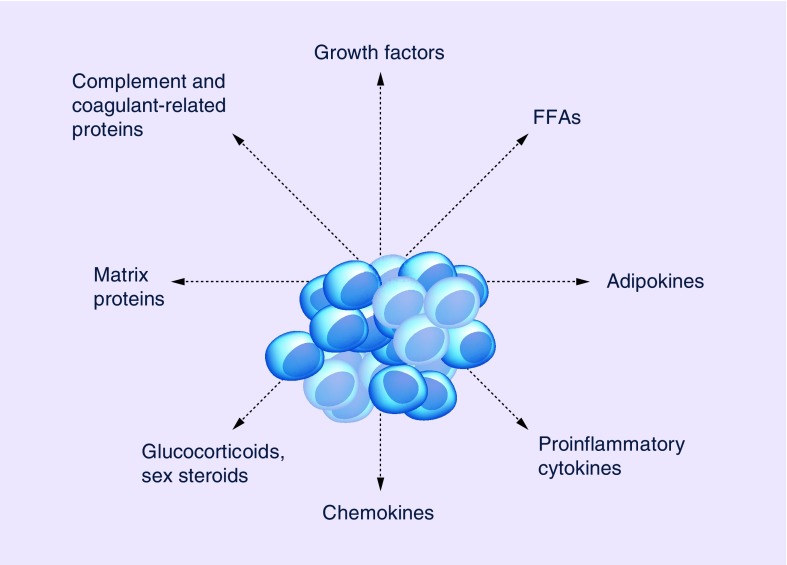
The evidence reviewed shows that multiple biological mechanisms underlie the association of obesity with prostate cancer (see Figure 1), which supports epidemiological and clinical findings.
Figure 1. . Adipocyte secreted factors with a role in prostate cancer development.
FFA: Free fatty acid.
Diet & prostate cancer
• Clinical evidence
Dietary factors that may be associated with an increased risk of both obesity and prostate cancer include positive energy balance, processed and unprocessed meat, saturated fat, trans fatty acid intake and total dietary fat intake [22,58–59]. Conversely, n-3 fatty acids may have a potentially protective effect against prostate cancer [60]. A pooled analysis of 45 observational studies showed no evidence of an association between dairy (RR: 1.06; 95% CI: 0.92–1.22), milk (RR: 1.06; 95% CI: 0.91–1.23), calcium (RR: 1.04; 95% CI: 0.90–1.15) and vitamin D (RR: 1.16; 95% CI: 0.98–1.38) intake and prostate cancer risk [61]. Of note, higher serum vitamin D levels have been associated not only with increased incidence of prostate cancer, but also with a decreased mortality in prostate cancer patients [62]. Evidence supporting the role of nutritional habits as a risk factor for prostate cancer includes the observation that countries with a higher dietary fat intake also present higher prostate cancer mortality rates [63]. In this regard, it is noteworthy that men migrating from Japan and China have an increased risk of prostate cancer, with second- and third-generation Chinese and Japanese Americans showing a similar risk to white American men [63]. Although migration studies suggest a positive correlation between changes in diet and prostate cancer risk, they also present a number of limitations, including the lack of quantification of dietary fat and the lack of appropriate controls for confounders. Prospective cohort studies do not suffer from such limitations. In one recently published meta-analysis of 19 different prospective cohort studies [59], no association of prostate cancer with total red meat consumption, fresh red meat consumption and measures of exposure to heterocyclic amine and heme iron was identified. Conversely, the standardized RR estimate for total prostate cancer and processed meat consumption was 1.05 (95% CI: 1.01–1.10). In another report including 52,683 prostate cancer patients with 4924 cases of advanced disease diagnosed in a cohort of 842,149 men, no significant association between seafood, total red meat, unprocessed red meat and processed meat was identified for all prostate cancer outcomes, although men in the highest red meat and processed meat intake subgroups presented a 17–19% increased risk of being diagnosed at an advanced stage than those in the lowest category [58]. Of note, an inverse association was identified for poultry intake, with a pooled RR for advanced and fatal cancers of men consuming ≥45 versus <5 g/day of 0.83 (95% CI: 0.70–0.99) and 0.69 (95% CI: 0.59–0.82), respectively. Furthermore, men who ate ≥25 versus <5 g/day of eggs presented a significant 14% increased risk of fatal and advanced cancers. It is also noteworthy that in this international cohort, positive associations of advanced and fatal cancers with egg and unprocessed red meat consumption, and inverse associations with poultry intake were limited to North American studies only. The relationship of dietary fat intake with prostate cancer mortality was explored in a prospective study [64] examining trans, animal, saturated, monounsaturated, polyunsaturated and vegetable intake in 4577 nonmetastatic prostate cancer patients enrolled in the Health Professionals Follow-up Study during the years 1986–2010. Crude rates per 1000 person-years for lethal prostate cancer in men in the lowest versus highest quartile of fat intake: 7.3 versus 7.6 for saturated fat; 6.4 versus 7.2 for monounsaturated fat; 5.8 versus 8.2 for polyunsaturated fat; 8.7 versus 6.1 for trans fat; 8.3 versus 5.7 for animal fat; and 4.7 versus 8.7 for vegetable fat. Of note, this study showed that using vegetable fat in order to replace 10% of energy intake from carbohydrate lowered the risk of lethal prostate cancer (HR: 0.71; 95% CI: 0.51–0.98; p = 0.04) and all-cause mortality (HR: 0.74; 95% CI: 0.61–0.88; p = 0.001). In another a prospective study [65] including 926 patients with nonmetastatic prostate cancer who were followed up for a median of 10 years after the completion of a dietary questionnaire, it was found that men who derived >5 and <5% of their daily caloric intake from saturated fat and from carbohydrate, respectively, presented a 1.8-fold increased risk of all-cause mortality (HR: 1.81; 95% CI: 1.20–2.74; p = 0.005) and a 2.8-fold increased risk of prostate cancer-specific mortality (HR: 2.78; 95% CI: 1.01–7.64; p = 0.05). In conclusion, although no strong association of a particular food or food class with prostate cancer has been reported, the reviewed studies indicate that saturated fat may increase the risk of prostate cancer-related death, while vegetable fat may exert a protective effect after a diagnosis of nonmetastatic prostate cancer.
• Biological mechanisms
The increased local and/or systemic flux of fatty acids derived from dietary intake may affect prostate cancer cell malignant phenotype. Fatty acids provide both energy and signaling molecules implicated in a number of pathologic and physiologic cellular processes. Several G-protein-coupled receptors (GPRs) have been identified as fatty acid sensors with nutrient-sensing capabilities by endocrine cells [66,67]. Moreover, free fatty acids bind to nuclear PPAR-γ regulating the expression of genes involved in glucose and lipids metabolism [68]. In cancer cells, fatty acids are essential to support cell growth, proliferation, differentiation and motility [69–72]. Hardy et al. [73] reported that oleate is able to promote breast cancer cells growth by activating Ca2+ signaling, Src proteins and PI3K/Akt via interaction with GPR40. Furthermore, Liu et al. [74] reported that oleate-mediated GPR40/ILK/Akt pathway activation is associated with the development of renal cell carcinoma. Prostate cancer is also highly dependent on fatty acid metabolism [69,75]. In fact, prostate cancer is a slowly proliferating tumor and the rates of glucose uptake and glycolysis are relatively low. This is due, at least in part, to the low expression of the primary glucose transporter GLUT1 [76]. Hagen et al. [77] showed different responses to fatty acid treatment in different prostate cancer cell lines. Yue et al. [78] revealed an accumulation of cholesterol ester-rich lipid droplets (LDs) in high-grade and metastatic prostate cancers. Interestingly, cholesterol esters accumulation correlated with androgen-independence and phosphate and tensin homolog loss. Moreover, the presence of cholesterol ester-rich lipid droplets seems to support the migratory and invasive capacities of prostate cancer cells and potentiates PI3K-dependent SREBP activity, which may enhance cancer aggressiveness.
Lipid metabolism in prostate cancer
During neoplastic progression, prostate cancer cells undergo adaptive metabolic changes in order to sustain their growth and proliferation [79]. The increased lipid biosynthesis required for cellular proliferation, membrane formation and cell signaling represents a critical event in metabolic reprogramming. An increased expression and activity of choline kinase, an enzyme involved in cell membrane phospholipids biosynthesis, has also been reported in prostate cancer, along with high levels of phosphatidylcholine, phosphatidylethanolamine and glycerophosphocholine. These findings are consistent with active membrane remodeling and cellular proliferation processes.
In addition, metabolic intermediates of de novo lipogenesis, including diacylglycerol, sphingosine 1-phosphate, phosphatidic acid and lysophosphatidic acid, act as second messengers in different signaling pathways regulating cell-to-cell communication, migration and invasion. De novo lipogenesis and cholesterogenesis are associated with the lipogenic phenotype of prostate cancer and are sustained by the conversion in the cytosol of citrate (derived from Krebs cycle in the mitochondria) to acetyl-CoA and oxaloacetate by ATP citrate lyase (ACLY). Acetyl-CoA is then transformed in malonyl-CoA by the enzyme acetyl-CoA carboxylase (ACC), and oxaloacetate is converted into pyruvate, which can re-enter the mitochondria for further utilization. Along with the NADPH generated in the pentose phosphate pathway, the NADPH synthesized by this pathway is able to provide the reducing equivalents required for reductive synthesis of fatty acids. Saturated fatty acids may then undergo other molecular modifications such as insertion of double bounds or elongation of the carbon chain, and can be used for energy, membrane integrity and cell signaling. In normal conditions, human cells preferentially use exogenous lipids for their metabolic requirements, whereas de novo synthesis is usually maintained at low levels. Instead, in cancer cells de novo lipogenesis and cholesterogenesis are highly activated, and in accordance with these findings, it has been demonstrated an increased expression of ACLY, ACC and fatty acid synthase (FAS) in prostate cancer cells [80,81]. In this context, it has been reported that SREBP-1 – a critical transcription factor for lipogenesis – is involved in the transcriptional regulation of androgen receptor and formation of fatty acids through an altered expression of ACLY, ACC and FAS. In addition, Huang et al. showed that SREBP-1-induced prostate cancer cell proliferation, migration and invasion by activating lipogenesis and through an increased expression of NADPH oxidase 5 [46]. Lipid metabolism is regulated in cancer cells by oncogenic signals. The LKB1–AMPK pathway acts as a cellular energy status sensor that protects cells from stresses caused by ATP depletion, thus deactivating ATP-consuming biosynthetic pathways. In fact, the activation of LKB1–AMPK axis blocks lipid metabolism via inhibition of ACC, FAS, ACLY and SREBP-1. In this scenario, it has recently been shown that loss of LKB1 expression is an early event in prostate cancer carcinogenesis and that an inverse correlation exists between the activity of the LKB1–AMPK pathway and the p38 MAPK cascade [82]. Moreover, the pivotal role of the LKB1–AMPK axis in controlling oncogenic pathways and cell metabolism is also due to its crosstalk with the PI3K–Akt, mTOR and MAPK pathways [83]. In particular, PI3K–Akt–mTORC1 pathway, which is activated in around 40% of primary and over 70% of metastatic prostate cancer, stimulates glucose uptake and promote glycolysis, providing more precursors for lipogenesis [84].
Consistently with such epidemiologic associations, cholesterol has been reported to stimulate prostate cancer growth both in vitro and in vivo [85]. Furthermore, reduced serum cholesterol levels were associated with lower intratumoral androgen levels and impaired tumor growth in xenograft mouse models of human prostate cancer [86], which underlines the role of steroid biosynthesis as an important biological mechanism linking cholesterol and prostate cancer.
Conclusion
In this review article, we aimed to explore currently available data about the relationship of prostate cancer incidence/biological aggressiveness with obesity, dietary factors and alteration in tumor lipid metabolism. Although the role of dietary factors remains uncertain, the preponderance of the evidence reviewed suggests that obesity does affect the risk of high-grade prostate cancer and has a detrimental effect on prostate cancer-specific mortality.
Future perspective
Interventions to tackle the obesity pandemic may be beneficial for primary prevention of prostate cancer. Increased attention toward proper diet and lifestyle should be given to patients undergoing androgen ablation treatment, due to the adverse metabolic effects of androgen deprivation. Diet modifications including increased consumption of vegetable fats should be considered in men undergoing radical treatment for localized prostate cancer, as such a simple and innocuous intervention may decrease the risk of disease recurrence. Finally, the rapidly expanding knowledge of the metabolic alterations involving lipid metabolism in prostate cancer cells has the potential to provide novel biological targets for prevention and treatment of prostate cancer.
EXECUTIVE SUMMARY.
Evidence obtained in large case–control studies suggests that obesity is associated with an increased risk of being diagnosed with high-grade prostate cancer and dying of the disease.
The increased risk of high-grade prostate cancer in obese men has a strong biological rationale, which includes systemic effects, such as the increased levels of IGF-1 and decreased levels of testosterone, as well as increased levels of inflammation and cytokines, and also local effects, such as larger prostates and periprostatic fat accumulation.
As far as dietary habits are concerned, saturated fat may increase the risk of prostate cancer-related death, while vegetable fat may exert a protective effect after a diagnosis of nonmetastatic prostate cancer.
Unlike other malignancies, prostate cancer is highly dependent on lipid metabolism, with free fatty acids being able to support cell growth, proliferation, differentiation and motility via binding to several biological targets, such as the nuclear PPAR-γ.
Prostate cancer cells present both intratumoral lipogenesis and steroidogenesis, which can provide druggable targets for prevention and treatment purposes.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Smith KB, Smith MS. Obesity statistics. Primary Care. 2016;43(1):121–135. doi: 10.1016/j.pop.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.De Sousa AG, Cercato C, Mancini MC, Halpern A. Obesity and obstructive sleep apnea–hypopnea syndrome. Obes Rev. 2008;9(4):340–354. doi: 10.1111/j.1467-789X.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 7.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J. Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lorenzo G, Buonerba C, Autorino R, De Placido S, Sternberg CN. Castration-resistant prostate cancer: current and emerging treatment strategies. Drugs. 2010;70(8):983–1000. doi: 10.2165/10898600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Buonerba C, Ferro M, Di Lorenzo G. Sipuleucel-T for prostate cancer: the immunotherapy era has commenced. Expert Rev. Anticancer Ther. 2011;11(1):25–28. doi: 10.1586/era.10.180. [DOI] [PubMed] [Google Scholar]
- 10.Buonerba C, Palmieri G, Di Lorenzo G. Docetaxel rechallenge in castration-resistant prostate cancer: scientific legitimacy of common clinical practice. Eur. Urol. 2010;58(4):636–637. doi: 10.1016/j.eururo.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Ferro M, Buonerba C, Terracciano D, et al. Biomarkers in localized prostate cancer. Future Oncol. 2016;12(3):399–411. doi: 10.2217/fon.15.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Cobelli O, Buonerba C, Terracciano D, et al. Urotensin II receptor on preoperative biopsy is associated with upstaging and upgrading in prostate cancer. Future Oncol. 2015;11(22):3091–3098. doi: 10.2217/fon.15.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferro M, Bruzzese D, Perdona S, et al. Prostate Health Index (Phi) and prostate cancer antigen 3 (PCA3) significantly improve prostate cancer detection at initial biopsy in a total PSA range of 2–10 ng/ml. PLoS ONE. 2013;8(7):e67687. doi: 10.1371/journal.pone.0067687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terracciano D, Bruzzese D, Ferro M, et al. Soluble interleukin-6 receptor to interleukin-6 (sIL6R/IL-6) ratio in serum as a predictor of high Gleason sum at radical prostatectomy. Oncol. Lett. 2011;2(5):861–864. doi: 10.3892/ol.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferro M, Bruzzese D, Perdona S, et al. Predicting prostate biopsy outcome: Prostate Health Index (PHI) and prostate cancer antigen 3 (PCA3) are useful biomarkers. Clin. Chim. Acta. 2012;413(15–16):1274–1278. doi: 10.1016/j.cca.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Perdona S, Di Lorenzo G, Autorino R, et al. Combined magnetic resonance spectroscopy and dynamic contrast-enhanced imaging for prostate cancer detection. Urol. Oncol. 2013;31(6):761–765. doi: 10.1016/j.urolonc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 17.De Cobelli O, Terracciano D, Tagliabue E, et al. Predicting pathological features at radical prostatectomy in patients with prostate cancer eligible for active surveillance by multiparametric magnetic resonance imaging. PLoS ONE. 2015;10(10):e0139696. doi: 10.1371/journal.pone.0139696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer – a dose–response meta-analysis of prospective studies. Ann. Oncol. 2012;23(7):1665–1671. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev. Res. (Phila.) 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Cobelli O, Terracciano D, Tagliabue E, et al. Body mass index was associated with upstaging and upgrading in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Urol. Oncol. 2015;33(5):201.e201–201.e208. doi: 10.1016/j.urolonc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998;68(6):1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 22.Di Sebastiano KM, Mourtzakis M. The role of dietary fat throughout the prostate cancer trajectory. Nutrients. 2014;6(12):6095–6109. doi: 10.3390/nu6126095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macinnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17(8):989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 24.Keating NL, O’malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J. Natl Cancer Inst. Monogr. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrated the association of cardiovascular disease and diabetes in a very large cohort of men.
- 25.Chavey C, Mari B, Monthouel MN, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J. Biol. Chem. 2003;278(14):11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 26.Putnam SD, Cerhan JR, Parker AS, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann. Epidemiol. 2000;10(6):361–369. doi: 10.1016/s1047-2797(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 27.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20 year follow-up study among 135006 Swedish construction workers. J. Natl Cancer Inst. Monogr. 1997;89(5):385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 28.Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br. J. Cancer. 2003;89(7):1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 1997;6(8):557–563. [PubMed] [Google Scholar]
- 30.Nilsen TI, Vatten LJ. Anthropometry and prostate cancer risk: a prospective study of 22,248 Norwegian men. Cancer Causes Control. 1999;10(4):269–275. doi: 10.1023/a:1008967330619. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard JS, Rohrmann S, Landis PK, et al. Association of prostate cancer risk with insulin, glucose, and anthropometry in the Baltimore longitudinal study of aging. Urology. 2004;63(2):253–258. doi: 10.1016/j.urology.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 32.Cantarutti A, Bonn SE, Adami HO, Gronberg H, Bellocco R, Balter K. Body mass index and mortality in men with prostate cancer. Prostate. 2015;75(11):1129–1136. doi: 10.1002/pros.23001. [DOI] [PubMed] [Google Scholar]
- 33.Blaya R, Thomaz LD, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. Aging Male. 2016 doi: 10.3109/13685538.2016.1154523. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Ryan CJ, Molina A, Li J, et al. Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: results from an analysis of a randomized Phase III trial. J. Clin. Oncol. 2013;31(22):2791–2798. doi: 10.1200/JCO.2012.45.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle P, Koechlin A, Bota M, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate specific antigen (PSA): a meta-analysis. BJU Int. 2016 doi: 10.1111/bju.13417. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Rescigno P, Buonerba C, Bellmunt J, Sonpavde G, De Placido S, Di Lorenzo G. New perspectives in the therapy of castration resistant prostate cancer. Curr. Drug Targets. 2012;13(13):1676–1686. doi: 10.2174/138945012803529956. [DOI] [PubMed] [Google Scholar]
- 37.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J. Natl Cancer Inst. Monogr. 2007;99(23):1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 38.Freedland SJ, Sun L, Kane CJ, et al. Obesity and oncological outcome after radical prostatectomy: impact of prostate-specific antigen-based prostate cancer screening: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. BJU Int. 2008;102(8):969–974. doi: 10.1111/j.1464-410X.2008.07934.x. [DOI] [PubMed] [Google Scholar]
- 39.Cox ME, Gleave ME, Zakikhani M, et al. Insulin receptor expression by human prostate cancers. Prostate. 2009;69(1):33–40. doi: 10.1002/pros.20852. [DOI] [PubMed] [Google Scholar]
- 40.Barnard RJ, Aronson WJ. Preclinical models relevant to diet, exercise, and cancer risk. Recent Results Cancer Res. 2005;166:47–61. doi: 10.1007/3-540-26980-0_4. [DOI] [PubMed] [Google Scholar]
- 41.Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol. Cell Endocrinol. 2012;351(2):269–278. doi: 10.1016/j.mce.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Flanagan J, Gray PK, Hahn N, et al. Presence of the metabolic syndrome is associated with shorter time to castration-resistant prostate cancer. Ann. Oncol. 2011;22(4):801–807. doi: 10.1093/annonc/mdq443. [DOI] [PubMed] [Google Scholar]
- 43.Somasundar P, Frankenberry KA, Skinner H, et al. Prostate cancer cell proliferation is influenced by leptin. J. Surg. Res. 2004;118(1):71–82. doi: 10.1016/j.jss.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Hoda MR, Popken G. Mitogenic and anti-apoptotic actions of adipocyte-derived hormone leptin in prostate cancer cells. BJU Int. 2008;102(3):383–388. doi: 10.1111/j.1464-410X.2008.07534.x. [DOI] [PubMed] [Google Scholar]
- 45.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J. Biol. Chem. 2003;278(43):42660–42667. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 46.Huang WC, Li X, Liu J, Lin J, Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol. Cancer Res. 2012;10(1):133–142. doi: 10.1158/1541-7786.MCR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65(6):1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 48.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58(6):1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 49.Shariat SF, Anwuri VA, Lamb DJ, Shah NV, Wheeler TM, Slawin KM. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J. Clin. Oncol. 2004;22(9):1655–1663. doi: 10.1200/JCO.2004.09.142. [DOI] [PubMed] [Google Scholar]
- 50.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br. J. Cancer. 2004;90(12):2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhindi B, Trottier G, Elharram M, et al. Measurement of peri-prostatic fat thickness using transrectal ultrasonography (TRUS): a new risk factor for prostate cancer. BJU Int. 2012;110(7):980–986. doi: 10.1111/j.1464-410X.2012.10957.x. [DOI] [PubMed] [Google Scholar]
- 52.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17(11):1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This model demonstrates that adipocytes play a role in promoting cancer metastasis.
- 53.Magi-Galluzzi C, Evans AJ, Delahunt B, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod. Pathol. 2011;24(1):26–38. doi: 10.1038/modpathol.2010.158. [DOI] [PubMed] [Google Scholar]
- 54.Van Roermund JG, Hinnen KA, Tolman CJ, et al. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011;107(11):1775–1779. doi: 10.1111/j.1464-410X.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 55.Laurent V, Guerard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ribeiro RJ, Monteiro CP, Cunha VF, et al. Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell Physiol. Biochem. 2012;29(1–2):233–240. doi: 10.1159/000337604. [DOI] [PubMed] [Google Scholar]
- 57.Llaverias G, Danilo C, Wang Y, et al. A western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am. J. Pathol. 2010;177(6):3180–3191. doi: 10.2353/ajpath.2010.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu K, Spiegelman D, Hou T, et al. Associations between unprocessed red and processed meat, poultry, seafood and egg intake and the risk of prostate cancer: a pooled analysis of 15 prospective cohort studies. Int. J. Cancer. 2016;138(10):2368–2382. doi: 10.1002/ijc.29973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bylsma LC, Alexander DD. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr. J. 2015;14:125. doi: 10.1186/s12937-015-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fradet V, Cheng I, Casey G, Witte JS. Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clin. Cancer Res. 2009;15(7):2559–2566. doi: 10.1158/1078-0432.CCR-08-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huncharek M, Muscat J, Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: a meta-analysis of 26,769 cases from 45 observational studies. Nutr. Cancer. 2008;60(4):421–441. doi: 10.1080/01635580801911779. [DOI] [PubMed] [Google Scholar]
- 62.Buschemeyer WC, 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur. Urol. 2007;52(2):331–343. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]; • Reviews the evidence about the association of obesity with prostate cancer.
- 63.Brawley OW, Knopf K, Thompson I. The epidemiology of prostate cancer part II: the risk factors. Semin. Urol. Oncol. 1998;16(4):193–201. [PubMed] [Google Scholar]
- 64.Richman EL, Kenfield SA, Chavarro JE, et al. Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA Intern. Med. 2013;173(14):1318–1326. doi: 10.1001/jamainternmed.2013.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Blarigan EL, Kenfield SA, Yang M, et al. Fat intake after prostate cancer diagnosis and mortality in the Physicians’ Health Study. Cancer Causes Control. 2015;26(8):1117–1126. doi: 10.1007/s10552-015-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The G-protein-coupled receptor 40 family (GPR40-GPR43) and its role in nutrient sensing. Biochem. Soc. Trans. 2006;34(Pt 5):770–773. doi: 10.1042/BST0340770. [DOI] [PubMed] [Google Scholar]
- 67.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 68.Marion-Letellier R, Savoye G, Ghosh S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016;785:44–49. doi: 10.1016/j.ejphar.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Zadra G, Photopoulos C, Loda M. The fat side of prostate cancer. Biochim. Biophys. Acta. 2013;1831(10):1518–1532. doi: 10.1016/j.bbalip.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zadra G, Priolo C, Patnaik A, Loda M. New strategies in prostate cancer: targeting lipogenic pathways and the energy sensor AMPK. Clin. Cancer Res. 2010;16(13):3322–3328. doi: 10.1158/1078-0432.CCR-09-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Navarro-Tito N, Soto-Guzman A, Castro-Sanchez L, Martinez-Orozco R, Salazar EP. Oleic acid promotes migration on MDA-MB-231 breast cancer cells through an arachidonic acid-dependent pathway. Int. J. Biochem. Cell Biol. 2010;42(2):306–317. doi: 10.1016/j.biocel.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Navarro-Tito N, Robledo T, Salazar EP. Arachidonic acid promotes FAK activation and migration in MDA-MB-231 breast cancer cells. Exp. Cell Res. 2008;314(18):3340–3355. doi: 10.1016/j.yexcr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 73.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60(22):6353–6358. [PubMed] [Google Scholar]
- 74.Liu Z, Xiao Y, Yuan Y, et al. Effects of oleic acid on cell proliferation through an integrin-linked kinase signaling pathway in 786-O renal cell carcinoma cells. Oncol. Lett. 2013;5(4):1395–1399. doi: 10.3892/ol.2013.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(3):230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 76.Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97(8):2035–2042. doi: 10.1002/cncr.11293. [DOI] [PubMed] [Google Scholar]
- 77.Hagen RM, Rhodes A, Ladomery MR. Conjugated linoleate reduces prostate cancer viability whereas the effects of oleate and stearate are cell line-dependent. Anticancer Res. 2013;33(10):4395–4400. [PubMed] [Google Scholar]
- 78.Yue S, Li J, Lee SY, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19(3):393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lucarelli G, Rutigliano M, Galleggiante V, et al. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev. Mol. Diagn. 2015;15(9):1211–1224. doi: 10.1586/14737159.2015.1069711. [DOI] [PubMed] [Google Scholar]
- 80.Swinnen JV, Heemers H, Van De Sande T, et al. Androgens, lipogenesis and prostate cancer. J. Steroid Biochem. Mol. Biol. 2004;92(4):273–279. doi: 10.1016/j.jsbmb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Ettinger SL, Sobel R, Whitmore TG, et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64(6):2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 82.Grossi V, Lucarelli G, Forte G, et al. Loss of STK11 expression is an early event in prostate carcinogenesis and predicts therapeutic response to targeted therapy against MAPK/p38. Autophagy. 2015;11(11):2102–2113. doi: 10.1080/15548627.2015.1091910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiacchiera F, Simone C. The AMPK–FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010;9(6):1091–1096. doi: 10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]
- 84.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–2231. [PubMed] [Google Scholar]
- 86.Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS ONE. 2012;7(1):e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]