Abstract
There are over 400 ongoing clinical trials using tumor-derived vaccines. This approach is especially attractive for many types of brain tumors, including glioblastoma, yet so far the clinical response is highly variable. One contributor to poor response is CD200, which acts as a checkpoint blockade, inducing immune tolerance. We demonstrate that, in response to vaccination, glioma-derived CD200 suppresses the anti-tumor immune response. In contrast, a CD200 peptide inhibitor that activates antigen-presenting cells overcomes immune tolerance. The addition of the CD200 inhibitor significantly increased leukocyte infiltration into the vaccine site, cytokine and chemokine production, and cytolytic activity. Our data therefore suggest that CD200 suppresses the immune system’s response to vaccines, and that blocking CD200 could improve the efficacy of cancer immunotherapy.
Keywords: : CD200 protein, checkpoint blockade, immunotherapy
Despite four decades of intense research into vaccine-based strategies for fighting cancer, the majority of immunotherapies against solid tumors still fail to achieve beneficial outcomes. This is especially true for the CNS tumor glioblastoma multiforme (GBM). A recent search on ClinicalTrials.gov revealed over 400 open clinical trials using tumor cells as a source of antigens to stimulate an anti-tumor response; 25 of these are directed toward CNS tumors.
The use of tumors as a source of tumor-associated antigens clearly has advantages; however, most cancers have robust mechanisms for evading the immune system [1]. Immune checkpoint inhibitory ligands and their receptors tightly control T-cell activation, maintaining self-tolerance and limiting immune-mediated collateral tissue damage. Checkpoint blockades such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 receptor (PD-1) [2] have been targeted in multiple clinical trials, demonstrating some success (reviewed in [1]) [3]. We have extensively studied another checkpoint blockade (CD200/CD200R) responsible for shutting down the immune system [4,5] making the CD200 blockade interaction an important target for cancer immunotherapy [6–8].
CD200 has been well characterized as an immunosuppressive protein that inhibits immune responses through its receptor [9–11]. In healthy individuals, CD200 is distributed on a wide variety of tissues, including B cells, activated T cells, certain vascular endothelia, kidney, placenta cells and neurons [12]. In contrast to the distribution of CD200 ligand, its receptor, CD200 receptor (CD200R), is mainly expressed on myeloid cells (monocytes, granulocytes, dendritic cells). CD200R is also expressed on T cells and B cells, inactivating leukocytes through negative immune signals [13–15]. High expression of CD200R has also been detected on differentiated central and effector memory T cells. CD200R expression is particularly apparent in polarized Th2 cells [16], resulting in the expansion of regulatory T cells [17–19].
CD200 is expressed on tumors such as chronic lymphocytic leukemia [11], multiple myeloma [6], acute myeloid leukemia [20], melanoma [21], ovarian cancer [22], metastatic small cell carcinoma [23], GBM [4] and on the murine glioma GL261 (Figure 1A). In addition, tumor progression and poor patient outcome have been shown to correlate with the presence of soluble CD200 [24]. Wong et al. [24] reported that soluble CD200 levels in the plasma of chronic lymphocytic leukemia patients correlate with tumor burden and disease state. In our Phase I vaccine trial, we demonstrated increasing levels of CD200 in the serum of our GBM and ependymoma immunotherapy patients upon tumor recurrence [4].
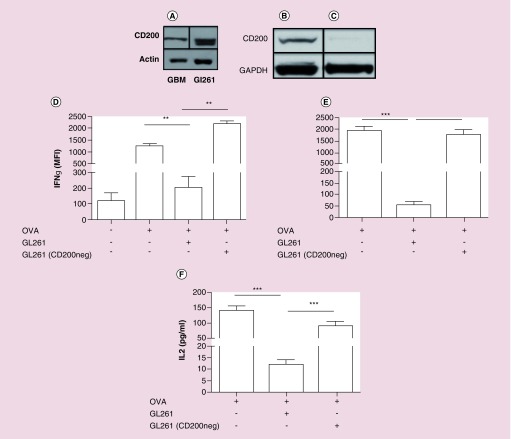
Figure 1. . Absorbing CD200 out of tumor-derived vaccines enhances immunogenicity.
(A) Human and mouse gliomas were analyzed by western analysis for CD200. (B & C) CD200 was absorbed out of murine GL261 tumor lysates and used to pulse (D) OT-1 splenocytes and (E) bone marrow derived dendritic cells with OVA as an immune stimulant with either wild-type GL261 or GL261 (CD200neg) tumor lysates. Error bars are ± SEM, asterisk represent a statistical significance *p < 0.05, **p = 0.005 or ***p = 0.0005 determined by unpaired t-test. Experiments are representative of three separate experiments.
OVA: Ovalbumin.
Absorbing CD200 out of tumor-derived vaccines enhances immunogenicity
Because CD200 is expressed on tumors, we hypothesized that we are suppressing the immune system with the tumor-derived vaccines designed specifically to induce an anti-tumor immune response. To test our hypothesis, we depleted CD200 from our tumor lysates using immunoprecipitation (Figure 1B & C). OT-I splenocytes were pulsed with ovalbumin (OVA) + GL261 tumor lysate (GL261) or GL261 depleted of CD200 (GL261 (CD200neg)). GL261 significantly suppressed the ability of OVA to induce an immune response (p = 0.009), which was reverted by depleting CD200 from the vaccine (p = 0.003) (Figure 1D). Because CD200 acts on antigen-presenting cells [19], we repeated this experiment with bone marrow-derived dendritic cells (DCs). Our experiments recapitulated the findings in Figure 1D that, compared with OVA alone, tumor lysates containing CD200 inhibited IFN-γ (p = 0.001) and IL-2 (p = 0.005) production, a result which was reversed by depleting CD200 (p = 0.001) (IFN-γ), p = 0.001 (Figure 1E) and (IL-2) (Figure 1F).
CD200 inhibitor blocks immune suppression from tumor-derived vaccines
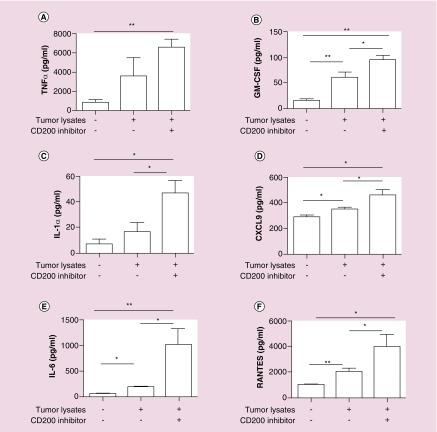
Targeting receptor–ligand interactions has become increasingly important, as indicated by CD200/CD200 receptor (CD200R) in leukemia cells and CD47/SIRP in many cancers cells [11,25–27]. We developed a peptide inhibitor targeting the CD200R isoform activation receptors [4]. Purified CD11b cells from wild-type mice were pulsed with tumor lysate containing CD200, with or without the CD200 inhibitor. In these experiments, with the exception of TNF-α and IL1α (p = 0.07 and p = 0.12 respectively), tumor lysates alone elicited a statistically significant cytokine response (p = 0.003 (GM-CSF), p = 0.012 (IL6), p = 0.02 (CXCL9) and p = 0.006 (RANTES) compared with no pulse controls. The CD200 inhibitor treatment group achieved a statistically significant enhanced immune response p = 0.004 (TNF-α), p = 0.001 (GM-CSF), p = 0.033 (IL1α), p = 0.015 (CXCL9), p = 0.001 (IL6) and p = 0.013 (RANTES) compared with no pulse control and p = 0.015 (GM-CSF), p = 0.023 (IL1α), p = 0.015 (CXCL9), p = 0.015 (IL6) and p = 0.046 (RANTES) compared with tumor lysate groups alone (Figures 2A–F). We observed enhanced secretion of TNF-α when adding the CD200 inhibitor to tumor lysates, however, these results failed to reach statistical significance (p = 0.069).
Figure 2. . CD200 peptide inhibitor blocks the suppressive properties of CD200.
(A–F) CD11b cells were isolated from C57BL/6 wild-type mice were pulsed with tumor lysates derived from wild-type Gl261 cells +/- the CD200 peptide inhibitor. Supernatants were analyzed for chemokine and cytokine secretion. Error bars are ± SEM, asterisk represent a statistical significance *p < 0.05 or **p = 0.005 determined by unpaired t-test. Experiments are representative of three separate experiments.
CD200 inhibitor enhances an antigen-specific response
To generate a tumor-specific immune response, CD8 T cells undergo priming by DC, the antigen-presenting cell most efficient at initiating potent CD8+ T-cell responses [28,29]. Currently, the efficacy of ex vivo derived DC immunotherapy is not well established for human cancers [30–33]. The limited success of these immunotherapies has been attributed to a variety of factors, including the preparation and administration of the vaccine, the disease stage of the participants in experimental trials, or the heterogeneous nature of most tumors. We suggest the failure to elicit an anti-tumor response is due to CD200 in tumor-derived vaccines used to activate DC.
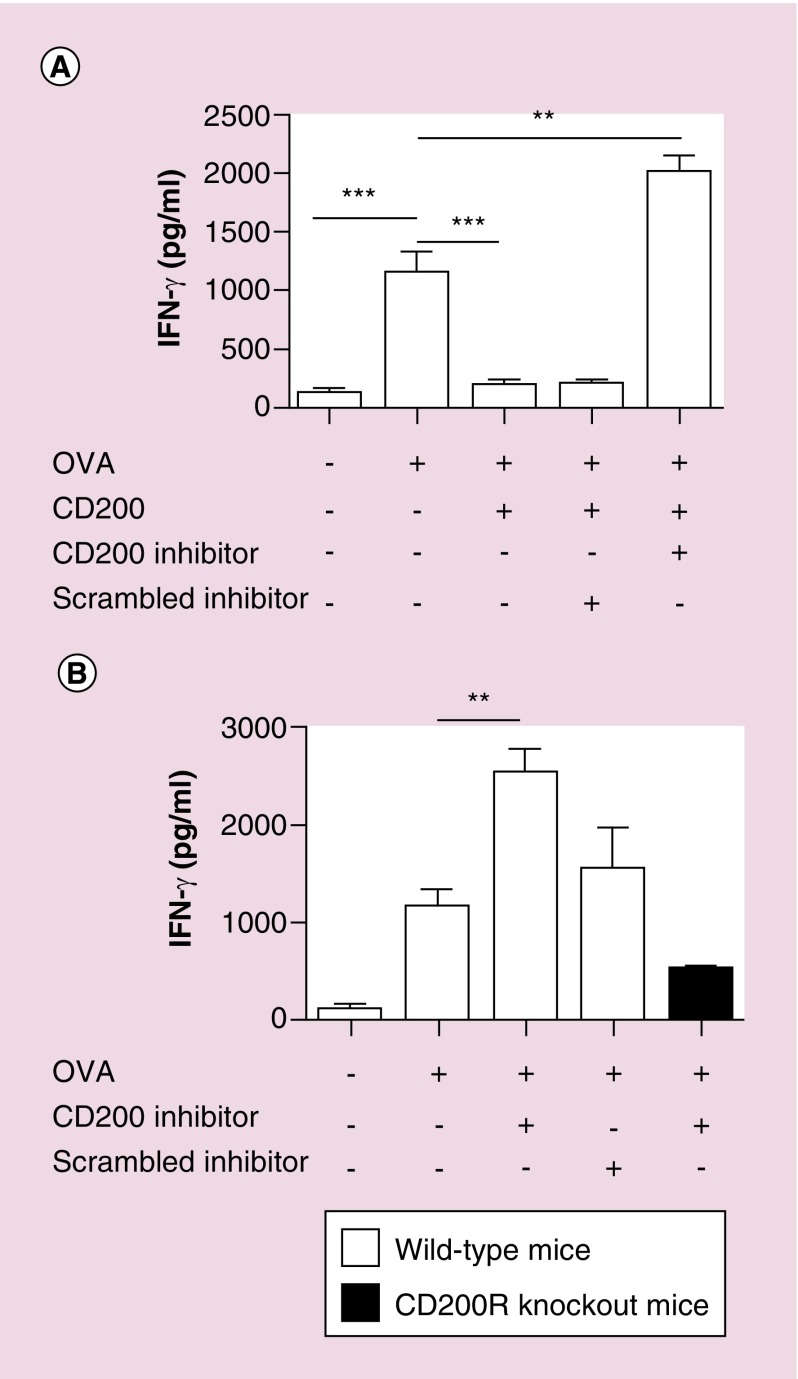
To test this, bone marrow-derived DC from wild-type mice were pulsed with OVA + CD200 with or without the CD200 inhibitor. Following 24 h incubation, cells were washed to remove any free inhibitor, then incubated with purified OT-I cells. As previously demonstrated in vivo [4], the CD200 inhibitor blocked the suppressive effects of CD200, reverting to an antigen-specific OVA immune response (Figure 3A). OVA significantly enhanced an IFN-γ response (p = 0.007), which was suppressed with the addition of CD200 (p = 0.009). The addition of the CD200 inhibitor overpowered the suppressive properties of the CD200 protein, significantly enhancing an immune response (p = 0.003), as compared with using OVA alone. Interestingly, in these experiments, we observed that cells pulsed with CD200 inhibitor + OVA significantly enhanced the immune response (p = 0.001) (Figure 3B) compared with OVA treated cells. These studies led us to hypothesize that the CD200 inhibitor activates antigen-presenting cells.
Figure 3. . CD200 inhibitor enhances an antigen-specific response.
(A & B) Bone marrow-derived dendritic cells from wild-type C57Bl/6 or CD200R KO mice were pulsed with OVA, OVA + CD200, OVA + CD200 + CD200 inhibitor or OVA + CD200 + scrambled inhibitor. Following 24 h incubation, cells were washed cells were washed, and purified OT-I CD8 T cells were added. Following 48 h incubation, supernatants were analyzed for IFN-γ production. Experiments are representative of three separate experiments. Error bars are ± SEM, asterisks represent a statistical significance *p < 0.05, **p = 0.005 or ***p = 0.0005 determined by unpaired t-test.
CD200 inhibitor modifies gene expression
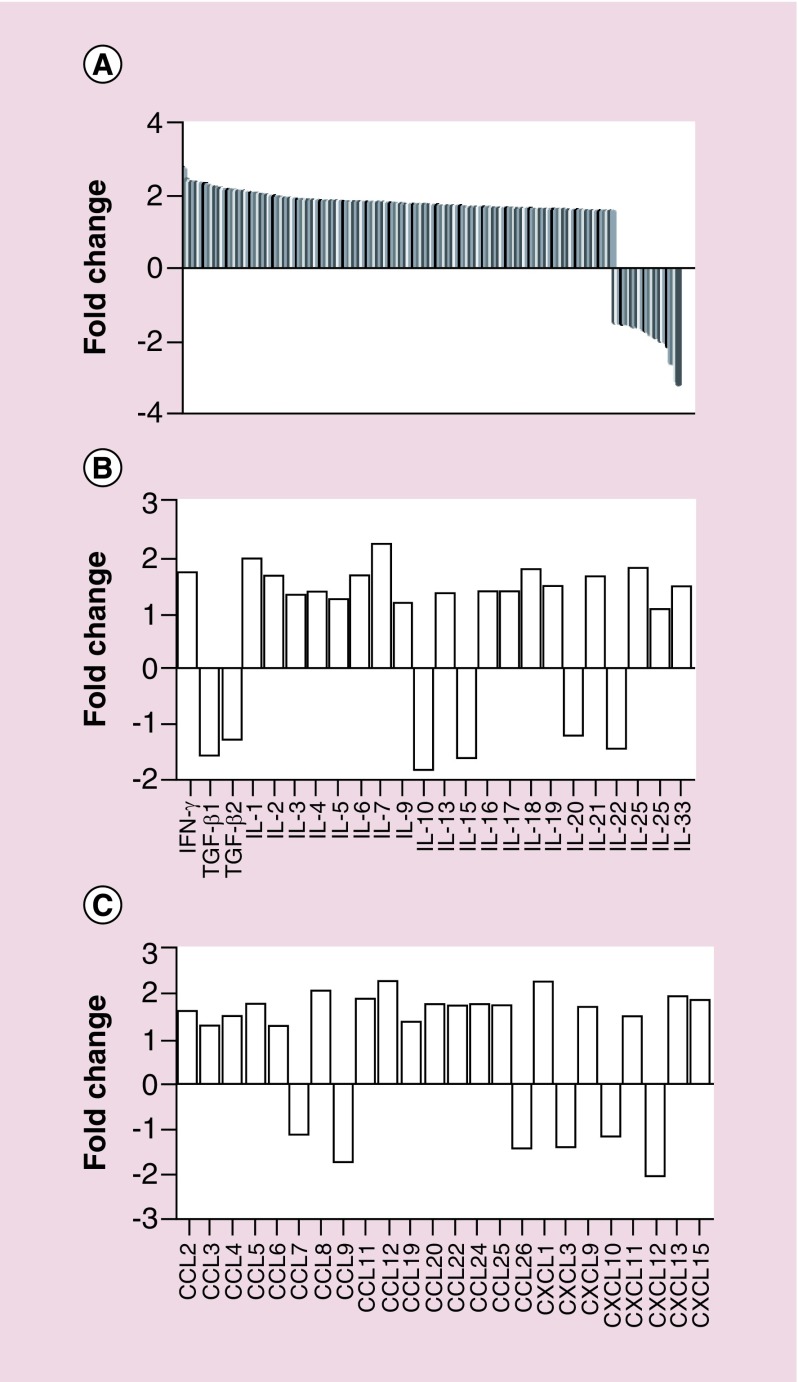
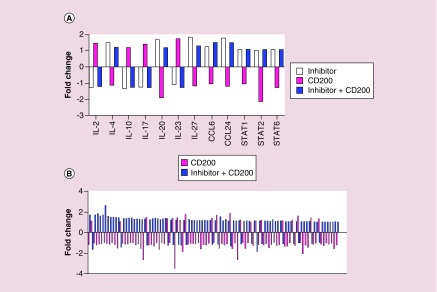
To test our hypothesis that the CD200 inhibitor activates antigen-presenting cells, CD11b cells from wild-type splenocytes were pulsed with CD200 protein, CD200 inhibitor or a combination of CD200 protein + CD200 inhibitor and analyzed by NanoString for 575 immune-related genes. All treatment groups were normalized to no pulse controls. In these experiments, 194 immune-related genes had a ± 1.5-fold change following pulsing with the CD200 inhibitor alone (Figure 4A–C).
Figure 4. . CD200 inhibitor modifies gene expression.
Purified CD11b cells isolated from wild-type C5Bl/6 mice were pulsed with CD200 inhibitor. RNA was isolated and analyzed by NanoString for 575 immune-related genes. Bars represent a (A) ± twofold change or (B & C) ± 1.5-fold change.
When we compared all three treatment groups, we observed that 98 genes within the CD200 protein group had an opposite response compared with genes within the CD200 inhibitor or CD200 protein + CD200 inhibitor treatment groups (Figure 5A & B). These experiments demonstrated that the CD200 inhibitor reversed the inhibitory signaling induced by the CD200 protein.
Figure 5. . CD200 inhibitor reverses CD200 protein inhibitory signals.
Purified CD11b cells isolated from wild-type C5Bl/6 mice were pulsed with a CD200 protein, CD200 inhibitor or a combination of CD200 protein and CD200 inhibitor. RNA was isolated and analyzed by NanoString for 575 immune-related genes. Bars represent a ± 1.5-fold change.
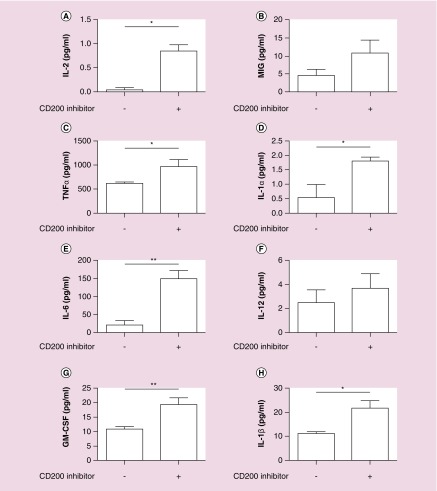
To determine if the CD200 inhibitor activated functional responses, DCs were pulsed with the CD200 inhibitor alone. These experiments revealed that the CD200 inhibitor activated DCs, statistically enhancing the production of IL-2, TNF-α, IL-1α, IL-6, GM-CSF and IL-1β (p = 0.01, p = 0.02, p = 0.04, p = 0.001, p = 0.004 and p = 0.02 respectively) (Figure 6A–H). We observed enhanced CXCL9 and IL-12 production, however, responses failed to reach statistical significance (p = 0.055 and p = 0.32, respectively).
Figure 6. . CD200 inhibitor stimulates dendritic cells.
(A–H) Bone marrow-derived dendritic cells from wild-type C57Bl/6 mice were pulsed with CD200 inhibitor. Following 48-h incubation, supernatants were analyzed for chemokine and cytokine production. Error bars are ± SEM, asterisk represent a statistical significance *p < 0.05 or **p = 0.005 determined by unpaired t-test.
CD200 inhibitor enhances leukocyte trafficking into the vaccine site
GM-CSF is often used in vaccines to enhance the infiltration of antigen-presenting cells into the vaccine site for antigen uptake and presentation [34]. We found that the CD200 inhibitor enhanced production of GM-CSF (Figure 6G) in vitro. Therefore, in the next set of experiments, non-tumor-bearing wild-type and CD200R KO mice were vaccinated with tumor lysates or CD200 inhibitor alone. Twenty-four hours later, mice were revaccinated with tumor lysates + CpG-ODN or tumor lysates + CpG-ODN + CD200 inhibitor, respectively. In one of the treatment groups, mice were vaccinated with the CD200 inhibitor 1 h prior to revaccination with tumor lysates + CD200 inhibitor (Figure 7A–C).
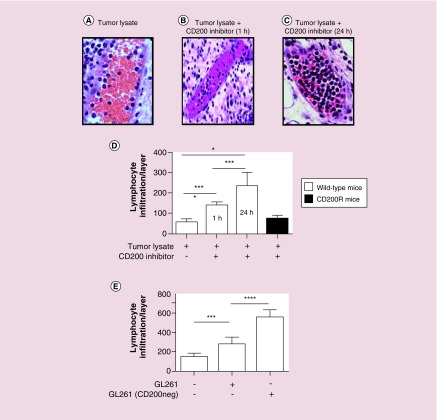
Figure 7. . CD200 inhibitor enhances leukocyte trafficking into the vaccine site.
Non-tumor-bearing C57Bl/6 or CD200R knockout mice were vaccinated with tumor lysates or CD200 inhibitor, either 1 or 24 h later, mice were revaccinated with (A) tumor lysates + CpG or (B) tumor lysates, CD200 inhibitor + CpG (1 h revaccination). (C) Tumor lysates, CD200 inhibitor + CpG (24-h revaccination). 6 h later, skin from the vaccine sites was harvested and analyzed by H&E staining. (D) Leukocytes within blood vessels in eight separate skin levels were counted. (E) In separate experiments, mice were vaccinated with wild-type GL261 lysates or GL261 lysates void of CD200. 24 h later, mice were revaccinated with either wild-type GL261 lysates or GL261 lysates void of CD200 + CpG. 6 h later, skin was harvested and leukocytes within blood vessels in eight separate skin levels were counted. Error bars are ± SEM, asterisks represent a statistical significance *p < 0.05, **p = 0.005 and ***p = 0.0005 determined by unpaired t-test.
6 h following revaccination, skin at the vaccine site was harvested and analyzed for leukocyte infiltration. No significant leukocyte infiltration was observed in saline vaccinated controls or in CD200R KO mice vaccinated with tumor lysates + CD200 inhibitor (data not shown). To quantify our results, vascular leukocytes from eight layers of tissue were counted (Figure 7D). These experiments demonstrated enhanced leukocyte infiltration into the vaccine site with as little as 1-h prevaccination with the CD200 inhibitor (p = 0.001; 1 h and p = 0.001 24 h) (Figure 7D). Moreover, knocking out the CD200 receptor failed to enhance leukocyte infiltration (p = 0.087).
These experiments demonstrated that while we were capable of eliciting an immune response using tumor-derived vaccines, the response failed to recruit antigen-presenting cells to the site of vaccination for antigen uptake. We next wanted to see how removing CD200 from tumor lysate vaccines influenced leukocyte infiltration. In these experiments, non-tumor-bearing wild-type mice were vaccinated with tumor lysate or tumor lysate void of CD200. 24 h later, mice were revaccinated with tumor lysate + CpG-ODN or tumor lysate void of CD200 + CpG-ODN, respectively (Figure 7E). As seen in the above experiments, we observed a significant infiltration of leukocytes into the site of vaccination (p = 0.004), however, removal of CD200 profoundly enhanced leukocyte infiltration (p = 0.0001) (Figure 7E).
CD200 inhibitor enhances an antitumor response
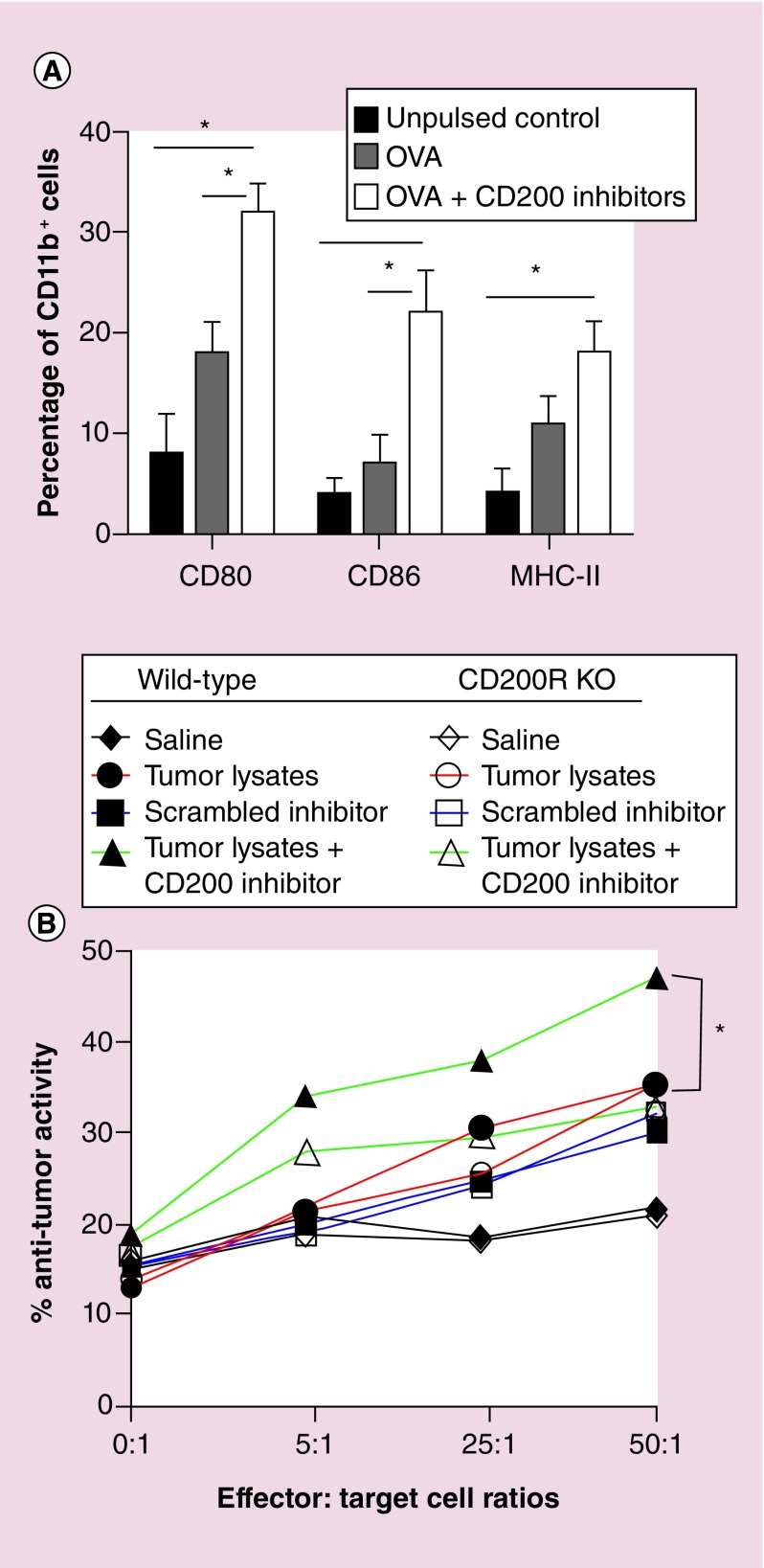
In the next set of experiments, we investigated the effects of CD200 inhibitor on the expression of costimulatory molecules. In these experiments, we used the OVA protein due to the SIINFEKL antigen’s ability to stimulate an immune response. Purified CD11b cells isolated from wild-type mice were pulsed with OVA or OVA + CD200 inhibitor. Following 24-h incubation, CD200 inhibitor significantly enhanced CD80, CD86 and MHC-II expression (p = 0.012, p = 0.028 and p = 0.038, respectively) as compared with no pulse controls (Figure 8A). CD200 inhibitor significantly enhanced the expression of CD80 and CD86 (p = 0.032 and 0.018, respectively) compared with the OVA alone treatment group.
Figure 8. . CD200 inhibitor enhances an antitumor response.
(A) Purified CD11b cells from wild-type C57Bl/6 cells were pulsed with OVA ± CD200 inhibitor. Forty-eight hours later, cells were analyzed for CD80/86 and MHC-II expression. (B) Tumor-bearing wild-type (solid symbols) or CD200 receptor knockout (CD200R KO) (open symbols) mice were vaccinated in the back of the neck with saline (black lines), wild-type GL261 tumor lysates (red lines), tumor lysates + scrambled CD200 inhibitor (blue lines) or tumor lysate + CD200 inhibitor (green lines). 20 days post vaccination, lymphocytes from cervical lymph nodes were harvested, incubated for 6 h with wild-type GL261 cells and analyzed for cytolytic activity. Asterisks represent statistical significance *p < 0.05 determined by two-way ANOVA.
To determine whether the use of our CD200 inhibitor would enhance functional responses, we used an in vivo cytolytic model to investigate the effect of CD200 inhibitor on an anti-tumor response. In these experiments, wild-type or CD200R KO mice underwent intracranial inoculation as described by Olin et al. [35]. Mice were vaccinated with tumor lysates with or without the CD200 inhibitor (Figure 8B). Scrambled inhibitor was used as a control. Twenty days post inoculation, lymphocytes from draining cervical lymph nodes were harvested and incubated with GL261 cells to initiate a tumoricidal response. Two-way ANOVA revealed a statistically significant enhancement of an anti-tumor response by lymphocytes with the addition of the CD200 inhibitor (p = 0.001) (Figure 8B).
Individual analysis by Student’s t-test revealed that tumor lysates in both wild-type and CD200R knockout mice with significantly enhanced anti-tumor responses (p = 0.001 and p = 0.001) at effector:target cell ratios of 25:1 and 50:1, respectively, as compared with the saline treatment group. In addition, the CD200 inhibitor group significantly enhanced anti-tumor responses (p = 0.001, p = 0.001 and p = 0.001) at effector:target cell ratios of 5:1, 25:1 and 50:1, respectively, as compared with the saline treatment group. In wild-type mice, the CD200 inhibitor treatment group exhibited significantly enhanced antitumor responses at effector:target cell ratios of 5:1, 25:1 and 50:1 (p = 0.0001, p = 0.026 and p = 0.003, respectively) as compared with the tumor lysate treatment group. In addition, there was a significantly enhanced anti-tumor response between CD200 inhibitor and CD200 scrambled inhibitor control treatment groups in wild-type mice at effector:target cell ratios of 5:1, 25:1 and 50:1 (p = 0.001, p = 0.0066 and p = 0.0018, respectively). We also observed significantly enhanced anti-tumor responses at effector:target cell ratios 5:1, 25:1 and 50:1 (p = 0.006, p = 0.016 and p = 0.006, respectively) between wild-type and CD200R KO mice in the CD200 inhibitor treatment group. No significant differences were observed between wild-type and CD200R KO mice treated with tumor lysates or the scrambled inhibitor. These experiments demonstrated the ability of our inhibitor to enhance an anti-tumor response when used in conjunction with a tumor-derived vaccines.
CD200 is upregulated on endothelial cells
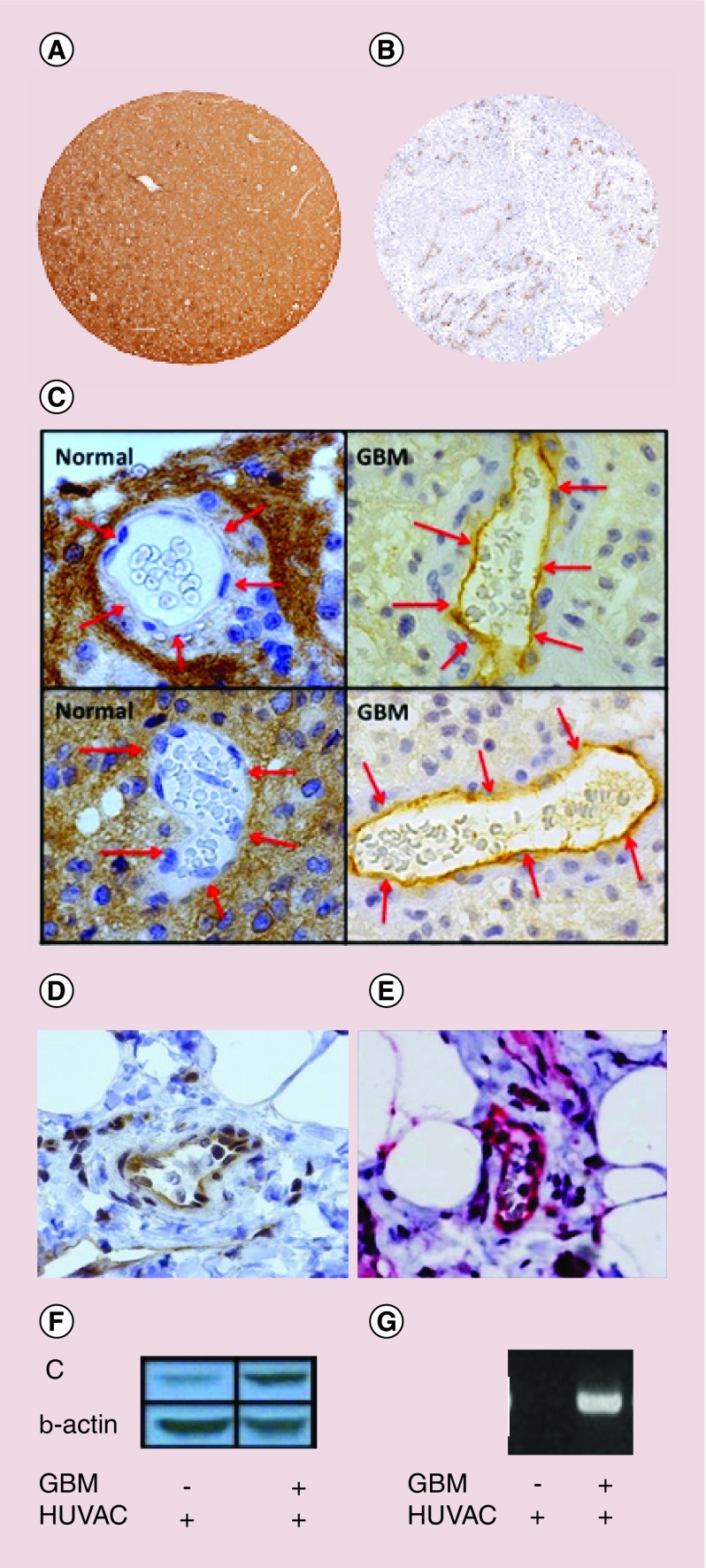
Inhibiting CD200/CD200R interactions has been suggested as a method to enhance immunotherapy [11,36–39]. A clinical trial sponsored by Alexin Pharmaceuticals (NCT00648739) developed a monoclonal anti-CD200 (ALXN6000) to block tumor-derived CD200 expressed on B-cell chronic lymphocytic leukemia and multiple myeloma cells from interacting with CD200R+ lymphocytes (clinicalTrials.gov) [39]. No results have been posted in clinicaltrials.gov. We do not anticipate that this method will be a very efficacious therapy. Twito et al. [40] has demonstrated that ‘A Disintegrin And Metalloprotease’ enzyme (ADAM28) sheds CD200 from B-cell chronic lymphocytic leukemia [40], which would invalidate the use of an antibody to block tumor-driven CD200–CD200R interactions. Our preliminary data correlate with Twito’s findings. We reported high transcription levels of CD200 in GBM [4], however, staining for CD200 protein revealed that, in contrast to normal CNS, GBM have low CD200 expression (Figure 9A & B) potentially due to secretion.
Figure 9. . CD200 is upregulated in vascular endothelial cells.
Tissues isolated from (A) normal human CNS or (B) glioblastoma multiforme (GBM) were analyzed for CD200 expression. (C) Vascular endothelial cells from normal tissue and GBM (D) breast tumor and (E) melanoma cells were analyzed for CD200 expression. Human endothelial cells were expanded on the bottom of a trans-well plate. GBM cells were placed on the top of the plates and incubated for 48 h. HUVAC were washed and analyzed by (F) western analysis and (G) RT-PCR for CD200 transcription.
To validate CD200 protein expression on GBMs, human GBM were analyzed for CD200 expression by western analysis. In contrast to normal CNS tissue, there was low expression of CD200 on the tumors. However, closer examination revealed that GBMs increase expression of CD200 on endothelial cells within the blood–brain barrier (Figure 9C). The same CD200 expression was seen in the vasculature of human breast carcinoma (Figure 9D) and melanoma (Figure 9E). To determine the ability of GBM to upregulate CD200, human endothelial cells (HUVAC) were placed on the bottom of a trans-well plate and human GBM was placed on the top. Following 72-h incubation, HUVAC cells were harvested and analyzed by western immunoblot (Figure 9F) and RT-PCR (Figure 9G) for CD200. These experiments demonstrated that GBM induces CD200+ endothelial cells.
Conclusion
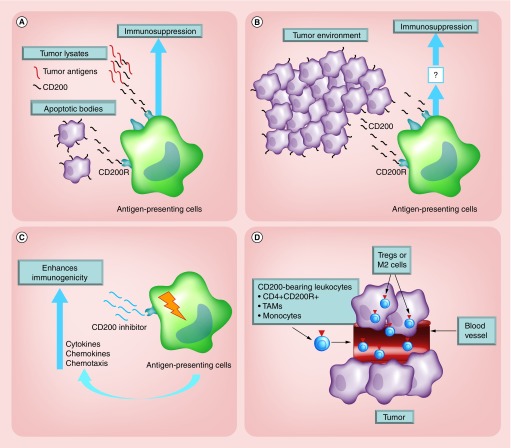
CD200 has been well described as immunosuppressive, making it a logical target for immunotherapy [4,8,11]. We have been extensively interrogating the multiple mechanisms by which CD200 inhibits the development of an antitumor response. We suggest that the CD200 in the tumor-derived vaccines and the CD200 protein secreted from the tumor micro-environment will inhibit the ability of antigen-presenting cells to mount an antitumor response (Figure 10A & B). We also argue that our CD200 peptide inhibitor, through the activation of a CD200 isoform receptor, reverses CD200-induced suppression (Figure 10C).
Figure 10. . Experimental models.
(A) CD200 from tumor lysates or apoptotic bodies used for vaccines binds to the CD200R on antigen presenting cells within the vaccine site inhibiting the development of an antitumor response. (B) CD200 is solubilized from tumors binding to CD200 inhibitory receptors on antigen-presenting cells in the draining lymph nodes inhibiting the development of an immune response. (C) Experimental model demonstrating how the CD200 inhibitor binds to the CD200 isoform activation receptors, over-riding the inhibitory signals of the CD200 protein. (D) CD200+ endothelial cells within the vasculature bind to CD200R+ lymphocytes, differentiating them into suppressor cell populations.
Our model is supported by studies reporting that CD200/CD200R interactions have been characterized as inhibitory receptors [26,41]. CD200R contains tyrosine motifs which signal through the recruitment of DOC2 to distinguish the CD200R from almost all other inhibitory receptors that have immunoreceptor tyrosine-based inhibition motifs [42]. However, additional CD200R-like proteins have recently been identified in mice and humans [13]. Four separate CD200 receptor genes have been identified [13]: CD200R1, CD200R2, CD200R3 and CD200R4 [41,43]. These receptors are predicted to be associated with DNAX activating protein, (DAP)12, known to potentiate and attenuate activation of leukocytes [13,44]. Although the CD200R isoforms have not been well characterized, Gorczynski et al. reported that specific peptide sequences within the CD200 protein act as antagonists. Gorczynski hypothesizes that these peptide sequences bind to one of the CD200R isoforms that normally contribute an activation signal [45].
Our data correlate with Gorczynski’s hypothesis. We suggest that our CD200 inhibitor is targeting one of the activation isoforms of the CD200 receptor. However, CD200 has multiple mechanisms of inducing immune suppression. Following close examination of CD200 immunohistochemistry, we have demonstrated that CD200 is upregulated on vascular endothelial cells (Figure 9C). CD200+ endothelial cells appear to be tumor-specific because the surrounding CNS does not express CD200 in the blood–brain barrier vasculature (data not shown). This is an important discovery because others have reported that tumor-CD200 expression differentiates CD4+CD200R+ cells into a suppressor T-regulatory population (reviewed in [46]) [17]. We suggest that CD200R-bearing leukocytes will interact with CD200+ endothelial cells to differentiate CD4+CD200R+ to regulatory T cells, leading to the development of an immunosuppressive tumor environment (Figure 10C).
Future perspective
Breaking CD200/CD200R interactions intensifies the success of antitumor therapy (reviewed in [46]). We developed a 13 amino acid CD200 peptide inhibitor that, given with tumor lysate, significantly enhances immunogenicity in our glioma model, as well as our breast carcinoma model [4]. We are now focusing our efforts on a mechanism to overcome the suppressive CD200+ endothelial cells (Figure 9C). We are developing a monoclonal anti-CD200R specific for the same epitope as our CD200 inhibitor, which we hope will block the differentiation of immune suppressor cells. We hypothesize that, following T-cell activation, systemic inoculation of the anti-CD200R will bind the CD200R on CD200R+ leukocytes. Our preliminary data suggest that blocking CD200R will allow CD200R leukocytes to enter the tumor micro-environment, escaping differentiation into their suppressive populations.
Executive summary.
Tumor-derived CD200 in vaccines inhibits an anti-tumor response
Tumor-derived vaccines are widely used for solid tumor immunotherapy.
Tumor-derived vaccines contain immunosuppressive proteins.
CD200/CD200R interaction is an immune checkpoint manipulated by tumors and suppressing an immune response, enhancing immune escape.
CD200 inhibitor blocks immune suppression from tumor-derived vaccines
CD200 peptide inhibitor blocks the suppressive effects of CD200 in tumor-derived vaccines.
CD200 inhibitor enhances leukocyte infiltration into the vaccination site.
CD200 inhibitor enhances immunogenicity
Tumor lysate combined with the CD200 inhibitor significantly enhances the development of an anti-tumor response.
CD200 inhibitor activates antigen-presenting cells
CD200 inhibitor acts as an agonist activating antigen-presenting cells, enhancing immune activation.
CD200 is upregulated on vascular endothelial blood vessel cells
Glioblastoma multiforme, breast tumors and melanoma upregulate CD200 on endothelial cells surrounding tumors, enhancing immune escape.
Conclusion
CD200 is a major limitation for the development of an anti-tumor response.
CD200 peptide inhibitor may be used to enhance solid tumor immunotherapy.
Acknowledgements
We thank the following people for their technical assistance and critique of this work: Mark Sanders, Director of the University Imaging Center, for his help with the figures and Ivory Paulk with her help in the laboratory. We also acknowledge a generous gift from Robert and Corinne Ferris.
Footnotes
Financial & competing interests disclosure
This work was supported by the National Cancer Institute R01CA154345 and the Randy Shaver Research and Community Fund. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Olin Mr, Pluhar Ge, Andersen Bm, Shaver R, Waldron NN, Moertel CL. Victory and defeat in the induction of a therapeutic response through vaccine therapy for human and canine brain tumors: a review of the state of the art. Crit. Rev. Immunol. 2014;34(5):388–432. doi: 10.1615/critrevimmunol.2014011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang DY, Sosman JA, Johnson DB. Combination Immunotherapy: an emerging paradigm in cancer therapeutics. Oncology (Williston Park) 2015;29(12):pii: 214815. [PubMed] [Google Scholar]
- 4.Moertel C, Xia J, Larue R, et al. CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J. ImmunoTher. Cancer. 2014;2:46. doi: 10.1186/s40425-014-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsay AG. Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity. Br. J. Haematol. 2013;162(3):313–325. doi: 10.1111/bjh.12380. [DOI] [PubMed] [Google Scholar]
- 6.Moreaux J, Veyrune JL, Reme T, De Vos J, Klein B. CD200: a putative therapeutic target in cancer. Biochem. Biophys. Res. Commun. 2008;366(1):117–122. doi: 10.1016/j.bbrc.2007.11.103. [DOI] [PubMed] [Google Scholar]
- 7.Gorczynski R, Boudakov I, Khatri I. Peptides of CD200 modulate LPS-induced TNF-alpha induction and mortality in vivo . J. Surg. Res. 2008;145(1):87–96. doi: 10.1016/j.jss.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Kretz-Rommel A, Bowdish KS. Rationale for anti-CD200 immunotherapy in B-CLL and other hematologic malignancies: new concepts in blocking immune suppression. Expert Opin. Biol. Ther. 2008;8(1):5–15. doi: 10.1517/14712598.8.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Liao KL, Bai XF, Friedman A. The role of CD200-CD200R in tumor immune evasion. J. Theor. Biol. 2013;328:65–76. doi: 10.1016/j.jtbi.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Gorczynski R, Chen Z, Khatri I, Yu K. sCD200 present in mice receiving cardiac and skin allografts causes immunosuppression in vitro and induces Tregs. Transplantation. 2013;95(3):442–447. doi: 10.1097/TP.0b013e3182754c30. [DOI] [PubMed] [Google Scholar]
- 11.Kretz-Rommel A, Qin F, Dakappagari N, et al. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J. Immunol. 2007;178(9):5595–5605. doi: 10.4049/jimmunol.178.9.5595. [DOI] [PubMed] [Google Scholar]
- 12.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102(2):173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 2003;171(6):3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 14.Rijkers ES, De Ruiter T, Baridi A, Veninga H, Hoek RM, Meyaard L. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol. Immunol. 2008;45(4):1126–1135. doi: 10.1016/j.molimm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Hoek RM, Ruuls SR, Murphy CA, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 16.Caserta S, Nausch N, Sawtell A, et al. Chronic infection drives expression of the inhibitory receptor CD200R, and its ligand CD200, by mouse and human CD4 T cells. PLoS One. 2012;7(4):e35466. doi: 10.1371/journal.pone.0035466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorczynski RM, Lee L, Boudakov I. Augmented Induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation. 2005;79(9):1180–1183. [PubMed] [Google Scholar]
- 18.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 19.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J. Immunol. 2006;176(1):191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- 20.Tonks A, Hills R, White P, et al. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21(3):566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 21.Petermann KB, Rozenberg GI, Zedek D, et al. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J. Clin. Invest. 2007;117(12):3922–3929. doi: 10.1172/JCI32163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol. Immunother. 2008;57(7):987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stumpfova M, Ratner D, Desciak EB, Eliezri YD, Owens DM. The immunosuppressive surface ligand CD200 augments the metastatic capacity of squamous cell carcinoma. Cancer Res. 2010;70(7):2962–2972. doi: 10.1158/0008-5472.CAN-09-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong KK, Brenneman F, Chesney A, Spaner DE, Gorczynski RM. Soluble CD200 is critical to engraft chronic lymphocytic leukemia cells in immunocompromised mice. Cancer Res. 2012;72(19):4931–4943. doi: 10.1158/0008-5472.CAN-12-1390. [DOI] [PubMed] [Google Scholar]
- 25.Akkaya M, Aknin ML, Akkaya B, Barclay AN. Dissection of agonistic and blocking effects of CD200 receptor antibodies. PLoS One. 2013;8(5):e63325. doi: 10.1371/journal.pone.0063325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorczynski RM. CD200 and its receptors as targets for immunoregulation. Curr. Opin. Invest. Drugs. 2005;6(5):483–488. [PubMed] [Google Scholar]
- 27.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. USA. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc. Natl Acad. Sci. USA. 1978;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 30.Soruri A, Zwirner J. Dendritic cells: limited potential in immunotherapy. Int. J. Biochem. Cell Biol. 2005;37(2):241–245. doi: 10.1016/j.biocel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Figdor CG, De Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat. Med. 2004;10(5):475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 32.O’neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104(8):2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 33.Olin M, Low W, Mckenna D, et al. Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induce a CD4+IL17+ response. J. Immunother. Cancer. 2014;2:4. doi: 10.1186/2051-1426-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine. 2015;33(51):7377–7385. doi: 10.1016/j.vaccine.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 35.Olin MR, Andersen BM, Zellmer DM, et al. Superior efficacy of tumor cell vaccines grown in physiologic oxygen. Clin. Cancer Res. 2010;16(19):4800–4808. doi: 10.1158/1078-0432.CCR-10-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorczynski RM, Clark DA, Erin N, Khatri I. Role of CD200 expression in regulation of metastasis of EMT6 tumor cells in mice. Breast Cancer Res. Treat. 2011;130(1):49–60. doi: 10.1007/s10549-010-1259-3. [DOI] [PubMed] [Google Scholar]
- 37.Gorczynski RM, Chen Z, Khatri I, Podnos A, Yu K. Cure of metastatic growth of EMT6 tumor cells in mice following manipulation of CD200:CD200R signaling. Breast Cancer Res. Treat. 2013;142(2):271–282. doi: 10.1007/s10549-013-2735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copland DA, Calder CJ, Raveney BJ, et al. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am. J. Pathol. 2007;171(2):580–588. doi: 10.2353/ajpath.2007.070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rygiel TP, Karnam G, Goverse G, et al. CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene. 2012;31(24):2979–2988. doi: 10.1038/onc.2011.477. [DOI] [PubMed] [Google Scholar]
- 40.Twito T, Chen Z, Khatri I, Wong K, Spaner D, Gorczynski R. Ectodomain shedding of CD200 from the B-CLL cell surface is regulated by ADAM28 expression. Leuk. Res. 2013;37(7):816–821. doi: 10.1016/j.leukres.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Gorczynski R, Chen Z, Kai Y, Lee L, Wong S, Marsden PA. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J. Immunol. 2004;172(12):7744–7749. doi: 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- 42.Hatherley D, Lea SM, Johnson S, Barclay AN. Structures of CD200/CD200 receptor family and implications for topology, regulation, and evolution. Structure. 2013;21(5):820–832. doi: 10.1016/j.str.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voehringer D, Rosen DB, Lanier LL, Locksley RM. CD200 receptor family members represent novel DAP12-associated activating receptors on basophils and mast cells. J. Biol. Chem. 2004;279(52):54117–54123. doi: 10.1074/jbc.M406997200. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat. Rev. Immunol. 2007;7(2):155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 45.Gorczynski R, Khatri I, Lee L, Boudakov I. An interaction between CD200 and monoclonal antibody agonists to CD200R2 in development of dendritic cells that preferentially induce populations of CD4+CD25+ T regulatory cells. J. Immunol. 2008;180(9):5946–5955. doi: 10.4049/jimmunol.180.9.5946. [DOI] [PubMed] [Google Scholar]
- 46.Holmannova D, Kolackova M, Kondelkova K, Kunes P, Krejsek J, Ctirad A. CD200/CD200R paired potent inhibitory molecules regulating immune and inflammatory responses; Part II: CD200/CD200R potential clinical applications. Acta Medica. 2012;55(2):59–65. doi: 10.14712/18059694.2015.56. [DOI] [PubMed] [Google Scholar]