Abstract
The emergence of immune ‘checkpoint inhibitors’ such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death receptor 1 (PD-1) has revolutionized treatment of solid tumors including melanoma, lung cancer, among many others. The goal of checkpoint inhibitor combination therapy is to improve clinical response and minimize toxicities. Rational design of checkpoint combinations considers immune-mediated mechanisms of antitumor activity: immunogenic cell death, antigen release and presentation, activation of T-cell responses, lymphocytic infiltration into tumors and depletion of immunosuppression. Potential synergistic combinations include checkpoint blockade with conventional (radiation, chemotherapy and targeted therapies) and newer immunotherapies (cancer vaccines, oncolytic viruses, among others). Reliable biomarkers are necessary to define patients who will achieve best clinical benefit with minimal toxicity in combination therapy.
Keywords: : checkpoint inhibitors, combination therapies, cytotoxic T-lymphocyte antigen 4 (CTLA-4), immunotherapy, malignancy, programmed death receptor-1 (PD-1) and ligand-1 (PD-L1)
Background
In the last decade, remarkable progress has been made in the clinical application of cancer immunotherapies exploiting the immune system's ability to identify and eradicate tumors, most notably the emergence of immune ‘checkpoint inhibitors’ blocking normally negative regulators of T-cell immunity such as cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death receptor 1 (PD-1). The US FDA approval of the CTLA-4 blocking antibody, ipilimumab (Bristol-Myers Squibb, Princeton, NJ) in melanoma and the PD-1 blocking antibodies in the treatment of advanced melanoma (pembrolizumab – Merck, Kenilworth, NJ; nivolumab – Bristol-Myers Squibb, Princeton, NJ), lung (pembrolizumab and nivolumab) and renal cancer (nivolumab) has ushered in great promise in treatment of a rapidly expanding spectrum of solid tumors also including ovarian cancer, bladder cancer, head and neck cancer and gastric cancer, among others [1–7].
Single agent PD-1 and CTLA-4 pathway blockade has demonstrated positive antitumor activity across multiple tumor types, but response rates still remain low and in minority of patients. There is now much focus on combining immunotherapies with conventional (radiation and chemotherapy) and other immunotherapies to improve clinical response rates and outcomes with combination therapy.
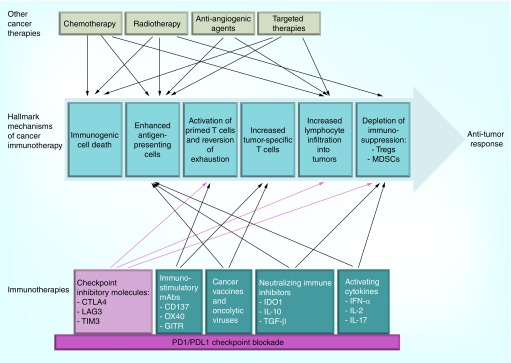
Rational checkpoint combinations should be designed in context of the hallmark mechanisms of cancer immunotherapy: immunogenic cell death; antigen release and presentation; priming of T-cell responses; enhancement of T-cell activity; infiltration into tumor tissues; and depletion of compensatory immunosuppression. Strategies must consider the compatibility and synergy of combination treatments – whether simultaneously or sequentially – to mediate antitumor efficacy or to reduce on-target side effects [8], while also tackling the immune-escape strategies of cancer (Figure 1). In this review, we highlight the rationale, evidence and challenges in designing synergistic combinations with checkpoint inhibitors.
Figure 1. . Building synergistic combinations on the foundation of PD-1 or PD-L1 blockade with both traditional and newer immune therapies.
Given the clinical success of PD-1–PD-L1 blockade in multiple solid cancers, the PD-1–PD-L1 pathway will probably become the foundation for immunotherapy combinations. Combinations of traditional therapies, including radiation and chemotherapy (top) and newer immunotherapies (bottom) are under clinical development. In developing combinations, therapies should be considered in context of the hallmark mechanisms of cancer immunotherapy: immunogenic cell death via apoptosis or necrosis; activation of professional antigen-presenting cells; activation of primed effector T cells to proliferate and exert their functions is counteracted by suppressive mechanisms in the tumor microenvironment that include Treg cells and MDSCs; increased a number of effector T lymphocytes that recognize tumor antigens; and infiltration of effector and suppressive leukocytes into tumors. Red arrows indicate mechanisms of PD-1–PD-L1 and other checkpoint inhibitors; blue arrows represent mechanisms of other immune and nonimmune therapy. The number of arrows is a demonstration of the strong potential for synergistic combinations [8,148].
MDSC: Myeloid-derived suppressor cell.
Reproduced with permission from [8] © Nature Publishing Group (2015).
Immune checkpoint molecules & their inhibitors
The clinical development of checkpoint blocking antibodies is built upon groundwork of the ‘two signal’ model of T-cell activation. In signal 1, the T-cell receptor (TCR) engages with the major histocompatibility complex (MHC). Signal 2 arises from costimulatory signal B7-CD28 on APCs [9–13]. Both signals are required for full T-cell response. Additional basic science researches have added layers of complexity to the understanding of T-cell activation and regulation: it is now understood that a variety of immunomodulatory signals, both costimulatory and coinhibitory, are needed to orchestrate an optimal antigen-specific immune response [13,14].
CTLA-4 monoclonal antibodies: ipilimumab & tremelimumab
CTLA-4 is mainly expressed on T helper and Treg cells, and acts in the first phase of immune response. Binding of CTLA-4 to its ligands B7–1 (also known as CD80) and B7–2 (also known as CD86) on APCs [9,10] leads to inhibition of T cells. Therefore, CTLA-4 blockade can amplify T-cell responses against tumors [13,14]. CTLA-4 blockade may also increase antitumor immunity through other mechanisms, such as partially depleting Treg cells in the tumor microenvironment, and interfering with the CTLA-4-mediated sequestration of co-stimulatory ligands [8,15].
The concept of targeting immune checkpoint receptors for cancer therapy was first pioneered by James Allison and colleagues in 1996 when CTLA-4 blockade was shown to lead to regression of tumors in animal models [16]. Following these studies, human CTLA-4 blocking antibodies, ipilimumab and tremelimumab, have been evaluated in multiple clinical trials, and both agents demonstrated durable responses in Phase I and II clinical studies in advanced melanoma with a similar toxicity profile. In Phase III clinical trials, ipilimumab demonstrated overall survival benefits for patients with advanced melanoma [17,18], leading to its FDA approval in March 2011. Both ipilimumab and tremelimumab are currently being evaluated in Phase II and III trials in patients with advanced mesothelioma, gastric cancer, NSCLC, bladder cancer and prostate cancer [19].
Programmed death-1 monoclonal antibodies: pembrolizumab & nivolumab
PD-1 is another key immune checkpoint receptor that acts as a negative regulator of T-cell function, and is more broadly expressed than CTLA-4. PD-1 is induced both on activated CD8+ T cells and Treg present in tumor microenvironment, and activated non-T-lymphocytes including B cells and natural killer (NK) cells [1]. PD-1 has two identified ligands: PD-L1 (also known as B7-H1; also has affinity for CD80) and PD-L2 (also known as B7-DC). PD-1 signaling contributes to T-cell exhaustion [20], and tumors may exploit this pathway, as PD-L1 is expressed on many cancers [1,21–22].
In clinical trials, at least two PD-1 blocking antibodies, pembrolizumab and nivolumab (Bristol-Myers Squibb), have demonstrated clinical activity in melanoma, as well as other solid tumors including non-small-cell lung cancer (NSCLC), renal cell cancer and head and neck cancers [1–6], leading to FDA approval of nivolumab and pembrolizumab in metastatic melanoma and NSCLC. Nivolumab is also FDA approved for patients with renal cell carcinoma. Under investigation are other PD-1 inhibitors (pidilizumab [CT-011]); PD-L1-specific agents (durvalumab [MEDI4736], atezolizumab [MPDL3280A] and MSB0010718C [NCT02155647, NCT01772004, NCT01943461]); and PD-L2-specific agents (rHIgM12B7 [NCT00658892]). The notable successes of monotherapy PD-1 and PD-L1 blockade suggest that these agents may become the preferred building blocks for checkpoint combinations in future combination strategies.
Other emerging immune checkpoints: LAG-3 & TIM-3
Lymphocyte activation gene 3 protein (LAG3; also known as CD223) and other inhibitory receptors are progressively expressed on T cells during the effector phases of a T-cell response [23]. It has been demonstrated that LAG3 can bind to MHC class II molecules and galectin-3 with functional consequences [24]. Interaction of LAG3–MHC class II allows inhibition of CD8+ effector T cells and enhancement of CD4+ Treg cells. LAG3 antibodies that do not block the LAG3–MHC class II interaction continue to enhance CD8+ effector T cells but do not inhibit Treg cell activation [25]. The expression of LAG3 on tumor-infiltrating Treg cells and cytotoxic T lymphocytes (CTLs) suggests possible involvement in immune evasion by tumors. Blocking LAG3 may therefore reverse T-cell exhaustion and enhance antitumor immunity [23]. BMS-986016, a LAG3-specific mAb, is in clinical development (NCT01968109 and NCT02061761).
T-cell immunoglobulin and mucin domain-containing 3 (TIM3; also known as HAVCR2) [26] is expressed on T helper 1 cells and CTLs, as well as innate immune cells such as dendritic cells (DCs) [27]. Commonly expressed in melanoma [28,29] and NSCLC, its function differs depending on the cell type on which it is expressed. Expression of TIM3 by tumor-infiltrating lymphocytes (TILs) is thought to keep the lymphocyte status inactive or even to induce apoptosis upon ligation to galectin-9 or other, still undefined, ligands [30].
Both LAG3 and TIM3 are frequently coexpressed with PD-1 on anergic or exhausted tumor-specific CD8+ T cells, and for this reason, dual blockade synergistically reverses anergy in preclinical models [25].
Combination checkpoint inhibition
Both CTLA-4 and PD-1 function as negative regulators, yet each plays a nonredundant role in modulating the cancer immune response. CTLA-4 is crucial to the attenuation and early activation of naïve and memory T cells, ‘priming cancer-specific T-cell immunity’, via interactions with ligands CD80 and CD84. In contrast, PD-1 plays a more prominent role in modulating T-cell activity in peripheral tissues via its interaction with PD-L1 and PD-L2. Korman and colleagues first explored this nonredundant synergistic combination in preclinical mouse models, and demonstrated that the combination of PD-1 and CTLA-4 blockade had synergistic antitumor activity in a mouse model of colon adenocarcinoma, MC38 [31]. These results were also shown by other groups [32].
The differing mechanisms of actions of CTLA-4 and PD-1 monoclonal antibodies (mAbs) can be better understood by defining the differences in chemical structure and expression of each immune checkpoint inhibitor antibody. Contrary to the most PD-1 and PD-L1 mAbs that are human IgG4, ipilimumab is a human IgG1 mAb and, for this reason, has a peculiar mechanism of action dependent both on agonism and cell depletion. Agonistic activity of ipilimumab depends on the multimerization of the mAb on the surface of the Fc receptor expressing cells, facilitating cross-linking of the antigen on the surface of the target cell (i.e., CD4+ T helper) and promoting their activation, proliferation and cytokine production; cell depletion, instead, depends on the ADCC principally directed to Treg cells. Nivolumab, pembrolizumab and other IgG4 mAbs have no agonistic or ADCC activity, and act primarily through the blocking of the interactions between ligand (PD-L1) and receptor (PD-1) [33].
Given these preclinical findings, clinical exploration was undertaken. In the Phase I clinical trial of ipilimumab and nivolumab, patients with metastatic melanoma [34,35] were treated with dose escalation of nivolumab added to different doses of ipilimumab. The maximum-tolerated dose was declared to have been exceeded at 3 mg per kg of each antibody, and impressive clinical responses were observed in patients treated with lower dose levels, which were selected for further studies. For patients who received concurrent ipilimumab and nivolumab across all doses, the 1- and 2-year overall survival rates were notable at 85 and 79%, respectively [35]. Combination therapy unfortunately did also lead to an increase in the frequency of adverse events (AEs) compared with prior experience with either antibody alone, in particular with nivolumab (for example, 61% of patients reported grade III AEs in the combination ipilimumab–nivolumab group). Interestingly, many of the grade III adverse events – in particular, those that led to establishing the maximum-tolerated dose – were asymptomatic laboratory abnormalities, such as asymptomatic elevation of lipase, amylase, alanine aminotransferase and/or aspartate aminotransferase.
Results from the Phase II study comparing ipilimumab plus nivolumab with ipilimumab alone were reported in 2015 [36]. In a study of 142 patients without prior systemic treatment, objective response rate was 61% for combination treatment versus 11% for ipilimumab alone. Of the patients with BRAF wild-type tumors, 22% experienced a complete remission in the combination arm; no complete remissions were observed in the ipilimumab-alone arm. Clinical benefit in the combination arm was independent of PD-L1 expression by tumor cells before treatment. Progression-free survival (PFS) was 4.4 months in patient who received ipilimumab alone, which is not yet reached for patients treated with the combination (hazard ratio [HR]: 0.40; p < 0.001). The AEs observed in this trial were similar to those reported in earlier Phase I trials [34].
These results were further supported in a Phase III trial, in which 945 patients were randomized to three arms: ipilimumab alone, nivolumab alone or the combination ipilimumab plus nivolumab [37]. The study was not statistically powered to compare PFS and response rate between nivolumab alone and the combination at time of publication. As data mature over the next several years, we anticipate comparative overall survival to be crucial to understanding the full clinical benefit of combination immunotherapy. Treatment-related AEs of grade III or above were significantly higher with combination therapy: 55% in the combination arm, 16% in nivolumab alone and 27% with ipilimumab alone [37].
On the basis of these promising results, the FDA approved the use of combination of nivolumab and ipilimumab in treatment for patients with unresectable or metastatic melanoma without a BRAF mutation. The combination of ipilimumab and nivolumab is being explored in other solid cancers in Phase III studies (renal cell carcinoma and NSCLC [38,39]), and earlier-phase clinical trials for various other tumor types (small-cell lung, triple-negative breast, pancreatic, gastric and bladder cancer). Very promising Phase I and II trials have been reported on increased activity of the combination of nivolumab and ipilimumab [40], durvalumab and tremelimumab [41] and pembrolizumab and ipilimumab [42] in NSCLC, with an overall response rate (ORR) around 40% across all the studies; however, in all these trials the higher rate of grade 3 and 4 AEs (around 20–30%) remains an important issue of the dual blockade.
Table 1 highlights selected key clinical trials in checkpoint inhibitor combinations. Combinations of PD-1-specific antibodies with other immune checkpoint inhibitors such as LAG3 or TIM3 are currently undergoing evaluation in preclinical and clinical setting [8,15,30,43].
Table 1. . Selected key published clinical trials of checkpoint inhibitor combinations.
| Disease | N | Phase | Treatment arms | Response rates (CR and PR) | Survival | Toxicities (Grade 3–4) | Ref. | |
|---|---|---|---|---|---|---|---|---|
|
Combinations of checkpoint inhibitors | ||||||||
| Ipi and nivo |
Advanced melanoma (untreated) |
945 |
3 |
Nivo alone vs ipi alone vs nivo plus ipi |
44% nivo; 19% ipi; 58% ipi plus ipi |
Not yet reported |
16.3% nivo; 28.3% ipi; 55% nivo plus ipi |
[37] |
| Ipi and nivo |
Advanced melanoma (untreated) |
142 |
2 |
Ipi alone vs ipi plus nivo |
11% ipi; 61% ipi plus nivo |
Median PFS: 4.4 months for ipi Not reached for ipi plus nivo |
24% ipi; 54% nivo plus ipi |
[36] |
| Ipi and nivo |
mRCC |
1070 |
3 |
Nivo plus ipi vs sunitinib |
Ongoing |
Ongoing |
Ongoing |
[38] |
| Ipi and nivo |
NSCLC |
75 |
2 |
Nivo alone vs nivo plus ipi |
Ongoing |
Ongoing |
Ongoing |
[39] |
|
Combination with other immunotherapies | ||||||||
| Ipi and GP100 vaccine |
Advanced melanoma (previously treated) |
676 |
3 |
Ipi alone vs ipi plus vaccine versus vaccine alone |
10.9% ipi; 5.7% ipi plus vaccine; 1.5% vaccine alone |
Median OS: 10.1 mos ipi 10.0 mos ipi vaccine 6.4 mos vaccine alone |
10–15% ipi; 3% gp100 alone |
[17] |
|
Combination with conventional therapies inducing immunogenic cell death | ||||||||
| Ipi and radiotherapy (single fraction) |
Post-docetaxel CRPC |
799 |
3 |
Ipi plus radiotherapy vs placebo plus radiotherapy |
NA |
Median OS: 11.2 mos for radiotherapy-ipi 10.0 mos placebo (p-value NS) |
26% ipi (irAEs); 3% placebo |
[44] |
| Ipi and dacarbazine |
Advanced- melanoma (untreated) |
502 |
3 |
Ipi plus dacarbazine vs dacarbazine alone |
15.2% ipi with dacarbazine; 10.3% dacarbazine alone |
Median OS: 11.2 mos ipi plus dacarbazine 9.1 mos dacarbazine |
56.3% ipi plus dacarbazine; 27.5% placebo |
[45] |
| Carboplatin plus paclitaxel and ipi |
NSCLC (untreated) |
204 |
2 |
Sequential or concurrent schedule vs control (68 sequential and 136 concurrent) |
32% irBORR ipi; 18% sequential chemotherapy control |
Median irPFS: 5.7 months for ipi 4.6 months sequential chemotherapy control (p = 0.05) |
15% phased ipi; 20 concurrent ipi; 6% control |
[46] |
| Ipi and bevacizumab | Advanced melanoma | 46 | 1 | Concurrent combination with escalating doses | 19.6% | Median OS: 25.1 months | NR | [47] |
Reproduced with permission from [8] © Nature Publishing Group (2015).
CR: Complete response; CRPC: Castrate-resistant prostate cancer; Ipi: Ipilimumab; irAEs: Immune-related adverse events; irBORR: Immune-response best overall response; irPFS: Immune-response progression-free survival; mos: Months; mRCC: Metastatic renal cell carcinoma; Nivo: Nivolumab; NR: Not responsive; NS: Not significant; NSCLC: Non-small-cell lung cancer; OS: Overall survival; PR: Partial response.
Enhancing tumor antigen release & presentation
Combining checkpoint inhibition with conventional therapies
Combining checkpoint inhibition with radiation therapy
Conventional treatment regimens – such as chemotherapy, radiotherapy, targeted therapy and ADCC (occasionally mediated by tumor-targeting antibodies) – can induce tumor cell death, allow the release of tumor antigens for presentation and thus in theory, prime the immune system. Additionally conventional anticancer therapies can deplete immunosuppressive cells such as Tregs and myeloid-derived suppressor cells (MDSCs) to enhance a latent antitumor immune response [48,49], thereby providing rationale behind checkpoint combination.
In addition to direct cytotoxic effects on cancer cells through cell-cycle arrest and apoptosis, radiation has been reported to stimulate antitumor immune responses. Proposed mechanisms include the release of tumor antigens and damage-associated molecular patterns (DAMPs), facilitation of tumor antigen uptake and cross-presentation by DCs and the induction of pro-inflammatory cytokines and chemokines which mediate the recruitment of T cells and DCs [44,50]. In mouse models, localized radiation therapy when combined with systemic checkpoint blockade resulted in the inhibition of systemic metastases [51]. Recently published data support the synergistic activity between radiation therapy, anti CTLA-4 and anti PD-L1 mAb, specifically the diversification of TCR repertoire of TILs in melanoma preclinical models [52]. In humans, anecdotal case studies demonstrated that patients treated with anti-CTLA-4 therapy and localized radiation therapy can result in regression not only of irradiated, but also of distant lesions in melanoma and lung cancer patients [53,54], a phenomena known as the abscopal effect.
Unfortunately, a Phase III study combining localized radiation with systemic CTLA-4 blockade in 799 patients with metastatic castration-resistant prostate cancer demonstrated prostate-specific antigen (PSA) declines, but no significant difference between the radiation + ipilimumab group and the radiation + placebo group in terms of overall survival in the primary analysis [55,56]. Since radiotherapy was not the experimental question in this trial, however, this trial did not formally test whether radiotherapy adds to the efficacy of ipilimumab. Trials are currently underway to evaluate radiotherapy in combination with immunotherapy including NCT 01689974, NCT 01970527, NCT 01557114, NCT02107755 and NCT01497808.
Combining checkpoint inhibition with chemotherapy
Like radiation, conventional chemotherapies have been shown to demonstrate the release of antigens and DAMPs, thus triggering immunogenic cell death [57]. In addition to direct cytotoxic effects on cancer cells, some chemotherapeutic agents can induce immunogenic cell death and activate antitumor immune response through other possible mechanisms: DCs activation and expression of costimulatory molecules; enhancement of cross-priming of CD8+ T cells; promotion of antitumor CD4+ T-cell phenotype; downregulation of MDSC and Treg activity; promotion of tumor cell death through lytic receptors or pathways (i.e., Fas, perforin, granzyme); increase in serum inflammatory cytokines and proinflammatory changes in tumor microenvironment [48,57–59].
These studies provide a rationale for the exploration of chemotherapy in combination with antibodies targeting costimulatory and coinhibitory receptors [60]. Ipilimumab and nivolumab have been explored in combination with chemotherapy in several trials (Table 1) [18,39,46,61]. An important Phase II trial in patients with stage IIIb/IV NSCLC showed that carboplatin-paclitaxel could be safely combined with ipilimumab. Interestingly a ‘phased regimen’ in which immunotherapy began after chemotherapy resulted in substantially improved PFS compared with chemotherapy alone [46].
Although in some studies the combination of chemotherapy and immunotherapy appears to be well-tolerated [18], in others, combinations have been associated with increased toxicity, in particular noted in combination of nivolumab with platinum-based chemotherapies in NSCLC, where grade 3–4 treatment-related AEs were reported in 45% of patients receiving checkpoint inhibition [39]. Potential immunosuppressive effects of chemotherapy render issues of dosing and timing critical. Among different schedules, the metronomic chemotherapy is the most promising one to be associated with checkpoint inhibitors, because of its immunomodulatory properties [62]. Overall, these findings suggest that while chemotherapy certainly presents an option for exploration in combination with immunotherapies, careful consideration needs to be given to determine appropriate dosing and sequencing of these agents and to establish whether such combinations would be more active and/or tolerable in specific cancer subtypes.
Combining checkpoint inhibition with targeted therapies
Tyrosine kinase inhibitors
There is accumulating evidence that tyrosine kinase inhibitors (TKIs), such as EGFR (erlotinib)- and HER2 (lapatinib)-directed therapies, may exert additional immunomodulatory effects that influence the outcome of immunotherapy. In a spontaneous gastrointestinal stromal tumor model responsive to therapy with imatinib, imatinib reduced tumor cell expression of indoleamine 2,3-dioxygenase (IDO) which was an immunomodulatory effect in addition to its direct cytotoxic effect on tumor cells [63]. This led to favorable changes in the tumor microenvironment, with an increase in the infiltrating CD8+ T cells and a decrease in Treg. The therapeutic effect of imatinib was augmented by combination with CTLA-4 blockade [63]. The same group has also demonstrated that imatinib therapy in gastrointestinal stromal tumor leads to polarization of tumor-associated macrophages to M2 phenotype (expected to favor tumor growth), as a result of interaction of macrophages with apoptotic tumor cells, highlighting a possible immune feedback mechanism that could also be targeted in order to improve the efficacy of combined immunotherapies [64].
In EGFR mutant NSCLC, the biologic rationale of combination of immune checkpoint inhibitors with EGFR inhibitors resides in overlapping antitumor activity independent from histology or KRAS and EGFR mutational status [7]. A growing body of evidence suggests that oncogenes may indirectly influence tumor microenvironment, regulating the release of ligands and cytokines. A correlation between EGFR activation and a composed signature of immunosuppression (manifested by the upregulation of PD-1, PD-L1, CTLA-4 and several tumor-promoting inflammatory cytokines) was reported. Globally considered, these findings suggest that concurrent inhibition of PD-1 and EGFR pathways may represent a rational and promising approach for EGFR-addicted NSCLC [65,66]. Remarkable clinical data exist about the combination of erlotinib and nivolumab in EGFR mutant, chemo-naive NSCLC, in a second-line setting after an anti-EGFR TKI (response rate [RR]: 20%, median progression-free survival [mPFS]: 30 weeks) [67]; and this is an area of very relevant ongoing research.
BRAF inhibitors
The rationale for this combination stems from preclinical studies that have demonstrated that RAF inhibition resulted in T-cell activation and proliferation, consistent with paradoxical activation of the MAPK pathway in BRAF wild-type T cells [68]. This effect was further potentiated by CTLA-4 blockade and resulted in the enhancement of antitumor activity of CTLA-4 blockade [69]. In a Phase I trial, the combination of vemurafenib and ipilimumab led to significant hepatotoxicity, requiring trial discontinuation [70,71]. Sequencing, as opposed to concurrent administration, of these agents may be preferable, but severe cutaneous toxicity was reported with patients who received vemurafenib shortly after ipilimumab [72]. Additionally, the combination of ipilimumab + dabrafenib + trametinib was not felt tolerable, although ipilimumab + dabrafenib may be more tolerable [73]. Until additional knowledge is obtained, the combination of ipilimumab and BRAF +/- MEK inhibitors is not recommended.
VEGF inhibitors
Tumor vasculature is known to exert immunosuppressive effects through variety of mechanisms, including decreasing the influx of lymphocytes and DCs in the tumors while increasing the intratumoral (i.t.) frequencies of Treg cells and MDSCs [74]. In an early phase clinical trial, ipilimumab has been combined with the VEGF inhibitor, bevacizumab [47], and demonstrated tolerability. The study of 46 advanced-stage melanoma patients demonstrated a notable 67% disease control rate and evidence of activated endothelium and increased T-cell tumor immune infiltration in biopsy samples. Median overall survival was >2 years. High-grade toxicity was more common in combination than either drug alone (hypophysitis, dermatitis, hepatitis), but was manageable in the study patients.
Other targeted agents
Other targeted inhibitors have been investigated in combination with immunotherapy. In a dose-escalation study of tremelimumab with sunitinib in metastatic renal cancer patients, combination therapy resulted in significant renal toxicity, requiring trial discontinuation [75]. On the other hand, the combination of nivolumab with sunitinib or pazopanib had durable responses, and although better tolerated, still was associated with hepatic and renal toxicities higher than single agent alone [76]. Combinations of tremelimumab with androgen deprivation in prostate cancer or aromatase inhibition in breast cancer appeared to also be well-tolerated with some evidence of preliminary clinical activity in both diseases [77].
That said, although some anti-VEGFR TKIs, like sunitinib or pazopanib, through decreasing STA3 signaling, are able to downregulate Treg cells and promote the formation of effector T helper 1 cells, not all the anti-VEGFR TKIs exert the same functions on immune system. For example, sorafenib seems to have immunosuppressive properties related to its inhibitory effects on MEK signaling [78].
Priming T-cell response
Combining with cancer vaccine therapy
Several different vaccination approaches have been explored to enhance the efficacy of immune checkpoint blocking antibodies. These include simple vaccine preparations consisting of specific peptides and proteins [79,80], as well as more complex strategies, such as engineered cellular vaccines, DC vaccines [81–83], virus-vectored vaccines [84,85] and oncolytic viruses. While combination of model antigen peptide vaccines with immunomodulatory antibodies in animal models led to robust induction of CTL responses [80,86], therapeutic efficacy was not seen in humans [17]. At present it is thus unclear whether peptide vaccination is sufficient to enhance the efficacy of immunomodulatory antibodies. Combination strategies are being directed to explore optimization via use of appropriate vaccine preparations and adjuvants, specifically the agents acting on the innate immunity such as DCs and TLR agonists [79,87].
In an attempt to bypass in vivo antigen processing, DC vaccines have been explored as a possible strategy to enhance the efficacy of immunomodulatory antibodies. In animal models, vaccination with peptide-antigen-loaded DCs, in combination with systemic CTLA-4 blockade, demonstrated increased efficacy than either treatment alone [81–83]. Another study explored a different strategy, where immature DCs were used for in situ vaccination by injection directly into irradiated tumor, in combination with systemic CTLA-4 blockade [88], resulting in the inhibition of distant tumor growth and improved animal survival. A Phase I trial of combination of MART-1 peptide-pulsed DC and tremelimumab has shown objective and durable tumor responses at the higher range of the expected response rate with either agent alone [89].
The synergistic efficacy of autologous modified tumor cellular vaccines with immunomodulatory antibodies [90–92] has also been explored in clinical trials. In these studies, allogeneic cancer cells transfected with GM-CSF (GVAX) have been evaluated in patients with metastatic pancreatic cancer and hormone-refractory prostate cancer in combination with ipilimumab [93]. In a prostate cancer trial, combination therapy resulted in 4 out of 16 patients achieving evidence of clinical benefit, as measured by PSA response or stabilization [94]. In the pancreatic cancer study, patients who received GVAX with ipilimumab demonstrated evidence of clinical benefit, three out of 15 patients having prolonged disease stabilization, and 7 out of 15 patients experiencing tumor marker declines [93]. These results warrant further clinical exploration.
Several studies have explored virus-vectored vaccines, as a means to augment the immune response to a specific antigen [84,85]. In preclinical models, combination of the recombinant vaccinia vector carrying the genes for CEA, B7.1, ICAM-1 and LFA-3 (rV-CEA-TRICOM) and recombinant fowlpox-boosted vaccines with systemic CTLA-4 blockade led to enhanced antitumor immunity [95]. In a Phase I trial of combination of poxviral-based PSA-TRICOM vaccine with ipilimumab in patients with metastatic castration-resistant prostate cancer [96], the use of the vaccine was not associated with increased rate of AEs and had some evidence of activity with PSA declines in 58% of the chemotherapy-naive patients [96].
Other studies have undertaken approaches combining immunomodulatory antibodies with activators of innate immune response, such as TLR agonists [97] and peginterferon alpha-2b [98], with marked efficacy seen in different tumor models. Intratumoral therapy with TLR agonists has shown promising results in the enhancement of therapeutic efficacy of immunomodulatory antibodies such as anti-CTLA-4, anti-PD-1 and anti-OX40 [99,100]. In a Phase I study, subcutaneous administration of TLR9 agonist in combination with tremelimumab in patients with melanoma and other advanced solid tumors [97] demonstrated good tolerability, with durable partial responses in 2 out of 17 melanoma patients [97].
Combining checkpoint inhibition with oncolytic viruses (T-VEC)
With the recent FDA approval of talimogene laherparepvec (T-VEC), a herpes simplex virus type 1-derived cancer vaccine for advanced melanoma patients, there is a renewed interest in oncolytic viruses in combination with checkpoint blockade. In both preclinical and clinical studies, it is clear that a robust and specific infection of tumor bed oncolytic viruses is achievable after intravenous infusion or i.t. injection using various platforms [101–103]. I.t. injection with oncolytic viruses in melanoma patients generated a systemic tumor antigen-specific T-lymphocyte response, as well as downregulation of Treg, suppressor CD8+ T cells and myeloid-derived suppressor cells in patients with clinical benefit [104,105].
T-VEC is the first oncolytic immunotherapy to demonstrate therapeutic and durable benefit in advanced solid cancers patients in a randomized study [106], and was just recently FDA approved in patients with unresectable melanoma. To date, most recent clinical trials with T-VEC have demonstrated a good safety profile in combination with chemotherapy, radiation, low-dose cyclophosphamide and targeted therapy [107–109]. In the clinic, the combination of ipilimumab and T-VEC is currently being tested in a Phase III trial to treat advanced metastatic patients (NCT01740297).
In an analogous study also performed in patients with advanced melanoma, intralesional injection of another oncolytic virus, coxsackievirus A21, similarly led to responses in the virus-injected and distant tumors [106]. The use of oncolytic viruses thus presents a highly attractive strategy for in situ vaccination, since this approach allows for potential immunization against multiple cancer antigens within the context of virus-induced proinflammatory microenvironment characterized by the release of pathogen-associated molecular patterns and DAMPs, necessary for efficient APC maturation and antigen presentation [110]. Checkpoint inhibitor combinations with oncolytic viruses is a promising strategy that remains to be tested fully.
Enhancing T-cell activity
Combining checkpoint inhibitors with immunostimulatory mAbs
T-cell dysfunction is characterized by general unresponsiveness to tumor antigens and is mediated in part through the upregulation of immune inhibitory receptors such as PD-1 and LAG-3, and is also potentially amenable to immunostimulatory mAbs. Several preclinical studies suggest that combination of checkpoint blocking antibodies with agonists of T-cell costimulatory receptors may act in a synergistic manner [92].
CD137 (also known as 4-1BB and TNFRSF9) is a T-cell and NK-cell costimulatory receptor, expressed at the cell surface following lymphocyte activation [15]. Upregulation of CD137 signaling has been shown to improve cytotoxic antitumor responses and T-cell survival [111]. Agonistic CD137-specific mAbs may also further enhance NK-cell-mediated ADCC [112]. A Phase I–II trial is underway to evaluate a combination of PD-1-blocking agents with CD137-specific mAbs, urelumab (NCT02253992). PF-05082566 is another agonistic CD137-specific mAb under evaluation in several malignancies. Although CD137-specific and PD-1-specific mAbs are well-tolerated as single agents, these studies will still need to be followed carefully for synergistic autoimmune toxicities.
Glucocorticoid-induced TNF receptor family-related protein (GITR; also known as TNFRSF18) is a costimulatory molecule that activates proliferation of CD4+ and CD8+ T cells and reverses Treg cell-mediated suppression of T cells [113]. Activating GITR may reverse Treg cell-mediated suppression, overcome self-tolerance and ultimately enhance antitumor immune responses [114]. Phase I evaluation of agonistic GITR-specific mAbs TRX518 and MK-4166 is ongoing (NCT01239134 and NCT02132754).
OX40 (also known as TNFRSF4) is a costimulatory receptor expressed primarily on activated CD4+ and CD8+ T cells. By promoting T-cell proliferation and survival, it enhances antitumor immune responses [115]. In preclinical mouse models, OX40 agonists have been shown to inhibit Treg cells, promote T-cell survival, thereby enhancing antitumor immunity [116]. While GITR is constitutively expressed on Tregs and is upregulated on CD4+ and CD8+ T cells after activation, OX40 is expressed only on activated T cells [8]. This different expression could explain also why anti-GITR mAbs can be tested as monotherapy or in combination with other immune checkpoint inhibitors, whereas an anti OX40 mAb should be preferably tested in combination with other immune checkpoint inhibitors able to activate effector T cells and induce OX40 expression [33]. Several trials are ongoing in patients with advanced-stage solid tumors to evaluate the combination of OX40-specific mAbs with checkpoint inhibitors (MEDI6469) plus tremelimumab or MEDI4737 (NCT02205333), and that of lirilumab with nivolumab (NCT01714739) or ipilimumab (NCT01750580).
Other costimulatory molecules under evaluation include CD40 (also known as TNFRSF5), as well as inducible costimulator, a costimulatory member of Ig receptor superfamily, which has been shown to significantly enhance the efficacy of CTLA-4 blockade in animal models [117].
Current knowledge of the crosstalk among coinhibitory and costimulatory receptors is limited. Multiple mechanisms for synergy are evident but the potential for increased AEs is also real: checkpoints inhibitors and costimulatory molecules often work on the same target cells. It is difficult to predict whether – and, if so, how – efficacy and safety of combinations in mouse models would translate to the human patients. Careful clinical monitoring of patient safety and toxicity is of paramount importance as these combinations are investigated in early phase clinical trials.
Targeting compensatory immune inhibitory mechanisms
Immune cells within the tumor microenvironment such as Tregs and MDSCs exert immunosuppression through a variety of mechanisms [103], and thus it has been proposed that depletion of such populations can work synergistically with checkpoint blockade.
Combining checkpoint inhibition with IDO inhibitors
IDO has been demonstrated to inhibit immune responses through several mechanisms, which include depletion of the essential amino acids and promotion of differentiation of FoxP3+ Tregs [118,119]. Expressed by many cancers, high levels of IDO expression have been associated with poor prognosis [120]. Holmgaard et al. demonstrated that upregulation of the IDO in the tumor microenvironment is a possible mechanism of resistance to anti-CTLA-4 immunotherapy [121]. In murine models, combination of small molecule inhibitors of IDO with CTLA-4-blocking antibodies resulted in significant enhancement of therapeutic efficacy; an effect that was also associated with significant increase in tumor-infiltrating lymphocytes [121]. Evaluation of combinations of IDO inhibitors and ipilimumab and pembrolizumab is currently being pursued in clinical trials, with preliminary data indicating promising activity of the combination in patients with metastatic melanoma [122,123].
Combining checkpoint inhibition with Treg depleting antibodies (anti-CD-25 antibodies, anti-CCR4 antibody)
CD25 is expressed on activated T effector lymphocytes, and depletion of Treg with CD25-targeting agents such as anti-CD25 antibody daclizumab [124] has a potential to target effector T cells as well. Sutmuller et al. demonstrated preclinically that the combination of CTLA-4 blockade and depletion of Treg with anti-CD25 antibody resulted in improved therapeutic efficacy of CTLA-4 blockade [125]. An alternative strategy has been suggested through the use of an anti-CCR4 antibody, which has been demonstrated to selectively deplete Treg from humans [126]. A Phase I study of the anti-CCR4 antibody mogamulizumab in patients with solid tumors is currently ongoing (NCT01929486), and a Phase II study has been reported for patients with relapsed T-cell lymphoma where clinical efficacy was seen [126].
Managing combination-associated toxicity
Clinical toxicities associated with CTLA-4 or PD-1 pathway blockade have been well-described and include a constellation of autoimmune or immune-related AEs (irAEs) [127,128]. Toxicities affect a variety of organ systems including the GI tract (colitis, diarrhea), lung (pneumonitis), endocrine system (hypophysitis, thyroiditis), liver (hepatitis) and skin (rash, pruritus), among others. While skin and gastrointestinal events usually appear within 1–2 cycle of dual blockade, hepatitis, pneumonitis and endocrine side effects are later irAEs. In the Phase III trial testing the combination of nivolumab and ipilimumab in melanoma patients, it was reported that median onset for treatment-related irAEs ranged from 5 weeks for skin AEs to 15 weeks for renal AEs [37].
In the Phase III study of the combination of ipilimumab and nivolumab in advanced melanoma [37], observed toxicities were within the already described spectrum of irAEs for monotherapy CTLA-4 or PD-1 checkpoint blockade. No distinctly new toxicities were described. However, frequency of irAEs and the number of patients with multiple irAEs were notably higher than previously described for either single checkpoint inhibitor alone. In some cases, toxicities may be severe and potentially life threatening in rare cases [129].
As with single therapy CTLA-4 and PD-1 blockade, irAEs are reversible when managed according to standard algorithms that make use of immunosuppressive medications such as steroids, or if refractory, infliximab for diarrhea (not hepatitis) or mycophenolate mofetil (hepatitis). Long-term effects of combination therapy, and whether a different range of immune-mediated toxic effects will manifest with chronic exposure are yet to be observed. Additional details about specific irAEs and management strategies have been recently reviewed [127]. Studies of combined PD-1 and CTLA-4 blockade in melanoma and other tumor types suggest that with sufficient clinical experience and appropriate management, immune checkpoint inhibitors can be safely given in combination to patients [38,39].
Biomarkers for predicting clinical benefits of checkpoint inhibitors in combination
Although immune checkpoint inhibitors have shown promising safety and efficacy, to date only a fraction of patients achieve long-term survival, with severe irAEs occurring on occasion. Identification of ‘baseline’ (pretreatment) and on-treatment biomarkers is needed to enable physicians to select individualized treatments for their patients, thereby maximizing clinical benefits and minimizing toxicities. At this time, no single immunologic or tumoral characteristic in a patient has been found to solely determine response to an immunotherapeutic agent, but several biomarkers are undergoing evaluation.
PD-L1 expression
Most biomarker investigations for PD-1/PD-L1 checkpoint inhibitors have focused on the tumor microenvironment, specifically immunohistochemical (IHC) expression of one of the ligands for PD-1 and PD-L1. In many tumor types including bladder cancer and melanoma, patients whose tumors express PD-L1, as detected by IHC, have numerically higher response rates to PD-1/PD-L1 blockade than patients who do not express PD-L1 [1,4,130].
However, many unresolved issues surround PD-L1 as a possible biomarker of response for anti-PD-1 and anti-PD-L1 antibodies [131], and variations exist with different tumors types. In RCC, higher levels of PD-L1 expression have been associated with poorer survival in renal cell cancer (RCC), but not necessarily treatment benefit; rather the relationship between PD-L1 expression and outcomes after treatment with nivolumab appears to depend on tumor type and histologic class. In nonsquamous NSCLC, PD-L1 has a predictive role for second-line nivolumab with an association between PD-L1 expression and benefit from anti-PD-1 treatment [132]. In squamous NSCLC, the expression of PD-L1 was neither prognostic nor predictive for benefit with nivolumab [4]. Patients who do not express PD-L1 can still have good responses to PD-1 therapy. It remains unclear whether differential expression levels of PD-L1 among various tumor types account for the somewhat different response rates observed.
Notably also, the value of predictive markers may change markedly with combination therapy. For instance, in melanoma biopsy samples taken from patients before treatment, PD-L1 expression was not predictive of overall response rate in patients undergoing concomitant CTLA-4 and PD-1 blockade [34,36–37]. It must be emphasized that testing PD-1 and PD-L1 expression in pretreatment biopsy samples provides only a single static evaluation of immune expression, and fails to capture dynamic changes that inevitably occur with immunotherapy treatment. Using biomarkers to guide the clinical management of combinations is an area that still needs to be more well-defined and warrants further study [8].
Tumor-infiltrating lymphocytes
The presence of TILs is associated with an ongoing immune response [133,134], and may predict a response to immunotherapy with PD1-specific or PD-L1-specific antibodies [135]. Clinical studies have shown that CTLA-4 inhibition increases the number of TILs in the tumor microenvironment, as assessed by IHC [136] and gene expression profiling [137]. These studies also showed possible associations with clinical activity relative to baseline biopsies in TILs, expression of immune-related genes and CD8+ T-cell/FOXP3+ T-cell ratios [136–138]. As only associations have been identified, however, these studies do not constitute identification of a definitive biomarker.
Biomarkers related to immunosuppression
Other immune cell types recruited to the tumor can suppress an anti-tumor immune response. Tumors are rendered resistant to attack owing to the expression of PD-L1 and the production of TGFβ, indoleamine 2,3-dioxygenase 1 (IDO1) and other immunosuppressive compounds and molecules (FOX-P3) by tumor-associated macrophages, immature tumor-associated DCs, Treg cells, IL-10-producing regulatory B cells, MDSCs and tumor cells themselves [139]. IHC and genetic profiling of the tumor microenvironment may therefore be used to categorize cancers according to their immunosuppressive mechanisms and thus may ultimately be used to explore agents targeting these immunosuppressive elements.
Genetic analysis with whole-exome sequencing, gene expression profiling
Emerging data, both in model systems and in patients, suggest that mutation-derived tumor antigens may serve as the primary targets of T cells activated by immune checkpoint blockade and vaccine therapy [140–143]. The molecular identity of antigens can be expressed by malignant cells, and recognized by host T cells is well-established [144]. However, it is also becoming increasingly clear that many of these shared antigens are expressed in the tumor microenvironment and also at some level by self-tissues, either in peripheral cells or in the thymus. This can lead to immunologic tolerance for the highest-avidity interactions between peptide and MHC–TCR complex, and limit therapeutic efficacy.
Gene expression profiling of the tumor samples has shown that patients with clinical activity had higher baseline expression of immune-related genes than those without clinical activity [2,145]. Ongoing whole-exome sequencing studies are also investigating whether mutant antigens are relevant to immunotherapy outcomes [146].
Conclusion & future perspectives
The emergence of checkpoint blockade in the last decade has ushered in great promise in the treatment of a rapidly expanding spectrum of solid tumors including NSCLC, renal cell cancer, ovarian cancer, bladder cancer, head and neck cancer and gastric cancer. However, even with immune checkpoint blockade, a substantial proportion of patients fails to derive clinical benefit. Combination strategies with conventional and immunotherapies are needed to increase clinical benefit and minimize adverse toxicities.
On the basis of currently available efficacy data, it is likely immunotherapy combinations will be built on the foundation of checkpoint inhibition in particular PD-1–PD-L1 blockade. Combinations should be developed in context of the hallmark mechanisms of immunotherapy: induction of immunogenic cell death in strategies of in situ vaccination as well as radiation and chemotherapy; priming of T-cell responses including vaccines and adoptive T-cell therapy directed to neoantigens presented by tumor cells; enhancement of T-cell activity with costimulatory and/or local proinflammatory agents; and targeting of compensatory immunosuppression in T-cell anergy and/or exhaustion. Best strategies will consider the compatibility and synergy of combination treatments – whether simultaneously or sequentially – to mediate antitumor efficacy and minimize toxicity.
The decision to move new combinations into the clinic should be informed by preclinical data in animal models and mechanistic immunologic evidence. The main issues regarding the ideation and conduction of clinical trial for immunotherapy are the choice of adequate end points, the use of modified statistical methods and search for predictive biomarkers [147], and are currently undergoing investigation. The art of finding synergistic combinations with checkpoint inhibition requires a dialogue between scientists in preclinical and clinical investigation for the next-generation of immune-oncology agents. By understanding the immunobiology present in specific patients, immune-related biomarkers may allow us to tailor immune therapies and combinations to achieve best clinical benefit.
Executive summary.
Immune ‘checkpoint inhibitors’ block negative regulators of T-cell immunity such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death receptor-1 (PD-1) and ligand 1 (PD-L1), unleashing an antitumor immune response.
-
The US FDA has approved several checkpoint inhibitors in the treatment of a variety of solid cancers including to date:
– CTLA-4 blocking antibody, ipilimumab (Bristol-Myers Squibb) in melanoma;
– PD-1 blocking antibodies in advanced melanoma (pembrolizumab – Merck; nivolumab – Bristol-Myers Squibb), lung (pembrolizumab and nivolumab) and renal cancer (nivolumab).
Single agent PD-1 and CTLA-4 checkpoint blockade has demonstrated antitumor activity across multiple solid tumor types, but responding patients are still in the minority, underscoring the need for combination strategies to increase clinical benefit and reduce toxicities.
Rational design of checkpoint inhibitor combinations considers potential synergy with different hallmark mechanisms of cancer immunotherapy including immunogenic cell death; antigen release and presentation; priming and activation of T-cell responses; reversal T-cell dysfunction; lymphocytic infiltration into tumors; and depletion of compensatory immunosuppression.
Great successes of checkpoint blockade, particularly of the PD-1–PD-L1 pathway, suggest that PD-1 inhibitors may become the preferred building blocks for future checkpoint combinations.
The FDA has approved the use of checkpoint combination nivolumab and ipilimumab in treatment for patients with unresectable or metastatic melanoma, and this combination anti-PD-1 and CTLA-4 blockade is being explored in other solid cancers such as renal cell carcinoma and NSCLC in Phase III studies.
-
Other potential synergistic combinations in ongoing investigation include:
– Checkpoint blockade with ‘traditional’ therapies inducing immunogenic cell death (radiation, chemotherapy and targeted molecular therapies);
– Combinations with agents that prime the immune response (cancer vaccines, oncolytic viruses);
– Combinations with immunostimulatory monoclonal antibodies (CD137 [4-IBB], CD357 [GITR], among others);
– Combinations with agents that reverse immunosuppression (indoleamine 2,3-dioxygenase inhibitors, anti-CD-25 antibodies and anti-CCR4 antibody).
-
Until additional knowledge is obtained, some checkpoint combinations are not recommended:
– Ipilimumab and BRAF +/- MEK inhibitors are not recommended due to significant hepatotoxicity observed;
– Tremelimumab and sunitinib in metastatic renal cancers due to significant nephrotoxicity.
Toxicities related to combination CTLA-4 and PD-1 blockade are similar to that of (but in higher frequency compared with) single agent checkpoint blockade, and include a constellation of tissue-specific inflammatory events referred to as immune-related adverse events, including colitis, pneumonitis, among others.
Immune-related adverse events are reversible when managed according to standard algorithms that make use of immunosuppressive medications such as steroids or if refractory, infliximab for diarrhea (not hepatitis) or mycophenolate mofetil (hepatitis).
No single immunologic or tumoral characteristic in a patient has been found to solely determine response to an immunotherapeutic agent, but several biomarkers are undergoing evaluation, including PD-L1 expression, tumor-infiltrating lymphocytes and gene expression profiling.
Footnotes
Financial & competing interests disclosure
MA Postow has received research support from Bristol-Myers Squibb and has served on advisory councils. MA Postow has also received honoraria from Bristol-Myers Squibb and Merck. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl Acad. Sci. USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seiwert TY, Burtness B, Weiss J, et al. A Phase 1 b study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV associated head and neck (H/N) cancer. J. Clin. Oncol. 2014;32(5s) Abstract 6011. [Google Scholar]
- 6.Rizvi NA, Garon EB, Patnaik A, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2014;32(5s) Abstract 8007. [Google Scholar]
- 7.Pilotto S, Kinspergher S, Peretti U, et al. Immune checkpoint inhibitors for non-small-cell lung cancer: does that represent a ‘new frontier’? Anticancer Agents Med. Chem. 2015;15(3):307–313. doi: 10.2174/1871520614666141110170259. [DOI] [PubMed] [Google Scholar]
- 8.Melero I, Berman DM, Aznar MA, Korman AJ, Perez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer. 2015;15(8):457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 9.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat. Rev. Immunol. 2002;2(6):439–446. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo . J. Exp. Med. 1987;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust. J. Exp. Biol. Med. Sci. 1975;53(1):27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 12.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 13.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013;94(1):25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer. 2007;7(2):95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 16.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 17.Hodi FS, O'day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 19.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 20.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 21.Flies DB, Sandler BJ, Sznol M, Chen L. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J. Biol. Med. 2011;84(4):409–421. [PMC free article] [PubMed] [Google Scholar]
- 22.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer response. Clin. Cancer. Res. 2013;19(19):5542. doi: 10.1158/1078-0432.CCR-13-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sierro S, Romero P, Speiser DE. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin. Ther. Targets. 2011;15(1):91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 24.Kouo T, Huang L, Pucsek AB, et al. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T Cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 2015;3(4):412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr. Opin. Immunol. 2012;24(2):213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4(11):1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 28.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fourcade J, Sun Z, Pagliano O, et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res. 2014;74(4):1045–1055. doi: 10.1158/0008-5472.CAN-13-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korman A, Chen B, Wang C, Wu L, Cardarelli P, Selby M. Activity of anti- PD-1 in murine tumor models: role of “host” PD-L1 and synergistic effect of anti-PD-1 and anti-CTLA-4. J. Immunol. 2007;178(S82):48.1–48.40. [Google Scholar]
- 32.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl Acad. Sci. USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015;14(8):561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first report of a successful immunotherapy combination in the clinic.
- 35.Sznol MEA. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) J. Clin. Oncol. 2014;32(Suppl. 5s) Abstract LBA9003. [Google Scholar]
- 36.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Presents the data of the first randomized controlled trial that compared ipilimumab plus nivolumab versus ipilimumab alone in patients with metastatic melanoma.
- 37.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammers HEA. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) J. Clin. Oncol. 2014;32(Suppl. 5s) Abstract 4504. [Google Scholar]
- 39.Antonia SJ, Gettinger SN, Chow LQ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: interim Phase I results. J. Clin. Oncol. 2014;32(Suppl.) Abstract 8023. [Google Scholar]
- 40.Al RNE. 16th World Conference on Lung Cancer. CO, USA: 6–9 September 2015. Safety and efficacy of first-line nivolumab and ipilimumab in non-small cell lung cancer. Presented at. Abstract ORAL 02.05. [Google Scholar]
- 41.Al AE. Phase Ib study of MEDI4736, a programmed cell death ligand-1 (PD-L1) antibody, in combination with tremelimumab, a cytotoxic T- lymphocyte-associated protein-4 (CTLA-4) antibody, in patients (pts) with advanced NSCLC. J. Clin. Oncol. 2015;33 Abstract 3014. [Google Scholar]
- 42.Al PE. Phase I study of pembrolizumab (pembro; MK-3475) plus ipilimumab (Ipi) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort. J. Clin. Oncol. 2015;33(Suppl.) Abstract 8011. [Google Scholar]
- 43.Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 46.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter Phase II study. J. Clin. Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]; •• This important Phase II trial in patients with stage IIIb/IV non-small-cell lung cancer showed that carboplatin-paclitaxel could be safely combined with ipilimumab. Interestingly a ‘phased regimen’ in which immunotherapy began after chemotherapy resulted in substantially improved progression-free survival compared with chemotherapy alone.
- 47.Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol. Res. 2014;2(7):632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesniere A, Apetoh L, Ghiringhelli F, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 2008;20(5):504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 2008;118(6):1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kroemer G, Zitvogel L. Abscopal but desirable: the contribution of immune responses to the efficacy of radiotherapy. Oncoimmunology. 2012;1(4):407–408. doi: 10.4161/onci.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer. Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 52.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first case report describing the abscopal effect in a metastatic melanoma patient.
- 54.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 2013;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter Phase I/II study. Ann. Oncol. 2013;24(7):1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, Phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 2011;8(3):151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 59.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol. Res. 2015;3(5):436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58(23):5301–5304. [PubMed] [Google Scholar]
- 61.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter Phase 2 trial. Ann. Oncol. 2013;24(1):75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 62.Andre N, Carre M, Pasquier E. Metronomics: towards personalized chemotherapy? Nat. Rev. Clin. Oncol. 2014;11(7):413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 63.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 2011;17(9):1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cavnar MJ, Zeng S, Kim TS, et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J. Exp. Med. 2013;210(13):2873–2886. doi: 10.1084/jem.20130875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilotto S, Molina-Vila MA, Karachaliou N, et al. Integrating the molecular background of targeted therapy and immunotherapy in lung cancer: a way to explore the impact of mutational landscape on tumor immunogenicity. Transl. Lung Cancer Res. 2015;4(6):721–727. doi: 10.3978/j.issn.2218-6751.2015.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al RE. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J. Clin. Oncol. 2014;32(Suppl. 5s) Abstract 8022. [Google Scholar]
- 68.Callahan MK, Masters G, Pratilas CA, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol. Res. 2014;2(1):70–79. doi: 10.1158/2326-6066.CIR-13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vella LJ, Pasam A, Dimopoulos N, et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol. Res. 2014;2(4):351–360. doi: 10.1158/2326-6066.CIR-13-0181. [DOI] [PubMed] [Google Scholar]
- 70.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 2013;368(14):1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 72.Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N. Engl. J. Med. 2012;366(9):866–868. doi: 10.1056/NEJMc1114329. [DOI] [PubMed] [Google Scholar]
- 73.Minor DR, Puzanov I, Callahan MK, Hug BA, Hoos A. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res. 2015;28(5):611–612. doi: 10.1111/pcmr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rini BI, Stein M, Shannon P, et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011;117(4):758–767. doi: 10.1002/cncr.25639. [DOI] [PubMed] [Google Scholar]
- 76.Ozono S. [Nivolumab (Anti-PD-1 antibody; ONO-4538/BMS-936558) in renal cancer] Gan To. Kagaku Ryoho. 2014;41(9):1077–1080. [PubMed] [Google Scholar]
- 77.Vonderheide RH, Lorusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer. Res. 2010;16(13):3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 78.Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111(12):5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 79.Sabbatini P, Tsuji T, Ferran L, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin. Cancer. Res. 2012;18(23):6497–6508. doi: 10.1158/1078-0432.CCR-12-2189. [DOI] [PubMed] [Google Scholar]
- 80.Ito D, Ogasawara K, Iwabuchi K, Inuyama Y, Onoe K. Induction of CTL responses by simultaneous administration of liposomal peptide vaccine with anti-CD40 and anti-CTLA-4 mAb. J. Immunol. 2000;164(3):1230–1235. doi: 10.4049/jimmunol.164.3.1230. [DOI] [PubMed] [Google Scholar]
- 81.Met O, Wang M, Pedersen AE, Nissen MH, Buus S, Claesson MH. The effect of a therapeutic dendritic cell-based cancer vaccination depends on the blockage of CTLA-4 signaling. Cancer Lett. 2006;231(2):247–256. doi: 10.1016/j.canlet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 82.Pedersen AE, Buus S, Claesson MH. Treatment of transplanted CT26 tumour with dendritic cell vaccine in combination with blockade of vascular endothelial growth factor receptor 2 and CTLA-4. Cancer Lett. 2006;235(2):229–238. doi: 10.1016/j.canlet.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Saha A, Chatterjee SK. Combination of CTL-associated antigen-4 blockade and depletion of CD25 regulatory T cells enhance tumour immunity of dendritic cell-based vaccine in a mouse model of colon cancer. Scand. J. Immunol. 2010;71(2):70–82. doi: 10.1111/j.1365-3083.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 84.Espenschied J, Lamont J, Longmate J, et al. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J. Immunol. 2003;170(6):3401–3407. doi: 10.4049/jimmunol.170.6.3401. [DOI] [PubMed] [Google Scholar]
- 85.Ko HJ, Kim YJ, Kim YS, et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67(15):7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 86.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J. Clin. Invest. 2002;109(5):651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63(12):3281–3288. [PubMed] [Google Scholar]
- 88.Son CH, Bae JH, Shin DY, et al. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J. Immunother. 2014;37(1):1–7. doi: 10.1097/CJI.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 89.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J. Clin. Oncol. 2009;27(7):1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 90.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc. Natl Acad. Sci. USA. 1998;95(17):10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4–1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS ONE. 2011;6(4):e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le DT, Dubenksy TW, Jr., Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin. Oncol. 2012;39(3):311–322. doi: 10.1053/j.seminoncol.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerritsen W, Van Den Eertwegh AJ, De Gruijl T, et al. Expanded Phase I combination trial of GVAX immunotherapy for prostate cancer and ipilimumab in patients with metastatic hormone-refractory prostate cancer (mHPRC) J. Clin. Oncol. 2008;26(Suppl.):5146. [Google Scholar]
- 95.Chakraborty M, Schlom J, Hodge JW. The combined activation of positive costimulatory signals with modulation of a negative costimulatory signal for the enhancement of vaccine-mediated T-cell responses. Cancer Immunol. Immunother. 2007;56(9):1471–1474. doi: 10.1007/s00262-007-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a Phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Millward M, Underhill C, Lobb S, et al. Phase I study of tremelimumab (CP-675 206) plus PF-3512676 (CPG 7909) in patients with melanoma or advanced solid tumours. Br. J. Cancer. 2013;108(10):1998–2004. doi: 10.1038/bjc.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J. Clin. Oncol. 2012;30(3):322–328. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113(15):3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Totterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J. Immunother. 2010;33(3):225–235. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 101.Adair RA, Roulstone V, Scott KJ, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 2012;4(138):138ra177. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 103.Donnelly OG, Errington-Mais F, Steele L, et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20(1):7–15. doi: 10.1038/gt.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaufman HL, Kim DW, Deraffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 105.Kaufman HL, Deraffele G, Divito J, et al. A Phase I trial of intralesional rV-Tricom vaccine in the treatment of malignant melanoma. Hum. Gene Ther. 2001;12(11):1459–1480. doi: 10.1089/104303401750298616. [DOI] [PubMed] [Google Scholar]
- 106.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 107.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14(8):559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 108.Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer. Res. 2010;16(15):4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 109.Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6(226):226ra232. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front. Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shuford WW, Klussman K, Tritchler DD, et al. 4–1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997;186(1):47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kohrt HE, Houot R, Goldstein MJ, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117(8):2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 2005;202(7):885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002;3(2):135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 115.Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunol. Rev. 2011;244(1):218–231. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur. J. Immunol. 2007;37(1):157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 117.Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J. Exp. Med. 2014;211(4):715–725. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front. Biosci. 2012;4:734–745. doi: 10.2741/e414. [DOI] [PubMed] [Google Scholar]
- 119.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol. Med. 2004;10(1):15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 120.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013;210(7):1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gibney GT, Hamid O, Gangadhar TC, et al. Preliminary results from a Phase 1/2 study of INCB024360 combined with ipilimumab (ipi) in patients (pts) with melanoma. J. Clin. Oncol. 2014;32(Suppl.) Abstract 301. [Google Scholar]
- 123.Omid H GT, Smith DC, Bauer TM, et al. Society for Melanoma Research (SMR) Congress. CA, USA: 18–21 November 2015. Preliminary data from a Phase 1/2 study of epacadostat (INCB024360) with pembrolizumab (pembro) in patients with advanced/metastatic melanoma. Presented at. [Google Scholar]
- 124.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. NY Acad. Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 125.Sutmuller RP, Van Duivenvoorde LM, Van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ogura M, Ishida T, Hatake K, et al. Multicenter Phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. 2014;32(11):1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 127.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am. Soc. Clin. Oncol. Educ. Book. 2015:76–83. doi: 10.14694/EdBook_AM.2015.35.76. doi:10.14694/EdBook_AM.2015.35.76. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 128.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 130.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013;31(34):4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE. 2015;10(6):e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 134.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]; •• This is a comprehensive study on the search for biomarkers following PD-1–PD-L1 blockade.
- 135.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl Acad. Sci. USA. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ji RR, Chasalow SD, Wang LS, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immun. 2012;61(7):1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang RR, Jalil J, Economou JS, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin. Cancer Res. 2011;17(12):4101–4109. doi: 10.1158/1078-0432.CCR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first report showing that response to CTLA4 blockade is strongly correlated with tumor mutational burden in patients with metastatic melanoma. It also shows that mutations giving rise to class I HLA-presented neoantigens predict treatment benefit.
- 140.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc. Natl Acad. Sci. USA. 2011;108(40):16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes how neoantigens can be used as personalized cancer vaccines in combination with immune checkpoint inhibitors.
- 142.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Rooij N, Van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 2013;31(32):e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brichard VG, Lejeune D. GSK's antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;25(Suppl. 2):B61–B71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 145.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16(4):399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 146.Boon T, Gajewski TF, Coulie PG. From defined human tumor antigens to effective immunization? Immunol. Today. 1995;16(7):334–336. doi: 10.1016/0167-5699(95)80149-9. [DOI] [PubMed] [Google Scholar]
- 147.Pilotto S, Carbognin L, Karachaliou N, et al. Moving towards a customized approach for drug development: lessons from clinical trials with immune checkpoint inhibitors in lung cancer. Transl. Lung Cancer Res. 2015;4(6):704–712. doi: 10.3978/j.issn.2218-6751.2015.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen H, Liakou CI, Kamat A, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc. Natl Acad. Sci. USA. 2009;106(8):2729–2734. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]