Abstract
Interferon-lambda (IFN-λ) is a new IFN type, related to IFN-α, that is commonly used in the clinic. However, significant side effects accompanying IFN-α treatment limit enthusiasm for IFN-α. In this review, we discuss the current landscape of IFN-α use in oncology and describe the biologic characteristics of IFN-λ. IFN-λ offers unique advantages, including a more tumor cell selective targeting, lower off-target binding and an ability to generate both innate and adaptive immune responses. IFN-λ has also demonstrated therapeutic benefit in murine cancer models. IFN-λ may be used in clinic as a single agent or in combination with other immunotherapy agents, such as immune checkpoint inhibitors. Further clinical trials will be needed to fully elucidate the potential of this novel agent in oncology.
Keywords: : IFN-α/λ combination, IFN-λ, IFN therapy, immune check point inhibitors
Interferon (IFN) is the largest cytokine family with a variety of pleiotropic functions that help shape the innate and adaptive immune response. IFN also mediates several intracellular signaling pathways that promote viral clearance and may be critical for the induction of host antitumor immunity. These functions have been highlighted by the inability to clear foreign pathogens or mediate tumor rejection in mice or patients with aberrant IFN signaling. Despite the importance of IFN in maintaining immune homeostasis, therapeutic applications have generally been limited and, especially in oncology, have had disappointing results. The identification of new IFNs and the availability of new and effective tumor immunotherapy agents have generated renewed interest in IFN and the potential to use these factors in combination immunotherapy regimens. IFN was independently discovered in the 1950s by Isaacs & Lindenmann [1] and Nagano & Kojima [2] through investigation of viral infections and the biology of viral interference. However, several new IFN members were identified more recently [3–5]. Early studies almost 60 years ago showed the induction of a soluble factor by cells infected with influenza viruses. This factor, which induced resistance to secondary viral infections, was called IFN. Currently, the IFNs are subdivided into three major classes: type I, type II and the lately described type III IFN [6–8]. This manuscript will briefly describe the types of IFNs, discuss their role in the treatment of cancer and introduce the more recently isolated IFN-λ with respect to its biology and potential as a cancer therapeutic.
Basic biology of IFNs
Type I IFN
Type I IFN is the largest cytokine subfamily comprising over 20 members. In addition to IFN-β, IFN-κ, IFN-ω and IFN-ε, 13 IFN-α subtypes were described in human. However, a variety of other type I IFNs such as limitin and IFN-τ do not exist in humans [6,9,10]. In humans, the genes that encode type I IFNs are mostly located on chromosome 9. All type I IFNs transduce cell signaling through the IFN-α/β receptor, formed by IFNAR1 and IFNAR2. After ligand and receptor binding, Jak1 and Tyk2 kinases are activated, inducing the formation of the IFN-stimulated gene factor 3 (ISGF3) transcription complex [6,10]. The ISGF3 complex includes activated STAT1/STAT2 and the IFN regulatory factor 9 (IRF9). However, non-STAT pathways involved in type I IFN signaling have also been described [11]. Currently, important efforts are focused on understanding the role of IFN-stimulated genes (ISGs) in controlling viral infections and other diseases [12,13].
Type II IFN
In contrast to type I IFN, only one type II IFN member (IFN-γ) is known. The gene encoding IFN-γ is positioned on chromosome 12 in humans. IFN-γ interacts with its receptor formed by IFNGR1 and IFNGR2, leading to JAK1 and JAK2 activation and subsequent induction of STAT1 dimerization and gene transcription [6,14–16]. However, JAK/STAT-independent signaling pathways have been reported, including the p38 and the phosphatidylinositol 3-kinase pathway cascades [17,18].
Type III IFN
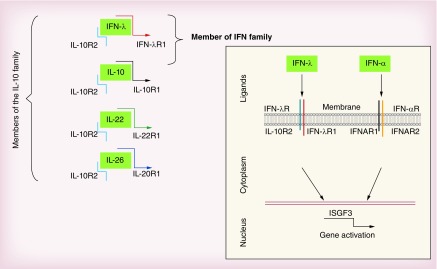
Type III IFN has only recently been discovered and includes four IFN-λ genes in humans which are clustered on chromosome 19 and encode highly homologous IFN-λ1, IFN-λ2, IFN-λ3 and IFN-λ4 proteins [6–8]. In contrast to humans, mice have only two functional genes located on chromosome 7 and encode the functional proteins, IFN-λ2 and IFN-λ3 [7,19]. The murine IFN-λ1 gene orthologue is a pseudogene [19]. However, no mouse IFN-λ4 gene orthologue has been reported. Although interacting with a distinct receptor, the IFN-λ receptor, all the IFN-λs induce similar signaling as type I IFN [3,4,19,20]. Type III IFNs use the specific receptor chain IFN-λR1, and IL-10R2, a receptor chain shared by IL-10 cytokine family members, IL-10, IL-22 and IL-26 [21,22]. For this reason, type III IFN is related to both IFN and IL-10 families (Figure 1). In contrast to IL-10R2, the expression of IFN-λR1 is under control in several cell types including, fibroblasts, adipocytes, endothelial and B cells [7]. Although interacting with unique and a distinct receptor from type I IFN, type III IFNs display quasi-identical cell signaling as seen with type I IFNs. This has been extensively reviewed elsewhere [23–26]. However the preferential expression of IFNLR1 in epithelial cells has driven particular interest in type III IFNs as playing a role in targeting viral clearance and causing disease in epithelial cells [25].
Figure 1. . IFN-λ: a member of two distinct cytokine families.
IFN-λ transduces cell signaling through IL-10R2 and IFN-λR1, the unique and specific receptor chain for all IFN-λs. By using IL-10R2 chain for their signaling, IFN-λs are close members of IL-10 family. However, by sharing similar signaling and activities as IFN-α/β, IFN-λs are also considered as members of the IFN family.
New paradigm of IFN receptor signaling
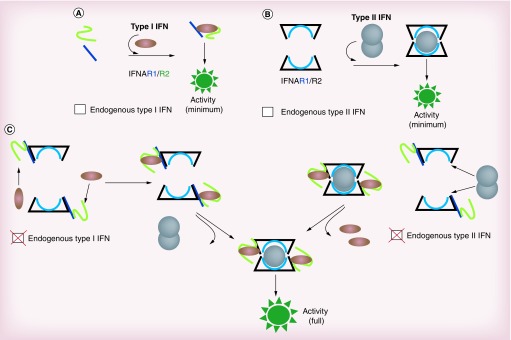
Although type I and II IFNs were extensively investigated, the precise contribution of each ligand and its respective receptor to the IFN response has not been definitively established. By using genomic deletions of each member of the type I and II IFN systems, we have recently demonstrated a critical role for endogenous IFN ligand receptors in the IFN response [27]. By omitting the endogenous IFN ligand receptors, the classical models of IFN receptor signaling underestimate the IFN response [28]. For this reason, we have proposed a new IFN model based on the clustering between the two IFN systems (Figure 2). In contrast to the classical IFN models, the new IFN model sheds lights on the impact of upstream receptor signaling on the IFN response and reveals new avenues for improving the use of IFNs in the clinic. Our new paradigm indicates that local concentration of the complex IFN/receptors rather than the number of receptors at the cell surface is crucial for generating optimal IFN activity. Therefore our paradigm may help design new products that can increase this local concentration and subsequently intensify IFN activity which is needed to improve IFN cancer therapy.
Figure 2. . New IFN receptor paradigm (external view of the cell surface).
(A) Traditional type I IFN system model. Type I IFN receptor is formed by two protein chains, IFNAR1 and IFNAR2. Upon the ligand (IFN-α/β) and receptor binding, cell signaling is triggered. (B) Traditional type II IFN (IFN-γ) system model. Type II IFN receptor is constituted by two protein chains, IFNGR1 and IFNGR2. IFN-γ binds as a dimer to the IFN-γ receptor heterodimer receptor and induces cell signaling. (C) A new IFN receptor paradigm. Constitutively produced IFN-α/β and IFN-γ enable the IFN receptors rearrangement and the induction of cell signaling amplification by the exogenous IFN.
Clinical application of IFNs
IFNs in clinical oncology
Current IFN cancer therapy involves IFN-α, the classical type I IFN tested several decades ago in experimental and clinical models [29–32]. IFN-α has been successfully used in oncology, particularly for hematological malignancies (hairy cell leukemia, chronic myeloid leukemia and follicular lymphoma) as well as some solid tumors (melanoma, renal carcinoma, hepatocellular carcinoma, infantile hemangioma and Kaposi sarcoma).
Hairy cell leukemia (HCL) is a highly treatable disease. IFN-α was successfully used for HCL treatment; however, with the development of more efficient chemotherapeutic agents, the standard treatment for this leukemia is not currently limited to IFN. IFN therapy is used for rare cases of resistance to chemotherapy.
Chronic myelogenous leukemia (CML) is a slowly progressing disease characterized by the presence of the Philadelphia chromosome in the majority of patients. Although current treatment for chronic phase CML is based on tyrosine kinase inhibitors (TKIs), IFN-α is still given to patients with CML who have been previously bone marrow transplanted or who have developed resistance to tyrosine kinase inhibitors (TKIs).
Follicular lymphoma is a hematologic neoplasm characterized by multiple remissions and relapses. IFN-α can often control this disease, and has been used for induction and as maintenance therapy. However, significant toxicities associated with IFN-α treatment coupled with a lack of overall survival benefit were recently reported [33].
In melanoma, the most aggressive skin cancer, clinical trials led to the approval of IFN-α for the adjuvant treatment of Stage II B and III disease. The Eastern Cooperative Oncology Group (ECOG) adjuvant melanoma trial E1684 was a randomized trial that compared 1 year of high-dose IFN-α treatment to observation in patients with Stage II B and III resected melanoma, and demonstrated prolonged relapse-free survival (RFS) and overall survival benefit in patients randomized to treatment with IFN-α [34]. Subsequent larger, randomized studies confirmed the relapse-free survival benefit but overall survival was inconsistently seen across these studies, perhaps due to trial design [35–37]. In these trials, the beneficial effect of IFN-α was optimal when the patients received a 1-year course of high dose therapy. Studies with low doses of IFN-α have not shown significant increase in overall survival in this setting [38–40].
Although IFN-α was approved by the US FDA and adopted by oncologists as the standard of care for the adjuvant therapy of Stage II B and III melanoma, important controversies remain. These include the high cost of drug administration, limited efficacy and adverse constitutional events associated with IFN-α treatment. Recently, the emergence of promising drugs, such as BRAF-targeted therapy and T-cell checkpoint inhibitors have led to intense interest in using these agents in the adjuvant setting. Randomized clinical trials are in progress in Europe and USA to test these agents against placebo, or IFN-α [41].
Before the introduction of the current antiretroviral therapy for Kaposi sarcoma, IFN-α was the first agent approved. Presently, IFN-α is occasionally used, particularly in combination with other anti-HIV drugs [42,43].
In hepatocellular carcinoma (HCC), IFN-α therapy appears to decrease recurrence after ablative therapies [44]. Several studies have demonstrated the suppressive effect of IFN-α in the development of HCC in patients with chronic hepatitis [45–48]. Thus IFN-α therapy for hepatitis B (HBV) and C (HCV) viral infections suppresses carcinogenesis and improves liver function [49–52]. Moreover, IFN-α therapy eliminates HCV mRNA, and reduces the development of HCC in patients with normalized transaminase levels [53]. Additionally, although a complete antitumor response is not achieved, IFN-α therapy suppresses HCC when compared with untreated cases [49,52,54].
Although the impact of IFN-α on survival in patients with advanced renal cell carcinoma is limited, IFN-α is still available alone or in combination with IL-2 and bevacizumab for the treatment of advanced renal cell carcinoma, especially for patients who have failed TKI therapy.
Hemangioma of infancy is one the most frequent tumors in children. Although it is not malignant, ulcerations or complications affecting vital organs can be life threatening in some patients. The standard first-line treatment for hemangioma has traditionally been surgery and corticosteroid therapy. However, the use of IFN-α in the management of hemangioma has achieved good results.
New relevance of type I IFN in tumor immunotherapy
Studies in mice models have shown the importance of host immune mechanisms in type I IFN antitumor response [55,56]. This has been well reviewed by Bellardelli and colleagues [57,58]. More recently, accumulative data from the clinic and preclinical studies indicate new relevance of type I IFN in tumor immunology, particularly in the context of the tumor microenvironment [59,60] and the recent development of T-cell checkpoint inhibitors [61,62].
Potential links between type I IFN gene expression signatures, T-cell infiltration and clinical outcome of cancer patients [63], have suggested new strategies for type I IFNs in tumor immunotherapy [64,65]. Induction of type I IFN by STING (stimulator of IFN genes), a cytosolic DNA sensing factor, suggested the potential role of STING pathway in promoting T-cell immune responses against cancer [66]. Although the impact of STING pathway in innate immune sensing of tumors has been demonstrated [67,68], cancer treatments with STING agonist such as 5,6-dimethylxanthenone-4-acetic acid (DMXAA) did not benefit human patients [69,70]. Apparently, the failure of DMXAA as anticancer agent in patients is attributed in part to its weak interaction with human STING, in contrast to the mouse analog. Current studies utilizing new methods for delivery of STING and STING agonists to the tumor microenvironment are in clinical development [71,72].
To promote the antitumor impact of IFN-α by overcoming tumor-mediated immune suppression, combination of IFN-α with T-cell checkpoint inhibitors is being tested in melanoma patients. Promising preliminary data suggested that such combinations may improve the clinical management of advanced melanoma beyond checkpoint inhibitor monotherapy [61]. An overall response rate of 24% with durable responses and downregulation of host immune suppressor mechanisms has been reported in Phase II trials in which advanced melanoma patients were treated with a combination of IFN-α and the anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) antibody, tremelimumab [73,74]. These studies suggest that the antitumor activity of IFN-α can be improved by inhibiting T-cell checkpoints. This may open new avenues in the interface between IFN therapy and the current development of cancer immunotherapy.
Overcoming the adverse effects of IFN
Since practically all the cells of the body respond to IFN-α, patients can develop numerous side effects during treatment [7,32]. Common adverse reactions include cytopenias (e.g., neutropenia, lymphopenia, thrombocytopenia), gastrointestinal dysfunction (e.g., nausea, vomiting, anorexia) and nervous system effects (e.g., fatigue, depression, suicidal ideation). Lymphopenia results from the myelosuppressive and inhibitory effects of IFN-α on lymphocytes in the periphery. The myelosuppresive effects dissipate once IFN-α treatment ends. Although the mechanisms of lymphopenia have been mostly elucidated [6,7,32], the mechanisms of side effects related to the other systems remain largely unknown. Most patients also experience common flu-like symptoms, especially in the first month of therapy. Although these symptoms usually persist for few hours after IFN-α administration, they often limit the amount of IFN-α, which can be used systemically. Thus, despite the therapeutic potential of IFN-α in cancer treatment, the toxicity profile often prevents completion of treatment and/or requires dose de-escalation. The development of new tools and approaches to overcome these side effects may have an impact on improving the risk/benefit profile of IFN-α therapy.
Development of IFN-λ as antitumor agent: preclinical data
In contrast to other IFN factors, only a few cell types respond to IFN-λ [19]. Interestingly, melanoma cells were among the few that responded well to IFN-λ. For this reason, we studied the potential antitumor activity of IFN-λ in the B16 mouse syngeneic model of melanoma. Due to the restricted cell targeting observed with IFN-λ, we hypothesized that IFN-λ therapy would be associated with therapeutic benefit without the off-target toxicity of IFN-α [7]. First, we engineered B16 cells to constitutively secrete IFN-λ. B16 melanoma cells transduced with IFN-λ showed upregulation of MHC class I at the cell surface. Next, in a tumor transplantation assay, we found that, in contrast to parental B16 melanoma cells, B16 cells constitutively producing IFN-λ were either rejected or grew at slower rates in C57BL/6 syngeneic mice.
To determine the indirect effects of IFN-λ in eliciting the antitumor activity of IFN-λ, we have generated B16 cells, resistant to IFN-λ response (B16.IFN-λRes) [19]. Similarly to IFN-λ-sensitive cells, significant antitumor effects were observed in C57BL/6 syngeneic mice transplanted with B16.IFN-λRes cells. Thus, host mechanisms appear to play a crucial role in prompting the antitumor effects of IFN-λ [19].
The antitumor activity of IFN-λ has been confirmed by independent groups in melanoma and in other tumor models. By using similar gene therapy approaches, Sato et al. demonstrated the antitumor activity of IFN-λ in the B16 mouse melanoma and CT26 colon adenocarcinoma models [75]. In these studies, treatment was associated with cell cycle arrest and apoptosis. An increase in caspase activity with induction of p21 and retinoblastoma protein (Rb) dephosphorylation was observed in cells transfected with IFN-λ2 cDNA [75].
Using similar methods, Numasaki et al. confirmed the antitumor activity of IFN-λ in MCA2005 fibrosarcoma cell tumor model [76]. Since MCA2005 fibrosarcoma cells were resistant to the direct effects of IFN-λ, different host mechanisms were investigated. The authors reported a role for NK cells, T cells and neutrophils in mediating the antitumor activity of IFN-λ. We and other groups have also validated the antitumor role of IFN-λ in other tumor models, including lung [77], hepatoma [78], esophagus [79,80], breast cancer [81], prostate [82] and colon cancer [83].
To mediate antitumor activity, IFN-λ acts directly on tumor cells and through induction of host antitumor immunity (Table 1). Direct antitumor effects of IFN-λ include inhibition of cell proliferation [19,77,84–87] and mitosis [19], while promoting cell apoptosis [75,77,79,80,83] and cell cycle arrest [19,75,80]. However, it seems that direct effects on tumor cells are not the major mechanism by which IFN-λ displays its antitumor activity. Involvement of host immune and anti-angiogenic mechanisms also appears to be important antitumor mechanisms of IFN-λ. It was first reported that IFN-λ played a role in suppressing tumor angiogenesis in the B16 mouse melanoma model by modulating the tumor microenvironment [19]. Durable antitumor immune responses have also been reported for IFN-λ [78]. These responses have largely been associated with induction of T-cell responses, as reported in the antitumor activity of IFN-λ in melanoma [75], fibrosarcoma [76], lung adenocarcinoma [79] and breast cancer [81]. However IFN-λ-induced innate immunity, largely represented by NK-cell induction, was demonstrated in numerous cancer models, including melanoma [75], fibrosarcoma [76], hepatoma [78], lung adenocarcinoma [79] and prostate adenocarcinoma [82]. A controversy about the direct or indirect effects of IFN-λ on NK cells was recently raised [82]. A direct effect of IFN-λ on NK cells has been suggested following the failure of transferred IFN-λR1-deficient NK cells in suppressing tumor growth in vivo [82]. However, no direct evidence has been reported in vitro, in accordance with our initial report [78] and other studies, demonstrating that IFN-λ was not directly acting on NK cells [88,89]. Innate immunity may also be mediated by macrophages and neutrophils, and there is one report suggesting IFN-λ may influence innate immunity against a fibrosarcoma tumor model through these cells [76].
Table 1. . Tumor targeting and mechanisms of action of IFN-λ. As indicated, the antitumor activity of IFN-λ has been demonstrated in several cancer models. Beside directs of IFN-λ on tumor cells, indirect effects resulting mostly from the modulation of innate immunity have been reported.
| Reported anti-tumor mechanisms of IFN-λ | Cancer models studied | Ref. |
|---|---|---|
| Anti-angiogenesis |
Melanoma |
[19] |
| Anti-proliferative | Melanoma | [19] |
| Lung adenocarcinoma | [77] | |
| Neuroendocrine cancer | [84] | |
| T lymphoma | [85] | |
| Colon cancer | [86] | |
| |
Hepatoma |
[87] |
| Cell cycle arrest | Melanoma | [75] |
| Colon cancer | [75] | |
| |
Esophageal carcinoma |
[80] |
| Antimitotic |
Melanoma |
[19] |
| Apoptosis | Melanoma | [75] |
| Colon cancer | [75,83] | |
| Lung adenocarcinoma | [77,79] | |
| |
Esophageal carcinoma |
[80] |
| Tumor microenvironment cells |
Melanoma |
[19] |
| NK cells | Melanoma | [75] |
| Fibrosarcoma | [76] | |
| Hepatoma | [78] | |
| Lung adenocarcinoma | [79] | |
| |
Prostate adenocarcinoma |
[82] |
| T cells | Melanoma | [75] |
| Fibrosarcoma | [76] | |
| Lung adenocarcinoma | [79] | |
| |
Breast cancer |
[81] |
| Neutrophils |
Fibrosarcoma |
[76] |
| Macrophages | Fibrosarcoma | [76] |
Potential clinical development of IFN-λ in oncology
Since the antitumor effects of IFN-λ have been reported in various tumor models by different groups, questions about the use of IFN-λ in clinical oncology continue to be raised. We believe that the beneficial use of IFN-λ as a new antitumor drug should be weighed in the context of the current IFN therapy and the emergence of other antitumor treatments. The first question that should be addressed immediately is: what can IFN-λ add to the current IFN-α-based classical IFN therapy? Is IFN-λ a future alternative to IFN-α, and better tolerated by patients? Based on the simplistic view of shared signaling between IFN-λ and IFN-α, and the restrictive effects of IFN-λ over IFN-α in relation to various cell types, early hypotheses suggest that IFN-λ may offer a potential solution to the issues related to side effects of current IFN-α-based therapy [7,90]. Some clinical achievements have been obtained with IFN-λ in the treatment of chronic hepatitis C patients [91,92]. In contrast to IFN-α, IFN-λ treatment induces more rapid viral suppression with lesser side effects [92]. Neutropenia and thrombocytopenia were uncommon in patients receiving IFN-λ [92], suggesting a potential clinical use of IFN-λ as alternative to IFN-α with hematologic toxicity. However to really determine the role of IFN-λ in oncology, randomized clinical trials are required.
In a comparative study between IFN-α and IFN-λ in a mouse hepatoma model, we demonstrated comparable antitumor activity, mediated through distinct antitumor mechanisms [78]. The combination of IFN-α and IFN-λ significantly improved the outcome of treatment for hepatoma in mice. In contrast to single agent IFN-α therapy, the IFN-α/λ combination therapy completely eradicated tumor growth [7,93]. By using either a gene therapy approach or direct injection of IFN-α and IFN-λ, we have demonstrated that around 50% of mice achieve complete responses [93]. Although the use of IFN-λ as an alternative to IFN-α can be introduced in cases of resistance to IFN-α treatment or intolerable side effects, IFN-λ may be suitable for clinical combination with IFN-α rather than as an alternative, to improve the quality and durability of antitumor response. In particular, combining IFN-λ with IFN-α may allow improved immunomodulatory effects with lower dosages of IFN-α, and reduced side effects. We found that the suppression of tumor growth induced by the IFN-α and IFN-λ in combination with one another was not based on synergistic biological activities between IFN-α and IFN-λ alone. Paradoxically, antagonistic interaction between IFN-α and IFN-λ may occur in some tumor models, in agreement with recent reports showing an upregulation of IFN-λR1 and resistance to IFN-α response [94].
Epithelial carcinomas of various organs appear to be differentially sensitive to IFN-λ [7]. Due to their sensitivity to IFN-λ, carcinomas may be among the most suitable targets for IFN-λ. Presently, IFN-λ presents unique advantages, such as tumor growth inhibition without hematologic toxicity [7,32,95]. In this regard, the advantages of IFN-λ over IFN-α might be useful in enhancing the therapeutic effects of T-cell checkpoint inhibitors, such as anti-CLTA-4, anti-PD-1 or anti-PD-L1 antibodies, which are among the most promising new immunotherapeutic drugs to reach the clinic, and are already in combinatory analysis with IFN-α [73,74]. IFN-λ may concomitantly decrease tumor burden and increase the antitumor immune response without inducing any significant myelosuppression or lymphopenia, which may allow more T cells to reach the tumor microenvironment.
Although we know that INF-λR1 governs IFN-λ functions, the impact of its restricted expression on cancer development remains poor understood. Many normal cell types such as fibroblasts, adipocytes, endothelial and B cells are unresponsiveness to IFN-λ [7,26,96]. However their counterpart transformed cells may gain sensitivity to IFN-λ and increase their aggressiveness. For example multiple myeloma cells which originate from B cells gain IFN-λ responsiveness and surprisingly their proliferation was induced by IFN-λ [97]. Furthermore, high responsiveness of transformed bladder cells to IFN-λ was associated with increased cell migration and invasion [98]. Therefore, although IFN-λ might favor cancer promotion or metastasis of some cancers, targeting IFN-λ axis remains crucial for developing new cancer therapies. Thus, the potential exists for broader application of IFN-λ as agonist or antagonist in clinical oncology, particularly in combination with IFN-α and T-cell checkpoint inhibitors. Further pursuit and understanding the precise mechanisms of IFN-λ in different cancers may be an important avenue in the therapeutic battle against cancer.
Future perspective
The IFN family plays an important role in maintaining immune system homeostasis and has been helpful in treatment of infectious disease and cancer. While IFN-α has been widely used in the clinic, treatment is complicated by relatively high rates of off-target toxicity. However new findings may help manage the adverse effects and increase the efficacy of IFN-α therapy. IFN-λ is a recently identified type III IFN and has already shown promising activity as cytokine therapy against cancer in a variety of murine tumor models. In addition to selective tumor cell targeting, leading to limited side effects, IFN-λ is emerging as an important inducer of innate and possible adaptive immunity. However in particular biological situations, it is not excluded that IFN-λ could act in opposite manner to IFN-α. Further investigations of IFN-λ and its interaction with IFN-α in the tumor microenvironment would be critical in generating new immunotherapies. Although cancer clinical trials have not yet been conducted, we propose that carcinomas in general and melanoma in particular may benefit from IFN-λ, and open new avenues to improve upon IFN therapy alone, and in combination with other immunotherapy agents.
Executive summary.
IFN-λ acts similarly than IFN-α by engaging different IFN receptor
Although structurally distinct, IFN-λ and IFN-α induce a quite similar cell signaling. Distinct receptors for IFN-λ and IFN-α are engaged to induce common IFN-λ/α signaling.
IFN-λ has selective cell targeting
In contrast to the other IFN classes, IFN-λ acts only on some cell types, mostly epithelial like cells.
The pattern of IFN-λ response has valuable advantages for limiting the adverse effects commonly reported with IFN therapy, based on IFN-λ.
IFN-λ exerts potent antitumor activity
In addition to melanoma, IFN-λ showed significant benefits in preclinical studies for several cancer models, including lung cancer and hepatocellular carcinoma.
Despite the potential promise of IFN-λ in cancer, no clinical trials of this agent have yet been conducted in oncology.
Antitumor mechanisms of IFN-λ
Although several studies showed the effects of IFN-λ in inhibiting cell proliferation and promoting cell apoptosis, it seems that IFN-λ exerts its antitumor activity mainly through host mechanisms.
Currently independent investigations demonstrated the crucial role of NK cells in eliciting the antitumor activity of IFN-λ.
IFN-λ & combination therapy
New evidences showed that concerted actions between IFN-λ and IFN-α can be crucial for tumor suppression and eradication, indicating new perspective of IFN therapy.
Because IFN-λ displayed less toxicity on immune cells than IFN-α, this IFN could be a best choice for a potential combination with immune checkpoint inhibitors.
Conclusion
IFN-λ is a new IFN with a high potential for cancer treatment.
Introducing IFN-λ for cancer therapy may open new avenues in oncology and revitalize the clinical use of IFN-α.
IFN-λ can be used as single agent or in combination with IFN-α.
Current studies have shown the potential for IFN-α in combination with immune checkpoint inhibitors modalities, and for these combinatory applications the role of IFN-λ may be particularly important for its limited lymphocytes toxicity.
By specifically acting on restricted cell types, IFN-λ has already revealed how it may be possible to dissect the beneficial from the toxic effects of IFN therapy.
Acknowledgements
We thank Dr Debra Laskin for her continuous support and helpful discussions. We thank Dr Karine Cohen Solal for critical reading and helpful discussions. We thank Dr Sidney Pestka, Dr Sergei Kotenko, Dr Jerome Langer and all collaborators for their direct and indirect support.
Footnotes
Authors’ contributions
All the authors (A Lasfar, H Gogas, A Zloza, HL Kaufman and JM Kirkwood) participated in writing the manuscript and designing the figures. A Lasfar coordinated all the efforts and submitted the manuscript after the approval of all the co-authors.
Financial & competing interests disclosure
We thank the Rutgers Cancer Institute of New Jersey for the financial support. H Gogas: Roche (consultant), Novartis (consultant), BMS (consultant), Amgen (consultant), Merck (consultant), Glaxo (consultant); HL Kaufman: Alkermes (Scientific Advisory Board), Amgen (Scientific Advisory Board, Clinical Trial Funding), BMS (Clinical Trial Funding), EMD Serono [Scientific Advisory Board (noncompensated), Clinical trial Funding], Merck [Scientific Advisory Board, Speaker's Bureau (funds returned to Rutgers University)], Prometheus [Scientific Advisory Board, Clinical Trial funding], Sanofi [Consulting services], Viralytics [Clinical Trial Funding]; John M Kirkwood: BMS (personal fees), Merck (personal fees), GSK (personal fees), Celgene (personal fees), Vical (personal fees), Ziopharm (personal fees). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B. Biol. Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 2.Nagano Y, Kojima Y. [Interference of the inactive vaccinia virus with infection of skin by the active homologous virus] C. R. Seances Soc. Biol. Fil. 1958;152(2):372–374. [PubMed] [Google Scholar]
- 3.Kotenko SV, Gallagher G, Baurin VV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003;4(1):69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]; • Following Mammalian Gene Collection publication by National Institute of Health in this report, along with [4], describes initial identification of human IFN-λs (1.2 and 3) or respectively IL-28A, IL-28B and IL-29.
- 4.Sheppard P, Kindsvogel W, Xu W, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 2003;4(1):63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]; • Following Mammalian Gene Collection publication by the National Institute of Health in this report, along with [3], describes initial identification of human IL-28A, IL-28B and IL-29 or respectively IFN-λs (1.2 and 3).
- 5.Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 2013;45(2):164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 7.Lasfar A, Abushahba W, Balan M, Cohen-Solal KA. Interferon lambda: a new sword in cancer immunotherapy. Clin. Dev. Immunol. 2011;2011:349575. doi: 10.1155/2011/349575. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports the biological and antitumor activities of IFN-λ and highlights common and distinct properties of IFN-λ and IFN-α.
- 8.Kotenko SV. IFN-lambdas. Curr. Opin. Immunol. 2011;23(5):583–590. doi: 10.1016/j.coi.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefevre F, Guillomot M, D'Andrea S, Battegay S, La Bonnardiere C. Interferon-delta: the first member of a novel type I interferon family. Biochimie. 1998;80(8–9):779–788. doi: 10.1016/s0300-9084(99)80030-3. [DOI] [PubMed] [Google Scholar]
- 10.Krause CD, Pestka S. Evolution of the Class 2 cytokines and receptors, and discovery of new friends and relatives. Pharmacol. Ther. 2005;106(3):299–346. doi: 10.1016/j.pharmthera.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 12.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoggins JW. Interferon-stimulated genes: roles in viral pathogenesis. Curr. Opin. Virol. 2014;6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19(21):2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 15.Bromberg JF. Activation of STAT proteins and growth control. Bioessays. 2001;23(2):161–169. doi: 10.1002/1521-1878(200102)23:2<161::AID-BIES1023>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 17.Kaur S, Uddin S, Platanias LC. The PI3’ kinase pathway in interferon signaling. J. Interferon Cytokine Res. 2005;25(12):780–787. doi: 10.1089/jir.2005.25.780. [DOI] [PubMed] [Google Scholar]
- 18.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 19.Lasfar A, Lewis-Antes A, Smirnov SV, et al. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66(8):4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]; •• Reports the characterization of the murine IFN-λ ligand/receptor system and constitutes the first report on the role of IFN-λ in cancer.
- 20.Hamming OJ, Terczynska-Dyla E, Vieyres G, et al. Interferon lambda 4 signals via the IFNlambda receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J. 2013;32(23):3055–3065. doi: 10.1038/emboj.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotenko SV, Langer JA. Full house: 12 receptors for 27 cytokines. Int. Immunopharmacol. 2004;4(5):593–608. doi: 10.1016/j.intimp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 23.Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunol. Rev. 2013;255(1):25–39. doi: 10.1111/imr.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann HH, Schneider WM, Rice CM. Interferons and viruses: an evolutionary arms race of molecular interactions. Trends Immunol. 2015;36(3):124–138. doi: 10.1016/j.it.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazear HM, Nice TJ, Diamond MS. Interferon-lambda: immune functions at barrier surfaces and beyond. Immunity. 2015;43(1):15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports the particular role of IFN-λ in mucosal surfaces and highlights the immune functions of IFN-λ against infections.
- 26.Stiff A, Carson Iii W. Investigations of interferon-lambda for the treatment of cancer. J. Innate Immun. 2015;7(3):243–250. doi: 10.1159/000370113. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Details the findings of different cancer studies investigating the role of IFN-λ as a potential antitumor agent.
- 27.Lasfar A, Cook JR, Cohen Solal KA, et al. Critical role of the endogenous interferon ligand-receptors in type I and type II interferons response. Immunology. 2014;142(3):442–452. doi: 10.1111/imm.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports the importance of endogenous IFN and IFN receptors in IFN response. It also reports a new paradigm for better explaining the IFN response.
- 28.Pestka S. The interferon receptors. Semin. Oncol. 1997;24(3 Suppl. 9):S9-18–S9-40. [PubMed] [Google Scholar]
- 29.Goldstein D, Laszlo J. The role of interferon in cancer therapy: a current perspective. CA Cancer J. Clin. 1988;38(5):258–277. doi: 10.3322/canjclin.38.5.258. [DOI] [PubMed] [Google Scholar]
- 30.Gresser I, Bourali C, Levy JP, Fontaine-Brouty-Boye D, Thomas MT. Increased survival in mice inoculated with tumor cells and treated with interferon preparations. Proc. Natl. Acad. Sci. USA. 1969;63(1):51–57. doi: 10.1073/pnas.63.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proc. Natl. Acad. Sci. USA. 1994;91(4):1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6(1):34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 33.Baldo P, Rupolo M, Compagnoni A, et al. Interferon-alpha for maintenance of follicular lymphoma. Cochrane Database Syst. Rev. 2010;20(1) doi: 10.1002/14651858.CD004629.pub2. CD004629. [DOI] [PubMed] [Google Scholar]
- 34.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin. Cancer Res. 2004;10(5):1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 35.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST. J. Clin. Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 36.Moschos S, Kirkwood JM. Present role and future potential of type I interferons in adjuvant therapy of high-risk operable melanoma. Cytokine Growth Factor Rev. 2007;18(5–6):451–458. doi: 10.1016/j.cytogfr.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Moschos S, Varanasi S, Kirkwood JM. Interferons in the treatment of solid tumors. Cancer Treat. Res. 2005;126:207–241. doi: 10.1007/0-387-24361-5_9. [DOI] [PubMed] [Google Scholar]
- 38.Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin. Oncol. 2002;29(3 Suppl. 7):18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- 39.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C. J. Clin. Oncol. 2000;18(12):2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 40.Kleeberg UR, Suciu S, Brocker EB, et al. Final results of the EORTC 18871/DKG 80ndash; 1 randomised Phase III trial. rIFN-alpha2b versus rIFN-gamma versus ISCADOR M versus observation after surgery in melanoma patients with either high-risk primary (thickness >3 mm) or regional lymph node metastasis. Eur. J. Cancer. 2004;40(3):390–402. doi: 10.1016/j.ejca.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J. Am. Acad. Dermatol. 2013;68(2):313–331. doi: 10.1016/j.jaad.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Safai B, Bason M, Friedman-Birnbaum R, Nisce L. Interferon in the treatment of AIDS-associated Kaposi's sarcoma: the American experience. J. Invest. Dermatol. 1990;95(Suppl. 6):S166–S169. doi: 10.1111/1523-1747.ep12875222. [DOI] [PubMed] [Google Scholar]
- 44.Shimomura S, Ikeda N, Saito M, et al. Long-term interferon therapy after radiofrequency ablation is effective in treating patients with HCV-associated hepatocellular carcinoma. Hepatol. Int. 2010;5(1):559–566. doi: 10.1007/s12072-010-9214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang S, Liu Y, Wang L, Duan C, Liu M. A meta-analysis and systematic review: adjuvant interferon therapy for patients with viral hepatitis-related hepatocellular carcinoma. World J. Surg. Oncol. 2013;11:240. doi: 10.1186/1477-7819-11-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzaferro V, Romito R, Schiavo M, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44(6):1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 47.Saito T, Chiba T, Suzuki E, et al. Effect of previous interferon-based therapy on recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. Int. J. Med. Sci. 2014;11(7):707–712. doi: 10.7150/ijms.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singal AK, Freeman DH, Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2010;32(7):851–858. doi: 10.1111/j.1365-2036.2010.04414.x. [DOI] [PubMed] [Google Scholar]
- 49.Lin SM, Yu ML, Lee CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J. Hepatol. 2007;46(1):45–52. doi: 10.1016/j.jhep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 50.Omata M, Yoshida H, Shiratori Y. Prevention of hepatocellular carcinoma and its recurrence in chronic hepatitis C patients by interferon therapy. Clin. Gastroenterol. Hepatol. 2005;3(10 Suppl. 2):S141–S143. doi: 10.1016/s1542-3565(05)00713-5. [DOI] [PubMed] [Google Scholar]
- 51.Vezali E, Aghemo A, Colombo M. Interferon in the treatment of chronic hepatitis C: a drug caught between past and future. Expert Opin. Biol. Ther. 2011;11(3):301–313. doi: 10.1517/14712598.2011.552906. [DOI] [PubMed] [Google Scholar]
- 52.Yu ML, Lin SM, Chuang WL, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir. Ther. 2006;11(8):985–994. [PubMed] [Google Scholar]
- 53.Ishikawa T. Secondary prevention of recurrence by interferon therapy after ablation therapy for hepatocellular carcinoma in chronic hepatitis C patients. World J. Gastroenterol. 2008;14(40):6140–6144. doi: 10.3748/wjg.14.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishikawa T, Higuchi K, Kubota T, et al. Combination PEG-IFN a-2b/Ribavirin therapy following treatment of hepatitis C virus-associated hepatocellular carcinoma is capable of improving hepatic functional reserve and survival. Hepatogastroenterology. 2012;59(114):529–532. doi: 10.5754/hge10867. [DOI] [PubMed] [Google Scholar]
- 55.Gresser I, Maury C, Carnaud C, De Maeyer E, Maunoury MT, Belardelli F. Anti-tumor effects of interferon in mice injected with interferon-sensitive and interferon-resistant Friend erythroleukemia cells. VIII. Role of the immune system in the inhibition of visceral metastases. Int. J. Cancer. 1990;46(3):468–474. doi: 10.1002/ijc.2910460324. [DOI] [PubMed] [Google Scholar]
- 56.Gresser I, Carnaud C, Maury C, et al. Host humoral and cellular immune mechanisms in the continued suppression of Friend erythroleukemia metastases after interferon alpha/beta treatment in mice. J. Exp. Med. 1991;173(5):1193–1203. doi: 10.1084/jem.173.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):119–134. doi: 10.1016/s1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 58.Rizza P, Moretti F, Capone I, Belardelli F. Role of type I interferon in inducing a protective immune response: perspectives for clinical applications. Cytokine Growth Factor Rev. 2015;26(2):195–201. doi: 10.1016/j.cytogfr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Dunn GP, Bruce AT, Sheehan KC, et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 2005;6(7):722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 60.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rafique I, Kirkwood JM, Tarhini AA. Immune checkpoint blockade and interferon-alpha in melanoma. Semin. Oncol. 2015;42(3):436–447. doi: 10.1053/j.seminoncol.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Highlights the beneficial combination of immune checkpoint blockade and IFN-α in melanoma.
- 62.Yang X, Zhang X, Fu ML, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25(1):37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linsley PS, Speake C, Whalen E, Chaussabel D. Copy number loss of the interferon gene cluster in melanomas is linked to reduced T cell infiltrate and poor patient prognosis. PLoS ONE. 2014;9(10):e109760. doi: 10.1371/journal.pone.0109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34(2):67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the role of IFN-α and STING pathway in immune response against cancer.
- 65.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin. Cancer Res. 2007;13(18 Pt 1):5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 66.Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat. Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Q, Man SM, Gurung P, et al. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J. Immunol. 2014;193(10):4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baguley BC, Ching LM. Immunomodulatory actions of xanthenone anticancer agents. BioDrugs. 1997;8(2):119–127. doi: 10.2165/00063030-199708020-00005. [DOI] [PubMed] [Google Scholar]
- 70.Lara PN, Jr, Douillard JY, Nakagawa K, et al. Randomized Phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011;29(22):2965–2971. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 71.Conlon J, Burdette DL, Sharma S, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 2013;190(10):5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao P, Ascano M, Zillinger T, et al. Structure-function analysis of STING activation by c[G(2’,5’)pA(3’,5’)p] and targeting by antiviral DMXAA. Cell. 2013;154(4):748–762. doi: 10.1016/j.cell.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarhini AA, Butterfield LH, Shuai Y, Gooding WE, Kalinski P, Kirkwood JM. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J. Immunother. 2012a;35(9):702–710. doi: 10.1097/CJI.0b013e318272569b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J. Clin. Oncol. 2012b;30(3):322–328. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J. Immunol. 2006;176(12):7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 76.Numasaki M, Tagawa M, Iwata F, et al. IL-28 elicits antitumor responses against murine fibrosarcoma. J. Immunol. 2007;178(8):5086–5098. doi: 10.4049/jimmunol.178.8.5086. [DOI] [PubMed] [Google Scholar]
- 77.Tezuka Y, Endo S, Matsui A, et al. Potential anti-tumor effect of IFN-lambda2 (IL-28A) against human lung cancer cells. Lung Cancer. 2012;78(3):185–192. doi: 10.1016/j.lungcan.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Abushahba W, Balan M, Castaneda I, et al. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol. Immunother. 2010;59(7):1059–1071. doi: 10.1007/s00262-010-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan Y, Zhang J, Liu Y, et al. Inhibition of lung adenocarcinoma transfected with interleukin 28A recombinant adenovirus (Ad-mIFN-lambda2) in vivo . Cancer Biother. Radiopharm. 2013;28(2):124–130. doi: 10.1089/cbr.2012.1247. [DOI] [PubMed] [Google Scholar]
- 80.Li Q, Kawamura K, Ma G, et al. Interferon-lambda induces G1 phase arrest or apoptosis in oesophageal carcinoma cells and produces anti-tumour effects in combination with anti-cancer agents. Eur. J. Cancer. 2010;46(1):180–190. doi: 10.1016/j.ejca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Burkart C, Arimoto K, Tang T, et al. Usp18 deficient mammary epithelial cells create an antitumour environment driven by hypersensitivity to IFN-lambda and elevated secretion of Cxcl. EMBO Mol. Med. 2013;5(7):967–982. doi: 10.1002/emmm.201201864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Souza-Fonseca-Guimaraes F, Young A, Mittal D, et al. NK cells require IL-28R for optimal in vivo activity. Proc. Natl Acad. Sci. USA. 2015;112(18):E2376–E2384. doi: 10.1073/pnas.1424241112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li W, Lewis-Antes A, Huang J, Balan M, Kotenko SV. Regulation of apoptosis by type III interferons. Cell Prolif. 2008;41(6):960–979. doi: 10.1111/j.1365-2184.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zitzmann K, Brand S, Baehs S, et al. Novel interferon-lambdas induce antiproliferative effects in neuroendocrine tumor cells. Biochem. Biophys. Res. Commun. 2006;344(4):1334–1414. doi: 10.1016/j.bbrc.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 85.Dumoutier L, Tounsi A, Michiels T, et al. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J. Biol. Chem. 2004;279(31):32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 86.Hui X, Chen H, Zhang S, et al. Antitumor activities of recombinant human interferon (IFN)-λ1 in vitro and in xenograft models in vivo for colon cancer. Cancer Lett. 2011;311(2):141–151. doi: 10.1016/j.canlet.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Tian S, Hui X, Fan Z, et al. Suppression of hepatocellular carcinoma proliferation and hepatitis B surface antigen secretion with interferon-λ1 or PEG-interferon-λ. FASEB J. 2014;28(8):3528–3539. doi: 10.1096/fj.14-250704. [DOI] [PubMed] [Google Scholar]
- 88.de Groen RA, Boltjes A, Hou J, et al. IFN-lambda-mediated IL-12 production in macrophages induces IFN-gamma production in human NK cells. Eur. J. Immunol. 2015;45(1):250–259. doi: 10.1002/eji.201444903. [DOI] [PubMed] [Google Scholar]
- 89.Morrison MH, Keane C, Quinn LM, et al. IFNL cytokines do not modulate human or murine NK cell functions. Hum. Immunol. 2014;75(9):996–1000. doi: 10.1016/j.humimm.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 90.Kirkwood JM, Bender C, Agarwala S, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J. Clin. Oncol. 2002;20(17):3703–3718. doi: 10.1200/JCO.2002.03.052. [DOI] [PubMed] [Google Scholar]; • Describes adverse effects of high-dose IFN-α treatment and also addresses the mechanisms and management of toxicities associated with current IFN therapy.
- 91.Muir AJ, Shiffman ML, Zaman A, et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52(3):822–832. doi: 10.1002/hep.23743. [DOI] [PubMed] [Google Scholar]
- 92.Muir AJ, Arora S, Everson G, et al. A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J. Hepatol. 2014;61(6):1238–1246. doi: 10.1016/j.jhep.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 93.Lasfar A, Abushahba W, de la Torre A, Castaneda I, Kotenko SV, Reuhl K. IFN therapy in TIB75 HCC model: combination of IFN-lambda and IFN-alpha induces complete remission. Hepatology. 2008;48(Suppl. 4):191. [Google Scholar]
- 94.Duong FH, Trincucci G, Boldanova T, et al. IFN-lambda receptor 1 expression is induced in chronic hepatitis C and correlates with the IFN-lambda3 genotype and with nonresponsiveness to IFN-alpha therapies. J. Exp. Med. 2014;211(5):857–868. doi: 10.1084/jem.20131557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108(10):3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 96.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo . PLoS Pathog. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novak AJ, Grote DM, Ziesmer SC, Rajkumar V, Doyle SE, Ansell SM. A role for IFN-lambda1 in multiple myeloma B cell growth. Leukemia. 2008;22(12):2240–2246. doi: 10.1038/leu.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports a gain of sensitivity of transformed B cells (multiple myeloma) to IFN-λ and the induction of proapoptotic activity.
- 98.Lee SJ, Lee EJ, Kim SK, et al. Identification of pro-inflammatory cytokines associated with muscle invasive bladder cancer; the roles of IL-5, IL-20, and IL-28A. PLoS ONE. 2012;7(9):e40267. doi: 10.1371/journal.pone.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports that beside its antitumor activity, IFN-λ can promote the migration and invasion of bladder cancer cells.